Abstract
Background
The challenge of early Tuberculosis (TB) infection among rural patients accessing HAART in a resource-limited setting with high HIV and TB burden has not been fully quantified.
Methods
This is a retrospective study nested within a prospective study of 969 patients consecutively initiated onto HAART at the CAPRISA AIDS Treatment programme in rural KwaZulu-Natal between January 2007 and December 2010. Patients were screened for clinical symptoms consistent with TB using a standardized checklist, and routine clinical investigations that included sputum microscopy and chest X-Ray diagnosis.
Results
Of 969 HIV-infected patients initiated on HAART, 173 (17.9%; 95% CI: 15.5 to 20.4) had active TB at HAART initiation. TB incidence rates were three fold higher in the first 3 months (early incident TB) following HAART initiation (11.5/100 person years (py); 95%CI: 7.1 to 17.5); compared to 4 – 24 months (late incident TB) post HAART initiation (3.2/100 py; 95%CI: 2.2 to 4.5; incidence rate ratio (IRR): 3.6; 95%CI: 2.0 to 6.4; p value <0.001). Immune status of patients at HAART initiation did not impact TB incidence rates in patients with CD4+ counts <50 (5.3/100) and >200 (4.9/100 py; p=0.81); cells/mm3. CD4+ count gains achieved 12 months post HAART initiation were significantly different in patients with early incident TB versus late incident TB; p=0.03.
Conclusion
Rural HIV treatment programmes in TB endemic settings experience high rates of TB irrespective of immunologic status of patients at HAART initiation, or duration on HAART.
Keywords: HIV, TB, tuberculosis, HAART, Africa, KwaZulu-Natal, KZN, South Africa
INTRODUCTION
Tuberculosis (TB) infection contributes substantially to morbidity and mortality among HIV positive patients. Globally, HIV associated TB peaked in 2005 at 1.39 million cases with approximately 15% incident cases and 480 000 deaths1. However, 2010 data indicated a continuing burden of HIV-associated TB with 1.1 million cases of TB of which 13% were incident cases and resulting in approximately 350 000 deaths2. KwaZulu-Natal, South Africa, is home to approximately 1.2 million HIV-infected individuals with 70% of TB patients co-infected with HIV3, and a TB notification rate of 1094 cases per 100,000 population4,5.
The use of highly active antiretroviral therapy (HAART) in HIV-infected patients reduces the risk of developing TB by 70–90%6–9, and is therefore a key strategy recommended by the World Health Organization (WHO) to prevent TB10. Studies from resource-limited settings show that HIV-infected patients have higher rates of TB compared to HIV uninfected patients, and with longer duration on HAART there is a decline in TB incidence rates11,12. Notwithstanding the evidence for TB screening and diagnosis, and HAART initiation early during TB therapy13–16; the burden of undiagnosed TB at HAART initiation and the number of new TB cases diagnosed during HAART in high HIV and TB settings have not been fully measured. In rural districts of KwaZulu-Natal where the TB and HIV epidemics converge most dramatically, the TB burden and its impact on patients at HAART initiation and during treatment remain poorly documented.
The aim of this study was to measure the prevalence and incidence rates of TB among patients accessing HAART in a rural community-based programme in a high TB and HIV prevalence setting in KwaZulu-Natal, South Africa, and explores its impact on therapeutic outcomes.
METHODS
Study population
We conducted a prospective cohort study among 969 HIV-positive patients initiated onto HAART at the Vulindlela CAPRISA AIDS Treatment programme between January 01, 2007 and December 31, 2010. This rural community outpatient clinic adjoins and receives patients from one of seven primary health care clinics in an area serving a community of about 400,000 people. Eligibility criteria were in accordance with South African Government HIV/AIDS treatment guidelines at the time17, 18. Patients were screened for clinical symptoms consistent with TB using a standardized checklist that asked about drenching night sweats, prolonged cough, fever and weight loss by either clinicians or professional nurses at every clinical visit. Patients that screened positive were referred to the adjacent government Primary Health Care Clinic for TB smear testing. Smear positive patients were initiated onto anti-TB therapy while sputum culture testing and antibiotic therapy was offered to all smear negative patients. Smear and culture negative patients with poor response to antibiotics, and those with suspected extra-pulmonary TB were referred to the local district hospital for chest X-Ray, abdominal ultrasonography or other invasive investigations such as lymph node aspirate, tissue biopsy etc. Access to TB microbiology services was variable and depended on, availability of skilled laboratory staff especially for culture and drug susceptibility testing (DST), and timeous communication of results to site staff and patients. Tuberculin Skin Testing and isoniazid preventive therapy (IPT) was not the currently available standard of care during the study period. Patients were seen monthly for the first 6 months and every three months thereafter unless clinically indicated. Routine demographic, laboratory and clinical data were recorded at baseline and at follow-up visits. Upon TB diagnosis, the following additional information was collected: date and method of diagnosis, TB type (extra-pulmonary tuberculosis (EPTB) or pulmonary tuberculosis (PTB)), TB drug susceptibility pattern, date of TB treatment initiation and completion. Patients who had both PTB and EPTB were then classified as having EPTB. TB was diagnosed and treated as per South African National TB Control Program guidelines19. All patients with complications or requiring further investigation and management were referred to the closest district level services about 30 km away.
For the purposes of this study, a patient with a ‘history of TB’ was defined as having completed TB treatment prior to HAART initiation. Patients with a TB history were classified as having a ‘recent history of TB’ if they had an episode of TB within the year prior to HAART initiation20. A ‘prevalent case of TB’ was defined as a patient who was already on TB treatment when HAART was initiated. ‘Incident TB cases’ were defined as new TB cases diagnosed after HAART initiation, and was further classified into “early incident TB” defined as a new TB diagnosis within 3 months of HAART initiation, and “late incident TB” defined as a new TB diagnosis occurring between 4 and 24 months post HAART initiation20. A recurrent episode of TB after TB treatment completion or cure for the TB episode present at baseline was regarded as an incident TB case in the prevalent TB group.
Statistical analysis
All reported p-values are two-sided. TB incidence was calculated as number of new TB cases after HAART initiation per 100 person-years (py) of follow up. Data are presented as proportions, means or medians where appropriate. Study duration for patients with no prevalent TB was calculated from HAART initiation date to date of TB diagnosis, or programme exit through LTFU, transfer out, or death. Patients with prevalent TB were included in the incidence calculation and their time on study calculated from the date treatment for prevalent TB was discontinued to either the date of a new TB diagnosis, or to programme exit through loss to follow-up (LTFU), transfer out, or death. All patient data were censored at 24 months of follow-up.
Poisson approximations were used to calculate confidence intervals (CIs) for TB incidence. Kaplan-Meier was used to construct survival curves. Incidence rate ratios (IRR) were used to identify factors associated with early and late incident TB. The following baseline covariates were used to assess factors associated with early and late incident tuberculosis: age, gender, weight, WHO stage HIV disease, number of previous TB episodes, CD4+ counts and viral load. Data was extracted from routinely collected data recorded on standardized case report forms captured on a customized database through datafax. Statistical analysis was performed using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA).
This study was approved by the Biomedical Research Ethics Committee, University of KwaZulu-Natal, ref no: E248/05.
RESULTS
Baseline demographic characteristics
The study comprised 969 HIV infected patients consecutively initiated on HAART between January 2007 and December 2010. The demographic and clinical characteristics at baseline are presented in Table 1. Approximately two-thirds of the cohort was female (67.8%). The mean age of patients was 34.3 years (SD± 9.6 years). The median follow-up time was 11 (IQR: 6.7 to 19.6) months, with 77.3 % (749/969) of patients still in active follow-up at 24 months post HAART initiation. The median baseline CD4+ count amongst patients with early incident TB; late incident TB; with no TB; and in the entire cohort were 101 (IQR 71–139); 112 (IQR 34–204); 131 (IQR 63–187); and 128 (IQR 61–186) cells/mm3 respectively. The median baseline CD4+ count overall, amongst patients with early incident TB; late incident TB; and with no TB was 128 (IQR 61–186); 101 (IQR 71–139); 112 (IQR 34–204) and 131 (IQR 63–187) cells/mm3 respectively. Approximately 50% of the cohort presented with clinically evident WHO stage 3 HIV disease.
Table 1.
Baseline characteristics of patients enrolled onto HAART
| Characteristics | Overall (N = 969) | No TB (N=745) | Prevalent TB (N=173) | Early incident TB (N=21) | Late incident TB (N=33) |
|---|---|---|---|---|---|
| Age (years), mean ± SD† | 34.3 ± 9.6 | 34.5±9.8 | 33.9±8.3 | 29.3±5.3 | 35.5±11.3 |
| Number of females, (%) n ‡ | (67.8) 651 | (69.2) 509 | (59.5) 103 | (81.0) 17 | (72.7) 24 |
| Weight (kilograms), mean ± SD* | 61.0 ± 13.5 | 62.0±14.1 | 56.9±10.6 | 62.4±12.6 | 58.1±11.4 |
| BMI (kg/m2) <18.5, (%) n ‡‡ | (13.4) 119 | (10.9) 75 | (23.3) 37 | (10.5) 2 | (17.2) 5 |
| Viral load (log copies/ml), mean ± SD** | 4.9 ± 0.9 | 4.9±0.9 | 5.3±0.9 | 5.2±1.1 | 5.1±0.7 |
| CD4+ cell count (cells/mm3), median (IQR)†† | 128 (61–186) | 139 (71–190) | 97 (41–147) | 104 (71–139) | 112 (34–204) |
| WHO Stage 3 of HIV Disease, (%) n *** | (59.3) 567 | (49.8) 365 | (96.0) 166 | (76.2) 16 | (71.9) 23 |
| Past history of TB at HAART initiation (%) (N=947) | (23.9) 226 | (18.3) 133 | (46.8) 80 | (23.8) 5 | (28.1) 9 |
| Recent past TB history, (%) n | (61.5) 139 | (46.6) 62 | (92.5) 74 | 1 (20.0) | 3 (33.3) |
| Remote past TB history, (%) n | (38.5) 87 | (53.4) 71 | (7.5) 6 | 4 (80.0) | 6 (66.7) |
Note: 3 patients who developed late incident TB had prevalent TB
SD = standard deviation, IQR = interquartile range, TB = tuberculosis
BMI =body mass index
10 patients had missing age
9 patients have missing gender
11 patients had missing weight
78 patients had missing BMI data
172 patients had missing viral load
86 patients had missing CD4+ count data
13 patients have missing WHO data
TB Status at HAART Initiation
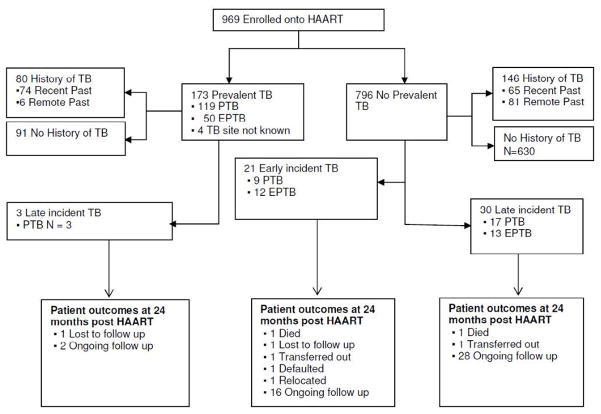
At baseline, all 969 patients were offered a TB symptom screening checklist. The diagnosis of active TB (prevalent TB group) was made in 173/969 patients (17.9%; 95% CI: 15.5 to 20.4). Two thirds of patients with baseline prevalent TB (68.6%) had PTB, 24.9% had EPTB; 4.0% had both PTB and EPTB; and 2.3% had TB with the site unspecified. Diagnosis of prevalent PTB (n=119) was made via sputum smear (n=54/119); chest X-Ray (n=54/65); clinical grounds only (n=9/11); and missing data on method of diagnosis (n=2). The diagnosis of prevalent EPTB (n=43) was made via diagnostic radiology (n=25), lymph node aspirate (n=4); joint aspirate (n=1), histology from biopsy specimen (n=2), on clinical grounds (n=5), unknown (n=6). Seven patients had both PTB and EPTB at baseline, diagnosed on sputum smear (n=1/7); chest X-Ray (n=2/7); clinical grounds only (n=2/7), diagnostic radiology (n=1/7); pleural tap (n=1/7). There were 226 patients with a past history of TB (80 in the prevalent TB group and 146 in the group without prevalent TB at baseline), of which 61.5% had an episode of TB within the year prior to HAART initiation (recent past history) (Figure 1). TB prevalence stratified by immune status is presented in Table 2. Prevalent TB was highest among patients with CD4+ counts < 50 cells/mm3 (25.1%, 47/187; 95% CI: 19.2 to 32.1).
Figure 1. Flow chart depicting TB burden in a community based HAART programme.
Definitions: Past history of TB: patient who completed TB treatment prior to HAART initiation; Recent history of TB: episode of TB within the year prior to HAART initiation; Remote history of TB: TB episode more than a year prior to HAART initiation; Prevalent TB: patient for whom treatment was ongoing during HAART initiation. Incident TB: new cases of TB diagnosed after HAART initiation which was further divided into ““early incident” TB” defined as a new TB diagnosis with 3 months of HAART initiation, and late incident TB defined as new TB diagnosis 4–24 months post HAART initiation.; PTB: pulmonary TB; EPTB: extra-pulmonary TB, including cases of both PTB and EPTB; CAT = The Centre for the AIDS Programme of Research in South Africa (CAPRISA) AIDS Treatment Programme
Notes: Past history of TB information was available for 947 individuals. Cohort included all patients enrolled onto HAART between January 2007 and December 2010; with patient follow-up time from enrolment date up until June 2011, when database was closed.
Table 2.
TB status stratified by CD4+ count
| Overall (N=969) | No TB (N=745) | Prevalent TB (N=173) | * Early incident TB (N=21) | ** Late incident TB (N=33) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline CD4+ count (cells/mm3) | (%) n | (%) n | Patients with prevalent TB/n | TB prevalence(95%CI) | Patients with incident TB/person years | Incidence rate per 100 person-years (95% CI) | Patients with incident TB/person years | Incidence rate per100 person-years (95% CI) |
| Missing | (8.9) 86 | (8.5) 63 | 17/86 | 19.8 (12.3 to 30.0) | 4/14.9 | 26.9 (7.3 to 68.8) | 2/75.6 | 2.6 (0.3 to 9.6) |
| CD4+ count <50/ mm3 | (19.3) 187 | (17.3) 129 | 47/187 | 25.1 (19.2 to 32.1) | 3/31.1 | 9.6 (2.0 to 28.2) | 9/195.1 | 4.6 (2.1 to 8.8) |
| CD4+ count 50–200/mm3 | (55.2) 535 | (56.8) 423 | 87/535 | 16.3 (13.3 to 19.7) | 12/104.2 | 11.5 (5.9 to 20.1) | 14/586.4 | 2.4 (1.3 to 4.0) |
| CD4+ count >200/mm3 | (16.6) 161 | (17.4) 130 | 22/161 | 13.7 (9.0 to 20.2) | 2/33.1 | 6.0 (0.7 to 21.8) | 8/170.8 | 4.7 (2.0 to 9.2) |
n = Number of patients
New TB diagnosis in the first 3 months after HAART initiation
TB incidence between 4–24 months post HAART initiation
Incident TB Infections
There were 54 new clinical TB cases identified after HAART initiation giving an overall TB incidence rate of 4.5 per 100 person-years (py) (95% CI: 3.3 to 5.8). There were 3 cases of incident TB in the group with prevalent TB at the time of HAART initiation. Early incident TB was three fold higher (11.5/100 py; 95%CI: 7.1 to 17.5) compared to late incident TB (3.2/100 py, 95%CI: 2.2 to 4.5; incidence rate ratio (IRR) 3.6, 95%CI: 2.0 to 6.4; p <0.001) (Figure 2). Diagnosis of incident PTB (N=29) was made via sputum smear (n=7/29); chest X-ray (n=19/29); sputum culture (n=1/3) and clinical grounds only (n=2/29). Diagnosis of incident EPTB (n=23) was made via diagnostic radiology (n=13/19), pleural tap (n=1/1), histology from biopsy specimen (n=1/1), clinical grounds only (n=5/23), unknown (n=3). Two patients with incident TB had both PTB and EPTB which was diagnosed by diagnostic radiology.
Figure 2.
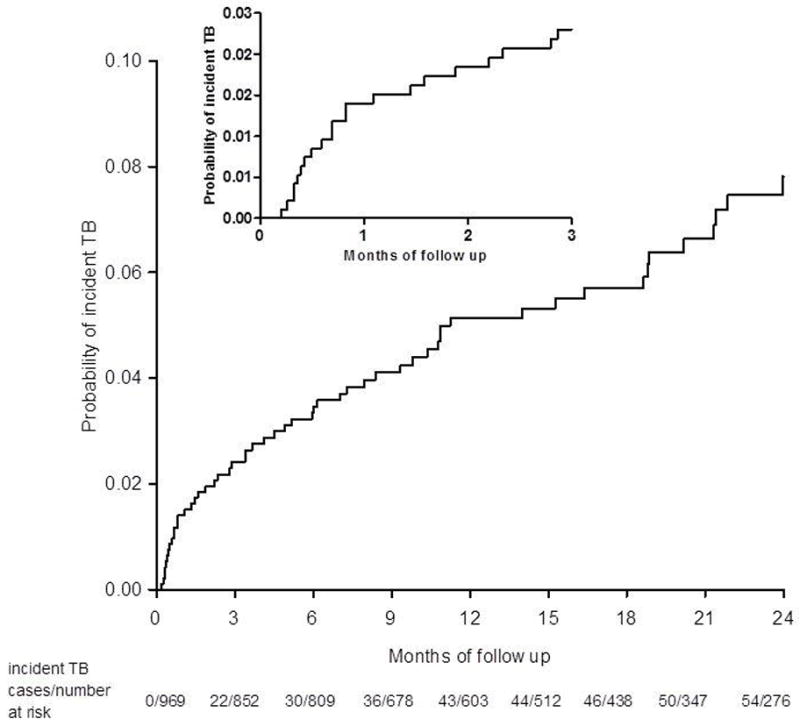
Kaplan-Meier estimates of cumulative probability of developing early incident TB
Risk factors for early and late incident TB
The rate of early incident TB was almost two-fold higher among female; 13.3 /100 py; (95%CI: 7.6 to 21.3) compared to male patients; 7.3/100 py; (95%CI: 2.0 to 18.8; IRR: 1.8, 95%CI: 0.6 to 7.4; p=0.21), and almost five-fold higher among patients aged between 24 and 34 years (17.8 /100 py, 95%CI: 10.0 to 29.4); compared to ≥35 years (3.8/100 py, 95%CI: 0.8 to 11.1; IRR: 4.7, 95%CI: 1.3 to 25.2; p=0.01) (Table 3). However, interaction between gender and age was not statistically significant (p=0.10). No other factors were found to be associated with incident TB.
Table 3.
Predictors of early and late incident TB
| Risk factor | *†Early TB | **Late TB | ||||
|---|---|---|---|---|---|---|
| Number with incident TB/person-years | Incidence (95%CI) | Incidence rate ratio | Number with incident TB/person-years | Incidence (95%CI) | Incidence rate ratio | |
| Age (years) | ||||||
| missing | 0/1.3 | - | 0/2.8 | |||
| <24 | 3/19.2 | 15.6 (3.2 to 45.6) | 4.1 (0.5 to 30.5) | 4/97.0 | 4.1 (1.1 to 10.6) | 1.1 (0.2 to 3.8) |
| 24–34 | 15/84.2 | 17.8 (10.0 to 29.4) | 4.7 (1.3 to 25.2) | 14/492.8 | 2.8 (1.6 to 4.8) | 0.8 (0.4 to 1.8) |
| 35+ | 3/78.7 | 3.8 (0.8 to 11.1) | reference | 15/435.2 | 3.4 (1.9 to 5.7) | reference |
|
| ||||||
| Gender | ||||||
| missing | 0/1.2 | - | 0/2.8 | |||
| Male | 4/54.5 | 7.3 (2.0 to 18.8) | reference | 9/326 | 2.8 (1.3 to 5.2) | reference |
| Female | 17/127.6 | 13.3 (7.6 to 21.3) | 1.8 (0.6 to 7.4) | 24/699 | 3.4 (2.2 to 5.1) | 1.2 (0.6 to 3.0) |
|
| ||||||
| BMI (kg/m2) | ||||||
| missing | 2/11.9 | 16.8 (2.0 to 60.7) | 4/64.6 | 6.2 (1.7 to 15.8) | ||
| <18.5 | 2/18.2 | 11.0 (1.3 to 39.7) | 0.9 (0.1 to 4.3) | 5/119.7 | 4.2 (1.4 to 9.7) | 1.6 (0.4 to 5.7) |
| 18.5–25 | 9/90.7 | 9.9 (4.5 to 18.8) | 0.8 (0.3 to 2.3) | 16/527.4 | 3.0 (1.7 to 4.9) | 1.2 (0.5 to 3.2) |
| >25 | 8/62.5 | 12.8 (5.5 to 25.2) | reference | 8/316.1 | 2.5 (1.1 to 5.0) | reference |
|
| ||||||
| WHO Clinical Stage of HIV Disease | ||||||
| missing | 0/1.8 | - | 1/5.6 | 17.8 (0.5 to 99.2) | ||
| 3 or 4 | 16/89.4 | 17.9 (10.2 to 29.1) | reference | 23/587.6 | 3.9 (2.5 to 5.8) | reference |
| 1 or 2 | 5/92.2 | 5.4 (1.8 to 12.7) | 0.3 (0.1 to 0.9) | 9/434.6 | 2.1 (0.9 to 3.9) | 0.5 (0.2 to 1.2) |
|
| ||||||
| Number of previous episodes of TB | ||||||
| missing | 0/3.3 | 1/14.4 | 7.0 (0.2 to 38.7) | |||
| 0 | 16/146.2 | 10.9 (6.3 to 17.8) | reference | 21/653.9 | 3.2 (2.0 to 4.9) | reference |
| 1 | 5/33.9 | 14.8 (4.8 to 34.4) | 1.3 (0.4 to 3.9) | 10/257.1 | 3.9 (1.9 to 7.2) | 1.2 (0.5 to 2.7) |
| 2 | - | - | 1/102.4 | 1.0 (0 to 5.4) | 0.3 (0 to 1.9) | |
|
| ||||||
| CD4+ count (cells/μL3) at HAART initiation | ||||||
| missing | 4/14.9 | 26.9 (7.3 to 68.8) | 2/75.6 | 2.6 (0.3 to 9.6) | - | |
| <50 | 3/31.1 | 9.6 (2.0 to 28.2) | 1.6 (0.2 to 19.1) | 9/195.1 | 4.6 (2.1 to 8.8) | 1.0 (0.3 to 2.9) |
| 50–200 | 12/104.2 | 11.5 (5.9 to 20.1) | 1.9 (0.4 to 17.6) | 14/586.4 | 2.4 (1.3 to 4.0) | 0.5 (0.2 to 1.4) |
| >200 | 2/33.1 | 6.0 (0.7 to 21.8) | reference | 8/170.8 | 4.7 (2.0 to 9.2) | reference |
|
| ||||||
| Viral load at HAART initiation(log copies/ml) | ||||||
| missing | 6/31.2 | 19.2 (7.1 to 41.8) | 8/210.4 | 3.8 (1.6 to 7.4) | - | |
| <5 | 4/80.7 | 5.0 (1.4 to 12.7) | 0.3 (0.1 to 1.1) | 13/387.8 | 3.4 (1.8 to 5.7) | 1.2 (0.5 to 2.9) |
| ≥ 5 | 11/71.5 | 15.4 (7.7 to 27.5) | reference | 12/429.6 | 2.8 (1.4 to 4.9) | reference |
Baseline immune status of patients did not impact overall TB incidence rates; patients with CD4+ counts <50 cells/ mm3 had TB incidence rates of 5.3/100 py (95% CI: 2.7 to 9.3) compared to 4.9/100 py (95%CI: 2.4 to 9.0) in patients with CD4+ counts >200 cells/mm3 (p=0.81). Additionally, baseline immune status did not impact rates of incident TB.
TB incidence rates 3 months post HAART initiation among patients with CD4+ counts < 50; between 50 and 200; and > 200 cells/mm3 were 4.6 (95%CI: 2.1 to 8.8); 2.4 (95%CI: 1.3 to 4.0); and 4.7 (95%CI: 2.0 to 9.2) per 100 py (Table 2).
Impact on therapeutic outcomes
There were 51 incident cases of TB among patients with no prevalent TB at baseline (26 PTB; 25 EPTB), and three incident cases of PTB among patients with prevalent TB at baseline. There was one case of multi-drug resistant TB in this cohort. Patients with incident PTB and EPTB had similar median baseline CD4+ counts, 106 vs. 129 cells/mm3 (p=0.57), and baseline log viral loads, 5.2 vs. 5.0 (p=0.19); and similar mean CD4+ count increases (p=0.17) and log viral load decreases (p=0.16) from baseline to 12 months post HAART initiation. The median (IQR) months on HAART to a new episode of TB was 3.7 (0.8 to 10.9) and 5.2 (1.6 to 10.8) in the PTB and EPTB groups respectively. Median CD4+ count change from baseline to 12 months post HAART initiation was significantly higher in patients experiencing early incident 167 (IQR 141–235) versus late incident 76 (IQR 32–187) cells/mm3 TB (p=0.03) (Supplemental Digital Content Table 4).
Mortality Rates
There were 57 deaths in this cohort with an overall mortality rate of 4.3 per 100 py (95% CI: 3.3 to 5.6). There were seven deaths among patients with baseline prevalent TB, mortality rate of 2.7 (95% CI: 1.1 to 5.6) per 100 py; compared to 50 deaths among 796 patients with no baseline prevalent TB, mortality rate of 4.7 (95% CI: 3.5 to 6.3) per 100 py (p=0.20) of follow-up. Patients with at least 1 episode of TB (either past history, prevalent or incident TB) had a lower mortality rate 3.4 (95% CI: 2.0 to 5.4) compared to patients with no TB, 4.5 (95% CI: 3.1 to 6.3) per 100 py (IRR: 0.8, 95%CI: 0.5 to 1.4; p=0.42) of follow-up. There was one death each in the early and late incident TB groups. The causes of death for 29 of 57 patients was known and this included PTB (n=4); EPTB (n=5); complications from severe gastroenteritis (n=6); trauma (n=2); natural causes (n=5); meningitis-unknown cause (n=2); lower respiratory tract infection (n=2); cerebro-vascular accident (n=1), intracranial lesion (n=1), complications from hyperlactatemia (n=1). Causes of death were not available in 28 patients as many of them died at home.
DISCUSSION
In a rural HIV treatment programme in a TB endemic setting, we found unacceptably high TB incidence rates irrespective of baseline immunologic status of patients at HAART initiation, or duration on HAART. We document TB incidence rates of 4.5/100 py of follow-up among HIV-infected patients receiving community-based HAART care in a rural setting. This incidence is higher than reports from other sub-Saharan countries and most other high TB burden settings2,21,22 and nearly 10-fold higher than reported among HIV negative patients from a setting with similar TB notification rates23. Thus, we provide further evidence for continued susceptibility of HAART-accessing patients to TB infection in a hyper-endemic TB setting sustained over 2 years post HAART initiation. This continued susceptibility to TB despite HAART likely points to on-going community-level and possibly nosocomial TB transmission.
We demonstrate an alarmingly high and consistent TB incidence (Table 2) among all baseline CD4+ count strata during study follow-up. The data show an inverse relationship between time on HAART and TB incidence, corroborating past studies, and likely due to greater TB-specific immune restoration with time spent on HAART6,24–26. Further, we highlight the need for close clinical observation in the first few months post HAART initiation by demonstrating highest rates of incident TB, likely due to “unmasked” infection, in the first three months post HAART initiation. Additionally, the continued high rate of new TB infections even at 24 months post HAART initiation is in keeping with published reports showing that, despite its decline with time on HAART, TB incidence among a HAART-accessing HIV population remains higher than in the general HIV-uninfected population27. We speculate that the high TB incidence rates observed in this study may be due to either impaired restoration of TB specific immunity when patients are severely immune-compromised (baseline CD4+ counts ≤200 cells/mm3) at HAART initiation, or due to high ongoing community level TB transmission. The incidence rates observed is most likely due to a combination of both these factors. Interestingly, although women carry a disproportionate burden of HIV in sub-Saharan Africa, and despite finding a two-fold higher rate of early incident TB in women compared to men; further analysis stratified by age and gender showed no statistically significant difference in risk for either early or late incident TB. Notwithstanding lower pre-HAART CD4+ counts among patients that developed early incident TB; therapeutic outcomes among patients at 12 months post HAART initiation was similar in all groups.
In contrast to our study, published literature demonstrates a far more substantial time dependant reduction in TB incidence among patients on HAART28–37. These studies report highest TB incidence during the first 3 months of HAART10 with a progressive reduction of all forms of TB during the first year of follow-up from 5.77/100 to 2.23/100 py38. Published meta-analysis data from developed country cohorts estimate a comparable effect of HAART on TB incidence despite differences in background risk of M. tuberculosis infection. These data provide an estimated TB incidence of 3 cases per 1000 py among patients accessing HAART; 10 fold lower compared to the TB incidence rates we found10. Findings similar to ours was reported in only one other study, conducted in a densely-populated urban informal settlement with a HIV sero-prevalence of 28% and TB notification rate of >1000/100000 population. In this study, TB incidence was reduced from 22.1 to 4.5/100 py among patients with a median baseline CD4+ count of 96 cells/mm3 (IQR 46–156), after approximately 3 years of HAART39.
The vast majority of TB episodes among patients with a previous TB history occurred in the 5-year period immediately prior to HAART initiation, a likely clinical feature of symptomatic HIV disease. We demonstrated a TB prevalence rate of 17.9% among HIV-infected patients initiating HAART, lower than previous reports from a South African community-based HAART program39. Other published studies from resource-limited settings report a high TB prevalence at HAART enrolment25,39 primarily among patients with CD4+ count <50 cells/mm3. However, data from this study shows high rates of prevalent TB especially in lower baseline CD4+ count strata, and not only among those severely immune compromised40. Among patients enrolling on HAART, one in four with CD4+counts <100 cells/mm3 and one in seven patients with CD4+counts ≥100 cells/mm3 had active TB. It is important to note however, that excluding active TB among HIV-infected patients is usually complex owing to high rates of smear-negative TB and difficulty of diagnosing asymptomatic or subclinical TB, common among patients accessing HAART41,42.
Unsurprisingly, there was no statistically significant difference in mortality rates among cases with known TB at baseline compared to those without. Mortality studies among HIV-infected patients conducted in this setting have repeatedly demonstrated high rates of undiagnosed TB responsible for as much as 79% of all deaths in HIV-infected patients43,44. Mortality rates were similar among patients with new episodes of EPTB as compared to those with new episodes of PTB; 2.3 (95% CI: 0.1 to 13.1) vs. 2.1 (95% CI: 0 to 11.6) per 100 py of follow-up. Interestingly, the mortality rate at 24 months in this study among patients with and without prevalent TB at baseline was very different to 5 year mortality rates of 4.84 and 2.62 per 100 person years of follow up among prevalent TB and TB free patients initiating ART in Cape Town South Africa45.
While it may be likely that most cases of early incident TB was due to immune reconstitution inflammatory syndrome (IRIS), it remains unclear as to what proportion of incident TB was due to “unmasking” versus “paradoxical” TB IRIS versus new TB infections. It is well understood that IRIS embodies an interpretive crisis since its clinical diagnosis is similar to TB treatment failure, drug toxicities, TB relapse or new AIDS defining illness46, and cannot be diagnosed by available tests47. Although consensus case definitions for TB IRIS have been formulated20, the translation of these guidelines to the bedside remains poor. It is likely that this study underestimated rates of TB IRIS due to the poor ability to diagnose this phenomenon. In addition, in the absence of chest x-rays, minimally symptomatic or asymptomatic pulmonary disease may be incorrectly attributed to HIV or to other opportunistic infections.
We acknowledge several limitations. This study was conducted in a hyper endemic HIV and TB setting which limits the generalizability of these findings to similar settings. Limited access to chest x-rays in this primary health care setting, especially for asymptomatic patients with low CD4+ counts may have led to significant underreporting of TB; which potentially limits the validity, and to a lesser extent the generalizability of the study. The reliance on TB clinical symptom screening; lack of use of a standardized algorithm for TB screening and diagnosis, together with limited access to TB microscopy services, further contributed to TB cases being missed. The findings from this study highlight the need for enhanced screening and the use of a diagnostic algorithm for TB in a setting with generalized TB and HIV co-epidemics. High rates of smear negative TB and long delays in TB diagnosis may have led to the misclassification of prevalent TB cases as incident TB, especially for episodes that occurred in the first 3 months post-HAART initiation. Data used was routinely collected programmatic data, and missing data elements may have led to an underestimation of the actual burden of TB in this setting. Additionally, TB outcome data was not always readily available for complicated patients referred for hospitalization or for those that were lost-to-follow-up. In contrast to published literature, we report low IRIS estimates, which may be due to the retrospective nature of this study and to the lack of standardised clinical tools for diagnosing IRIS. Molecular strain typing would have been extremely useful in determining if cases of recurrent TB in this cohort were due to relapse or reinfection particularly in patients with early incident TB and in the 3 cases with incident and recurrent TB, but given the lack of cost-effective but unavailable tools such as chest x-rays in this setting, such endeavours were unfortunately unrealistic. It is important to note that our health care facility implemented standard WHO TB infection control measures, and data for this study was collected prior to the programmatic implementation of IPT. The impact of IPT on TB prevalence and incidence in community based HAART programmes remains to be explored, however, in this cohort it is important to note that starting patients on IPT with HAART may result in many patients with active TB disease having initiated IPT.
CONCLUSION
Our study on the prevalence and incidence of TB in a rural, community-based HIV treatment programme has described a disproportionately high TB burden in patients accessing HAART. Our findings highlight the urgent need to implement TB preventive therapy and TB infection control practices in HAART programmes in endemic settings. TB incidence by baseline CD4+ count is highest among patients with CD4+ <50 cells/mm3,40; however, we also observed high TB incidence rates at higher CD4+ counts. Therefore, despite apparent immunologic recovery among HAART-accessing HIV patients, high TB incidence is still a potent threat, possibly reflecting community and nosocomial TB transmission. The availability of point-of-care diagnostic assays such as GeneXpert that readily diagnose TB while patients queue for services will facilitate TB case finding prior to the start of HAART, thereby reducing TB related morbidity and mortality commonly found among patients newly enrolled into HAART programmes in TB endemic setting.
Supplementary Material
Acknowledgments
Funding
Patient care in the CAPRISA AIDS Treatment project is supported by the KwaZulu-Natal Department of Health, the Global Fund to fight AIDS, Tuberculosis and Malaria and the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the US National Institutes for Health’s Comprehensive International Program of Research on AIDS grant (CIPRA, grant # AI51794). KN was supported by the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, grant # D43 TW000231). AB was supported by funds from the Oxford University Department of Public Health. The funding sources listed here did not have any role in the analysis or preparation of the data in this manuscript, nor was any payment received by these or other funding sources for this manuscript.
We gratefully acknowledge the contributions of the CAPRISA AIDS Treatment team for providing clinical care of study patients. We thank the KwaZulu-Natal Department of Health, the staff of the Umgungundlovu district health office and the nurses at the Mafakhathini Primary Care Clinic for their professional support and for clinical care of patients. The mentorship and oversight provided by Professor Salim Abdool Karim and Quarraisha Abdool Karim of CAPRISA was invaluable, without which this project would not have been possible.
Footnotes
Presentations at meetings: This has been presented as an oral abstract at the 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2013), Kuala Lumpur, Malaysia, 30 June - 3 July 2013; Abstract number A-581-0078-00125.
Financial disclosure: All Authors report no conflict of interest
Conflicts of Interest
All authors declare no conflicts of interest.
Authors Contributions
KN conceived the study. KN and AB designed and conducted the study. KN, QAK, SSAK, AB, NYZ and KN prepared, extracted and analyzed the data. JF, MU, FK and PK provided ongoing support, management of clinical care and study co-ordination. KN, QAK, SSAK, AB, and KN wrote the manuscript. KN, QAK, SSAK, AB, KN, and NYZ interpreted the data. All authors approved submission of this manuscript.
References
- 1.WHO. Global tuberculosis control - epidemiology, strategy, financing. WHO Report 2009. Geneva, Switzerland: 2009. [Google Scholar]
- 2.W.H.O. Global Tuberculosis Control 2011. Geneva, Switzerland: 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 3.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009 Sep 12;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008 Jan 2;22(1):139–144. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 5.HST. [Accessed December 2010.];Reported cases of TB (all types) (per 100 000) http://www.hst.org.za/healthstats/16/data.
- 6.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002 Jun 15;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 7.Girardi E, Sabin CA, d’Arminio Monforte A, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005 Dec 15;41(12):1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 8.Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PloS one. 2007;2(9):e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002 Feb 15;34(4):543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 10.Collaboration H-C. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012 May;54(9):1364–1372. doi: 10.1093/cid/cis203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Harries AD, Williams BG, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011 May;15(5):571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardi E, Antonucci G, Vanacore P, et al. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur Respir J. 2004 Jul;24(1):11–17. doi: 10.1183/09031936.04.00109303. [DOI] [PubMed] [Google Scholar]
- 13.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010 Feb 25;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011 Oct 20;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. The New England journal of medicine. 2011 Oct 20;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. The New England journal of medicine. 2011 Oct 20;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D.o.H. National Antiretroviral Treatment Guideline, National Department of Health South Africa 2004. 2004 http://www.kznhealth.gov.za/arv/arv5.pdf.
- 18.D.o.H. The South African Antiretroviral Treatment Guidelines 2010. 2010 http://www.uj.ac.za/EN/CorporateServices/ioha/Documentation/Documents/ART%20Guideline.pdf.
- 19.DoH, South, Africa. National Tuberculosis Management Guidelines 2009. 2009 http://familymedicine.ukzn.ac.za/Libraries/Guidelines_Protocols/TB_Guidelines_2009.sflb.ashx.
- 20.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet infectious diseases. 2008 Aug;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middelkoop K, Bekker LG, Myer L, et al. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. American journal of respiratory and critical care medicine. 2010 Oct 15;182(8):1080–1085. doi: 10.1164/rccm.201004-0598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, et al. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clinical & developmental immunology. 2011;2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. The Journal of infectious diseases. 2007 Aug 15;196 (Suppl 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 24.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009 Jul;13(7):862–867. [PMC free article] [PubMed] [Google Scholar]
- 25.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007 Mar 30;21(6):713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet MM, Pinoges LL, Varaine FF, et al. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS. 2006 Jun 12;20(9):1275–1279. doi: 10.1097/01.aids.0000232235.26630.ee. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005 Dec 2;19(18):2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 28.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 29.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009 Sep 15;49(6):965–972. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 30.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008 Oct 1;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi BH, Giganti M, Mulenga PL, et al. CD4+ response and subsequent risk of death among patients on antiretroviral therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009 Sep 1;52(1):125–131. doi: 10.1097/QAI.0b013e3181ab6d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger S, Petoumenos K, Kamarulzaman A, et al. Long-term patterns in CD4 response are determined by an interaction between baseline CD4 cell count, viral load, and time: The Asia Pacific HIV Observational Database (APHOD) J Acquir Immune Defic Syndr. 2009 Apr 15;50(5):513–520. doi: 10.1097/qai.0b013e31819906d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Graviss EA. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003 Dec;7(12):1178–1185. [PubMed] [Google Scholar]
- 34.Moore DM, Harris R, Lima V, et al. Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):357–363. doi: 10.1097/QAI.0b013e3181b62933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanjako D, Kiragga A, Ibrahim F, Castelnuovo B, Kamya MR, Easterbrook PJ. Sub-optimal CD4 reconstitution despite viral suppression in an urban cohort on antiretroviral therapy (ART) in sub-Saharan Africa: frequency and clinical significance. AIDS Res Ther. 2008;5:23. doi: 10.1186/1742-6405-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash D, Katyal M, Brinkhof MW, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008 Nov 12;22(17):2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawn SD, Wood R. Incidence of tuberculosis during highly active antiretroviral therapy in high-income and low-income countries. Clin Infect Dis. 2005 Dec 15;41(12):1783–1786. doi: 10.1086/498308. [DOI] [PubMed] [Google Scholar]
- 38.Dembele M, Saleri N, Carvalho AC, et al. Incidence of tuberculosis after HAART initiation in a cohort of HIV-positive patients in Burkina Faso. Int J Tuberc Lung Dis. 2010 Mar;14(3):318–323. [PubMed] [Google Scholar]
- 39.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006 Aug 1;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 40.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PloS one. 2010;5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbett EL, Bandason T, Cheung YB, et al. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS medicine. 2007 Jan;4(1):e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005 May 15;40(10):1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 43.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS medicine. 2010 Jun;7(6):e1000296. doi: 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinson NA, Karstaedt A, Venter WD, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007 Oct 1;21(15):2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PloS one. 2013 Feb;8(2):e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. The Journal of infectious diseases. 2009 Feb 1;199(3):437–444. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 47.Leone S, Nicastri E, Giglio S, Narciso P, Ippolito G, Acone N. Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: a systematic review. Int J Infect Dis. 2010 Apr;14(4):e283–291. doi: 10.1016/j.ijid.2009.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.