Abstract
The purpose of this study was to test the hypotheses that development of mature vimentin+/α-smooth muscle actin+/desmin+ (V+A+D+) myofibroblasts from corneal fibroblasts is regulated by transforming growth factor (TGF) β and platelet-derived growth factor (PDGF); and that myofibroblast development in vitro follows a similar developmental pathway as it does in vivo. Mouse corneal stromal fibroblasts (MSF) were isolated from the corneas of Swiss Webster mice and cultured in serum-free media augmented with DMEM/F12 and varying doses of TGFβ (0.1 to 2.0 ng/ml), with and without mouse PDGF-AA and/or PDGF-BB (2.0 ng/ml), to study the transition of the MSF to V+A+D+ myofibroblasts. The mean percentage of vimentin+, α-SMA+ and desmin+ cells was determined at each time point (2 to 15 days), with each growth factor concentration. MSF in vitro were noted to undergo the same developmental transition from V+A−D− to V+A+D− to V+A+D+ myofibroblasts as precursors undergo in vivo. TGFβ at a dose of 0.5 ng/ml and 1.0 ng/ml with 2.0 ng/ml PDGF-AA and 2.0 ng/ml PDGF-BB in DMEM/F12 serum-free media was optimal for the development of V+A+D+ myofibroblasts. This study defines optimal in vitro conditions to monitor the development of MSF into myofibroblasts. The combined effects of TGFβ and PDGF promote the full development of V+A+D+ myofibroblasts from MSF.
Introduction
Myofibroblasts are important modulators of the development of opacity (haze) following corneal surgery, injury, and infection (Masur et al., 1996; Jester et al., 1999; Mohan et al., 2003). Prior studies have shown that these cells can develop from either keratocyte-derived or bone marrow-derived cells and that cytokines such as transforming growth factor (TGF) β, platelet-derived growth factor (PDGF), and interleukin (IL)-1 have important roles in the development and death of these cells (Boström et al., 1996; Masur et al., 1996; Jester et al., 1999 and 2002; Kaur et al., 2009; Singh et al., 2011; Singh et al., 2012). For example, Jester and coworkers (2002) demonstrated that myofibroblast differentiation in rabbit keratocytes requires synergistic growth factor/integrin signaling involving TGFβ, PDGF, and the fibronectin receptor. Masur and coworkers (1996) demonstrated the density dependence of myofibroblast development from fibroblast precursors and also showed that TGF-beta receptor expression and smad2 localization are cell density dependent in fibroblast precursors to myofibroblasts (Petridou, et al., 2000). Epithelial cells and stromal cells, including myofibroblasts themselves (autocrine modulation), produce TGFβ and PDGF cytokines in corneas and other tissues in vivo (Masur et al., 1996; Zhang and Phan, 1999; Jester, et al., 1999; Thannickal, et al, 2004; Kaur et al., 2009; Saika, et al., 2010; Singh, et al., 2011 and 2012; Wilson, 2012: Tandon, et al., 2010).
Myofibroblasts have been shown to have variable cell phenotypes based on immunohistochemical staining of filaments and a classification system has been proposed for these cells (Schmitt-Graff, Desmouliere and Gabbiani, 1994; Kohnen et al., 1996). Thus, myofibroblasts that express only vimentin are termed V-type myofibroblasts, those that express vimentin and desmin are called VD-type myofibroblasts, those that express vimentin, SMA, and desmin are called VAD-type myofibroblasts, those that express vimentin and SMA are called VA-type myofibroblasts, and those that express vimentin and myosin are called VM-type myofibroblasts. Previous studies demonstrated that corneal myofibroblasts proceed through a developmental sequence from vimentin+/α-smooth muscle actin−/desmin− (V+A−D−) precursors to V+A+D− intermediate cells to mature V+A+D+ myofibroblasts in vivo (Chaurasia, et al., 2009). The purpose of the present study was to determine whether MSF undergo a similar develomental sequence to differentiated myofibroblasts in vitro that is modulated by TGFβ and PDGF.
Methods
Isolation of mouse corneal stromal fibroblasts (MSF)
The methods described by Yoshida and coworkers (2005) were modified to isolate mouse stromal fibroblasts (MSF) under serum-free conditions for use in subsequent experiments testing the effects of growth factors or plasmids that produce inhibitory factors on myofibroblast development. Briefly, corneas were removed from Swiss Webster mouse eyes (Pel Freeze, Rogers, AR) and the Descemet’s-endothelium complex was stripped away with 0.12 mm forceps. The remaining stroma and epithelium was incubated in 5 mg/ml of dispase II (Roche Diagnostics, Indianapolis, IN) at 4°C overnight. Lo ose epithelium was removed and the corneal stromal discs were cut into small segments and digested in 0.05% trypsin (Sigma, St. Louis, MO) for 30 minutes at 37°C, followed by incubation with 78 U/ml collagenase (Sigma) and 38 U/ml hyaluronidase (Sigma) for 30 minutes at 37°C. Stromal cells were mechanic ally dissociated into single cells and cultured in “augmented DMEM/F12” [DMEM/F12 (1:1) supplemented with 20 ng/ml epidermal growth factor (Sigma), 10 ng/ml of fibroblast growth factor 2 (Sigma), B27 supplement (Invitrogen, Carlsbad, CA), and 103 U/ml leukemia inhibitory factor (Chemicon International Inc., Temecula, CA)] at a density of 5 X 105 cells/ml in a 5% CO2 incubator at 37°C. Initial culture was performed in 35-mm dishes and cells were sub-cultured into 25-cm2 culture flasks. The spheres were sub-cultured in 75 cm2 culture flasks after 7 to 14 days. The medium was changed every 3 to 5 days, along with added growth factors.
Immunocytochemistry
Immunocytochemistry was performed as described previously (Yoshida, et al., 2005; Singh, et al., 2011). In brief, mouse corneal sphere cells and cells freshly isolated from mouse cornea were attached to glass slides using a cytospin preparation (Auto Smear CF-120; Sakura, Tokyo, Japan) and then fixed in 4% paraformaldehyde for 15 minutes at 4°C. Cells were washed twice with PBS for one minute with gentle agitation. Cells were treated with permeabilization solution [0.25% Triton® X-100 (Sigma, St. Louis, MO)] in PBS for five minutes at room temperature with gentle agitation and subsequently washed twice in PBS for one minute with gentle agitation. Cells were resuspended in blocking solution [5% BSA (Sigma, St. Louis, MO) PBS, pH 7.4] and incubated for one hour at room temperature with gentle agitation. Cells were washed twice with PBS for one minute with gentle agitation. Cells were stained with anti-vimentin antibody (sc-7557, goat antibody, Santa Cruz Biotech, Santa Cruz, CA) diluted 1:50 in phosphate buffered saline with 1% BSA, anti-α-smooth muscle actin (ab5694, chicken antibody, Abcam, San Francisco, CA) diluted 1:50 in 1% BSA and/or anti-desmin antibody (sc-7559, goat antibody, Santa Cruz Biotech) diluted 1:200 in 1% BSA for 90 minutes and then incubated with the respective secondary antibodies (A-11058; Alexa Fluor® 594 donkey anti-goat IgG, Invitrogen or A11039; Alexa Fluor 488 goat anti-chicken IgG, Invitrogen) for 60 minutes. Immunocytochemistry controls were performed by omitting primary antibodies. Cover slips were mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA) to allow visualization of all nuclei in the cells. All immunocytochemistry experiments were performed at least three times in triplicate to insure results were consistent. The slides were viewed and photographed with a Leica (Leica Microsystems, Buffalo Grove, IL) DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software (Surrey, BC, Canada).
In vitro transfection protocol
Sphere cells were used after six days of culture in serum-free modified medium. On the day of transfection, sphere cells were washed once with serum-free growth medium without antibiotics and cells were seeded at a density of 2–3 × 106 cells per well in 0.8 ml of serum-free growth medium without antibiotics. Lipofectamine® LTX & Plus Reagent (Catalog no-15338-100, Invitrogen, CA, USA) was used according to the manufacturer’s instructions for the transfection assay. The plasmid vectors used for the transfection assay were pGFP.TGFRBKDEL and empty pCMV control vector (obtained from Dr. B.K. Ambati, Moran Eye Center, UT, USA). The TGFβ-KDEL vector interferes with TGFβ signaling through anomalous sorting of cytokine bound to the expressed altered receptor (Singh et al., 2011). α-SMA was analyzed at six days after transfection and culture of cells in presence or absence of 1.0 ng/ml TGFβ (T7039, Sigma, St. Louis, MO, USA) with 2.0 ng/ml PDGF-AA or PDGF-BB (CYT-590 or CYT-590, ProSpec-Tany Technogene Ltd., PO Box 6591, East Brunswick, NJ).
Quantification of cells
The total number of cell nuclei and the number of α-smooth muscle actin+ or desmin+ cells per slide were counted in randomly selected, noncontiguous 400X microscopic fields using an analysis program in Image Pro (assisted by the staff of the Imaging Core, Lerner Research Institute, The Cleveland Clinic). The results were expressed as the percentage of α-smooth muscle actin+ or Desmin+ cells/total DAPI positive sphere cells quantified real-time at the microscope.
Statistical analysis
Statistical comparisons between the different experimental conditions were performed using analysis of variance (ANOVA). p < 0.05 was considered statistically significant. Results are expressed as the mean ± standard error.
Results
Effect of TGFβ and PDGF on α-SMA expression
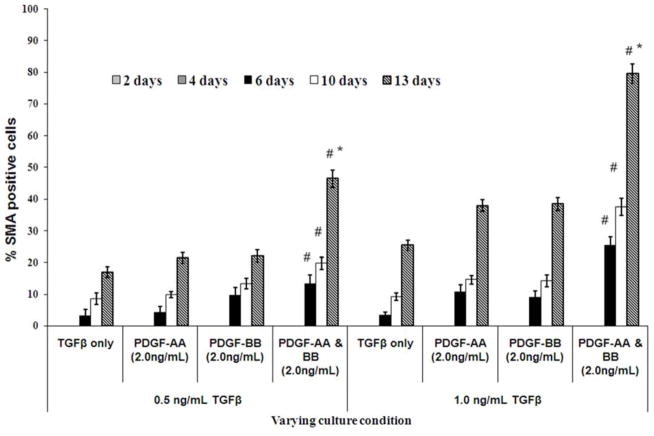
All MSF isolated for these studies were vimentin+ and continued to express vimentin when they were α-SMA+ and/or desmin+ (not shown). When corneal fibroblasts were cultured in the presence of varying concentrations (0.1 ng/ml to 2.0 ng/ml) of TGFβ and/or PDGF-AA or PDGF-BB, in serum free media, a gradual differentiation of V+A−D− corneal fibroblasts into α-SMA+ myofibroblasts was observed (Fig. 1). Representative images for α-SMA expression are shown in Fig. 2A–E. The expression of α-SMA+ myofibroblasts was monitored from day two to day 13, as shown in Fig. 1. The first α-SMA+ myofibroblasts were observed after six days of culture in presence of either 0.5 ng/ml or 1.0 ng/ml TGFβ with or without PDGF-AA and/or PDGF-BB in the culture media. More than 80% of corneal fibroblast cells become α-SMA+ after 13 days of culture in presence of 1.0 ng/ml TGFβ and PDGF-AA and PDGF-BB compared to corneal fibroblasts cultured with 0.5 ng/ml TGFβ and PDGF-AA and PDGF-BB (p < 0.01) where it was observed that approximately 50% of cells became α-SMA+. Thus, the presence of PDGF-AA and PDGF-BB in the medium significantly increased α-SMA+ myofibroblast generation at 13 days in culture compared with either isoform of PDGF alone with 0.5 ng/ml or 1.0 ng/ml TGFβ. More than 99% of corneal fibroblasts transitioned to α-SMA+ myofibroblasts within six days when 2.0 ng/ml TGFβ was present in the culture with or without PDGF-AA and/or PDGF-BB (results not shown).
Fig. 1.
Quantification of the %SMA-positive cells when incubated in serum-free DMEM modified media in presence of varying concentrations of TGFβ and/or PDGF-AA and/or PDGF-BB for different time intervals. Quantification of %SMA myofibroblasts was performed using a repeated measure ANOVA (mean ± SE). Note that when corneal fibroblasts are cultured with either 0.5 ng/ml or 1.0 ng/ml TGFβ, the greatest increase in SMA+ myofibroblasts is noted when both PDGF-AA and PDGF-BB are also present in the culture medium. *Indicates the result is significant compared to 6 and 10 days culture with both TGFβ and PDGFAA and PDGF-BB at p ≤0.01. The percentage of SMA+ myofibroblasts increased significantly (p < 0.001) when corneal fibroblasts are culture for 13 days, as compared to when the stromal fibroblasts were cultured for 6 days with TGFβ and/or PDGF in the culture media. # Indicates that the results are significant for SMA expression when TGFβ, PDGF-AA and PDGF-BB all are present together in the culture media compared to when culture media contain only TGFβ with or without 2.0ng/ml PDGF-AA and/or 2.0ng/ml PDGF-BB (p≤0.05). Quantitation was performed real-time at the microscope using Image Pro software.
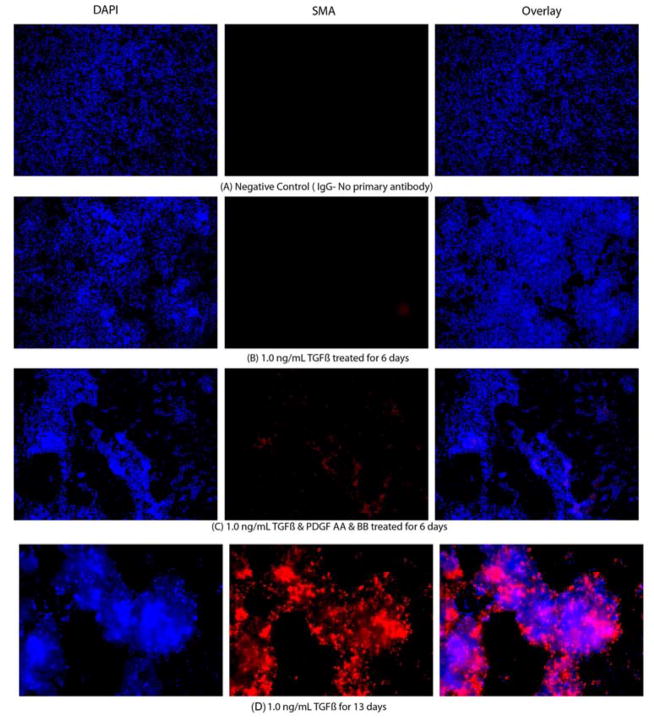
Fig. 2.
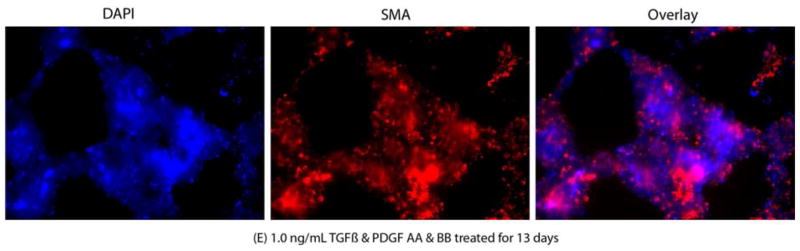
Representative SMA immunocytochemistry (IHC) images of mouse stromal fibroblasts (MSF) cultured in serum-free medium under varying culture conditions. The cultures were maintained for 15 days and small aliquots were taken in triplicate for performing IHC. The cultures were maintained in triplicate for quantitative analysis of IHC images. The left lane shows the nuclei of all cells stained blue with DAPI. The middle lane shows SMA+ cells stained red. The right lane is an overlay of the DAPI and SMA staining. A. MSF cultured (stained with non-specific IgG); B. MSF cultured in presence of 1.0 ng/ml TFGβ for 6 days; C. MSF cultured in presence of 1.0 ng/ml TFGβ and PDGF AA and PDGF BB for 6 days; D. MSF cultured in presence of 1.0 ng/ml TFGβ for 13 days; E. MSF cultured in presence of 1.0 ng/ml TFGβ and PDGF AA and PDGF BB for 13 days. Magnifications 400X.
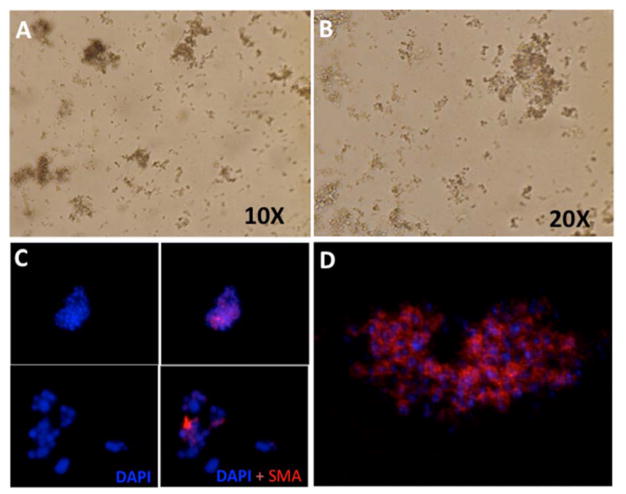
Phase contrast microscopy was used to monitor survival of the sphere cells and demonstrate that spheres were maintained in the supplemented media (Fig. 3A and 3B). Also, small and large spheres were monitored for the α-SMA myofibroblast marker (Fig. 3C). α-SMA staining was also monitored using confocal microscopy, as shown in Fig. 3D, and the results were identical to those noted with light microscopy.
Fig. 3.
Corneal fibroblast “sphere cells.” A and B. Phase contrast microscopic image of corneal fibroblast spheres in serum-free culture media at 10X and 20X magnification. C. Sphere cells expressing myofibroblast marker: Immunocytochemical analysis showed expression of α-SMA (red) in spheres. Blue is the nuclei of cells counterstained with DAPI. D. Confocal microscopic image of single sphere cells expressing α-SMA (Average projection in Z-plane).
Effect of TGFβ and PDGF on desmin expression
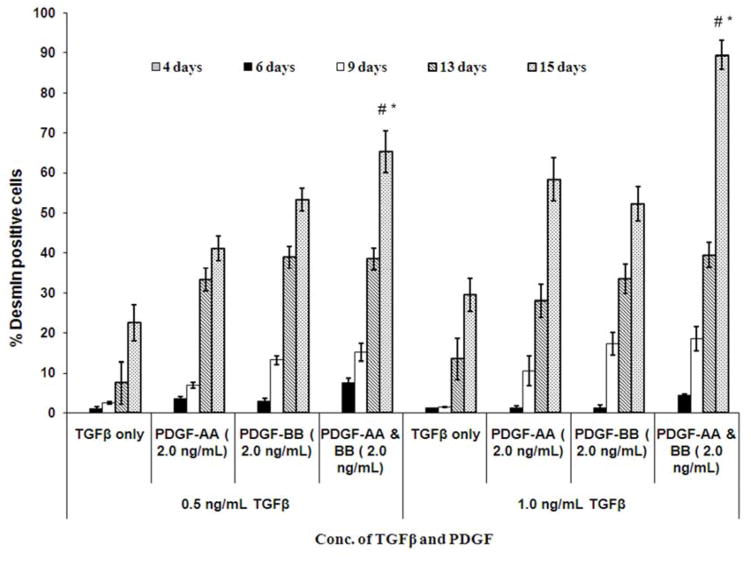
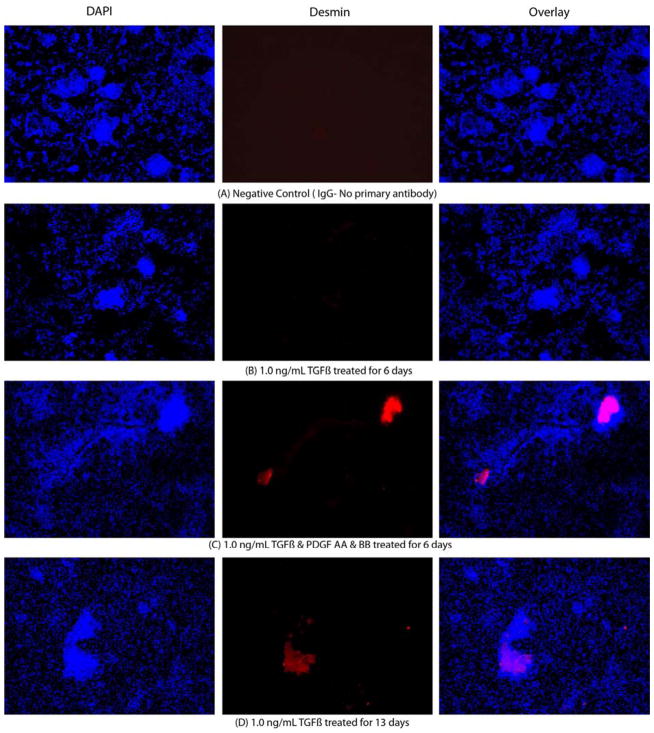
A gradual increase in desmin expression over time was observed when corneal fibroblasts were cultured in presence of varying concentrations of TGFβ, with or without PDGF-AA and PDGF-BB in serum-free medium (Fig. 4). Representative images of desmin expression are shown in Fig. 5A–E. More than 40% of the corneal fibroblasts expressed significant desmin only after 13 days of culture in presence of TGFβ, as they transitioned from immature (V+A+D−) to mature (V+A+D+) myofibroblasts. Greater than 60% of sphere cells expressed desmin after 15 days in the presence of 0.5 ng/ml or 1.0 ng/ml TGFβ when both 2.0 ng/ml PDGF-AA and 2.0 ng/ml PDGF-BB were present in the medium (Fig. 4). However, if TGFβ was increased to 2.0 ng/ml, along with 2.0 ng/ml PDGF-AA or 2.0 ng/ml PDGF-BB, over 90% of sphere cells expressed desmin after six days of culture (Fig. 5E). Thus, the effect of TGFβ on corneal fibroblast maturation into immature V+A+D− myofibroblasts and further into mature V+A+D+ myofibroblasts is augmented by the presence of PDGF-AA or PDGF-BB in the culture medium.
Fig. 4.
Quantification of % desmin-positive cells when incubated in serum-free DMEM modified media in presence of varying concentrations of TGFβ and/or PDGF-AA and/or PDGF-BB for different time intervals. Quantification of %desmin+ mouse stromal fibroblasts was performed using a repeated measure ANOVA (mean ± SE). *Indicates the result is significant compared to 6 and 10 days culture with both TGFβ and PDGFAA and PDGF-BB (p ≤0.01). The percentage of desmin+ myofibroblasts increased significantly (p < 0.01) when corneal fibroblasts were culture for 13 days, as compared to when the stromal fibroblasts were cultured for 6 days with TGFβ and/or PDGF in the culture media. # indicates that the results are significant for desmin expression when TGFβ, PDGF-AA and PDGF-BB are all present in the culture media compared to when the culture media contains only TGFβ with or without 2.0 ng/ml PDGF-AA and/or 2.0 ng/ml PDGF-BB (p≤0.05). Note that the quantitation was performed real-time at the microscope using Image Pro software.
Fig. 5.
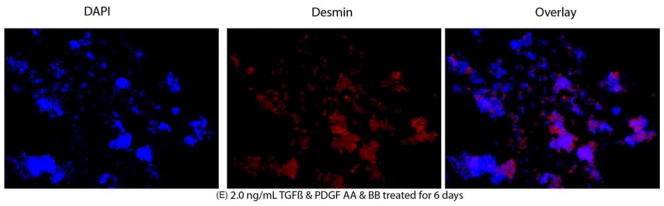
A–E. Representative desmin Immunocytochemistry of mouse stromal fibroblasts (MSF) cultured in a serum-free media under varying culture condition. The left lane shows the nuclei of all cells stained blue with DAPI. The middle lane shows desmin+ cells stained red. The right lane is an overlay of the DAPI and desmin staining. A. MSF immunohistochemistry with control non-specific IgG. B. MSF cultured in presence of 1.0 ng/ml TFGβ for 6 days. C. MSF cultured in presence of 1.0 ng/ml TFGβ and PDGF-AA and PDGF-BB for 6 days. D. MSF cultured in presence of 1.0 ng/ml TFGβ for 13 days. E. MSF cultured in presence of 2.0 ng/ml TFGβ and PDGF-AA and PDGF-BB for 13 days. Magnification 400X.
Effect of TGFβ signaling blockade on α-SMA+ myofibroblasts development
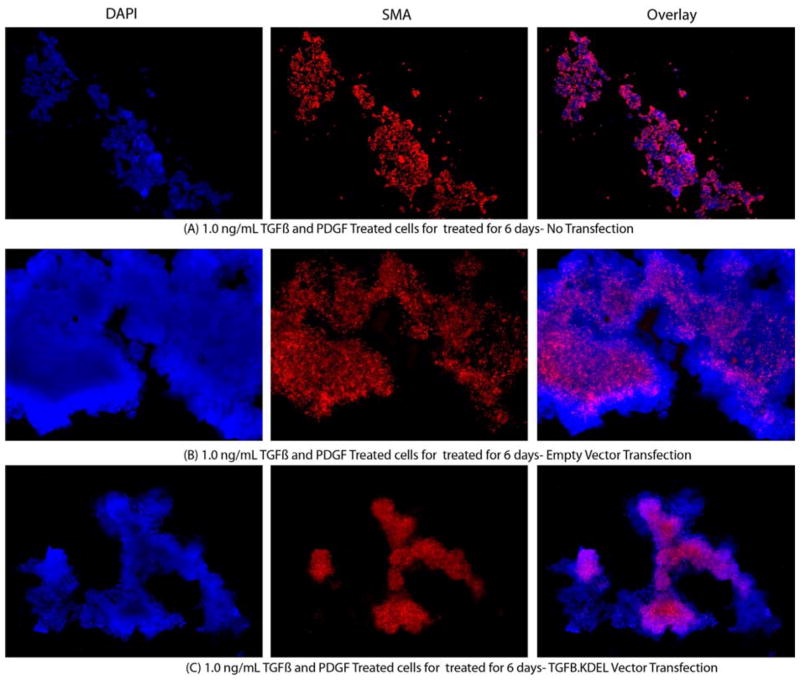
Primary corneal fibroblast sphere cells transfected with the TGRBR.KDEL plasmid vector expressed significantly lower α-SMA compared to control cells or empty vector-transfected sphere cells when cultured in a serum-free medium with 1.0 ng/ml TGFβ and 2.0 ng/ml PDGF-AA for six days after transfection (Fig. 6 and 7). α-SMA expression was not significantly different in sphere fibroblast cells transfected with empty plasmid vector compared to un-transfected cells when cultured in a serum-free medium with 1.0 ng/ml TGFβ and 2.0 ng/ml PDGF-AA for six days.
Fig. 6.
Representative α-SMA immunocytochemistry images of mouse stromal fibroblasts (MSF) cultured in a serum-free media with 1.0 ng/ml TGFβ and PDGF for 6 days after transfection with different vectors. The left lane shows the nuclei of all cells stained blue with DAPI. The middle lane shows α-SMA+ sphere cells stained red. The right lane is an overlay of the DAPI and α-SMA staining. A. Control MSF cultured without transfection. B. MSF cultured after transfection with empty vector. C. MSF cultured after transfection with TGRBR.KDEL vector. Magnification 400X.
Fig. 7.
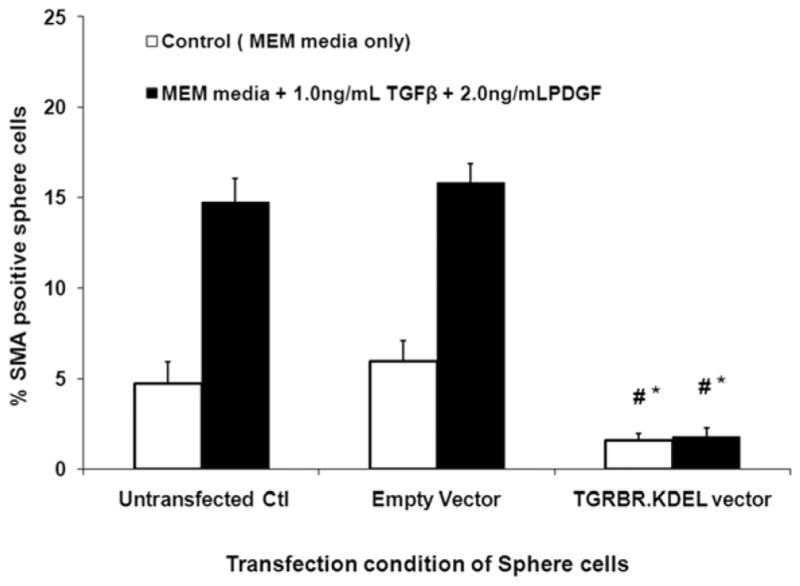
Quantification of % SMA mouse stromal fibroblasts marker cultured in a serum-free media along with 1.0 ng/ml TGFβ and 2.0 ng/ml PDGF-AA/BB. The % SMA+ MSF myofibroblasts decreases significantly when they were transfected with TGRBR.KDEL vector compared to un-transfected control cells or empty vector-transfected cells. * indicates the results were significant compared to un-transfected control ((p≤0.01). # indicates the results were significant compared to empty pCMV vector-transfected controls (p≤0.01).
Discussion
TGFβ has been shown to have a critical role in the generation and maintenance of corneal stromal myofibroblasts (Funderburgh et al., 2001; Bourlier et al., 2012; Andrianifahanana et al, 2013; Weber et al, 2013). Studies by Jester and coworkers (2002) showed that TGFβ induces keratocyte proliferation and myofibroblast differentiation through activation of a PDGF autocrine loop. PDGF blocked in the stroma confirmed a role for PDGF in myofibroblast generation and suggested that in rabbit cells PDGF acts at a specific point of transition from V+A−D− to V+A+D− myofibroblasts (Kaur et al, 2009).
The focus of this study was to characterize the role of TGFβ and PDGF in myofibroblast development in vitro from V+A−D− corneal stromal fibroblast precursors. Myofibroblasts are stromal cells that may develop during the corneal wound healing process, depending on the type of injury, that have traditionally been identified through their expression of α-smooth muscle actin (Masur, et al., 1996; Jester et al., 1999; Stramer et al., 2003). This marker is useful to detect mature myofibroblasts in the corneal stroma, since other cell types in the cornea do not express α-SMA. However, recent studies have found that the earliest stromal precursors to myofibroblasts in the cornea express vimentin (V), but not α-SMA or desmin, another later marker of cell development (Chaurasia et al., 2009). Thus, corneal myofibroblasts go through a sequential change in phenotype from V+A−D− to V+A+D− to V+A+D+ cells. A similar process of myofibroblast differentiation has been found to occur in other tissues and organs (Schmitt-Graff, Desmouliere, and Gabbiani, 1994; Kohnen et al., 1996). Once developed, the myofibroblasts that express all three markers persist in the anterior stroma until such time as they undergo apoptosis, likely when the epithelial basement membrane is regenerated and its barrier function is restored, and epithelium-derived TGFβ levels in the anterior corneal stroma fall (Torricelli, et al., 2013).
It is likely that myofibroblast precursor cells begin the developmental transition in all corneas that have epithelial and epithelial basement membrane injury where TGFβ, and possibly PDGF, from the corneal epithelium penetrate into the anterior stroma at high levels. These precursor cells would not be detected by staining for α-SMA in the early post-injury period since they only begin to express α-SMA after about one to two weeks of exposure to TGFβ. In many corneas, the epithelial basement membrane is regenerated and re-establishes barrier function to TGFβ penetration into the stroma. In these corneas, once stromal TGFβ levels fall, myofibroblast precursors likely halt development and undergo apoptosis (Wilson, 2012). In some corneas, however, especially those with higher levels of injury like photorefractive keratectomy for high myopia, where the epithelial basement membrane is not normally regenerated (Torricelli et al, 2013), high levels of TGFβ continue to penetrate into the anterior stroma and the myofibroblast precursors complete their development and excrete large quantities of disorganized extracellular matrix materials. These persistent mature myofibroblasts, and the extracellular matrix they secrete, comprise the anterior stromal opacity referred to as haze. Once generated, this haze can persist for years or even decades, although the haze does resolve over time in many corneas—presumably because the epithelial basement membrane finally regenerates, and the myofibroblasts die, and their secreted extracellular matrix is removed by resident keratocytes.
Studies in mice have shown that myofibroblasts in the cornea can also develop from bone marrow-derived cells that migrate into the corneal stroma after epithelial injury (Barbosa et al, 2010; Singh et al, 2012). The results of those studies suggested that some corneal myofibroblasts associated with haze after PTK are primarily derived from bone marrow-derived cells, although the factors that favor bone marrow vs. stromal cell origin of myofibroblast precursors are unknown but likely important in determining the wound healing response to different injuries to the cornea.
The role of TGFβ blockade was also studied using vectors designed from human TGF receptor II domains 2 and 3 sequences that are 93% concordant with mouse TGFβ II (and which bind mouse TGFβ), along with the KDEL sequence. The pGFP.TGFRBKDEL vector effectively blocked TGFβ-mediated mouse myofibroblast development in situ after irregular PTK (Singh et al, 2011) and was shown to also inhibit the developmental transition of corneal precursor cells to mature myofibroblasts in the present study.
The current study established the optimal concentration and time required to convert corneal fibroblasts to myofibroblasts via the V+A−D− precursor to V+A+D− immature myofibroblast to V+A+D+ mature myofibroblast developmental pathway. A dose of 0.5 ng/ml to 1.0 ng/ml of TGFβ, along with 2.0 ng/ml PDGF AA and PDGF BB, in DMEM/F12 serum-free media was found to be the optimal condition to study this transformation (Fig. 1 and Fig. 2). It is hoped that defining these conditions will facilitate other studies that probe myofibroblast development such as the specific changes in gene expression and cell functions modulated by TGFβ and PDGF in vimentin+ corneal fibroblasts as they transition from V+A−D− to V+A+D− to V+A+D+ myofibroblasts.
Highlights.
Mature α-smooth muscle actin+ and desmin+ myofibroblasts develop from vimentin+ corneal fibroblasts in vitro
TGFβ and PDGF are important regulators of myofibroblast development
TGFβ blockade inhibits myofibroblast development in vitro
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. We thank B.K. Ambati, Moran Eye Center, UT, USA for providing the pGFP.TGFRBKDEL and empty pCMV vector. We also acknowledge Vandana Agrawal for her technical expertise in these studies.
Footnotes
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrianifahanana M, Wilkes MC, Gupta SK, Rahimi RA, Repellin CE, Edens M, Wittenberger J, Yin X, Maidl E, Becker J, Leof EB. Profibrotic TGFβ responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J. 2013 doi: 10.1096/fj.12-224907. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010;91:92–6. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, Bouloumié A. TGF beta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7:e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–9. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–8. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor (beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–57. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for corneal crystallins. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. 2009;88:960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnen G, Kertschanska S, Demir R, Kaufmann P. Placental villous stroma as a model system for myofibroblast differentiation. Histochem Cell Biol. 1996;105:415–429. doi: 10.1007/BF01457655. [DOI] [PubMed] [Google Scholar]
- Masur S, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Petridou S, Maltseva O, Spanakis S, Masur SK. TGF-beta receptor expression and smad2 localization are cell density dependent in fibroblasts. Invest Ophthalmol Vis Sci. 2000;41:89–95. [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Sumioka T, Okada Y, Miyamoto T, Shirai K, Kitano A, Tanaka S. Transforming growth factor beta signal transduction: a potential target for maintenance/restoration of transparency of the cornea. Eye Contact Lens. 2010;36:286–289. doi: 10.1097/ICL.0b013e3181eef01c. [DOI] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp Eye Res. 2012;98:1–8. doi: 10.1016/j.exer.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGFβ and PDGF-B blockade on corneal myofibroblast development in mice. Exp Eye Res. 2011;93:810–7. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–46. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Toews GB, White ES, Lynch JP, Martinez FJ. Mechanisms of pulmonary fibrosis, Ann. Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophth Vis Sci. 2013;54:4026–33. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta (1) Am J Respir Cell Mol Biol. 1999;21:658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]