Abstract
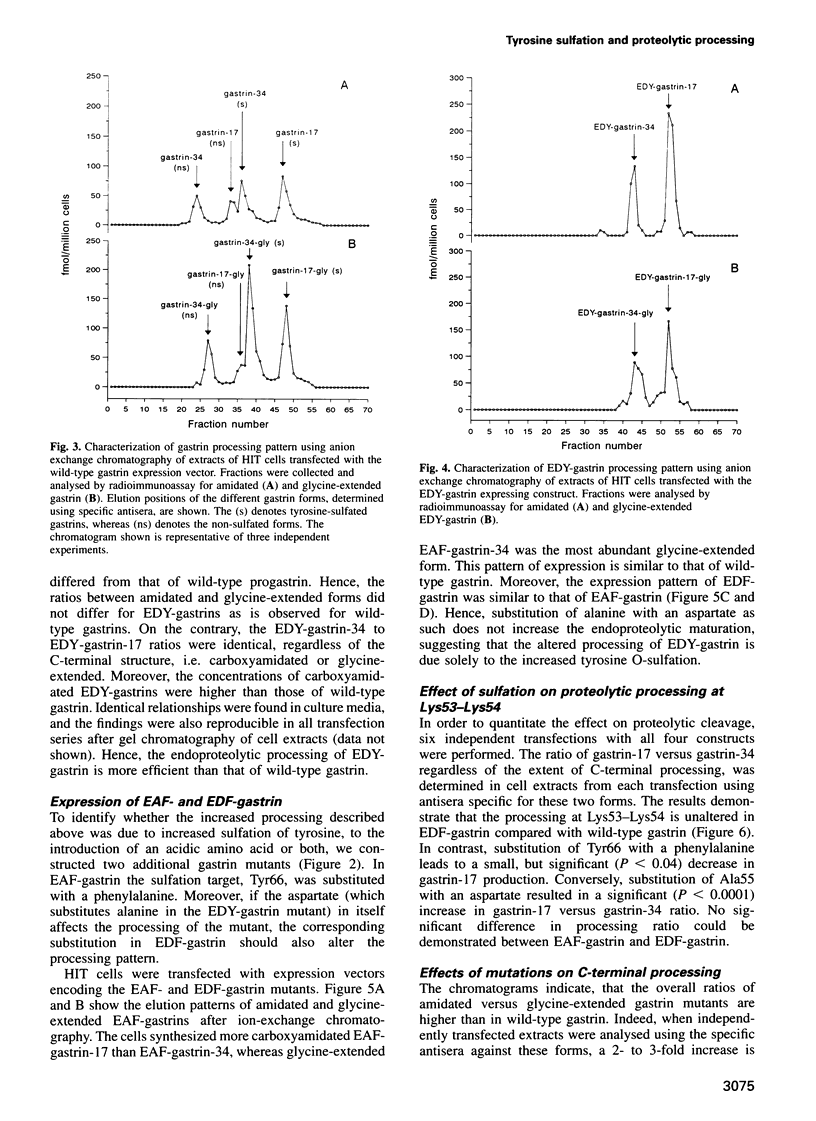
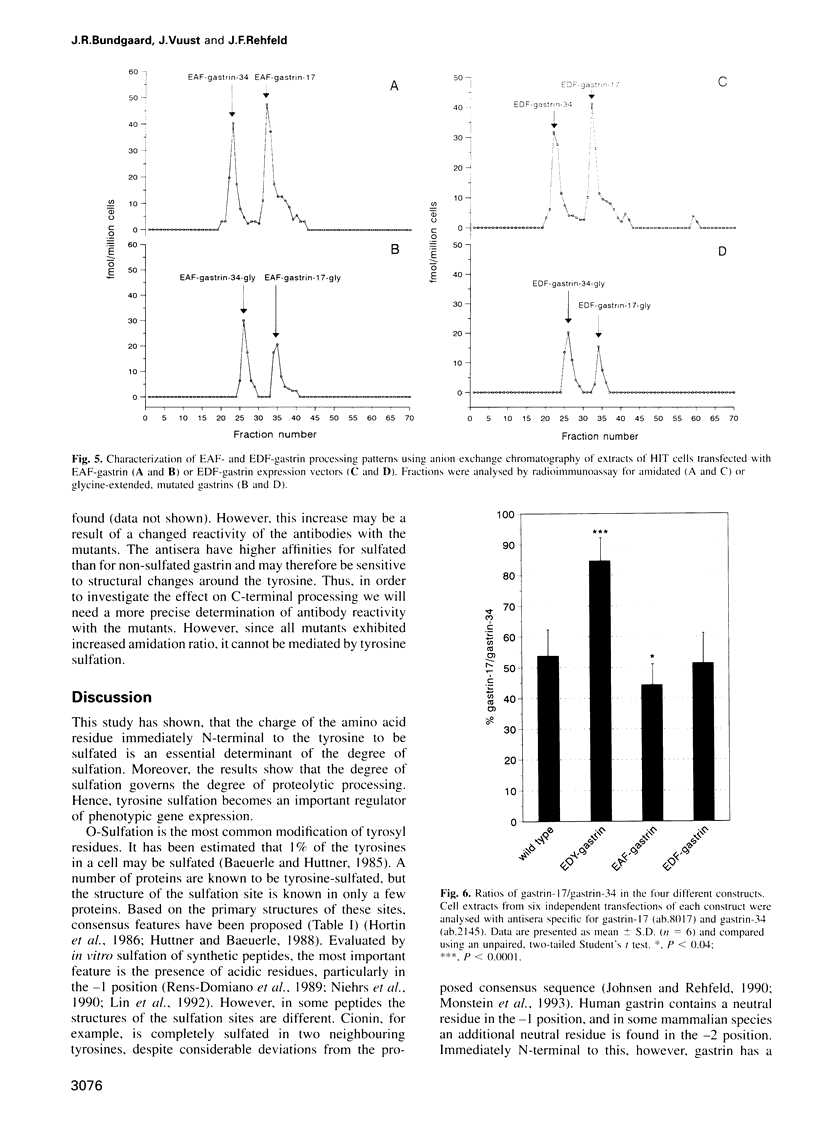
Tyrosine O-sulfation is a common post-translational modification of secretory and membrane proteins. The biological function of sulfation is known in only a few proteins, where it appears to enhance protein-protein interactions. Based on known sequences around sulfated tyrosines, a consensus sequence for prediction of target tyrosines has been proposed. However, some proteins are tyrosine sulfated at sites that deviate from the proposed consensus. Among these is progastrin. It is possible that the deviation explains the incomplete sulfation characteristic for bioactive gastrin peptides. In order to test this hypothesis, we have performed site-directed mutagenesis of the gastrin gene followed by heterologous expression in an endocrine cell line. The results show that substitution of the alanyl residue immediately N-terminal to the sulfated tyrosine with an acidic amino acid promotes the sulfation of gastrin peptides. Hence, the study supports the proposed consensus sequence for tyrosine sulfation. Importantly, however, the results also reveal that complete sulfation increases the endoproteolytic maturation of progastrin. Thus, our study suggests an additional function for tyrosine sulfation of possible general significance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. N. Species variation in the tyrosine sulfation of mammalian gastrins. Gen Comp Endocrinol. 1985 Apr;58(1):44–50. doi: 10.1016/0016-6480(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Andersen B. N., Stadil F. Sulfation of gastrin in Zollinger--Ellison sera: evidence for association between sulfation and proteolytic processing. Regul Pept. 1983 Jul;6(3):231–239. doi: 10.1016/0167-0115(83)90141-6. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation of yolk proteins 1, 2, and 3 in Drosophila melanogaster. J Biol Chem. 1985 May 25;260(10):6434–6439. [PubMed] [Google Scholar]
- Baker R. T., Board P. G. The human ubiquitin gene family: structure of a gene and pseudogenes from the Ub B subfamily. Nucleic Acids Res. 1987 Jan 26;15(2):443–463. doi: 10.1093/nar/15.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardram L., Rehfeld J. F. Production and evaluation of monospecific antibodies for a processing-independent sequence of human progastrin. Scand J Clin Lab Invest. 1989 Apr;49(2):173–182. doi: 10.3109/00365518909105418. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C. Inhibition of pro-cholecystokinin (CCK) sulfation by treatment with sodium chlorate alters its processing and decreases cellular content and secretion of CCK 8. Neuropeptides. 1994 Mar;26(3):195–200. doi: 10.1016/0143-4179(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Brand S. J., Andersen B. N., Rehfeld J. F. Complete tyrosine-O-sulphation of gastrin in neonatal rat pancreas. 1984 May 31-Jun 6Nature. 309(5967):456–458. doi: 10.1038/309456a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Hansen L., Rehfeld J. F. Ileal expression of gastrin and cholecystokinin. In search of a related hormone. FEBS Lett. 1994 Apr 25;343(2):115–119. doi: 10.1016/0014-5793(94)80301-3. [DOI] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Hille A., Braulke T., von Figura K., Huttner W. B. Occurrence of tyrosine sulfate in proteins--a balance sheet. 1. Secretory and lysosomal proteins. Eur J Biochem. 1990 Mar 30;188(3):577–586. doi: 10.1111/j.1432-1033.1990.tb15438.x. [DOI] [PubMed] [Google Scholar]
- Hille A., Huttner W. B. Occurrence of tyrosine sulfate in proteins--a balance sheet. 2. Membrane proteins. Eur J Biochem. 1990 Mar 30;188(3):587–596. doi: 10.1111/j.1432-1033.1990.tb15439.x. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Alpha-carboxyamidation of antral progastrin. Relation to other post-translational modifications. J Biol Chem. 1987 Dec 15;262(35):16953–16957. [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Measurement of precursors for alpha-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem. 1986 Jan;152(1):119–126. doi: 10.1016/0003-2697(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F., Schwartz T. W. Impaired alpha-carboxyamidation of gastrin in vitamin C-deficient guinea pigs. FEBS Lett. 1986 Feb 3;196(1):151–154. doi: 10.1016/0014-5793(86)80231-9. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J., Stone S. R., Donella-Deana A., Pinna L. A. The effect of substituting phosphotyrosine for sulphotyrosine on the activity of hirudin. Eur J Biochem. 1990 Feb 22;188(1):55–59. doi: 10.1111/j.1432-1033.1990.tb15370.x. [DOI] [PubMed] [Google Scholar]
- Hortin G. L., Farries T. C., Graham J. P., Atkinson J. P. Sulfation of tyrosine residues increases activity of the fourth component of complement. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1338–1342. doi: 10.1073/pnas.86.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G., Folz R., Gordon J. I., Strauss A. W. Characterization of sites of tyrosine sulfation in proteins and criteria for predicting their occurrence. Biochem Biophys Res Commun. 1986 Nov 26;141(1):326–333. doi: 10.1016/s0006-291x(86)80372-2. [DOI] [PubMed] [Google Scholar]
- Huang S. C., Yu D. H., Wank S. A., Mantey S., Gardner J. D., Jensen R. T. Importance of sulfation of gastrin or cholecystokinin (CCK) on affinity for gastrin and CCK receptors. Peptides. 1989 Jul-Aug;10(4):785–789. doi: 10.1016/0196-9781(89)90114-9. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Jensen S., Borch K., Hilsted L., Rehfeld J. F. Progastrin processing during antral G-cell hypersecretion in humans. Gastroenterology. 1989 Apr;96(4):1063–1070. doi: 10.1016/0016-5085(89)91624-7. [DOI] [PubMed] [Google Scholar]
- Johnsen A. H., Rehfeld J. F. Cionin: a disulfotyrosyl hybrid of cholecystokinin and gastrin from the neural ganglion of the protochordate Ciona intestinalis. J Biol Chem. 1990 Feb 25;265(6):3054–3058. [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Expression of human gastrin gene in normal and gastrinoma tissues. Gene. 1986;50(1-3):345–352. doi: 10.1016/0378-1119(86)90338-0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyte A., van Schijndel H. B., Niehrs C., Huttner W. B., Verbeet M. P., Mertens K., van Mourik J. A. Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J Biol Chem. 1991 Jan 15;266(2):740–746. [PubMed] [Google Scholar]
- Lin W. H., Larsen K., Hortin G. L., Roth J. A. Recognition of substrates by tyrosylprotein sulfotransferase. Determination of affinity by acidic amino acids near the target sites. J Biol Chem. 1992 Feb 15;267(5):2876–2879. [PubMed] [Google Scholar]
- Monstein H. J., Thorup J. U., Folkesson R., Johnsen A. H., Rehfeld J. F. cDNA deduced procionin. Structure and expression in protochordates resemble that of procholecystokinin in mammals. FEBS Lett. 1993 Sep 27;331(1-2):60–64. doi: 10.1016/0014-5793(93)80297-8. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Beisswanger R., Huttner W. B. Protein tyrosine sulfation, 1993--an update. Chem Biol Interact. 1994 Jun;92(1-3):257–271. doi: 10.1016/0009-2797(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Huttner W. B. Purification and characterization of tyrosylprotein sulfotransferase. EMBO J. 1990 Jan;9(1):35–42. doi: 10.1002/j.1460-2075.1990.tb08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C., Huttner W. B., Rüther U. In vivo expression and stoichiometric sulfation of the artificial protein sulfophilin, a polymer of tyrosine sulfation sites. J Biol Chem. 1992 Aug 5;267(22):15938–15942. [PubMed] [Google Scholar]
- Niehrs C., Kraft M., Lee R. W., Huttner W. B. Analysis of the substrate specificity of tyrosylprotein sulfotransferase using synthetic peptides. J Biol Chem. 1990 May 25;265(15):8525–8532. [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen C. P., Johnsen A. H. Post-poly(Glu) cleavage and degradation modified by O-sulfated tyrosine: a novel post-translational processing mechanism. EMBO J. 1995 Jan 16;14(2):389–396. doi: 10.1002/j.1460-2075.1995.tb07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I. Pituitary gastrins. Different processing in corticotrophs and melanotrophs. J Biol Chem. 1981 Oct 25;256(20):10426–10429. [PubMed] [Google Scholar]
- Rehfeld J. F., de Magistris L., Andersen B. N. Sulfation of gastrin: effect on immunoreactivity. Regul Pept. 1981 Sep;2(5):333–342. doi: 10.1016/0167-0115(81)90037-9. [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S., Hortin G. L., Roth J. A. Sulfation of tert-butoxycarbonylcholecystokinin and other peptides by rat liver tyrosylprotein sulfotransferase. Mol Pharmacol. 1989 Oct;36(4):647–653. [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986 Aug 12;25(16):4622–4628. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- Suchanek G., Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977 Mar;74(3):975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Desmond H., Pauwels S., Gregory H., Young J., Dockray G. J. The human gastrin precursor. Characterization of phosphorylated forms and fragments. Biochem J. 1988 Dec 15;256(3):951–957. doi: 10.1042/bj2560951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Berglund L., Boel E., Norris F., Norris K., Rehfeld J. F., Marcker K. A., Vuust J. Structure of a human gastrin gene. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1067–1069. doi: 10.1073/pnas.81.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solinge W. W., Rehfeld J. F. Radioimmunoassay for sequence 38-54 of human progastrin: increased diagnostic specificity of gastrin-cell diseases. Clin Chim Acta. 1990 Nov 15;192(1):35–46. doi: 10.1016/0009-8981(90)90269-x. [DOI] [PubMed] [Google Scholar]



