Abstract
Recently we demonstrated that docosahexaenoic acid (DHA) is highly neuroprotective when animals were allowed to survive during one week. This study was conducted to establish whether the neuroprotection induced by DHA persists with chronic survival. Sprague-Dawley rats underwent 2 h of middle cerebral artery occlusion (MCAo) and treated with DHA or saline at 3 h after MCAo. Animals received neurobehavioral examination (composite neuroscore, rota-rod, beam walking and Y maze tests) followed by ex vivo magnetic resonance imaging and histopathology at 3 weeks. DHA improved composite neurologic score beginning on day 1 by 20%, which persisted throughout weeks 1–3 by 24–41% compared to the saline-treated group. DHA prolonged the latency in rota-rod on weeks 2–3 by 162–178%, enhanced balance performance in the beam walking test on weeks 1 and 2 by 42–51%, and decreased the number of entries in the Y maze test by 51 % and spontaneous alteration by 53 % on week 2 compared to the saline-treated group. DHA treatment reduced tissue loss (computed from T2-weighted images) by 24% and total and cortical infarct volumes by 46% and 54% compared to the saline-treated group. These results show that DHA confers enduring ischemic neuroprotection.
Keywords: Docosahexaenoic acid, experimental stroke, middle cerebral artery occlusion, behavior, rota-rod test, beam walking test, Y maze test, ex vivo MRI
1. Introduction
Stroke is a leading cause of death and disability in the United States. Annually, 795,000 people experience new or recurrent stroke and 41% of stroke patients die [1]. Even though some patients survive stroke, they suffer serious long-term disabilities such as a locomotion, sensory, vision, language and cognition. Thus, maximal enhancement of behavioral function accompanied with the reduction of infarction is a major goal of post-stroke therapy with a promise better quality of life for stroke survivors.
Docosahexaenoic acid (DHA; 22:6, n=3) is a member of the essential omega-3 fatty acid family and is concentrated in the membranes of the central nervous system [2]. DHA is well known as a robust neuroprotectant against experimental stroke [3–5]. Recently we demonstrated that DHA improves behavioral function, decreases infarct volume, promotes cell survival in the ischemic penumbra as well as resolution of cerebral edema in one week of survival after focal cerebral ischemia in rats [3–5]. In addition, therapeutic window shows that DHA is neuroprotective when administered up to 5 h after experimental stroke during 7 days survival period [3]. The dose-response study in rats with transient focal cerebral ischemia showed that a 5 mg/kg dosage was highly neuroprotective [3–5]; thus, this dose was applied in this study.
The objective of the present study was to test the hypothesis that DHA-induced neuroprotection endures in animals allowed to survive for three weeks after focal ischemic insult. The effect of DHA treatment was investigated using a battery of different behavioral tests in conjunction with ex vivo magnetic resonance imaging (MRI) and histopathology.
2. Material and methods
2.1. Animal Preparation
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Louisiana State University Health Sciences Center, New Orleans. Male Sprague-Dawley rats (290 to 330 g; Charles River Laboratory, Wilmington, MA, USA) were fasted overnight with free access to water before surgical procedure. Anesthesia was induced by inhalation of 3% of isoflurane in a mixture of 70% nitrous oxide and 30% oxygen. During the procedure, isoflurane was maintained at 1% in the same ratio of nitrous oxide and oxygen. Orally intubated rats were paralyzed by injection of pancuronium bromide (0.5 mg/kg, i.v.) and then mechanically ventilated during the surgical procedure. A catheter was inserted in the right femoral vein and then removed after infusion of the drug. The femoral arterial catheter was not implanted and we did not measure arterial blood gases and glucose, to avoid functional impairment of the hindlimb. Rectal (CMA/150 Temperature Controller, CMA / Microdialysis AB, Stockholm, Sweden) and cranial (left temporalis muscle; Omega Engineering, Stamford, CT, USA) temperatures were monitored and maintained at 37.0 to 37.5 °C during surgical procedures. Rectal temperature and body weight were closely monitored during the three-week survival period.
2.2. Focal cerebral ischemia model
The right middle cerebral artery (MCA) was occluded for 2 h by intraluminal filament, as we previously described [6]. Briefly, the right common carotid artery (CCA) was exposed through an incision in the neck. The CCA was isolated from surrounding nerves. The distal external carotid artery (ECA) and pterygopalatine arteries were tied. A 4-cm of 3-0 monofilament nylon suture coated with poly-Lysine was introduced via the proximal ECA into the internal carotid artery and MCA. After 2 h of MCAo, rats were anesthetized with the same anesthetic combination and intraluminal filaments were removed. The animals were allowed to survive for three weeks with free access to water and food.
2.3. Treatment
The agents (DHA; 5mg/kg, Cayman, Ann Arbor, MI; n=12) or vehicle (0.9% saline; n=8) were administered intravenously into the femoral vein at 3 h after onset of MCAo.
2.4. Behavioral Tests
Rats were pre-trained for the rota-rod, beam walking, and Y maze tests for three consecutive days before MCAo procedure. Animals that failed to accomplish the requirements of the tests during pre-training period were excluded from the study. All behavioral tests were performed by an investigator blinded to the treatment groups.
2.4.1. Total neurologic score
A composite neurological battery was conducted during MCAo (at 60 min) and then on days 1, 2, 3 and weeks 1, 2, and 3 after treatment. The battery consisted of two tests: (1) the postural reflex test to evaluate upper body posture when the animal is suspended by the tail, and (2) forelimb placing test to assess sensorimotor integration in forelimb placing responses to visual, tactile and proprioceptive stimuli [6]. Total neurologic score was graded on a scale from 0 (normal) to 12 (maximal deficit), as we previously described [6]. Only animals with a high-grade neurological deficit (10 or greater) were included in the study.
2.4.2. Rota-rod test
Rota-rod test was used to evaluate the motor function after cerebral ischemia [7]. In the pre-training period, animals were trained to run for 5 min at 16 RPM on a rota-rod (ENV-575, Med Associate, Inc., Albans, VT, USA). On weeks 1, 2 and 3 after treatment, the rota-rod test consisted of five trials per day on each test day. Each trial recorded the time to fall during a 5-min session at the speed of 16 RPM. A 15-min break was given between the five trials.
2.4.3. Beam walking test
The beam walking test was used to assess motor coordination, integration [8] and balance performance [9] after focal cerebral ischemia. Rats were pre-trained to cross the beam (100 cm × 2.5 cm × 2.5 cm; 60 cm above floor) voluntarily without a slip. The test was conducted three times per day on weeks 1, 2 and 3 after treatment. Motor coordination and integration were assessed by counting the number of half and full slips on the ipsilateral and contralateral (stroke) sides, when the rat is traversing the beam for 3 min [10]. A step that slipped outside of beam was considered a full slip, while touching the side of the beam was defined as a half slip [11]. Balance performance on the beam was graded as follows: 0 = Balances with steady posture; 1 = Grasps side of beam; 2 = Hugs beam and 1 limb falls down from beam; 3 = Hugs beam and 2 limbs fall down from beam; 4 = Attempts to balance on beam but falls off > 40 sec; 5 = Attempts to balance on beam but falls off > 20 sec; 6 = Falls off, no attempt to balance or hang on beam < 20 sec [9]. Rats were given a 5-min break between trials.
2.4.4. Y maze test
The Y maze test was used to evaluate cognitive function, especially working memory in a new environment [12]. During the pre-training period, rats were allowed to explore the Y maze (Stoelting, Wood Dale, IL, USA) for 30 min. On weeks 1, 2 and 3 after treatment, the number of entries, spontaneous alteration and percentage of alteration were recorded for 8 min three times per test day. Entry is defined as a complete placement of hind paws within the arm of the maze. Spontaneous alteration is considered when a rat visits a new arm and does not return to one of previously-visited two arms. The percentage of alteration was calculated as follows: [Number of spontaneous alteration / (Number of entry - 2)] × 100 [12]. Rats were given a 10-min break between trials.
2.5. Ex vivo Magnetic Resonance Imaging (MRI)
At three weeks after completion of the behavioral tests, brains were removed after transcardiac perfusion with 0.9% saline followed by 4% paraformaldehyde. High resolution ex vivo MRI data of brains were obtained using an 11.7 T Bruker Advance 8.9 cm horizontal bone instrument equipped with an 89 mm (ID) receiver coil (Bruker Biospin, Billerica, MA, USA). Lesion volume, tissue loss, left and right hemispheres areas and ventricles size were quantified from high resolution T2 weighted images (T2WI). Residual hemisphere volume on each side was calculated by subtraction of ventricle volume from the volume of the hemisphere on each side. Tissue loss was defined as the subtraction of residual volume of the lesion hemisphere from residual volume of the non-lesion hemisphere.
2.6. Histopathology
Histopathology was performed after completion of ex vivo MRI. Cryoprotected brains were cut into twenty-micron-thick sections in the coronal plane and stained with thionine (Nissl). Brain sections were then digitized (MCID core imaging software; InterFocus Imaging Ltd., Cambridge, England) at nine standardized coronal levels (bregma levels: +5.2, +2.7, +1.2, −0.3, −1.3, −1.8, −3.8, −5.0 and –7.3 mm) [13] using a CCD camera (QICAM Fast 1394, QIMAGING, British Columbia, Canada). An investigator blinded to the experimental groups then outlined the zone of the cortical and subcortical infarction as well as the left and right hemispheres of each section. Infarct volume was calculated as the integrated product of the cross-sectional area and inter-sectional distance. Brain sections were imaged on a motorized microscope BX61VS (Olympus, Japan) at 20 × objective.
2.7. Statistical analysis
Repeated measures analysis of variance (ANOVA), followed by Bonferroni procedure to correct multiple comparisons was performed. Two-tailed Student’s t-tests were used for two-group comparisons. One-way ANOVA followed by Dunnett’s test was used in the secondary analysis of the final behavioral endpoints. Data are presented as mean values ± SEM. Differences at p < 0.05 were considered statistically significant.
3. Results
3.1. Physiological variables
There were no significant differences in rectal and cranial (temporalis muscle) temperatures and body weight between DHA (n=12) and saline (n=8) treated groups during the three-week survival period (Table 1). All animals lost body weight during the first week, but regained it afterward (Table 1).
Table 1.
Physiological variables
| Saline (n=8) |
DHA (n=12) |
|
|---|---|---|
| Before MCAo (15 minutes) | ||
| Cranial Temprature (°C) | 36.8 ± 0.2 | 36.6 ± 0.2 |
| Rectal Temperature (°C) | 37.2 ± 0.2 | 37.2 ± 0.2 |
| Body Weight (g) | 301 ± 9 | 317 ± 5 |
| During MCAo (15 minutes) | ||
| Cranial Temprature (°C) | 37.1 ± 0.2 | 37.1 ± 0.1 |
| Rectal Temperature (°C) | 37.4 ± 0.3 | 37.7 ± 0.2 |
| After treatment (15 minutes) | ||
| Cranial Temprature (°C) | 36.9 ± 0.2 | 36.8 ± 0.2 |
| Rectal Temperature (°C) | 37.6 ± 0.1 | 37.5 ± 0.1 |
| After treatment (Day 1) | ||
| Rectal Temperature (°C) | 38.2 ± 0.4 | 38.1 ± 0.2 |
| Body Weight (g) | 273 ± 8 | 290 ± 4 |
| After treatment (Day 2) | ||
| Rectal Temperature (°C) | 37.2 ± 0.2 | 37.8 ± 0.2 |
| Body Weight (g) | 255 ± 10 | 278 ± 7 |
| After treatment (Day 3) | ||
| Rectal Temperature (°C) | 36.7 ± 0.3 | 37.6 ± 0.1 |
| Body Weight (g) | 246 ± 8 | 277 ± 8 |
| After treatment (Week 1) | ||
| Rectal Temperature (°C) | 37.4 ± 0.3 | 37.2 ± 0.3 |
| Body Weight (g) | 270 ± 11 | 297 ± 12 |
| After treatment (Week 2) | ||
| Rectal Temperature (°C) | 37.3 ± 0.5 | 37.7 ± 0.1 |
| Body Weight (g) | 312 ± 20 | 346 ± 12 |
| After treatment (Week 3) | ||
| Rectal Temperature (°C) | 37.6 ± 0.3 | 37.6 ± 0.2 |
| Body Weight (g) | 357 ± 23 | 383 ± 10 |
Values are mean ± SEM
MCAo; Middle cerebral artery occlusion
3.2. Behavioral tests
3.2.1. Composite neurologic score
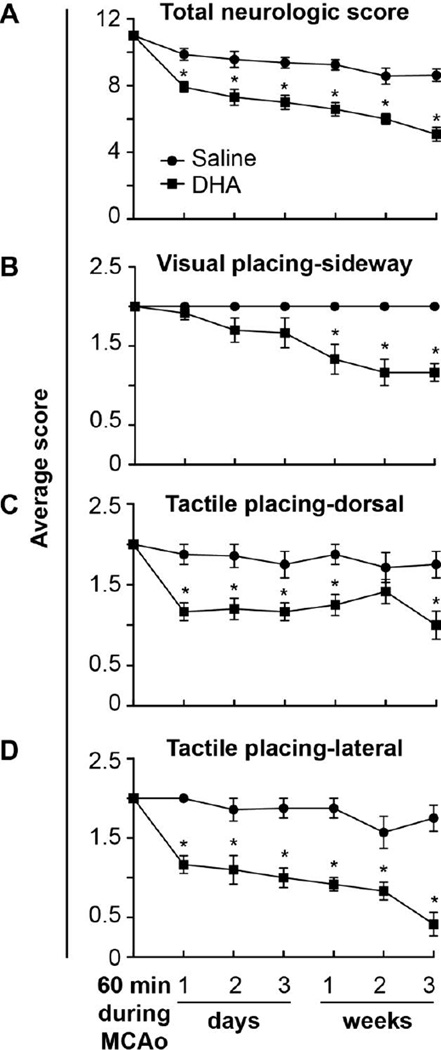
The saline-treated group showed a continuous severe composite neurologic score despite moderate improvement throughout the three-week survival period. Rats treated with DHA showed an improved composite neurologic score compared to the saline group during the three-week survival period (Fig 1A). Significant improvement of visual (sideway), tactile (dorsal and lateral) forelimb placing reaction was observed in DHA-treated rats during the three-week survival period compared to the vehicle group (Fig. 1B–D). Rats treated with DHA tended to have an improved postural reflex, visual placing-forward and proprioceptive reflex, but this did not reach statistical significance.
Figure 1.
Behavioral outcome after stroke. Panel A shows total neurologic score (normal = 0, maximal deficit = 12) during 60 min of MCAo and various time after MCAo. Panels B and D show time course of recovery of forelimb placing reactions to visual and tactile (dorsal and lateral) stimuli (normal = 0, maximal deficit = 2). DHA treatment improved total neurological score and placing reaction, compared to the saline-treated group. Values shown are means ± SEM (saline group, n=8 and DHA group, n=12) * significantly different from corresponding saline group (P < 0.05; repeated measures ANOVA followed by Bonferroni tests).
3.2.2. Rota-rod
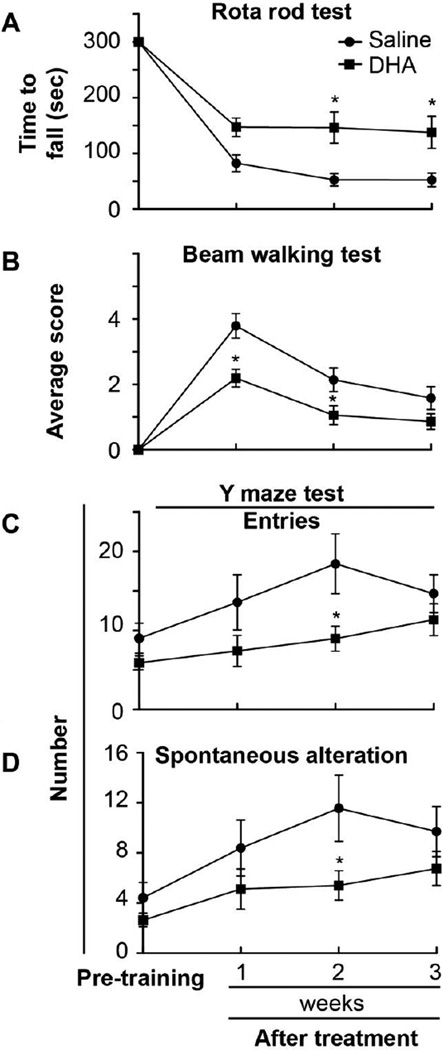
The rats treated with saline continued to show a decrease in the time it took to fall of the rota-rod during the three-week survival period. Treatment with DHA prolonged the time to fall throughout the three weeks, but was only statistically significant on week 2 and 3 compared to the saline-treated group (Fig 2A).
Figure 2.
Motor coordination, balancing and cognitive tests after stroke. Rota-rod (Panel A), beam walking (Panel B) and Y maze (Panels C and D) tests on weeks 1, 2, and 3 after stroke. Motor coordination, balance performance (normal = 0, maximal deficit = 6), and cogntive function are improved by DHA treatment compared to saline group. Data are means ± SEM (saline group, n=8 and DHA group, n=12). * significantly different from saline group (P <0.05, repeated measures ANOVA followed by Bonferroni tests)
3.2.3. Beam walking test
Vehicle-treated rats showed increased number of half and full slips on the contralateral (stroke) and ipsilateral sides on weeks 1, 2 and 3. DHA treatment lowered the number of full slips (0.2 ± 0.1 vs. 0.7 ± 0.3) and increased the number of half slips (0.6 ± 0.1 vs. 0.1 ± 0.1) on the ipsilateral side, compared to the saline-treated rats on week 1.There were no differences in the number of half and full slips between DHA- and saline-treated animals on the stroke-affected side throughout the three-week survival period. Balance performance was severely impaired in the vehicle group throughout the three-week survival period (Fig 2B). In contrast, rats treated with DHA showed significantly-improved balance performance compared to the saline group for weeks 1 and 2 (Fig 2B). Secondary analysis of the balance performance, comparing pre-training vs. week three time point, reviled, that the vehicle group was impaired throughout the three weeks survival period despite the spontaneous improvement. Contrary, the balance performance of DHA treated group was almost recovered to the level of pre-training period on week 3 (p < 0.01, One-way ANOVA followed by Dunnett’s test; Fig 2B).
3.2.4. Y maze
The number of entries, spontaneous alterations and percentage of alterations were increased in saline-treated rats on weeks 1, 2, and 3 (Fig 2C and D). Treatment with DHA lowered the number of entries and spontaneous alterations compared to saline-treated rats throughout the three-week survival period, but was only statistically significant on week 2 (Figs. 2C and D). There was no difference of percentage in alteration between the groups.
4. Ex vivo magnetic resonance imaging
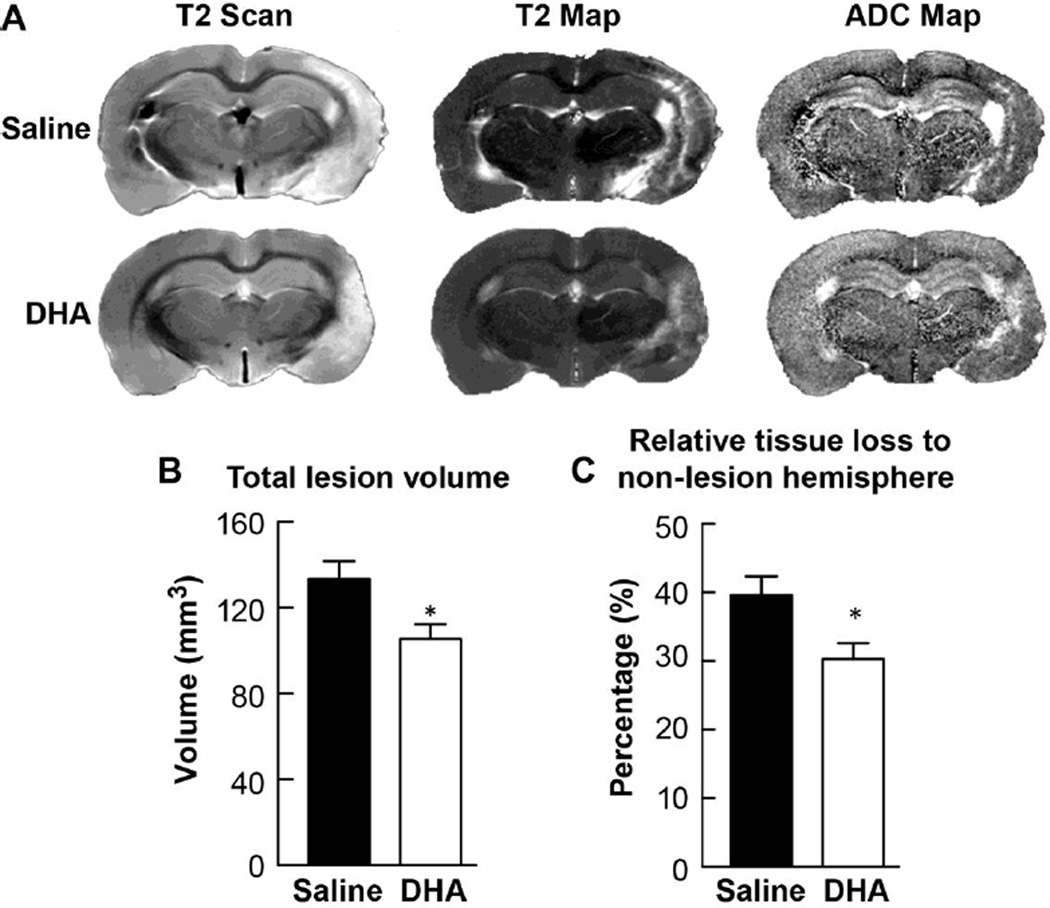
Representative T2WI and ADC maps from saline- and DHA-treated rats are presented in Figure 3A. T2 hyperintensities were observed in the cortex and striatum of saline-treated rat. In contrast, DHA-treated rat had a smaller lesion. ADC maps displayed no differences between groups. Analysis of T2WI data on week 3 demonstrated that treatment with DHA attenuated the lesion volume by 20.9 % (Fig 3B) and decreased the relative tissue loss to the non-lesion hemisphere by 23.4% (Fig 3C) compared to the saline-treated group. There were no differences in ventricle size and residual volumes in right hemisphere between groups on week 3.
Figure 3.
Ex vivo MRI at three weeks after stroke. Panel A shows representative reconstructed T2 and ADC maps of rat brains treated with DHA or saline. T2 hyperintensities were observed in the cortex and subcortex of the saline-treated rat. In contrast, the DHA-treated animal had smaller lesions that were mostly located in the cortical area. Panels B and C present total lesion volume and relative tissue loss, computed from T2WI. Significant reduction of total lesion volume and relative tissue loss to non-lesion hemisphere was present in the DHA-treated group compared to the corresponding saline group. Data are means ± SEM. (saline group, n=8 and DHA group, n=6). * significantly different from saline group (P< 0.05, repeated measures ANOVA followed by Bonferroni tests)
5. Histopathology
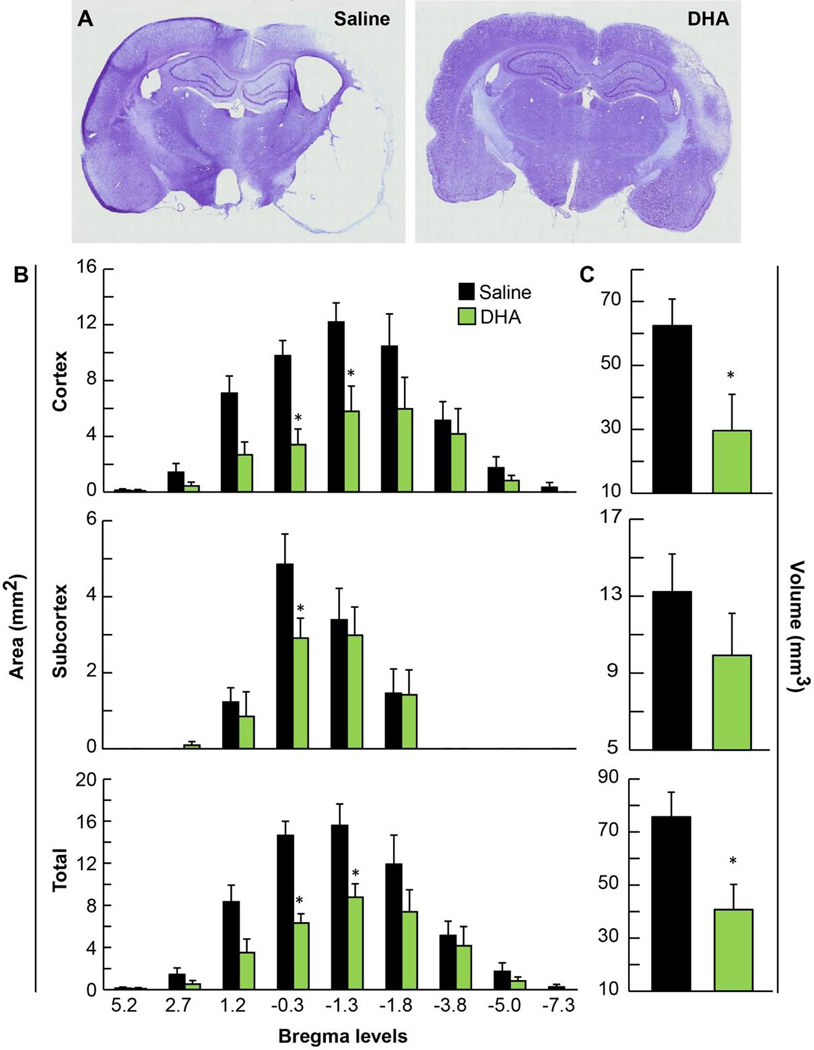
Large zones of infarct, involving cortical and subcortical regions from a saline-treated rat on week 3, are presented in Figure 4A. In contrast, smaller cortical and subcortical infarct areas were observed in the DHA-treated animal (Fig 4A). Treatment with DHA reduced cortical and total infarct areas at multiple coronal levels compared to saline treatment (Fig 4B). Maximal protection by DHA treatment occurred within the cortex (by 53.9 %), compared to saline treatment (Fig 4C). Also DHA treatment ameliorated subcortical infarct volume, but this amelioration was not significant. Total infarct volume was decreased by DHA treatment (by 46.2 %) compared to saline-treated group (Fig 4C). Six animals died during the experiment: 3 in the saline group (died on days 1 and 13) and 3 rats in the DHA group (died on days 1, 2 and 6).
Figure 4.
Histopathology at three weeks after stroke. Panel A shows representative computer generated Nissl-stained brain sections of rats treated with saline or DHA. The saline-treated rat shows extensive lesion with development of cavities in the cortex and subcortex. In contrast, the DHA-treated rat has a smaller infarction in the cortical and subcortical areas. Panels B and C present cortical, subcortical and total infarct areas and volumes. Significant reduction of cortical and total infarct areas and volumes were present in DHA-treated animals compared to the saline group. Data are means ± SEM. * significantly different from vehicle group (P< 0.05, repeated measures ANOVA followed by Bonferroni tests).
Discussion
The results of this study establish that systemic administration of DHA confers marked and enduring neuroprotection, as assessed together by neurobehavioral, ex vivo MRI and histological methods, in animals followed for three weeks after focal cerebral ischemia. Neurobehavioral improvement began within one day after insult and endured for three weeks. The ex vivo MRI and histological data in DHA-treated animals were concordant with the behavioral outcome. These results are very encouraging, because the neuroprotective efficacy shown with short survival periods may not necessarily persist during longer survival periods.
Recent research revealed that the omega-3 fatty acid found in fish oil decreases the risk of stroke and coronary heart disease [14]. DHA and its precursor, linolenic acid (18:3, n-3) are processed in the liver, which then delivers DHA to the central nervous system through the blood stream [15]. DHA is necessary to maintain proper physical conformation of ion channels, receptors and transporters in membranes in cells involved in memory, synaptic membrane biogenesis and function and neuroprotection [16]. After ischemic insult, free DHA, which is released from the cell membrane, leads to depletion of omega-3 polyunsaturated fatty acids in the brain. Depletion of DHA induces extensive damage of sensory, behavioral and cognitive function [17].
Neurobehavioral deficits are important indicators of stroke development in humans as well as in experimental cerebral ischemia models. Several behavioral tests have been applied to ischemia research in regards to clinical criteria. A recent report has called attention to the importance of long-term functional assessment after stroke to improve translation from bench to bedside [18]. Sensorimotor deficit, a distinguished aspect of neurological function, was accurately evaluated in our study by composite neurological score [6]. The applied battery consisted of two tests for evaluating upper body posture and assessing sensorimotor integration in forelimb placing responses to visual, tactile and proprioceptive stimuli [6]. Recently we demonstrated the improvement of the composite neurological function in animals treated with DHA, which survived for one week after focal cerebral ischemia [3–5]. The results from our study showed that DHA treatment significantly improved composite neurological score on day 1 (by 20%), day 2 (by 24%), day 3 (by 25%) and continued through week 1 (by 29%), week 2 (by 30%) and week 3 (by 41%) compared to the vehicle group.
In addition to the composite neurological score, we conducted other behavioral tests focused on motor coordination/integration, balance and cognitive function after focal cerebral ischemia. The rota-rod is a well-established method used to evaluate motor function because failure of coordination and integration of limb movement leads to animals falling off the rota-rod [19].
Beneficial effect of DHA treatment on locomotor activity was showed after global cerebral ischemia in gerbils (200 mg/kg, once a day for 10 weeks) [20] and neonatal hypoxic-ischemic brain injury in rats (maternal supplementation of DHA + eicosapentaenoic acid, 15 mg in every gram of regular diet, given from the second day of pregnancy to 14 days of parturition) [21]. Contrary, one study reported the detrimental effects of very low dose of DHA (500 nmol/kg, administered i.p. at 60 min after reperfusion) on the accelerated rota-rod performance after transient focal cerebral ischemia [22].
Our data demonstrated that intravenous administration of DHA (5 mg/kg, i.v.) prolonged the time to fall from the rota-rod with fixed speed by 178% on week 2 and by 163% on week 3. In addition, DHA treatment showed the improvement of the balance performance in the beam walking test by 42% on week 1 and by 51% on week 2 compared to the saline-treated group.
Stroke patients have twice the risk of developing dementia, resulting in vascular lesions, white matter changes or a combination of all of these [23]. A consistent dietary supplementation of DHA was found to be beneficial for memory function in short-term [24] and long-term survival in global cerebral ischemia [25–27],as well as in neonatal hypoxic brain injury models [23]. Our data demonstrates that DHA treatment improves cognitive function using a Y maze by lowering the number of entries by 51% and the number of spontaneous alterations by 53% compared to saline-treated rats on week 2.
Restoration of behavioral function is highly associated with the reduction of lesion volume. We had shown recently that DHA treatment significantly attenuates lesion volume accompanied with resolution of edema using in vivo MRI during a seven-day survival period after focal cerebral ischemia [3]. Independent work performed in the same period showed that DHA treatment also reduced lesion volume using in vivo MRI on day 1 after MCAo [28]. Our ex vivo T2WI data revealed that administration of DHA reduced total lesion volume and the relative tissue loss to the non-lesion hemisphere compared to saline treatment on week 3. Brain edema and water mobility were not different between groups on week 3 because most edema was resolved within seven days after stroke.
Our previous studies showed a significant decreases of infarct volume with DHA treatment in rats that survived for seven days after focal cerebral ischemia [3–5]. The histological analysis from our current study showed that treatment with DHA reduced cortical infarct by 54% and total infarct volumes by 46% on week 3.
The mechanism of DHA neuroprotection is still not completely understood. Previous studies showed that DHA salvages neurons and astrocytes within the infarcted areas [3, 4]. DHA rescues more neurons by protecting astrocytes, which have a critical role in the maintenance and protection of neurons through secretion of growth and neurotrophic factors. Also DHA downregulates activation of microglia in the infarcted regions [3]. Microglia-activated response to injury mediates intrinsic neuroinflammation that results during apoptosis and phagocytosis of debris [29]. There is evidence for an anti-inflammatory effect of DHA during ischemic injury [3]. Furthermore, DHA treatment emphasizes beneficial role in the cellular survival cascade after focal cerebral ischemic injury by upregulation of p 473 and p 308 Akt phosphorylation and S6 and pGSK in GSK signaling [5]. Pan et al. [31] suggested that suppression of cytotoxic factor production accompanied with promotion of pro-survival cascade mediated by ERK and / or Bcl-2 enhances neuroprotection by DHA pretreatment in focal cerebral ischemia. Along with the anti-inflammatory and pro-survival effect, the anti-oxidant effect of DHA was shown by a decrease of lipid peroxidation in ischemic injury using F2-isoprostanes, which is a reliable marker for oxidative stress [28]. Also consistent administration of Ethyl-DHA demonstrated an anti-oxidant property in a global ischemic gerbil model by free radical scavenging, inhibition of lipid peroxidation, prevention of GSH depletion and lowering of GSH-Px and CAT activity [20]. These mechanisms provide a possible explanation for restoration of behavioral functions and attenuation of lesions following cerebral ischemic insult.
In summary, this study demonstrates the persistence of neuroprotection by single intravenous administration of DHA with an improvement of behavioral outcomes accompanied by attenuation of lesions in chronic survival after experimental stroke. These results show great promise for translation as a therapeutic option in the treatment of stroke patients.
Acknowledgment
This study was supported by R01 NS046741 (NGB) and R01 NS065786 (LB) from the National Institute of Neurological Disorders and Stroke. We thank Kristal Atkins for technical assistance with behavioral tests; Sonny Kim and Kamalakar Ambadipudi for MRI acquisition and analysis assistance; Darlene Guillot and Ryan Labadens for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare there is no conflict of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan N. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Khoutorova L, Atkins K, Eady T, Hong S, Lu Y, et al. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl Stroke Res. 2011;2:33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat Model of transient focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG. Docosahexaenoic Acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One. 2012;7:e46151. doi: 10.1371/journal.pone.0046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD, Hsu CY. Middle cerebral artery occlusion in the rat by intraluminal suture: neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1623. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Pardridge WM. Blood–brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111:227–229. doi: 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Ohlsson A-L, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26:644–649. doi: 10.1161/01.str.26.4.644. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 10.Schallert T, Woodlee M, Fleming S. Disentangling multiple types of recovery from brain injury. Pharmacology of cerebral ischemia. 2002:201–216. [Google Scholar]
- 11.Metz GA. Behavioral testing in rodent models of stroke. Neuromethods. 2010;47:199–212. [Google Scholar]
- 12.Wahl F, Allix M, Plotkine M, Boulu R. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- 13.Konig JFR, Klippel RA. The Rat Brain, a Stereotaxic Atlas of the forebrain and lower parts of the brain stem. In: Lange B, Grunwald U, editors. Williams & Wilkins; 1963. with technical assistance from. [Google Scholar]
- 14.Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am J Clin Nutr. 2008;88:1618–1625. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott B, Bazan N. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci USA. 1989;8:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazan N. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;12:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 18.Freret T, Schumann-Bard P, Boulouard M, Bouet V. On the importance of long-term functional assessment after stroke to improve translation from bench to bedside. Exp Transl Stroke Med. 2011;3:1–5. doi: 10.1186/2040-7378-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkaya M, Endres M. Behavioral testing in mouse models of stroke. In: Dirnagl U, editor. Rodent Models of Stroke. Humana Press; 2010. pp. 179–197. [Google Scholar]
- 20.Cao D-H, Xu J-F, Xue R-H, Zheng W-F, Liu Z-L. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils. Pharmacol Biochem Behav. 2004;79:651–659. doi: 10.1016/j.pbb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 Polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic–ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D-Y, Pan H-C, Yen Y-J, Wang C-C, Chuang Y-H, Chen S-Y, et al. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28:1220–1229. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Leys D, Hénon H, Mackowiak-Cordoliani M-A, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 24.Okada M, Amamoto T, Tomonaga M, Kawachi A, Yazawa K, Mine K, et al. The chronic administration of docosahexaenoic acid reduces the spatial cognitive deficit following transient forebrain ischemia in rats. Neuroscience. 1996;71:17–25. doi: 10.1016/0306-4522(95)00427-0. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes JS, Mori MA, Ekuni R, Oliveira RMW, Milani H. Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutr Res. 2008;28:798–808. doi: 10.1016/j.nutres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Plamondon H, Roberge M-C. Dietary PUFA supplements reduce memory deficits but not CA1 ischemic injury in rats. Physiol Behav. 2008;95:492–500. doi: 10.1016/j.physbeh.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Correia Bacarin C, Mori MA, Dias Fiuza Ferreira E, Valério Romanini C, Weffort de Oliveira RM, Milani H. Fish oil provides robust and sustained memory recovery after cerebral ischemia: Influence of treatment regimen. Physiol Behav. 2013;119:61–71. doi: 10.1016/j.physbeh.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Chauveau F, Cho T-H, Perez M, Guichardant M, Riou A, Aguettaz P, et al. Brain-targeting form of docosahexaenoic acid for experimental stroke treatment: MRI evaluation and anti-oxidant impact. Curr Neurovasc Res. 2011;8:95–102. doi: 10.2174/156720211795495349. [DOI] [PubMed] [Google Scholar]
- 29.Danton GH, Dietrich WD. Inflammatory Mechanisms after Ischemia and Stroke. J NeuropatholExp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]