Abstract
Objective
To describe risk factors for scar in eyes treated with ranibizumab or bevacizumab for neovascular age-related macular degeneration (AMD).
Design
Prospective cohort study within a randomized clinical trial.
Participants
Patients with no scar on color fundus photography (CFP) or fluorescein angiography (FA) at enrollment in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT).
Methods
Eyes were assigned to ranibizumab or bevacizumab treatment and to 1 of 3 dosing regimens for 2 years. Masked readers assessed CFP and FA. Baseline demographic characteristics, visual acuity, morphologic features on photography and optical coherence tomography (OCT), and genotypes associated with AMD risk were evaluated as risk factors using adjusted hazard ratios (aHRs) and associated 95% confidence intervals (CIs). Scars were classified as fibrotic with well-demarcated elevated mounds of yellowish white tissue or nonfibrotic with discrete flat areas of hyperpigmentation with varying amounts of central depigmentation.
Main Outcome Measures
Scar formation.
Results
Scar developed in 480 of 1059 eyes (45.3%) by 2 years. Baseline characteristics associated with greater risk of scarring were predominantly classic choroidal neovascularization (CNV) (aHR, 3.1; CI, 2.4–3.9) versus occult CNV, blocked fluorescence (aHR, 1.4; CI, 1.1–1.8), foveal retinal thickness >212 μm (aHR, 2.4; CI, 1.7–3.6) versus <120 μm, foveal subretinal tissue complex thickness >275 μm (aHR, 2.4; CI, 1.7–3.6) versus ≤75 μm, foveal subretinal fluid (aHR, 1.5; CI, 1.1–2.0) versus no subretinal fluid, and subretinal hyperreflective material (SHRM) (aHR, 1.7; CI, 1.3–2.3) versus no SHRM. Eyes with elevation of the retinal pigment epithelium had lower risk (aHR, 0.6; CI, 0.5–0.8) versus no elevation. Drug, dosing regimen, and genotype had no statistically significant association with scarring. Fibrotic scars developed in 24.7% of eyes, and nonfibrotic scars developed in 20.6% of eyes. Baseline risk factors for the scar types were similar except that eyes with larger lesion size or visual acuity <20/40 were more likely to develop fibrotic scars.
Conclusions
Approximately half of eyes enrolled in CATT developed scar by 2 years. Eyes with classic neovascularization, a thicker retina, and more fluid or material under the foveal center of the retina are more likely to develop scar.
Subretinal and retinal scarring are associated with profound vision loss and are natural outcomes of neovascular age-related macular degeneration (nvAMD).1–4 Because untreated choroidal neovascularization (CNV) progresses from a neovascular bundle to a variably mixed fibrovascular structure and eventually culminates in a scar, it causes local destruction of photoreceptors, retinal pigment epithelium (RPE), and choroidal blood vessels, leading to permanent alteration in macular morphology and reduction in vision. Eyes that develop fibrosis after photodynamic therapy for CNV have poor vision outcomes.5 Scar that develops after radiotherapy for nvAMD has been described.6,7 However, treatment patterns for nvAMD have changed in the past decade, and nearly all patients now receive treatment with intravitreal injections of drugs that target vascular endothelial growth factor (VEGF).8 Although anti-VEGF treatment generally stabilizes or improves visual acuity, scar formation has been identified as one of the causes of visual acuity loss after treatment.9
The factors associated with scarring after anti-VEGF therapy have not been described. In the Comparison of Age-related Macular Degeneration Treatments Trials (CATT), a multicenter clinical trial sponsored by the National Eye Institute, approximately 1200 patients were treated with the anti-VEGF drugs ranibizumab and bevacizumab and followed closely with visual acuity testing, optical coherence tomography (OCT), color fundus photography (CFP), and fluorescein angiography (FA). We describe the morphologic features of scars that evolve after anti-VEGF treatment, their incidence through 2 years of treatment, and associated baseline risk factors.
Methods
Enrollment and Follow-up of Subjects
Between February 2008 and December 2009, 1185 patients were enrolled in CATT through 43 clinical centers in the United States. Each patient had untreated active CNV secondary to age-related macular degeneration (AMD) in 1 eye, designated as the study eye. Inclusion and exclusion eligibility criteria and baseline morphologic features have been described previously.10 Key inclusion criteria included age ≥50 years and visual acuity between 20/25 and 20/320 in the study eye. At study entry, active CNV was considered present when both leakage on FA and fluid on time-domain OCT were documented through central image review.11,12 The neovascular complex or fluid needed to be under the fovea. At enrollment, scar at the foveal center was an exclusion criterion, but eyes with nonfoveal scarring that was <50% of the total CNV lesion were eligible. Patients were randomly assigned to treatment with intravitreal injections of ranibizumab or bevacizumab to 1 of 3 dosing regimens for the 2 years of the study: monthly injections, monthly evaluation with injection only when signs of active neovascularization were present (pro re nata [PRN]), or monthly evaluation for 1 year followed by PRN injections for 1 year. Patients were examined approximately every 28 days.10 Stereoscopic CFP, FA, and OCT scans were obtained at baseline, 1 year, and 2 years. Eyes receiving PRN therapy had monthly OCT scans. An institutional review board associated with each center approved the clinical trial protocol. All patients provided written inforemed consent. The study was compliant with Health Insurance Portability and Accountability Act regulations. The CATT was registered with ClinicalTrials.gov (NCT00593450).
Assessment of Images
Methods used to grade digital CFP, FA, and OCT scans in CATT have been described previously.11,12 At baseline, images were assessed for the following features: type of CNV; presence of contiguous hemorrhage, serous pigment epithelial detachment, or blocked fluorescence that was not due to hemorrhage; pathology at the foveal center; presence of CNV or scar in the fellow eye; and presence of geographic atrophy in both the study eye and the fellow eye. The area of CNV and the total CNV lesion was measured using Image J (available at http://rsbweb.nih.gov/ij/; Rasband WS, ImageJ, US National Institutes of Health, Bethesda, MD, 1997e2012). Identification of scar was based only on CFP and FA characteristics. Two broad scar categories were identified: fibrotic scars and nonfibrotic scars.
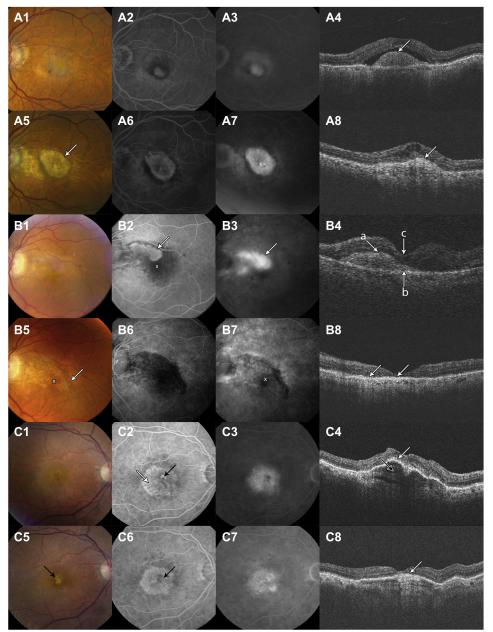
Fibrotic scars were defined as obvious white or yellow mounds of fibrous-appearing tissue that were well defined in shape and appeared solid on color stereo images. Figure 1A shows CNV at baseline with a dome-shaped subretinal hyperreflective material (SHRM) developing into a yellowish-brown solid fibrotic scar with a smaller and irregular SHRM at year 2. Hyperfluorescence due to tissue staining or blocked fluorescence of the underlying choroid was present on FA. When fibrotic scars were admixed with active neovascularization, there was leakage on angiography (Fig 1A).13 Other imaging modalities (e.g., OCT) may reveal characteristics that are not discernible on CFP and FA. Figure 1B illustrates classic CNV that does not involve the foveal center on FA, but SHRM on the OCT extends under the fovea. At 2 years, the CFP shows the developing fibrotic scar extending into the foveal center and beyond the baseline CNV area (Fig 1B5). The scar is hypofluorescent early and stains minimally in the late-phase FA (Fig 1B6, B7). The OCT scan at year 2 shows flattening of SHRM (Fig 1B8) overlying retinal thinning, loss of the photoreceptor outer segments, ellipsoid zone, and external limiting membrane. This case illustrates that in some cases the extent of classic CNV at baseline is better visualized by OCT than FA and may be an important feature in predicting whether a developing fibrotic scar is likely to involve the foveal center. Furthermore, the loss of outer retinal layers highlights the anatomic reasons for poor visual acuity typically associated with scar formation.
Figure 1.
Development of fibrotic scar from classic choroidal neovascularization (CNV). A, Choroidal neovascularization at baseline developing into a fibrotic scar at 2 years. B, Classic CNV seen on color fundus photography and fluorescein angiography (FA) at baseline does not extend into the foveal center (white X), whereas baseline optical coherence tomography shows subretinal hyperreflective material under the fovea (B4, white arrows). Color fundus photographs and FA at 2 years show a fibrotic scar extending into the foveal center and beyond the baseline CNV. C, Baseline early FA (C2) shows leakage (black arrow) that characterizes classic CNV within a large occult CNV lesion (C2, white arrow). Optical coherence tomography shows retinal pigment epithelium (RPE) elevation with a hyperreflective “onion peel” appearance in the sub-RPE space that corresponds to the occult lesion (C4, black arrow). At 2 years, there is a small yellow fibrotic scar (C5 , black arrow) at the site of the baseline classic CNV. There is no fibrous scarring in the area of occult CNV. Optical coherence tomography shows flattening of the RPE elevation (C8, white arrow) and a persisting “onion-peel” appearance in the subretinal space.
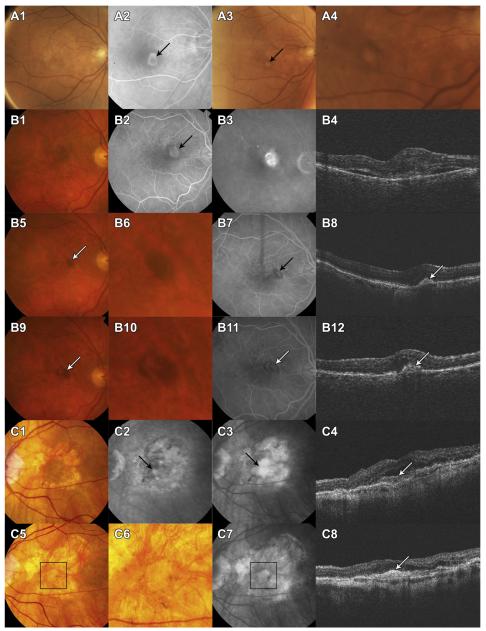
Nonfibrotic scars were typically flat, small, well-circumscribed areas of pigmentation with varying degrees of central hypopigmentation on CFP images (Fig 2). The peripheral pigmentary changes in these scars often followed the outline of the previously active CNV lesion. The hypopigmented area was flat, and choroidal vessels were not visible. Hyperfluorescence of the depigmented area appeared early on FA and persisted or increased in intensity in the late phase. Hypofluorescence on FA surrounding the hyperfluorescence corresponded to the pigmented borders apparent on CFP. In rare instances, flat yellowish areas with or without clearly demarcated hyperpigmented borders in the area of baseline CNV were present and classified as nonfibrotic scars (Fig 2C1–C8).
Figure 2.
Development of nonfibrotic scars. A, Classic choroidal neovascularization (CNV) (A2) at baseline. At 2 years, color fundus photography (CFP) (A3, A4) shows a circumscribed small area of hypopigmentation surrounded by a ring of dark pigmentation. B, Classic CNV at baseline (B1–4). Dark pigmentation in the area of the baseline CNV (B5 and B6, white arrow) at 1 year. Optical coherence tomography shows retinal pigment epithelium (RPE) elevation with hyperreflective material in the sub-RPE space. At 2 years, an area of hypopigmentation is seen within the darkly pigmented area. C, Baseline CFP shows several areas of geographic atrophy around the foveal center. Fluorescein angiogram shows classic CNV (complement component 3) and optical coherence tomography shows subretinal fluid overlying an area of subretinal hyperreflective material (C4, white arrow). There is increased signal penetration into the choroid. At 2 years, the CFP shows a small, yellow, flat scar in the area of baseline CNV, closely resembling the geographic atrophy (within the black square on C5). However, unlike the adjacent areas of geographic atrophy, in this region, the choroidal vessels are not visible. Optical coherence tomography shows thickened hyperreflective material (C8). The first 2 examples (A1-A4 and B1-B12) demonstrate the typical appearance of a nonfibrotic scar with signet-shaped hyperpigmentation surrounding an area of hypopigmentation. The last example (C1-C8) shows a flat scar without pigmentation that seems to be an uncommon presentation of nonfibrotic scar.
The location of fluid (intraretinal, subretinal, and sub-RPE); thickness at the foveal center of the retina, subretinal fluid, and subretinal tissue complex; presence of SHRM; RPE elevation; epiretinal membrane; and vitreomacular attachment were determined from OCT B-scan cross-sections. Time-domain OCT was performed at baseline through 1 year, and time-domain OCT or spectral-domain OCT was performed during the second year. The SHRM, RPE, and RPE elevation, excluding subretinal fluid, comprised the subretinal tissue complex.
Candidate Risk Factors
Candidate risk factors for scarring included baseline demographic characteristics, history of cigarette smoking, hypertension, diabetes, dietary supplementary use, cancers, hypercholesterolemia, osteoarthritis, anti-VEGF drug and regimen, visual acuity in the study eye and fellow eye, and glaucoma, as well as the morphologic features graded by the reading centers. Five single nucleotide polymorphisms (SNPs) previously associated with risk and progression of AMD were evaluated as risk factors for incident scar: (1) complement factor H Y402H (rs1061170), (2) age-related maculopathy susceptibility 2 (also called LOC387715) A69S (rs10490924), (3) high temperature requirement factor A1 (rs11200638), (4) complement component 3 R80G (rs2230199), and (5) toll-like receptor 3 (rs3775291).
Statistical Methods
Only subjects without scar at baseline were included. Each candidate risk factor, except for the SNPs, was first evaluated by univariate analysis (without adjustment for any other risk factors) using a discrete time Cox proportional hazard model for time to scar. The predictors with a P < 0.20 in the univariate analysis were included in a multivariate Cox proportional hazard model so that the independent effect of each predictor could be assessed. Treatment regimen was included as a time-dependent covariate to accommodate the treatment regimen re-randomization at 1 year for the patients treated monthly. The final multivariate model was created by applying a backward selection procedure that retained only those predictors with a P < 0.05, with the exception of drug and regimen groups, which were included in all multivariate models. Adjusted hazard ratios (aHRs) for scar development during 2 years and their 95% confidence intervals (CIs) were calculated on the basis of the final multivariate models. Similar analyses were performed separately for fibrotic scars and nonfibrotic scars. The association of scar formation and the number of risk alleles for each specific SNP was assessed with a logistic regression model that included age, sex, and smoking status. P values for the genetic analysis were adjusted to control for the false discovery rate.14 All data analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC).
Results
After excluding patients with scar at baseline (n = 46) and those without gradable photographs at both 1 and 2 years because of death, missed visits, or poor photograph quality (n = 80), there were 1059 patients eligible for scar risk factor analysis (Fig 3, available at http://aaojournal.org). At the end of 1 year, 339 eyes (32.0%) had developed a scar, and after 2 years of anti-VEGF therapy, 480 eyes (45.3%) had developed a scar.
The results of the univariate analysis are shown in Tables 1 to 3 (available at http://aaojournal.org). By univariate analysis, the risk factors associated with increased risk of scar were poor baseline visual acuity in the study eye; larger baseline CNV area; minimally and predominantly classic CNV; blocked fluorescence on angiography; hemorrhage associated with the lesion (included hemorrhage within and contiguous with the lesion, measured as approximate disc areas); greater retinal thickness, subretinal tissue complex thickness at the foveal center; and presence of intraretinal and subretinal fluid and SHRM. Factors associated with lower risk of scar were worse visual acuity in the fellow eye, retinal angiomatous proliferation (RAP) and geographic atrophy in the study eye, sub-RPE fluid, and RPE elevation. Systemic diseases, such as diabetes, hypertension, and hypercholesterolemia, were not associated with scar formation.
In the multivariate final model, several baseline features independently predicted scarring (Table 4). Eyes with predominantly classic CNV on FA (aHR, 3.1; 95% CI, 2.4–3.9) and minimally classic CNV on FA (aHR, 2.3; 95% CI, 1.8–3.0) had higher risk when compared with eyes with only occult CNV. This is illustrated in Figure 1C, where a small area of baseline classic CNV within a large occult CNV lesion (C2, white arrow) develops into a small yellow fibrotic scar at year 2. No scar development can be seen on CFP or FA in the area of the baseline occult CNV. The CNV lesions with blocked fluorescence had a higher risk when compared with CNV lesions without blocked fluorescence (aHR, 1.4; 95% CI, 1.1–1.8). Eyes with retinal thickness at the foveal center >212 μm on OCT (aHR, 2.4; 95% CI, 1.7–3.6) or retinal thickness between 120 and 212 μm (aHR, 1.6; 95% CI, 1.1–2.3) had higher risk than eyes with retinal thickness <120 μm. Risk of scarring increased with greater subretinal tissue complex thickness (P < 0.0001). Eyes with subretinal fluid in the foveal center had higher risk compared with eyes with no subretinal fluid (aHR, 1.5; 95% CI, 1.1–2.0). Risk was greater for eyes with SHRM (aHR, 1.7; 95% CI, 1.3–2.3) and less for eyes with RPE elevation (aHR, 0.6; 95% CI, 0.5–0.8). The frequency of scar development was similar for the 2 anti-VEGF drugs, bevacizumab compared with ranibizumab (aHR, 1.2; 95% CI, 0.96–1.4), and for the dosing regimens, PRN compared with monthly (aHR, 0.9; 95% CI, 0.8–1.1).
Table 4.
Multivariate Analysis for Incidence of Scar within 2 Years
| Baseline Characteristics | Subjects Included in the Final Model (N=1010) |
Subjects with Scar at Week 52 or 104, n (%) |
Adjusted Hazard Ratio (95% CI) |
P Value* |
|---|---|---|---|---|
| Lesion type | ||||
| Occult only | 614 | 183 (29.8) | 1.00 | <0.0001 |
| Minimally classic | 144 | 90 (62.5) | 2.30 (1.76–3.00) | |
| Predominantly classic | 252 | 188 (74.6) | 3.05 (2.40–3.86) | |
| Blocked fluorescence | ||||
| No | 872 | 364 (41.7) | 1.00 0.02 | |
| Yes | 138 | 97 (70.3) | 1.38 (1.07–1.78) | |
| Retinal thickness at foveal center (μm) | ||||
| <120 | 104 | 35 (33.7) | 1.00 | <0.0001 |
| 120–212 | 542 | 228 (42.1) | 1.61 (1.12–2.33) | |
| >212 | 364 | 198 (54.4) | 2.44 (1.67–3.56) | |
| Subretinal tissue complex thickness at foveal center (μm) | ||||
| >0–≤75 | 246 | 77 (31.3) | 1.00 | <0.0001 |
| >75–≤160 | 244 | 122 (50.0) | 1.68 (1.24–2.27) | |
| >160–≤275 | 257 | 134 (52.1) | 1.89 (1.39–2.57) | |
| >275 | 263 | 128 (48.7) | 2.45 (1.78–3.37) | |
| Subretinal fluid | ||||
| No fluid | 175 | 70 (40.0) | 1.00 | 0.014 |
| Fluid not at foveal center | 476 | 230 (48.3) | 1.08 (0.82–1.44) | |
| Fluid in foveal center | 359 | 161 (44.9) | 1.45 (1.08–1.96) | |
| RPE elevation | ||||
| No | 133 | 90 (67.7) | 1.00 | 0.0005 |
| Yes | 877 | 371 (42.3) | 0.63 (0.49–0.82) | |
| SHRM | ||||
| No | 235 | 58 (24.7) | 1.00 | 0.0005 |
| Yes | 775 | 403 (52.0) | 1.71 (1.26–2.30) | |
| Drug | ||||
| Ranibizumab | 525 | 227 (43.2) | 1.00 | 0.14 |
| Bevacizumab | 485 | 234 (48.3) | 1.15 (0.96–1.39) | |
| Regimen† | ||||
| Monthly for 2 yrs | 252 | 111 (44.1) | 1.00 | 0.44 |
| Monthly yr 1, PRN yr 2 | 252 | 122 (48.4) | – | |
| PRN for 2 yrs | 506 | 228 (45.1) | 0.93 (0.76–1.13) |
CI = confidence interval; PRN = pro re nata; RPE = retinal pigment epithelium; SHRM = subretinal hyperreflective material.
P values are from a time-dependent Cox proportional hazard model.
Regimen was a time-dependent variable with the value of monthly or PRN.
Fibrotic Scars and Nonfibrotic Scars
At the end of 2 years of anti-VEGF therapy, 262 patients (24.7%) developed fibrotic scar; 205 (19.4%) developed fibrotic scar by 1 year, and an additional 57 (5.4%) developed fibrotic scar by the end of 2 years. The cumulative incidence rates of fibrotic scar at 1 and 2 years were 0.19 (95% CI, 0.17–0.22) and 0.26 (95% CI, 0.24–0.29), respectively. At the end of 2 years, 218 subjects (20.6%) developed nonfibrotic scar; 134 (12.7%) developed non-fibrotic scar by 1 year, and an additional 84 (7.9%) developed nonfibrotic scar by the end of 2 years. The cumulative incidence rates of nonfibrotic scar at 1 and 2 years were 0.13 (95% CI, 0.11–0.15) and 0.24 (95% CI, 0.21–0.27), respectively.
Several OCT characteristics at year 2 were associated with fibrotic and nonfibrotic scarring at the foveal center. The OCT characteristics among eyes with fibrotic scar at the foveal center, nonfibrotic scar at the foveal center, and no scar or an extrafoveal scar are quantified in Table 5. Eyes with geographic atrophy at the foveal center were excluded from this analysis because their thickness measurements could be abnormally low. Mean thickness of the retina and subretinal fluid at the foveal center was similar among the 3 groups. The mean thickness of the subretinal tissue complex at the foveal center was greatest (168 μm; standard error [SE], 8.7) for eyes with fibrotic scars, less (148 μm; SE, 14.5) for eyes with nonfibrotic scars, and least (119 μm; SE, 4.1) for eyes with no scar at the foveal center (P < 0.0001). Intraretinal fluid at the foveal center was more common in eyes with fibrotic scar (65%) than in eyes with nonfibrotic scars or no scar (46.3% and 48.1%, respectively; P < 0.0001). Subretinal fluid was less common in eyes with fibrotic scar (24%) than in eyes with nonfibrotic or no scar (46.3% and 38.8%, respectively; P < 0.004). Sub-RPE fluid was less common in eyes with fibrotic scar (20.7%) and nonfibrotic scar (25.9%) than in eyes with no scar at the foveal center (41.9%; P < 0.001). Mean visual acuity score in letters at the end of 2 years was 57.6 (~20/80) among 150 eyes with a fibrotic scar at the foveal center, 67.5 (~20/50) among 54 eyes with a nonfibrotic scar at the foveal center, and 71.8 (~20/40) among 680 eyes with no scar in the foveal center (P < 0.001).
Table 5.
Association of Optical Coherence Tomography Characteristics at 2 Years with Foveal Center Scar at 2 Years (N=884*)
| Scarring at the Foveal Center at 2 Years |
||||
|---|---|---|---|---|
| Optical Coherence Tomography Characteristics | Fibrotic Scar (n=150) | Nonfibrotic Scar (n=54) | No Scar (n=680) | P Value |
| Thickness at foveal center, μm, mean (SE) | ||||
| Retina | 165 (5.88) | 152 (9.85) | 163 (2.92) | 0.58 |
| Subretinal fluid | 4.51 (2.27) | 11.7 (3.80) | 10.7 (1.32) | 0.13 |
| Subretinal tissue complex | 168 (8.70) | 148 (14.5) | 119 (4.08) | <0.0001 |
| Fluid at foveal center, n (%) | ||||
| Any | 118 (78.7) | 43 (79.6) | 508 (74.7) | 0.27 |
| Intraretinal | 98 (65.3) | 25 (46.3) | 327 (48.1) | <0.0001 |
| Subretinal | 36 (24.0) | 25 (46.3) | 264 (38.8) | 0.004 |
| Sub-RPE | 31 (20.7) | 14 (25.9) | 285 (41.9) | <0.0001 |
| Visual acuity at yr 2, mean (SE) | 57.6 (1.34) | 67.5 (2.23) | 71.8 (0.63) | <0.0001 |
RPE = retinal pigment epithelium; SE = standard error.
Eyes with foveal center geographic atrophy (n = 63) at week 104 were excluded.
Presence of a scar in the fellow eye at baseline did not substantially increase the risk of scarring in the study eye. At 2 years, among 133 patients with a scar in the fellow eye at baseline, 36 (27%) had a fibrotic scar, 17 (13%) had a nonfibrotic scar, and 80 (60%) had no scar in the study eye. In contrast, among 926 patients who did not have a fibrotic scar in the fellow eye at baseline, fibrotic scar developed in 226 (24%), nonfibrotic scar developed in 201 (22%), and no scar developed in 499 (54%; P = 0.06).
Risk factors for fibrotic and nonfibrotic scar development relative to those without any scarring are presented in Table 6. All of the factors identified for the combined group of fibrotic and nonfibrotic scars were identified as risk factors for fibrotic scars alone, plus 2 additional features were identified. Worse initial visual acuity and larger lesion size were associated with increased risk of fibrotic scars compared with eyes without any scarring. Some of the factors identified for the combined group of scars were not significantly associated with the risk of nonfibrotic scars alone (Table 6, italics). However, the hazard ratios were in the same direction (i.e., >1.00 or <1.00) as the hazard ratios for fibrotic scars. Although a larger area of CNV was associated with increased risk of fibrotic scar, it was associated with decreased risk of nonfibrotic scar. Eyes with classic CNV and eyes with SHRM were at higher risk of both types of scar. There was minimal association between a scar of either type and drug or treatment regimen.
Table 6.
Multivariate Analysis for Incidence of Fibrotic Scar and Nonfibrotic Scar within 2 Years
| Fibrotic Scar vs. No Scar |
Nonfibrotic Scar vs. No Scar |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics |
Subjects
(N=799) |
Fibrotic
Scar, n (%) |
Adjusted Hazard
Ratio (95% CI) * |
P Value * |
Subjects
(N=765) |
Nonfibrotic
Scar, n (%) |
Adjusted Hazard
Ratio (95% CI) * |
P Value * |
| Baseline visual acuity in study eye | ||||||||
| 20/25–40 | 290 | 45 (15.5) | 1.00 | 0.004 | 333 | 87 (26.1) | 1.00 | 0.33 |
| 20/50–80 | 307 | 103 (33.6) | 1.61 (1.10–2.35) | 280 | 76 (27.1) | 0.91 (0.66–1.25) | ||
| 20/100–160 | 152 | 76 (50.0) | 2.14 (1.42–3.22) | 121 | 43 (35.5) | 1.12 (0.77–1.62) | ||
| 20/200–320 | 50 | 26 (52.0) | 1.52 (0.86–2.68) | 31 | 7 (22.6) | 0.55 (0.25–1.22) | ||
| Baseline area of CNV (disc areas) | ||||||||
| ≤1 | 296 | 88 (29.7) | 1.00 | 0.019 | 331 | 121 (36.6) | 1.00 | 0.04 |
| >1–≤2 | 173 | 57 (33.0) | 1.70 (1.16–2.50) | 152 | 36 (23.7) | 0.77 (0.52–1.15) | ||
| >2–≤4 | 174 | 50 (28.7) | 1.42 (0.95–2.14) | 152 | 28 (18.4) | 0.60 (0.39–0.93) | ||
| >4 | 88 | 23 (26.1) | 1.24 (0.73–2.12) | 77 | 11 (14.3) | 0.48 (0.25–0.91) | ||
| Missing | 68 | 32 (47.1) | 2.03 (1.25–3.31) | 53 | 17 (32.1) | 1.12 (0.66–1.88) | ||
| Lesion type | ||||||||
| Occult only | 516 | 85 (16.5) | 1.00 | <0.0001 | 532 | 98 (18.4) | 1.00 | <0.0001 |
| Minimally classic | 109 | 55 (50.5) | 2.76 (1.92–3.97) | 89 | 35 (39.3) | 2.39 (1.61–3.56) | ||
| Predominantly classic | 174 | 110 (63.2) | 4.14 (2.84–6.03) | 144 | 80 (55.6) | 3.11 (2.23–4.32) | ||
| Blocked fluorescence | ||||||||
| No | 694 | 186 (26.8) | 1.00 | 0.0004 | 690 | 179 (25.9) | 1.00 | 0.41 |
| Yes | 105 | 64 (61.0) | 1.84 (1.32–2.58) | 75 | 34 (45.3) | 1.18 (0.79–1.75) | ||
| Retinal thickness at foveal center (μm) |
||||||||
| <120 | 87 | 18 (20.7) | 1.00 | <0.0001 | 86 | 17 (19.8) | 1.00 | 0.08 |
| 120–212 | 421 | 112 (26.3) | 1.67 (0.99–2.80) | 433 | 117 (27.0) | 1.58 (0.95–2.65) | ||
| >212 | 286 | 120 (42.0) | 2.73 (1.60–4.66) | 245 | 79 (32.2) | 1.83 (1.07–3.11) | ||
| Subretinal tissue complex thickness at foveal center (μm) |
||||||||
| >0–≤75 | 202 | 33 (16.3) | 1.00 | <0.0001 | 214 | 45 (21.0) | 1.00 | 0.11 |
| >75–≤160 | 180 | 58 (32.2) | 1.57 (1.00–2.48) | 189 | 65 (34.4) | 1.58 (1.07–2.34) | ||
| >160–≤275 | 194 | 71 (36.6) | 2.08 (1.32–3.26) | 186 | 63 (33.9) | 1.44 (0.96–2.14) | ||
| >275 | 223 | 88 (39.5) | 3.11 (1.96–4.94) | 175 | 40 (22.9) | 1.18 (0.76–1.83) | ||
| Subretinal fluid | ||||||||
| No fluid | 139 | 34 (24.5) | 1.00 | 0.012 | 141 | 36 (25.5) | 1.00 | 0.74 |
| Fluid not at foveal center | 371 | 125 (33.7) | 1.03 (0.68–1.54) | 352 | 106 (30.1) | 1.16 (0.79–1.71) | ||
| Fluid at foveal center | 289 | 91 (31.5) | 1.60 (1.04–2.46) | 271 | 71 (26.2) | 1.12 (0.75–1.68) | ||
| RPE elevation | ||||||||
| No | 97 | 54 (55.7) | 1.00 | <0.0001 | 79 | 36 (45.6) | 1.00 | 0.07 |
| Yes | 702 | 196 (27.9) | 0.50 (0.36–0.70) | 682 | 175 (25.7) | 0.71 (0.49–1.03) | ||
| SHRM | ||||||||
| No | 204 | 27 (13.2) | 1.00 | 0.008 | 208 | 31 (14.9) | 1.00 | 0.004 |
| Yes | 595 | 223 (37.5) | 1.82 (1.17–2.83) | 557 | 182 (32.7) | 1.78 (1.20–2.64) | ||
| Drug | ||||||||
| Ranibizumab | 428 | 130 (30.4) | 1.00 | 0.85 | 396 | 98 (24.8) | 1.00 | 0.16 |
| Bevacizumab | 371 | 120 (32.4) | 1.03 (0.78–1.34) | 369 | 115 (31.2) | 1.22 (0.93–1.60) | ||
| Regimen† | ||||||||
| Monthly for 2 yrs | 205 | 64 (31.2) | 1.00 | 0.13 | 189 | 48 (25.4) | 1.00 | 0.81 |
| Monthly for 1 yr, PRN for 2 yrs | 197 | 67 (34.0) | – | 186 | 55 (29.6) | – | ||
| PRN for 2 yrs | 397 | 119 (30.0) | 0.81 (0.62–1.07) | 390 | 110 (28.2) | 1.04 (0.78–1.39) | ||
CI = confidence interval; CNV = choroidal neovascularization; PRN = pro re nata; RPE = retinal pigment epithelium; SHRM = subretinal hyperreflective material.
Note: All results not in italics are from the final multivariate model. The results in italics are from the multivariate model with adjustment of all variables in the final model.
From a time-dependent Cox proportional hazards model.
Regimen was a time-dependent variable with a value of monthly or PRN.
There was no significant relationship between scar development and the 5 SNPs that were evaluated. A stepwise analysis also failed to show a significant interaction among the number of risk alleles present. Adjusting for age, sex, and smoking habits did not alter these results (Table 7).
Table 7.
Association of Genotype with the Incidence of Scar within 2 Years (N = 797)
|
Subjects at Risk,
n |
Scar at Week 52
or 104, n (%) * |
Univariate Analysis
|
Adjusted Analysis
|
||
|---|---|---|---|---|---|
| SNP § | Genotype | Hazard Ratio (95% CI) † | Hazard Ratio (95% CI) ‡ | ||
| CFH rs1061170 | CC | 258 | 125 (48.5) | 1.01 (0.76–1.34) | 1.02 (0.77–1.36) |
| TC | 370 | 164 (44.3) | 0.90 (0.69–1.18) | 0.91 (0.70–1.19) | |
| TT | 169 | 82 (48.5) | 1.00 | 1.00 | |
| Linear trend P | 0.88 | 0.83 | 0.77 | ||
| Adjusted P∥ | 0.78 | ||||
| ARMS2 rs10490924 | TT | 163 | 76 (46.6) | 1.06 (0.80–1.42) | 1.06 (0.79–1.38) |
| GT | 371 | 177 (47.7) | 1.11 (0.88–1.40) | 1.09 (0.86–1.38) | |
| GG | 263 | 118 (44.9) | 1.00 | 1.00 | |
| Linear trend P | 0.65 | 0.60 | 0.64 | ||
| Adjusted P∥ | 0.78 | ||||
| HTRA1 rs11200638 | AA | 155 | 74 (47.7) | 1.10 (0.83–1.48) | 1.10 (0.82–1.47) |
| AG | 371 | 176 (47.4) | 1.10 (0.87–1.39) | 1.10 (0.87–1.39) | |
| GG | 271 | 121 (44.7) | 1.00 | 1.00 | |
| Linear trend P | 0.49 | 0.45 | 0.48 | ||
| Adjusted P∥ | 0.78 | ||||
| C3 rs2230199 | GG | 55 | 27 (49.1) | 1.06 (0.71–1.59) | 1.08 (0.72–1.62) |
| CG | 303 | 131 (43.2) | 0.84 (0.67–1.04) | 0.85 (0.68–1.07) | |
| CC | 439 | 213 (48.5) | 1.00 | 1.00 | |
| Linear trend P | 0.41 | 0.40 | 0.52 | ||
| Adjusted P∥ | 0.78 | ||||
| CC | 397 | 172 (43.3) | 0.81 (0.57–1.16) | 1.00 (0.70–1.44) | |
| TLR3 rs3775291 | TC | 326 | 162 (49.7) | 0.99 (0.69–1.41) | 0.82 (0.57–1.18) |
| TT | 74 | 37 (50.0) | 1.00 | 1.00 | |
| Linear trend P | 0.10 | 0.08 | 0.08 | ||
| Adjusted P∥ | 0.48 | ||||
| No. of risk alleles | 0–2 | 85 | 47 (55.3) | 1.00 | 1.00 |
| 3 | 112 | 46 (41.1) | 0.67 (0.45–1.01) | 0.67 (0.44–1.01) | |
| 4 | 154 | 70 (45.5) | 0.76 (0.53–1.11) | 0.76 (0.52–1.10) | |
| 5 | 173 | 81 (46.8) | 0.81 (0.56–1.16) | 0.82 (0.57–1.17) | |
| 6 | 136 | 65 (47.8) | 0.86 (0.59–1.25) | 0.85 (0.58–1.25) | |
| ≥7 | 137 | 62 (45.3) | 0.76 (0.52–1.11) | 0.76 (0.52–1.12) | |
| Linear trend P | 0.71 | 0.75 | 0.78 | ||
| Adjusted P∥ | 0.78 |
ARMS2 = age-related maculopathy susceptibility 2; C3 = complement 3; CI = confidence interval; CFH = complement factor H; HTRA1 = HtrA serine peptidase 1; TLR3 = toll-like receptor 3 gene.
Linear trend P value is from a logistic regression model with genotype coded as 0, 1, and 2 risk alleles.
Linear trend P value is from proportional hazards model with genotype coded as 0, 1, and 2 risk alleles.
Linear trend P value is from proportional hazard models adjusted for age, gender, and smoking status.
The risk alleles are C for CFH, T for ARMS2, A for HTRA1, G for C3, and C for TLR3.
The multiple testing adjusted P values were calculated using the approach of false discovery rate.
Discussion
After 1 year, scar developed in one third of the eyes treated with anti-VEGF drugs, and by 2 years, approximately half the eyes developed scar. We identified baseline characteristics that predicted scar formation: classic CNV, blocked fluorescence on FA, increased retinal thickness, foveal subretinal fluid, and SHRM. The type of anti-VEGF therapy and dosing regimen did not strongly influence scar development. Moreover, commonly described AMD genotypes were not associated with increased risk of scarring.
In our study, it was important to characterize the nature of the scar observed on CFP and FA because these specific features had predictive value of visual acuity and did not always conform to definitions specified in earlier studies.7,15,16 Fibrotic scars were relatively easy to recognize as raised mounds of white or yellowish tissue that were well defined in shape and appeared solid on color stereo images.13 Nonfibrotic scars were typically flat, depigmented lesions with varying amounts of signet-shaped peripheral dark pigmentation that conformed to the baseline CNV area. On OCT, nonfibrotic scars, as defined by CFP and FA, often had hyperreflective material in a subretinal or sub-RPE location that would be consistent with fibrosis. Also, the foveal center thickness of the subretinal tissue complex in eyes with a nonfibrotic scar was between the thickness of eyes with fibrotic scars and those with no scar, that is, those having foveal CNV, fluid, or no pathology.
We avoided using terms such as “disciform scar” and “atrophic scar,” which were used in some reports.13–15 Disciform scar implies a disc-shaped, circular fibrotic scar,17–19 an appearance that was rarely seen on CATT photographic images after 1 or 2 years of anti-VEGF therapy. In a previous study, “atrophic scar” was used to describe flat or slightly concave areas of uniformly depigmented RPE with well-defined borders through which large choroidal vessels are visible.16 These atrophic scars stained but did not exhibit fluorescein leakage, and their FA characteristics did not correspond to geographic atrophy. In atrophic scars, hyperfluorescence did not begin early as it does in RPE window defects (because there may be a “thin” layer of RPE or fibrous tissue present) and did not fade in the late phase of the angiogram. This presentation is different from nonfibrotic scars that appear as discrete areas of dark hyperpigmentation at the site of the baseline CNV, with varying amounts of central depigmentation with no visible choroidal vessels and the appearance of early hyperfluorescence on FA.
To our knowledge, this report is the first from a large-scale, prospective study to describe in detail the incidence and risk of scars that develop from CNV lesions after PRN or monthly ranibizumab and bevacizumab intravitreal injections. Previous histopathologic and clinical studies of eyes with disciform scars secondary to AMD have documented loss of the majority of the overlying photoreceptors and outer nuclear layer and poor visual function.2,20–22
Descriptions of scars in reports from other studies of anti-VEGF treatments have not been detailed or have a short follow-up period and a relatively small number of eyes.23–27 Reports from MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration) and ANCHOR (ANti-VEGF Antibody for the Treatment of Predominantly Classic CHORoidal Neovascularization in AMD Trial) noted that visual acuity loss was not statistically associated with leakage, hemorrhage, or fibrosis in ranibizumab-treated eyes.16 Our study found that visual acuity was lowest in eyes that developed fibrotic scar involving the foveal center.
An important question that has prognostic and therapeutic implications is whether intravitreal anti-VEGF therapy alters the formation of retinal scar in eyes with nvAMD. The ability of VEGF to regulate scar tissue formation has not been studied extensively. In a study that used fetal and adult wound-healing murine models, scar-free fetal wounds had lower VEGF levels and were less vascular than fibrotic fetal wounds, and the scar-free phenotype was converted to a scar-forming phenotype when exogenous VEGF was added.28 When VEGF was neutralized in adult wounds, vascularity was reduced, and scar formation was decreased. Bevacizumab is reported to have antifibrotic activity that reduces scar formation in glaucoma filtration surgery.29–31 Furthermore, as noted earlier, large disciform scars were rarely seen after 2 years anti-VEGF therapy in CATT. The rarity of large fibrotic scars in CATT suggests that extensive fibrosis is aborted or delayed by anti-VEGF therapy. Paradoxically, in eyes with proliferative diabetic retinopathy treated with anti-VEGF injections, there is increased fibrosis caused by imbalances between connective tissue growth factor and VEGF.32,33 These data suggest that VEGF plays a diverse role in the wound repair process.
The type of CNV, as determined by FA at baseline, predicted scar formation. Scars were least likely to develop in eyes with occult CNV only. When occult CNV is admixed with the classic type, the risk doubles. The risk triples when the angiographic phenotype subset is composed predominantly of classic CNV. It is possible that intravitreal anti-VEGF treatment decreases scar formation in purely occult lesions by confining the CNV to the sub-RPE space, thereby stopping progression to classic CNV. Stevens et al34 reported evidence suggesting that classic CNV increases the risk of development of fibrosis in AMD. The greater propensity for classic CNV to transform into a scar is illustrated in Figure 1C, where the minimally classic lesion’s small, centrally located classic CNV changes into a fibrotic scar in the CFP at 2 years, whereas the larger occult portion of the CNV lesion, although active, does not transform to a CFP-visible scar.
The CATT included eyes treated only with anti-VEGF monotherapy. However, in a recent phase II study, eyes were treated with ranibizumab, with or without anti—platelet-derived growth factor therapy (Dugal PU, Reichel E, Boyer DS, et al. Phase 2b trial results show effectiveness of combination therapy. Retina Times. Fall 2012). The results showed that combination therapy more effectively than monotherapy improved visual acuity and better eliminated SHRM. In the present study, we found that SHRM was associated with scar formation. Accordingly, in the future, it will be of interest to determine whether combination therapy similarly reduced the rate of fibrotic or nonfibrotic scar as one explanation for the better visual acuity observed in that study.
Several studies have suggested that CNV sequelae, such as subretinal hemorrhage, are associated with fibrovascular scarring.35 For example, in one retrospective study, progressive visual acuity loss occurred in 41 eyes of 40 patients with subfoveal subretinal hemorrhage that comprised more than 50% of the CNV lesion.36 In that report, it was not clear whether the scar formation was directly a result of the more extensive hemorrhage. In contrast, in another study of eyes with nvAMD treated with anti-VEGF therapy, subfoveal fibrosis developed in the absence of significant subfoveal hemorrhage.37 Likewise, hemorrhage was not associated with the development of scar in CATT. However, blocked fluorescence, defined as blockage of fluorescence that was not associated with pigment or hemorrhage, was a strong baseline predictor of scar formation. The blocked fluorescence could have been the result of deep sub-RPE hemorrhage or deep fibrosis not visible on color photographs.
In a previous CATT report, we described a strong association between decreased visual acuity at the end of 1 year and the presence of intraretinal fluid but not subretinal or sub-RPE fluid on OCT.1 Thus, it is surprising that subretinal fluid is independently associated with scar formation by the end of 2 years because scar was also associated with decreased visual acuity at 2 years. It is possible that with a longer period of observation, subretinal fluid also could affect visual acuity through scar formation.
In our study, we did not see a significant association of RAP with scar after adjusting for other factors, although the univariate analysis (Table 1, available at http://aaojournal.org) showed that eyes with RAP at baseline developed fewer scars when compared with eyes that did not have RAP. This observation could be related to the relatively smaller RAP lesions or their common association with occult CNV, which forms fewer scars than classic CNV. A relatively small study that followed the natural history of RAP lesions for a mean duration of 20 months found subretinal fibrosis on FA and red-free photographs in 10 of 16 eyes (62%),38 but a more recent study reported that only one third of the RAP lesions treated with three 0.5-mg intravitreal injections of ranibizumab and followed up for 3 years developed retinal scarring.39 Although further investigation is warranted, these data suggest that anti-VEGF treatment prevents scar formation from RAP lesions.
We did not find an association between scar and genotypes for 5 SNPs (complement factor H, age-related maculopathy susceptibility 2, high temperature requirement factor A1, complement component 3, and toll-like receptor 3) that are known to have strong associations with the development of AMD. Further studies to investigate the expression of connective tissue growth factor and other fibrosis-associated cytokines, such as transforming growth factor ß, in eyes with nvAMD and exploration of association with known genomic predictors of excessive scar formation, such as keloids, may reveal genetic variants that may mitigate development of scar in nvAMD.40
In conclusion, a thorough understanding of the presenting morphology, history, and subsequent morbidity of nvAMD targeted by intravitreal anti-VEGF injections is essential to predict outcomes. Angiographic characteristics, such as the classic CNV phenotype, blocked fluorescence, and larger CNV lesions at baseline, and OCT characteristics, such as greater retinal thickness and subretinal tissue complex thickness, foveal subretinal fluid, and SHRM, predict increased risk of scar formation. Because scar formation is strongly associated with poor visual outcomes, investigation of potential treatments that reduce the formation of macular scar in AMD could prevent the loss of visual function.
Supplementary Material
Acknowledgments
Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. ClinicalTrials.gov number, NCT00593450.
Footnotes
Presented at: Association for Research in Vision and Ophthalmology, May 5–9, 2013, Seattle, Washington.
Financial Disclosure(s): The author(s) have made the following disclosure(s): C.A.T. receives research support through Duke University from Genentech and royalties through Duke University from Alcon Laboratories. G.J.J. has a consultancy relationship with Heidelberg Engineering, has active or pending grants from Regeneron, is a scientific advisory board member for Bayer and Pfizer, and is a consultant for Neurotech. Other members of the writing committee have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Jaffe GJ, Martin DF, Toth CA, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2013;120:1860–70. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressler NM, Frost LA, Bressler SB, et al. Natural course of poorly defined choroidal neovascularization associated with macular degeneration. Arch Ophthalmol. 1988;106:1537–42. doi: 10.1001/archopht.1988.01060140705039. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Pauleikhoff D. Neovascular age-related macular degeneration: natural history and treatment outcomes. Retina. 2005;25:1065–84. doi: 10.1097/00006982-200512000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Sivaprasad S, Saleh GM, Jackson H. Does lesion size determine the success rate of photodynamic therapy for age-related macular degeneration? Eye (Lond) 2006;20:43–5. doi: 10.1038/sj.eye.6701787. [DOI] [PubMed] [Google Scholar]
- 6.AMDRT Research Group The Age-Related Macular Degeneration Radiotherapy Trial (AMDRT): one year results from a pilot study. Am J Ophthalmol. 2004;138:818–28. doi: 10.1016/j.ajo.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Trikha R, Morse LS, Zawadzki RJ, et al. Ten-year follow-up of eyes treated with stereotactic fractionated external beam radiation for neovascular age-related macular degeneration. Retina. 2011;31:1303–15. doi: 10.1097/IAE.0b013e318203ee46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis LH, Hammill BG, Qualls LG, et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 Medicare beneficiaries. Am J Ophthalmol. 2012;153:1116–24. doi: 10.1016/j.ajo.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SY, Oubraham H, Uzzan J, et al. Causes of unsuccessful ranibizumab treatment in exudative age-related macular degeneration in clinical settings. Retina. 2012;32:1480–5. doi: 10.1097/IAE.0b013e318240a516. [DOI] [PubMed] [Google Scholar]
- 10.CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunwald JE, Daniel E, Ying GS, et al. CATT Research Group. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:1634–41. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCroos FC, Toth CA, Stinnett SS, et al. CATT Research Group. Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119:2549–57. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser PK, Blodi BA, Shapiro H, Acharya NR, MARINA Study Group Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–75. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol) 1995;57:289–300. [Google Scholar]
- 15.Beaumont PE, Kang HK. Lesion morphology in age-related macular degeneration and its therapeutic significance. Arch Ophthalmol. 2006;124:807–12. doi: 10.1001/archopht.124.6.807. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Shapiro H, Tuomi L, et al. MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–30. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 18.Zauberman H, Ivry M, Sachs U. The macular vessels in pre-disciform and disciform senile macular degeneration. Am J Ophthalmol. 1970;70:498–504. doi: 10.1016/0002-9394(70)90882-2. [DOI] [PubMed] [Google Scholar]
- 19.Gregor Z, Bird AC, Chisholm IH. Senile disciform macular degeneration in the second eye. Br J Ophthalmol. 1977;61:141–7. doi: 10.1136/bjo.61.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–35. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Sadda S, Pearlman J, et al. Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina. 2002;22:471–7. doi: 10.1097/00006982-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hogg R, Curry E, Muldrew A, et al. Identification of lesion components that influence visual function in age related macular degeneration. Br J Ophthalmol. 2003;87:609–14. doi: 10.1136/bjo.87.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 25.Heier JS, Boyer D, Nguyen QD, et al. CLEAR-IT 2 Investigators The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor Trap-Eye dosed as-needed after 12-week fixed dosing. Ophthalmology. 2011;118:1098–106. doi: 10.1016/j.ophtha.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–7. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Unver YB, Yavuz GA, Bekiroğlu N, et al. Relationships between clinical measures of visual function and anatomic changes associated with bevacizumab treatment for choroidal neovascularization in age-related macular degeneration. Eye (Lond) 2009;23:453–60. doi: 10.1038/eye.2008.349. [DOI] [PubMed] [Google Scholar]
- 28.Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2000;8:579–90. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill EC, Qin Q, Van Bergen NJ, et al. Antifibrotic activity of bevacizumab on human Tenon’s fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2010;51:6524–32. doi: 10.1167/iovs.10-5669. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50:5217–25. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- 31.Memarzadeh F, Varma R, Lin LT, et al. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009;50:3233–7. doi: 10.1167/iovs.08-2441. [DOI] [PubMed] [Google Scholar]
- 32.Van Geest RJ, Lesnik-Oberstein SY, Tan HS, et al. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–90. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper EJ, de Smet MD, van Meurs JC, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124:1457–62. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- 34.Stevens TS, Bressler NM, Maguire MG, et al. Occult choroidal neovascularization in age-related macular degeneration. A natural history study. Arch Ophthalmol. 1997;115:345–50. doi: 10.1001/archopht.1997.01100150347006. [DOI] [PubMed] [Google Scholar]
- 35.Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age—related macular degeneration. Ophthalmologica. 1999;213:97–102. doi: 10.1159/000027400. [DOI] [PubMed] [Google Scholar]
- 36.Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–9. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- 37.Hwang JC, Del Priore LV, Freund KB, et al. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42:6–11. doi: 10.3928/15428877-20100924-01. [DOI] [PubMed] [Google Scholar]
- 38.Viola F, Massacesi A, Orzalesi N, et al. Retinal angiomatous proliferation: natural history and progression of visual loss. Retina. 2009;29:732–9. doi: 10.1097/IAE.0b013e3181a395cb. [DOI] [PubMed] [Google Scholar]
- 39.Rouvas AA, Chatziralli IP, Theodossiadis PG, et al. Long-term results of intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy, and intravitreal triamcinolone with photodynamic therapy for the treatment of retinal angiomatous proliferation. Retina. 2012;32:1181–9. doi: 10.1097/IAE.0b013e318235d8ce. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima M, Chung S, Takahashi A, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42:768–71. doi: 10.1038/ng.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.