Abstract
The acceptance of estradiol signaling through receptors found in the cell membrane, as well as, the nucleus has provided for a re-examination of timing and location of estradiol actions on neural circuits mediating sexual receptivity (lordosis). Estradiol membrane signaling involves the transactivation of metabotropic glutamate receptors (mGluR) that transduce steroid information through PKC signaling cascades producing rapid activation of lordosis regulating circuits. It has been known for some time that estradiol initially produces an inhibition of the medial preoptic nucleus (MPN). We have demonstrated that underlying this inhibition is estradiol acting in the arcuate nucleus to induce β-endorphin release which inhibits the MPN through a μ-opioid receptor mechanism. This transient inhibition is relieved by either subsequent progesterone treatment or longer exposure to higher doses of estradiol to facilitate lordosis behavior. We review recent findings about estradiol membrane signaling inducing dendritic spine formation in the arcuate nucleus that is critical for estradiol induction of sexual receptivity. Moreover, we discuss the evidence that in addition to ERα, several other putative membrane estrogen receptors facilitate lordosis behavior through regulation of the arcuate nucleus. These include the GRP30 and the STX activated Gq-mER. Finally, we report on the importance of GABA acting at GABAB receptors for estradiol membrane signaling that regulates lordosis circuit activation and sexual receptivity.
Keywords: progesterone, MOR, β-endorphin, NPY, actuate nucleus, membrane estrogen receptor, ERα
INTRODUCTION
Research with steroid hormones especially in the context of reproduction has always been about understanding effects in terms of timing and dose. It became clear very quickly that in order to induce female sexual receptivity, a delay was needed between estradiol treatment and resulting behavior. It became standard practice to prime female rodents with a long-lasting estradiol, estradiol benzoate (EB) and wait approximately 48 hours before testing for behavior (1). Experiments examining the timecourse of estradiol action established that lordosis behavior could not be elicited due to a delay between estradiol treatment of ovariectomized (OVX) animals and lordosis behavior. Based on work done in the periphery (especially the chick oviduct), it was hypothesized that estradiol was inducing the synthesis of new proteins. In the late 1970s and early 1980s using blockers of transcription and translation, a number of investigators showed the importance of estradiol-induced protein synthesis for reproductive behavior (2–4). These results dovetailed nicely with the emerging concept of neuropeptides as transmitters in the brain. Soon it became clear that reproduction was heavily dependent on neuropeptide signaling apart from gonadotropin releasing hormone (GnRH; 5), the releasing factor of luteinizing hormone (LH) and follicle stimulating hormone (FSH). Included among these reproductively important neuropeptides were the endogenous opioid peptides: methionine enkephalin, leucine-enkephalin and β-endorphin (β-END; 4, 6–20), cholecystokinin (CCK; 21–23), galanin (24) and neuropeptide Y (NPY; 25). As with testing for sexual receptivity (26–29), the standard model was to treat animals with EB, wait 48 hours and then analyze neuropeptide levels, or antagonize their receptors to see the effect on lordosis behavior.
In the intact rodent, the rise of circulating estradiol is followed by progesterone, a key event for proceptive behaviors that also facilitates the receptive behaviors. The levels of estradiol in the intact rat are insufficient to induce lordosis without progesterone (30–33). Interestingly, several groups noted that OVX females were absolutely refractory to progesterone for approximately 16–24 hours after EB priming (29, 34, 35). Estradiol was shown to induce the expression of progesterone receptors (PR), without which, progesterone was not effective at inducing proceptive or receptive behavior (36–41). At present, it is not clear if estradiol is acting at a nuclear estrogen receptor (ER) or whether membrane ER (mER) contributes to the expression of PR. In OVX rats, low EB doses, which themselves were not effective at inducing behavior (e.g., 2 µg) could be augmented by progesterone. In practice, 500 µg progesterone is often used, but careful analysis revealed that as little as 100 µg is sufficient to induce maximal levels of lordosis behavior (42, 43). Powers demonstrated the mediobasal hypothalamus was the most sensitive site for progesterone action for augmenting estradiol to induce sexual receptivity (44), as is the ventromedial nucleus of the hypothalamus (VMH). The arcuate nucleus of the hypothalamus (ARH) is also a lordosis regulating region where estradiol and progesterone act (45–47).
Over the past 15 years, we have been investigating the temporal and dose-dependent effects of estradiol signaling. These experiments utilized both estradiol-only and estradiol + progesterone induced lordosis behavior as a behavioral read-out of the lordosis regulating circuitry of the hypothalamus and limbic system (48–50). More recently, we identified an important part of this larger circuit that extends from the ARH to the medial preoptic nucleus of the hypothalamus (MPN) and then to the VMH, which is critical for steroid induction of behavior (34, 51, 52; reviewed in 53). Our results indicate that it is in the ARH that estradiol has its initial actions, which are mediated by membrane-initiated signaling. The MPN receives input from the accessory olfactory system through the posterodorsal medial amygdala along with limbic input from the bed nucleus of the stria terminalis. The VMH is the final common pathway from the integrative circuits in the hypothalamus and limbic system to the periaquaductal grey (PAG), reticular formation and vestibular nuclei. In turn, these brainstem regions project to spinal motoneurons innervating trunk and neck musculature needed for the lordosis posture (reviewed in 54).
STEROID ACTIVATION OF SEXUAL RECEPTIVITY
As we discussed in a 2008 review (55), female sexual behavior can be divided into three components: attractivity, proceptivity and receptivity (56). Most laboratories study proceptive and receptive behaviors. Proceptive behaviors are solicitations that underlie the motivation to copulate and function to entice the male. They present as hopping, darting and ear wiggling. The motivation to copulate has been studied using pacing chambers that allowed the female to control the interactions with the male (57–61). The ability to copulate with a male regardless of her motivational state was defined by Beach as sexual receptivity, physically manifested as the lordosis reflex (31, 56; reviewed in 62). In many species and especially in rodents, the lordosis reflex is a stereotypic arching of the back, elevation of the hindquarters, dorsiflexion of the tail and extension of the neck. This posture is a measure of sexual receptivity that is quantified as the lordosis quotient, defined as the number of lordosis divided by the number of mounts × 100. In practice, the male is often allowed to mount the female 10 times. The lordosis reflex is elicited by appropriate hormonal priming: estradiol and progesterone in the intact female, and stimulation of mechanoreceptors along the flanks, the area around the tail and especially the perineum (54). These tactile stimuli are provided by a mounting male and have been shown to excite both the VHM and ARH (46, 63, 64).
In the intact cycling rat, the sequential release of estrogens and progesterone from the ovary tightly regulates sexual receptivity. Sexual receptivity can be induced in an OVX rat by: treatment with EB 30 to 48 hours prior to testing followed by progesterone 4 hours prior to testing (34, 42, 65); treatment with unesterified estradiol-17β at 24 and 12 hours prior to testing followed by progesterone 4 hours prior to testing (66, 67); and treatment with EB alone for 6 days prior to testing (EB alone; 68–70). Alternatively, a sufficiently large estradiol dose can be administered without supplemental progesterone. While both steroid treatments induce sexual receptivity, the mechanisms through which sexual receptivity is induced appear to be different: 1) A higher dose of estradiol only is needed to induce sexual receptivity when given alone compared to when estradiol is given with a subsequent dose of progesterone (28; reviewed in 27). 2) Repetitive estradiol treatment result in a ramping of increasing sexual receptivity until maximal levels of sexual receptivity are achieved, whereas, repeated estradiol + progesterone treatments produces consistent maximal levels of sexual receptivity (71, 72). 3) Estradiol-only sexual receptivity is independent of the activation of progesterone receptors (73) since antagonism of progesterone receptors or progesterone synthesis does not inhibit estradiol-only induced sexual receptivity (26, 62, 74). 4) The onset of sexual receptivity in the estradiol-only treated rat occurs later compared to estrogen and progesterone treated rats. 5) Rats treated with estradiol-only remain sexually receptive for longer periods of time (29; reviewed in 27). In summary, progesterone treatment transiently augments the estrogenic induction of the lordosis and eventually inhibits many of the estradiol-induced effects terminating the behavior (72).
Interestingly, sexual receptivity lags behind the administration of estradiol. This refractory period lasts approximately 20–24 hours, but at these early time points, the rat requires progesterone supplementation of the estradiol treatment (34, 39). One tidy explanation is that estradiol induces the expression of progesterone receptors which requires approximately 16 hours (39, 41, 75, 76) which are needed for progesterone action. However, in addition to inducing progesterone receptors during this refractory period, estradiol rapidly induces the inhibition of medial preoptic neuronal activity (77). Indeed, in rats treated with a single large dose of estradiol or smaller doses given repeatedly facilitate sexual receptivity lordosis behavior induced by estradiol-only is delayed until 30–48 hours after initial treatment (1, 29).
The control of female reproduction requires the coordination of sexual receptivity with the production of a viable oocyte. The primary stimulus regulating reproductive behavior and ovulation is the increasing levels of estradiol that peak on proestrus. Interestingly, in the intact animal, the rise of ovarian progesterone is coincident with the luteinizing hormone (LH) surge, which occurs several hours after the female becomes receptive (72, 78). Moreover, adrenal progesterone is probably not involved since exogenous estradiol facilitates lordosis in OVX and adrenalectomized (OVX/ADX) rats (71). Although a possible explanation is that estradiol induced progesterone synthesis in the hypothalamus would activate estradiol-induced progesterone receptors in the hypothalamus stimulating behavior before the levels of peripheral progesterone are significantly elevated (53, 74, 79). However, when OVX/ADX rats were treated with 10 µg EB and then 48 hours later with free estradiol (50 µg 17 β-estradiol; 80), blockade of progesterone receptors or steroidogenesis did not attenuate sexual receptivity, but did block proceptive behaviors (53, 74). These data demonstrate that neuroprogesterone has a role in initiating proceptive behaviors, but lordosis behavior is not dependent on de novo synthesis of neuroprogesterone. Moreover, these results are consistent with the long-standing idea that progesterone is responsible for inducing proceptive behaviors, such as hop-darting and ear-wiggling (81). Consequentially, it appears that neither progesterone nor progesterone receptors are needed for estradiol-only induced lordosis suggesting that a different circuit is activated compared with the one activated by estradiol plus progesterone as previously suggested. This is supported by the findings that estradiol- only facilitation of lordosis was blocked by antagonism of the orphanin FQ-opioid receptor-like receptor system, whereas estradiol plus progesterone facilitation was not blocked (82, 83).
Progesterone has another important function vis-a-vis receptive behavior; it “resets” the lordosis regulating circuits in the brain. Sequential treatment of OVX animals with estradiol and progesterone facilitates lordosis and then terminates the behavior (33, 84, 85). This relatively sharp cessation of lordosis is not seen in OVX animals made receptive by estradiol alone (86). Perhaps more importantly, females treated with 3–5 µg EB once every 4 or up to 10 days have an increased lordosis quotient with each subsequent treatment until maximally receptive (71, 87). Repeated treatment once every four days with 2 µg EB produces constant minimal levels of lordosis behavior (34), and subsequent progesterone treatment induces maximal sexual receptivity. There are intriguing data suggesting that lordosis behavior that is induced by estradiol and progesterone is dependent on dopamine activation of the progesterone receptor through the D1 dopamine receptor (88–90) but the mechanism has not been established. Progesterone receptors A and B are found in the plasma membrane, but it is unlikely that progesterone receptor-D1 receptor transactivation occurs since D1 and progesterone receptors A and B do not co-immunoprecipitate (91). It is likely that the D1 and progesterone receptor signaling pathways act within a given lordosis neurocircuit, potentially through progesterone receptor directly interacting through the Src kinase pathway within the cytoplasm (92, 93).
ARH to MPN to VMH Circuit
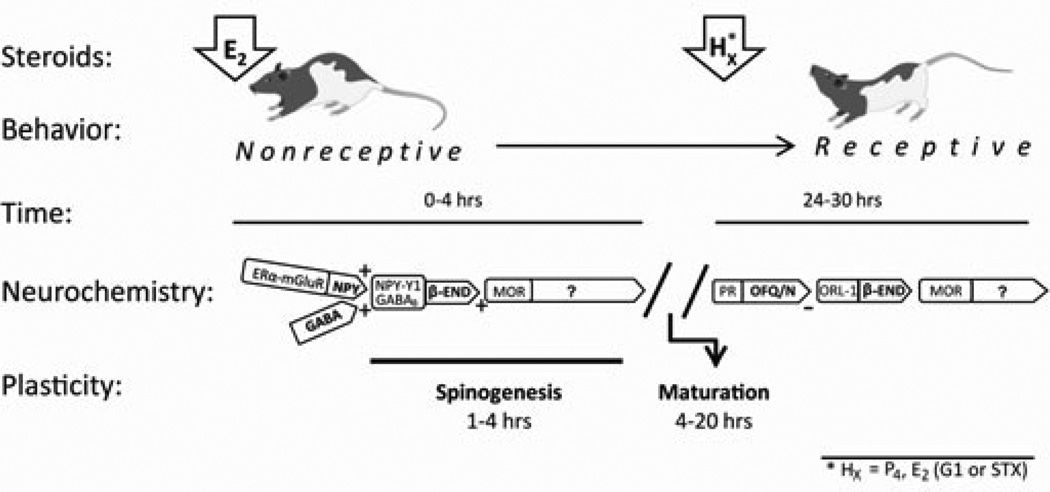
The ARH to MPN neural circuit provides an excellent opportunity to examine the temporal patterns of steroid signaling that regulate sexual receptivity (Figure 1). The major player in this circuit is the endogenous opioid system, and in particular β-endorphin (β -END) and its receptor, the μ-opioid receptor, (MOR; 94, 95). β -END is one of several posttranslational products expressed in proopiomelanocortin (POMC) neurons in the ARH. One population of POMC neurons projects to the periventricular nucleus and participate in the regulation of food intake (96–98). Another POMC neuron population regulates sexual behavior and is distinguished by its projection to the MPN, neuronal morphology, and sensitivity to MOR agonists and ATP-sensitive potassium (KATP) channel modulators (94–99). Activation, particularly by endogenous ligands, induces MOR internalization into early endosomes – the initial mechanism of desensitization or down regulation (100–106). Thus, MPN MOR internalization is a measure of activation of this inhibitory lordosis circuit (34, 94, 107, 108). Introduction of MOR agonists into the MPN rapidly and robustly inhibit lordosis behavior in maximally receptive females (8, 34, 109), which is associated with internalization. The reversal of estradiol-induced MOR internalization produces a facilitation of sexual receptivity (34, 94, 107, 108, 110, 111; Figure 1).
Figure 1.
A schematic of the timecourse of events initiated by estradiol (E2) and progesterone (P4) leading to sexual receptivity in the rat. This timeline begins with an ovariectomized rat treated with E2 to activate β-endorphin (β-END) neurons in the arcuate nucleus of the hypothalamus (ARH). In the ARH, estrogen receptor-α (ERα), trafficked to the cell membrane by E2, transactivates type 1a metabotropic glutamate receptors (mGluR1a) stimulating the release of NPY and GABA onto β-END neurons (activating NPY-Y1 and GABAB receptors, respectively). The relatively rapid, and membrane ERα-dependent, action leads to the release of β-END onto medial preoptic nucleus (MPN) neurons expressing μ-opioid receptors (MORs) – inhibiting lordosis behavior for approximately 20–24 hrs. Simultaneously acting through membrane ERα complexed with mGluR1a (mERα-mGluR1a), E2 induces the formation of dendritic spines in the ARH. While these spines are immature and probably not functional, without this spinogenesis lordosis behavior is not induced by E2. In addition to the activation of MOR and spinogenesis, during this initial phase after E2, the appropriate proteins, neuropeptides and receptors are transcribed. The initial “priming” phase is dependent on E2, but the behavioral “triggering” phase (24–30 post E2) that follows is dependent on either E2 or progesterone (P4). This activation requires GABA and GABAB receptors. For behavior to ensue, P4 (or E2) must relieve the MOR inhibition and induce functional spines. In the ARH, acting through it cognate receptor ORL-1, orphanin FQ/nociception (OFQ/N) inhibits β-END release relieving the MOR blockade of the ventromedial hypothalamus As with the initial action of E2, GABAB receptor activation is critical. While P4 is the signal for triggering lordosis behavior in the intact rat, experimentally, E2 acting through GPR30 can substitute for P4 and trigger lordosis.
MPN MOR activation/internalization fluctuates throughout the estrous cycle coincident with sexual receptivity – activated during diestrous days 1 and 2, deactivated on the evening of proestrus when the rat is sexually receptive and reactivated on the morning of estrus when she is no longer receptive (112) suggesting that the activity of this circuit is important for timing the onset and termination of sexual receptivity. This is mimicked in OVX rats by the appropriate steroid treatment. In OVX rodents, MPN MORs are found on the cell surface indicating an inactive circuit (107). Within 20 minutes of estradiol treatment, the release of β-END activates MPN MOR, which is maintained for at least 48 hours in rats that receive a priming dose of estradiol that does not induce sexual receptivity (34, 82). Blocking estradiol-induced β-END with naloxone or progesterone facilitates lordosis behavior (34, 107, 113, 114). Membrane impermeable estradiol-biotin conjugate injected into the ARH induced internalization of MPN MOR, establishing that membrane-initiated estradiol signaling was responsible for the early and rapid activation of this circuit (108). In ERαKO mice the estradiol-induced MPN MOR activation was abrogated reinforcing that ERα was the necessary ER (110). Further, estradiol was fully able to induce MOR internalization in ERβKO mice (110).
In the ARH, membrane ERα (mERα) and metabotropic glutamate receptor-1a (mGluR1a) receptor are expressed in ARH neurons and form a signaling complex (mERα-mGluR1a; Figure 1; 108, 115). The mERα transactivation of mGluR1a can occur with or without glutamate (108). As expected, blocking the mGluR1a abrogated estradiol induced MOR activation as well as the ability of a high dose of estradiol to induce sexual receptivity (108). Conversely, activating mGluR1a concurrently with the priming dose of estradiol facilitated lordosis compared to the estradiol only treated females (108). mERα-mGluR1a signaling activates the PKCθ signaling pathway to induce internalization of MPN MOP and actively inhibit lordosis (115). Blocking ARH PKC activity inhibits both estradiol and mGluR1a agonist induced MPN MOR activation (115). Further, ARH infusions of a PKC antagonist 30 minutes prior to estradiol administration inhibit facilitation of lordosis (115). Although estradiol activates the POMC neuron output from the ARH, specifically which neurons mediate the estradiol membrane signaling has not been completely elucidated. Evidence points to estradiol regulation of neurons or neural circuits that converge on the POMC/β-END neurons. In the rat, estradiol appears to be acting through an NPY neuron. In vivo, ERα mRNA is expressed in approximately 10–20% of ARH NPY neurons (116, 117). Using an immortalized NPY neuronal cell line (N–38), we showed mERα expression, estradiol activation of PKCθ and calcium mobilization (118). Such results are consistent with an estradiol-induced activation of NPY-Y1 receptors on MPN-projecting POMC neurons, which inhibit lordosis behavior (25, 94). Moreover, a NPY-Y1 receptor agonist injected into the ARH induces MPN MOR internalization (94).
An intriguing possibility is that mERα-mGluR1a signaling acts as an estrogen sensor (119). The response of the circuit determines whether the amount of estradiol is sufficient to induce sexual receptivity or insufficient to induce behavior. In the intact rat, the level of estradiol falls into the latter category and requires progesterone to facilitate lordosis behavior. The initial activation ARH-MPN lordosis inhibitory circuit prevents the rat from copulating prior to the other priming effects of ovarian hormones that are inducing uterine development and ovulation so that they are coordinated with sexual behavior. Treatment of OVX rats with > 5 µg EB will induces lordosis, but requires estradiol signaling to switch signs: from inhibiting to inducing behavior. In vivo, the levels of mERα in the ARH are differentially regulated by estradiol dose (120). In cultured hypothalamic neurons, estradiol regulates membrane levels of ERα transiently increasing their levels and then down regulating mERα, which decreases membrane signaling (121). In OVX rats, high levels of EB initially activate MPN MOR for at least 24 hours (107). However by 48 hours, MPN MOR are deactivated and lordosis is facilitated even while significant levels of estradiol remain in the circulation (82, 107, 122). Low levels of estradiol are unable to switch-off the β-END-MPN MOR circuit by themselves and maintain inhibition of sexual receptivity (34, 82, 123). In addition to more estradiol or subsequent progesterone treatment, neuroactive messengers can turn off the MPN MOR inhibition and facilitate lordosis (reviewed in 62).
GABA
GABAB receptors mediate both initial and sustained estradiol–induced activation of β-END release into the MPN (Figure 1). GABAB receptor blockade prior to EB treatment blocked estradiol-induced MPN MOR activation. When GABAB receptors are blocked 30 hours post-EB, estradiol-induced MPN MOR internalization/activation is reversed and lordosis behavior facilitated (124). These results indicate that GABA signaling through GABAB receptors is important for estradiol membrane-initiated activation of the ARH lordosis microcircuit that induces β-END neurotransmission (Figure 1). Interestingly, GABAB receptors are also needed at the time of estradiol treatment. Inhibition of GABAB receptors in the ARH blocked the estradiol-induced MPN MOR activation. This transient MOR-mediated inhibition is necessary for lordosis behavior, as initially demonstrated by (14–17, 125) and may be a circuit-levels explanation of the findings that GABA transmission in the mediobasal hypothalamus was essential for the facilitation of lordosis behavior. Knockdown of the enzymes, GAD65 and GAD67, prevented GABA synthesis and facilitation of lordosis (126). Thus, by preventing GABA synthesis, GABAB receptors would not be activated, preventing down-stream actions of estradiol - attenuating lordosis behavior. These results indicate that estradiol-induced MOR activation is maintained at least in part by GABAB signaling. Antagonizing GABAB receptors thirty hours after estradiol priming mimics the action of progesterone in this circuit (124). Whether progesterone acts through silencing GABAB receptors will require further study, but is an intriguing possibility.
Spinogenesis
The idea that estradiol regulates synaptic interactions is a very old one (127). While the most dramatic effects of estradiol on neuronal morphology occur during development, significant steroid regulation of dendritic structure also occurs in adulthood (128, 129; reviewed in 130). In the VMH, a nucleus intimately associated with sexual receptivity, estradiol increased spine density and dendritic branching (131–134). Estradiol also reduced the length of long primary dendrites that extend laterally out of the VMH the potential site of afferents from the MPN that are inhibited by β-END (51). In the context of the lordosis regulating circuit, as MOR inhibition wears off or is blocked with progesterone, excitatory afferents contact newly formed dendritic spines, stimulating the VMH.
Recently, we demonstrated estradiol-induced morphological plasticity in the ARH (135–137). As in the VMH, estradiol treatment induced dendritic spines within 4 hours of estradiol treatment. Once induced, spine density did not change for 48 hours, but the spine morphology suggested a process of maturation (Figure 1). In the ARH, the newly formed spines were filapodial and only slowly took on a more mature morphology (136). Filapodial spines are considered immature, unstable and nonfunctional (138). Moreover, filapodial spines are highly labile, rapidly appearing and disappearing during intense neural activity until they are stabilized by contacting an appropriate presynaptic partner (139–141). Mushroom-shaped spines are thought to be mature, stable and functional. The larger heads of mushroom-shaped spines contain an extensive protein rich structure, known as the postsynaptic density, which is composed of receptors and anchoring proteins that allow for efficient synaptic transmission. Stabilization involves receptors recruited into the spine membrane and anchored at the postsynaptic specialization by scaffold proteins (reviewed in 142). In the ARH, mushroom-shaped spines appeared some 20 hours after estradiol treatment when the post-estradiol refractory period expires and lordosis behavior can be elicited with progesterone treatment (Figure 1).
Spinogenesis requires the rearrangement of β-actin underlying filapodial outgrowth. In the ARH, this increase in β-actin immunoreactivity is correlated with direct observation of an increase in spines demonstrated with Golgi staining (136). Pharmacological inhibition of β-actin polymerization with cytochalasin D prevents spinogenesis. Actin remodeling is regulated by group I mGluR induced cell signaling (143, 144). Thus, we hypothesized that the estrogenic regulation of spinogenesis involves the ERα-mGluR1a signaling complex and modulates actin dynamics through phosphorylation of molecules important for spine formation including cofilin, an actin depolymerizing factor (for review see 145–147). Cofilin must be deactivated for formation of filamentous actin, which occurs when it is phosphorylated. Within an hour of estradiol treatment, phosphorylated cofilin (p-cofilin) levels are increased in the ARH (136). Estradiol-induced phosphorylation of cofilin is attenuated by antagonism of mGluR1a implicating the mERα-mGluR1a complex in the cell signaling. Cofilin deactivation allows for the establishment of new spines (148, 149). Thus, in the final analysis, estradiol membrane-initiated signaling regulated the actin cytoskeleton inducing the formation of filapodial dendritic spines in the ARH. Deactivated p-cofilin has been implicated in stabilizing long term potentiation (LTP) through the expansion of synaptic contacts (150) suggesting that estradiol regulation of cofilin activity may explain the generation and maturation of dendritic spines associated with lordosis behavior. Injection of estradiol-primed rats with cytochalasin D into the ARH prevents the formation of spines and abrogates the lordosis behavior induced in non-cytochalasin treated controls (136). One seductive hypothesis is that estradiol rapidly induces labile spines that require another stimulus that stabilizes them (151; reviewed in 152). This is supported by the observation in the hippocampus, where estradiol is paired with an LTP protocol results in an increase in connectivity (153). In the ARH and the VMH, estradiol provides the initial spinogenesis, but the nature of the second stimulus remains to be elucidated.
MEMBRANE ESTROGEN RECEPTORS REGULATING SEXUAL RECEPTIVITY
It has become clear that estradiol has actions that are mediated at the membrane to initiate cell signaling and transcription as well as directly at the nucleus to modulate transcription (154, 155). Although there is increasing evidence that a number of putative ERs may participate in the estradiol induction of lordosis behavior, only the ERα appears to be critical. Knock down of ERα expression inhibits facilitation of sexual receptivity (156–160) and prevents the estrogenic activation of the lordosis regulating circuitry in the hypothalamus (110). Overwhelming evidence suggests that the same ER responsible for nuclear-initiated signaling also mediate membrane-initiated signaling by interacting with metabotropic glutamate receptors (mGluR; 161). Indeed, we have demonstrated the importance of membrane ERα-mGluR signaling in ARH neurons in order to produce female sexual receptivity (108). More recently, using primary cultures of embryonic hypothalamic NPY neurons (162), the presence of membrane ERα and estradiol regulation of ERα and mGluR1a levels on the cell membrane were demonstrated (118). These NPY neurons appear to be the point of initial estradiol stimulation of hypothalamic circuits regulating lordosis reflex (94, 108, 115; Figure 1).
As described above, estradiol rapidly activates ARH neurons releasing NPY. In immortalized N-38 neurons, estradiol rapidly induced levels of free cytoplasmic calcium ([Ca2+]i) and the phosphorylation of PKCθ (118), necessary steps in the activation of lordosis behavior. mGluR1a antagonism blocked both of these estradiol actions indicating the transactivation of mGluR1a by ERα (163) as described for embryonic hypothalamic neurons and adult astrocytes (121, 164). Significantly, estradiol regulated levels of membrane ERα levels through PKC activation in N-38 neurons. Estradiol increased the levels of ERα in the cell membrane and the activation of PKCθ paralleled the ERα insertion into the cell surface, an action blocked with bisindolylmaleimide, a PKC pathway inhibitor. This same novel PKC has been implicated in regulating membrane estradiol actions that facilitate sexual receptivity (115). This type of PKC regulation has been observed for other membrane receptors, as well. For example, stimulating PKC induces a rapid delivery of N-methyl-d-aspartate (NMDA) glutamate receptors to the cell membrane involving exocytosis and the soluble NSF-associated protein SNAP (165).
Interestingly, ERα is present on the N-38 cell membrane as both a full length (66 kDa) and a slice variant, ERαΔ4 (52 kDa), as seen in other hypothalamic neurons and astrocytes (118, 121, 136, 166, 167). Such a splice variant mRNA has been described in brain homogenates (168), but these studies demonstrated that the ERαΔ4 is targeted to the membrane. The deletion of exon 4 precludes the transcription of a protein with a nuclear translocation signal and potentially unable to bind estradiol since the alternatively spliced exon codes for part of the ligand binding domain. Our results indicate that it is the full-length ERα that interacts with the mGluR1a to initiate cell signaling, the function of this spice variant is at present unclear. It is become more evident that in addition to the full length ERα, other splice variants are present in the brain. For example, some authors suggest that an alternatively spliced ERα missing exon 7 (ERαΔ7) is the most common variant (169). Although, we did not detect the ERαΔ7 protein in our hypothalamic, cultured neuron or astrocyte preparations either by western blot or PCR for the alternatively spliced mRNA, other regions of the brain may have enriched expression of such ERα variants (118).
GPR30
A rather unexpected finding was that the ER antagonist, ICI 182,780, elicited cell signaling and receptor trafficking when given in the absence of exogenous estradiol. Such startling findings have been reported in other preparations (170–172), as well as in vivo where ICI 182,780 facilitated lordosis behavior in estradiol-primed nonreceptive rats (123). However, when N-38 neurons were sequentially treated with ICI 182,780 and estradiol, both effects were attenuated (118). One possibility is that ICI 182,780 is activating another ER or that the antagonist structurally alters ERα protein to influence signaling (173). In hippocampal neurons, it has been suggested that GPR30 (also called GPER) is the ER (172). Indeed, in nonreceptive estradiol-primed rats, the activation of GPR30 with either its agonist, G-1, or free estradiol facilitated lordosis within 30 minutes which was blocked by the GPR30 antagonist, G15 (Figure 1; 174). However, the idea that GPR30 is an ER remains controversial (175). GPR30 does not mediate estrogenic responses in reproductive organs in mice (176–178) and while GRP30 has been located in a number of different cells (179–183), surface biotinylation, a method for labeling membrane proteins, does not reveal GRP30, suggesting that GPR30 is not present on cell membranes of hypothalamic neurons or astrocytes (166, 167, 184). Since estradiol can access intracellular receptors as easily as those on the cell membrane, the location of GPR30 on the smooth endoplasmic reticulum, rather than on the cell membrane, may be moot. Indeed, the putative GPR30 agonist, G1, lethargically increased [Ca2+]i in adult hypothalamic astrocytes, and stimulated progesterone synthesis (184). In cultured hypothalamic neurons, estradiol and G1 induced calcium oscillations, which ICI 182780 not only did not inhibit but caused oscillations itself. But the estradiol and G1 effects were blocked after treatment with GPR30 siRNA (185). While these data are provocative, in breast cancer cells, G1 appears to activate an ERα splice variant, ERα36 which is preferentially targeted to the cell membrane (186). Thus, it remains to be established whether GPR30 is a unique ER in the brain or increases the expression of ERα and its splice variants and thus signals through these ERs.
Gq-mER
Another putative receptor that has been implicated in estradiol membrane signaling is activated by the tamoxifen analogue, STX (187). Kelly and colleagues named this STX-activated protein the Gq-mER based on its ability to induce phosphorylation of a novel PKC, PKCδ, and uncouple an inwardly rectifying potassium channel flux (GIRK) in guinea pig ARH (188). While the receptor has not been structurally characterized or its gene cloned, the present results indicate that Gq-mER behaves like a membrane ER. STX has no affinity for the classical ERs or for GPR30 (reviewed in 189). STX is blocked by ICI 182,780 and has a pharmacological profile similar to the ERα agonist PPT (4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (184, 188, 190). As with estradiol or E-BSA, site-specific injections of STX into the ARH induced MOR internalization and stimulated sexual receptivity (191). Similarly, the mGluR1a antagonist, LY367,385, blocked the ability of STX to induce MOR internalization in the medial preoptic nucleus (Figure 1). While the nature of the STX activation of the mGluR1a must await the characterization of the Gq-mER, these results are consistent with the emerging idea that there are several membrane ERs involved in the estradiol activation of circuits in the CNS that signal through mGluR (155, 191).
CONCLUSIONS
The neural control of lordosis behavior appeared to be well worked out at the end of the last century. A convincing model had been worked out involving steroid regulated transcription of proteins and neuropeptides throughout several dispersed neural circuits that controlled this reflexive behavior. However, in the first years of the current millennium, our understanding of the mechanisms of estrogenic action and even the lordosis-regulating circuit has undergone significant revision. It became increasingly clear that estrogens had both immediate and longer-term actions. The former affected cell signaling and the latter mediated transcriptional regulation. Studies, using various techniques, established that immediate estrogen actions were mediated by membrane ERs. Although a number of putative mERs have been implicated in estradiol membrane signaling, the best evidence is that nuclear ERα through transactivation of mGluR1a mediates estrogenic actions related to the induction of sexual receptivity. The mERα-mGluR1a complex is trafficked to the membrane in association with the scaffold protein CAV-1. Within the circuit, the evidence points to the ARH where mERα induces dendritic spines and the activation of NPY-Y1 receptors on POMC neurons that release β-END in the MPN producing a transient inhibition of lordosis behavior mediated by MOR. Relief of this inhibition allows the expression of sexual receptivity. In the intact rodent, progesterone in the ARH inhibits β-END neurons through the deactivation of excitatory circuits and activation of a combination of inhibitory circuits that releases the MPN MOR inhibition. During this active inhibition, estrogen-dependent gene transcription occurs, including the expression of progesterone receptors and proteins needed for dendritic spine stabilization. Thus, both the immediate, membrane-initiated cell signaling and the direct nuclear estrogen action are required for full sexual receptivity.
Acknowledgements
This work was supported by NIH Grants DA013185 & HD042635 to PM and HD058638 to KS.
LITERATURE CITED
- 1.Boling FL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinol. 1939;25:359–364. [Google Scholar]
- 2.Rainbow T, Davis P, McEwen B. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 3.Rainbow TC, McGinnis MY, Davis PG, McEwen BS. Application of anisomycin to the lateral ventromedial nucleus of the hypothalamus inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1982;233:417–423. doi: 10.1016/0006-8993(82)91217-3. [DOI] [PubMed] [Google Scholar]
- 4.Parsons B, Rainbow TC, Pfaff DW, McEwen BS. Hypothalamic protein synthesis essential for the activation of the lordosis reflex in the female rat. Endocrinology. 1982;110(2):620–624. doi: 10.1210/endo-110-2-620. [DOI] [PubMed] [Google Scholar]
- 5.Wu TJ, Glucksman MJ, Roberts JL, Mani SK. Facilitation of lordosis in rats by a metabolite of luteinizing hormone releasing hormone. Endocrinology. 2006;147(5):2544–2549. doi: 10.1210/en.2005-1646. [DOI] [PubMed] [Google Scholar]
- 6.Hammer RP, Cheung S. Sex steroid regulation of hypothalamic opioid function. In: Micevych PE, Hammer RP, editors. Neurobiological Effects of Sex Steroid Hormones. New York, NY: Cambridge University Press; 1995. pp. 143–159. [Google Scholar]
- 7.Hammer RP. Mu-opiate receptor binding in the medial preoptic area is cyclical and sexually dimorphic. Brain Res. 1990;515:187–192. doi: 10.1016/0006-8993(90)90595-3. [DOI] [PubMed] [Google Scholar]
- 8.Pfaus JG, Pfaff DW. Mu-, delta-, and kappa-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm Behav. 1992;26(4):457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- 9.Priest CA, Borsook D, Hyman SE, Pfaff DW. Estrogen and stress interact to regulate the transcriptional activity of a proenkephalin promoter-beta-GAL fusion gene in the hypothalamus of transgenic mice. Soc Neurosci Abs. 1995;21:1364. [Google Scholar]
- 10.Quinones-Jenab V, Ogawa S, Jenab S, Pfaff DW. Estrogen regulation of preproenkephalin messenger RNA in the forebrain of female mice. J Chem Neuroanat. 1996;12(1):29–36. doi: 10.1016/s0891-0618(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 11.Romano GJ, Bonner TI, Pfaff DW. Preprotachykinin gene expression in the mediobasal hypothalamus of estrogen-treated and ovariectomized control rats. Experimental Brain Research. 1989;76:21–26. doi: 10.1007/BF00253619. [DOI] [PubMed] [Google Scholar]
- 12.Romano GJ, Harlan RE, Shiverst BD, Howells RD, Pfaff DW. Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol Endocrinol. 1988;2:1320–1328. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 13.Romano GJ, Mobbs CV, Howells RD, Pfaff DW. Estrogen regulation of proenkephalin gene expression in the ventromedial hypothalamus of the rat: Temporal qualities and synergism with progesterone. Brain Res Mol Brain Res. 1989;5:51–58. doi: 10.1016/0169-328x(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 14.Torii M, Kubo K. The effects of intraventricular injection of beta-endorphin on initial estrogen action to induce lordosis behavior. Physiol Behav. 1994;55(1):157–162. doi: 10.1016/0031-9384(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 15.Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: the effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195(3):167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- 16.Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212(1):68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- 17.Torii M, Kubo K, Sasaki T. Facilitatory and inhibitory effects of beta-endorphin on lordosis in female rats: relation to time of administration. Horm Behav. 1999;35(3):271–278. doi: 10.1006/hbeh.1999.1526. [DOI] [PubMed] [Google Scholar]
- 18.Pfaus JG, Gorzalka BB. Opioids and sexual behavior. Neurosci Biobehav Rev. 1987;11(1):1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- 19.Pfaus JG, Gorzalka BB. Selective activation of opioid receptors differentially affects lordosis behavior in female rats. Peptides. 1987;8(2):309–317. doi: 10.1016/0196-9781(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 20.Pfaff DW. Luteinizing hormone-releasing factor potentiates lordosis behavior in hypophysectomized ovariectomized female rats. Science. 1973;182(117):1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- 21.Bloch GJ, Dornan WA, Babcock AM, Gorski RA, Micevych PE. Effects of site-specific CNS microinjection of cholecystokinin on lordosis behavior in the male rat. Physiology and Behavior. 1989;46(4):725–730. doi: 10.1016/0031-9384(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 22.Babcock AM, Block GJ, Micevych PE. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol Behav. 1988;43(2):195–199. doi: 10.1016/0031-9384(88)90237-5. [DOI] [PubMed] [Google Scholar]
- 23.Bloch GJ, Babcock AM, Gorski RA, Micevych PE. Effects of cholecystokinin on male copulatory behavior and lordosis behavior in male rats. Physiology and Behavior. 1988;43:351–357. doi: 10.1016/0031-9384(88)90198-9. [DOI] [PubMed] [Google Scholar]
- 24.Bloch GJ, Butler PC, Kohlert JG. Galanin microinjected into the medial preoptic nucleus facilitates female- and male-typical sexual behaviors in the female rat. Physiol Behav. 1996;59(6):1147–1154. doi: 10.1016/0031-9384(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 25.Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117(6):2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- 26.Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45(2):152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- 27.Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. New York: Plenum Press; 1985. pp. 183–227. [Google Scholar]
- 28.Pfaff DW. Nature of sex hormone effects on rat sex behavior: Specificity of effects and individual patterns of response. J Comp Physiol Psychol. 1970;73:349–358. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- 29.Quadagno DM, McCullough J, Langan R. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3(3):175–179. doi: 10.1016/0018-506x(72)90029-3. [DOI] [PubMed] [Google Scholar]
- 30.Powers JB. Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiol and Behav. 1970;5:831–835. doi: 10.1016/0031-9384(70)90167-8. [DOI] [PubMed] [Google Scholar]
- 31.Beach FA. Hormones and Behavior. New York: Paul B Hoeber; 1948. [Google Scholar]
- 32.Young WC. The hormones and mating behavior. Baltimore: Willims and Willims; 1961. [Google Scholar]
- 33.Sodersten P, Eneroth P. Estradiol and progesterone in the control of sexual receptivity in female rats. Scand J Psychol. 1982;(Suppl 1):127–132. doi: 10.1111/j.1467-9450.1982.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 34.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21(15):5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green R, Luttge WG, Whalen RE. Induction of receptivity in ovariectomized rats by a single intravenous injection of estradiol-17-B. Physiol Behav. 1970;5:137–141. doi: 10.1016/0031-9384(70)90056-9. [DOI] [PubMed] [Google Scholar]
- 36.Shughrue PJ, Lubahn DB, NegroVilar A, Korach KS, Merchenthaler I. Responses in the brain of estrogen receptor alpha-disrupted mice. Proc Natl Acad Sci U S A. 1997;94(20):11008–11012. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. Journal of Comparative Neurology. 2000;427(2):185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Parsons B, MacLusky NJ, Kreiger MS, McEwen BS, Pfaff DW. The effects of long-term estrogen exposure on the induction of sexual behavior and measurements of brain estrogen and progestin receptors in the female rat. Horm Behav. 1979;13:301–313. doi: 10.1016/0018-506x(79)90047-3. [DOI] [PubMed] [Google Scholar]
- 39.Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107(3):774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- 40.McGinnis MY, Parsons B, Rainbow TC, Krey LC, McEwen BS. Temporal relationship between cell nuclear progestin receptor levels and sexual receptivity following intravenous progesterone administration. Brain Res. 1981;218(1–2):365–371. doi: 10.1016/0006-8993(81)91315-9. [DOI] [PubMed] [Google Scholar]
- 41.Parsons B, Rainbow TC, Pfaff DW, McEwen BS. Oestradiol, sexual receptivity and cytosol progestin receptors in rat hypothalamus. Nature. 1981;292(5818):58–59. doi: 10.1038/292058a0. [DOI] [PubMed] [Google Scholar]
- 42.Whalen RE. Estrogen-progesterone induction of mating in female rats. Horm Behav. 1974;5(2):157–162. doi: 10.1016/0018-506x(74)90040-3. [DOI] [PubMed] [Google Scholar]
- 43.Fadem BH, Barfield RJ, Whalen RE. Dose-response and time-response relationships between progesterone and the display of patterns of receptive and proceptive behavior in the female rat. Horm Behav. 1979;13(1):40–48. doi: 10.1016/0018-506x(79)90033-3. [DOI] [PubMed] [Google Scholar]
- 44.Powers JB. Facilitation of lordosis in ovariectomized rats by intracerebral progesterone implants. Brain Res. 1972;48:311–325. doi: 10.1016/0006-8993(72)90186-2. [DOI] [PubMed] [Google Scholar]
- 45.Pfaus JG, Manitt C, Coopersmith CB. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiology & Behavior. 2006;89(5):627–636. doi: 10.1016/j.physbeh.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Pfaus JG, Marcangione C, Smith WJ, Manitt C, Abillamaa H. Differential induction of Fos in the female rat brain following different amounts of vaginocervical stimulation: modulation by steroid hormones. Brain Res. 1996;741(1–2):314–330. doi: 10.1016/s0006-8993(96)00985-7. [DOI] [PubMed] [Google Scholar]
- 47.Georgescu M, Sabongui C, Del Corpo A, Marsan L, Pfaus JG. Vaginocervical stimulation induces Fos in glutamate neurons in the ventromedial hypothalamus: attenuation by estrogen and progesterone. Horm Behav. 2009;56(4):450–456. doi: 10.1016/j.yhbeh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Micevych PE, Ulibarri C. Development of the limbic-hypothalamic cholecystokinin circuit: A model of sexual differentiation. Dev Neurosci. 1992;14(1):11–34. doi: 10.1159/000111643. [DOI] [PubMed] [Google Scholar]
- 49.Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54(3):347–354. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Ulibarri C, Micevych PE. Role of perinatal estrogens in sexual differentiation of the inhibition of lordosis by exogenous cholecystokinin. Physiol and Behav. 1993;54:95–100. doi: 10.1016/0031-9384(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 51.Sinchak K, Garcia BL, Bowlby R, Charukulvanich P, Garcia MP, Sanathara NM. Mu-opioid receptor neurons and opioid receptor-like receptor neurons in the medial preoptic nucleus project to the region of the ventromedial nucleus of the hypothalamus. San Diego: Society for Neuroscience; 2010. [Google Scholar]
- 52.Polovin G, Bowlby R, Garcia BL, Thach V, Tea P, Seng H, Sinchak K. Subpopulation of m-opioid receptor neurons in the medial preoptic nucleus express estrogen receptor-a and opioid receptor-like receptor-1. New Orleans, LA, USA: Society for Neuroscience; 2012. [Google Scholar]
- 53.Micevych PE, Dewing P. Membrane-initiated estradiol signaling regulating sexual receptivity. Frontiers in Endocrinology. 2011;2:26. doi: 10.3389/fendo.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaff DW, Schwartz-Giblin S, McCarthy M, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press, Ltd; 1994. pp. 107–220. [Google Scholar]
- 55.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290(1–2):44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7(1):105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 57.Coria-Avila GA, Pfaus JG. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148(3):623–632. doi: 10.1016/j.neuroscience.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 58.Mendelson SD, Gorzalka BB. An improved chamber for the observation and analysis of the sexual behavior of the female rat. Physiol Behav. 1987;39(1):67–71. doi: 10.1016/0031-9384(87)90345-3. [DOI] [PubMed] [Google Scholar]
- 59.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum the female rat during paced copulatory behavior. Behav Neurosci. 1995;109(2):354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 60.Pfaus JG, Smith WJ, Coopersmith CB. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I. A correlational and factor analysis and the effects of ovarian hormones. Horm Behav. 1999;35(3):224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- 61.Yang LY, Clemens LG. Influence of male-related stimuli on female postejaculatory refractory period in rats. Physiol Behav. 1998;63(4):675–682. doi: 10.1016/s0031-9384(97)00523-4. [DOI] [PubMed] [Google Scholar]
- 62.Micevych P, Sinchak K. The Neurochemistry of Limbic-Hypothalamic Circuits Regulating Sexual Receptivity. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd ed. New York: Springer; 2007. pp. 151–193. [Google Scholar]
- 63.Flanagan LM, Pfaus JG, Pfaff DW, McEwen BS. Induction of FOS immunoreactivity in oxytocin neurons after sexual activity in female rats. Neuroendocrinology. 1993;58(3):352–358. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- 64.Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RB, Pfaff DW. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624(1–2):253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- 65.Hardy DF, Debold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiology & Behavior. 1971;7(4):643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- 66.Clark AS, Roy EJ. Behavioral and cellular responses to pulses of low doses of estradiol-17 beta. Physiol Behav. 1983;30(4):561–565. doi: 10.1016/0031-9384(83)90221-4. [DOI] [PubMed] [Google Scholar]
- 67.Sodersten P, Eneroth P, Hansen S. Induction of sexual receptivity in ovariectomized rats by pulse administration of oestradiol-17 beta. J Endocrinol. 1981;89(1):55–62. doi: 10.1677/joe.0.0890055. [DOI] [PubMed] [Google Scholar]
- 68.Blasberg ME, Clark AS. Anabolic-androgenic steroid effects on sexual receptivity in ovariectomized rats. Horm Behav. 1997;32(3):201–208. doi: 10.1006/hbeh.1997.1422. [DOI] [PubMed] [Google Scholar]
- 69.Dohanich GP, Clemens LG. Inhibition of estrogen-activated sexual behavior by androgens. Horm Behav. 1983;17(4):366–373. doi: 10.1016/0018-506x(83)90046-6. [DOI] [PubMed] [Google Scholar]
- 70.Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci. 1992;106(4):690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- 71.Bloch GJ, Babcock AM, Gorski RA, Micevych PE. Cholecystokinin stimulates and inhibits lordosis behavior in female rats. Physiol Behav. 1987;39(2):217–224. doi: 10.1016/0031-9384(87)90012-6. [DOI] [PubMed] [Google Scholar]
- 72.Sodersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to receptivity in intact and ovariectomized rats. J Endocrinol. 1981;89(1):45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- 73.Mani SK, Blaustein JD, Omalley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31(3):244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 74.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res Rev. 2008;57(2):470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsons B, McEwen BS, Pfaff DW. A discontinuous schedule of estradiol treatment is sufficient to activate progesterone-facilitated feminine sexual behavior and to increase cytosol receptors for progestins in the hypothalamus of the rat. Endocrinology. 1982;110(2):613–619. doi: 10.1210/endo-110-2-613. [DOI] [PubMed] [Google Scholar]
- 76.Moguilewsky M, Raynaud JP. The relevance of hypothalamic and hypophyseal progestin receptor regulation in the induction and inhibtion of sexual behavior in the female rat. Endocrinol. 1979;105:516–522. doi: 10.1210/endo-105-2-516. [DOI] [PubMed] [Google Scholar]
- 77.Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101(1):67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- 78.Moss RL. Relationship between the central regulation of gonadotropins and mating behavior in female rats. Adv Behav Biol. 1974;11:55–76. doi: 10.1007/978-1-4684-3069-1_3. [DOI] [PubMed] [Google Scholar]
- 79.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78(1):29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 80.Parsons B, Rainbow TC, Snyder L, McEwen BS. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology. 1984;39(1):25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- 81.Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horm Behav. 1980;14(1):65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- 82.Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Horm Behav. 2011;60(5):540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51(3):406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goy RW, Phoenix CH, Young WC. Inhibitory action in the corpus luteum on the hormonal induction of estrous behavior in the guinea pig. Gen Comp Endocrinol. 1966;6(2):267–275. doi: 10.1016/s0016-6480(66)80014-x. [DOI] [PubMed] [Google Scholar]
- 85.Nadler RD. A biphasic influence of progesterone on sexual receptivity of spayed female rats. Physiol Behav. 1970;5(1):95–97. doi: 10.1016/0031-9384(70)90019-3. [DOI] [PubMed] [Google Scholar]
- 86.Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res. 2004;153(2):295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 87.Butler PC, Mills RH, Bloch GJ. Inhibition of lordosis behavior in male and female rats by androgens and progesterone. Horm Behav. 2001;40(3):384–395. doi: 10.1006/hbeh.2001.1703. [DOI] [PubMed] [Google Scholar]
- 88.O'Malley BW, Schrader WT, Mani S, Smith C, Weigel NL, Conneely OM, Clark JH. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 89.Mani SK, Mitchell A, O'Malley BW. Progesterone receptor and dopamine receptors are required in Delta(9)-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci U S A. 2001;98(3):1249–1254. doi: 10.1073/pnas.031563998. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auger AP, Moffatt CA, Blaustein JD. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology. 1997;138(1):511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- 91.Mahavongtrakul M, Phan J, Sinchak K. Progesterone receptors A and B are found on the plasma membrane of the arcuate nucleus of the hypothalamus but do not complex with dopamine D1 receptor to facilitate lordosis. San Diego, CA. USA: Society for Neuroscience; 2013. [Google Scholar]
- 92.Lima-Hernandez FJ, Beyer C, Gomora-Arrati P, Garcia-Juarez M, Encarnacion-Sanchez JL, Etgen AM, Gonzalez-Flores O. Src kinase signaling mediates estrous behavior induced by 5beta-reduced progestins, GnRH, prostaglandin E2 and vaginocervical stimulation in estrogen-primed rats. Horm Behav. 2012;62(5):579–584. doi: 10.1016/j.yhbeh.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Flores O, Beyer C, Gomora-Arrati P, Garcia-Juarez M, Lima-Hernandez FJ, Soto-Sanchez A, Etgen AM. A role for Src kinase in progestin facilitation of estrous behavior in estradiol-primed female rats. Horm Behav. 2010;58(2):223–229. doi: 10.1016/j.yhbeh.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y–Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24(4):947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheung S, Hammer R. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in the arcuate neurons that innervate the medial preoptic are of the rat. Neuroendocrinology. 1995;62:283–292. doi: 10.1159/000127015. [DOI] [PubMed] [Google Scholar]
- 96.Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci. 2000;20(17):6707–6713. doi: 10.1523/JNEUROSCI.20-17-06707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacobowitz DM, O'Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci U S A. 1978;75(12):6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56(6):1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 99.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144(4):1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 100.Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: Substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92(7):2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1977;74(8):3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arden JR, Segredo V, Wang Z, Lameh J, Sadaee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65(4):1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- 103.Keith D, Murray S, Zaki P, Chu P, Lisson D, Kang L, Aimi J, Evans C, von Zastrow M. Rapid endocytosis of opioid receptors: Differential regulation by opioid peptide and morphine. Soc Neurosci Abstr. 1995;21:1353. [Google Scholar]
- 104.Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53(3):377–384. [PubMed] [Google Scholar]
- 105.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Bio Chem. 1996;271(32):19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 106.Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human mu opioid receptor. Journal of Neuroscience. 1995;15(3 Pt 2):2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sirinathsinghji DJS. Regulation of lordosis behavior in the female rat by corticotropin-releasing factor, beta-endorphin/corticotropin and luteinizing hormone-releasing hormone neuronal systems in the medial preoptic area. Brain Res. 1986;375:149–156. doi: 10.1016/0006-8993(86)90957-1. [DOI] [PubMed] [Google Scholar]
- 110.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71(6):802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 111.Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16(15):1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 112.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27(2):197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 113.Acosta-Martinez M, Etgen AM. Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Horm Behav. 2002;41(1):88–100. doi: 10.1006/hbeh.2001.1741. [DOI] [PubMed] [Google Scholar]
- 114.Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64(1–2):64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 115.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149(12):5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sar M, Sahu A, Crowley WR, Kalra SP. Localization of neuropeptide-Y immunoreactivity in estradiol-concentrating cells in the hypothalamus. Endocrinology. 1990;127(6):2752–2756. doi: 10.1210/endo-127-6-2752. [DOI] [PubMed] [Google Scholar]
- 117.Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. Journal of Comparative Neurology. 1999;411(2):346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 118.Dominguez R, Dewing P, Kuo J, Micevych P. Membrane-initiated estradiol signaling in immortalized hypothalamic N-38 neurons. Steroids. 2013 doi: 10.1016/j.steroids.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33(4):342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinology. 2013 doi: 10.1210/en.2013-1235. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. The Journal of Neuroscience. 2010;30(38):12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mana A, Garcia BL, Fuentes KN, Sinchak K. Differential activation/deactivation of lordosis circuit is dependent on estradiol dosage. Chicago: Society for Neuroscience; 2009. [Google Scholar]
- 123.Garcia BL, Mana A, Kim A, Sinchak K. Antagonism of estrogen receptors facilitates sexual receptivity through opioid circuits in the arcuate nucleus of the hypothalamus and the medial preoptic nucleus in estradiol primed non-receptive female rats. San Diego: Society for Neuroscience; 2010. [Google Scholar]
- 124.Sinchak K, Dewing P, Ponce L, Gomez L, Christensen A, Berger M, Micevych P. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Horm Behav. 2013;64(1):136–143. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Torii M, Kubo K, Sasaki T. Differential effects of beta-endorphin and Met- and Leu-enkephalin on steroid hormone-induced lordosis in ovariectomized female rats. Pharmacology, Biochemistry and Behavior. 1997;58(4):837–842. doi: 10.1016/s0091-3057(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 126.McCarthy MM, Masters DB, Rimvall K, Schwartz-Giblin S, Pfaff DW. Intracerebral administration of antisense oligodeoxynucleotides to GAD65 and GAD67 mRNAs modulate reproductive behavior in the female rat. Brain Res. 1994:209–220. doi: 10.1016/0006-8993(94)91019-7. [DOI] [PubMed] [Google Scholar]
- 127.Matsumoto A, Arai Y. Synaptogenic effect of estrogen on the hypothalamic arcuate nucleus of the adult female rat. Cell Tissue Res. 1979;198(3):427–433. doi: 10.1007/BF00234187. [DOI] [PubMed] [Google Scholar]
- 128.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 129.Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215(3–4):187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33(4):331–341. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51(5):530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- 132.Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447(3):234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- 133.Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20(4):1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432(3):329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 135.Christensen A, Bentley GE, Cabrera R, Ortega HH, Perfito N, Wu TJ, Micevych P. Hormonal regulation of female reproduction. Horm Metab Res. 2012;44(8):587–591. doi: 10.1055/s-0032-1306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31(48):17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153(8):3872–3877. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 139.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420(6917):812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 140.Parnass Z, Tashiro A, Yuste R. Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons. Hippocampus. 2000;10(5):561–568. doi: 10.1002/1098-1063(2000)10:5<561::AID-HIPO6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 141.Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 142.Micevych P, Christensen A. Membrane-initiated estradiol signaling regulates the central nervous system. Front Neuroendocrinol. 2012;33(4):329–330. doi: 10.1016/j.yfrne.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Cruz-Martin A, Crespo M, Portera-Cailliau C. Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice. PLoS One. 2012;7(2):e32446. doi: 10.1371/journal.pone.0032446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99(3):1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanchez AM, Flamini MI, Polak K, Palla G, Spina S, Mannella P, Genazzani AD, Simoncini T. Actin cytoskeleton remodelling by sex steroids in neurones. J Neuroendocrinol. 2012;24(1):195–201. doi: 10.1111/j.1365-2826.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- 146.Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J Neurobiol. 2004;58(1):103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- 147.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35(1):121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 149.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 150.Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci. 2007;27(30):8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105(38):14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31(45):16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29(41):12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30(3):315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96(2):103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138(1):507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 157.Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835(1):80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 158.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31(3):232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 159.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 160.Ogawa S, Gordan JD, Taylor J, Lubahn D, Korach K, Pfaff DW. Reproductive functions illustrating direct and indirect effects of genes on behavior. Horm Behav. 1996;30(4):487–494. doi: 10.1006/hbeh.1996.0052. [DOI] [PubMed] [Google Scholar]
- 161.Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19(6):413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145(1):393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- 163.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25(20):5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150(3):1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4(4):382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 166.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29(48):15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154(4):1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, Crews D. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proceedings of the National Academy of Sciences, USA. 1993 Aug;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ishunina TA, Swaab DF. Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol. 2008;24(2):93–98. doi: 10.1080/09513590701705148. [DOI] [PubMed] [Google Scholar]
- 170.Dudley MW, Sheeler CQ, Wang H, Khan S. Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action. Proc Natl Acad Sci U S A. 2000;97(7):3696–3701. doi: 10.1073/pnas.040558197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Robertson JA, Zhang Y, Ing NH. ICI 182,780 acts as a partial agonist and antagonist of estradiol effects in specific cells of the sheep uterus. J Steroid Biochem Mol Biol. 2001;77(4–5):281–287. doi: 10.1016/s0960-0760(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 172.Zhao L, O'Neill K, Brinton RD. Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J Pharmacol Exp Ther. 2006;319(3):1124–1132. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 173.Lupien M, Jeyakumar M, Hebert E, Hilmi K, Cotnoir-White D, Loch C, Auger A, Dayan G, Pinard GA, Wurtz JM, Moras D, Katzenellenbogen J, Mader S. Raloxifene and ICI182,780 increase estrogen receptor-alpha association with a nuclear compartment via overlapping sets of hydrophobic amino acids in activation function 2 helix 12. Mol Endocrinol. 2007;21(4):797–816. doi: 10.1210/me.2006-0074. [DOI] [PubMed] [Google Scholar]
- 174.Long NP, Chhorvann S, Sinchak K. In estradiol primed rats subsequent free estradiol rapidly facilitates lordosis through G-protein coupled receptor 30 (GPR30) New Orleans: Society for Neuroscience; 2012. [Google Scholar]
- 175.Otto C, Fuchs I, Altmann H, Klewer M, Schwarz G, Bohlmann R, Nguyen D, Zorn L, Vonk R, Prelle K, Osterman T, Malmstrom C, Fritzemeier KH. In vivo characterization of estrogen receptor modulators with reduced genomic versus nongenomic activity in vitro. J Steroid Biochem Mol Biol. 2008;111(1–2):95–100. doi: 10.1016/j.jsbmb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 176.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80(1):34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 177.Levin ER. Minireview: Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25(3):377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romano N, Lee K, Abraham IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149(11):5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12(21):6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 180.Kuo WH, Chang LY, Liu DL, Hwa HL, Lin JJ, Lee PH, Chen CN, Lien HC, Yuan RH, Shun CT, Chang KJ, Hsieh FJ. The interactions between GPR30 and the major biomarkers in infiltrating ductal carcinoma of the breast in an Asian population. Taiwan J Obstet Gynecol. 2007;46(2):135–145. doi: 10.1016/S1028-4559(07)60007-2. [DOI] [PubMed] [Google Scholar]
- 181.Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114(3):465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196(4):386 e1–386 e9. doi: 10.1016/j.ajog.2007.01.004. discussion e9–11. [DOI] [PubMed] [Google Scholar]
- 183.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 184.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. Journal of Neuroscience. 2010;30(39):12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23(3):349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23(29):9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73(9–10):985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 191.Christensen A, Micevych P. A novel membrane estrogen receptor activated by STX induces female sexual receptivity through an interacation with mGluR1a. Neuroendocrinology. 2013 Apr 6; doi: 10.1159/000351077. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]