Abstract
Background
Mohawk (Mkx) is a homeodomain-containing transcription factor that is expressed in various mesoderm-derived tissues, particularly in developing tendons. In this study, we investigate the exact expression pattern and functions of Mkx in forelimbs.
Methods
We analyzed the forelimbs of Mkx knockout mice (from embryonic day [E] 18.5 to postnatal day [P] 28-week) by using knocked-in Venus signals, Masson trichrome staining, and hematoxylin and eosin (H&E) staining.
Results
We detected Venus signals in forelimb tendons, pulleys, and volar plates (VPs) in P21 mice. In-depth histological analysis showed that compared to the wild-type mice, the Mkx knockout mice showed significant hypoplasia in the flexor digitorum profundus (FDP) tendons from E18.5. The VPs and pulleys appeared normal until P0, however, by P14, they became increasingly thicker in Mkx-null mice compared to wild-type mice. The fiber alignment was particularly disrupted in VPs of Mkx-null mice.
Conclusions
These results suggest that Mkx is an important regulator of the differentiation of VPs and pulleys, as well as of tendon differentiation.
Introduction
Tendons are dense connective tissues of mesodermal origin, which connect and transmit force from muscles to bones. There is one flexor tendon for the thumb and two for each of the other fingers1. Flexor tendon injuries such as lacerations or ruptures are common and are challenging for orthopedic surgeons 2.
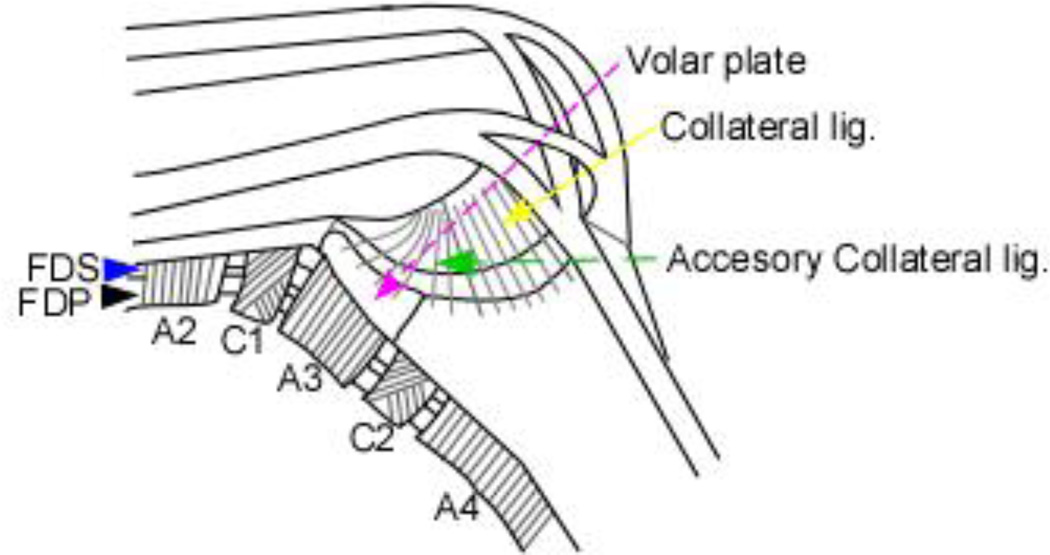
The functions of tendons are supported by several peritendinous tissues, such as pulleys and volar plates (VPs). VPs are small fibrocartilaginous structures located in the proximal interphalangeal (PIP) joints (Fig. 1) 3. VPs can be injured by hyperextension, sometimes resulting in dorsal dislocation of the phalanx or major joint instability4, such as in PIP joint fracture-dislocations5. Even with adequate treatment, this injury can result in chronic pain, stiffness, and swelling 6,7. Although tendon and VP injuries are major clinical issues for orthopedic surgeons, the molecular mechanisms underlying their development are not completely understood.
Figure 1. Schematic representation of the volar plate (VP), flexor digitorum profundus (FDP) tendons, flexor digitorum superficialis (FDS) tendons, annular pulleys (A2-A4), and cruciform pulleys (C1, C2).
Pink dashed arrow, VP. Black arrowhead, FDP. Blue arrowhead, FDS. Yellow arrow, collateral ligament. Green dashed arrow, accessory collateral ligament. (Modified from Ueba Y. Hand anatomy and its function. 2010.3)
To establish new therapies for these injuries, knowledge regarding tendon biology is required. To date, two transcription factors, scleraxis (Scx) and mohawk (Mkx), have been identified as critical regulators of tendon development. Previous studies have shown that Scx is essential for the initiation of tendon and tendon sheath differentiation8–10, whereas Mkx plays an important role in tendon maturation11,12. Scx, a basic helix–loop–helix (bHLH) transcription factor expressed in tendon progenitors8,9, is required for the proper development of limb tendons by promoting collagen synthesis in tendons10. Col1a1, Col1a2, aggrecan, and tenomodulin (Tnmd) genes are the key targets of the Scx-dependent transcription10,13–15. The Mkx homeobox gene, also known as iroquois-related homeobox like 1 (Irxl1), is a homeodomain-containing transcription factor that belongs to the three amino acid loop extension (TALE) superclass of atypical homeodomain proteins, which have three extra amino acid residues between the first and second helices of their homeodomains16–18. Mkx encodes a 353-amino-acid protein with a putative homeodomain motif highly similar to those of the invertebrate iroquois and vertebrate Irx subfamily members16–20. Biochemical studies have shown that Mkx has transcriptional repressor activity21,22.
We previously identified Mkx as a transcription factor expressed in developing tendons by constructing a whole-mount in situ hybridization database, termed “EMBRYS” (http://embrys.jp/embrys/html/MainMenu.html)23. The initial characterization of mouse Mkx showed a dynamic transcription pattern in various mesoderm-derived tissues, such as the dorsomedial lip and the ventrolateral lip of somites, limb buds, endocardia, kidneys, and male gonads11,16,18. In the somite and limb bud, the expression of Mkx is observed in the progenitors of skeletal muscles, tendons, and cartilage16–18.Our group, along with two other groups, generated Mkx knockout mice to investigate the functions of Mkx and reported the critical function of Mkx in the development of tendons, showing that tendons of Mkx-null mice were significantly hypoplastic throughout the body11,12, 24. It was also found that the mRNA levels of Col1a1 and Col1a2, which encode type I collagen, as well as those of decorin (Dcn), fibromodulin (Fmod), and Tnmd were lower in Mkx-null mice than in their wild-type littermates11,12.
However, the role of Mkx in peritendinous tissues is largely unknown. In this study, by analyzing gene-targeted mice, we found that the Mkx transcription factor is required for VP and pulley maturation, as well as for tendon development.
Materials and methods
Venus knock-in Mkx-deletion mice
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the National Institute for Child Health and Development. Venus, an improved green fluorescent protein (GFP) gene, was inserted in mice heterozygous for exon 2 deletion of the Mkx gene (Mkx+/−), as described previously12. The Mkx+/− mice were intercrossed to generate Mkx−/− homozygous mice.
Immunohistochemistry
For immunohistochemical analysis of Venus signals, forelimbs from postnatal day (P) 21-mice were dissected and fixed with 4% paraformaldehyde in phosphate-buffered saline PBS) at 4 °C for 1 h. The tissues were embedded in optimum cutting temperature (O.C.T.) compound (Sakura Finetek) and frozen rapidly in liquid nitrogen. The specimens were sectioned at 7 µm. Cryosections were air-dried and blocked with Blocking One (Nacalai Tesque) for 1 h. The sections were then incubated at 4 °C overnight with polyclonal rabbit anti-GFP antibody (MBL), rinsed, and incubated for 1 h with Alexa Fluor 488 conjugates of anti-rabbit IgG antibody (Molecular Probes) and DAPI (4′ 6-diamidino-2-phenylindole) for staining of the cell nuclei. High-magnification images of the fluorescent signals were captured using a BX53 compound microscope (Olympus). These experiments were performed with at least three independent samples to confirm their reproducibility.
Histological analysis
For histological analysis, forelimbs harvested from embryos (E18.5) and mice (from P0 to 7 months) were collected at predetermined stages (P0, 1-, 2-, 8-, 24- and 28-weeks) and fixed in 4% paraformaldehyde in PBS at 4°C overnight. Tissues were decalcified in 20% formic acid and 20% citric acid, dehydrated through graded methanol, embedded in paraffin, and sectioned at 7-µm thickness, followed by staining with hematoxylin and eosin (H&E).
Forelimbs from P21-mice were dissected and fixed with 4% paraformaldehyde in PBS at 4 °C for 1 h. The tissues were embedded in O.C.T. compound (Sakura Finetek) and frozen rapidly in liquid nitrogen. Specimens were sectioned at 7 µm. Cryosections were stained with Masson trichrome.
Statistical analysis
The data were processed using the StatView J-4.5 software. Values have been reported in terms of mean ± standard deviation (SD). Student's t-test was used to determine the level of significance. The probability level accepted for significance was P < 0.05.
Results
Expression of Mkx in the forelimbs of mice
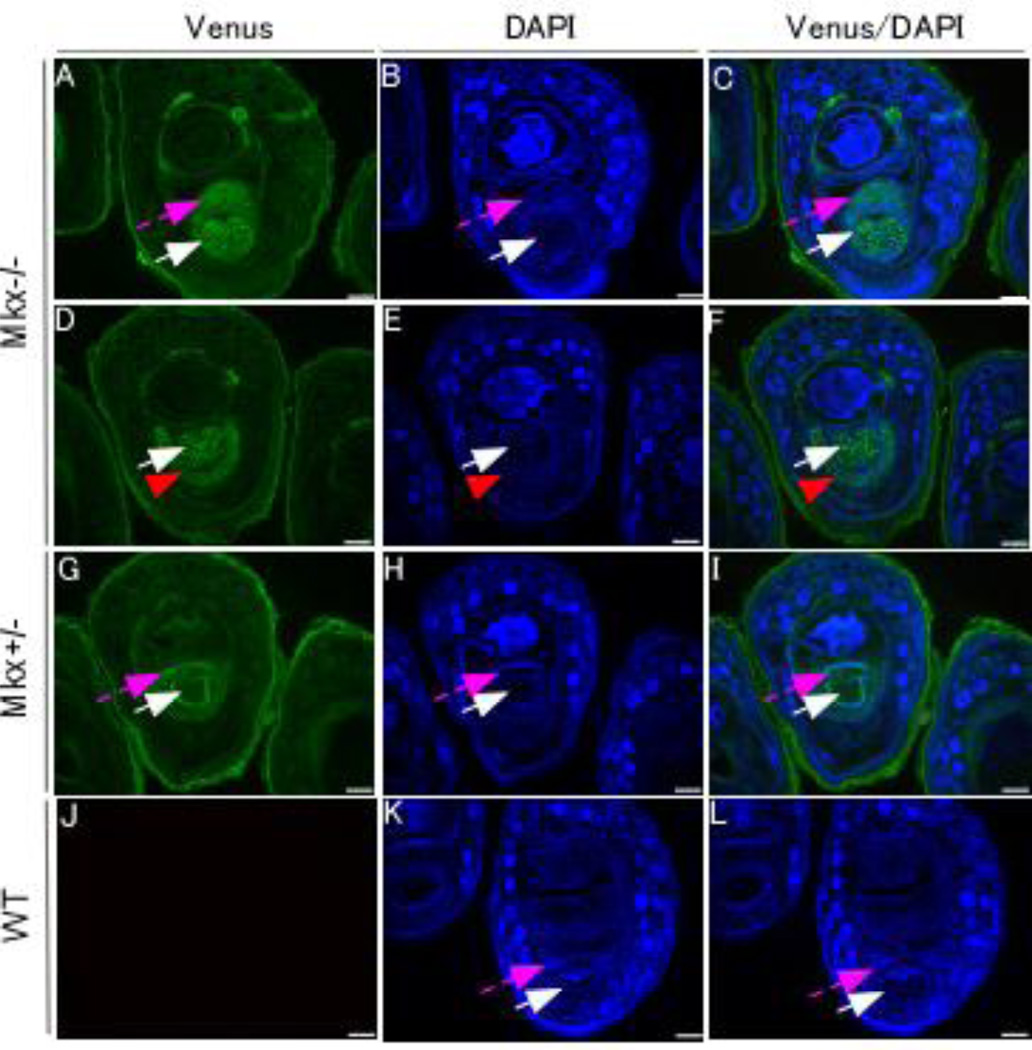
Previous studies have shown the expression patterns of Mkx in embryos or adult tendons11,12. Here, we focused on examining Mkx expression in the postneonatal stages in forelimbs. Interestingly, Venus expression, which recapitulates Mkx expression, was strongly detected in VPs of P21-Mkx−/− (Fig. 1, Fig. 2 A–C) and Mkx+/− mice (Fig. 2 G–I) in addition to tendons (Fig. 2 A–I) and pulleys (Fig. 2 D–F). Conversely, no signals were detected in the wild-type littermates (Fig. 2 J–L). We also confirmed that the Venus signals were detected in the VPs, pulleys, and tendons of 7-month-old Mkx−/− and Mkx+/− mice, while no signals were detected in the wild-type littermates (data not shown).
Figure 2. Venus signals were detected in flexor digitorum profundus (FDP) tendons, pulleys, and the VPs in P21 Venus knock-in Mkx+/− and −/− mice.
(A, D, G, and J)Venus signals of Mkx−/−, Mkx+/−, and wild-type (WT) digits at P21. (B, E, H, and K) DAPI (4′6-diamidino-2-phenylindole) staining of a section of Mkx−/−, Mkx+/−, and WT digits at P21. (C, F, I, and L) Merge of a section of Mkx−/−, Mkx+/−, and WT digits at P21. Pink dashed arrows, VPs. White arrows, FDP tendons. Red arrowheads, pulleys. Scale bar, 100 µm.
Mkx−/− mutant mice have forelimb tendon defects
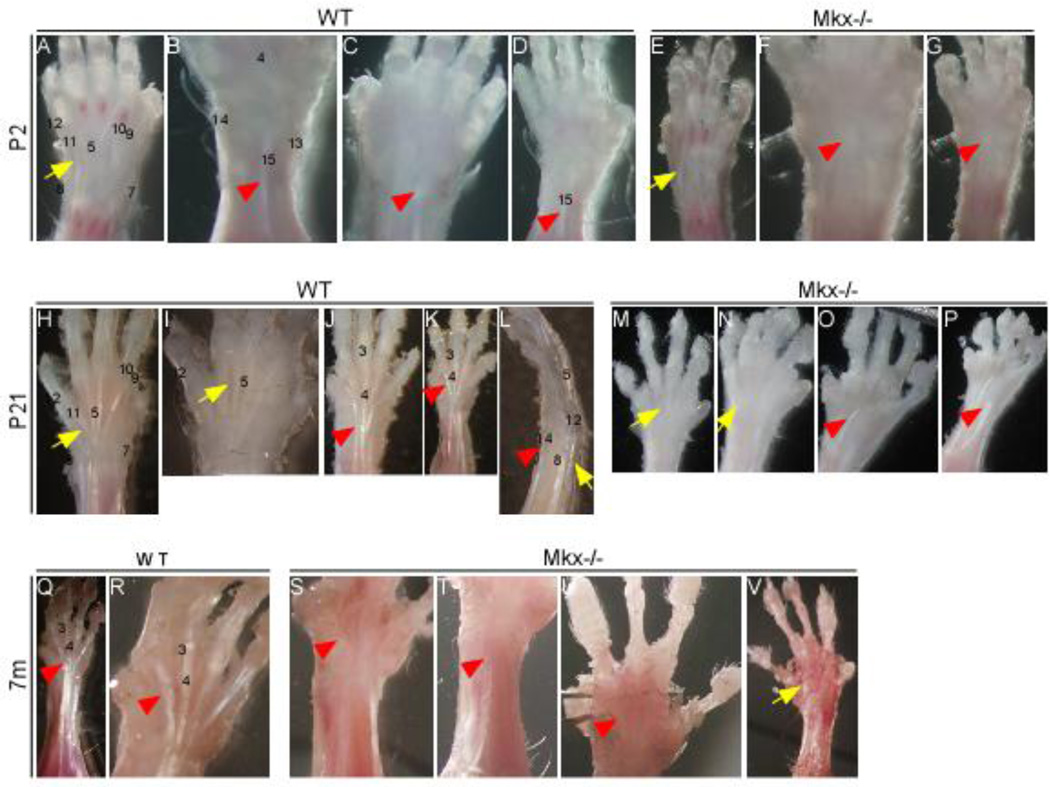
To investigate the function of Mkx in tendon formation, we analyzed the tendons of Mkx-null mice. As previously described11,12,24, tendons throughout the body, such as Achilles, tail, back, and patellar tendons are hypoplastic in adult Mkx-null mice. Here, we performed detailed analysis of forelimbs tendons in Mkx-null mice from the postneonatal to the adult stages. This analysis showed that both the extensor and flexor digitorum profundus (FDP) tendons of P2 (Fig. 3 E–G), P21 (Fig. 3 M–P), and 7-month-old (Fig. 3 S–V) Mkx-null mice were dramatically hypoplastic compared with those of the wild-type littermates (Fig. 3 A–D, H–L, Q–R).
Figure 3. Significant hand tendon defects are observed in Mkx-null mice.
The extensor tendons and flexor digitorum profundus (FDP), in P2, P21, and 7-month-old Mkx-null mice were dramatically reduced compared to those of the wild-type littermates. (A–G) Tendon appearance of wild-type (WT) (A–D) and mutant (E–G) digits at P2. (H–P) Tendon appearance of wild-type (WT) (H–L) and mutant (M–P) digits at P21. (Q–V) Tendon appearance of wild-type (WT) (Q, R) and mutant (S–V) digits at 7 months. Red arrowheads, FDP tendons. Yellow arrows, extensor tendons. The numbering of the tendons refers to that introduced by Watson et al. 25 as follows: 3, flexor digitorium sublimis (FDS); 4, flexor digitorium profundus (FDP); 5, extensor digitorium communis (EDC); 7, extensor pollicis; 8, extensor carpi ulnaris; 9, extensor carpi radialis longus; 10, extensor carpi radialis brevis; 11, extensor digiti quarti; 12, extensor digiti quinti; 13, flexor carpi radialis; 14, flexor carpi ulnaris; 15, palmaris longus.
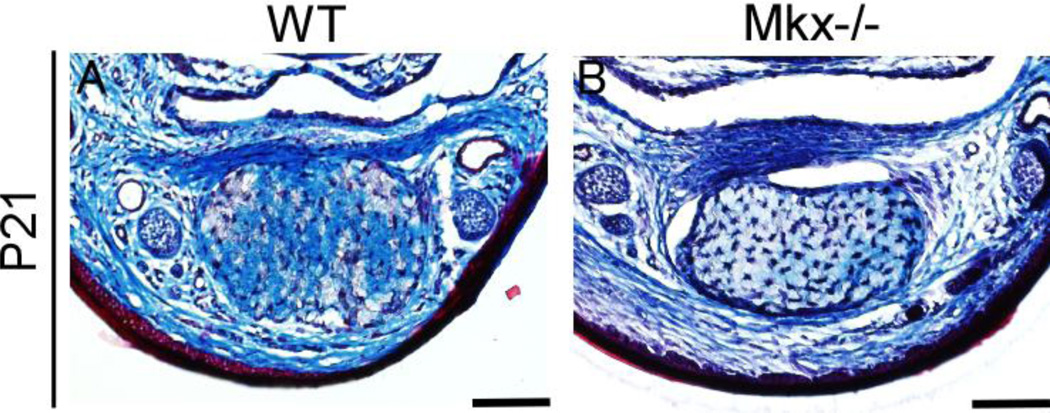
To further confirm the tendon phenotype observed in Mkx-null mice, we performed Masson trichrome staining of the forelimbs of P21 mice (Fig. 4) and H&E staining of the forelimbs of P0-, P14-, 8-, 24- and 28-week-old mice, and found that the FDP tendons of Mkx-null mice were smaller than those of the wild-type counterparts (Fig. 5–A, Fig. 6–A).
Figure 4. VP is composed of collagen.
Masson trichrome staining of the flexor digitorium profundus (FDP) tendon and volar plates (VP) in wild-type (A) and Mkx-null mice at P21 (B). Scale bar, 100 µm.
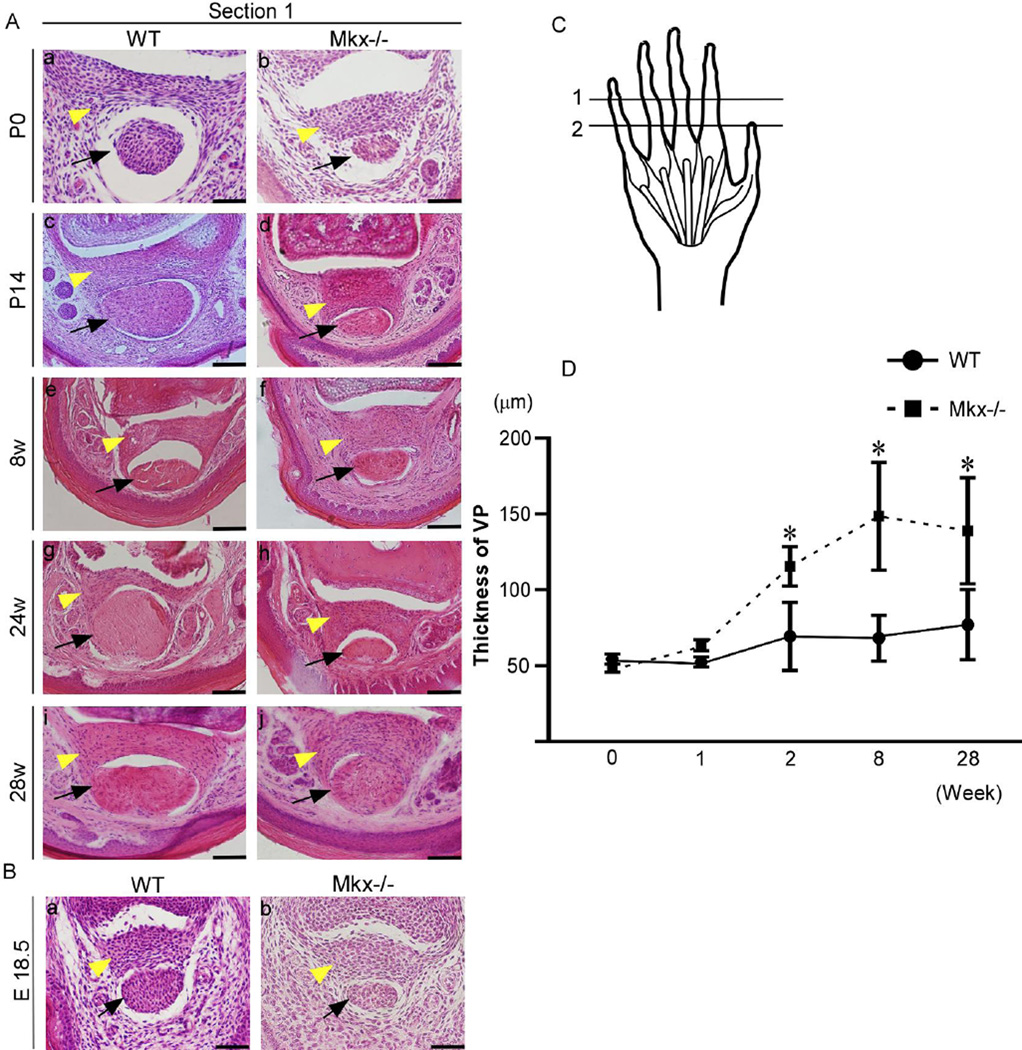
Figure 5. Tendon mass decreases in Mkx-null mice since E18.5. Volar plates (VPs) are normal in embryonic Mkx-null mice and become thick in Mkx-null mice after P14.
(A) Hematoxylin and eosin (H&E) staining of the flexor digitorium profundus (FDP) tendon and VP in wild-type (a, c, e, g, and i) and Mkx-null mice (b, d, f, h, and j), in the time range from P0 to 28 weeks. Scale bars are 50 µm in a and b and 100 µm in c–j. (B) H&E staining of the FDP tendon and VP in E18.5 wild-type (a) and Mkx-null mice (b). Scale bar, 50 µm. Yellow arrowheads, VPs. Black arrows, FDP tendons. (C) Section levels are marked by black lines: (1) proximal interphalangeal (PIP) joint, (2) middle of the proximal phalanx (level of the A2 pulley). The sections were taken at level 1. (D) Comparison of the thickness of VPs between Mkx-null mice and wild-type littermates. *P < 0.05, n = 5.
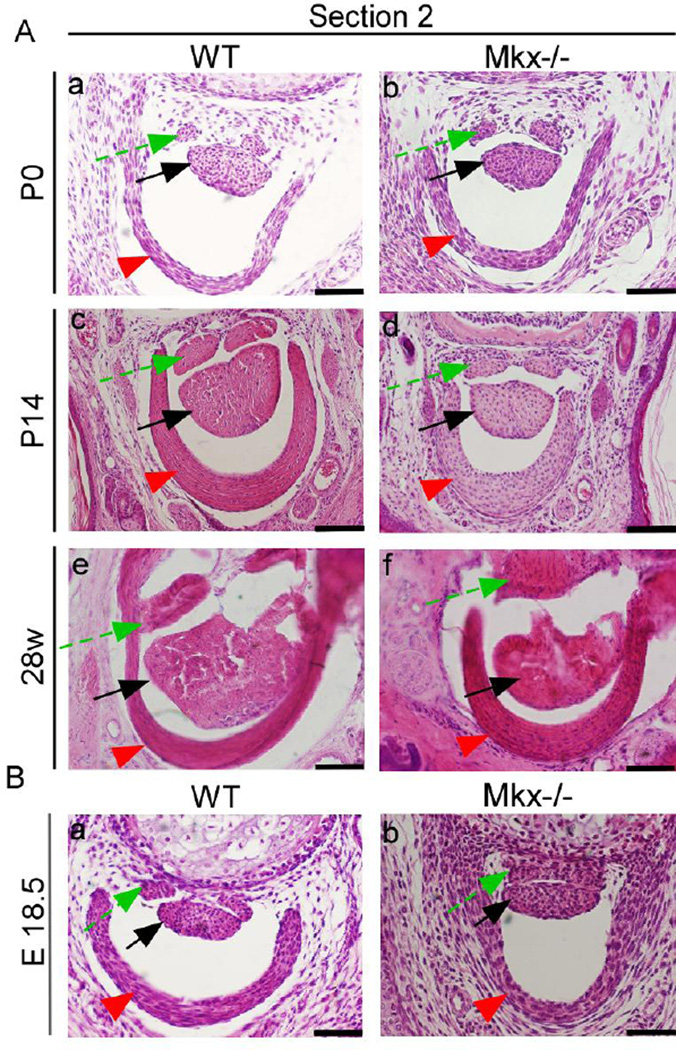
Figure 6. Pulleys are normal in embryonic Mkx-null mice and become thick in Mkx-null mice after P14.
(A) Hematoxylin and eosin (H&E) staining of the flexor digitorium profundus (FDP) tendon, flexor digitorum sublimis (FDS) tendon, and pulleys in wild-type (a, c, and e) and Mkx-null mice (b, d, and f) in the time range from P0 to 28 weeks. Scale bars are 50 µm in a and b and 100 µm in c–f. (B) H&E staining of the FDP tendon, FDS tendon, and pulley in E18.5 wild-type (a) and Mkx-null mice (b). Scale bar, 50 µm. Green dashed arrows, FDS tendons. Red arrowheads, pulleys. Black arrows, FDP tendons. The sections were taken at level 2 (for details, see the caption of Fig. 5-C).
To determine whether tendon defects in Mkx-knockout mice are also observed in embryonic stages, we performed H&E staining of the forelimbs of Mkx-null embryos at E18.5. The size of the FDP tendons in Mkx-null embryos was also smaller than that in the wild-type littermates (Fig. 5–B, Fig. 6–B). Taken together, Mkx plays a critical role in hand tendon differentiation in the development stages.
Abnormality in VPs and pulleys in Mkx−/− mutant mice
Next, we observed two section levels as described by Watson et al. 25: one was the PIP level, in which we observed the FDP and the VP; the other was the level of the center of the proximal phalanx, in which we observed the FDP, the flexor digitorum superficialis (FDS), and the A2 pulley (Fig. 5–C). The pulleys and VPs appeared normal at the embryonic stage (Fig. 5–B, Fig. 6–B) and P0 in Mkx-null mice (Fig. 5–A, a and b ; Fig. 6–A, a and b). However, compared to the VPs of the wild-type littermates, the VPs became thicker in Mkx-null mice by P14 (Fig. 5–A, c and d). We found that 8, 24 and 28-week-old Mkx null mice also had much thicker VPs (Fig. 5–A, e–j; Fig. 5–D). Comparison with the results for the wild-type littermates showed that pulleys as well as VPs became thicker in Mkx-null mice by P14 (Fig. 6–A, c and d). Twenty eight-week-old Mkx-null mice also had thicker pulleys Fig. 6–A, e and f).
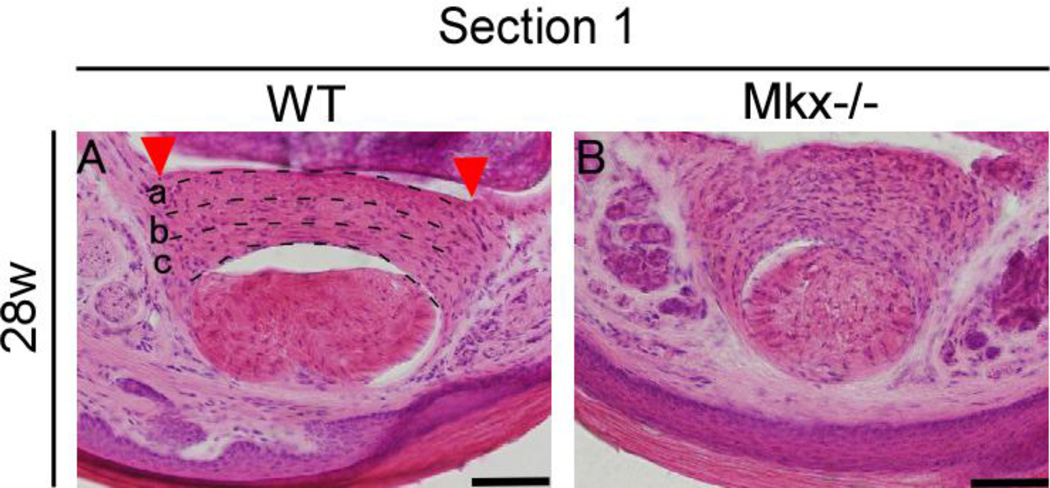
In wild-type mice, VPs are complex structures with three separate layers (Fig. 5–A a, c, e, g, and i; Fig. 7–A). In the palmar-most layer (Fig.7–A, c), fibers run transversely. It has been reported that the fibers of this layer are in continuity with the accessory collateral ligament4. The second layer (Fig. 7–A, b), which is the central core of the plate, has both longitudinal and transverse fibers that create a tight “basket-weave” pattern. The dorsal-most layer (Fig. 7–A, a) has fibers that run longitudinally, that is, the same direction as the flexor tendon. We also observed that the dorsal surface of the VP forms the floor of the PIP joint capsule (Fig. 7–A) and that the palmar surface of the VP forms the floor of the flexor tendon sheath. Interestingly, the VP of Mkx-null mice was composed of fibers whose orientation was quite disorganized (Fig. 5–A, b, d, f, h, and j; Fig. 7–B).
Figure 7. Enlarged view of Fig. 5-A, i and j. Volar plates (VPs) are complex structures with three separate layers in wild-type mice. In Mkx-null mice, the orientation of the fibers of the VPs is disorganized.
(A) Hematoxylin and eosin (H&E) staining of the flexor digitorium profundus (FDP) tendon and VP in 28-week-old wild-type mice. a, The dorsal-most layer. b, The second layer. c, The palmar-most layer. (B) H&E staining 355 of the FDP tendon and VP in 28-week-old Mkx-null mice.
Thus, in addition to regulating tendon growth, Mkx plays an important role in the differentiation and homeostasis of the VPs and pulleys.
Discussion
The VPs of the PIP joints are fibrocartilaginous structures26 with three distinct layers27. Because VPs have the specific orientation of the collagen bundles, they are thought to resist the tension created by hyperextension and lateral torsional strain4.
In this study, we observed the expression of Mkx in VPs and pulleys as well as in tendons. Although VPs and pulleys in Mkx-null mice appeared grossly normal until P0, they became thicker in Mkx-null mice by P14 compared to those in wild-type littermates. Detailed histological analysis showed that the VPs of Mkx-null mice had markedly disorganized fibers and did not have three distinct layers, suggesting that the VPs in Mkx-null mice may be easily injured by hyperextension and lateral torsional strain, and result in dorsal dislocation of the phalanx or major joint instability. These data suggest that this morphologic difference might be due to mechanical stress or degenerative changes after birth. In this regard, we recently reported the potential role of Mkx in human tendon/ligament tissue homeostasis 28. Since Mkx expression in VPs and pulleys was observed in adult mice, Mkx may also be involved in VP and pulley homeostasis and regeneration.
Pulleys are composed of two layers of fibrocartilaginous tissues. Snapping or locking of the thumb or fingers is caused by the thickening of the digit’s A1 pulley 29. Although the etiological factors of these diseases remain unclear, overload on pulleys is known to cause fibrocartilaginous metaplasia. In this regard, it would be of interest to test Mkx expression in such conditions. We also observed that Mkx was expressed in the annulus fibrosus of the intervertebral disc (data not shown), suggesting that Mkx is also related to the differentiation of other parts of fibrocartilaginous components.
Taken together, our results indicate that Mkx is a regulator not only of tendon differentiation, but also of VP and pulley development and homeostasis. Understanding how Mkx regulates peritendinous tissues will likely provide an important insight into the etiological factors of VPs- and pulley-related diseases and can lead to further development of the related treatment.
Footnotes
Author contributions
N.O., Y.I., Y.T. and H.A. designed the study; N.O., Y.I. and M.I. performed the research; N.O., Y.I., M.L., S.T., H.N. and H.A. analyzed the data; and N.O. and H.A. wrote the paper.
Conflict of interests
The authors declare no conflict of interest.
Contributor Information
Naoko Onizuka, Email: Nnok@aol.com, Department of Systems BioMedicine, National Center for Child Health and Development, (2-10-1, Okura, Setagaya, Tokyo 157-0074, Japan, telephone number : +81-3-3416-0181 fax number : +81-3-3416-2222); Department of Orthopaedic Surgery, School of Medicine, Keio University, (35 Shinanomachi, Shinjuku, Tokyo 160-8582, Japan telephone number : +81-3-3353-1211).
Yoshiaki Ito, Email: yoshito.syst@tmd.ac.jp, Department of Systems BioMedicine, Tokyo Medical and Dental University, (1-5-45, Yushima, Bunkyou-ku, Tokyo 113-8510, Japan, telephone number : +81-3-3813-6111).
Masayo Inagawa, Email: inagawa-m@ncchd.go.jp, Department of Systems BioMedicine, National Center for Child Health and Development, (2-10-1, Okura, Setagaya, Tokyo 157-0074, Japan. telephone number : +81-3-3416-0181, fax number : +81-3-3416-2222).
Hiroyuki Nakahara, Email: nakahara@scripps.edu, Molecular and Experimental Medicine, The Scripps research Institute, (10550 North Torrey Pines Road, La Jolla, CA 92037, telephone number : +1-858-784-1000).
Shuji Takada, Email: s-takada@ncchd.go.jp, Department of Systems BioMedicine, National Center for Child Health and Development, (2-10-1, Okura, Setagaya, Tokyo 157-0074, Japan, telephone number : +81-3-3416-0181 fax number : +81-3-3416-2222).
Martin Lotz, Email: mlotz@scripps.edu, Molecular and Experimental Medicine, The Scripps research Institute, (10550 North Torrey Pines Road, La Jolla, CA 92037, telephone number : +1-858-784-1000).
Yoshiaki Toyama, Email: toyama@z6.keio.jp, Department of Orthopaedic Surgery, School of Medicine, Keio University, (35 Shinanomachi, Shinjuku, Tokyo 160-8582, Japan, telephone number : +81-3-3353-1211).
Hiroshi Asahara, Email: asahara.syst@tmd.ac.jp, Department of Systems BioMedicine, Tokyo Medical and Dental University, (1-5-45, Yushima, Bunkyou-ku, Tokyo 113-8510, Japan, telephone number : +81-3-3813-6111); Department of Systems BioMedicine, National Center for Child Health and Development, (2-10-1, Okura, Setagaya, Tokyo 157-0074, Japan, telephone number : +81-3-3416-0181 fax number : +81-3-3416-2222); Molecular and Experimental Medicine, The Scripps research Institute, (10550 North Torrey Pines Road, La Jolla, CA 92037, telephone number : +1-858-784-1000).
References
- 1.Doyle JR. Anatomy of the flexor tendon sheath and pulley system: a current review. J Hand Surg Am. 1989;14:349–351. doi: 10.1016/0363-5023(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 2.Griffin M, Hindocha S, Jordan D, Saleh M, Khan W. An overview of the management of flexor tendon injuries. Open Orthop J. 2012;6:28–35. doi: 10.2174/1874325001206010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueba Y. Hand anatomy and its function. Fifth edition. Kyoto: Kinpodo; 2010. (in Japanese) [Google Scholar]
- 4.Williams EH, McCarthy E, Bickel KD. The histologic anatomy of the volar plate. J Hand Surg Am. 1998;23:805–810. doi: 10.1016/S0363-5023(98)80154-8. [DOI] [PubMed] [Google Scholar]
- 5.Calfee RP, Sommerkamp TG. Fracture-dislocation about the finger joints. J Hand Surg Am. 2009;34:1140–1147. doi: 10.1016/j.jhsa.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Dionysian E, Eaton RG. The long-term outcome of volar plate arthroplasty of the proximal interphalangeal joint. J Hand Surg Am. 2000;25:429–437. doi: 10.1016/s0363-5023(00)70026-8. [DOI] [PubMed] [Google Scholar]
- 7.Deitch MA, Kiefhaber TR, Comisar BR, Stern PJ. Dorsal fracture dislocations of the proximal interphalangeal joint: surgical complications and long-term results. J Hand Surg Am. 1999;24:914–923. doi: 10.1053/jhsu.1999.0914. [DOI] [PubMed] [Google Scholar]
- 8.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 9.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 10.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Watson SS, Lan Y, Keene DR, Ovitt CE, Liu H, Schweitzer R, Jiang R. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol Cell Biol. 2010;30:4797–4807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, Nishida K, Akimoto T, Takahashi M, Miyaki S, Asahara H. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A. 2010;107:10538–10542. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Watanabe H, Nifuji A, Yamada Y, Olson EN, Noda M. Overexpression of a single helix-loop-helix-type transcription factor, scleraxis, enhances aggrecan gene expression in osteoblastic osteosarcoma ROS17/2.8 cells. J Biol Chem. 1997;272:29880–29775. doi: 10.1074/jbc.272.47.29880. [DOI] [PubMed] [Google Scholar]
- 14.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol. 2009;47:188–195. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DM, Arredondo J, Hahn K, Valente G, Martin JF, Wilson-Rawls J, Rawls A. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev Dyn. 2006;235:792–801. doi: 10.1002/dvdy.20671. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Liu W, Maltby KM, Lan Y, Jiang R. Identification and developmental expression analysis of a novel homeobox gene closely linked to the mouse Twirler mutation. Gene Expr Patterns. 2006;6:632–636. doi: 10.1016/j.modgep.2005.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi JK, Bruneau BG. Irxl1, a divergent Iroquois homeobox family transcription factor gene. Gene Expr Patterns. 2007;7:51–56. doi: 10.1016/j.modgep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DM, Beres BJ, Wilson-Rawls J, Rawls A. The homeobox gene Mohawk represses transcription by recruiting the sin3A/HDAC co-repressor complex. Dev Dyn. 2009;238:572–580. doi: 10.1002/dvdy.21873. [DOI] [PubMed] [Google Scholar]
- 22.Bilioni A, Craig G, Hill C, McNeill H. Iroquois transcription factors recognize a unique motif to mediate transcriptional repression in vivo. Proc Natl Acad Sci U S A. 2005;102:14671–14676. doi: 10.1073/pnas.0502480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama S, Ito Y, Ueno-Kudoh H, Shimizu H, Uchibe K, Albini S, Mitsuoka K, Miyaki S, Kiso M, Nagai A, Hikata T, Osada T, Fukuda N, Yamashita S, Harada D, Mezzano V, Kasai M, Puri PL, Hayashizaki Y, Okado H, Hashimoto M, Asahara H. A systems approach reveals that the myogenesis genome network is regulated by the transcriptional repressor RP58. Dev Cell. 2009;17:836–848. doi: 10.1016/j.devcel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura W, Machii M, Xue X, Sultana N, Hikosaka K, Sharkar MT, Uezato T, Matsuda M, Koseki H, Miura N. Irxl1 mutant mice show reduced tendon differentiation and no patterning defects in musculoskeletal system development. Genesis. 2011;49:2–9. doi: 10.1002/dvg.20688. [DOI] [PubMed] [Google Scholar]
- 25.Watson SS, Riordan TJ, Pryce BA, Schweitzer R. Tendons and muscles of the mouse forelimb during embryonic development. Dev Dyn. 2009;238:693–700. doi: 10.1002/dvdy.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowers WH, Wolf JW, Jr, Nehil JL, Bittinger S. The proximal interphalangeal joint volar plate. I. An anatomical and biomechanical study. J Hand Surg Am. 1980;5:79–88. doi: 10.1016/s0363-5023(80)80049-9. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Hashizume H, Inoue H, Ogura T. Collagen framework of the volar plate of human proximal interphalangeal joint. Acta Med Okayama. 1994;48:101–108. doi: 10.18926/AMO/31103. [DOI] [PubMed] [Google Scholar]
- 28.Nakahara H, Hasegawa A, Otabe K, Ayabe F, Matsukawa T, Onizuka N, Ito Y, Ozaki T, Lotz MK, Asahara H. Transcription factor mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 2013;65:2081–2089. doi: 10.1002/art.38020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore JS. Flexor tendon entrapment of the digits (trigger finger and trigger thumb) J Occup Environ Med. 2000;42:526–545. doi: 10.1097/00043764-200005000-00012. [DOI] [PubMed] [Google Scholar]