Abstract
The kidney atrophies in patients with advanced chronic kidney disease (CKD) but factors influencing kidney size in normal adults are less clear. To help define this we measured kidney volumes on contrast-enhanced CT images from 1344 potential kidney donors (ages 18 to 75 years). Cortical volume per body surface area progressively declined in both genders with increased age. Statistically, this was primarily dependent on the age-related decline in glomerular filtration rate (GFR). Independent predictors of increased cortical volume per body surface area were male gender, increased GFR, increased 24-hour urine albumin, current smoker, and decreased high-density lipid cholesterol. Medullary volume per body surface area increased with age in men while it increased with age in women until age 50 followed by a subsequent decline. Independent predictors of increased medullary volume per body surface area were older age, male gender, increased GFR, increased 24-hour urine albumin, increased serum glucose, and decreased serum uric acid. Thus, while cortical volume declines with age along the same biological pathway as the age-related decline in GFR and albuminuria some CKD risk factors are actually associated with increased cortical or medullary volume among relatively healthy adults. Underlying hypertrophy or atrophy of different nephron regions may explain these findings.
Keywords: kidney cortical volume, kidney medullary volume, aging, kidney function, CKD risk factors
INTRODUCTION
In persons with advanced chronic kidney disease (CKD), the kidney has been known to atrophy[1], particularly the cortex[2]. Kidney volume has been recently suggested as a useful surrogate for kidney function in CKD patients[3]. Although these prior investigations establish an association of decreased kidney volume with decreased kidney function among patients with advanced CKD, the association of kidney volume with kidney function and CKD risk factors in patients with mild or no CKD is less clear. Indeed, some risk factors for CKD, namely obesity and diabetes, have been associated with increased kidney volume [4–6].
The relationship of kidney volume with normal aging has been uncertain. Relatively small studies in potential kidney donors have not found evidences of kidney volume decline with age by computed tomography (CT) scan, though few subjects over the age of 60 years were included[6, 7]. Conversely, imaging studies in populations with clinical indications for their CT scan and older adults do find a decline in kidney parenchymal volume with age [7–10]. The reasons for the discrepant findings are unclear. The cortex and medulla contain different nephron segments that may hypertrophy or atrophy in the setting of different CKD risk factors and markers. Separate analyses of cortical and medullary volumes focusing on the renal parenchyma (e.g., exclusion of sinus fat and the renal pelvis from volume calculations) may help clarify the clinical factors that associate with kidney parenchymal volume.
Because potential kidney donors undergo a standardized rigorous evaluation including a renal CT angiogram (CTA), they are useful for characterizing clinical-pathological correlations in a population not selected on disease [11–13]. While patients with known CKD and significant comorbidity are excluded from the donor evaluation, patients with subclinical disease (e.g., microalbuminuria) and some CKD risk factors (e.g., hypertension) often undergo evaluation. A unique benefit of contrast enhanced CT scanning is the ability to distinguish cortex from medulla. So the objective of this study was to accurately measure the cortical, medullary, and total parenchymal volume in a large sample of potential kidney donors and determine their association with age, kidney function and CKD risk factors.
RESULTS
Study sample
There were 1487 potential kidney donors with an analyzed CTA between 2000 and 2008 at the Mayo Clinic. Of these, 9 were excluded for diabetes (fasting glucose > 126) [14], 24 were excluded for movement artifact, 103 were excluded for incomplete kidney coverage, 1 was excluded for scout image only (solitary kidney), and 7 were excluded for polycystic kidneys[15], leaving 1344 donors for the study sample. Of these, 1192 were segmented for bilateral kidney cortical volume and medullary volume. The remaining 152 potential donors only had segmentation for the total parenchymal volume due to inadequate cortical-medullary differentiation. Table 1 lists the clinical characteristics for potential donors. Larger potential donors (male or higher BSA) tended to have inadequate cortical-medullary differentiation, likely due to more X-ray beam attenuation by surrounding tissue. Since body size is both a CKD risk factor and confounded with CT image quality [15], we did not exclude potential donors with inadequate cortical-medullary differentiation in our analyses of total parenchymal volume. As shown in Table 2, kidney volumes had a satisfactory inter- and intra-test reproducibility.
Table 1.
Characteristics of potential kidney donors with contrast enhanced CT imaging.
| Characteristic | Potential donors with adequate cortical-medullary differentiation (n=1192) Mean ± SD or N (%) |
Potential donors with inadequate cortical- medullary differentiation (n=152) Mean ± SD or N (%) |
|---|---|---|
| Demographic | ||
| Age, y | 44±12 | 45±12 |
| Over age 60 years | 118(10%) | 16(11%) |
| Men | 477(40%) | 82(54%) * |
| White | 1064(89%) | 138(91) |
| Body surface area, m2 | 1.9±0.2 | 2.0±0.3 * |
| Kidney function | ||
| mGFR, ml/min/1.73m2 | 102±19 | 102±19 |
| Hyperfiltration, mGFR >130ml/min/1.73m2 | 85(7.3%) | 13(8.8%) |
| 24-h urine albumin excretion, mg | 7.2±15 | 8.2±9.7 |
| Albuminuria, 24-h urine albumin >30mg | 35(3%) | 7(5%) |
| CKD risk factors | ||
| Current smoker | 244(20%) | 28(18%) |
| Past smoker | 281(24%) | 28(19%) |
| BMI, kg/m2 | 28.3 ±5.3 | 29.2±6.9 |
| Obesity, BMI>30kg/m2 | 402(34%) | 54(36%) |
| Systolic BP, mmHg | 124±15 | 126±17 |
| Diastolic BP, mmHg | 74±10 | 75±10 |
| Hypertension | 176(15%) | 18(12%) |
| Serum glucose, mg/dl | 95±8 | 95±10 |
| Total cholesterol, mg/dl | 199±39 | 193±36 |
| HDL cholesterol, mg/dl | 57±17 | 53±13 *† |
| LDL cholesterol, mg/dl | 116±33 | 115±32 |
| Uric acid, mg/dl | 5.3±1.3 | 5.5±1.4 |
| Renal Cysts | ||
| Any cortical cyst >10mm in diameter | 96(8%) | 21(14%)*† |
| Any medullary cyst >10mm in diameter | 55(5%) | 9(6%) |
Note:
p<0.05 by Oneway Anova test or Fisher exact test.
p>0.05 with age-gender-BSA adjustment.
Abbreviations: BSA=body surface area; BMI=body mass index; BP=blood pressure; HDL=high-density lipoprotein; LDL=low-density lipoprotein; mGFR =measured glomerular filtration rate.
Table 2.
The median inter-test coefficient of variation (CV) of kidney volume measurements in 23 potential kidney donors who underwent a second contrast-enhanced CT scan (a mean 2.4 years apart) and median intra-test CV of kidney volume measurements in 24 potential donors by two different imaging technologists.
| Kidney parenchymal volume | Cortical volume | Medullary volume | |
|---|---|---|---|
| Inter-test CV | 2.9% | 6.2% | 6.9% |
| Intra-test CV | 3.7% | 2.4% | 3.6% |
Kidney volume with age
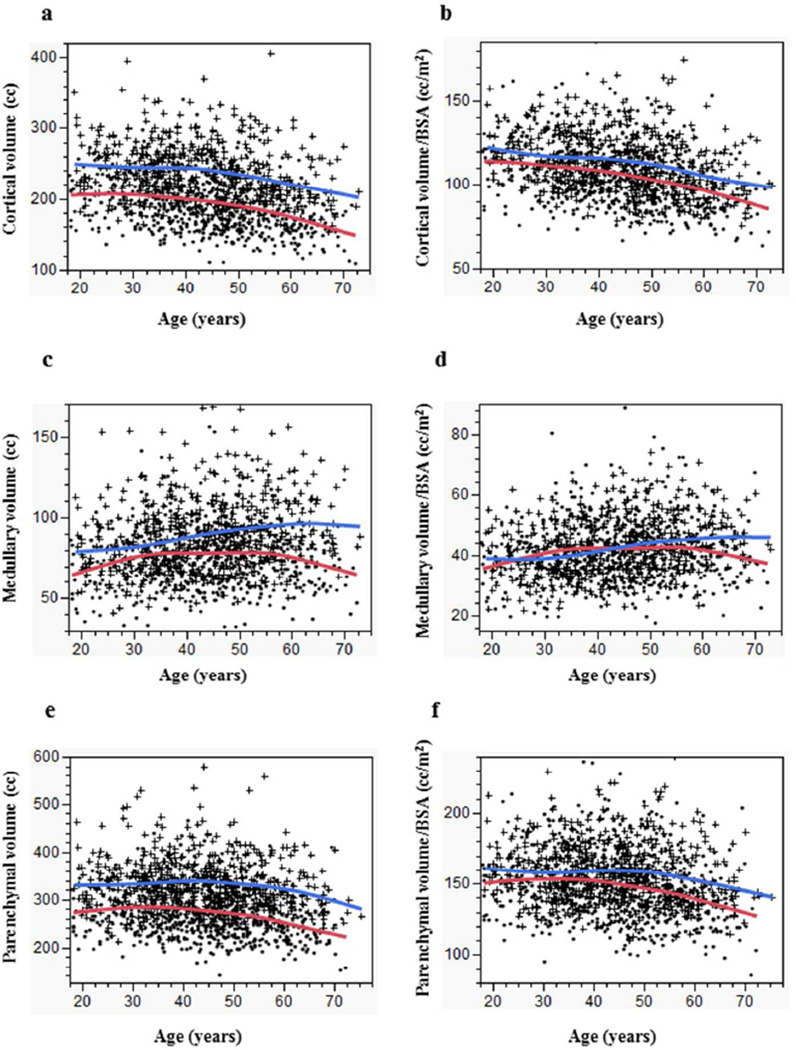
The median kidney cortical volume among 1192 potential donors was 211 cc (10th–90th percentiles 157–268 cc). As shown in Table 3 and Figure 1a, men had larger kidney cortical volume than women (p<0.01 for all age groups). Cortical volume and cortical volume per BSA progressively declined with age but more steeply after age 50 years (Figure 1a, Figure 1b, Table 4). The rate of decline did not differ by gender (p=0.15, test for age × gender interaction). Cortical volume had a stronger correlation with BSA (r=0.62, p<0.01) (Online Figure a) compared to BMI (r=0.29, p < 0.01).
Table 3.
The median (10%, 90%) kidney cortical, medullary volume and kidney parenchymal volume (cc) among potential kidney donors by age group.
| Age group | Cortical volume | Medullary volume | Parenchymal volume | |||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Overall (n=1184–1334) | 190(151, 243) | 234(189, 295) | 76(55, 99) | 86(64, 120) | 269(213, 339) | 325(267, 402) |
| 18–29y (n=157–170) | 204(161, 250) | 244(190, 300) | 72(50, 91) | 77(57, 111) | 272(224, 343) | 322(263, 401) |
| 30–39y (n=308–349) | 200(157, 253) | 241(200, 295) | 76(57, 106) | 82(61, 112) | 283(224, 351) | 331(271, 397) |
| 40–49y (n=354–401) | 193(154, 245) | 240(188,294) | 79(54, 100) | 88(64, 127) | 272(219, 340) | 335(264, 416) |
| 50–59y (n=255–290) | 180(146, 225) | 218(186,297) | 78(57, 102) | 91(67, 125) | 262(212, 325) | 321(271, 404) |
| 60–69y (n=110–124) | 163(133, 203) | 207(172,274) | 70(54, 92) | 96(77,126) | 230(196, 285) | 307(260, 405) |
Figure 1.
Age related changes in kidney volumes. (a) kidney cortical volume, (b) kidney cortical volume per BSA, (c) kidney medullary volume, (d) kidney medullary volume per BSA, (e) kidney parenchymal volume, and (f) kidney parenchymal volume per BSA by age (curves are smoother fit) in men (blue curve, cross) and women (red curve, closed circle).
Table 4.
Change in kidney cortical, medullary, and parenchymal volume by age.
| Kidney Volumes | Age < 50 years | Age ≥ 50 years | Test for change in slope before and after age 50 years* |
|---|---|---|---|
| Cortical Volume | cc per decade (p-value) | cc per decade (p-value) | p-value |
| Women | −5.6(0.006) | −17.7(<0.0001) | 0.002 |
| Men | −4.5(0.07) | −17.9(0.004) | 0.04 |
| Cortical volume per BSA | cc/m2 per decade (p-value) | cc/m2 per decade (p-value) | p-value |
| Women | −3.7(0.0002) | −7.8(<0.0001) | 0.04 |
| Men | −3.2(0.002) | −9.2(0.001) | 0.07 |
| Medullary volume | cc per decade (p-value) | cc per decade (p-value) | p-value |
| Women | 2.8(0.008) | −6.1(0.006) | 0.0001 |
| Men | 5.1(0.0004) | 0.7(0.83) | 0.25 |
| Medullary volume per BSA | cc/m2 per decade (p-value) | cc/m2 per decade (p-value) | p-value |
| Women | 1.4(0.01) | −2.6(0.04) | 0.003 |
| Men | 2.0(0.0006) | 0.2(0.88) | 0.41 |
| Parenchymal volume | cc per decade (p-value) | cc per decade (p-value) | p-value |
| Women | −2.7(0.32) | −22.6(<0.001) | 0.0002 |
| Men | 1.4(0.68) | −22.7(0.002) | 0.004 |
| Parenchymal volume per BSA | cc/m2 per decade (p-value) | cc/m2 per decade (p-value) | p-value |
| Women | −2.2(0.07) | −9.8(<0.001) | 0.005 |
| Men | −0.8(0.51) | −10.3(0.0008) | 0.01 |
Based on modeling age as a linear spline with a knot at age 50 years.
The median kidney medullary volume among 1192 potential donors was 80 cc (10th–90th percentiles 57–110 cc). As shown in Table 3 and Figure 1c, men had larger kidney medullary volume than women (p<0.01 for all age groups). Medullary volume and medullary volume per BSA increased with age in men, while in women it increased with age until age 50 years with a subsequent decline (Figure 1c, Figure 1d, Table 4). These changes differed by gender (p=0.009, test for age × gender interaction). Medullary volume had a stronger correlation with BSA (r=0.45, p<0.01) (Online Figure b) compared to BMI (r=0.20, p<0.01).
Kidney parenchymal volume was obtained through the sum of cortex and medulla volume or direct measurement for potential donors where inadequate cortical-medullary differentiation. The median kidney parenchymal volume among 1344 potential donors was 292 cc (10th–90th percentiles 225–373 cc). As shown in Table 3 and Figure 1e, men had larger kidney parenchymal volume than women (p<0.01 for all age groups). Kidney parenchymal volume and parenchymal volume per BSA was relatively stable with age until age 50 years with a subsequent decline (Figure 1e, Figure 1f, Table 4). These changes differed by gender (p=0.01, test for age × gender interaction). Parenchymal volume had stronger correlation with BSA (r=0.68, p<0.01) (Online Figure c) than with BMI (r=0.36, p<0.01).
Kidney volume with kidney function and CKD risk factors
As shown in Table 5, the independent predictors of increased cortical volume were younger age, male gender, increased measured glomerular filtration rate (mGFR), increased 24-h urine albumin, current smoker, increased BMI, increased serum glucose, and decreased HDL cholesterol. Increased cortical volume per BSA had similar independent associations with male gender, increased mGFR, increased 24-h urine albumin, current smoker and decreased HDL cholesterol. Age was not an independent predictor of cortical volume per BSA (even when modeling age as a linear spline with a knot at age 50 years with age × gender interaction terms). BMI was negatively associated with increased cortical volume per BSA but there is relatively strong correlation between BMI and BSA(r=0.66, p<0.001).
Table 5.
Predictors of kidney cortical volume and cortical volume per body surface area (BSA) among 1192 potential donors.
| Characteristics | Cortical volume | Cortical volume /BSA | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| *Age, 10y | −10.5 | <.0001 | −2.3 | 0.009 | −4.7 | <.0001 | −0.7 | 0.09 |
| Male | 44.8 | <.0001 | 34.9 | <.0001 | 8.6 | <.001 | 5.0 | <.0001 |
| GFR, SD | 21.9 | <.0001 | 18.7 | <.0001 | 10.7 | <.0001 | 9.8 | <.0001 |
| 24 h urine albumin, doubling | 5.1 | <.0001 | 2.4 | <.0001 | 1.7 | <.0001 | 1.0 | 0.0002 |
| Current smoker | 15 | <.0001 | 9.9 | <.0001 | 9.3 | <.0001 | 6.3 | <.0001 |
| Past smoker | 1.0 | 0.73 | - | - | - | - | - | - |
| BMI, SD | 15 | <.0001 | 12.4 | <.0001 | −0.6 | 0.24 | −1.2 | 0.009 |
| Diastolic BP, SD | 5.1 | <.0001 | 1.3 | 0.17 | - | - | - | - |
| Serum glucose, SD | 7.6 | <.0001 | 2.6 | 0.01 | 0.3 | 0.65 | 0.8 | 0.13 |
| Total cholesterol, SD | −2.4 | 0.06 | - | - | - | - | - | - |
| HDL cholesterol, SD | −16.2 | <.0001 | −3.4 | 0.001 | −3.1 | <.0001 | −1.4 | 0.003 |
| LDL cholesterol, SD | 1.5 | 0.24 | - | - | - | - | - | - |
| Serum uric acid, SD | 13.7 | <.0001 | 0.24 | 0.83 | - | - | - | - |
β indicates the change in volume (cc) with characteristic.
Multivariate associations were not substantively different with age modeled as a linear spline with a knot at 50 years with age × gender interaction terms.
Hyperfiltration (GFR >130ml/min/1.73m2) was associated with cortical volume (48 cc larger, p<0.001), even after age-gender-BSA adjustment (37 cc larger, p<0.001). Albuminuria (24-hr urine albumin>30mg) was associated with cortical volume (28 cc larger, p=0.004), even after age-gender-BSA adjustment (24 cc larger, p<0.001). Obesity (BMI>30 kg/ m2) was associated with cortical volume (25 cc larger, p<0.001) even after age-gender adjustment (25cc larger, p<0.001).
Kidney medullary volume correlated with GFR (r=0.20, p<0.001), although the association was weaker compared to cortical volume (r=0.49, p<.001). As shown in Table 6, the independent predictors of increased medullary volume were older age, male gender, increased mGFR, increased 24-h urine albumin, increased BMI, increased serum glucose, and decreased serum uric acid. Increased medullary volume per BSA had similar independent associations with older age, male gender, increased mGFR, increased 24-h urine albumin, increased serum glucose, and decreased serum uric acid. Uric acid was positively associated with increased medullary volume in unadjusted analysis due to the higher uric acid levels and larger medullary volumes in men compared to women.
Table 6.
Predictors of kidney medullary volume and medullary volume per body surface area (BSA) among 1192 potential donors.
| Characteristics | Medullary volume | Medullary volume /BSA | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| *Age, 10y | 1.6 | 0.002 | 3.7 | <.0001 | 1.1 | <.0001 | 2.2 | <0.001 |
| Male | 11.9 | <.0001 | 13.7 | <.0001 | 0.4 | 0.43 | 1.9 | 0.005 |
| GFR, SD | 4.3 | <.0001 | 5.4 | <.0001 | 2.0 | <.0001 | 2.9 | <.0001 |
| 24 h urine albumin, doubling | 1.2 | 0.003 | 0.9 | 0.01 | 0.3 | 0.09 | 0.4 | 0.02 |
| Current smoker | 2.2 | 0.14 | - | - | - | - | - | - |
| Past smoker | 2.8 | 0.05 | 1.1 | 0.41 | - | - | - | - |
| BMI, SD | 4.4 | <.0001 | 4.9 | <.0001 | −1.0 | 0.0006 | −0.5 | 0.09 |
| Diastolic BP, SD | 2.1 | 0.0005 | 0.3 | 0.66 | - | - | - | - |
| Serum glucose, SD | 4.4 | <.0001 | 1.7 | 0.01 | 0.9 | 0.004 | 0.7 | 0.04 |
| Total cholesterol, SD | −0.7 | 0.26 | - | - | - | - | - | - |
| HDL cholesterol, SD | −2.2 | 0.0002 | 0.3 | 0.67 | - | - | - | - |
| LDL cholesterol, SD | −0.1 | 0.84 | - | - | - | - | - | - |
| Serum uric acid, SD | 1.7 | 0.005 | −3.1 | <.0001 | −1.7 | <.0001 | −1.9 | <.0001 |
β indicates the change in volume (cc) with characteristic.
Multivariate associations were not substantively different with age modeled as a linear spline with a knot at 50 years with age × gender interaction terms.
Hyperfiltration was associated with medullary volume (9.5 cc larger, p=0.0002), even after age-gender-BSA adjustment (10.7cc larger, p<0.001). Albuminuria was not associated with medullary volume (2.7 cc larger, p=0.46). Obesity was associated with medullary volume (8.4 cc larger, p<0.001) even after age-gender adjustment (8.4cc larger, p< 0.001).
Kidney parenchymal volume correlated with GFR (r=0.45, p<0.001). As shown in Table 7, the independent predictors of increased parenchymal volume were male gender, increased mGFR, increased 24-h urine albumin, current smoker, increase BMI, increased serum glucose, decreased HDL cholesterol, and decreased serum uric acid. Older age was borderline associated with increased parenchymal volume in multivariate analysis. Increased parenchymal volume per BSA had similar associations with male gender, increased mGFR, increased 24-h urine albumin, and current smoker. Older age was statistically associated with increased kidney parenchymal volume per BSA in the multivariate analysis. Findings were similar using GFR in ml/min instead of GFR in ml/min/1.73 m2 in the multivariate analyses except that with adding BSA as a predictor to the model, BMI was not associated or was negatively associated with kidney volumes (Online Table).
Table 7.
Predictors of kidney parenchymal volume and parenchymal volume per body surface area (BSA) among 1344 potential donors.
| Characteristics | Kidney parenchymal volume | Kidney parenchymal volume /BSA | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| *Age, 10y | −8.6 | <.0001 | 2.2 | 0.05 | −3.5 | <0.0001 | 1.7 | 0.0008 |
| Male | 59.5 | <.0001 | 51.5 | <.0001 | 9.5 | <.0001 | 7.9 | <.0001 |
| GFR, SD | 26.9 | <.0001 | 24.7 | <.0001 | 12.9 | <.0001 | 12.8 | <.0001 |
| 24 h urine albumin, doubling | 8.1 | <.0001 | 4.4 | <.0001 | 2.4 | <.0001 | 1.9 | <.0001 |
| Current smoker | 16.9 | <.0001 | 12.2 | <.0001 | 11.0 | <.0001 | 7.9 | <.0001 |
| Past smoker | 4.6 | 0.24 | - | - | - | - | - | - |
| BMI, SD | 22.0 | <.0001 | 19.4 | <.0001 | −0.8 | 0.20 | −1.0 | 0.07 |
| Diastolic BP, SD | 9.0 | <.0001 | 1.2 | 0.31 | - | - | - | - |
| Serum glucose, SD | 11.5 | <.0001 | 2.9 | 0.03 | 0.9 | 0.19 | 0.83 | 0.16 |
| Total cholesterol, SD | −3.1 | 0.052 | - | - | - | - | - | - |
| HDL cholesterol, SD | −20.4 | <.0001 | −3.0 | 0.02 | −2.5 | <0.001 | 0.17 | 0.75 |
| LDL cholesterol, SD | 1.7 | 0.29 | - | - | - | - | - | - |
| Serum uric acid, SD | 17 | <.0001 | −2.9 | 0.04 | −0.8 | 0.21 | −1.1 | 0.06 |
β indicates the change in volume (cc) with characteristic.
Multivariate associations were not substantively different with age modeled as a linear spline with a knot at 50 years with age × gender interaction terms.
Hyperfiltration was associated with kidney parenchymal volume (57cc larger, p<0.0001) even after age-sex-BSA adjustment (49cc larger, p<0.001). Albuminuria was associated with parenchymal volume (44cc larger, p<0.001) even after age-sex-BSA adjustment (33cc larger, p<0.001). Obesity was associated with kidney parenchymal volume (36cc larger, p<0.001) even after age-gender adjustment (35cc larger, p<0.001).
Kidney volume with renal cysts
After age-sex-BSA adjustment, presence of any cortical cyst >10mm in diameter was associated with medullary volume (4.5 cc larger, p=0.03) and kidney parenchymal volume (8.2 cc larger, p=0.05), but not cortical volume (4.9 cc larger, p=0.16), After age-sex-BSA adjustment, presence of any medullary cyst >10mm in diameter was not statistically significantly associated with cortical volume (5.3 cc larger, p=0.23), medullary volume (3.0 cc larger, p=0.24), or kidney parenchymal volume (9.0 cc larger, p=0.10). Adding presence of any cyst >10mm to the multivariate models did not substantively change any of the associations of kidney volumes with clinical characteristics.
Independence of GFR decline with age from kidney volume changes
The decline in cortical volume per BSA (−4.7 cc/m2 per decade, p<0.001) with age was largely attenuated after adjustment for GFR (−1.2 cc/m2 per decade, p=0.001) and no longer statistically significant with further adjustment for CKD risk factors (gender, 24-h urine albumin, current smoker, BMI, glucose, and HDL cholesterol). Alternatively, we assessed whether the decline in GFR with age was independent of kidney volumes. GFR declined with age by −6.8 (95% CI −6.0 to −7.6) ml/min/1.73 m2 per decade, and this decline changed with adjustment for cortical volume per BSA −4.2 (95% CI −3.4 to −5.0) ml/min/1.73 m2 per decade, medullary volume per BSA −7.6 (95% CI −6.8 to −8.4) ml/min/1.73 m2 per decade, or kidney parenchymal volume per BSA −5.2 (95% CI −4.6 to −5.8) ml/min/1.73 m2 per decade. The decline in GFR with age remained statistically significant with adjustment for cortical volume per BSA and the other CKD risk factors (gender, 24-h urine albumin, current smoker, BMI, glucose, and HDL cholesterol) −4.7 (95% CI –3.9 to –5.6) ml/min/1.73 m2 per decade. The impact of BSA adjustment on unstandardized GFR (ml/min) decline with age was minimal. GFR declined by −8.3 ml/min per decade unadjusted, by −7.5 ml/min per decade adjusted for BSA, by −4.3 ml/min per decade adjusted for cortical volume, and by −4.7 ml/min per decade adjusted for cortical volume and BSA.
DISCUSSION
This study separately characterized the clinical characteristics that associate with kidney cortex, medulla, and parenchymal volume in relatively healthy adults and found several novel findings. First, with older age, cortical volume progressively declined in both genders while medullary volume increased in men but only initially increased in women followed by a subsequent decline. Second, the age-related decline in cortical volume was dependent on the age-related decline in kidney function and CKD risk factors, but the age-related decline in GFR was only partially attenuated by the cortical volume decline and CKD risk factors. Third, cortical and medullary volume had different associations with CKD risk factors suggesting that hypertrophy or atrophy can differ between nephron segments in their interaction with CKD risk factors. Finally, while a decrease in cortical volume is often seen in advanced CKD, increased cortical volume may be an early marker of CKD risk since it associates with hyperfiltration and albuminuria.
Our results showed kidney cortical volume per BSA decreased with aging, and this was dependent on GFR decline, albuminuria, and increased CKD risk factors with older age. Underlying nephrosclerosis (glomerulosclerosis and tubular atrophy) may explain this association by leading to both cortical volume loss and GFR reduction [16, 17]. It was also evident that cortical volume decline only partially accounted for GFR decline with aging. Since nephrons unaffected by nephrosclerosis can hypertrophy and hyperfilter to compensate for sclerosed nephrons, GFR decline with age may not be fully reflected in the cortical volume decline with age [17].
Unlike cortical volume, medullary volume increased with age, except in women over the age of 50 years. The reason for this increase and the gender difference are not entirely clear. There are important functional and structural changes of medulla during aging and with kidney injury. Medullary fibrosis increases with age and hypertension[18]; renomedullary interstitial cells show antihypertensive action by secretion of prostaglandins [19]; and hypoxia of the medulla may increase susceptibility to acute and chronic renal injury[20]. Autopsy studies suggest medullary fibrosis increases with age throughout the medulla, but only the fibrosis in inner medulla associates with decreased kidney weight[18]. This may indicate hypertrophy or hyperplasia of nephron segments in the outer stripe of medulla. Alternatively, age-related global glomerulosclerosis is most severe in the superficial cortex. This may increase perfusion to the juxtamedullary nephrons with the longest loops of Henle that may hypertrophy and increase medullary volume[21]. Eventually, atrophy from nephrosclerosis could have a net effect of medullary volume loss, and this may occur earlier in women than in men. Women are known to have fewer nephrons than men [22, 23] and may have less compensatory reserve. Furthermore, this finding may be related to the post-menopausal loss of the protective effects of estrogen, which leads to loss of renal microvessels [24].
Another prior study also using potential kidney donors did not find kidney parenchymal volume to decline with age but few subjects older than 60 years were included[6]. Our data clarifies that while kidney parenchymal volume is stable with aging in younger adults, there is still a decline with age in the older adults who are ostensibly healthy. Further, the competing cortical volume decline and medullary volume increase with age explains why the total parenchymal volume changes little with age in younger adults even though cortical nephrosclerosis on renal biopsy occurs with aging even in young adults [16].
Our data suggested increased cortical volume may be an indicator and a pathway of early kidney injury since it associates with hyperfiltration and albuminuria. Diabetics are known to develop enlarged kidneys with hyperfiltration in early diabetic nephropathy [25]. This process may be more universal than just diabetes as evidenced by our findings in a non-diabetic population. Hypertension associates with hyperfiltration[26, 27], but our study did not find an association between increased kidney volume and blood pressure independent of other clinical characteristics. Prior studies associated current cigarette smoking with hyperfiltration and proteinuria, whereas past smokers did not have abnormal kidney function [28, 29]. Our study extends these findings by showing an increased kidney volume with current smoking, but not in past smokers. Obesity has been previously associated with increased kidney parenchymal volume[6] and hyperfiltration[30–32]. Glomerulomegaly is one of the common features of obesity related glomerulopathy[33]. The association of obesity with increased cortical volume indicates that obesity-related glomerulomegaly with tubular hypertrophy might contribute to the volume increase in cortex at the early kidney injury. Studies in subjects without kidney disease showed lower HDL cholesterol associated with hyperfiltration[34]. Our data of the association of lower HDL cholesterol with increased cortical volume suggest HDL cholesterol plays a protective role in the cortical changes of early CKD. Treatment of low HDL cholesterol level, together with smoking cessation and body weight control may help to prevent the increased cortical volume and underlying hypertrophy of nephrons that reflect early kidney injury.
Different from cortical volume per BSA, increased medullary volume per BSA independently associated with increased glucose and decreased serum uric acid. As a product of purine nucleotide cycle, production of uric acid is more active in medulla than in cortex [35]. Studies showed serum uric acid is elevated in CKD and may be a marker for CKD progression [36, 37]. Hyperuricemia may lead to more damage and atrophy in the segments of nephrons in medulla than in cortex. Renal medulla may be the predominant site of several hyperglycemia-induced alterations in biochemical cellular pathways [38] and glycogen is shunted into the synthesis of medullary glycosaminoglycans during anti-diuresis [39]. Indeed, changes in tubulointerstitial structures have been evident during the early stages in diabetes [40, 41]. Given the relatively healthy population in our study, the association of increased serum glucose with increased medullary volume, but not cortical volume, suggest that glucose-related structural changes in the medulla might precede those in the cortex.
While we excluded detected cysts from the measurement of cortical and medullary volume, small undetected cysts could potentially contribute to increased kidney volumes. Indeed, patients with cortical cysts did have an increase in their medullary and kidney parenchymal volumes, possibly due to undetected cysts. However, detectable cysts did not account for any of the association of kidney volumes with kidney function and CKD risk factors. Thus, it is unlikely that undetected cysts explain the associations between kidney volumes and clinical characteristics.
A limitation of this study was relatively few subjects ages 70 years and older since the study population was potential kidney donors. We did not control the hydration status of donors undergoing CTAs and hydration may potentially affect kidney volumes. However, these were all healthy adults without acute illness at the time of their CT scans and diuretic use (3.9% of donors) was not associated with cortical, medullary, or total parenchymal kidney volume (p≥0.15 for all). As a cross-sectional study, the temporal relationship between changes in kidney volume and changes in kidney function is unclear.
In conclusion, our study provides new insights in the association of kidney cortical and medullary volume with aging, kidney function, and CKD risk factors. Increased kidney volume associates with hyperfiltration, albuminuria, and CKD risk factors even among a relatively healthy population. Increased kidney volume may reflect underlying glomerulomegaly and tubular hypertrophy. The reasons for gender differences between age related changes in the cortex and medulla volumes requires further study. The clinical role, if any, of cortical volume or medullary volume in evaluating kidney health and risk for CKD deserves further exploration. Future work is also needed to understand the relationship between the macro-anatomy of the kidney with the micro-anatomy on renal biopsy.
METHODS
Study population
This study was approved by the institutional review board at the Mayo Clinic, Rochester Minnesota. All potential kidney donors were identified from 2000 to 2008 at the Mayo Clinic and were included if they had research authorization in accordance with Minnesota State law [42]. Potential kidney donors underwent a renal CTA scan and a standardized laboratory and clinical evaluation [42]. Kidney function and CKD risk factors were obtained through abstraction of electronic charts at the time of the donor evaluation and prior to donor nephrectomy (if done).
Multidetector CT examination
As described previously [15, 42], 4-channel MDCT scanner (Qxi; GE Medical Systems, www3.gehealthcare.com/en) with slice thickness of 1.25mm and 7.5 mm/rotation was used in 2000–2005 and 64-channel MDCT scanner (Sensation 64; Siemens Medical Solutions, www.medical.siemens.com) with 64 × 0.6 collimation, a pitch of 1.2, and 23 mm/rotation was used in 2005–2008 to acquire CTA examinations. CT Images from the angiogram phase were downloaded into a local workstation for processing.
CT scan review and kidney volume measurement
We estimated the kidney cortical and medullary volume using software (ITK-SNAP version 1.1, www.itksnap.org, Figure 2) [43] to semi-automatically segment the cortex and medulla from transverse images obtained during the angiogram (arterial) phase. Contrast enhancement from the arcuate artery to afferent arterioles defined the cortex. Segmentation was based on active contour evolution using an energy minimizing spline algorithm. Cortex and medulla were segmented separately by using appropriate intensity value thresholds to create a feature image. Automated segmentation was initiated by placing spherical bubbles on the feature image (cortex or medulla). Hand editing ensured exclusion of any non-parenchymal regions, including sinus fat, cysts, kidney stones, scars or vessels missed by the software. Kidney volumes were measured in a random order by a trained imaging technologist masked to other characteristics of the donor. Images with movement artifact, polycystic kidneys, or incomplete kidneys in the imaging window were excluded. While the volumes measured excluded detectable cysts, the presence of any cortical or medullary cyst greater than 10 mm in diameter was specifically determined as described elsewhere [15]. For contrast images with inadequate cortex from medulla differentiation, full kidney segmentation with exclusion of sinus fat, cysts and other non-parenchymal tissues was performed and kidney parenchymal volume was measured directly.
Figure 2.
Example of kidney cortex (yellow) and medulla (blue) segmentation using software ITK-SNAP in (a) and (b) a 3-dimensional view, (c) and (d) in a coronal view, (e) and (f) in a sagittal view, and (g) and (h) in a transversal view.
Clinical and laboratory evaluation of potential kidney donors
All potential kidney donors underwent a prescreening by a transplant nurse on a telephone interview before the clinic visit [15, 42] to exclude disorders that would preclude the donation (e.g., known CKD, diabetes, or cardiovascular disease). The remaining potential donors underwent a pre-scheduled battery of tests and evaluations over a 2-day period. These included a contrast enhanced abdominal CT scan, iothalamate clearance measurement (GFR in ml/min/1.73 m2), 24-hour urine albumin excretion, and CKD risk factors. These CKD risk factors were mostly components of metabolic syndrome and included blood pressure (BP), serum glucose, total cholesterol, LDL cholesterol, HDL cholesterol and uric acid. Body mass index (BMI) and body surface area (BSA) were calculated from height and weight [44, 45]. Obesity was defined as BMI >30 kg/m2. Hypertension was defined as use of antihypertensive therapy for high blood pressure or systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at the initial evaluation. Hyperfiltration was defined by GFR>130ml/min/1.73m2 and albuminuria was defined by 24-h urine albumin excretion > 30 mg.
Reproducibility of kidney volume measurements
Occasionally potential kidney donors will undergo two separate evaluations as they may not be selected for the first kidney donation and the recipient may require another allograft. We searched the medical record of all our potential kidney donors to identify those with two pre-donation CT scans. The cortex and medulla were segmented for each of the two CT Scans and compared (inter-test CV). Finally we had two imaging technologists independently segment the same CT images (intra-test CV).
Statistical analysis
Cortical and medullary volumes were summed between both kidneys and kidney parenchymal volume was the sum of cortical and medullary volume. Scatter plots were generated to explore the relationship of kidney volumes with age, kidney function, and CKD risk factors. Linear regression models were used to identify which of these clinical characteristics associated with cortex, medulla, or whole parenchymal kidney volume. A linear spline with a knot at age 50 years was used to determine whether age related changes in kidney volumes differed between younger and older adults. Multivariate models assessed independence of clinical characteristics that associated with kidney volumes. Age × gender interaction was also assessed. Only clinical characteristics that associated with kidney volume in the univariate analysis were included in the multivariate models, except when redundant. Independent predictors for kidney volume were also assessed for their associations with kidney volume per BSA. The relationship between kidney cysts and non-cyst parenchymal volume were analyzed in separate models. All statistical analyses were performed using JMP, version 9.0 (SAS institute, www.jmp.com).
Supplementary Material
ACKNOWLEDGEMENT
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Footnotes
DISCLOSURE
All the authors declared no financial disclosure or conflict of interests.
Contributor Information
Xiangling Wang, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Terri J. Vrtiska, Department of Radiology, Mayo Clinic
Ramesh T. Avula, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Leah R. Walters, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Harini A. Chakkera, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Walter K. Kremers, Biomedical Statistics and Informatics, Mayo Clinic
Lilach O. Lerman, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
Andrew D. Rule, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic
REFERENCES
- 1.Buturovic-Ponikvar J, Visnar-Perovic A. Ultrasonography in chronic renal failure. European journal of radiology. 2003;46(2):115–122. doi: 10.1016/s0720-048x(03)00073-1. [DOI] [PubMed] [Google Scholar]
- 2.Paivansalo M, Huttunen K, Suramo I. Ultrasonographic findings in renal parenchymal diseases. Scandinavian journal of urology and nephrology. 1985;19(2):119–123. doi: 10.3109/00365598509180238. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, et al. Assessing renal parenchymal volume on unenhanced CT as a marker for predicting renal function in patients with chronic kidney disease. Academic radiology. 2012;19(6):654–660. doi: 10.1016/j.acra.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Pantoja Zuzuarregui JR, Mallios R, Murphy J. The effect of obesity on kidney length in a healthy pediatric population. Pediatr Nephrol. 2009;24(10):2023–2027. doi: 10.1007/s00467-009-1202-1. [DOI] [PubMed] [Google Scholar]
- 5.Paivansalo MJ, et al. Effect of hypertension, diabetes and other cardiovascular risk factors on kidney size in middle-aged adults. Clin Nephrol. 1998;50(3):161–168. [PubMed] [Google Scholar]
- 6.Johnson S, et al. Determinants and functional significance of renal parenchymal volume in adults. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(1):70–76. doi: 10.2215/CJN.00030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney international. 2012;82(3):270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gourtsoyiannis N, et al. The thickness of the renal parenchyma decreases with age: a CT study of 360 patients. AJR. American journal of roentgenology. 1990;155(3):541–544. doi: 10.2214/ajr.155.3.2117353. [DOI] [PubMed] [Google Scholar]
- 9.Emamian SA, et al. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR. American journal of roentgenology. 1993;160(1):83–86. doi: 10.2214/ajr.160.1.8416654. [DOI] [PubMed] [Google Scholar]
- 10.Glodny B, et al. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC urology. 2009;9:19. doi: 10.1186/1471-2490-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rule AD, et al. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012;59(5):611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz EC, et al. Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant. 2011;26(8):2695–2700. doi: 10.1093/ndt/gfq769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz EC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5(3):431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes--2011. Diabetes care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rule AD, et al. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(5):611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rule AD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152(9):561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rule AD, et al. Association of kidney function and metabolic risk factors with density of glomeruli on renal biopsy samples from living donors. Mayo Clin Proc. 2011;86(4):282–290. doi: 10.4065/mcp.2010.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haggitt RC, Pitcock JA, Muirhead EE. Renal medullary fibrosis in hypertension. Human pathology. 1971;2(4):587–597. doi: 10.1016/s0046-8177(71)80072-2. [DOI] [PubMed] [Google Scholar]
- 19.Muirhead EE, et al. Renomedullary interstitial cells (RIC), prostaglandins (PG) and the antihypertensive function of the kidney. Prostaglandins. 1973;3(5):581–594. doi: 10.1016/0090-6980(73)90096-8. [DOI] [PubMed] [Google Scholar]
- 20.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. The New England journal of medicine. 1995;332(10):647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 21.Samuel T, et al. Determinants of glomerular volume in different cortical zones of the human kidney. Journal of the American Society of Nephrology : JASN. 2005;16(10):3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 22.Hughson MD, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69(4):671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 23.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21(6):898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 24.Urbieta-Caceres VH, et al. Age-dependent renal cortical microvascular loss in female mice. Am J Physiol Endocrinol Metab. 2012;302(8):E979–E986. doi: 10.1152/ajpendo.00411.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derchi LE, et al. Ultrasonographic imaging and Doppler analysis of renal changes in non-insulin-dependent diabetes mellitus. Academic radiology. 1994;1(2):100–105. doi: 10.1016/s1076-6332(05)80826-8. [DOI] [PubMed] [Google Scholar]
- 26.Okada R, et al. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27(5):1821–1825. doi: 10.1093/ndt/gfr651. [DOI] [PubMed] [Google Scholar]
- 27.Palatini P, et al. Factors associated with glomerular hyperfiltration in the early stage of hypertension. Am J Hypertens. 2012;25(9):1011–1016. doi: 10.1038/ajh.2012.73. [DOI] [PubMed] [Google Scholar]
- 28.Maeda I, et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(10):2462–2469. doi: 10.2215/CJN.00700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noborisaka Y, et al. The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environmental health and preventive medicine. 2012 doi: 10.1007/s12199-012-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chagnac A, et al. Glomerular hemodynamics in severe obesity. American journal of physiology. Renal physiology. 2000;278(5):F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 31.Bosma RJ, et al. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney international. 2004;65(1):259–265. doi: 10.1111/j.1523-1755.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 32.Wuerzner G, et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56(2):303–312. doi: 10.1053/j.ajkd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Kambham N, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney international. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 34.Krikken JA, Gansevoort RT, Dullaart RP. Lower HDL-C and apolipoprotein A–I are related to higher glomerular filtration rate in subjects without kidney disease. Journal of lipid research. 2010;51(7):1982–1990. doi: 10.1194/jlr.M005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepinski J, et al. The purine nucleotide cycle activity in renal cortex and medulla. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1989;14(4):307–309. doi: 10.1016/s0272-6386(89)80209-4. [DOI] [PubMed] [Google Scholar]
- 36.Fassett RG, et al. Biomarkers in chronic kidney disease: a review. Kidney international. 2011;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 37.Siu YP, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Nordquist L, Palm F. Diabetes-induced alterations in renal medullary microcirculation and metabolism. Current diabetes reviews. 2007;3(1):53–65. doi: 10.2174/157339907779802120. [DOI] [PubMed] [Google Scholar]
- 39.Darnton SJ. The conversion of injected glucose into renal glycogen and mucopolysaccharides. An autoradiographic study of rabbits in various states of hydration. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1969;102(2):273–282. doi: 10.1007/BF00335505. [DOI] [PubMed] [Google Scholar]
- 40.Pollock JS, Carmines PK. Diabetic nephropathy: nitric oxide and renal medullary hypoxia. American journal of physiology. Renal physiology. 2008;294(1):F28–F29. doi: 10.1152/ajprenal.00525.2007. [DOI] [PubMed] [Google Scholar]
- 41.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(8):3049–3056. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz EC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(3):431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yushkevich PA, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. discussion 312-3. [PubMed] [Google Scholar]
- 45.Flegal KM, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.