Abstract
Background
Cockroach allergy is a key contributor to asthma morbidity in children living in urban environments.
Objective
We sought to document immune responses to cockroach allergen and provide direction for the development of immunotherapy for cockroach allergy.
Methods
Four pilot studies were conducted: (1) an open-label study to assess the safety of cockroach sublingual immunotherapy (SLIT) in adults and children; (2) a randomized, double-blind biomarker study of cockroach SLIT versus placebo in adults; (3) a randomized, double-blind biomarker study of 2 doses of cockroach SLIT versus placebo in children; and (4) an open-label safety and biomarker study of cockroach subcutaneous immunotherapy (SCIT) in adults.
Results
The adult SLIT trial (n = 54; age, 18–54 years) found a significantly greater increase in cockroach-specific IgE levels between the active and placebo groups (geometric mean ratio, 1.92; P < .0001) and a trend toward increased cockroach-specific IgG4 levels in actively treated subjects (P = .09) but no evidence of functional blocking antibody response. The pediatric SLIT trial (n = 99; age, 5–17 years) found significant differences in IgE, IgG, and IgG4 responses between both active groups and the placebo group but no consistent differences between the high- and low-dose groups. In the SCIT study the treatment resulted in significant changes from baseline in cockroach IgE, IgG4, and blocking antibody levels. The safety profile of cockroach immunotherapy was reassuring in all studies.
Conclusions
The administration of cockroach allergen by means of SCIT is immunologically more active than SLIT, especially with regard to IgG4 levels and blocking antibody responses. No safety concerns were raised in any age group. These pilot studies suggest that immunotherapy with cockroach allergen is more likely to be effective with SCIT.
Keywords: Cockroach, immunotherapy, sublingual immunotherapy, subcutaneous immunotherapy, inner city asthma
It has been convincingly demonstrated over the past 2 decades that the combination of cockroach allergy and cockroach exposure is one of the most important factors contributing to the high morbidity seen in inner-city children with asthma.1,2 Consequently, one of the major initiatives of the National Institute of Allergy and Infectious Diseases–sponsored Inner-City Asthma Consortium (ICAC) has been to develop treatment strategies that target cockroach allergy as an immune-based therapeutic approach to asthma. To accomplish this goal, a standardization trial of German cockroach allergen extracts compared 3 cockroach extracts to establish biological potency and to determine an optimal surrogate in vitro test of biological potency.3 An eventual ICAC goal is to conduct multicenter efficacy trials of cockroach immunotherapy for inner-city asthma. Treatment of children with asthma living in the inner city poses a number of significant risks, one of which is the potential for anaphylaxis during immunotherapy. Given these concerns, sublingual immunotherapy (SLIT) has been the focus of this program because of the growing body of literature supporting its efficacy and safety profile with other common allergens.4–6 However, before a definitive trial could be designed and implemented, it was deemed essential to gather data on the safety of cockroach SLIT, as well as on the dose and route of administration needed to achieve the greatest likelihood of efficacy. In addition, the ICAC has chosen to examine how the SLIT approach would compare with subcutaneous immunotherapy (SCIT), at least at the level of immunologic activity. To that end, 4 pilot clinical trials have now been conducted and involve a total of 190 children and adults. We now report the findings of these 4 phased studies, focusing on both safety and the capacity of SLIT and SCIT to generate immune responses and the direction for future trials.
RESULTS
SCSS
Of the 9 adult SCSS participants, 7 reported adverse events that were likely or possibly treatment related. Reported symptoms included oral or throat pruritus in 5, nonurticarial rash in 3, and nausea in 1. All were graded as mild or moderate except for 1 subject who graded throat irritation and cough as severe on days 2 and 3. No symptoms required treatment or adjustments in dosing.
In the 8- to 17-year-old group, 2 of the 9 participants were treated with an antihistamine for oral pruritus, but none required dosing adjustments. One participant opted to discontinue treatment after experiencing a reaction following her first maintenance dose. This was a 16-year-old girl who tolerated the dose escalation on day 1 but experienced oral pruritus, increased salivation, and a single episode of vomiting after taking the same dose on the second day.9 Treatment was not required, and all symptoms completely resolved within minutes.
In the 5- to 7-year-old group, one of the 9 participants reported increased nasal congestion at the completion of treatment, 1 reported mild pruritus of his nose and eyes during dose escalation, and a third had a single facial hive and erythema on his neck during dose escalation. After treatment with cetirizine, the skin manifestations resolved. He returned the next day to complete the dose escalation and the 2-week treatment period without incident.
Adherence, as assessed based on home diaries and measurement of the amount of residual extract returned, appeared good, aside from 1 adult who returned a vial with less residual extract than anticipated and 2 pediatric subjects who returned vials with more extract than expected.
BioCSI
Fifty-four subjects were enrolled, including 38 female and 16 male subjects (age range, 18–54 years; Table II). Cockroach skin test wheals ranged from 3 to 12 mm (median, 6 mm), and cockroach-specific IgE levels ranged from 0.36 to 150 kU/L (median, 2 kU/L). Twenty-eight subjects were randomized to active treatment, and 26 were randomized to placebo. There were no significant baseline differences between the groups, except for lower cockroach IgG4 levels in the placebo group (Table II). Forty-one subjects completed the entire study, with 4 lost to follow-up, 8 discontinued because of noncompliance with visits or dosing, and 1 discontinued because of a possible adverse reaction (a placebo-treated subject).
TABLE II.
BioCSI and BioCSI2 demographic and baseline characteristics
| BioCSI
|
BioCSI2
|
||||
|---|---|---|---|---|---|
| Placebo | Extract | Extract, high dose | Extract, low dose | Placebo | |
| No. of participants | 26 | 28 | 30 | 31 | 28 |
|
| |||||
| Deactivated from study | 3 (10%) | 3 (10%) | 7 (25%) | ||
|
| |||||
| Female sex | 17 (65%) | 21 (75%) | 9 (30%) | 9 (29%) | 8 (29%) |
|
| |||||
| Age (y) | 36 (18–54) | 27.5 (18–50) | 10 (4–17) | 11 (5–17) | 11 (5–17) |
|
| |||||
| Diagnosis of asthma | 18 (69%) | 20 (71%) | 25 (83%) | 28 (90%) | 23 (82%) |
|
| |||||
| Diagnosis of rhinitis | 24 (92%) | 22 (79%) | 20 (67%) | 21 (68%) | 23 (82%) |
|
| |||||
| Cockroach-specific IgE (kU/L) | 2.9 (0.4–113.3) | 1.9 (0.4–150) | 6.7 (0.4–349) | 4.9 (0.44–174) | 9.2 (0.41–98.6) |
|
| |||||
| Cockroach-specific IgG (mg/mL) | 4.1 (2.0–33.8) | 4.3 (2.0–20.6) | 5.1 (2.0–24.9) | ||
|
| |||||
| Cockroach-specific IgG4 (mg/mL)* | 0.08 (0.02–1.03) | 0.19 (0.01–3.6) | 0.12 (0.00–7.5) | 0.14 (0.00–3.5) | 0.47 (0.00–2.6) |
|
| |||||
| Allergen-IgE complex binding to B cells (%)† | 130.9 (48.4–383.7) | 118.5 (79.3–436.1) | 84.9 (5.0–426.4) | 78.3 (6.2–421.7) | 80.8 (16.9–201.5) |
|
| |||||
| German cockroach wheal size (mm) | 6 (3–11) | 6 (3–12) | 5 (3–12) | 5 (3.5–12) | 5.8 (4–11.5) |
Values are counts (percentages) or medians (ranges).
In BioCSI IgG4 levels were significantly lower in the BioCSI placebo group at baseline (P = .007); no other values differed between the treatment groups (P > .05). No BioCSI2 values differed between the treatment groups (P > .05).
Blocking antibody activity measured as the percentage of allergen-IgE complexes bound to B cells after addition of participant’s serum; values can be higher than 100% when the participant’s serum contains anti-cockroach IgE antibodies that increase the formation of complexes to greater than control levels.
The primary outcome was the change from baseline in serum cockroach-specific IgE levels over the 6 months of treatment. For the intention-to-treat analysis, at least 1 posttreatment IgE measurement was available for 23 of 28 actively treated and 25 of 26 placebo-treated subjects. Group results are displayed in Table III and Fig 1, demonstrating an almost 2-fold difference in posttreatment cockroach-specific IgE levels between the active and placebo groups (P < .0001). When individual subjects were examined, 9 (32%) of 23 actively treated and 1 (4%) of 25 placebo-treated subjects had at least a 3-fold increase in specific IgE levels from baseline (see Fig E1 in this article’s Online Repository at www.jacionline.org). A statistically significant increase from baseline values in the active group was present at month 1 and continued throughout the study period.
TABLE III.
Biomarker treatment effect summary
| Study | Outcome | Treatment group changes*
|
Treatment effects†
|
||||
|---|---|---|---|---|---|---|---|
| Placebo | Low-dose extract‡ | High-dose extract‡ | Low-dose extract vs placebo | High-dose extract vs placebo | Low-dose extract vs high-dose extract | ||
| BioCSI | IgE (kU/L) | 1.12 (.18) | 2.16 (<.0001) | — | 1.92 (<.0001) | — | — |
|
| |||||||
| IgG4 (mg/mL) | 0.93 (.16) | 1.06 (.30) | — | 1.14 (.09) | — | — | |
|
| |||||||
| Allergen-IgE complex binding to B cells (%)§ | 3.0 (.78) | 11.9 (.29) | — | 8.9 (.57) | — | — | |
|
| |||||||
| BioCSI2 | IgE (kU/L) | 1.18 (.14) | 2.69 (<.0001) | 1.98 (<.0001) | 2.28 (<.0001) | 1.68 (.001) | 0.74 (.04) |
|
| |||||||
| IgG (mg/mL) | 1.04 (.36) | 1.14 (.001) | 1.32 (<.0001) | 1.10 (.11) | 1.27 (<.0001) | 1.15 (.01) | |
|
| |||||||
| IgG4 (mg/mL) | 1.03 (.85) | 1.45 (.01) | 1.57 (.003) | 1.40 (.13) | 1.52 (.06) | 1.08 (.70) | |
|
| |||||||
| Allergen-IgE complex binding to B cells (%)§ | 5.7 (.46) | 19.2 (.007) | −7.2 (.30) | 13.5 (.20) | −12.9 (.21) | −26.4 (.008) | |
Changes in outcome measures for baseline level versus treatment period. For IgE, IgG, and IgG4, values are geometric mean ratios. For the allergen-IgE complex binding to B cells, values are arithmetic differences in percentage of binding. Numbers in parentheses are P values.
Effect comparison between given treatments. For IgE, IgG, and IgG4, values are geometric mean ratios. For the allergen-IgE complex binding to B cells, values are arithmetic differences.
The low dose contained 4.2 μg of Bla g 2 and 84 μg of Bla g 1 per day. The high dose contained 16.8 μg of Bla g 2 and 336 μg of Bla g 1 per day. The BioCSI study did not include a high-dose arm.
Blocking antibody activity measured as the percentage of allergen-IgE complexes bound to B cells after addition of the participant’s serum.
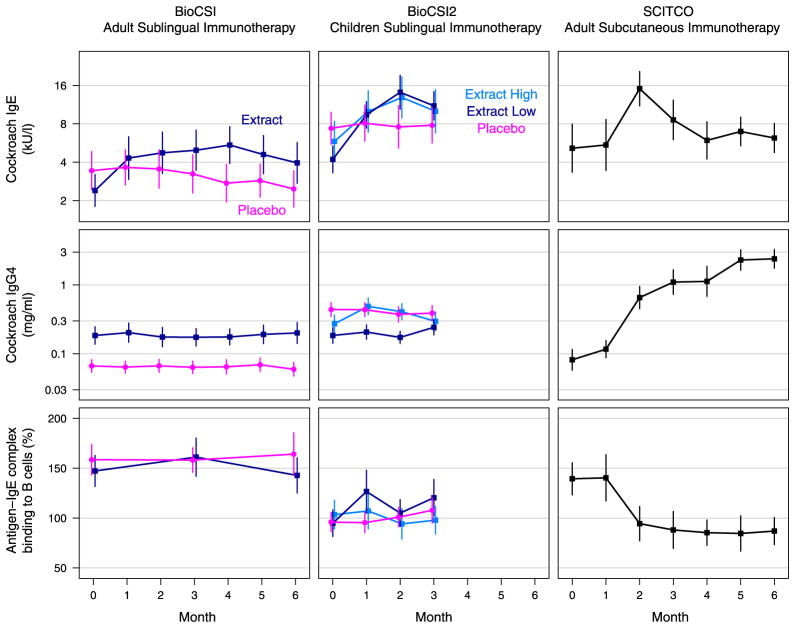
FIG. 1.
IgE and IgG4 levels and blocking antibody activity responses by study group. Average* biomarker levels by treatment arm in BioCSI (total n = 54, column 1), BioCSI2 (n = 89, column 2), and SCITCO (n = 10, column 3). Treatment effects for BioCSI and BioCSI2 are detailed in Table I. All 3 biomarkers showed significant change from baseline during the 6-month treatment period in SCITCO. *Geometric means are plotted for IgE and IgG4 levels and arithmetic means for blocking antibody activity.
There was a trend toward increased IgG4 responses in actively treated subjects (P = .09, Table III and Fig 1). Other secondary outcomes, including blocking antibody responses, which were represented as IgE-FAB (Table III and Fig 1), and skin test responses, which were assessed by using end point titration (data not shown), were not significantly affected by therapy. The IgE-FAB response correlated with the IgG4 response (rs = 0.57, P = .004).
Minor adverse events were common, but the therapy was overall well tolerated. In total, 93% of actively treated and 88% of placebo-treated subjects reported at least 1 adverse event, of which 16.4% were characterized as definitely or probably related to treatment. The most common adverse events were throat irritation (43% of actively treated subjects and 46% of control subjects) and general, ear, oral, and eye pruritus (57% for actively treated subjects and 77% for placebo-treated subjects). One subject was removed from the study after an urticarial reaction, although after unblinding, it was determined that she was receiving placebo. Self-reported adherence was 95%, with no significant differences between active treatment and placebo or between responders and nonresponders in the active treatment group.
BioCSI2
Ninety-nine subjects were enrolled, including 30 in the high-dose group, 31 in the low-dose group, and 28 in the placebo group. Baseline characteristics were similar among the groups, except for higher cockroach IgG4 levels in the placebo group (Table II). Eighty-nine subjects were included in the intention-to-treat analysis, which required at least 1 postbaseline cockroach IgE measurement. Both the high- and low-dose active treatment groups exhibited significant increases in cockroach IgE compared with the placebo group (P < .01, Table III and Fig 1). However, comparison of the high- and low-dose groups revealed greater IgE responses in the low-dose group (P =.04). When individual subjects were examined, 13 (43%) of 30 in the high-dose group, 12 (40%) of 30 in the low-dose group, and 3 (11%) of 28 in the placebo group had at least a 3-fold increase in specific IgE levels from baseline values (see Fig E2 in this article’s Online Repository at www.jacionline.org). In agreement with BioCSI, the low-dose extract treatment did not achieve significant increases in cockroach-specific IgG4 levels. Also, cockroach IgG levels did not increase. In those receiving the high-dose treatment, we observed modest but significant increases in both cockroach IgG and IgG4 levels (Table III and Fig 1), but the effect of the high dose was not stronger than that of the low dose. As in BioCSI, active treatment did not result in significant inhibition of the FAB assay, and the high-dose extract did not offer any advantage in this regard. In fact, the only effect we saw in the FAB assay was a paradoxical increase in B-cell binding in the low-dose group (Table III).
More stringent adherence assessments were applied in this protocol, including both participant reports and vial weights. We defined adherence as taking 75% or more of the prescribed extract (based on measuring the weight of the returned study drug bottles) and being in the maintenance phase for at least 45 days. With these criteria, we found that 15 of 43 receiving the high dose and 27 of 46 receiving the low dose (combining the active and placebo groups) met this per-protocol definition (P = .02) of adherence. However, study outcomes were unchanged when only the “treatment-compliant” subset was examined in a per-protocol analysis (data not shown).
Similar to the BioCSI study, 343 nonserious adverse events were reported among 78 of the 99 participants; 12.5% were characterized as definitely or probably related to treatment, but there were no differences among any of the 3 treatment groups, and no adverse events required dosing adjustments or study discontinuation. Eight participants experienced oral pruritis, tongue pruritus, or both during the study: 6 receiving the low active extract dose, 1 receiving the high dose, and 1 receiving placebo.
SCITCO
Eleven subjects were enrolled because 1 subject was replaced early in the study as a result of scheduling issues. Of the 10 evaluable subjects, 8 were female, the median age was 37.5 years (age range, 24–52 years), 7 had asthma, and 6 had allergic rhinitis. The median cockroach SPT wheal was 6.75 mm (range, 3–9 mm), and the median cockroach IgE level was 3.8 kU/L (range, 0.9–24.9 kU/L).
The primary outcome in this study was safety, and no treatment-related serious or severe adverse events were reported. Three events rated as moderate in severity were all localized injection-site reactions, and another 147 events rated as mild in severity were judged to be probably or possibly related to treatment. These consisted primarily of minor injection-site reactions or symptoms consistent with the participants’ underlying allergic rhinitis. No adjustments in dosing were required as a result of an adverse event.
Significant changes from baseline in cockroach-specific IgE levels (1.78-fold increase, P = .02), IgG4 levels (12.95-fold increase, P < .001), and FAB activity (43% inhibition of B-cell binding, P < .001) were detected (Fig 1). Five participants exhibited at least a 3-fold increase in cockroach IgE levels, whereas all 10 participants had at least a 2-fold increase in cockroach IgG4 levels (see Figs E3 and E4 in this article’s Online Repository at www.jacionline.org).
DISCUSSION
The studies described in this report represent the outcome of the first stage of a National Institute of Allergy and Infectious Diseases–sponsored program under the ICAC to develop immunotherapy for cockroach allergy, one of the most important culprits of asthma morbidity in the US urban, low-income population. Immunotherapy against cockroach allergens could potentially be applied for both treatment and prevention of asthma in children and for treatment of asthma in adults. To date, there have been no controlled trials of SLIT or SCIT using German cockroach extracts and only 1 controlled study of American cockroach SCIT that demonstrated clinical and immunologic effects.10 As noted, the ICAC program began with a trial comparing 3 commercially available cockroach extracts3 and then proceeded to the phase I and phase I/II trials described in this report. The goal is to eventually conduct a phase III trial of cockroach immunotherapy through either the sublingual or subcutaneous route. Data on safety and dosing were needed to reach this stage. In addition, the decision to proceed with either sublingual or subcutaneous treatment required comparative data. Because both approaches necessitate a prolonged treatment period before clinical outcomes can be obtained, we selected another strategy to avoid major delays and to reduce the cost of drug development under limited resources. In this regard we chose to measure biomarkers that reflect the biologic activity of treatment. We fully recognize that no such marker predicts efficacy, but a combined examination of a series of markers that are known to change with allergen immunotherapy offers an overall impression of the biologic activity of a treatment modality.
In the phase I SCSS trial we demonstrated an early and reassuring safety profile for cockroach SLIT in a study that began in adults and progressed in a stepwise fashion to children as young as 5 years of age. Although 1 subject was unable to complete treatment because of a reaction characterized by oral symptoms and vomiting, all others tolerated the therapy, with only 1 patient reporting severe symptoms and none experiencing a serious adverse event, even though a majority of subjects were required to have a high level of cockroach sensitivity. The safety profile was similar in the 2 BioCSI studies; although these studies did not require any specific degree of cockroach sensitivity, they allowed enrollment of patients with slightly more symptomatic asthma.
The main objective of the BioCSI trial was to determine whether the dose chosen for cockroach SLIT was immunologically active, as assessed based on a variety of in vitro biomarkers, as well as based on skin test reactivity. Although we found a statistically significant group difference in our primary outcome (ie, cockroach-specific IgE levels), only a minority of participants had substantial changes in this outcome when compared with those observed in well-designed and clinically effective studies using grass SLIT.7 The fact that no changes were seen in any other biomarker was of further concern.
There were several possible explanations for the observed modest and inconsistent immunologic response, including dose, adherence, lower responsiveness in adults compared with children, and even the possibility that the cockroach extract was not sufficiently antigenic. Therefore the BioCSI2 and SCITCO studies were developed to address several of these concerns. In BioCSI2 we used a 4-fold higher dose of cockroach antigen in children and assessed adherence more carefully. Although we detected slightly greater IgG4 responses in this pediatric study, IgE responses were very similar to those in BioCSI, and no greater response in any parameter was found in those participants treated with the higher dose of cockroach extract. Blocking antibody responses were undetectable with the higher dose, whereas there was an unexpected increase in FAB activity in the low-dose group. This immune-based change paralleled the observed early increase in IgE levels, which suggests that low-dose SLIT might have induced functional IgE that actually enhanced FAB activity, an undesirable effect that disappeared at the higher dose. Although our results clearly suggested that adherence is a significant concern with this mode of therapy, especially when asking participants to comply with twice daily dosing, the outcomes were similar when the “adherent” participants were examined separately.
The SCIT trial was designed as an early safety study but also as a proof of concept that consistent, allergen immunotherapy-related immunologic changes can be induced with German cockroach extract. Findings with regard to safety were reassuring because no severe reactions were seen. Mild reactions to SCIT were common, but they did not affect dosing. SCIT resulted in more vigorous and consistent immunologic responses by using the same extracts as in the SLIT studies. Although overall IgE responses were similar to those seen in the SLIT studies, IgG4 responses and the reduction in FAB activity, both of which have been shown in some studies to bear modest relationships to clinical outcomes, were clearly more vigorous with SCIT.11,12
Even with the higher dose tested in the BioCSI2 study, it is still possible that the SLIT dose of cockroach was not adequate for a consistent immune response. Unfortunately, sublingual dosing is limited by the potency of the extract and the volume that can be held in the sublingual space. In the initial study comparing the relative potencies of 3 commercially available German cockroach extracts, it was concluded that these nonstandardized extracts were all of relatively low potency.3 Although the major allergen content of the maintenance dose used in BioCSI, approximately 3.3 μg of Bla g 2, was at least 3-fold lower than the dust mite SLIT dose used by Bush et al,7,13 we anticipated a more consistent response in the high-dose BioCSI2 group. It is also noteworthy that a number of study participants experienced oral pruritus on sublingual administration, indicating that low extract activity might not be a major factor. If the daily dose was adequate, one could argue that the duration of therapy was not sufficient to demonstrate an effect. Again, we consider this unlikely because of the published experience from other SLIT trials and because our findings indicated a clear plateau of the IgE response by the third month of treatment.7 A final possibility is that our subject selection was not appropriate because we did not require a minimum level of cockroach sensitivity above what is commonly considered “positive” and did not include any challenge procedures to confirm clinical reactivity. In addition, we did not assess cockroach exposure at baseline or during the study, a factor that could have had effects on the serologic markers under study. However, none of these elements have been consistently incorporated into SLIT trials, and there is no evidence that their use would have improved the outcomes of our studies. All participants in our studies had both a positive skin test result and at least a class I specific IgE antibody level against German cockroach extract to avoid potential problems with nonspecificity of diagnosis.
Our results are consistent with a number of other studies of SLIT. Although some studies, especially those using standardized grass pollen extracts, have shown more consistent responses for the same serologic biomarkers used in our studies, other SLIT studies using a variety of aeroallergens have not detected consistent immunologic changes.14,15 In addition, several studies in which no immunologic effects were found demonstrated significant clinical responses, and studies in which immunologic effects were found did not necessarily predict clinical outcomes. We intentionally did not assess clinical responses in our studies because the duration of treatment was not sufficient to make any conclusions in this regard. It is also important to note that our outcomes relied primarily on antibody responses and did not include analyses of cellular changes.
In summary, in these early studies of cockroach SLIT and SCIT, we have not identified any safety problems that would raise concerns as to the continuation of research with cockroach allergen immunotherapy. We also found that German cockroach SLIT induces significant changes in serum specific IgE levels, although somewhat inconsistently. Cockroach SCIT induces overall more substantial immunologic changes, especially in IgG4 and FAB inhibition. These pilot studies provide the foundation to proceed with this program that might eventually lead to a new therapy for cockroach allergy, especially focused on patients living in inner-city environments, in which asthma is common and severe and cockroach is a dominant allergen with regard to sensitization, exposure, and disease activity. Moreover, the lessons learned in these studies to evaluate the potential effectiveness of a particular antigen for immunotherapy point to the need to more fully evaluate many aspects of this treatment to achieve the greatest possible benefit.
METHODS
Protocol synopses
SCSS
SCSS was an open-label single-site trial of German cockroach allergen extract administered through the sublingual route. It was primarily designed to study the safety of this therapy. The objective of this protocol was to assess whether treatment with cockroach SLIT using the per-protocol allergen extract doses is safe. The primary outcome measure was the rate of related adverse events and serious adverse events in the course of treatment.
Twenty-seven cockroach-sensitive participants (5–55 years of age) with a history of perennial allergic rhinitis with or without asthma for a minimum of 1 year before study entry were studied. They were all enrolled from the Baltimore site. To study safety in older participants before proceeding to children, participants were enrolled in the study by age, with those 18 through 55 years of age enrolled first, those 8 through 17 years of age enrolled second, and those 5 through 7 years of age enrolled last. Each age group had 9 participants, with at least 3 participants having asthma within each age group. Each age group was also enrolled by level of cockroach sensitization based on skin test wheal size. Three of the 9 participants had low-to-moderate sensitization, and 6 had a high level of sensitization (skin test wheal size response of ≥6 mm, which is greater than that elicited by the saline control). Each subject underwent a 1-day, 8-dose escalation to the maintenance dose, followed by a daily dose for 14 days, with the first 2 doses administered under observation and the remaining 12 treatments self-administered at home.
BioCSI
BioCSI was a randomized multicenter trial comparing glycerinated German cockroach allergen extract administered through the sublingual route with placebo. The primary objective of this protocol was to determine whether cockroach SLIT would induce a 3-fold group mean difference in levels of cockroach IgE, a biomarker of allergen immunotherapy, compared with placebo when measured over 6 months. In addition, the safety of the therapy was monitored.
Fifty-four inner-city adults (age, 18–55 years) with a history of perennial allergic rhinitis, asthma, or both and documented allergic sensitivity to German cockroach were studied in this trial. They were enrolled from 4 major urban areas in the United States (Baltimore, Boston, Chicago, and Denver). Participants were randomized 1:1 to receive either:
glycerinated German cockroach allergen extract administered through the sublingual route with a maximum study dose (MSD) of 420 μL daily (3 pumps daily) or
placebo at 420 μL daily (3 pumps daily).
Escalation to the maintenance dose of 420 μL daily was accomplished in 1 study visit in most cases but could be continued on a second day, if needed. After reaching the MSD, participants took the investigational agent at home daily and were seen in the clinic every month for 6 months. Blood was collected each month for assessment of cockroach-specific IgE levels and other biomarkers of allergen immunotherapy.
BioCSI2
BioCSI2 was a randomized multicenter trial comparing 2 doses of glycerinated German cockroach allergen extract administered through the sublingual route with placebo. The primary objective of this protocol was to determine whether the high dose of cockroach SLIT would induce a 3-fold group mean difference in levels of cockroach IgE, a biomarker of allergen immunotherapy, compared with placebo when measured over 3 months. The study also examined the effect of the low dose of SLIT on cockroach-specific IgE levels. In addition, the safety of the therapy was monitored.
Ninety-nine inner-city children (age, 5–17 years) with a history of perennial allergic rhinitis, asthma, or both and documented allergic sensitivity to German cockroach were studied in this trial. They were enrolled from 5 major urban areas in the United States (Baltimore, Chicago, Cincinnati, Dallas, and Detroit). Participants were randomized to 1 of 4 treatment groups:
glycerinated German cockroach allergen extract administered through the sublingual route with an MSD of 420 μL daily (3 pumps daily);
glycerinated German cockroach allergen extract administered through the sublingual route with an MSD of 840 μL twice daily (6 pumps twice daily);
placebo at 420 μL daily (3 pumps daily); and
placebo at 840 μL twice daily (6 pumps twice daily).
Escalation to the low dose of 420 μL daily was accomplished in 1 study visit in most cases but could be continued on a second day, if needed. Escalation to the high dose of 840 μL twice daily took place in weekly intervals over 2 to 4 weeks. After reaching their assigned dose, participants took the investigational agent at home daily (once or twice, depending on the group) and were seen in the clinic every 2 weeks for 3 months. Blood was collected each month for assessment of cockroach-specific IgE levels and other biomarkers of allergen immunotherapy.
SCITCO
SCITCO was an open-label single-site trial of German cockroach allergen extract administered by means of subcutaneous injection. It was primarily designed to study the safety of this therapy. The objective of this protocol was to assess whether treatment with cockroach SCIT using the per-protocol allergen extract doses is safe. The primary outcome measure was the rate of related adverse events and serious adverse events in the course of treatment.
Ten adults (age, 18–55) years with a history of perennial allergic rhinitis, asthma, or both who were also cockroach sensitive were studied in this trial. They were all enrolled from the Baltimore site. Participants received escalating doses of subcutaneous cockroach extract twice per week over 11 weeks until they reached the maintenance dose of 0.6 mL of a 1:20 concentration of extract. Participants were then followed for 15 weeks as they received weekly injections at the maintenance dose. Blood was collected each month for assessment of cockroach-specific IgE levels and other biomarkers of allergen immunotherapy.
Supplementary Material
TABLE I.
Summary of study design for the 4 cockroach immunotherapy protocols
| Treatment | SCSS
|
BioCSI
|
BioCSI2
|
SCITCO
|
|---|---|---|---|---|
| SLIT | SLIT | SLIT | SCIT | |
| Design | Open label, single site | DBPC, multicenter | DBPC, low and higher dose, multicenter | Open label, single site |
|
| ||||
| CR antigen dose per day | Bla g 2: 4.2 μg Bla g 1: 50 μg |
Bla g 2: 4.2 μg Bla g 1: 50 μg |
Low dose Bla g 2: 4.2 μg Bla g 1: 50 μg High dose: Bla g 2: 16.8 μg Bla g 1: 202 μg |
Bla g 2: 6 μg Bla g 1: 120 μg |
|
| ||||
| Primary outcome | Adverse events | Change in cockroach-specific IgE level | Change in cockroach-specific IgE level | Adverse events |
|
| ||||
| Treatment duration | 14 d | 6 mo | 3 mo | 6 mo |
|
| ||||
| Ages of participants | Children and adults | Adults | Children | Adults |
|
| ||||
| Sample size | 27 | 54 | 89 | 10 |
CR, Cockroach; DBPC, double-blind, placebo-controlled.
Clinical implications.
These 4 pilot studies provide the basis for larger-scale efficacy studies of immunotherapy for cockroach allergy, especially for patients living in urban areas in which cockroach exposure is a major contributor to asthma morbidity.
Acknowledgments
Supported in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract nos. NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, and HHSN2722010000521 and from the National Center for Research Resources and National Center for Advancing Translational Sciences, National Institutes of Health, under grants RR00052, 1UL1RR025771, UL1 RR024982, and UL1 TR000077-04. Immunologic extracts were donated for some studies by Greer Pharmaceuticals (Lenoir, NC).
Abbreviations used
- BioCSI
Biomarkers of Cockroach Sublingual Immunotherapy
- BioCSI2
Biomarkers of Cockroach Sublingual Immunotherapy 2
- FAB
Facilitated allergen binding
- ICAC
Inner-City Asthma Consortium
- SCIT
Subcutaneous immunotherapy
- SCITCO
Subcutaneous Immunotherapy in Cockroach-sensitive Adults
- SCSS
Sublingual Cockroach Safety Study
- SLIT
Sublingual immunotherapy
- SPT
Skin prick test
Footnotes
Disclosure of potential conflict of interest: R. A. Wood has consultant arrangements with the Asthma and Allergy Foundation of America, is employed by Johns Hopkins University, has received grants from the National Institutes of Health (NIH), and receives royalties from UpToDate. E. C. Matsui, G. Hershey, and G. T. O’Connor have received grants from the NIH. R. Gruchalla has received a grant and travel support from the National Institute of Allergy and Infectious Disease (NIAID) and has consultant arrangements with the US Food and Drug Administration. A. H. Liu is on the Data Monitoring Committee for a large clinical trial for GlaxoSmithKline, has consultant arrangements with DBV, and has received speakers’ honoraria from Merck. J. A. Pongracic has received a grant, travel support, and payment for writing and reviewing the manuscript from the University of Wisconsin and is employed by the Pediatric Faculty Foundation of the Ann & Robert H. Lurie Children’s Hospital of Chicago. E. Zoratti has received a grant and travel support from the NIAID. M. Granada has received a grant from the NIH and has received payment for lectures from Forest Labs. S. R. Durham has consultant arrangements from Stallergenes, Circassia, Merck, and ALK-Abelló; has received grants from Novartis, Stallergenes, and Letti; has received payment for lectures from Merck Sharp and Dohme; and has received payment for manuscript preparation from ALK-Abelló. W. W. Busse has received grants from the NIH/NIAID and the National Heart, Lung, and Blood Institute; is a board member for Merck; has consultant arrangements with Amgen, Novartis, GlaxoSmithKline, MedImmune, and Genentech; is on the data monitoring boards for Boston Scientific and Genentech; is on the study oversight committee for ICON; and receives royalties from Elsevier. The rest of authors declare that they have no relevant conflicts of interest.
References
- 1.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 3.Slater JE, James R, Pongracic JA, Liu AH, Sarpong S, Sampson HA, et al. Biological potency of German cockroach allergen extracts determined in an inner city population. Clin Exp Allergy. 2007;37:1033–9. doi: 10.1111/j.1365-2222.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 4.Esch RE, Bush RK, Peden D, Lockey RF. Sublingual-oral administration of standardized allergenic extracts: phase 1 safety and dosing results. Ann Allergy Asthma Immunol. 2008;100:475–81. doi: 10.1016/S1081-1206(10)60474-7. [DOI] [PubMed] [Google Scholar]
- 5.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008;358:2259–64. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121:512–8. e2. doi: 10.1016/j.jaci.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–9. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–6. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava D, Gaur SN, Arora N, Singh BP. Clinico-immunological changes post-immunotherapy with Periplaneta americana. Eur J Clin Invest. 2011;41:879–88. doi: 10.1111/j.1365-2362.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–47. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 12.Shamji MH, Ljorring C, Francis JN, Calderon MA, Larché M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–26. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 13.Bush RK, Swenson C, Fahlberg B, Evans MD, Esch R, Morris M, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011;127:974–81. e1–7. doi: 10.1016/j.jaci.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;(12):CD002893. doi: 10.1002/14651858.CD002893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT) Allergy. 2011;66:740–52. doi: 10.1111/j.1398-9995.2011.02583.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.