Abstract
Hematopoietic stem cell (HSC) transplantation has curative potential for patients with hematological malignancies. Clinically, HSCs derived from mobilized peripheral blood are used more frequently than bone marrow. However, current standard mobilizing agents yield grafts that may not contain sufficient HSCs. Here, using murine models, we discovered that FLT3L synergized with Plerixafor to mobilize phenotypically defined HSCs, and their combination (FP) was superior to G-CSF alone or in combination with Plerixafor (GP). Additionally, FP mobilized more Treg cells, NK cells, and plasmacytoid dendritic cells compared with G-CSF alone or GP. Both syngeneic and allogeneic grafts mobilized by FP led to long-term survival in transplanted mice. Collectively, FP represents a promising novel and potent mobilization regimen with potential clinical application in both the autologous and allogeneic transplantation settings.
Keywords: Cell mobilization, Transplantation, FLT3L, Plerixafor
Introduction
Hematological malignancies contribute to almost 10% of the mortalities caused by cancers in the United States [1]. Hematopoietic stem cell transplantation (HSCT) is a well-established treatment with curative potential for a variety of hematological cancers. In addition to bone marrow transplantation (BMT), mobilized peripheral blood provides a rich source of HSCs for transplantation. Successful HSCT depends on a mobilization regimen that provides an adequate number of HSCs for engraftment but is less likely to provoke transplant related complications. However, current mobilization regimens occasionally do not mobilize sufficient numbers of cells into the peripheral blood, or the mobilized immune cells often lead to post transplant complications, such as graft-versus-host disease (GVHD).
The CXCR4 antagonist Plerixafor (Mozobil) induces safe and rapid mobilization of clinical grafts capable of promoting robust hematopoietic recovery in HSCT patients [2]. However, the number of hematopoietic stem/progenitor cells (CD34+ cells) mobilized by Plerixafor alone is far lower than G-CSF, the current gold standard mobilizing regimen, suggesting that combining Plerixafor with other agents may be more effective for clinical applications. FLT3 ligand (FLT3L) is a stem-cell specific growth factor which expands and may also mobilize stem cells in mice after administration for ten days, either as a single agent or in combination with other molecules such as IL-8 and G-CSF [3, 4]. Most recently, FLT3L has been introduced into a clinical trial, demonstrating that the administration of FLT3L on a consecutive 5- or 10-day schedule effectively mobilizes CD34+ HSCs in healthy donors [5]. Here we tested the effect of the combination of two clinical grade drugs, FLT3L and Plerixafor, on stem cell mobilization, and evaluated the transplantation outcomes of the mobilized cells in both syngeneic and allogeneic murine models. Our data demonstrate that the combination of FLT3L and Plerixafor is a promising regimen for mobilizing adequate stem cells and other cell subsets that lead to better engraftment and survival following both syngeneic and allogeneic transplantation.
Materials and Methods
Mice
BALB/c and C57BL/6 mice were 8- to10-week-old and purchased from National Cancer Institute-Frederick. All animal work was approved by The Ohio State University Animal Care and Use Committee, and mice were treated in accordance with the institutional guidelines for animal care.
Cell Mobilization
C57BL/6 mice were injected intraperitoneally (i.p.) with recombinant human FLT3L (Celldex Therapeutics or Miltenyi Biotec) (350 μg/kg/day) for 10 consecutive days or G-CSF (Amgen) (150 μg/kg/day) for 5 consecutive days. Twelve hours after the last dose of FLT3L or G-CSF, mice were injected i.p. with PBS or Plerixafor (AMD3100, Sigma) (5 mg/kg) and sacrificed 1 hour later. The peripheral blood was collected retroorbitally while the mice were under deep anesthesia, as described previously [6]. In detail, mice were anesthetized by i.p. injection with 100 uL of a mixture of ketamine (54.3 mg/mL) and xylazine (9.1 mg/mL). Blood was collected from the retro-orbital venous plexus through a microcapillary heparinized tube, followed by euthanization of the mice while they were still deeply anesthetized. The red blood cells (RBC) were depleted by RBC lysis buffer. The remaining cells were subjected to flow cytometric analysis or transplantation. The capacity of human FLT3L to bind murine Flt3 receptors and Plerixafor to block murine Cxcr4 receptor has been previously reported [7-10].
Transplantation
For syngeneic transplantation, C57BL/6 mice were subjected to 9 Gy of irradiation delivered in two fractions separated by 4 hours using RS 2000 X-Ray Irradiator (Rad Source Technologies, Suwanee GP). Four hours later, each mouse was transplanted via tail vein with 2 × 105 mobilized RBC-depleted peripheral blood cells (PBC) from C57BL/6 mice. For allogeneic transplantation, the recipient BALB/c mice were subjected to a lethal irradiation (delivered as aforementioned) and injected with 8 × 105 mobilized RBC-depleted PBC per mouse isolated from C57BL/6 mice.
The rationale of using these cell numbers for transplantation is based on previous studies demonstrating that about 90% of mice survived when syngeneically infused with 1 × 106 peripheral nucleated cells mobilized by G-CSF [11, 12], but only around 17% of mice survived if 2.5 × 105 cells mobilized by G-CSF were transplanted [12]. Therefore, for syngeneic transplants, 2 × 105 mobilized cells were used in our experiments to explore whether the combination of FLT3L and Plerixafor has an advantage over G-CSF with a greater mobilizing potential. For allogeneic transplants, a four-fold higher number of cells (i.e. 8 × 105 donor cells isolated from C57BL/6 mice) were administered i.v. into each BALB/c recipient, considering the likely graft rejection and GVHD complication in the allogeneic model. The lethally irradiated mice were housed in a sterile environment, and baytril antibiotic was provided in the drinking water to prevent infection-caused mortality. Following transplantation, mice were monitored daily and the phenotypes of the dying mice were recorded.
Colony formation assay
Colony formation assays were performed using MethoCult® GF M3434 (StemCell Technologies, Vancouver, Canada) according to the manufacturer's instructions. Briefly, 4.6 × 104 of mobilized white blood cells were resuspended in 0.3 mL of Iscove's Modified Dulbecco's Media (IMDM, Life Technologies, Grand Island, NY) with 2% FBS and added to 3 mL of MethoCult®. The vortexed mixture was then dispensed into three 35 mm dishes at 1.1 mL per plate, which were placed into a 100 mm culture dish and incubated at 37°C, 5% CO2 for 12 days. Colonies were counted on an inverted microscope (Carl Zeiss Microscopy Thornwood, NY).
Flow cytometric analysis
The mAbs (supplementary data) reactive with murine cell antigens were purchased from BD Biosciences or eBioscience. Cell preparation and analysis were performed as previously described [13]. Mouse HSCs were defined as Lin-Sca-1+c-Kit+ (LSK) cells, and Lin- (lineage negative) cells were gated as shown in Supplemental Figure 1. The immune subsets were gated as CD3-NK1.1+ for NK cells, CD3+CD25+Foxp3+ for Treg cells, B220-CD11c+CD11b+ for cDCs and CD11b-CD11clowPDCA1+SiglecH+ for pDCs.
Statistics
For continuous endpoints, Student's t test was used to compare two independent groups. Measurements such as frequency and absolute number LSK cells, colony formation, and absolute number of Treg cells, were logarithmically transformed to normalize the data and stabilize the variance. A one-way ANOVA model was applied to multiple comparisons, and a two-way ANOVA model was performed to evaluate the synergistic effect between FLT3L and Plerixafor. Log-rank test was used to compare survival curves. All tests were two sided. P values were adjusted for multiple comparisons by Holms' procedure. A P- value <0.05 was considered as statistically significant.
Results
FLT3L and Plerixafor combination effectively mobilized Lin-Sca-1+c-Kit+ (LSK) cells into peripheral blood
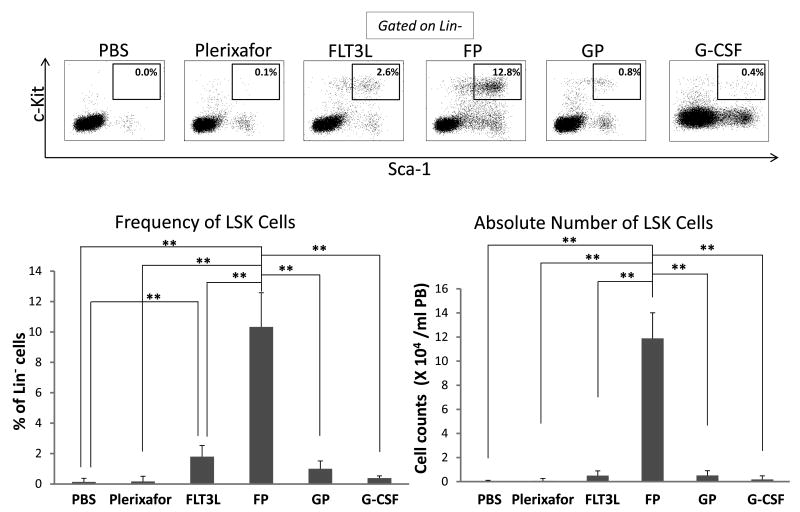
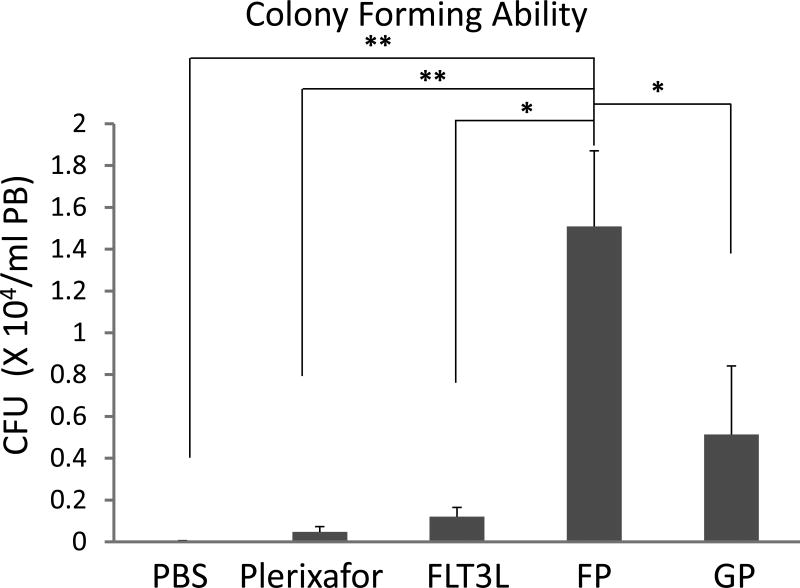
To test the effect of the combination of FLT3L and Plerixafor (FP) on HSC mobilization, both the white blood cell (WBC) counts and HSCs (Lin-Sca-1+c-Kit+, LSK cells, a hematopoietic stem cell-enriched population) in the peripheral blood were analyzed in mice subjected to various mobilization regimens, including the combination of G-CSF and Plerixafor (GP), one of the strongest mobilizing regimens currently used in the clinic. Cell counts showed that mice in the GP group had the highest WBC counts, and the FP group the second highest (Supplemental Figure 2). Compared to PBS-treated mice, Plerixafor-treated mice had modest upregulation of LSK cells, while FLT3L-treated mice mobilized significantly more LSK cells into the blood (Figure 1A). However, the FP combination mobilized significantly higher frequency and greater absolute number of LSK cells, reaching up to 6-fold and 12-fold higher than those of FLT3L alone, respectively (Figures 1B and 1C). Statistical analysis revealed that FLT3L and Plerixafor showed a synergistic mobilizing effect (P < 0.05, Supplemental Figure 3). Although the mice from the GP group had the highest WBC counts, the absolute number of LSK cells was similar to FLT3L alone, since the majority of WBC cells mobilized by GP were neutrophils (Supplemental Figure 4). We also compared the LSK cells mobilized by FP with those by G-CSF alone, which is the regimen most frequently used in the clinic, and data demonstrated that FP mobilized significantly more LSK cells (Figures 1A, 1B and 1C P < 0.05). Colony formation assays revealed that cells mobilized by FP contained significantly more colony-forming units (CFU) than any other regimens, including GP, FLT3L alone, and Plerixafor alone (Figure 1D), and a synergistic effect was also observed between FLT3L and Plerixafor (Supplemental Figure 5, P < 0.01).
Figure 1. FLT3L and Plerixafor combination effectively mobilized Lin-Sca-1+c-Kit+ (LSK) cells into peripheral blood.
(A-C) Peripheral blood cells in mice treated with indicated mobilizing regimens were harvested, subjected to red blood cell depletion, and stained for HSCs (Lin-Sca-1+c-Kit+, LSK cells). The representative data from 1 out of 9 mice with similar results are shown in (A), and the summary data of 3 mice in one of three experiments with similar data for the frequency (B) and the absolute number (B) of LSK cells are also shown. FP indicates the combination of FLT3L and Plerixafor, and GP denotes the combination of G-CSF and Plerixafor. (D) Mobilized peripheral blood cells were prepared as in (A) and subjected to colony formation assay. Shown are the summary data of 3 mice for the colony forming units (CFU) larger than 200 cells. For all panels, * indicates P < 0.05, ** indicates P < 0.01, and error bars represent S.D. FP, the combination of FLT3L and Plerixafor; GP, the combination of G-CSF and Plerixafor.
FLT3L and Plerixafor combination effectively mobilized NK cells, Treg cells and DCs into peripheral blood
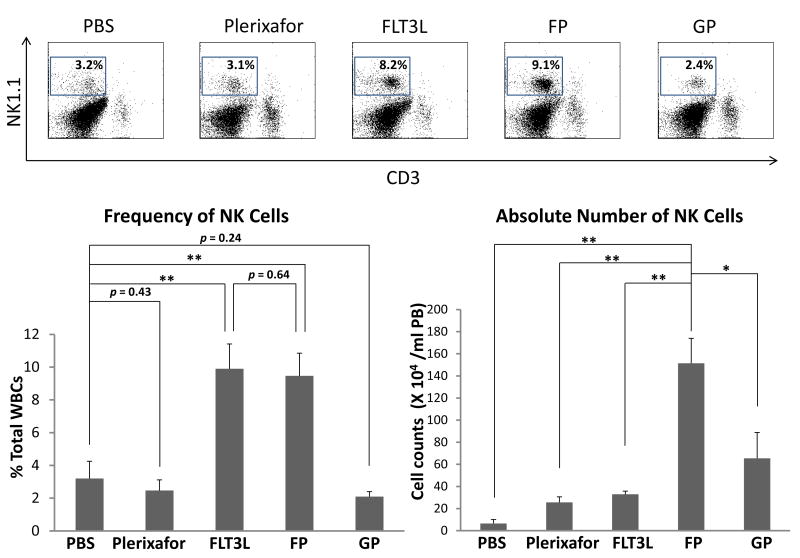
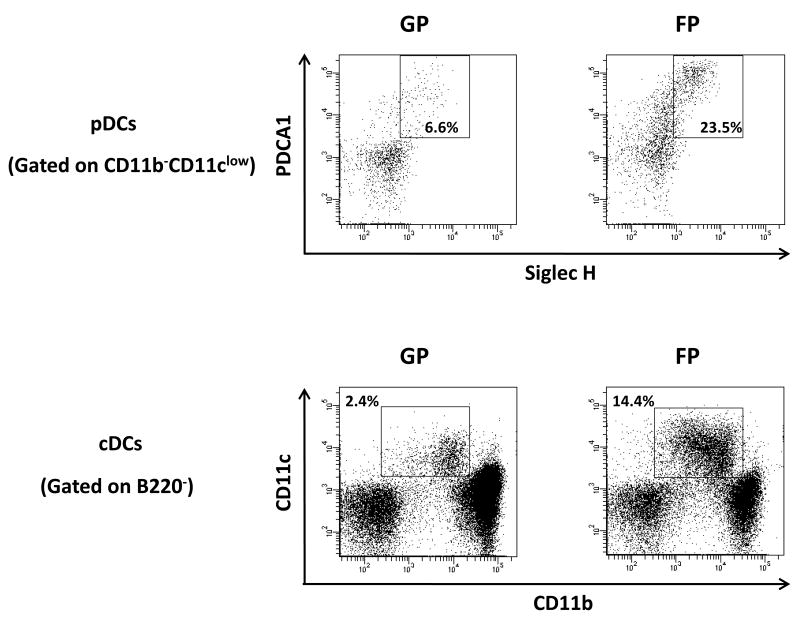
In addition to LSK cells, other immune cell subsets were also assessed. Mobilization with FP or FLT3L alone significantly increased natural killer (NK, CD3-NK1.1+) cell percentages to the same extent (Figures 2A and 2B), consistent with previous reports of induction of NK cell expansion by FLT3L [14]. Importantly, FP administration resulted in much more dramatic increase of NK cells in absolute number than FLT3L alone or any other regimens (Figure 2B, right panel). Although none of the mobilizing agents induced a significant increase of Treg cell frequency (supplemental Figure 6), FP led to significantly higher absolute number of Treg cells in mobilized blood than the other regimens (Figure 2C). In agreement with previous findings that FLT3L is able to expand dendritic cells (DCs) both in vivo and in vitro [15, 16], the two major subsets of DCs, plasmacytoid DC (pDCs) and conventional DC (cDCs), were both significantly increased in proportion for the FP group when compared with GP (Figure 2D).
Figure 2. FLT3L and Plerixafor combination effectively mobilized NK cells, Treg cells and DCs into peripheral blood.
(A, B) Peripheral blood cells in mice treated with indicated mobilizing regimens were harvested, subjected to red blood cell depletion, stained for NK cells, and subjected to flow cytometric analysis. Shown are the representative (A) and the summary (B) data of 3 mice in one of three experiments with similar results. (C) Mobilized peripheral blood cells were prepared as in (A) and subjected to flow cytometric analysis for Treg cells. Shown are the summary data of 3 mice for the absolute number of Treg cells. (D) GP- and FP-mobilized peripheral blood cells from 3 mice were pooled together respectively and stained for cDCs (B220-CD11b+CD11c+) and pDCs (CD11b-CD11clowPDCA+SiglecH+). For all panels, * indicates P < 0.05, ** indicates P < 0.01, and error bars represent S.D. FP, the combination of FLT3L and Plerixafor; GP, the combination of G-CSF and Plerixafor.
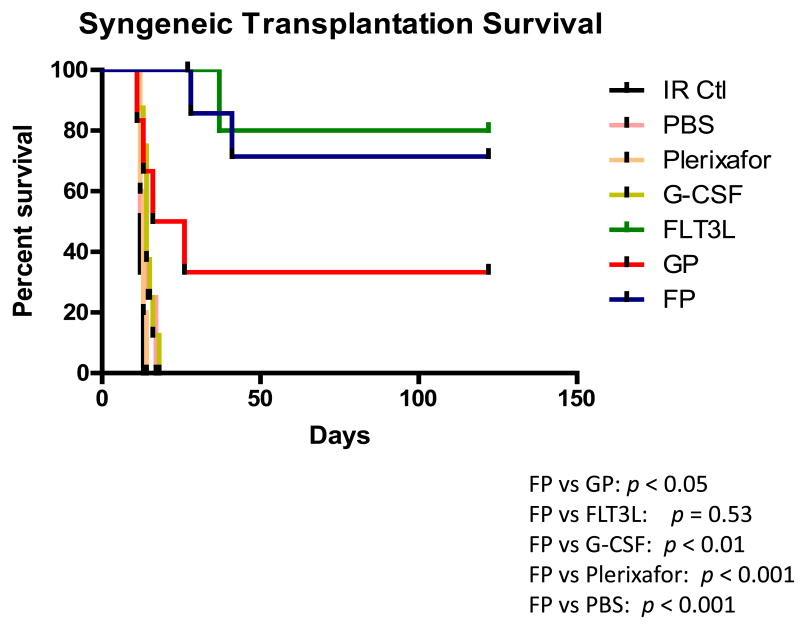
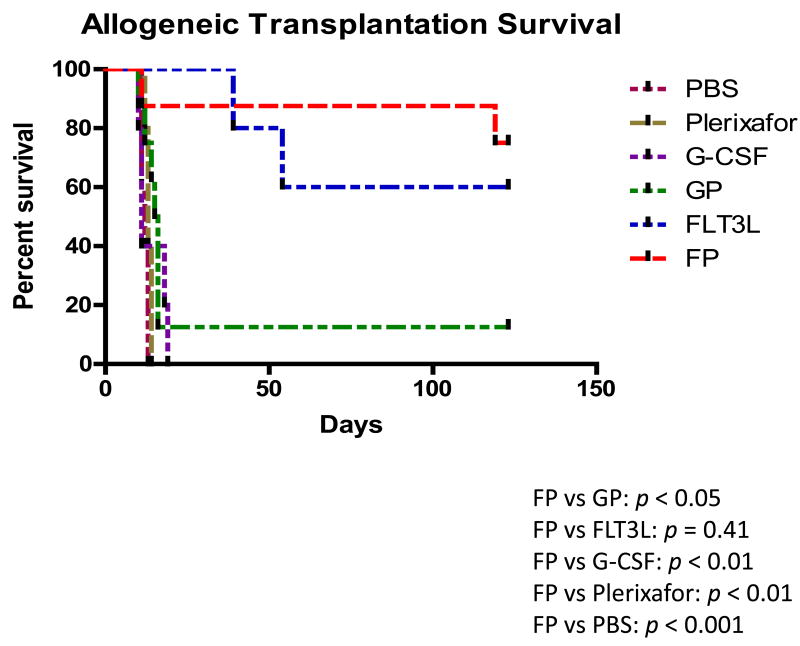
FLT3L and Plerixafor combination-mobilized grafts significantly enhanced survival of mice in both syngeneic and allogeneic setting
To test the clinical potential of FP-mobilized grafts, cells mobilized by different regimens were transfused into lethally irradiated syngeneic mice. Mice receiving grafts mobilized by PBS, Plerixafor alone or G-CSF alone died within 21 days, probably due to failure to reconstitute hematopoietic cells, as the frequencies of progenitor or LSK cells were much lower in these groups (Figure 1A). This indicates that fewer progenitor or LSK cells were transplanted for these groups when a consistent number of mobilized cells were infused for each group. In contrast, mice receiving FLT3L or FP-mobilized products survived 100% at day 21, and the survival rate maintained at approximately 70% as far out as 4 months after transplantation (Figure 3A). The engraftment of the cells mobilized by FP was significantly superior to that of the cells mobilized by GP (P < 0.05), with the latter having a survival rate of only 35% at 4 months post-transplant. An examination of bone marrow in the surviving mice also showed that mice receiving FP grafts contained a higher frequency of LSK cells than those receiving GP grafts (supplemental Figure 7).
Figure 3. FLT3L and Plerixafor combination-mobilized grafts significantly enhanced survival of mice in both syngeneic and allogeneic setting.
Survival analysis of lethally irradiated C57BL/6 mice (A) or BALB/c mice (B) receiving peripheral blood (PB) cells from C57BL/6 mice mobilized by the indicated regimens. Irradiated mice without any cell infusion (IR Ctl) served as a control group. A total of 2 × 105 (A) or 8 × 105 (B) PB cells were transplanted for each recipient. Each group contains 5-8 mice. Log-rank test was used to compare survival curves. FP, the combination of FLT3L and Plerixafor; GP, the combination of G-CSF and Plerixafor. P values in (A): FP vs. GP, p < 0.05, FP vs. FLT3L, p = 0.53, FP vs. G-CSF, p < 0.01, FP vs. Plerixafor, p < 0.001, FP vs. PBS, p < 0.001; P values in (B): FP vs. GP, p < 0.05, FP vs. FLT3L, p = 0.41, FP vs. G-CSF, p < 0.01, FP vs. Plerixafor, p < 0.01, FP vs. PBS, p < 0.001.
Next we explored the transplantation efficacy of the FP-mobilized cells in an allogeneic transplantation model. Lethally irradiated BALB/c mice were transplanted with peripheral blood cells mobilized by the different regimens from C57BL/6 mice. Based on previous reports [11], more mobilized peripheral blood cells were transplanted in this MHC-mismatched model than in the above syngeneic model (8 × 105 vs. 2 × 105 cells per recipient). All recipients of grafts mobilized by PBS, Plerixafor alone or G-CSF alone died within 3 weeks after transplantation; while 80% and 66% of mice receiving grafts mobilized by FP or FLT3L alone survived to 4 months post-transplantation, respectively (Figure 3B). The survival rate of the FP groups was significantly higher than that observed in the GP group (12.5% alive at 4 months post-transplantation, P < 0.05). At 4 months post-transplantation, the mice in the FP group that remained healthy were sacrificed and found to have both long-term HSCs (LT-HSC, CD135-CD34- LSK) and short-term HSCs (ST-HSC, CD135-CD34+LSK) [17] originated from donor C57BL/6 mice (H2Kb) dominating in bone marrow (supplemental Figure 8), suggesting that their superior survivals may be due to the better hematopoietic reconstitution resulting from enhanced stem cell mobilization by this regimen.
Discussion
Since mobilized peripheral blood stem cells were demonstrated to have clinical advantages over bone marrow stem cells for HSCT [18], extensive efforts have been invested in the pursuit of optimal mobilizing regimens. Currently G-CSF based regimens (alone or in combination of chemotherapy or other agents) are the most common methods used to mobilize HSCs for autologous transplantation. However, poor mobilization still remains an important problem. In a study of 976 patients who went through mobilization, 29.7% failed to achieve sufficient numbers of stem cells [19]. Here we report the combination of FLT3L and Plerixafor (FP) as a novel regimen that is able to induce significantly more robust HSC mobilization, when compared to the current strongest regimen in clinic, the combination of G-CSF and Plerixafor. In fact, FP leads to 6-fold increase in frequency and 12-fold increase in absolute number of phenotypically defined stem cells mobilized into peripheral blood (Figure 1). A recent clinical trial has shown the safety and efficiency of FLT3L alone in CD34+ stem cell mobilization in healthy donors [5]. Considering the synergistic effect between FLT3L and Plerixafor reported here, it's tempting to speculate the likelihood of circumventing mobilization failure issue using this regimen in poor mobilizers. And indeed, the lethally irradiated syngeneic mice were rescued with a small amount of mobilized grafts by FP but not the regimens currently used in the clinic, i.e., G-CSF alone or in combination of Plerixafor (Fig 3A), further demonstrating the stem cell mobilizing potential of this regimen.
The allogeneic transplantation setting, on the other hand, requires a sufficient number of HSCs as well as other cell subsets to help overcome allogeneic transplantation-related complications, such as GVHD and engraftment failure due to allograft rejection. Here we showed that FP-mobilized grafts significantly improved the survival of mice after allogeneic transplantation (Fig 3B). The superiority of FP to other regimens may be explained by more HSCs as well as more NK cells, Treg cells and DCs that were mobilized into peripheral blood to suppress acute GVHD (aGVHD). NK cells have been demonstrated to induce an antitumor response while suppressing the development of GVHD in mice [20]. Treg cells are important immune regulatory cells which have also been shown to generate protective effects against GVHD following allogeneic transplantation [21]. As a result, FP-mobilized grafts might favor a better outcome in MHC-mismatched transplantation compared with grafts mobilized by other regimens. Although cDCs are generally considered to enhance GVHD [22], the simultaneous increase of pDCs may attenuate the effect of cDCs, as pDCs have been shown to inhibit GVHD [23, 24]. Indeed, in our allogeneic model, compared to mice from control groups of PBS, G-CSF alone, and GP, mice transplanted with peripheral blood cells mobilized by FP had lower incidence of aGVHD (characterized by hunched back, inactivity, diarrhea, ruffled fur, and over 10% weight loss). It is also noteworthy that compared to the cells mobilized by other regimens, those mobilized by FP had consistently higher numbers of monocytes (Gr1medCD11b+; Supplemental Figure 4), a population that has been increasingly reported to be involved in both autologous [25] and allogeneic [26] stem cell transplantation as monocytic myeloid-derived suppressor cells (MDSCs) [27]. In allogeneic stem cell transplantation, MDSCs might also mitigate GHVD as NK and Treg cells do. This could be another explanation for the survival advantage observed in mice infused with FP-mobilized cells, although the role of this population in transplantation requires further study. Therefore, we believe that the immune cell subsets and the HSCs mobilized by FP may act together to contribute to the beneficial transplantation outcome in the allogeneic mouse model that we used.
In summary, in these preclinical studies we describe a novel potent cell mobilization regimen combining FLT3L and Plerixafor, which may have broad clinical applications in both autologous and allogeneic transplantation. Future clinical trials are warranted to further explore the potential of this regimen.
Supplementary Material
Acknowledgments
This project was supported in part by grants from NIH (OD018403 to H.C.M. and J.Y.; CA155521 to J.Y.), a 2012 scientific research grant from the National Blood Foundation (to J.Y.), and an institutional research grant (IRG-67-003-47) from the American Cancer Society (to J.Y.). The authors would like to sincerely thank Dr. David M. Lucas for his careful and critical reading of this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: HCM is an employee of Celldex Therapeutics. The other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 3.Brasel K, McKenna HJ, Charrier K, et al. Flt3 ligand synergizes with granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor to mobilize hematopoietic progenitor cells into the peripheral blood of mice. Blood. 1997;90:3781–3788. [PubMed] [Google Scholar]
- 4.de Kruijf EJ, Hagoort H, Velders GA, et al. Hematopoietic stem and progenitor cells are differentially mobilized depending on the duration of Flt3-ligand administration. Haematologica. 2010;95:1061–1067. doi: 10.3324/haematol.2009.016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anandasabapathy N, Hurley A, Breton G, et al. A Phase 1 Trial of the Hematopoietic Growth Factor CDX-301 (rhuFlt3L) in Healthy Volunteers. Biol Blood Marrow Transplant. 2013;19:S112–S113. [Google Scholar]
- 6.Ferraro F, Lymperi S, Mendez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 8.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 9.Gerlach LO, Skerlj RT, Bridger GJ, et al. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem. 2001;276:14153–14160. doi: 10.1074/jbc.M010429200. [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass B, Uharek L, Zeis M, et al. Allogeneic peripheral blood progenitor cell transplantation in a murine model: evidence for an improved graft-versus-leukemia effect. Blood. 1997;90:1694–1700. [PubMed] [Google Scholar]
- 12.Zeng D, Dejbakhsh-Jones S, Strober S. Granulocyte colony-stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: impact on blood progenitor cell transplantation. Blood. 1997;90:453–463. [PubMed] [Google Scholar]
- 13.Yu J, Mao HC, Wei M, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guimond M, Freud AG, Mao HC, et al. In vivo role of Flt3 ligand and dendritic cells in NK cell homeostasis. J Immunol. 2010;184:2769–2775. doi: 10.4049/jimmunol.0900685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strobl H, Bello-Fernandez C, Riedl E, et al. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–1434. [PubMed] [Google Scholar]
- 17.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz N, Linch DC, Dreger P, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 19.Gertz MA. Current status of stem cell mobilization. Br J Haematol. 2010;150:647–662. doi: 10.1111/j.1365-2141.2010.08313.x. [DOI] [PubMed] [Google Scholar]
- 20.Olson JA, Leveson-Gower DB, Gill S, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markey KA, Banovic T, Kuns RD, et al. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood. 2009;113:5644–5649. doi: 10.1182/blood-2008-12-191833. [DOI] [PubMed] [Google Scholar]
- 23.Toubai T, Malter C, Tawara I, et al. Immunization with host-type CD8{alpha}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner. Blood. 2010;115:724–735. doi: 10.1182/blood-2009-06-229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banovic T, Markey KA, Kuns RD, et al. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol. 2009;182:912–920. doi: 10.4049/jimmunol.182.2.912. [DOI] [PubMed] [Google Scholar]
- 25.P LF, Ansell Kerryn. Autograft Monocytes: The Bad Humors of Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation. J Stem Cell Res Ther. 2013;S3 [Google Scholar]
- 26.Mougiakakos D, Jitschin R, von Bahr L, et al. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. 2013;27:377–388. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.