Abstract
Multiple studies have shown that sub-therapeutic appointment adherence and medication adherence are associated with worse clinical outcomes for people living with HIV disease. Thus, poor appointment and medication adherence diminish individual and community HIV control and transmission. Yet not enough is known about interventions that can improve retention in HIV care. The purpose of this study was to test an intervention to improve retention and/or medication adherence in a public clinic in the Deep South. One hundred participants with retention or medication adherence difficulties were randomized to either a six-month intervention or usual care, and followed longitudinally for one year. The intervention was multidimensional, based on the Information-Motivation-Behavioral Skills (IMB) Model. The intervention addressed information about HIV and the importance of retention/adherence, motivation to be retained and/or adhere to medications, and the behavioral skills needed to manage and maintain these healthy behaviors in a combination of face-to-face and telephone sessions. The proportion of those with at least one visit in each 4-month block (third) of the year increased in those with minimal exposure to the intervention (three out of eight intervention contacts) as compared to those with less intervention exposure (p = .098). Those with at least this minimal exposure averaged a significantly higher number of thirds that included a clinic visit as compared to those with less intervention exposure (p = .013). The intervention did not demonstrate a significant effect on medication adherence, though this is contradictory to a previous study testing a version of this intervention designed to address only medication adherence. Further study to increase uptake of the intervention is needed to increase its efficacy.
Keywords: HIV, medication adherence, patient care, retention, IMB
Introduction
Antiretroviral treatment (ART) for HIV has transformed HIV into a manageable, chronic disease (Lucas, Chaisson, & Moore, 1999) and may even offer additional benefits of controlling transmissibility of the virus to others (Cohen, McCauley, & Gamble, 2012). Although the first ART regimens were difficult to manage in many ways, current regimens are less toxic, have fewer pills, and less frequent dosing. Despite advances in availability and regimen burdens of ART, failure to adhere sufficiently to one's ART regimen continues to have dire consequences. Additionally, the influence of poor adherence to clinical care (poor retention) on morbidity and mortality has become well-recognized (Giordano et al., 2007; Mugavero, Hui-Yi Lin, et al., 2009).
Self-directed behaviors leading to positive health outcomes in people living with HIV (PLWH) vary considerably among patients enrolled in HIV care. The Deep South, considered to be the six Southeastern United States: Georgia, South Carolina, North Carolina, Alabama, Mississippi, and Louisiana (Whetten & Reif, 2006), has alarmingly high rates of delayed entry into HIV care, having less-than-optimal retention in clinical care and sub-optimal ART adherence. (Krawczyk, Funkhouser, Kilby, Kaslow, et al., 2006; Krawczyk, Funkhouser, Kilby, & Vermund, 2006; Mugavero, Lin, et al., 2009; Napravnik et al., 2006; Reif, Geonnotti, & Whetten, 2006; Reif, Whetten, Lowe, & Ostermann, 2006).
Treatment Adherence
While much work has been done in the past 20 years to improve support for medication adherence, an aspect of adherence that has only recently begun to be addressed in the literature is adherence to clinical care, otherwise known as retention. The problem of poor retention in HIV care has been found to be associated with poor health outcomes and mortality (Giordano et al., 2007; Giordano, Hartman, Gifford, Backus, & Morgan, 2009; Mugavero et al., 2007; Naar-King et al., 2007).
Information-Motivation-Behavioral Skills (IMB) Model
The Information-Motivation-Behavioral Skills (IMB) model for medication adherence was chosen as a conceptual framework for this research because of its attention to the multidimensional nature of adherence, as well as its attention to the impact of contextual variables, such as mental health issues and environmental issues that impact access and adherence. According to this model, information about HIV and medications interacts with an individual's motivation to take medications to influence the performance of behavioral skills related to adherence (Amico, 2011; Fisher, Fisher, Amico, & Harman, 2006; Fisher, Fisher, & Harman, 2003; Kalichman et al., 2001). In addition, contextual variables such as stigma, medication access, poverty, homelessness, substance abuse, and other environmental influences have been described as moderating the effect of the personal factors on adherence.
There are few intervention that have shown effectiveness in improving retention in HIV care but strategies are recommended (Thompson et al., 2012) and needed (Gardner, McLees, Steiner, del Rio, & Burman, 2011). To address this gap we developed a Motivational Interviewing (MI)-based intervention called the CLIMB intervention, CLIMB has been tested before in relation to adherence to HIV medications, and showed a moderate effect size in a small pilot study (Konkle-Parker, Erlen, Dubbert, & May, 2012) The purpose of this study was to test its effectiveness in retention in care as measured by the number of thirds of the year that contained a medical visit, three measures of medication adherence, and change in self-reported Information, Motivation, and Behavioral Skills levels for both phenomena.
Research Methods
Sample and Procedure
One hundred participants were recruited by convenience sampling at a large public clinic at a medical center in the southern region of the United States. This number of participants was chosen as a feasible number for an exploratory trial, not based on a power analysis. All patients who came to their clinic appointment for 11 months, or approximately 1200 patients, were screened until the target sample was enrolled. The study was approved by the University of Mississippi Medical Center (UMMC) Institutional Review Board, and participants signed an informed consent, and received a stipend for their time in data collection. To be eligible for participation, HIV-positive individuals who were 18 years or older must have had a documented medication adherence or retention problem.
Recruitment occurred between August 2009 and June 2010, with last follow-up in August 2011. Participants were randomized to control or experimental group using the Study360 (www.almedtrac.com) Study Management system, stratified by gender, race, and salient treatment adherence issue, (i.e. medication or visit adherence), in order to ensure adequate representation in both control and treatment groups. Participants who experienced both medication and appointment adherence problems were asked to identify the more problematic issue. In this sample, 52 (52%) considered visit adherence to be their primary adherence issue, but most considered both to be important issues, and therefore exploratory counseling in both adherence issues was done with all participants. Those in the control condition received only routine usual care, which in this clinic is directed by each clinical provider; those randomized to intervention received usual care as well.
Intervention
A clinical research coordinator trained in MI facilitated the intervention while receiving ongoing feedback from an MI expert. For quality assurance purposes, excerpts from a random sample of the face-to-face audio-recorded MI sessions were coded by an external MI expert using the MI Treatment Integrity (MITI 3.0). (Moyers, Martin, Manuel, Miller, & Ernst, 2007) Throughout the course of the study, the interventionist was consistently rated in a range from Beginner to Expert proficiency in Global Ratings and Behavior Counts.
The interventionist provided HIV education at a 6th grade reading level using an educational book created for this purpose in a one-on-one educational session that typically lasted 20-30 minutes though could be expanded according to the need of the participant. Personal motivation was addressed through MI. Social motivation was addressed through a video portraying other HIV-positive individuals discussing their own difficulties and successes related to retention and medication adherence. Behavioral skills were addressed through the distribution of adherence-enhancing devices, including pillboxes, reminder watches, and calendars for noting appointments, as well as training on patient-provider communication skills.
Based on previous work done by the first author (Konkle-Parker, Erlen, & Dubbert, 2010; Konkle-Parker et al., 2012), the interventions consisted of two face-to-face sessions and six telephone calls. Strategies used in the intervention are listed in Table 1.
Table 1.
Strategies incorporated in Intervention
| Information | Motivation | Behavioral Skills | |
|---|---|---|---|
| 1: Face to Face | Basic HIV education | a)rapport-building and setting the foundation for MI – participant personal goals, the impact of HIV and treatment adherence issue(s) on those goals, and identification of ideas and plans for change; b) introduction of the concept of a “Self Care Buddy,” described as a person of their choice who could work with them to manage their treatment adherence; |
a) distribution of adherence-enhancing devices which included pill boxes, watches with alarms, and pocket or wall calendars; b) problem-solving around the adherence barriers; c) education on patient-provider communication. |
| 2: Face to Face | c)a video that showed peers demonstrating medication and visit adherence strategies, to promote social motivation; d) feedback from data collected at baseline: self-reported depression, substance use, adherence, and Information, Motivation, and Behavioral Skills (IMB) regarding medication or visit adherence. e)most recent CD4 cell count and HIV RNA plasma viral load |
||
| 3 – 8 Telephone | Further exploration of current concerns with medicines and appointments, current challenges and supports for medication and visit adherence, anticipated plan of action for coping with adherence issues, patient-provider communication, and feedback from the most recent data collection and lab values. | ||
MI = Motivational Interviewing
Data Collection
Data were collected at clinic visits if possible, in order to facilitate participation. The baseline visit included enrollment and randomization; the second was 4-6 months after enrollment at approximately the end of the intervention, and the last data collection occurred at a clinic visit approximately 12 months after enrollment. Data were collected using an audio-supported computer-assisted self-interview (ACASI) in order to minimize social desirability bias, after instruction for participants who were not computer-literate. Table 2 describes the instruments used in data collection and sample items.
Table 2.
Data collection instruments
| INSTRUMENT | CHARACTERISTIC MEASURED | INSTRUMENT INFORMATION |
|---|---|---|
| Short Test of Functional Health Literacy Assessment (STOFHLA) Numeracy scale (Nurss, Parker, Williams, & Baker, 2001) | Health literacy | Participant reads a labeled medicine bottle and responds to questions such as, “If you take your first tablet at 7:00 a.m., when should you take the next one?” or reads an appointment card and respond to questions such as “When is your next appointment?” Fourteen questions answered correctly or not totaled 0 - 14, indicating low, marginal, and adequate health numeracy. |
| Sayles Internalized HIV stigma scale (Sayles et al., 2008) | Internalized HIV stigma | Participants were asked how frequently they felt stigmatized on a 5-point scale ranging from “None of the Time” to “All of the Time.” Sample items include “HIV is different than other diseases like cancer because people with HIV are judged” and “I am concerned that if I go to the HIV clinic someone I know might see me.” Cronbach's α = .86 in this sample. |
| Social Provision Scale (Cutrona & Russell, 1987) | Social support | Participants were asked about their agreement with 24 items on a 4-point scale ranging from “Strongly Disagree” to “Strongly Agree.” Sample items include “There is no one I can turn to in times of stress” and “No one needs me to take care of them.” Overall Cronbach's α =.66. Individual subscales ranged from α =.55 for Reliable Alliance to α =.74 for Guidance. |
| Alcohol Use Disorders Identification Test – Current (Adewuya, 2005) and Drug Use Disorders Identification Test – Current (Berman, Bergman, Palmstierna, Schlyter, & Daar, 2005) | Substance use | Sample questions include “How often do you have a drink containing alcohol?” and “Do you use more than one type of drug on the same occasion?” Responses ranged from 0 - 12 for the AUDIT-C and 0 – 16 for the DUDIT-C, with normed cut-off scores for hazardous drinking or drug use. |
| Patient Health Questionnaire Depression Scale (Spitzer, Kroenke, Williams, & Patient Health Questionnaire Primary Care Study Group, 1999) | Depressive symptoms | Sample symptoms include “Little interest or pleasure in doing things” and Trouble concentrating on things, such as reading the newspaper or watching television.” Scores range from 0 to 36 and were used to compute presence or absence of major depression according to diagnostic criteria. |
| CASE Adherence rating scale (Mannheimer et al., 2006) | Medication adherence | Rated on a 6-point rating scale |
| Visual analogue scale of prescribed ARV medicines taken in the previous month (Amico et al., 2006) | Medication adherence | Scores ranged from 0 - 100 |
| LW-IMB-AAQ (Center for Health Intervention and Prevention, 2007) | Information, Motivation, and Behavioral Skills (IMB) deficits for medication adherence | LW-IMB-AAQ used average scores for each domain, measuring agreement with statements on a 5-point scale, ranging from “Strongly Agree” to “Strongly Disagree.” Sample items included “Skipping a few of my HIV medications from time to time would not really hurt my health” and “I am worried that the HIV medications I have been prescribed will hurt my health.” Cronbach's α = .65 for Information, .79 for Motivation, and .87 for Behavioral Skills, |
| IMB Engagement in Care Questionnaire (unpublished) | Adherence to care | The IMB Engagement In Care measure was developed for this study by the first two authors, using an adaptation of the previously validated LW-IMB-AAQ. It used summed scores in each domain, scoring from 5-15 for Information, 5-50 for Motivation, and 5-50 for Behavioral Skills. Sample items included “I know how important it is to go to my HIV clinic appointment every 3-4 months, even if I feel good” and “I worry about other people seeing me going into the clinic or waiting there for my appointment.” Cronbach's α = .47 for Information, .60 for Motivation, and .82 for Behavioral Skills in this sample. |
| Electronic Medical Record (EMR) | Visit attendance | Kept visits |
| EMR | CD4 and HIV RNA viral load | |
| Pharmacy refill | Medication Adherence by refill | Date of refill at pharmacies, excluding automatic shipments |
A total of 416 tracked clinic visits were evaluated during the year prior to the study and 441 during the year on study. Visits were characterized in thirds for the year pre- and post-enrollment, corresponding to 1-122 days, 123-243 days, and 244-366 days pre- or post-enrollment.
Data Analysis
Two primary outcomes were evaluated: adherence to care, or visit constancy, while on study (defined as at least one kept HIV medical visit in each third of the year following baseline assessment) and ART adherence (VAS scores of 90 or greater; pharmacy refill estimated 90% or greater adherence [days covered] for the full year post baseline). Effects of CLIMB were evaluated first using randomly assigned study arm (CLIMB vs control), followed by an evaluation of the effect of having received at least 3 intervention sessions (otherwise known as minimal exposure), vs 0 – 2 intervention sessions. Analyses on visit constancy used independent groups ANCOVA on number of thirds of the year in which an HIV appointment occurred during the year on study while controlling for pre-intervention adherence to HIV visits in thirds, and logistic regression to evaluate visit constancy on study. These analyses were conducted for assigned study arm and specific to those who received minimL exposure versus those who did not. Despite normalcy in the distribution of visit constancy, because this variable is essentially a count of events, we confirmed ANCOVA results with generalized estimation equations using the same predictors but specifying a Poisson distribution and AR(1) structure.
Analyses on self-reported adherence used generalized linear modeling with robust estimators of optimal adherence at each of the three assessment intervals examining CLIMB versus control and minimal exposure to CLIMB versus less than that. Pharmacy refill-based optimal adherence was evaluated using logistic regression. All analyses were conducted in SPSS v18.0.
Results
Participant Characteristics
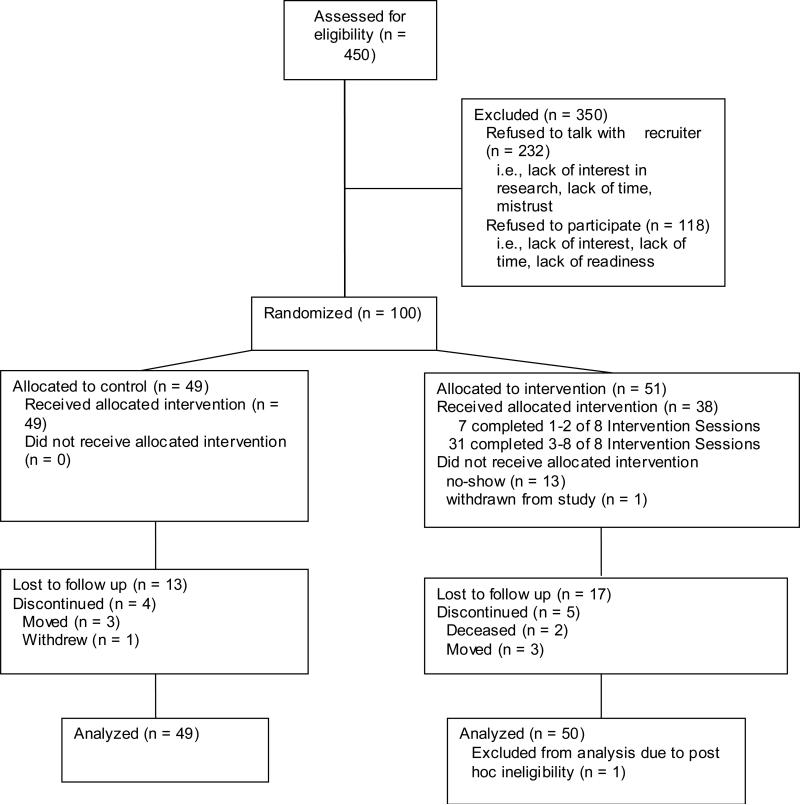
Of the 450 patients eligible for the study, 218 agreed to talk with the recruiter (See Figure 1). From this pool, 100 were enrolled in the study, with 49 randomized to control and 51 to experimental group. Reasons for non-involvement primarily were lack of time, lack of interest in being involved in research, and lack of interest in addressing their adherence issue. One experimental participant was subsequently dis-enrolled due to an unrelated issue, arriving at 99 in the sample pool. Of these, 49 (49%) were male. Ninety (90%) were African American, and 52 (52%) reported visit adherence as their most salient adherence issue.
Figure 1.
Recruitment and Retention Patterns of Study Participants
As indicated in Table 3, over a quarter of the sample scored in the major-depression range (29%) and almost half were categorized as having “hazardous” level of drinking (42%). At baseline, study arms differed in report of any current drug use, with 51% of those in the control arm and 22% of those in the intervention arm reporting use (X2(n=92) 8.81, p=.003). Pre-intervention retention from clinic records indicated that 61% (n=60) did not have at least one visit in each third of the year prior to baseline (53% of those in the control arm and 68% of those in the intervention arm). For baseline medication adherence, according to VAS scores dichotomized to 90% or greater, 65% reported less than 90% adherence, which did significantly differ at baseline between study arms (76% with <90% adherence in the control condition vs 57% reporting this in the intervention condition; X2(n=95) 3.96, p=.046). Study completion variables, such as number of assessments completed, did not significantly differ by arm. Overall retention for all measurement occasions (a total of three) was 40%, with 70% providing at least one follow-up measure. All analyses presented below were repeated using any drug use as a control variable and did not change the profile of results.
Table 3.
Difference between groups at baseline
| Full Sample (99) | Control Arm (50) | Intervention Arm (49) | p for Difference between arms at baseline | Less than 3 sessions (72) | 3 or more sessions (27) | p for Difference between exposure groups at baseline | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female | 51% (51) | 56% (26) | 47% (25) | .353 | 47% (34) | 63% (17) | .163 |
| Male | 48% (48) | 44% (20) | 53% (28) | 53% (38) | 37% (11) | ||
| Age M (SD) | 37.44 (8.96) | 37.85 (9.18) | 37.09 (8.83) | .679 | 36.56 (9.01) | 39.81 (8.52) | .11 |
| Of Color | 92% (91) | 94% (32) | 91% (48) | .629 | 92% (66) | 7% (5) | .826 |
| Major Depression | 29% (29) | 30% (14) | 28% (15) | .816 | 28% (20) | 33% (9) | .589 |
| Hazardous Drinking | 42% (42) | 44% (20) | 42% (22) | .843 | 40% (29) | 48% (13) | .480 |
| Any reported drug use | 35% (32) | 51% (21) | 22% (11) | .003 | 43% (29) | 12% (3) | .006a |
| ≥90 Adherence by VAS | 35% (33) | 24% (10) | 43%(23) | .046 | 37% (25) | 30% (8) | .510 |
| A visit in each third of year prior to baseline | 39% (39) | 46% (21) | 34% (18) | .235 | 42% (30) | 33% (9) | .450 |
| Number of thirds with HIV care in year prior to baseline | 2.07 (.85) | 2.13 (.88) | 2.02 (.82) | .651 | 2.08 (.89) | 2.04 (.81) | .810 |
| Assessments completed | |||||||
| Baseline only | 29 (29%) | 33% (15) | 26% (14) | 38% (27) | 7% (2) | ||
| Baseline plus 1 | 30% (30) | 22% (10) | 38% (20) | .224 | 31% (22) | 30% (8) | .005 |
| Baseline plus 2 | 40% (40) | 46% (21) | 36% (19) | 32% (23) | 63% (17) |
Fisher exact p
Nine (9%) participants withdrew from the study for reasons such as death, re-location to another city, state, or clinic and 25 (25%) were lost to follow-up. Thirty-three (33%) missed the second data collection (V=2), and 25 (25%) missed the third data collection visit. For those randomized to CLIMB, 19 (37%) completed 0-2 intervention sessions, and 31 (61%) completed 3-8 intervention sessions, which the research team considered to be minimal exposure, including both face-to-face sessions and at least one telephone session.
Visit Constancy
Evaluation of visit constancy for the year following baseline assessment indicated that randomization to intervention condition did not significantly predict constancy defined dichotomously. Analyses of those with minimal CLIMB exposure demonstrated a trend towards a greater proportion of individuals with 100% visit constancy on study while controlling for pre-study constancy rates (p = .098). Thirty percent of participants across both control and intervention arms with minimal exposure as compared to only 15% of those who had less exposure had 100% visit constancy. The difference in the continuous measure (number of thirds in which an HIV care appointment was kept) was significant between the minimal exposure group and those with less exposure controlling for pre-intervention constancy (p = .013). As indicated in Table 4, all groups generally deteriorated on the constancy measures, however those with at least minimal exposure deteriorated less than those who did not. The results of these analyses were similar when controlling for the identified baseline non-equivalence variable (any drug use at baseline) and also if analyses focused exclusively on the year on study without controlling for retention in the prior year. The results were similarly significant when using GEE with Poisson distribution.
Table 4.
Outcomes for Retention in HIV care for year on study
| Condition | Had a visit in each third of prior year | Had a visit in each third of year on study | B | SE | Exp(B) | 95% CI | p |
|---|---|---|---|---|---|---|---|
| Control (n=49) | 46% (21) | 20% (10) | −.263 | .516 | .769 | [.280, 2.115] | .611 |
| Intervention (n=50) | 34% (18) | 18% (9) | |||||
| < 3 intervention contacts (n= 72) | 42% (30) | 15% (11) | .898 | .541 | 2.456 | [.851, 7.084] | .096 |
| ≥ 3 intervention contacts (n=27) | 33% (9) | 30% (8) |
| Condition | Mean (SD) # visits in prior year | Mean (SD) # visits in year on study | F | df | p |
|---|---|---|---|---|---|
| Control (n=46) | 2.13 (.88) | 1.54 (1.09) | .104 | 1,99 | .748 |
| Intervention (n=53) | 2.02 (.82) | 1.58 (0.95) | |||
| < 3 intervention contacts (n= 72) | 2.08 (.89) | 1.42 (1.03) | 6.409 | 1,99 | .013* |
| > 3 intervention contacts (n=27) | 2.04 (.81) | 1.96 (.85) |
P < .05
ART Adherence
Self-reported ART adherence by VAS over the three assessments (Table 5) did not significantly differ by study arm, with each arm generally reducing adherence over time, or by intervention exposre. This was also the case based on pharmacy refill data that was available for 69 participants (Table 6). Analyses repeated for the adherence rating scale suggested the same general profile of results.
Table 5.
Self-reported VAS Medication Adherence over Time
| Condition | ≥90% Adherence* | B | SE | Wald | 95% CI | p | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6m | 12m | ||||||
| Control (n=49) | 71% | 65% | 64% | .387 | .7437 | .271 | [−1.070, 1.845] | .602 |
| Intervention (n=50) | 60% | 49% | 58% | |||||
| < 3 intervention contacts (n= 72) | 63% | 57% | 61% | .068 | .7609 | .008 | [−1.423, 1.559] | .929 |
| ≥ 3 intervention contacts (n=27) | 70% | 54% | 60% | |||||
Estimated proportions from generalized linear model
Table 6.
Pharmacy refills on study (n = 69)
| Condition | Optimal Refill Adherence (≥90%) | X2 | p | Exp(B) | 95% CI |
|---|---|---|---|---|---|
| Control (n=40) | 12% (5) | .163 | .686a | 1.289 | [.375, 4.439] |
| Intervention (n=45) | 16% (7) | ||||
| < 3 intervention contacts (n= 62) | 16% (10) | .765 | .382b | .495 | [.100, 2.454] |
| ≥ 3 intervention contacts (n=23) | 9% (2) | ||||
Fisher exact= 0.762
Fisher exact= 0.499
Discussion
The purpose of this study was to pilot test an intervention based on the Information-Motivation-Behavioral Skills (IMB) model for its effectiveness in improving adherence to HIV clinical care and HIV medications in a sample of individuals who had shown difficulty with one or both of these issues. The intervention was designed to be one that could be replicated in a poorly resourced clinical setting as it involved only two face-to-face one-on-one sessions and six telephone contacts with a trained interventionist . The uptake of the intervention was variable, with one-third of the participants randomized to intervention taking part in only 0-2 contacts, one-third in 3-5 contacts, and one-third in 6-8 contacts. The minimal dose to have exposure to all the important aspects of the intervention was determined to be three out of the eight contacts. Those participants who had a minimal dose tended to have a higher proportion who were fully retained in care as defined by having a clinical visit in all of the 4-month blocks of the year on study as compared to those individuals who had less exposure. In addition, those who experienced the minimal exposure had significantly less deterioration of retention than those who did not have that amount of exposure. There was no increase in medication adherence, however; contrary to the results of previous work testing this intervention in a sample of individuals who had recently started or restarted HIV medication therapy, where there was a trend toward improved medication adherence (Konkle-Parker et al., 2012).
Visit Constancy
This intervention appeared to make a difference in retention, when the participant engaged in a sufficient dose. Although visit constancy did not increase from baseline, it stayed virtually the same in those who engaged in a minimal dose of the intervention, and declined markedly in those who did not. Only 61% of those randomized to intervention obtained the minimal dose level
Medication adherence
The intervention, even at a minimal dose level, did not demonstrate a significant effect on medication adherence in this sample of individuals with pre-existing medication adherence and/or retention problems, measured by the Adherence Rating Scale, VAS, or pharmacy refill count. This result needs more exploration to determine the reasons for these contradictory findings compared to a previous trial (Konkle-Parker et al., 2012). In that trial, a nurse practitioner with experience in HIV carried out the intervention, and the study targeted individuals who newly started or restarted HIV medicines rather than the chronically non-adherent.
The participants in the sample were individuals who experienced adherence and retention difficulties, some for a very long time, and many with very disordered lives and multiple priorities other than caring for their HIV disease. One can speculate that there was an important subsection of this sample of individuals who were not in a stage of readiness to change, and that the intervention did little to advance them from that position to one where they were more ready/able to change to more adherent behavior. Indeed, the originators of the IMB model recognized that the model would not hold up in situations where modifying variables were present, such as severe mental illness, lack of access to the medications, or substance abuse. This research team would suggest that those with multiple competing priorities such as food insecurity, inconsistent shelter, children in great need, and seeking stability in a chaotic life may make HIV disease management a low priority, and the utilization of a focus on individual information, motivation, and behavioral skills may not be sufficiently salient to show an impact.
Limitations
There are several important limitations to this study. These include the small sample of 99 participants with pre-existing medication adherence and retention problems from a single clinic at an academic medical center in the Deep South. As previously mentioned, the sample size was chosen for feasibility, not for sufficient power, and thus significance of findings may have been missed or underestimated.
These findings may not be generalizable to individuals at a different stage in disease management, in a different setting, or a different region of the country. In addition, the intervention was delivered by a single interventionist. As such, we are unable to disentangle effects emanating from the interventionist from effects of the intervention.
As with any research study, the Hawthorne effect caused simply by being in a research study could have improved visit adherence, especially since the intervention group had more sustained contact with research staff than the control group.
Implications
An individual-level intervention, given initially in person and then continued by telephone, can be successful in preventing deterioration of retention to HIV care, if the participant engages sufficiently. In addition, seeking out individuals who have a readiness to change so that they are able to utilize the concepts of the intervention may also improve its efficacy. The staff time outlay makes the design of this intervention replicable in many low-resourced clinics, though the necessary MI training and continued training and reinforcement of the principles may be out of reach in some circumstances. Targeting the intervention on those who are ready to change may improve the efficiency of the efforts.
Because of the lack of efficacy in medication adherence, and the significant time commitment to training and reinforcement required in MI, this intervention would benefit from additional aspects to increase its value. Considerations could include using Short Messaging Service (SMS) text messages for outreach and patient-centered messages to support phone or face-to-face interactions. In addition, a clinic is already using MI for other purposes would benefit from this intervention structure without much additional outlay of resources.
Acknowledgements
This research was funded by a grant from the National Institute of Mental Health, R34MH84670
REFERENCES
- Adewuya AO. Validation of the Alcohol Use Disorders Identification Test (AUDIT) as a screening tool for alcohol-related problems among Nigerian university students. Alcohol and alcoholism. 2005;40(6):575–577. doi: 10.1093/alcalc/agh197. [DOI] [PubMed] [Google Scholar]
- Amico KR. A situated-Information Motivation Behavioral Skills Model of Care Initiation and Maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. Journal of Health Psychology. 2011 doi: 10.1177/1359105311398727. doi: 10.1177/1359105311398727. [DOI] [PubMed] [Google Scholar]
- Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, Fisher JD. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- Amico KR, Konkle-Parker DJ. IMB Engagement In Care Instrument. 2007 unpublished instrument. [Google Scholar]
- Berman AH, Bergman H, Palmstierna T, Schlyter F, Daar ES. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in Criminal Justice and Detoxification Settings and in a Swedish Population Sample. Eur Addict Res. 2005;1:22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- Center for Health Intervention and Prevention The LifeWindows Information- Motivation-Behavioral Skills ART Adherence Questionnaire (LW-IMB-AAQ) 2007 Retrieved from http://www.chip.uconn.edu/int/F_LWIMBARTQuestionnaire.pdf.
- Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS. 2012;7(2):99–105. doi: 10.1097/COH.0b013e32834f5cf2. doi: 10.1097/COH.0b013e32834f5cf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. In: Jones WH, Perlman D, editors. Advances in personal relationships. JAI Press; Greenwich, CT: 1987. pp. 37–67. [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman J. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Fisher WA, Fisher JD, Harman J. The Information-Motivation-Behavioral Skills Model: A General Social Psychological approach to understanding and promoting health behavior. In: Suls J, Wallston KA, editors. Social Psychological Foundations of Health and Illness. Blackwell Publishing; Malden, MA: 2003. pp. 82–106. [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Gifford AL, White AC, Jr., Suarez-Almazor ME, Rabeneck L, Hartman C, Morgan RO. Retention in care: a challenge to survival with HIV infection. Clinical Infectious Diseases. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. [Evaluation Studies Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. HIV Clinical Trials. 2004;5(2):74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of Retention in HIV Care Among a National Cohort of US Veterans. HIV Clinical Trials. 2009;10(5):299–305. doi: 10.1310/hct1005-299. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Rompa D, DiFonzo K, Simpson D, Austin J, Luke W, Buckles J. HIV treatment adherence in women living with HIV/AIDS: research based on the Information-Motivation-Behavioral Skills model of health behavior. Journal of the Association of Nurses in AIDS Care. 2001;12(4):58–67. doi: 10.1016/S1055-3290(06)60217-3. [DOI] [PubMed] [Google Scholar]
- Konkle-Parker DJ, Amico KR, Henderson HM. Barriers and Facilitators to Engagement in HIV Clinical Care in the Deep South: Results From Semi-Structured Patient Interviews. Journal of the Association of Nurses in AIDS care. 2011;22(2):90–99. doi: 10.1016/j.jana.2010.06.002. doi: 10.1016/j.jana.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker DJ, Erlen JA, Dubbert PM. Lessons learned from an HIV adherence pilot study in the Deep South. Patient Education and Counseling. 2010;78(1):91–96. doi: 10.1016/j.pec.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the Deep South. J Am Acad Nurse Pract. 2012;24(8):488–498. doi: 10.1111/j.1745-7599.2012.00712.x. doi: 10.1111/j.1745-7599.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. Southern Medical Journal. 2006;99(5):472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby JM, Vermund SH. Delayed access to HIV diagnosis and care: Special concerns for the Southern United States. AIDS Care. 2006;18(Supplement 1):S35–S44. doi: 10.1080/09540120600839280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. [Research Support, U.S. Gov't, P.H.S.]. Annals of Internal Medicine. 1999;131(2):81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- Mannheimer SB, Mukherjee R, Hirschhorn LR, Dougherty J, Celano SA, Ciccarone D, Finkelstein R. The CASE adherence index: A novel method for measuring adherence to antiretroviral therapy. [Multicenter Study Research Support, U.S. Gov't, P.H.S.]. AIDS Care. 2006;18(7):853–861. doi: 10.1080/09540120500465160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.0 (MITI 3.0) University of New Mexico Center on Alcoholism, Substance Abuse and Addictions (CASAA); 2007. [Google Scholar]
- Mugavero M, Hui-Yi Lin, Willig JH, Westfall AO, Ulett KB, Routman JS, Allison JJ. Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clinical Infectious Diseases. 2009;48:248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero M, Lin H-Y, Allison JJ, Giordano TP, Willig JH, Raper JL, Saag MS. Racial disparities in HIV virologic failure: Do missed visits matter? Journal of Acquired Immune Deficiency Syndrome. 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero M, Lin H-Y, Allison JJ, Willig JH, Chang P-W, Marler M, Saag MS. Failure to establish HIV care: characterizing the “no show” phenomenon. Clinical Infectious Diseases. 2007;45(1):127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care and STDs. 2007;21(Supplement 1):S–40-48. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- Napravnik S, Eron JJ, Jr., McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(Suppl 1):S45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- Nurss JR, Parker RM, Williams MV, Baker DW. TOFHLA: Test of Functional Health Literacy in Adults. Second ed. Peppercorn Books & Press; Snow Camp, NC: 2001. [Google Scholar]
- Reif S, Geonnotti KL, Whetten K. HIV infection and AIDS in the Deep South. American Journal of Public Health. 2006;96(6):970–973. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Whetten K, Lowe K, Ostermann J. Association of unmet needs for support services with medication use and adherence among HIV-infected individuals in the southeastern United States. AIDS Care. 2006;18(4):277–283. doi: 10.1080/09540120500161868. [DOI] [PubMed] [Google Scholar]
- Rumptz MH, Tobias C, Rajabiun S, Bradford J, Cabral H, Young R, Cunningham WE. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care and STDs. 2007;21(Supplement 1):S–30-39. doi: 10.1089/apc.2007.9989. [DOI] [PubMed] [Google Scholar]
- Sayles JN, Hays RD, Sarkisian CA, Mahajan AP, Spritzer KL, Cunningham WE. Development and psychometric assessment of a multidimensional measure of internalized HIV stigma in a sample of HIV-positive adults. AIDS and Behavior. 2008;12(5):748–758. doi: 10.1007/s10461-008-9375-3. doi: http://dx.doi.org/10.1007/s10461-008-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Patient Health Questionnaire Primary Care Study Group Validation and Utility of a Self-report Version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Nachega JB. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Annals of Internal Medicine. 2012:E–419. doi: 10.7326/0003-4819-156-11-201206050-00419. doi: 10.1059/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, BG G. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- Whetten K, Reif S. Overview: HIV/AIDS in the deep south region of the United States. AIDS Care. 2006;18(Suppl 1):S1–5. doi: 10.1080/09540120600838480. [Editorial] [DOI] [PubMed] [Google Scholar]
- Whetten K, Reif S, Lowe K, Eldred L. Gender differences in knowledge and perceptions of HIV resources among individuals living with HIV in the Southeast. Southern Medical Journal. 2004;97(4):342–349. doi: 10.1097/01.SMJ.0000118902.64603.A5. [DOI] [PubMed] [Google Scholar]
- Williams B, Amico KR, Konkle-Parker DJ. Qualitative Assessment of Barriers and Facilitators to HIV Treatment. Journal of the Association of Nurses in AIDS Care. 2011;22(4):307–312. doi: 10.1016/j.jana.2010.11.001. doi: 10.1016/j.jana.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]