Abstract
The discovery of a growth hormone receptor antagonist (GHA) was initially established via expression of mutated GH genes in transgenic mice. Following this discovery, development of the compound resulted in a drug termed pegvisomant, which has been approved for use in patients with acromegaly. Pegvisomant treatment in a dose dependent manner results in normalization of IGF-1 levels in most patients. Thus, it is a very efficacious and safe drug. Since the GH/IGF-1 axis has been implicated in the progression of several types of cancers, many have suggested the use of pegvisomant as an anti-cancer therapeutic. In this manuscript, we will review the use of mouse strains that possess elevated or depressed levels of GH action for unraveling many of GH actions. Additionally, we will describe experiments in which the GHA was discovered, review results of pegvisomant’s preclinical and clinical trials, and provide data suggesting pegvisomant’s therapeutic value in selected types of cancer.
Keywords: Growth hormone receptor antagonist (GHA), GHA, Somavert, pegvisomont, GHR−/− mice, FaGHRKO, cancer
1. Introduction
Although definitions differ, translational research typically refers to basic science research that is effectively “translated” into clinical benefits. This review describes several decades of research at the “bench” in the area of growth hormone (GH). Much of the bench discoveries originated through the study of several strains of genetically engineered mice with altered GH induced intracellular signaling. Analysis of these mice has led to a better understanding of the basic function of GH as well as its fundamental and tissue-specific role in various physiological processes. The discovery of the GH receptor antagonist (GHA) originated in mice, which resulted in a drug Somavert® (pegvisomant for injection) for the treatment of patients with acromegaly. In this review, we will summarize the general phenotypes of select mouse lines that have extreme alterations in GH action and will highlight data referring to cancer using these mice. An overview of the basic phenotypes of these mice is provided in Table 1 and pictures of the mice are shown in Figures 1 and 2. We will also compare the general features of these mice to cognate clinical populations. Finally, we will conclude by describing how pegvisomant may have applications directed toward several types of cancer.
Table 1.
Phenotypic comparison of genetically modified mice produced in our laboratory with alterd activity of GH
| Mouse Model | bGHa | GHR−/−b | GHAc | FaGHRKOd |
|---|---|---|---|---|
| Level of GH signaling | ↑↑ | none | ↓ | ↓fat only |
| Growth/Body Weight | ↑↑ | ↓↓ | ↓ | ↑↑ |
| Body Composition (Adult) | ||||
| % Lean Mass | ↑↑ | ↓↓ | ↓ | ↔ |
| % Fat Mass | ↓↓ | ↑ | ↑↑ | ↑↑ |
| GH/IGF-1 Axis | ||||
| GH | ↑↑ | ↑ | ↑ | ↔ |
| IGF-1 | ↑↑ | ↓↓ | ↓ | ↑/↔ |
| IGFBP-3 | ↑ | ↓ | ↓ | ↔ |
| Plasma levels | ||||
| Glucose | ↑/↔ | ↓ | ↔ | ↔ |
| Insulin | ↑ | ↓↓ | ↔ | ↔ |
| Leptin | ↓ | ↑ | ↑↑ | ↔/↑ |
| Adiponectin | ↓ | ↑↑ | ↑ | ↓/↔ |
| Reproduction | ||||
| Sexual Maturity (Time) | ↓↓ | ↑ | n.d. | n.d. |
| Fertility | ↓ | n.d. | n.d. | |
| Morbidity | ||||
| Tumor Incidence | ↑ | ↓↓ | n.d. | n.d. |
| Cardiac/Vascular | ↑ | ↔ | n.d. | n.d. |
| Deficits | ||||
| Kidney Impairments | ↑ | ↓ | ↓ | n.d. |
| Lifespan | ↓ | ↑ | ↔ | n.d. |
n.d. = not determined
(Bartke et al., 1998; Berryman et al., 2004; Berryman et al., 2006; Cecim et al., 1995; Lubbers et al., 2013; Olsson et al., 2003; Olsson et al., 2005; Palmer et al., 2009; Palmiter et al., 1982; Palmiter et al., 1983)
as reviewed in (List et al., 2011)
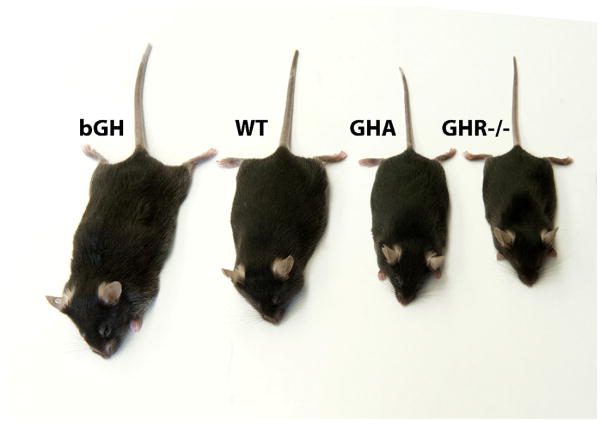
Fig. 1. Photo of mice with altered GH action in the same genetic background (C57BL/6J).
A giant bGH transgenic mouse (mouse on left), wild-type mouse (2nd mouse from the left), a dwarf GHA transgenic mouse (3rd mouse from the left) and a dwarf GHR−/− mouse (4th mouse from the left). These mice represent elevated, normal, decreased and absent levels of GH action, respectively.
Fig. 2. Photo of mice with removal of GHR specifically in adipose tissue.

A wild-type mouse is shown on the left and a FaGHRKO mouse is shown on the right. FaGHRKO mice are obese demonstrating a direct action of GH on adipose tissue (List et al., 2013).
2. GH excess
2.1. Genetically engineered mice with excess GH
Over thirty years ago, a transgenic mouse that expressed rat GH was first described (Palmiter et al., 1982). In subsequent years, multiple research groups have generated and characterized various species of GH transgenes in mice (e.g. ovine GH, human GH, bovine GH). While GH transgene expression results in a fairly uniform phenotype, there are some differences among the various transgenic lines that are likely due to diverse levels of GH expression as well as the species of the transgene. Regarding the latter point, human GH can bind to both GH and prolactin receptors whereas rat, bovine and ovine GH bind exclusively to the GH receptor. Thus, overexpression of human GH in mice results in a physiological state in which both GH receptors and prolactin receptors are simultaneously activated whereas rat, bovine, or ovine GH transgene expression elucidate the specific role of GH action.
GH transgenic mice have a phenotype that resembles the conditions found in acromegaly and will be more fully outlined in the subsequent section. As expected GH transgenic mice have elevated plasma levels of GH and insulin like growth factor-1 (IGF-1) (Kopchick et al., 1999). These mice also exhibit accelerated somatic growth starting around three weeks of age and remain larger than littermate controls through their adult life (Kaps et al., 1999; Knapp et al., 1994; Palmer et al., 2009; Palmiter et al., 1982) (Figure 1) The extreme elevation in circulating GH also results in hyperinsulinemia despite euglycemia (Kopchick et al., 1999; Palmer et al., 2009) that is likely due to GH’s diabetogenic effect. Reports detailing body composition of GH transgenic mice are inconsistent, but the discrepancy appears to be primarily due to the age of the mice when examined. Data in young GH transgenic mice are the most conflicting with male (≤ 3 months of age) and female mice (≤ 6 months of age) showing increases in fat mass in some studies (Eckstein et al., 2002; Kaps et al., 1999; Palmer et al., 2009; Pomp et al., 1996) and decreases in fat mass in others (Pomp et al., 1996). Again, the level or species of transgene expression may contribute to these discordant findings. Regardless, in adulthood (older than 4–6 months of age in males and > 6 months of age in females), GH transgenic mice are consistently reported to be lean with a significant reduction in the mass of all adipose depots (Berryman et al., 2004; Palmer et al., 2009). Transgenic mice also have altered adipokine and cytokine levels with reduced leptin and adiponectin (total as well as high molecular weight forms) levels as well as increased resistin in the circulation and TNF-α, and IL-6 levels in adipose tissue as compared to control mice (Berryman et al., 2004; Lubbers et al., 2012; Wang et al., 2007). The importance of GH in nutrient partitioning is highlighted by studies in which diet is altered in these mice. That is, GH transgenic mice fed a high fat diet are resistant to diet-induced obesity and gain substantial lean mass with the additional calorie intake (Berryman et al., 2006; Olsson et al., 2005). In contrast, calorie restriction improves their insulin levels and alters lipid metabolism (Wang et al., 2007). Importantly, many of the reports, such as data generated for body composition (Palmer et al., 2009), show gender differences corresponding to transgene expression with females having a less dramatic phenotype than male transgenic animals.
GH transgenic mice show signs of premature aging and have drastically reduced (~50%) lifespans compared to littermate controls (Bartke, 2003; Wolf et al., 1993). The cause of their premature death is multifactorial. Tissue-specific pathological organ damage has been noted as a result of elevated GH levels. Examples include glomerulosclerosis and triglyceride deposition in the kidney, resulting in marked kidney damage (Doi et al., 1991; Kumar et al., 2011). There is also evidence of impaired cardiac and vascular function (Bohlooly et al., 2005; Bollano et al., 2000; Izzard et al., 2009; Miquet et al., 2011) as well as lipid abnormalities (Frick et al., 2001). Moreover, most GH sensitive tissues are enlarged in proportion to the larger size of the mouse, but several tissues, such as liver and spleen, experience disproportionate increases in size, which likely influences organ function (Liu et al., 1995; Machado et al., 2005; Orian et al., 1989; Quaife et al., 1989; Shea et al., 1987; Yang et al., 1993a; Yang et al., 1993b). Finally, GH transgenic mice have greater incidence of tumors. For example, hGH transgenic mice have early onset of mammary tumors (Cecim et al., 1994) although the role of prolactin versus GH signaling in these mice has not been resolved. Further, the hepatomegaly in these transgenic mice is linked to hypertrophy and hyperplasia of hepatocytes, which appears to contribute to an increase the frequency of tumors and dysregulation of serveral oncogenic pathways in this tissue (Miquet et al., 2013; Quaife et al., 1989). The chronic expression of IGF-1 results in a different phenotype, suggesting that the elevation of GH has a direct impact on several of the pathologies seen in GH transgenic mice (Quaife et al., 1989).
2.2. Excess GH in humans (acromegaly/gigantism)
Acromegaly is a hormonal disorder characterized by chronically elevated levels of circulating GH resulting in subsequent elevated levels of IGF-1. Typically, the condition is caused by a GH-secreting pituitary adenoma. As such, the treatment of acromegaly usually aims at removing the tumor with subsequent control of tumor growth as well as normalizing GH and IGF-1 levels. If GH and IGF-1 are in excess during childhood prior to closure of the epiphyseal growth plate and left untreated, then excessvie growth or gigantism results. GH excess occurring in in adulthood results in enlargement of extremities along with thickening of the skin, insulin resistance, sleep apenea, and cardiac abnomalities. The condition results in significant morbidity and increased mortality, as reviewed (Melmed, 2009; Melmed et al., 2009; Sherlock et al., 2011). The clinical presentation of these conditions share many characteristics with GH transgenic mice, including elevations in the activity of the GH/IGF-1 axis, increased lean mass and reduced fat mass, impaired glucose homeostasis and increased incidence of select cancers. However, there are important distinctions. First, the cause of chronic excess of GH in acromegaly, as stated above, is usually due to autonomous GH secretion from a pituitary adenoma whereas, in GH transgenic mice, the GH excess is due to transgene insertion resulting in ectopic GH secretion. Second, the level of circulating GH is often markedly greater in transgenic mice than what is observed in acromegaly. Finally, the transgenic mice have excessive GH gene expression for most of their postnatal life and, thus, resemble the excessive somatic growth associated with acromegalic-induced gigantism. Regardless, GH transgenic mice provide a valuable means to evaluate the impact of GH excess on specific tissues and organ systems.
3. GH resistance
3.1. Genetically engineered mice with disruption of the GHR gene
In attempts to better understand the numerous in vivo actions of GH, our laboratory generated the GH receptor gene disrupted mouse (GHR−/−) in the mid 1990’s (Zhou et al., 1997) (Figure 1). They also are termed global GHR−/− mice. Since these mice are completely resistant to GH action, the unique phenotype of these mice provides key evidence as to the actions of GH. Thus, they have been and continue to be used to study the various activities of GH. In fact, since their creation, this mouse has been used in over 100 published studies (List et al., 2011).
As might be expected, GHR−/− mice have an overall phenotype that is opposite to that of the GH transgenic mice previously described. They have a pronounced decrease in body size with extremely low circulating IGF-1 despite elevated GH levels (Zhou et al., 1997). Organ weights are proportionally decreased with the exception of liver and kidney, which are disproportionally smaller, and brain and select adipose depots, which are disproportionally larger (Berryman et al., 2010; Coschigano et al., 2003a). In addition to the obvious effects on body size, GHR−/− mice have many other unique attributes that highlight the numerous roles of GH on metabolism (List et al., 2011). For example, GHR−/− mice are exceptionally insulin sensitive with low levels of circulating insulin (Al-Regaiey et al., 2005; Bartke et al., 2004; Berryman et al., 2006; Bonkowski et al., 2009; Coschigano et al., 2003a; Dominici et al., 2000; Egecioglu et al., 2006; Hauck et al., 2001; Liu et al., 2004; Panici et al., 2009). Fasting blood glucose levels are decreased at younger ages in GHR−/− mice compared to controls but tend to normalize at advanced ages (Al-Regaiey et al., 2005; Liu et al., 2004). Also, GHR−/− mice are obese with a lifelong increase in percent body fat (Berryman et al., 2004; Berryman et al., 2006; Berryman et al., 2010). Consistent with their increased adiposity, GHR−/− mice have elevated levels of leptin (Al-Regaiey et al., 2005; Berryman et al., 2004; Egecioglu et al., 2006). Adiponectin levels (both total and high molecular weight forms) also are increased in GHR−/− mice, which is counterintuitive since adiponectin levels are usually negatively correlated with increased adiposity (Al-Regaiey et al., 2005; Berryman et al., 2004; Lubbers et al., 2012; Nilsson et al., 2005). However, since adiponectin is also positively associated with improved insulin sensitivity, this adipokine appears to be more highly correlated with insulin sensitivity in a GH resistant state. Another trait of the GHR−/− mice that may in part explain the “healthy obesity” is that the adipose tissue is not uniformly increased, but rather almost exclusively increased in the subcutaneous white adipose tissue (WAT) depot (Berryman et al., 2004; Berryman et al., 2010). The concept that subcutaneous WAT might be healthier than other WAT depots has been supported by a number of studies and has been recently reviewed (Lee et al., 2013). Perhaps, the most striking observation from the GHR−/− mice is that they are extremely long-lived (Coschigano et al., 2003a). In fact, GHR−/− mice are officially recognized as the world’s longest-lived laboratory mouse (http://www.methuselahfoundation.org/). The precise mechanisms involved in lifespan extension seen in GHR−/− mice are the focus of many laboratories (including our own) in the aging field. However, reduction in neoplastic diseases are a major factor as 83% of WT mice die from neoplastic disease compared to 42% (49% reduction) of the GHR−/− mice (Ikeno et al., 2009), as more fully described in the subsequent section on pegvisomant and cancer. In addition to decreased cancer, GHR−/− mice also appear to be protected from diabetes (type 1 and 2). For example, streptozotocin-induced type 1 diabetes induction results in glomerulosclerosis, increases in glomerular volume, and increases in the ratio of mesangial area to total glomerular area in wild-type mice while GHR−/− mice show none of these pathological changes (Bellush et al., 2000). Futhermore, GHR−/− mice on a high-fat diet have lower levels of insulin and glucose compared to WT mice on a high-fat diet despite a larger increase in adiposity (Berryman et al., 2006).
In attempts to further understand the health benefits of the GHR−/− mice, our laboratory and others have recently generated and characterized various tissue-specific GHR gene disrupted mice using the Cre-LoxP system. Deletion of GHR in liver results in decreased circulating IGF-1, elevated GH, insulin resistance and hepatic steatosis (Fan et al., 2009). Two separate groups have deleted GHR in skeletal muscle and have reported different results. Using the Mef-2c promoter/enhancer to drive Cre recombinase expression, Mavalli and colleagues (Mavalli et al., 2010) observed peripheral adiposity and impaired glucose metabolism. However, when the muscle creatine kinase (MCK) promoter/enhancer is used, Vijayakumar and colleagues (Vijayakumar et al., 2012) reported reduced adiposity and improved glucose metabolism. Tissue specific disruption of GHR in pancreatic β-cell results in impaired insulin secretion (Wu et al., 2011). In our laboratory, we generated fat specific GHR disruption (FaGHRKO mice), which have a unique phenotype (List et al., 2013) (Figure 2). They have a normal GH/IGF-1 axis and glucose homeostasis, but are severely obese. Furthermore, FaGHRKO mice do not have increased adiponectin levels which are seen in global GHR−/− mice, suggesting that GH does not act directly on adipose tissue to regulate adiponectin as previously suggested (List et al., 2013). Thus, the removal of GHR in specific tissues allows researchers to better evaluate organ-specific actions of GH. Collectively, these results demonstrate that disruption of GHR in specific tissues can dramatically influence glucose homeostasis and adiposity. Nonetheless, no single tissue specific deletion of the GHR results in an overall phenotype that resembles those of GHR−/− mice and implies some level of important tissue crosstalk for the unique phenotype observed in these global GHR−/− mice. Finally, the incidence and progression of neoplastic disease has not been reported in any of these GHR tissue specific gene disrupted mice.
3.2. Disruption of GHR in humans (Laron Syndrome)
Laron syndrome (LS; also known as GH insensitivity or resistance syndrome) is a genetic disorder most commonly caused by mutations in the GHR gene; however, mutations in other genes in the GH/GHR induced signaling pathway have also been described (Hwa et al., 2007; Hwa et al., 2011; Laron et al., 1993; Rosenfeld et al., 2007). Thus, the GHR−/− mouse generated in our laboratory serves as an excellent model of LS in humans and a thorough comparison has been the subject of a recent book (Laron and Kopchick, 2011). GHR−/− mice possess many similar traits to the human condition. These similarities include dwarfism, decreased IGF-1, increased GH, GH resistance, delayed sexual development, decreased muscle mass, decreased bone mineral density, decreased cranial bone size, hypoglycemia at younger ages, increased adiposity with greater increases in subcutaneous WAT, increased leptin and adiponectin levels and astonishingly, a resistance to diabetes and cancer (Bellush et al., 2000; Guevara-Aguirre et al., 2011; Ikeno et al., 2009; Laron and Kopchick, 2011).
There are a few potential differences between individuals with LS and GHR−/− mice regarding glucose metabolism and longevity. These differences appear to depend on the particular population of LS individuals evaluated. Laron and colleagues suggest that LS individuals tend to develop hyperinsulinemia at advanced ages (Laron and Kopchick, 2011) while GHR−/− mice have low levels of insulin throughout life. This difference may partially explain the discrepency in lifespan. As stated earlier, GHR−/− mice are extremely long-lived, while individuals with LS appear to have normal lifespans (Kopchick and Laron, 2011). Unfortunately, longevity studies in individuals with LS have limitations as this syndrome is extremely rare, making it difficult to study, and individuals with LS have a higher incidence of accidental and alcohol related deaths (Guevara-Aguirre et al., 2011). However, a recent study, which reports on a group of LS individuals from Ecuador, which have been studied for over 22 years, shows that individuals with LS have dramatic reduction in insulin levels, improved insulin sensitivity, with significantly decreased HOMA-IR scores, and, as stated above, have no cases of cancer or diabetes (Guevara-Aguirre et al., 2011). Thus, it appears that humans with LS actually are indeed resistant to diabetes and cancer similar to the GHR−/− mice.
4. GH Receptor Antagonist (GHA)
4.1. Development of the GHA
In 1991, our laboratory first described mice that express a GHA transgene (Chen et al., 1991a; Chen et al., 1991b; Chen et al., 1991c). The GHA transgene was a mutated bovine (b) GH gene in which the codon for glycine at position 119 was replaced with a larger amino acid, namely arginine, as described in detail below. Expression of the mutated GH transgene decreased GH action, resulting in dwarf mice (Figure 1), and was subsequently determined to be a classic receptor antagonist (Okada et al., 1992).
The fortuitous discovery of GHA is worthy of discussion as it not only has provided a better understanding of how GH interacts with its receptor but also has provided a novel mouse strain to assess the impact of a reduction in GH action in an animal system. However, most importantly, the discovery and characterization of GHA resulted in the development of an innovative drug, pegvisomant, used for the treatment of patients with acromegaly. This subject will be more thoroughly described later in this review. The GHA and resulting mice helped unravel several basic features of the GH/GHR interaction. For example, GHA helped to establish that the stoichometry of GH:GHR is 1:2 (Cunningham et al., 1991; de Vos et al., 1992) and that two distinct regions of a single GH molecule interact with one receptor homodimer. At the time, one of the regions in GH responsible for binding to the GHR, referred to as site 1, had already been identified and shown to have a high affinity for the GHR (Bass et al., 1991; Cunningham et al., 1989; Cunningham et al., 1991; Cunningham and Wells, 1989; Fuh et al., 1992; Pearce et al., 1996). However, the second GH site remained obscure despite some hint that the region responsible for binding at Site 2 might be located in helix 3 since this region had previously been implicated in bone growth (Hara et al., 1978). A thorough assessment of the amino acids within helix 3 revealed that this helix had an ‘imperfect’ amphipathic configuration due to three amino acids. We hypothesized that engineering a perfect amphipathic configuration for helix 3 might result in greater growth promoting activity. Thus, we engineered a mutated bGH gene in which the three amino acid were converted to amino acids that would result in a perfect amphipathic helix (Q117L, G119R and A122D). Surprisingly, instead of augmenting GH’s growth-promoting activity, the perfect amphipathic amino acid substitutions in helix 3 antagonized the function of GH in vitro and in vivo (Chen et al., 1991a; Chen et al., 1991b; Chen et al., 1990). Subsequently, only the G119R substitution was shown to be responsible for the GH antagonistic effect (Chen et al., 1991c). The mutation of the glycine codon in this helix allows the GHA site 1 to bind properly to the preformed GHR dimer on the surface of target tissues, but prevents proper or functional binding at GH’s Site 2. This improper interaction fails to induce intracellular GHR signaling (Chen et al., 1991a; Chen et al., 1991c; Chen et al., 1990). Thus, GHA acts as a competitive inhibitor of endogenous GH or a classic receptor antagonist. For human GH, additional work revealed that similar antagonist effects could be achieved by substituting the corresponding glycine with lysine at position 120 (Chen et al., 1994). The study of GHA transgene expression in mice (GHA mice) has been helpful in understanding not only the potential impact of GH deficiency but also provides the foundational work for developing the GHA into a pharmaceutical agent.
4.2. Genetically engineered mice expressing the GHA transgene
The phenotype of GHA mice is generally intermediate between that of WT and GHR−/− mice. For example, GHA mice are dwarf (Chen et al., 1991a; Chen et al., 1991c; Chen et al., 1990), but not as dramatic as seen in GHR−/− mice (Coschigano et al., 2003a). Interestingly, while notably dwarf in early life, the body weight of male GHA mice gradually catches up to that of control mice (Berryman et al., 2013; Coschigano et al., 2003a). However, the increase in body weight in later adult life is due to marked increases in adiposity and not due to an increase in body length or lean tissue (Berryman et al., 2013). Like GHR−/− mice, GHA mice exhibit a disproportionate increase in the subcutaneous fat depot (Berryman et al., 2004; Yakar et al., 2004) although other fat pads are also significantly increased in older GHA mice as compared to controls (Berryman et al., 2013).
Circulating levels of several factors have been reported in these mice. Serum IGF-1 and IGFBP-3 are reduced by ~80% and 30%, respectively compared to controls (Coschigano et al., 2003a). Glucose homeostasis is moderately improved in young mice with low to normal plasma levels of glucose and insulin (Coschigano et al., 2003a; Yakar et al., 2004). However, there is evidence of increasing insulin levels with advancing age in male GHA mice (Berryman et al., 2013; Coschigano et al., 2003a). Finally, adipokine levels are dramatically alterd in GHA mice. Like GHR−/− mice, total and high molecular weight adiponectin levels as well as leptin levels are significantly increased (Berryman et al., 2004; Berryman et al., 2013; Lubbers et al., 2013; Yakar et al., 2004). Notably, the leptin levels surge in later life (by 18 months) in GHA mice reaching levels ~5–7 fold higher than littermate controls (Berryman et al., 2013), which does not appear to occur in GHR−/− mice (unpublished results). The relevance of this increase in the context of relatively consistent production of other adipokines is a subject of current investigation. Regardless, these collective data reveal an important effect of age on the overall phenotype of GHA mice.
Only one study has reported lifespan data for GHA mice (Coschigano et al., 2003a). Although this study reported a propensity for GHA mice to live longer than littermate controls, especially for females, the difference was not significant. Noteworthy is the fact that GHA mice are an exception to other mouse strains with decreases in GH action that experience major improvements in lifespan, such as the aforementioned GHR−/− mice. However, it should also be mentioned that GHA mice, with their severe obesity especially with advancing age (Berryman et al., 2013), evidently do not live shorter than littermate controls. Thus, expression of the antagonist appears to confer some resilency to the morbidities that are commonly associated with an obese state. Regardless, GHA mice are valuable for lifespan studies as they offer both an exception to the dogma that decreases in GH signaling increases lifespan and they defy the common pathologies that accompany obesity. Moreover, GHA mice could be considered a more clinically relevant mouse line than GHR−/− mice; afterall, repression of GH action is pharmacologically achievable with the use of a GHA (Somavert®, pegvisomant for injection) whereas total repression of GH action, as in GHR−/− mice, is not and would not be clinically desirable.
5. Pegvisomant
5.1. Development of pegvisomant
The discovery of the GHA via the generation of the GHA transgenic mouse and confirmation in vitro led to the exciting speculation as to whether GHA could be used in clinical settings where a decrease in GH action was desirable. The obvious initial indication would be one where there are elevated serum GH levels, such as that found in patients with acromegaly. For a GHA to be effective, it would have to be present in serum at much higher levels than endogenous GH in order to effectively block the GHR. This posed a major problem in that the serum half-life of GH and the GHA is approximately 15 minutes (Veldhuis et al., 2002), and endogenous GH is released in a pulsatile manner thoughout the day. Therefore, the half-life of GHA would have to be greatly extended to continually block endogenous GH signaling. Since the serum half-life of GH was known to increase via conjugation of 5-kDa polyethylene glycol (pegylation, PEG) moieties (Clark et al., 1996), the same modification was investigated as a method to improve the pharmacokinetics of GHA. PEG binds to the primary amine groups in lysine residues as well as the N-terminus of proteins, thus increasing the size of the protein and reducing its clearance from serum. However, it was critical to avoid steric hindrance that could be caused by the addition of PEG molecules to lysine residues in regions of the protein that are important for GHR binding. In the case of GHA, there were two lysine residues at positions 168 and 172 that are critical for binding of site 1 to the GHR.. In order to preserve binding affinity of PEG-G120K, these residues were mutated to K168A and K172R so as to prevent pegylation at these sites. Other modifications were made to increase PEG-G120K’s binding affinity and to increase the potency of the drug. Six mutations resulting in the following amino acid substitutions, H18D, H21N, R167N, D171S, E174S and I179T, were introduced into that molecule and had previously been shown to increase the binding affinity at site 1 (Cunningham et al., 1991; Pradhananga et al., 2002). These site 1 amino acid substitutions also abolish the ability of the molecule to bind to the prolactin receptor (Goffin et al., 1999), thus reducing off-target pharmacologic effects.
The resulting recombinant protein, pegvisomant (Somavert® pegvisomant for injection), consists of a G120K molecule that is conjugated to PEG- at 4–5 sites and contains the above-mentioned 8 amino acid changes (Figure 3). Pegylation increases the serum half-life to approximately 100 hours (Muller et al., 2004) and acts as an effective GHA despite a 20-fold reduced affinity for the GH receptor due to the pegylation (Pradhananga et al., 2002).
Fig. 3. Pegvisomant – the amino acid sequence.
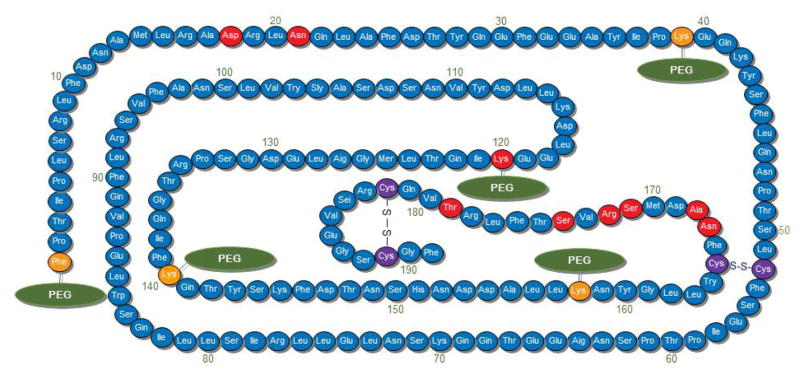
Five pegylation sites are indicated with a green PEG notation. The corresponding Lys residues are depicted in orange (except the Lys at position 120 which is shown in red). Other amino acid changes found in pegvisomant that differ from wild type hGH are displayed in red. Importantly, Lys at postion 120 replaces Gly. It is this crucial amino acid substitution that generates the GHA.
5.2. Characterization of pegvisomant in vivo
In mice, very high doses of pegvisomant (10–20 times that normally given to treat acromegaly in humans) can reduce IGF-1 levels up to 70% (Kopchick et al., 2002). Also, GHA (as noted above) expressed in transgenic mice is effective at inhibiting GHR activity. However, human (h) GHA fails to antagonize GHR activity in GH-deficient dwarf rats when co-infused with GH (Mode et al., 1996). We are still puzzled by this observation and do not have an explanation.
Two studies designed to demonstrate the safety and effectiveness of pegvisomant in primates were conducted in rhesus monkeys. In the first study, a weekly bolus of 1 mg/kg of pegvisomant was injected subcutaneously into ovariectomized monkeys (Wilson, 1998). Serum IGF-1 levels were 61% lower in the pegvisomant injected animals compared to controls after 3 days and demonstrated maximal depression 10 days post injection. IGF-1 levels normalized to that of controls 14 days post injection. In the follow-up study, five ovariectomized monkeys were given the same dose of pegvisomant (Wilson, 2000). Within 24 hours of pegvisomant administration, IGF-1 levels began to fall, continued to decline through day 5, resulting in an overall 78% decline. These studies demonstrated that pegvisomant was well tolerated and effective in inhibiting endogenous GH action in primates.
5.3. Clincal use of pegvisomant/Somavert
5.3.1. Phase I-III clinical trials
Once pegvisomant was shown to be safe in primates and an effective suppressor of IGF-1, clinical trials in humans commenced. In a phase I, placebo-controlled study, 36 healthy young men received single subcutaneous dose of pegvisomant at 0.03, 0.1, 0.3 or 1 mg/kg (Thorner et al., 1999). Pegvisomant caused no serious adverse events at any of the above doses. IGF-1 levels were decreased in a dose dependent manner for the 0.3 and 1.0 mg/kg doses. Maximum suppression for the 1 mg/kg pegvisomant dosage was observed 5 days post-injection with IGF-1 being reduced to nearly 50% of baseline levels. There were no substantial changes in circulating GH levels at any of the doses given. However, there was an extremely small, yet significant rise, in mean GH levels for the 1 mg/kg dose after 48hr in one of two GH quantification assays utilized, which may indicate reduced negative IGF-1 feedback on the pituitary.
The first study reporting treatment of acromegalic patients with pegvisomant was presented at The Endocrine Society’s Annual Meeting in 1998 (van der Lely et al., 1998). Approximately two thirds of the patients had previously received surgery, radiotherapy and octreotide treatment. Medical treatment was withdrawn for three weeks from all patients prior to receiving pegvisomant. In this double blind, placebo-controlled phase II study, 46 patients with acromegaly were given placebo or a 30mg/week or 80mg/week dose of pegvisomant. The drug was well tolerated, and no patients were dropped due to adverse events. Total IGF-1 levels were reduced by 16% in patients receiving 30mg/week while total IGF-1 levels were reduced by 31% in those receiving 80mg/week after six weeks of treatment. In addition, free IGF-1 levels in patients receiving the 80mg/week dose was reduced by 47% after 6 weeks of treatment.
A phase III clinical trial, treating patients with acromegaly established the short term safety and efficacy of pegvisomant (Trainer et al., 2000). In this randomized, double-blind, placebo-controlled study, 112 patients with acromegaly received daily subcutaneous doses of pegvisomant for 12 weeks. Patients were selected whose serum IGF-1 level were greater than 1.3 times the upper level of the normal age-adjusted range and who had withdrawn from the use of other drugs. Patients were randomly selected to receive a daily dose of 10, 15 or 20 mg of pegvisomant or placebo. The drug was well tolerated, with only one patient classified as experiencing a serious adverse reaction. This patient had elevated levels of alanine and aspartate aminotransferases in the serum that returned to normal within eight weeks of discontinuation of treatment. All three treatment groups demonstrated a dose dependent decrease in serum IGF-1, IGFBP-3 and acid-labile subunit levels. Normalization of serum IGF-1 levels was achieved in 38% of patients receiving 10 mg/day and 82% of those receiving 20mg/day. In addition, the IGF-1 levels were normalized rapidly, with 75% of the maximal reductions occurring within the first two weeks of treatment. While three patients at the 20 mg/day dose did not achieve normal IGF-1 levels, they all showed substantial decreases in IGF-1 levels. Also, there was a dose-dependent increase in serum GH levels, coinciding with the decrease in serum IGF-1 levels. This reflects the negative feedback loop that helps regulate GH secretion by the somatotrophs of the pituitary. Evaluation of typical symptoms associated with acromegaly revealed improvement in the treatment groups with regards to decreases in soft tissue swelling, excessive perspiration and fatigue.
5.3.2. Initial long-term trials
While the phase III trial demonstrated the short term safety and efficacy of pegvisomant, questions regarding its long-term use remained. To address this issue, a new study enlisted a cohort of 160 patients for pegvisomant treatment of an average of 425 days (van der Lely et al., 2001). As in the above study, other medical interventions were discontinued and patients had serum IGF-1 level at least 1.3 times the upper limit of the normal range. Patients received initial doses of 10 mg/day of pegvisomant and that dose was increased or decreased in increments of 5 mg/day (with a maximum dose of 40 mg/day) until IGF-1 level normalization was achieved. For data analysis, patients were divided into groups receiving pegvisomant for 6 months, 12 months, or 18 months. Patients in the 12 month group were also included in the 6 month group while patients in the18 month group were included in both the 6 and 12 month groups. Remarkably, serum IGF-1 levels were normalized in 97% of patients (n=90) treated with pegvisomant for 12 months or more. Similar to results in the short-term Phase III clinical trial study, the negative feedback loop that helps regulate GH secretion from the pituitary resulted in an increase in serum GH levels in all patient groups, rising by 12.5 ug/L in the 6 and 12 month group and by 14.2 ug/L in the 18 month group. GH levels returned to baseline levels within 30 days in patients withdrawn from pegvisomant and not placed on alternative medical therapy, demonstrating that pegvisomant treatment was responsible for the elevated GH levels. Antibodies to pegvisomant were detected in roughly 17% of patients, although none showed signs of tachyphylaxis.
Serum insulin and glucose concentrations decreased in all three treatment groups demonstrating an improvement in parameters of insulin resistance. This is extremely important since nearly one third of untreated acromegalics develop type 2 diabetes. The improvement of these important metabolic parameters via pegvisomant treatment of acromegalic individuals are benefits not observed when a somatostatin analog is used (Barkan et al., 2005). The effect of pegvisomant treatment on pituitary tumor growth was assessed by magnetic resonance imaging. During the early human clinical trials using pegvisomant, the fear of increases in pituitary tumor size was apparent since similar scenarios occurred when dealing with Nelson’s syndrome following bilateral adrenalectomy (Nelson et al., 1960). It should be noted that bilateral adrenalectomy completely ablates cortisol from the individual while pegvisomant merely normalizes IGF-1 levels. The mean tumor volume was unchanged for the 131 patients treated with pegvisomant for which scans were available. Subsequent studies with more patients and longer-term follow up have demonstrated that pegvisomant treatment is not associated with tumor progression.
5.3.3. The long term trial: ACROSTUDY
Due to the new mechanism of action for pegvisomant, the Food and Drug Administration (FDA) and European Medicines Agency (EMEA) required an ongoing observational survey to determine pegvisomant’s long-term efficacy and safety. This study, carried out by Pfizer Inc, was started in 2004 under the name ACROSTUDY (Trainer, 2009).
As of February 2009, more than 792 patients had been included in the study with a mean of 3.3 years of pegvisomant treatment. The majority of patients were receiving daily injections of pegvisomant, and 67% were not undergoing additional treatment for acromegaly. After 12 months of treatment, average serum IGF-1 levels dropped from 518 ng/ml to 277 ng/ml. Normalization of serum IGF-1 levels was relatively constant at 62% regardless of the length of treatment. The 62% normalization rate was unexpectedly low given that the results from the phase III study demonstrated a 97% normalization rate (van der Lely et al., 2001). The reason for this discrepancy is believed to be due to a failure of physicians to increase the dose of pegvisomant in patients who do not show IGF-1 normalization (Trainer, 2009). This is evidenced by the fact that four out of five patients with higher than normal IGF-1 levels after three years of treatment remained on a dose of 20 mg/day or lower. Therefore, it is believed that IGF-1 normalization in the vast majority of patients can be achieved by vigilant pegvisomant dose titration. In addition, those patients taking other medical therapies in combination with pegvisomant did not exhibit increased IGF-1 normalization rates compared to those on IGF-1 monotherapy.
Of the more than 792 patients, adverse events were reported in 142 patients and 56 of those were attributed to pegvisomant therapy. The most common adverse event, which occurred in 29 patients (3.7%), was abnormal elevation of liver enzymes. Liver enzyme levels were returned to normal in 10 of these patients, half of which remained on pegvisomant and several of whom stopped treatment and subsequently restarted on pegvisomant. The 56 reported serious adverse events occurred in a total of 46 patients. Thirteen of these were attributed to pegvisomant, with 9 due to elevated liver enzymes. Importantly, no patient treated with pegvisomant showed any evidence of sustained liver damage.
Pituitary tumor growth was reported as a serious adverse event in three patients. 411 patients underwent pre-treatment and post-treatment MRI imaging to investigate what effect, if any, pegvisomant has on pituitary tumor volume. Of these patients, 70 patients had a change in tumor size during treatment, with 22 showing an increase and 31 showing a decrease. Of those with lower tumor volume, 26 had been either previously treated with radiotherapy or were on combination therapy (either somatostatin analogue, dopamine agonist or both). It is believed that these additional treatments are the likely cause of tumor shrinkage. Of those with an increase in tumor volume, 6 had previously received radiation therapy and two had discontinued somatostatin analogue treatment. Tumor growth was observed before the initiation of pegvisomant treatment in two of the 22 patients. In 11 patients, additional review was unable to confirm a suspected increase in tumor size by the investigator.
6. GH/IGF-1 association with cancer
Decades of research have clearly established a central role of the GH/IGF-1 axis in postnatal growth. More recently, considering the ability of GH to promote cell proliferation and angiogenesis and inhibit apoptosis, many have turned their attention of the ability of GH/IGF-1 to be involved in several types of cancers. In that regard, a recent pathway-based approach of genome-wide association studies (GWAS) identified GH induced signaling pathways as the third highest pathway associated with breast cancer susceptibility (Menashe et al., 2010). Therefore, it is possible that inhibitors of this pathway, such as GHA, may prove to be effective therapeutics to inhibit the growth and/or progression of numerous cancers.
6.1. Human epidemiological studies
Numerous studies in humans have provided evidence that the GH/IGF-1 axis plays a role in the development and/or progression of human cancers. This literature is considerable and will not be reviewed here. However, we will point out three examples. A study depicting the association of height and cancer in over 300 individuals found a significant increase in the incidence of colon, breast and prostate cancer in taller individuals (Gunnell et al., 2001). Similarly, studies of individuals with elevated GH levels due to acromegaly indicated that there is a moderate risk in developing colorectal cancer (Melmed, 2009; Renehan and Brennan, 2008). As previously stated, Laron individuals have an inactivating mutation within their GHR gene, and as such, are insensitive to GH action. Data from 2 cohorts of Laron patients strongly implicate GH action in human cancer. In one cohort of 230 Laron patients, none of the individuals developed cancer (Steuerman et al., 2011). In the second cohort located in Ecuador, out of a population of 99 Laron patients, only 1 patient exhibited a malignancy that turned out to be non-lethal in contrast to a 17% cancer incidence in control individuals (Guevara-Aguirre et al., 2011). Thus, there is an extraordinary and exciting reduction in the incidence of cancer in Laron Syndrome patients.
6.2. Evidence from animal studies
As discussed previously, a study by Ikeno (Ikeno et al., 2009) detailed the overall reduced occurance of neoplasms in GHR−/− mice throughout their lifespan and complements that seen in Laron patients. A longevity study was performed on GHR−/− mice and their WT littermates. Histopathological examination was performed on the mice following their death. While neoplastic lesions are found in more than 90% of WT mice, such lesions are found in only 68% of the GHR−/− mice. In addition, the tumor burden (number of different types of tumors) in the GHR−/− mice is reduced by 47% compared to WT mice and shows that the overall tumor incidence is reduced in GHR−/− mice. As for the cause of death, 83% of WT mice die from neoplastic disease while only 42% (49% reduction) of the GHR−/− mice die from such lesions.
There is mounting evidence from animal studies that implicate GH in oncogenesis, as recently reviewed by Chhabra, Waters and Brooks (Chhabra et al., 2011). However, mammary cancers are perhaps the best studied (Perry et al., 2008). A study utilizing the GHA mouse model demonstrated that inhibition of the GH action could provide striking protection from breast cancer in vivo (Pollak et al., 2001). In this study, 8week-old GHA and control mice were given a weekly gavage of the carcinogen, dimethylbenz(a)anthracene (DMBA) and monitored for tumor incidence. At the end of the 39 week study period, 68% of GHA animals remained without tumors, while only 32% of control animals were tumor free.
Another study utilizing the Spontaneous Dwarf Rat (SDR), which has an inactivating mutation in the GH gene Ghdr/dr, was conducted to assess GH signaling in the progression of mammary carcinogenesis (Swanson and Unterman, 2002). Female SDR and control rats were subjected to chemical carcinogenesis with N-methyl-N-nitrosourea (MNU) or DMBA and observed for 180 days. All of the wild-type rats (n = 10) developed multiple mammary cancers (5.3/rat) while only 3 SDR rats (n = 15) developed cancers (0.2/rat). A subsequent study examined the effect of GH administration to SDR along with MNU treatment (Shen et al., 2007). In this study, SDR and control rats were injected with GH or vehicle following MNU exposure. Tumor occurrence did not occur in SDR while 80% of control animals and the GH-treated SDR group developed tumor at two months following MNU exposure. Importantly, cessation of GH injections from SDR rats resulted in the rapid regression of all tumors and subsequent resumption of GH injections resulted in a rapid resurgence of tumors at the original tumor sites. This work demonstrated that SDR rats treated with GH become vulnerable to mammary carcinogenesis and that these advanced rat mammary cancers are dependent on GH for their survival. In a similar study, SDR rats were injected with MNU and treated with GH with or without estradiol (E2) and progesterone (P4), with E2 and P4 alone, or with IGF-1(Thordarson et al., 2004). As demonstrated above, the GH-treated SDR rats exhibited a 100% tumor incidence by 10 weeks of treatment, while only 50% of the IGF-1 treated SDR rats developed tumors by week 14. As expected, SDR animals treated with E2 + P4 had no tumors, while, paradoxically, GH + E2 + P4 treated animals also showed almost no tumor incidence. This suggests that steroid treatment blocked the action of GH possibly by reducing serum IGF-1 levels.
Our GHR−/− mouse model has also been used to examine the role of the GH/IGF-1 axis in mammary carcinogenesis. GHR−/− animals were crossed to C3(1)/TAg mice, a mouse line that develops estrogen receptor α negative mammary cancers (Zhang et al., 2007). While TAg/GHR+/+ animals developed an average of nearly 10 tumors per animal, TAg/GHR−/− animals only showed ~3 tumors per animal. Even more striking than the number of tumors was the tumor size. TAg/GHR+/+ tumors grew to more than 10 times the volume of those found in GHR disrupted animals. These experiments demonstrate that the disruption of the GH/IGF-1 axis significantly retards TAg-driven mammary carcinogenesis, and once again, suggest that antagonists of GH induced signaling may be an effective strategy to inhibit the progression of estrogen-independent breast cancer. A similar study was used to examine the role of the GH/IGF in prostate carcinogenesis (Wang et al., 2005). In this study, GHR−/− mice were again crossed with C3(1)/TAg mice. The resulting mice develop prostate neoplasia as a result of large T antigen expression that progresses to invasive prostate carcinoma. Tag/GHR+/+ and Tag/GHR−/− mice were generated and sacrificed at 9 months of age. While seven of eight Tag/GHR+/+ mice developed prostate neoplasias, only one of eight Tag/GHR−/− mice developed such lesions suggesting that disruption of GH signaling confers resistance to prostate carcinogenesis.
6.3. Use of pegvisomant in cancer xenographs
The above studies as well as many confirming in vitro studies argue for the use of pegvisomant for selected cancers. In an early study, pegvisomant was examined for its impact on cancer cell xenografts in athymic nude mice (Divisova et al., 2006). Treatment of WT FVB/N mice with pegvisomant severely reduced mammary development and reduced mammary gland GH and IGF-1 signaling. In this study, human breast cancer cells (MCF-7) were grown as subcutaneous xenografts in the thoractic mammary fat pad until they reached a volume of approximately 100–200mm3. The mice were then randomized and injected daily with pegvisomant (250mg/kg) or vehicle. MCF-7 xenografts stopped growing upon pegvisomant treatment while MCF-7 control animals showed a steady increase in tumor size. In another study, pegvisomant was analyzed for its ability to inhibit the growth of human colorectal carcinoma cell lines in nude mice (Dagnaes-Hansen et al., 2004). In this study, Colo205 and HT-29 cell xenographs were grown in nude mice until they reached a volume of 90–105mm3 and 60–70mm3, respectively. The mice were then separated into 2 groups and injected every other day with either saline or pegvisomant at 60mg/kg. After 16 days of injections, pegvisomant treatment caused a 39% reduction in tumor volume and a 44% reduction in tumor weight in the nude mice with the COLO 205 colorectal cancer. However, pegvisomant had no effect on the HT-29 colorectal cancer and suggests the inhibition of GH/IGF-1 may represent a treatment for some colorectal cancers. In a third study, pegvisomant was assessed for antitumor activity against human meningiomas in nude mice (McCutcheon et al., 2001). Primary cultures of human meningioma tumors were xenografted into athymic mice. Mice were then injected with pegvisomant or vehicle for 8 weeks. Following treatment tumor growth was assessed. The mean tumor size in the vehicle injected cohort increased from 284mm3 to 350mm3 while those in the pegvisomant treated group decreased from 291mm3 to 198mm3. This study complemented their earlier work which demonstrated that the growth rate of GHR expressing primary meningioma cultures was decreased via the blockade of the GH/IGF-1 axis by B2036 (a nonpegylated version of pegvisomant) and suggested that the down-regulation of this axis may represent a potent therapeutical approach for this disease (Friend et al., 1999).
6.4. Additional human cancer studies
The role of the GH/IGF-1 axis has been studied on glioma cell lines (Friend et al., 2001). In one study both the GH and IGF-1 receptors were found to be expressed in 5 immortalized glioma cell lines. Treatment of two of these lines (U251 and U373) with IGF-1 resulted in increased mitogenesis as assessed by thymidine uptake. U251 cells were then xenografted into immunocompromised (scid) mice that had been crossed with the lit mouse strain. 10 of scid/lit heterozygous mice and 10 scid/lit homozygous were injected with 106 U251 and 20 more injected with U373 cells. Five mice developed tumors in each group. The initial tumor volume in the U251 lit/+ and lit/lit mice was 269 and 215 mm3, and volumes after 8 weeks were 2,589 and 1,819 mm3, respectively. Initial tumor volumes for U373 cells were 324 and 266mm3 in the lit/+ and lit/lit mice, respectively, and after 8 weeks, the volumes were 526 and 599 mm3, respectively. These results were not statistically significantly and suggest that a low GH/IGF-1 environment does not inhibit the growth of these tumors in vivo.
The possibility of the GH acting to promote growth in an autocrine/paracrine manner has also been reported by Lobie and colleagues. In one study, human endometrial carcinoma cell lines, RL95-2 and AN3, were stably transfected with a plamid designed to express human (h) GH (Pandey et al., 2008). Both AN3-vector (control cells) and AN3-hGH cell lines expressed equivalent levels of the GHR while AN3-hGH secreted detectable levels of hGH into the culture medium. The AN3-hGH cells were found to grow significantly faster over a 14 day time period compared to control AN3-vector containing cells. The AN3-hGH cells also demonstrated enhanced anchorage-independent cell growth as indicated by colony formaton in soft agar. Similar results were found with the RL95-2 cell line. Both control and RL95-2-hGH expressed the GHR, and both cell lines express endogenous hGH. However, RL95-2-hGH produced and secreted increased levels of hGH into the culture medium. Once again, the total cell number of RL95-2-hGH cells increased much faster than control cells over a 14 day period and anchorage-independent growth was also increased. In addition, antagonism of GH signaling by B2036 resulted in a reduction in oncogenic cell characteristics. When xenografted into athymic mice, RL95-2-hGH cells tumors grew significantly faster than RL95-2-vector tumors. In a second study, the autocrine effects of hGH on primary human mammary carcinoma cultures was examined. Primary cultures that were negative for hGH mRNA were highly sensitive to exogenous hGH stimulation. As expected, since they there is no autocrine hGH stimulation in these cells, they were not affected by B2036. In contrast, B2036 significantly inhibited the proliferation of primary cultures that were positive for hGH mRNA. These cells theoretically would produce autocrine hGH.
7. Conclusion
The mouse strains generated in our laboratory with either enhanced or repressed GH action have elucidated much about the in vivo functions of GH including metabolic and adipogenic effects and possible contributions to oncogenesis. The generation and characterization of the GHA mouse strain resulted in the discovery of a new class of drugs, namely the GHA, of which Somavert® (pegvisomant for injection) was developed. It is currently used in the treatment of patients with acromegaly. Considering the body of evidence from in vitro and in vivo studies and human epidemiological reports, there is a growing belief that the GH/IGF-1 signaling pathway is capable of promoting growth of selected types of cancer. As such, inhibitors of this pathway using agents such as pegvisomant may prove to be an effective therapeutic intervention for certain cancer indications. Hopefully, future clinical trials will determine whether pegvisomant is of value in the treatment of these cancers.
Highlights.
Exess GH leads to poor health outcomes
Removal of GH action is associated with resistance to diabetes and cancer
The GHA was discovered using transgenic mice that express mutated GH genes
Somavert® (pegvisomant for injection) is an approved drug for acromegaly
GHA may have applications for selected cancer indications
Acknowledgments
Research Support: This work was supported by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by NIH grants P01AG031736, AG032290 and DK083729, by the AMVETS, by the Diabetes Institute at Ohio University.
Abbreviations
- GH
growth hormone
- GHA
growth hormone receptor antagonist
- TNF-α
tumor necrosis factor alpha
- IL-6
interleukin-6
- IGF-1
insulin-like growth factor-1
- GHR−/−
growth hormone receptor gene disrupted mice
- MCK
muscle creatine kinase
- FaGHRKO
Fat specific growth hormone receptor knockout mice
- WAT
white adipose tissue
- LS
Laron Syndrome
- bGH
bovine growth hormone
- PEG
polyethylene glycol
- DMBA
dimethylbenz (a) anthracene
- SDR
spontaneous dwarf rat
- MNU
N-methyl-N-nitrosourea
- E2
estradiol
- P4
progesterone
Footnotes
The authors declare no conflict of interest except for JJK who discovered GHA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Barkan AL, Burman P, Clemmons DR, Drake WM, Gagel RF, Harris PE, Trainer PJ, van der Lely AJ, Vance ML. Glucose homeostasis and safety in patients with acromegaly converted from long-acting octreotide to pegvisomant. J Clin Endocrinol Metab. 2005;90:5684–5691. doi: 10.1210/jc.2005-0331. [DOI] [PubMed] [Google Scholar]
- Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT. Does growth hormone prevent or accelerate aging? Exp Gerontol. 1998;33:675–687. doi: 10.1016/s0531-5565(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of Soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res. 2004;36:550–558. doi: 10.1055/s-2004-825796. [DOI] [PubMed] [Google Scholar]
- Bass SH, Mulkerrin MG, Wells JA. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci U S A. 1991;88:4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–168. doi: 10.1210/endo.141.1.7284. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A Dwarf Mouse Model With Decreased GH/IGF-1 Activity That Does Not Experience Life-Span Extension: Potential Impact of Increased Adiposity, Leptin, and Insulin With Advancing Age. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlooly YM, Olsson B, Bruder CE, Linden D, Sjogren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahren B, Ohlsson C, Oscarsson J, Tornell J. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes. 2005;54:51–62. doi: 10.2337/diabetes.54.1.51. [DOI] [PubMed] [Google Scholar]
- Bollano E, Omerovic E, Bohlooly-y M, Kujacic V, Madhu B, Tornell J, Isaksson O, Soussi B, Schulze W, Fu ML, Matejka G, Waagstein F, Isgaard J. Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology. 2000;141:2229–2235. doi: 10.1210/endo.141.6.7486. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cecim M, Bartke A, Yun JS, Wagner TE. Expression of human, but not bovine, growth hormone genes promotes development of mammary tumors in transgenic mice. Transgenics. 1994;1:431–437. [Google Scholar]
- Cecim M, Fadden C, Kerr J, Steger RW, Bartke A. Infertility in transgenic mice overexpressing the bovine growth hormone gene: disruption of the neuroendocrine control of prolactin secretion during pregnancy. Biol Reprod. 1995;52:1187–1192. doi: 10.1095/biolreprod52.5.1187. [DOI] [PubMed] [Google Scholar]
- Chen NY, Chen WY, Kopchick JJ. A growth hormone antagonist protects mice against streptozotocin induced glomerulosclerosis even in the presence of elevated levels of glucose and glycated hemoglobin. Endocrinology. 1996;137:5163–5165. doi: 10.1210/endo.137.11.8895392. [DOI] [PubMed] [Google Scholar]
- Chen WY, Chen N, Yun J, Wagner TE, Kopchick JJ. In vitro and in vivo studies of the antagonistic effects of human growth hormone analogs. J Biol Chem. 1994;269:15892–15897. [PubMed] [Google Scholar]
- Chen WY, White ME, Wagner TE, Kopchick JJ. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology. 1991a;129:1402–1408. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wight DC, Chen NY, Coleman TA, Wagner TE, Kopchick JJ. Mutations in the third alpha-helix of bovine growth hormone dramatically affect its intracellular distribution in vitro and growth enhancement in transgenic mice. J Biol Chem. 1991b;266:2252–2258. [PubMed] [Google Scholar]
- Chen WY, Wight DC, Mehta BV, Wagner TE, Kopchick JJ. Glycine 119 of bovine growth hormone is critical for growth-promoting activity. Mol Endocrinol. 1991c;5:1845–1852. doi: 10.1210/mend-5-12-1845. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A. 1990;87:5061–5065. doi: 10.1073/pnas.87.13.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone-IGF-1 axis in cancer. Expert Review of Endocrinology & Metabolism. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- Clark R, Olson K, Fuh G, Marian M, Mortensen D, Teshima G, Chang S, Chu H, Mukku V, Canova-Davis E, Somers T, Cronin M, Winkler M, Wells JA. Long-acting growth hormones produced by conjugation with polyethylene glycol. J Biol Chem. 1996;271:21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin and IGF-1 levels and increased lifespan. Endocrinology. 2003a;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003b;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Jhurani P, Ng P, Wells JA. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989;243:1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991;254:821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Dagnaes-Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A. Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res. 2004;24:3735–3742. [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- Divisova J, Kuiatse I, Lazard Z, Weiss H, Vreeland F, Hadsell DL, Schiff R, Osborne CK, Lee AV. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;98:315–327. doi: 10.1007/s10549-006-9168-1. [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Kimata K, Peten EP, Yamada Y, Striker GE. Glomerulosclerosis in mice transgenic for growth hormone. Increased mesangial extracellular matrix is correlated with kidney mRNA levels. J Exp Med. 1991;173:1287–1290. doi: 10.1084/jem.173.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Lochmuller EM, Koller B, Wehr U, Weusten A, Rambeck W, Hoeflich A, Wolf E. Body composition, bone mass and microstructural analysis in GH-transgenic mice reveals that skeletal changes are specific to bone compartment and gender. Growth Horm IGF Res. 2002;12:116–125. doi: 10.1054/ghir.2002.0272. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, Bergstrom G, Svensson L, Oscarsson J, Tornell J, Bohlooly YM. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2006;290:E317–325. doi: 10.1152/ajpendo.00181.2005. [DOI] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, Digirolamo DJ, Kopchick JJ, Leroith D, Trucco M, Sperling MA. Liver-specific Deletion of the Growth Hormone Receptor Reveals Essential Role of GH Signaling in Hepatic Lipid Metabolism. J Biol Chem. 2009 doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999;48:377–382. doi: 10.2337/diabetes.48.2.377. [DOI] [PubMed] [Google Scholar]
- Frick F, Bohlooly YM, Linden D, Olsson B, Tornell J, Eden S, Oscarsson J. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab. 2001;281:E1230–1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- Friend KE, Khandwala HM, Flyvbjerg A, Hill H, Li J, McCutcheon IE. Growth hormone and insulin-like growth factor-I: effects on the growth of glioma cell lines. Growth Horm IGF Res. 2001;11:84–91. doi: 10.1054/ghir.2000.0183. [DOI] [PubMed] [Google Scholar]
- Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg. 1999;91:93–99. doi: 10.3171/jns.1999.91.1.0093. [DOI] [PubMed] [Google Scholar]
- Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992;256:1677–1680. doi: 10.1126/science.256.5064.1677. [DOI] [PubMed] [Google Scholar]
- Goffin V, Bernichtein S, Carriere O, Bennett WF, Kopchick JJ, Kelly PA. The human growth hormone antagonist B2036 does not interact with the prolactin receptor. Endocrinology. 1999;140:3853–3856. doi: 10.1210/endo.140.8.7047. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–342. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- Hara K, Hsu Chen CJ, Sonenberg M. Recombination of the biologically active peptides from a tryptic digest of bovine growth hormone. Biochemistry (Mosc) 1978;17:550–556. doi: 10.1021/bi00596a028. [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- Hwa V, Camacho-Hubner C, Little BM, David A, Metherell LA, El-Khatib N, Savage MO, Rosenfeld RG. Growth hormone insensitivity and severe short stature in siblings: a novel mutation at the exon 13-intron 13 junction of the STAT5b gene. Horm Res. 2007;68:218–224. doi: 10.1159/000101334. [DOI] [PubMed] [Google Scholar]
- Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best Pract Res Clin Endocrinol Metab. 2011;25:61–75. doi: 10.1016/j.beem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard AS, Emerson M, Prehar S, Neyses L, Trainer P, List EO, Kopchick JJ, Heagerty AM. The cardiovascular phenotype of a mouse model of acromegaly. Growth Horm IGF Res. 2009 doi: 10.1016/j.ghir.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kaps M, Moura AS, Safranski TJ, Lamberson WR. Components of growth in mice hemizygous for a MT/bGH transgene. J Anim Sci. 1999;77:1148–1154. doi: 10.2527/1999.7751148x. [DOI] [PubMed] [Google Scholar]
- Knapp JR, Chen WY, Turner ND, Byers FM, Kopchick JJ. Growth patterns and body composition of transgenic mice expressing mutated bovine somatotropin genes. J Anim Sci. 1994;72:2812–2819. doi: 10.2527/1994.72112812x. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annu Rev Nutr. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Laron Z. Laron Syndrome - From Man to Mouse. Berlin: Springer; 2011. [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23:623–646. doi: 10.1210/er.2001-0022. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Brosius FC, 3rd, Menon RK. The glomerular podocyte as a target of growth hormone action: implications for the pathogenesis of diabetic nephropathy. Curr Diabetes Rev. 2011;7:50–55. doi: 10.2174/157339911794273900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z, Blum W, Chatelain P, Ranke M, Rosenfeld R, Savage M, Underwood L. Classification of growth hormone insensitivity syndrome [editorial] J Pediatr. 1993;122:241. doi: 10.1016/s0022-3476(06)80120-4. [DOI] [PubMed] [Google Scholar]
- Laron Z, Kopchick J. Laron syndrome - from man to mouse lessons from clinical and experimental experience. Berlin; New York: Springer; 2011. p. 1.p. xiv.p. 531. online resource. [Google Scholar]
- Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: mplication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27:524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Striker LJ, Phillips C, Chen NY, Chen WY, Kopchick JJ, Striker GE. Growth hormone expression is required for the development of diabetic glomerulosclerosis in mice. Kidney Int Suppl. 1995;51:S37–38. [PubMed] [Google Scholar]
- Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216:363–374. doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers ER, List EO, Jara A, Sackmann-Sala L, Cordoba-Chacon J, Gahete M, Kineman RD, Boparai R, Bartke A, Kopchick J, Berryman DE. Adiponectin in mice with altered growth hormone action: links to insulin sensitivity and longevity? The Journal of Endocrinology. 2012 doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado MO, Hirata RD, Sellitti DF, Iotti R, Iotti A, Cusumano AM, Riordan GP, Coschigano KT, Kopchick JJ, Zuhl I, Nguyen N, Hirata MH, Doi SQ. Growth hormone promotes glomerular lipid accumulation in bGH mice. Kidney Int. 2005;68:2019–2028. doi: 10.1111/j.1523-1755.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, Friend KE. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94:487–492. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- Melmed S. Acromegaly pathogenesis and treatment. The Journal of clinical investigation. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A. Guidelines for acromegaly management: an update. The Journal of clinical endocrinology and metabolism. 2009;94:1509–1517. doi: 10.1210/jc.2008-2421. [DOI] [PubMed] [Google Scholar]
- Menashe I, Maeder D, Garcia-Closas M, Figueroa JD, Bhattacharjee S, Rotunno M, Kraft P, Hunter DJ, Chanock SJ, Rosenberg PS, Chatterjee N. Pathway analysis of breast cancer genome-wide association study highlights three pathways and one canonical signaling cascade. Cancer Res. 2010;70:4453–4459. doi: 10.1158/0008-5472.CAN-09-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquet JG, Freund T, Martinez CS, Gonzalez L, Diaz ME, Micucci GP, Zotta E, Boparai RK, Bartke A, Turyn D, Sotelo AI. Hepatocellular alterations and dysregulation of oncogenic pathways in the liver of transgenic mice overexpressing growth hormone. Cell Cycle. 2013;12:1042–1057. doi: 10.4161/cc.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquet JG, Giani JF, Martinez CS, Munoz MC, Gonzalez L, Sotelo AI, Boparai RK, Masternak MM, Bartke A, Dominici FP, Turyn D. Prolonged exposure to growth hormone impairs insulin signaling in the heart. J Mol Endocrinol. 2011 doi: 10.1530/JME-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode A, Tollet P, Wells T, Carmignac DF, Clark RG, Chen WY, Kopchick JJ, Robinson IC. The human growth hormone (hGH) antagonist G120RhGH does not antagonize GH in the rat, but has paradoxical agonist activity, probably via the prolactin receptor. Endocrinology. 1996;137:447–454. doi: 10.1210/endo.137.2.8593788. [DOI] [PubMed] [Google Scholar]
- Muller AF, Kopchick JJ, Flyvbjerg A, van der Lely AJ. Clinical review 166: Growth hormone receptor antagonists. J Clin Endocrinol Metab. 2004;89:1503–1511. doi: 10.1210/jc.2002-022049. [DOI] [PubMed] [Google Scholar]
- Nelson DH, Meakin JW, Thorn GW. ACTH-producing pituitary tumors following adrenalectomy for Cushing’s syndrome. Ann Intern Med. 1960;52:560–569. doi: 10.7326/0003-4819-52-3-560. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Binart N, Bohlooly YM, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005;331:1120–1126. doi: 10.1016/j.bbrc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Okada S, Chen WY, Wiehl P, Kelder B, Goodman HM, Guller S, Sonenberg M, Kopchick JJ. A growth hormone (GH) analog can antagonize the ability of native GH to promote differentiation of 3T3-F442A preadipocytes and stimulate insulin-like and lipolytic activities in primary rat adipocytes. Endocrinology. 1992;130:2284–2290. doi: 10.1210/endo.130.4.1547740. [DOI] [PubMed] [Google Scholar]
- Olsson B, Bohlooly YM, Brusehed O, Isaksson OG, Ahren B, Olofsson SO, Oscarsson J, Tornell J. Bovine growth hormone-transgenic mice have major alterations in hepatic expression of metabolic genes. Am J Physiol Endocrinol Metab. 2003;285:E504–511. doi: 10.1152/ajpendo.00444.2002. [DOI] [PubMed] [Google Scholar]
- Olsson B, Bohlooly YM, Fitzgerald SM, Frick F, Ljungberg A, Ahren B, Tornell J, Bergstrom G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146:920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- Orian JM, Lee CS, Weiss LM, Brandon MR. The expression of a metallothionein-ovine growth hormone fusion gene in transgenic mice does not impair fertility but results in pathological lesions in the liver. Endocrinology. 1989;124:455–463. doi: 10.1210/endo-124-1-455. [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353–1360. doi: 10.1210/en.2008-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Pandey V, Perry JK, Mohankumar KM, Kong XJ, Liu SM, Wu ZS, Mitchell MD, Zhu T, Lobie PE. Autocrine human growth hormone stimulates oncogenicity of endometrial carcinoma cells. Endocrinology. 2008;149:3909–3919. doi: 10.1210/en.2008-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Wang F, Bonkowski MS, Spong A, Bartke A, Pawlikowska L, Kwok PY, Masternak MM. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce KH, Jr, Ultsch MH, Kelley RF, de Vos AM, Wells JA. Structural and mutational analysis of affinity-inert contact residues at the growth hormone-receptor interface. Biochemistry (Mosc) 1996;35:10300–10307. doi: 10.1021/bi960513b. [DOI] [PubMed] [Google Scholar]
- Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE. The contribution of growth hormone to mammary neoplasia. J Mammary Gland Biol Neoplasia. 2008;13:131–145. doi: 10.1007/s10911-008-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br J Cancer. 2001;85:428–430. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp D, Oberbauer AM, Murray JD. Development of obesity following inactivation of a growth hormone transgene in mice. Transgenic Res. 1996;5:13–23. doi: 10.1007/BF01979918. [DOI] [PubMed] [Google Scholar]
- Pradhananga S, Wilkinson I, Ross RJ. Pegvisomant: structure and function. J Mol Endocrinol. 2002;29:11–14. doi: 10.1677/jme.0.0290011. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124:40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Brennan BM. Acromegaly, growth hormone and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22:639–657. doi: 10.1016/j.beem.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol Metab. 2007;18:134–141. doi: 10.1016/j.tem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Shea BT, Hammer RE, Brinster RL. Growth allometry of the organs in giant transgenic mice. Endocrinology. 1987;121:1924–1930. doi: 10.1210/endo-121-6-1924. [DOI] [PubMed] [Google Scholar]
- Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, Unterman TG, Mehta RG, Swanson SM. Advanced rat mammary cancers are growth hormone dependent. Endocrinology. 2007;148:4536–4544. doi: 10.1210/en.2007-0513. [DOI] [PubMed] [Google Scholar]
- Sherlock M, Woods C, Sheppard MC. Medical therapy in acromegaly. Nat Rev Endocrinol. 2011;7:291–300. doi: 10.1038/nrendo.2011.42. [DOI] [PubMed] [Google Scholar]
- Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- Swanson SM, Unterman TG. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23:977–982. doi: 10.1093/carcin/23.6.977. [DOI] [PubMed] [Google Scholar]
- Thordarson G, Semaan S, Low C, Ochoa D, Leong H, Rajkumar L, Guzman RC, Nandi S, Talamantes F. Mammary tumorigenesis in growth hormone deficient spontaneous dwarf rats; effects of hormonal treatments. Breast Cancer Res Treat. 2004;87:277–290. doi: 10.1007/s10549-004-9504-2. [DOI] [PubMed] [Google Scholar]
- Thorner MO, Strasburger CJ, Wu Z, Straume M, Bidlingmaier M, Pezzoli SS, Zib K, Scarlett JC, Bennett WF. Growth hormone (GH) receptor blockade with a PEG-modified GH (B2036- PEG) lowers serum insulin-like growth factor-I but does not acutely stimulate serum GH. J Clin Endocrinol Metab. 1999;84:2098–2103. doi: 10.1210/jcem.84.6.5732. [DOI] [PubMed] [Google Scholar]
- Trainer PJ. ACROSTUDY: the first 5 years. Eur J Endocrinol. 2009;161(Suppl 1):S19–24. doi: 10.1530/EJE-09-0322. [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi: 10.1016/s0140-6736(01)06844-1. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Lamberts SW, Barkan A, Panadya N, Besser GM, Trainer P, Bonnert V, Melmed S, Clemmons D, Rose R, Vance ML, Thorner MO, Zib K, Davis RJ, Bennett W, Scarlett JA. A six week, double blind, placebo controlled study of a growth hormone antagonist, B2036-PEG (Trovert) in acromegalic patients. 80th Annual Meeting of the Endocrine Society; New Orleans, LA. 1998. p. 57. [Google Scholar]
- Veldhuis JD, Bidlingmaier M, Anderson SM, Evans WS, Wu Z, Strasburger CJ. Impact of experimental blockade of peripheral growth hormone (GH) receptors on the kinetics of endogenous and exogenous GH removal in healthy women and men. J Clin Endocrinol Metab. 2002;87:5737–5745. doi: 10.1210/jc.2001-011885. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A, Wu Y, Sun H, Li X, Jeddy Z, Liu C, Schwartz GJ, Yakar S, LeRoith D. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103. doi: 10.2337/db11-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- Wang Z, Prins GS, Coschigano KT, Kopchick JJ, Green JE, Ray VH, Hedayat S, Christov KT, Unterman TG, Swanson SM. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology. 2005;146:5188–5196. doi: 10.1210/en.2005-0607. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Effects of estradiol and exogenous insulin-like growth factor I (IGF-I) on the IGF-I axis during growth hormone inhibition and antagonism. J Clin Endocrinol Metab. 1998;83:4013–4021. doi: 10.1210/jcem.83.11.5279. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Insulin-like growth factor I (IGF-I) replacement during growth hormone receptor antagonism normalizes serum IGF-binding protein-3 and markers of bone formation in ovariectomized rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1557–1562. doi: 10.1210/jcem.85.4.6522. [DOI] [PubMed] [Google Scholar]
- Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68:71–87. doi: 10.1016/0047-6374(93)90141-d. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu C, Sun H, Vijayakumar A, Giglou PR, Qiao R, Oppenheimer J, Yakar S, LeRoith D. Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121:2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113:96–105. doi: 10.1172/JCI200417763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Striker LJ, Kopchick JJ, Chen WY, Pesce CM, Peten EP, Striker GE. Glomerulosclerosis in mice transgenic for native or mutated bovine growth hormone gene. Kidney Int Suppl. 1993a;39:S90–94. [PubMed] [Google Scholar]
- Yang CW, Striker LJ, Pesce C, Chen WY, Peten EP, Elliot S, Doi T, Kopchick JJ, Striker GE. Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone. Studies in transgenic mice. Lab Invest. 1993b;68:62–70. [PubMed] [Google Scholar]
- Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, Hedayat S, Christov KT, Ray VH, Unterman TG, Swanson SM. Inhibition of estrogen-independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis. 2007;28:143–150. doi: 10.1093/carcin/bgl138. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]