Abstract
Objective
To compare actual and perceived causes of fever in northern Tanzania.
Methods
In a standardized survey, heads of households in 30 wards in Moshi, Tanzania, were asked to identify the most common cause of fever for children and for adults. Responses were compared to data from a local hospital-based fever etiology study that used standard diagnostic techniques.
Results
Of 810 interviewees, the median (range) age was 48 (16, 102) years and 62.8% were females. Malaria was the most frequently identified cause of fever, cited by 56.7% and 43.6% as the most common cause of fever for adults and children, respectively. In contrast, malaria accounted for 2.0% of adult and 1.3% of pediatric febrile admissions in the fever etiology study. Weather was the second-most frequently cited cause of fever. Participants who identified a non-biomedical explanation such as weather as the most common cause of fever were more likely to prefer a traditional healer for treatment of febrile adults (OR 2.7, p<0.001). Bacterial zoonoses were the most common cause of fever among inpatients, but no interviewees identified infections from animal contact as the most common cause of fever for adults; 0.2% identified these infections as the most common cause of fever for children.
Conclusions
Malaria is perceived to be a much more common cause of fever than hospital studies indicate whereas other important diseases are under-appreciated in northern Tanzania. Belief in non-biomedical explanations of fever is common locally and has important public health consequences.
Keywords: Africa, fever, malaria, beliefs, Tanzania
INTRODUCTION
Community understanding of the etiologies of disease in sub-Saharan Africa can differ markedly from western biomedical explanations (Sabuni 2007, Iriso et al. 2000). Such local perceptions of illnesses can influence healthcare seeking behavior with important public health consequences (Chibwana et al. 2009, Comoro et al. 2003). In sub-Saharan Africa, where fever is a common health complaint (Feikin et al. 2011), particular attention has been paid to cultural understanding of fever and its causes (Agyepong and Manderson 1994, Molyneux et al. 1999).
Many studies investigating local perceptions of fever have been conducted in settings where malaria is highly endemic and have focused on the ability of mothers to recognize the symptoms of malaria in children (Tarimo et al. 2000, Comoro et al. 2003). These studies have shown that malaria is widely recognized across sub-Saharan Africa as the most important cause of fever (Malik et al. 2006, Tarimo et al. 2000). In fact, in many settings respondents did not distinguish between malaria and the syndrome of fever itself (Ahorlu et al. 1997, Pilkington et al. 2004).
The past decade has witnessed a marked decline in malaria-related illness in many parts of sub-Saharan Africa, with multiple countries reporting reductions in the number of malaria cases by at least 50% (WHO 2009, Murray et al. 2012). Consequently, an increasing number of people reside in locations where malaria prevalence is low, yet little has been done to assess beliefs about febrile illness in such settings. An understanding of community perceptions of fever is needed to shed light on healthcare seeking behavior in low malaria transmission areas and also to explore the ways in which such perceptions may influence the widespread problem of malaria overdiagnosis (Chandler et al. 2008a).
In addition to malaria, studies have demonstrated that many people in sub-Saharan Africa consider non-biomedical explanations of fever to be important causes of febrile disease in their community. These studies have identified a range of non-biomedical explanations for fever, including supernatural causes, such as witchcraft, and more material causes originating in the body, the environment, or social relations, such as teething, over-exposure to the sun, or alcohol (Winch et al. 1996, Sabuni 2007). Some studies suggest that individuals are more likely to seek care from traditional healers if they believe the fever to have supernatural origins (Fosu 1981, Comoro et al. 2003). However, little is known about how beliefs in non-supernatural but non-biomedical causes of fever, such as weather, affect healthcare seeking behavior.
Community perceptions about causes of fever are best evaluated in the context of local disease prevalence data so that direct comparisons can be made and important discrepancies between actual and perceived causes can be identified. However, such comparisons have rarely been possible. To investigate local perceptions of the causes of fever in a low malaria prevalence setting, we surveyed heads of households in northern Tanzania, where disease prevalence data from a recent study of fever etiologies were readily available.
METHODS
Study location
This survey was conducted in Moshi Urban (population ~ 144,000) and Moshi Rural (population ~ 402,000) Districts of northern Tanzania, an area characterized by low malaria transmission intensity (Hay et al. 2009, Oesterholt et al. 2006). While the Chagga tribe predominates, many ethnic groups are present in the study area.
Selection of households
Thirty of the 45 wards in Moshi Urban and Rural Districts were selected randomly in a population-weighted fashion, including 22 wards from Moshi Rural District and 8 wards from Moshi Urban District. A starting point in each selected ward was chosen randomly by dropping a pencil on a map or, when no map was available, was chosen randomly while touring the ward on foot by a member of the study team who was not previously familiar with the area. A direction was similarly arbitrarily chosen, and the first 27 households along that direction from the starting point were included in the survey.
Community survey design
Before the community survey, focus group discussions were held to generate a list of possible fever causes to be presented to survey participants. To ensure that the list of causes of fever reflected the full spectrum of local beliefs, focus group discussions were held in two wards in Moshi Rural District and in one ward in Moshi Urban District. Each focus group consisted of 10 community members who represented the sexes, a broad range of ages, and a variety of occupations approximately equally. Focus group members were asked to identify common causes of fever in the Moshi area. While leading the focus group discussions, researchers ensured that participants identified only direct causes of fever. For example, when ‘rain’ was mentioned as a cause of fever, study team members asked participants to clarify whether rain was believed to cause fever directly or indirectly, perhaps by leading to increased numbers of mosquitoes or via some supernatural means. Any direct cause that was identified by more than one member of any focus group was included as an option in the community survey. In some cases, similar responses were grouped into a single option. For example, exposure to sun, hot weather, rain, cold weather, and dust were grouped together as ‘weather.’ Biomedical causes of fever ascertained in recently conducted hospital-based studies of fever etiology and not identified in focus groups were grouped together and included among the survey options as ‘infectious diseases acquired from contact with animals’ and ‘other infectious diseases.’
Community survey administration
The community survey was conducted from 13 June 2011 through 22 July 2011. Tanzanian high school graduates who received specific training in study procedures administered the survey to heads of households in the 27 selected homes in each ward. A head of household was defined as an adult who was responsible for the care of other members of the household. After obtaining informed consent, study team members administered a standardized questionnaire in Swahili, the local language. The questionnaire comprised questions about demographics, socioeconomic status, and healthcare seeking behavior. Respondents were also asked ‘What are the three most common causes of fever among children?’ and ‘Of these, which is the most common cause?’ The same questions were then asked regarding adults. Children were defined as individuals younger than 13 years to be consistent with the definition used in the hospital-based fever etiology study. Because the Swahili word for fever, homa, encompasses a broad range of symptoms, the research assistants clarified that these questions specifically referred to fever characterized by elevated body temperature, joto la mwili. Respondents were asked to choose these causes of fever from the list of suggested options described above or to choose ‘other’ if their answer did not appear on the list.
Local disease prevalence data
Given the absence of data regarding fever etiologies in outpatient settings, local disease prevalence data were taken from the previously published results of a year-long etiology of fever study conducted among inpatients at two hospitals in Moshi (Biggs et al. 2011, Prabhu et al. 2011, Hertz et al. 2012, Crump et al. 2011a, b, 2013; Bouley et al. 2012). In instances where diagnostic test results were not available for all participants of the etiology of fever study, the proportion of participants who tested positive for a given infection was applied to the whole study population to extrapolate numbers of persons for uniform comparison.
Data analysis
Data were entered using Cardiff Teleform 9.0 (Cardiff Inc., Vista, CA) and analyzed using JMP 8.0 (SAS, Cary, NC). Descriptive statistics are presented as medians, ranges, and interquartile ranges (IQR) for continuous variables and as proportions for categorical variables. Mann-Whitney U tests were used to compare differences in medians for continuous data. Pearson’s chi-square was used to compare categorical data. A continuous socioeconomic status variable (SES score) was developed by performing a principal component analysis (Vyas and Kumaranayake 2006) using 17 variables related to ownership of assets, housing characteristics, and access to infrastructure. The SES score was used as an approximate measure of the socioeconomic status of the household. Urban residence was defined as residence within Moshi Urban District. For purposes of data analysis, weather, teething, pain, alcohol, and witchcraft were all classified as non-biomedical explanations of fever. Of these, weather, teething, pain, and alcohol were classified as non-supernatural. Although the association between weather and teething is controversial, multiple well-designed studies have failed to identify an independent association between teething and true fever (Wake et al. 2000, Tighe and Roe 2007, Ramos-Jorge et al. 2011, Macknin et al. 2000), and the dangers of misattributing fevers that occur during the teething period to tooth eruption rather than infectious disease have been well-described (Lloyd 1996). Hence it was decided to classify teething as a non-biomedical explanation of fever.
Ethical approval
This study was approved by the Kilimanjaro Christian Medical Centre Research Ethics Committee, the Tanzania National Institutes for Medical Research National Research Ethics Coordinating Committee, and institutional review boards of Duke University Health System and the International Vaccine Institute.
RESULTS
A total of 810 heads of households were interviewed; their sociodemographic features are described in Table 1. The median (range) age of respondents was 48 (16, 102) years, and 509 (62.8%) were females.
Table 1.
Sociodemographic features of heads of households surveyed in Moshi, 2011
| n/N | (%) | |
|---|---|---|
| (N=810) |
||
| Urban residence | 216/810 | (26.7) |
| Age median (range), years | 48(16, 102) | -- |
| Female | 509/810 | (62.8) |
| Chagga tribe | 607/810 | (74.9) |
| Literate | 729/810 | (90.0) |
| Completed primary school | 632/810 | (78.0) |
| Completed secondary school | 111/810 | (13.7) |
| SES score, median (IQR) | −0.5 (−1.2, 1.0) | -- |
| Knows cause of malaria | 764/808 | (94.6) |
| Tested for HIV | 428/810 | (52.8) |
| Owns bednet | 548/810 | (67.7) |
| Drinks untreated water | 642/803 | (80.0) |
The overall prevalence of various etiologies of fever among inpatients in the Moshi area, as previously described (Biggs et al. 2011, Hertz et al. 2012, Prabhu et al. 2011, Crump et al. 2011a,b,2013) , are displayed in Table 2. The most common etiologies of fever among both children and adults were infectious diseases acquired from animal contact, such as leptospirosis and Q fever. Malaria was an uncommon cause of inpatient fever.
Table 2.
Actual etiologies of fever among febrile pediatric and adult inpatients in northern Tanzania, 2007–2008a
| Etiology of fever among pediatric inpatients |
% | Etiology of fever among adult inpatients |
% |
|---|---|---|---|
| Infectious diseases from animal contactb | 20.2 | Infectious disease from animal contactd | 33.3 |
| Other infectious diseasesc | 13.2 | Other infectious diseasese | 25.1 |
| Typhoid fever | 1.3 | Typhoid fever | 6.5 |
| Malaria | 1.3 | Malaria | 2.0 |
| Acute HIV | 0.1 |
Data from (Hertz et al. 2012, Biggs et al. 2011, Prabhu et al. 2011, Crump et al. 2011a, Crump et al. 2011b)
Leptospirosis 7.7%, spotted fever group rickettsioses 7.4%, Q fever 2.6%, Brucellosis 2.0%
Arboviral infections 10.2%, invasive bacterial diseases other than Salmonella Typhi 2.1%, invasive fungal diseases 0.9%
Leptospirosis 10.1%, spotted fever group rickettsioses 8.7%, Q fever 7.9%, Brucellosis 5.3%, typhus group rickettsioses 1.0%
Invasive bacterial diseases other than Salmonella Typhi 10.7%, arboviral infections 5.7%, invasive fungal diseases 5.2%, invasive mycobacterial infections 3.5%
Beliefs about fever in children
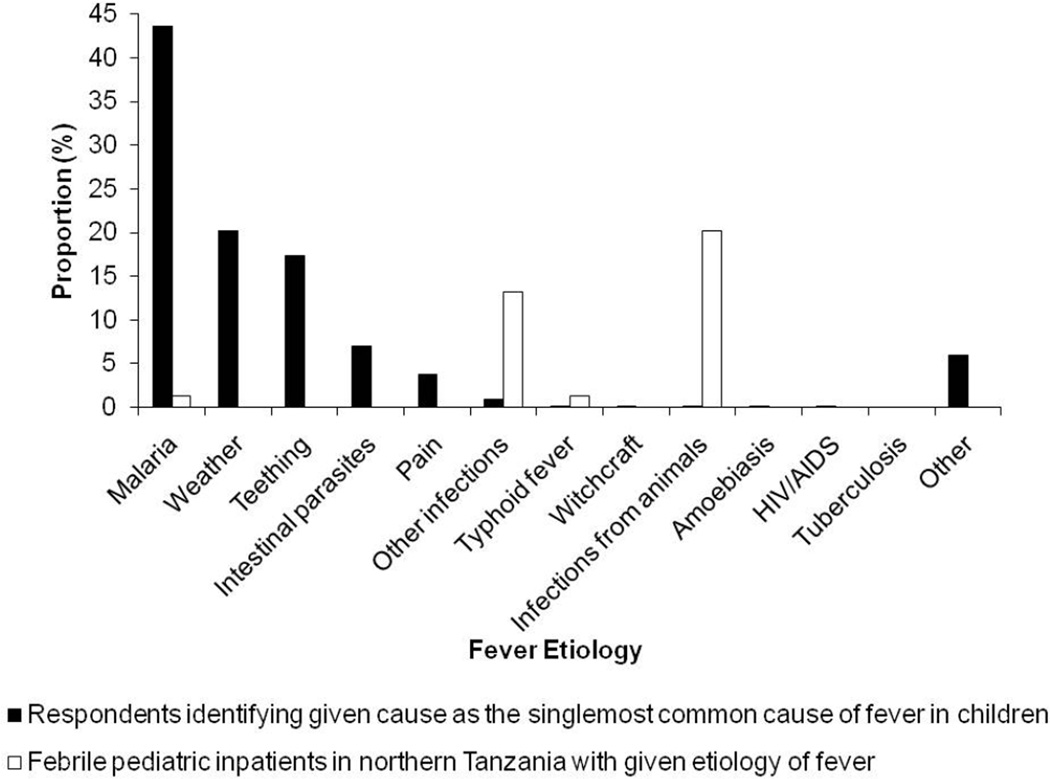
The most frequently cited perceived causes of fever in children were malaria, weather, and teething, identified by 618 (76.3%), 503 (62.1%), and 459 (56.7%) respondents, respectively (Table 3). Figure 1 compares the actual prevalence of various fever etiologies among pediatric inpatients in Moshi with the responses of heads of households when asked to identify the single-most common cause of childhood fever. Malaria was the most common response, cited by 353 (43.6%) respondents, but was present in 6 (1.3%) febrile pediatric inpatients. Infectious diseases from animal contact, which caused 94 (20.2%) fevers among pediatric inpatients, were identified by 2 (0.2%) respondents. Weather and teething, which were not considered to be an actual etiology of fever in any pediatric inpatient, were identified as the most common cause of fever by 164 (20.2%) and 141 (17.4%) participants, respectively.
Table 3.
Perceived common causes of fever in children identified by heads of households in Moshi, 2011a
| Perceived cause of fever | # of Respondents | % |
|---|---|---|
| (N=810) |
||
| Malaria |
618 | 76.3 |
| Weather | 503 | 62.1 |
| Teething | 459 | 56.7 |
| Intestinal parasites | 306 | 37.8 |
| Pain | 110 | 13.6 |
| Other infectious diseases | 97 | 12.0 |
| Typhoid fever | 63 | 7.8 |
| Witchcraft | 22 | 2.7 |
| Amoebiasis | 22 | 2.7 |
| HIV/AIDS | 10 | 1.2 |
| Tuberculosis | 7 | 0.9 |
| Infectious diseases from animal contact | 7 | 0.9 |
| Other | 206 | 25.4 |
In response to the question, ‘What are three most common causes of fever in children?’
Figure 1.
Actual etiologiesa and perceived causesb of fever among children in northern Tanzania
a Actual etiologies of fever among febrile pediatric inpatients in northern Tanzania, 2007–2008 (N=467) (Biggs et al. 2011, Prabhu et al. 2011, Hertz et al. 2012, Crump et al. 2011a, Crump et al. 2011b)
b According to residents of Moshi, 2011, in response to the question, ‘What is the most common cause of fever among children?’ (N=810)
Beliefs about fever in adults
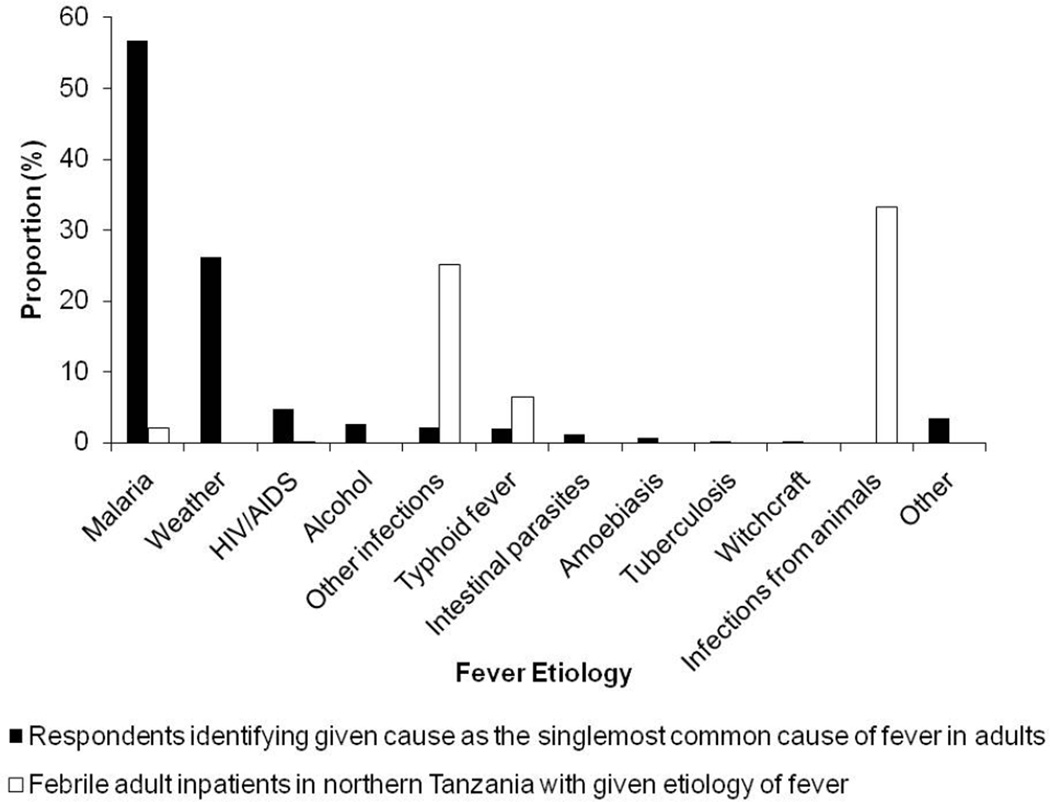
The most frequently identified causes of fever in adults were malaria, weather, and typhoid fever, which were selected by 766 (94.6%), 564 (69.6%), and 185 (22.8%) respondents, respectively (Table 4). Figure 2 compares the actual prevalences of fever etiologies among adult inpatients in Moshi to the perceived causes of fever cited by heads of households when asked to identify the single- most common cause of fever in adults. Malaria, which was the actual etiology of fever in 8 (2.0%) adult inpatients, was identified as the single-most common cause of adult fever by 459 (56.7%) of respondents. Infectious diseases from animal contact, which were the most common actual etiology of fever in adult inpatients, were not cited by any participant as the single-most common cause of adult fever.
Table 4.
Perceived common causes of fever in adults identified by heads of households in Moshi, 2011a
| Cause of Fever | # of Respondents | % |
|---|---|---|
| (N=810) |
||
| Malaria |
766 | 94.6 |
| Weather | 564 | 69.6 |
| Typhoid fever | 185 | 22.8 |
| Other infectious diseases | 167 | 20.6 |
| HIV/AIDS | 137 | 17.0 |
| Intestinal parasites | 133 | 16.4 |
| Alcohol | 115 | 14.2 |
| Amoebiasis | 102 | 12.6 |
| Tuberculosis | 33 | 4.1 |
| Infectious diseases from animal contact | 5 | 0.6 |
| Witchcraft | 4 | 0.5 |
| Other | 219 | 27.0 |
In response to the question, ‘What are the three most common causes of fever in adults?’
Figure 2.
Actual etiologiesa and perceived causesb of fever among adults in northern Tanzania
a Actual etiologies of fever among febrile adults inpatients in northern Tanzania, 2007–2008 (N=403) (Biggs et al. 2011, Prabhu et al. 2011, Hertz et al. 2012, Crump et al. 2011a, Crump et al. 2011b)
b According to residents of Moshi, 2011, in response to the question, ‘What is the most common cause of fever among adults?’ (N=810)
Correlates of belief in non-biomedical explanations of fever
Table 5 compares the sociodemographic features of participants who identified a non-biomedical explanation as the single-most common cause of fever in children to all other respondents. Belief in a non-biomedical explanation of childhood fever was associated with being a member of the Chagga tribe (Odds Ratio [OR] 1.6, p=0.004) and lower socioeconomic status (p<0.001), whereas urban residence (OR 0.44, p<0.001), completion of primary school (OR 0.47, p<0.001), completion of secondary school (OR 0.55, p=0.006), and ownership of a bednet (OR 0.67, p=0.007) were negatively associated. Heads of household who cited a non-biomedical explanation as the most common cause of fever in children were also significantly more likely to identify a traditional healer as the best treatment option for children with elevated body temperatures for more than three days (OR 2.9, p=0.001). This difference persisted when comparing heads of household who cited a nonsupernatural, non-biomedical explanation such as weather, teething, or pain as the most common cause of fever to all other respondents. Twenty-eight (14.9%) of these heads of households chose a traditional healer as the best treatment option for children with elevated body temperatures for more than three days, whereas only 14 (5.6%) of all other respondents preferred a traditional healer (OR 3.0, p=0.001).
Table 5.
Sociodemographic features and healthcare seeking behavior of participants who identified a non-biomedical explanationa as the most common cause of fever in children, Moshi, 2011
| Identified a non-biomedical cause as the most common cause of fever in children (N=338) |
All other participants (N=472) | OR (95% CI) |
p-value | |||
|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | |||
| Urban residence | 60/338 | (17.8) | 156/472 | (33.1) | 0.44 (031–0.61) | <0.001* |
| Age, median (range), years | 49 (19, 98) | 47 (16, 102) | -- |
0.147 | ||
| Female | 205/338 | (60.7) | 304/472 | (64.4) | 0.85 (0.64–1.1) | 0.275 |
| Chagga tribe | 271/338 | (80.2) | 336/472 | (71.2) | 1.6 (1.2–2.3) | 0.004* |
| SES score, median (IQR) | −0.66 (−1.4, 0.08) | −0.39 (−1.1, 1.5) | -- | <0.001* | ||
| Completed primary school | 238/338 | (70.4) | 394/472 | (83.5) | 0.47 (0.34–0.66) | <0.001* |
| Completed secondary school | 33/338 | (9.8) | 78/472 | (16.5) | 0.55 (0.35–0.84) | 0.006* |
| Tested for HIV | 172/338 | (50.9) | 256/472 | (54.2) | 0.87 (0.66–1.2) | 0.346 |
| Drinks untreated water | 268/335 | (80.0) | 374/468 | (79.9) | 1.0 (0.71–1.4) | 0.976 |
| Have a bednet at home | 211/338 | (62.4) | 337/472 | (71.4) | 0.67 (0.49–0.90) | 0.007* |
| No antibacterials or antimalarials at home | 315/338 | (93.2) | 431/472 | (91.3) | 1.3 (0.77–2.2) | 0.328 |
| Knows cause of malaria | 308/338 | (91.1) | 456/470 | (97.0) | 0.32 (0.16–0.60) | <0.001* |
| Treatment at traditional healer first choice for child with feverb | 7/188 | (3.7) | 5/249 | (2.0) | 1.9 (0.59–6.0) | 0.277 |
| Treatment at traditional healer first choice for child with elevated body temperature > 3 daysb | 28/188 | (14.9) | 14/249 | (5.6) | 2.9 (1.5–5.8) | 0.001* |
Non-biomedical explanations were weather, teething, pain, and witchcraft
Question only asked of participants with children at home (N = 437)
Table 6 presents the sociodemographic features of respondents who identified a nonbiomedical explanation as the most common cause of fever in adults. Such participants were significantly more likely to be older (p=0.003), members of the Chagga tribe (OR 2.5, p<0.001), and of lower socioeconomic status (p=0.005). They were significantly less likely to reside in an urban area (OR 0.22, p<0.001), to own a bednet (OR 0.57, p<0.001), or to have completed primary (OR 0.35, p<0.001) or secondary (OR 0.53, p=0.012) school. Heads of household who cited a non-biomedical explanation as the most common cause of fever in adults were significantly more likely to identify a traditional healer as the best treatment option both for adults with fever (OR 2.7, p<0.001) and for adults with elevated body temperatures for more than three days (OR 3.2, p<0.001). These differences persisted when comparing heads of households who cited a non-supernatural, nonbiomedical explanation such as weather or alcohol to all other respondents: 34 (14.5%) and 33 (14.1%) of these heads of households identified a traditional healer as the best treatment option for an adult with fever and with an elevated body temperature for more than three days, respectively, compared to 34 (5.9%) and 28 (4.9%) of all other respondents (OR 2.7, p<0.001 and OR 3.2, p <0.001, respectively).
Table 6.
Sociodemographic features and healthcare seeking behavior of participants who identified a non-biomedical explanationa as the most common cause of fever in adults, Moshi, 2011
| Identified a non-biomedical cause as the most common cause of fever in children (N=235) |
All other participants (N=575) | OR (95% CI) |
p-value | |||
|---|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | |||
| Urban residence | 24/235 | (10.2) | 192/575 | (33.4) | 0.22 (0.14–0.36) | <0.001* |
| Age, median (range), years | 50 (22, 98) | 46 (16, 102) | -- |
0.003* | ||
| Female | 139/235 | (59.2) | 370/575 | (64.4) | 0.80 (0.59– 1.1) | 0.165 |
| Chagga tribe | 201/235 | (85.5) | 406/575 | (70.6) | 2.5 (1.6– 3.7) | <0.001* |
| SES score, median (IQR) | −0.61 (−1.4, 0.08) | −0.43 (−1.2, 1.3) | -- | 0.005* | ||
| Completed primary school | 151/235 | (64.3) | 481/575 | (83.7) | 0.35 (0.25–0.50) | <0.001* |
| Completed secondary school | 21/235 | (8.9) | 90/575 | (15.7) | 0.53 (0.32– 0.87) | 0.012* |
| Tested for HIV | 113/235 | (48.1) | 315/575 | (54.8) | 0.76 (0.54–1.0) | 0.083 |
| Drinks untreated water | 195/233 | (83.7) | 447/570 | (78.4) | 1.4 (0.95–2.1) | 0.091 |
| Have a bednet at home | 138/235 | (58.7) | 410/575 | (71.3) | 0.57 (0.42– 0.79) | <0.001* |
| No antibacterials or antimalarials at home | 222/235 | (94.5) | 524/575 | (91.1) | 1.7 (0.89–3.1) | 0.110 |
| Knows cause of malaria | 209/235 | (88.9) | 555/573 | (96.9) | 0.26 (0.14–0.49) | <0.001* |
| Treatment at traditional healer first choice for adult with fever | 34/235 | (14.5) | 34/575 | (5.9) | 2.7 (1.6–4.4) | <0.001* |
| Treatment at traditional healer first choice for adult with elevated body temperature > 3 days | 33/235 | (14.0) | 28/575 | (4.9) | 3.2 (1.9–5.4) | <0.001* |
Non-biomedical explanations were weather, alcohol, and witchcraft
DISCUSSION
Our findings demonstrate a substantial discrepancy between actual etiologies and perceived causes of fever in northern Tanzania. Such discrepancies are troubling because they create the potential for real and treatable causes of febrile illness to be overlooked by patients and their caregivers. In particular, community members considered malaria to be the single-most common cause of fever despite malaria being a relatively uncommon cause of inpatient fever. This discordance is important because community misperceptions about malaria prevalence may contribute to malaria over-diagnosis, a widespread problem in sub-Saharan Africa (Chandler et al. 2008b, Hume et al. 2008), by shaping patient expectations and therefore influencing clinician decision-making. In one study conducted in northern Tanzania, a quarter of physicians reported being afraid that patients might complain if they were not treated for malaria (Chandler et al. 2008a). Although the problem of malaria over-diagnosis has complex causes, policy makers should consider the role of community misperceptions when trying to reduce excessive rates of inappropriate malaria diagnosis.
Beyond influencing clinician decision-making, the discrepancy between the actual and perceived prevalence of malaria may have other notable public health implications. Individuals who believe that malaria is a leading cause of fever may be more likely to self-medicate with antimalarials than others. Thus, communities in which there is a large discrepancy between the actual and perceived prevalence of malaria may experience higher levels of inappropriate antimalarial usage resulting in wasted health resources and delay of appropriate care. Self-treatment with antimalarials is a common practice across sub-Saharan Africa (Deressa et al. 2003, Hodel et al. 2009), and in Tanzania, pharmacies routinely dispense antimalarials without a prescription (Kagashe et al. 2011). In such a setting, community misperceptions about malaria prevalence may lead to inappropriate use of expensive first-line artemisinin-based combination therapy (O'Connell et al. 2011) and delay in appropriate treatment for other febrile diseases.
In addition to the overemphasis on malaria among survey respondents, we found under-appreciation of other infectious diseases, such as bacterial zoonoses. Bacterial zoonoses are increasingly identified as a major cause of illness in sub-Saharan Africa (Bertherat et al. 1999, Steinmann et al. 2005, Crump et al. 2013), and prevention and treatment strategies are underdeveloped. However, programs to address bacterial zoonoses may be more difficult to design and implement than those for malaria. Because community appreciation of non-malaria etiologies of fever is low, successful public health interventions will likely need to incorporate a large component of education.
After malaria, the most commonly cited perceived cause of fever in both adults and children was weather. Other non-biomedical explanations, such as teething, alcohol, and witchcraft, were also cited frequently. Such non-biomedical explanations for fever have been identified in numerous studies across sub-Saharan Africa. Indeed, weather, teething, witchcraft, and alcohol are perceived to be important causes of fever in many countries in the region, including Nigeria, Ghana, the Democratic Republic of Congo, Malawi, Gabon, Uganda, and Tanzania (Adetunji 1991), Ahorlu et al. 1997, Chibwana et al. 2009, Pilkington et al. 2004, Sabuni 2007, Kengeya-Kayondo et al. 1994, Winch et al. 1996). Although educational programs would need to be tailored to individual communities, common themes can be identified that are likely to be relevant to many settings within the continent.
Our results demonstrate that individuals who believe in non-biomedical explanations of fever are significantly more likely to seek care from a traditional healer for febrile adults and children. The finding that individuals who perceive witchcraft to be an important cause of fever tend to prefer traditional healers is consistent with the results of prior studies involving focus groups and semi-structured interviews (Chibwana et al. 2009, Pilkington et al. 2004). However, the finding that those who believe in non-biomedical, non-supernatural explanations for fever such as weather are also more likely to prefer traditional healers for treatment of fevers is important. Belief in such causes of fever, which is common in sub-Saharan Africa, may be substantially affecting healthcare seeking behavior across the region. Efforts to educate communities about biomedical explanations of fever may therefore increase use of conventional healthcare services for febrile episodes.
This study had several limitations. First, the beliefs of heads of household interviewed in this study may not be representative of the beliefs of the entire community. In particular, younger residents and males were not well represented among survey respondents. Furthermore, the actual etiologies of fever as determined from the inpatient etiology of fever study may not be representative of the spectrum of febrile diseases in the community, some of which may not routinely result in hospitalization (Crump et al. 2003). Moreover, tuberculosis was not evaluated comprehensively as part of the etiology of fever study, making comparisons between community perceptions and actual prevalence difficult for this infection. Additionally, the term ‘infectious diseases from contact with animals’ may not have been interpreted by some respondents as including contact with animal products or excreta, making this term an imperfect descriptor of bacterial zoonoses. As with all survey-based studies, participants’ answers may have been subject to social acceptability bias. In particular, regarding the distinction between supernatural and other nonbiomedical causes, some respondents may have been reluctant to identify the cause of fever that they truly believed to be most common, preferring instead to give an answer they perceived to be more acceptable to the researchers. The use of young, relatively educated interviewers to administer the surveys may have heightened social acceptability bias, but the use of focus group discussions to draw out understandings of the causes of disease before developing the household survey options attempted to minimize this bias.
In summary, we found substantial discrepancies between the actual etiologies and perceived causes of fever among community members in northern Tanzania. As a growing proportion of febrile episodes in sub-Saharan Africa occurs in low malaria transmission settings, the overemphasis on malaria and under-appreciation of other important infectious diseases found here merit the attention of policy makers across the region. Most importantly, our findings highlight an important knowledge gap regarding common etiologies of fever that merits attention in community health educational programs. Many fever-related educational interventions in sub-Saharan Africa to date have focused on malaria (Nkuo Akenji et al. 2005, Eriksen et al. 2010, Okeke and Uzochukwu 2009), but these findings suggest that much more comprehensive fever education programs are needed. Addressing knowledge and beliefs around febrile illness may have many potential benefits, such as a reduction in malaria over-diagnosis, attenuation of inappropriate use of antimalarial drugs, more rapid initiation of appropriate management for non-malaria febrile diseases, improved receptivity of community members to public health interventions for locally important infectious diseases, and an increase in prompt presentation to conventional healthcare services for febrile illnesses.
ACKNOWLEDGEMENTS
We thank Frank R. Mhina, Edward S. Mshana, Yusuf S. Msuya, Daniel F. Ngowi, and Tumsifu G. Tarimo for administering questionnaires; Enock J. Kessy, Alphonse S. Mushi, and Evaline M. Ndosi for assisting in data entry; and Carol F. Sangawe for driving. We acknowledge the Hubert-Yeargan Center for Global Health at Duke University and Kilimanjaro Christian Medical Centre for critical infrastructure support. This research was funded by the Typhoid Fever Surveillance in sub-Saharan Africa Program; the US National Institutes of Health; and by the UK Biotechnology and Biological Sciences Research Council.
REFERENCES
- Adetunji JA. Response of parents to five killer diseases among children in a Yoruba community, Nigeria. Soc Sci Med. 1991;32:1379–1387. doi: 10.1016/0277-9536(91)90198-l. [DOI] [PubMed] [Google Scholar]
- Agyepong IA, Manderson L. The diagnosis and management of fever at household level in the Greater Accra Region, Ghana. Acta Trop. 1994;58:317–330. doi: 10.1016/0001-706x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Ahorlu CK, Dunyo SK, Afari EA, Koram KA, Nkrumah FK. Malaria-related beliefs and behaviour in southern Ghana: implications for treatment, prevention and control. Trop Med Int Health. 1997;2:488–499. [PubMed] [Google Scholar]
- Bertherat E, Renaut A, Nabias R, Dubreuil G, Georges-Courbot MC. Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon. Am J Trop Med Hyg. 1999;60:610–615. doi: 10.4269/ajtmh.1999.60.610. [DOI] [PubMed] [Google Scholar]
- Biggs HM, Bui DM, Galloway RL, et al. Leptospirosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2011;85:275–281. doi: 10.4269/ajtmh.2011.11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley AJ, Biggs HM, Stoddard RA, et al. Brucellosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;87:1105–1111. doi: 10.4269/ajtmh.2012.12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CI, Jones C, Boniface G, Juma K, Reyburn H, Whitty CJ. Guidelines and mindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study. Malar J. 2008a;7:53. doi: 10.1186/1475-2875-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CI, Mwangi R, Mbakilwa H, Olomi R, Whitty CJ, Reyburn H. Malaria overdiagnosis: is patient pressure the problem? Health Policy Plan. 2008b;23:170–178. doi: 10.1093/heapol/czm046. [DOI] [PubMed] [Google Scholar]
- Chibwana AI, Mathanga DP, Chinkhumba J, Campbell CH., Jr Socio-cultural predictors of health-seeking behaviour for febrile under-five children in Mwanza-Neno district, Malawi. Malar J. 2009;8:219. doi: 10.1186/1475-2875-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoro C, Nsimba SE, Warsame M, Tomson G. Local understanding, perceptions and reported practices of mothers/guardians and health workers on childhood malaria in a Tanzanian district--implications for malaria control. Acta Trop. 2003;87:305–313. doi: 10.1016/s0001-706x(03)00113-x. [DOI] [PubMed] [Google Scholar]
- Crump JA, Morrissey AB, Nicholson WL, et al. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Ramadhani HO, Morrissey AB, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011a;16:830–837. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Ramadhani HO, Morrissey AB, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011b;52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Youssef FG, Luby SP, et al. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis. 2003;9:539–544. doi: 10.3201/eid0905.020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deressa W, Ali A, Enqusellassie F. Self-treatment of malaria in rural communities, Butajira, southern Ethiopia. Bull World Health Organ. 2003;81:261–268. [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Mujinja P, Warsame M, et al. Effectiveness of a community intervention on malaria in rural Tanzania - a randomised controlled trial. Afr Health Sci. 2010;10:332–340. [PMC free article] [PubMed] [Google Scholar]
- Feikin DR, Olack B, Bigogo GM, et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One. 2011;6:e16085. doi: 10.1371/journal.pone.0016085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosu GB. Disease classification in rural Ghana: framework and implications for health behaviour. Soc Sci Med B. 1981;15:471–482. doi: 10.1016/0160-7987(81)90021-1. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Gething PW, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz JT, Munishi OM, Ooi EE, et al. Chikungunya and Dengue Fever among Hospitalized Febrile Patients in Northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel EM, Kabanywanyi AM, Malila A, et al. Residual antimalarials in malaria patients from Tanzania--implications on drug efficacy assessment and spread of parasite resistance. PLoS One. 2009;4:e8184. doi: 10.1371/journal.pone.0008184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume JC, Barnish G, Mangal T, Armazio L, Streat E, Bates I. Household cost of malaria overdiagnosis in rural Mozambique. Malar J. 2008;7:33. doi: 10.1186/1475-2875-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriso R, Accorsi S, Akena S, et al. 'Killer' canines: the morbidity and mortality of ebino in northern Uganda. Trop Med Int Health. 2000;5:706–710. doi: 10.1046/j.1365-3156.2000.00625.x. [DOI] [PubMed] [Google Scholar]
- Kagashe GA, Minzi O, Matowe L. An assessment of dispensing practices in private pharmacies in Dar-es-Salaam, Tanzania. Int J Pharm Pract. 2011;19:30–35. doi: 10.1111/j.2042-7174.2010.00075.x. [DOI] [PubMed] [Google Scholar]
- Kengeya-Kayondo JF, Seeley JA, Kajura-Bajenja E, et al. Recognition, treatment seeking behaviour and perception of cause of malaria among rural women in Uganda. Acta Trop. 1994;58:267–273. doi: 10.1016/0001-706x(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Lloyd S. Teething in babies: separating fact from fiction. Prof Care Mother Child. 1996;6:155–156. [PubMed] [Google Scholar]
- Macknin ML, Piedmonte M, Jacobs J, Skibinski C. Symptoms associated with infant teething: a prospective study. Pediatrics. 2000;105:747–752. doi: 10.1542/peds.105.4.747. [DOI] [PubMed] [Google Scholar]
- Malik EM, Hanafi K, Ali SH, Ahmed ES, Mohamed KA. Treatment-seeking behaviour for malaria in children under five years of age: implication for home management in rural areas with high seasonal transmission in Sudan. Malar J. 2006;5:60. doi: 10.1186/1475-2875-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux CS, Mung'Ala-Odera V, Harpham T, Snow RW. Maternal responses to childhood fevers: a comparison of rural and urban residents in coastal Kenya. Trop Med Int Health. 1999;4:836–845. doi: 10.1046/j.1365-3156.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Nkuo Akenji TK, Ntonifor NN, Ching JK, et al. Evaluating a malaria intervention strategy using knowledge, practices and coverage surveys in rural Bolifamba, southwest Cameroon. Trans R Soc Trop Med Hyg. 2005;99:325–332. doi: 10.1016/j.trstmh.2003.12.016. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Gatakaa H, Poyer S, et al. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malar J. 2011;10:326. doi: 10.1186/1475-2875-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterholt MJ, Bousema JT, Mwerinde OK, et al. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J. 2006;5:98. doi: 10.1186/1475-2875-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke TA, Uzochukwu BS. Improving childhood malaria treatment and referral practices by training patent medicine vendors in rural south-east Nigeria. Malar J. 2009;8:260. doi: 10.1186/1475-2875-8-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington H, Mayombo J, Aubouy N, Deloron P. Malaria, from natural to supernatural: a qualitative study of mothers' reactions to fever (Dienga, Gabon) J Epidemiol Community Health. 2004;58:826–830. doi: 10.1136/jech.2003.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu M, Nicholson WL, Roche AJ, et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. 2011;53:e8–e15. doi: 10.1093/cid/cir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Jorge J, Pordeus IA, Ramos-Jorge ML, Paiva SM. Prospective longitudinal study of signs and symptoms associated with primary tooth eruption. Pediatrics. 2011;128:471–476. doi: 10.1542/peds.2010-2697. [DOI] [PubMed] [Google Scholar]
- Sabuni LP. Dilemma with the local perception of causes of illnesses in central Africa: muted concept but prevalent in everyday life. Qual Health Res. 2007;17:1280–1291. doi: 10.1177/1049732307307864. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Bonfoh B, Peter O, Schelling E, Traore M, Zinsstag J. Seroprevalence of Qfever in febrile individuals in Mali. Trop Med Int Health. 2005;10:612–617. doi: 10.1111/j.1365-3156.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- Tarimo DS, Lwihula GK, Minjas JN, Bygbjerg IC. Mothers' perceptions and knowledge on childhood malaria in the holendemic Kibaha district, Tanzania: implications for malaria control and the IMCI strategy. Trop Med Int Health. 2000;5:179–184. doi: 10.1046/j.1365-3156.2000.00537.x. [DOI] [PubMed] [Google Scholar]
- Tighe M, Roe MF. Does a teething child need serious illness excluding? Arch Dis Child. 2007;92:266–268. doi: 10.1136/adc.2006.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- Wake M, Hesketh K, Lucas J. Teething and tooth eruption in infants: A cohort study. Pediatrics. 2000;106:1374–1379. doi: 10.1542/peds.106.6.1374. [DOI] [PubMed] [Google Scholar]
- Winch PJ, Makemba AM, Kamazima SR, et al. Local terminology for febrile illnesses in Bagamoyo District, Tanzania and its impact on the design of a community-based malaria control programme. Soc Sci Med. 1996;42:1057–1067. doi: 10.1016/0277-9536(95)00293-6. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2009. Geneva, Switzerland: WHO Press; 2009. [Google Scholar]