Abstract
Rationale and Objectives
Earlier initiation of antiretroviral therapy (ART) in HIV-tuberculosis(TB) is associated with increased immune reconstitution inflammatory syndrome (IRIS). The severity, frequency and complications of TB IRIS were evaluated in A5221, a randomized trial of earlier ART (within 2 weeks after TB treatment initiation) vs. later ART (8-12 weeks after TB treatment) in HIV-infected patients starting TB treatment.
Methods and Measurements
In 806 participants, TB IRIS was defined using published clinical criteria. Cases were classified as severe(hospitalization/death), moderate(corticosteroid use/invasive procedure), or mild(no hospitalization/procedures/steroids). Fisher's Exact, Wilcoxon, and log rank tests were used for comparisons.
Main Results
TB IRIS occurred in 61 (7.6%) patients: 10.4% in earlier vs. 4.7% later ART, 11.5% with CD4+ < 50 vs. 5.4% CD4+ ≥ 50 cells/mm3. The CD4+/ART arm interaction was significant, p=0.014, with 44.3% of TB IRIS occurring with CD4+ < 50 and earlier ART. TB IRIS occurred sooner with earlier vs. later ART initiation, at a median of 29 vs. 82 days after TB treatment initiation (p<0.001). IRIS manifestations included lymphadenopathy(59.0%), constitutional symptoms(54.1%), and radiographic changes(41.0%); CNS TB IRIS was uncommon (6.6%). TB IRIS was mild in 27.9%, moderate in 41.0%, and severe in 31.1%. No TB IRIS-associated deaths occurred. IRIS management required ≥ 1 invasive procedures in 34.4%, hospitalization in 31.1% and corticosteroids in 54.1%.
Conclusions
TB IRIS was more frequent with earlier ART initiation and CD4+ <50 cells/mm3. As ART is implemented earlier in HIV-TB co-infection, programs will require the diagnostic capabilities, clinical resources and training necessary to manage TB IRIS.
Keywords: HIV/AIDS, tuberculosis, immune reconstitution inflammatory syndrome (IRIS), paradoxical reaction
Background
Antiretroviral therapy (ART) must be started during tuberculosis (TB) treatment in human immunodeficiency virus (HIV)-TB co-infected patients to reduce mortality[1]. ART should be started as early as two weeks after tuberculosis treatment initiation in patients with low CD4+ cell counts to decrease mortality and AIDS complications[2-4]. However, co-treatment of HIV and tuberculosis is complicated by drug-drug interactions, overlapping medication toxicities, programmatic challenges of implementing earlier ART and an increased risk of paradoxical immune reconstitution inflammatory syndrome (IRIS). Paradoxical TB IRIS refers to recurrence or worsening clinical features of tuberculosis despite effective tuberculosis treatment, in contrast to unmasking IRIS, which is a new presentation of unrecognized tuberculosis that is “unmasked” after initiation of ART[5].
Paradoxical TB IRIS incidence increases when ART is started in close proximity to TB treatment and in those with low CD4+ cell counts[6]. Thus, increased rates of TB IRIS are anticipated as programs scale up implementation of earlier ART in TB co-infection to reduce AIDS progression and death[7].
To inform clinicians and programs providing ART in HIV-TB co-infected patient, this planned secondary analysis was conducted to characterize the frequency, presentation, severity and risk factors for TB IRIS within the AIDS Clinical Trials Group (ACTG) A5221 STRIDE study. The STRIDE study was an open label, randomized study comparing ART started earlier (within 2 weeks after TB treatment initiation) vs. later (8-12 weeks after TB treatment initiation) in HIV-infected participants with CD4+ cell counts < 250 cells/mm3 receiving treatment for suspected tuberculosis[2].
Methods
Study population
The STRIDE study enrolled HIV-infected ART-naïve participants with confirmed or probable tuberculosis, stratified by screening CD4+ cell count of < or ≥ 50 cells/ mm3; eligibility criteria for the STRIDE study are described in detail elsewhere[2]. Confirmed TB was defined as detection of acid fast bacilli (AFB) in sputum smear or lymph node specimen, or a positive culture for Mycobacterium tuberculosis from sputum, lymph node, or another sterile site. Probable TB required clinician's assessment that signs and symptoms warranted empiric TB treatment. Participants were required to have received 1-14 days of rifamycin-based TB treatment at the time of study entry. Study-provided ART was 600 mg of efavirenz daily (Stocrin, donated by Merck) and a fixed-dose combination of emtricitabine 200 mg daily and tenofovir disoproxil fumarate 300 mg daily (Truvada, donated by Gilead Sciences). Participants were followed for 48 weeks after study entry. The study protocol was approved by an institutional review board or ethics committee at each participating site. The National Institutes of Health funded this study and provided study oversight.
TB IRIS definition and severity index
Paradoxical TB IRIS was defined as the presence of at least one major or two minor TB IRIS criteria, from the International Network for the Study of HIV-Associated IRIS (INSHI) case definition[8]. Major TB IRIS criteria were new or worsening: 1) lymph nodes, cold abscesses, or other focal tissue involvement, 2) radiologic features of tuberculosis, 3) central nervous system tuberculosis, or 4) serositis (pleural effusion, ascites or pericardial effusion). Minor TB IRIS criteria were new or worsening: 1) constitutional symptoms (fever, night sweats, weight loss), 2) respiratory symptoms (cough, dypnea, stridor), or 3) abdominal pain accompanied by peritonitis, hepatomegaly, splenomegaly or abdominal adenopathy. The STRIDE TB IRIS case definition did not require concomitant ART administration nor demonstration of initial response to TB treatment. Site investigators were not blinded to treatment arm allocation when evaluating possible TB IRIS. All suspect TB IRIS cases were reported on a standardized case report form (see Supplemental Digital Content) and were confirmed by an independent reviewer blinded to treatment allocation (CAB). TB IRIS was managed as per local standard of care and not specified in the study protocol. A study-defined TB IRIS severity index was applied as follows: severe–IRIS complicated by hospitalization or death, moderate–IRIS necessitating corticosteroid use or invasive procedure for evaluation and/or symptom management (such as lymph node drainage), or mild–IRIS cases without hospitalization, steroid use, or invasive procedures.
Statistical analysis
The primary endpoint of this secondary analysis was time from the date of TB treatment initiation to the date of TB IRIS diagnosis. Participants who died or were lost to follow-up or completed follow-up without a TB IRIS diagnosis were censored at their last clinic visit. Failure-time plots were calculated using the Kaplan-Meier method[9]. Cox proportional hazards models, stratified by screening CD4+, examined associations between baseline covariates (age, male sex, race/ethnicity, HIV RNA, body mass index (BMI), presence of AIDS-defining illness other than TB, randomized treatment strategy, and confirmed vs. probable TB, and South Africa vs. elsewhere, South America vs. elsewhere) and time to TB IRIS [10]. Covariates that were univariately significant (p<0.1) were examined in a multivariate Cox proportional hazards analysis. Using the backward elimination method, the final stratified multivariate model contained only significant covariates. Since participants often died or withdrew from study before a TB IRIS diagnosis, a competing risks analysis was completed as a sensitivity analysis. Because a stratified competing risks analysis could not be done using available software, the multivariate model as per the non-stratified Cox proportional hazards analysis was fit using proportional hazards regression modeling of sub-distribution functions in competing risks ([11]).Between-group differences were assessed using Fisher's Exact, proportion, Wilcoxon rank-sum, exact ordered Wilcoxon, and log rank tests. Interactions were assessed using logistic regression. These tests were two-sided with a 5% Type 1 error rate. Incidence of TB IRIS was calculated using standard epidemiological methods.
Results
From September 2006 through August 2009, 806 STRIDE participants were enrolled from 26 sites on 4 continents. TB IRIS occurred in 7.6% (61/806) (Table 1), with an overall incidence 9.0/100 person-years (PY). The proportion with TB IRIS was twice as high in those randomized to earlier ART vs. later ART (10.4% vs. 4.7%) and in those with screening CD4+ cell count < 50 vs. ≥ 50 cells/mm3 (11.5% vs. 5.4%) (Table 2); there was a significant interaction between CD4+ cell count strata and ART arm (earlier vs. later, p=0.014). Nearly half of TB IRIS cases (44.3% (27/61)) occurred in participants with screening CD4+< 50 randomized to earlier ART. In those with screening CD4+ ≥ 50, there was no difference in TB IRIS incidence between earlier and later ART (5.7% vs. 5.0%, proportion test p= 0.85).
Table 1.
Participant characteristics
| TB IRIS | |||||
|---|---|---|---|---|---|
| Characteristic [median (Q1, Q3) or N (%)] | Total (N=806) | Yes (N=61) | No (N=745) | P-Value | |
| Baseline age (years) | 34 (29, 41) | 33 (28, 41) | 34 (29, 41) | 0.67a | |
| Sex | Male | 501 | 44 (8.9%) | 457 (91.2%) | 0.10b |
| Female | 305 | 17 (5.6%) | 288 (94.4%) | ||
| Race | Black | 634 | 50 (7.9%) | 584 (92.1%) | 0.78b |
| White | 66 | 5 (7.6%) | 61 (92.4%) | ||
| Asian | 55 | 2 (3.6%) | 53 (96.4%) | ||
| Other | 51 | 4 (7.8%) | 47 (92.2%) | ||
| Ethnicity | Non-Hispanic | 670 | 42 (6.3%) | 628 (93.7%) | 0.004 b |
| Hispanic | 136 | 19 (14.0%) | 117 (86.0%) | ||
| Site of enrollment | Other Africac | 333 | 6 (1.8%) | 327 (98.2%) | <0.001 b |
| South Africa | 221 | 33 (14.9%) | 188 (85.1%) | ||
| South America | 161 | 16 (9.9%) | 145 (90.1%) | ||
| Asia | 52 | 2 (3.8%) | 50 (96.2%) | ||
| North America | 39 | 4 (10.3%) | 35 (89.7%) | ||
| Baseline CD4+ count (cells/mm3)d | 77 (36, 145) | 45 (24, 81) | 82 (39, 146) | <0.001 a | |
| CD4+ count at ART start (cells/mm3)e | 76 (34, 146) | 40 (23, 77) | 79 (36, 150) | <0.001 a | |
| Baseline HIV RNA (log™ copies/mL)f | 5.4 (5.0, 5.8) | 5.7 (5.4, 5.9) | 5.4 (5.0, 5.8) | <0.001 a | |
| Baseline Body Mass Index (BMI, kg/m2) | 19.2 (17.5, 21.4) | 20.2 (18.3, 22.4) | 19.2 (17.4, 21.3) | 0.065a | |
| Presence of AIDS-defining illness other than TB at randomization | 55 | 6 (10.9%) | 49 (89.1%) | 0.30b | |
| TB at randomization | Confirmed | 374 | 47 (12.6%) | 327 (87.4%) | <0.001 b |
| Probable/No TB | 432 | 14 (3.2%) | 418 (96.8%) | ||
| Days on TB treatment at ART startg | 14 (10, 70) | 12 (8, 65) | 24 (10, 70) | 0.028 a | |
Wilcoxon rank-sum test with continuity correction.
Fisher's Exact test.
Other Africa included Botswana, Kenya, Malawi, Uganda, Zambia, and Zimbabwe.
Nine participants had missing baseline CD4+ count; screening CD4+ count were used in this summary.
Due to missing data (ART not initiated), sample sizes were N= 765, N = 60, and N = 705, respectively.
Due to missing HIV RNA data, sample sizes were N= 803, N = 61, and N = 742, respectively.
Due to missing data (ART not initiated), sample sizes were N=783, N = 61, and N = 722, respectively.
Table 2.
TB IRIS cases by treatment strategy and CD4+ strata
| Earlier ART 10.4% (42/405) | Later ART 4.7% (19/401) | |
|---|---|---|
| CD4+ < 50 11.5% (33/285) | 18.8% (27/144) | 4.3% (6/141) |
| CD4+ > 50 5.4% (28/521) | 5.7% (15/261) | 5.0% (13/260) |
Note: Significant interaction between CD4+ strata and treatment strategy, logistic regression p=0.014.
TB IRIS manifestations and timing
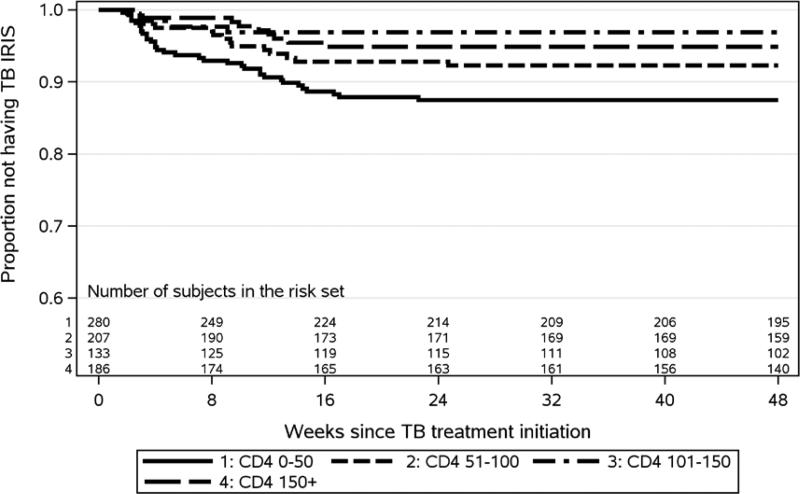
Of the total 61 TB IRIS cases, 42 (69%) occurred in the earlier ART arm and 19 (31%) in the later ART arm. The majority (93.4% (57/61)) of TB IRIS cases met one or more major TB IRIS criteria, while 6.6% (4/61) met only two or more minor criteria for TB IRIS (Table 3). The most common manifestations of TB IRIS were new or worsening lymphadenopathy (59.0%) and radiographic features of TB (41.0%), with infrequent serositis (8.2%) and CNS manifestations (6.6%). Minor TB IRIS criteria of constitutional (54.1%) and respiratory (34.4%) symptoms were also frequent. The majority of IRIS cases occurred in the first 4 months (Figure 1) after TB treatment initiation (median 57 days (Q1, Q3 22, 84)) and presented significantly earlier in participants randomized to earlier ART vs. later (median 29 vs. 82 days, p<0.001) (Table 4). Four TB IRIS cases occurred prior to ART initiation in the later ART arm; no IRIS cases occurred prior to ART in the earlier arm. Among those who initiated ART prior to experiencing TB IRIS, median time to TB IRIS after ART initiation was 16 days (Q1, Q3 10, 49), 18 days with earlier and 15 days with later ART initiation (p=0.28). TB IRIS symptoms lasted a median of 87 days (Q1, Q3 44, 139) with a non-significant difference between earlier and later ART initiation (median 92 vs. 70 days, respectively, p=0.79).
Table 3.
Characteristics of TB IRIS
| Treatment Strategy | |||
|---|---|---|---|
| Characteristic | Total (N=61) | Earlier ART (N=42) | Later ART (N=19) |
| Major TB IRIS criteria met (1 or more) | 57 (93.4%) | 38 (90.5%) | 19 (100.0%) |
| Only minor criteria met (2 or more) | 4 (6.6%) | 4 (9.5%) | 0 (0.0%) |
| Major TB IRIS Criteria | |||
| Lymphadenopathy or other focal tissue involvement | 36 (59.0%) | 24 (57.1%) | 12 (63.2%) |
| Radiologic features of TB | 25 (41.0%) | 17 (40.5%) | 8 (42.1%) |
| Infiltrates | 20 | 13 | 7 |
| Adenopathy | 13 | 10 | 3 |
| Effusion | 4 | 3 | 1 |
| Serositis | 5 (8.2%) | 2 (4.8%) | 3 (15.8%) |
| CNS tuberculosis | 4 (6.6%) | 3 (7.1%) | 1 (5.3%) |
| Minor TB IRIS Criteria | |||
| Constitutional symptoms (fever, night sweats, weigh loss) | 33 (54.1%) | 20 (47.6%) | 13 (68.4%) |
| Respiratory symptoms (cough, dyspnea, stridor) | 21 (34.4%) | 17 (40.5%) | 4 (21.1%) |
| Abdominal pain | 11 (18.0%) | 8 (19.0%) | 3 (15.8%) |
| Management | |||
| Steroids prescribed | 33 (54.1%) | 23 (54.8%) | 10 (52.6%) |
| Invasive procedure | 21 (34.4%) | 15 (35.7%) | 6 (31.6%) |
| Hospitalization | 19 (31.1%) | 13 (31.0%) | 6 (31.6%) |
| TB treatment interruption | 3 (4.9%) | 3 (7.1%) | 0 (0.0%) |
| ART treatment interruption | 3 (4.9%) | 3 (7.1%) | 0 (0.0%) |
Figure 1. Kaplan-Meier plot of time-to-TB IRIS diagnosis from TB treatment initiation by baseline CD4+ category (log rank p-value = 0.004).
The number of TB IRIS cases within each baseline CD4+ category were 34 in CD4+ 0-50, 14 in CD4+ 51-100, 7 in CD4+ 100-150 and 6 in CD4+ >150.
Table 4.
Time to IRIS occurrence and resolution
| Treatment Strategy | CD4+ Stratum (cells/mm3) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic [Median (Q1, Q3)] | Total (N=61) | Earlier ART (N=42) | Later ART (N=19) | P-valuea | CD4+ < 50 (N=33) | CD4+ > 50 (N=28) | P-valuea |
| Days on TB meds at time of TB IRIS diagnosis | 57.0 (22.0, 84.0) | 28.5 (20.0, 69.0) | 82.0 (65.0, 99.0) | <0.001 | 29.0 (22.0, 82.0) | 65.5 (31.5, 84.5) | 0.39 |
| Days on ART at time of TB IRIS diagnosisb | 16.0 (10.0, 49.0) | 18.0 (10.0, 59.0) | 15.0 (7.0, 23.0) | 0.28 | 18.0 (10.0, 43.0) | 15.5 (7.0, 54.0) | 0.86 |
| Days until TB IRIS resolvedc | 86.5 (43.5, 138.5) | 92.0 (46.0, 134.0) | 69.5 (42.0, 149.0) | 0.79 | 81.0 (35.0, 141.0) | 104.0 (46.0, 136.0) | 0.96 |
Wilcoxon rank-sum test with continuity correction.
Due to missing data (TB IRIS diagnosed before ART initiation), sample sizes were N= 57, N = 42, N = 15, N= 33, and N = 24, respectively.
Due to missing TB IRIS resolution data, sample sizes were N= 56, N = 38, N = 18, N= 29, and N = 27, respectively.
TB IRIS Management and Severity
More than half of participants (54.1% (33/61)) with TB IRIS received corticosteroids to manage symptoms, with median treatment duration of 15 days (Q1, Q3 7, 32). Twenty-one participants (34.4%) with TB IRIS required one or more invasive procedures to aid in diagnosis or to manage complications, such as abscess drainage. These procedures included fine needle aspiration (10), outpatient abscess drainage (3), surgical abscess drainage (1), lymph node biopsy (5), lumbar puncture (2), thoracentesis (1), liver biopsy (1) and surgical pleural drainage (2). Hospitalization was required in 31.1% (19/61) of participants with TB IRIS; no deaths were attributed to TB IRIS. TB IRIS infrequently led to TB treatment interruption (>7 days) (3/61) or HIV treatment interruption (>3 days) (3/61); 2 participants interrupted both ART and TB treatment. Applying the study-defined TB IRIS severity index, severe TB IRIS requiring hospitalization occurred in 19/61 (31.1%), moderate TB IRIS (steroids and/or invasive procedure) in 25/61 (41.0%), and mild TB IRIS in 17/61 (27.9%) (Table 5, see Supplemental Digital Content). There was no difference in TB IRIS severity with earlier vs. later ART or with CD4+ < 50 vs. ≥ 50 cells/mm3 (exact ordered Wilcoxon p=0.84 and 0.17, respectively).
Predictors of TB IRIS
Participants developing TB IRIS had significantly lower baseline CD4+ counts (45 vs. 82 cells/mm3, p<0.001), higher baseline HIV RNA (5.7 vs. 5.4 log10 copies/mL, p<0.001), and started ART earlier in relationship to TB treatment (12 vs. 24 days, p=0.028) (Table 1). TB IRIS occurred significantly more frequently in participants with confirmed TB compared to those with only probable TB (47/374 (12.6%) vs. 14/432 (3.2%), p<0.001). TB IRIS occurred in 14.9% (33/221) of participants enrolled from South Africa compared to lower proportions among those from other African countries (1.8% (6/333)), South America (9.9% (16/161)), Asia (3.8% (2/52)) and North America (10.3% (4/39)). Hispanic ethnicity was significantly associated with a two-fold higher occurrence of TB IRIS compared to non-Hispanic ethnicity (14.0% (19/136) vs. 6.3% (42/670), p=0.004).
The time-to-TB IRIS from TB treatment initiation was significantly shorter for those with baseline CD4+< 50 cells/mm3 compared to other CD4+ categories (p=0.004; Figure 1) and for earlier ART compared to later (p=0.002; Figure 2, see Supplemental Digital Content). In the stratified multivariate Cox proportional hazards model, earlier ART initiation was significantly associated with a shorter time to TB IRIS compared to later ART initiation. The hazard ratio (HR) of developing TB IRIS was 2.47 (90% CI 1.56, 3.91, p=0.001) for earlier ART vs to later ART, after adjusting for male sex (HR 1.80, 90% CI 1.09, 2.98, p=0.054), enrollment from South Africa vs. elsewhere (HR 2.78, 90% CI 1.79, 4.32, p<0.001), baseline HIV RNA (HR 1.83, 90% CI 1.24, 2.71, p=0.011), baseline BMI (HR 1.07, 90% CI 1.01, 1.13, p=0.066), and confirmed vs. probable TB at entry (HR 3.16, 90% CI 1.90, 5.24, p<0.001). We also considered the competing risks of death and lost to follow-up; the results of the competing risks analysis were not different from the Cox analysis.
Discussion
In HIV co-infected patients on TB treatment, TB IRIS occurred in 7.6%, with TB IRIS occurring twice as frequently in those with CD4+ counts < 50 cells/mm3 vs. ≥ 50, and in those starting earlier ART (within 2 weeks after TB treatment initiation) compared to those starting later ART. However, in those with CD4≥ 50, earlier initiation of ART did not increase rate of TB IRIS compared to those starting later, indicating that the increased risk of TB IRIS with earlier ART is concentrated in those with very low CD4+ cell counts of < 50 cells/mm3. TB IRIS was not significantly more severe with earlier ART or in those with CD4+ < 50 cells/mm3. Notably, there were no deaths associated with TB IRIS in this series.
As programs globally implement earlier ART in TB co-infection to reduce AIDS progression and death, increased rates of TB IRIS are an expected consequence. As ART implementation is increasingly task shifted from physicians to nurses and other non-physician providers in resource-limited settings[12], appropriate training for health care workers in recognition and management of TB IRIS will be key, as well as access to experienced clinicians to aid in the management of severe and/or complex cases. Providers need the tools and training necessary to diagnose and manage TB IRIS, which includes access to diagnostic testing to exclude other AIDS-related diagnoses as well as drug-resistant TB, which can each present similarly to TB IRIS[13]. Access to invasive procedures may also be necessary, as one-third of STRIDE participants with TB IRIS underwent procedures ranging from fine needle aspiration to thoracotomy as part of IRIS diagnosis and/or management. The absolute number of invasive procedures was low (21/806); however, this number does not take into account the procedures performed on those in whom TB IRIS was suspected, but ultimately excluded. Once diagnosed, TB IRIS was addressed in the outpatient setting for the majority of patients (69%), and there were no deaths attribute to TB IRIS, indicating that TB IRIS was generally a manageable condition. However, considerable resources were used during TB IRIS management, with inpatient care utilized in 31.1% of participants and corticosteroids used in over half, with concomitant monitoring for steroid-related complications. TB IRIS episodes were of moderate (41.0%) or severe (31.1%) intensity in the majority of participants experiencing TB IRIS, and lasted a median of nearly three months, leading to ongoing clinical resources to monitor and manage patients.
Finally, programs may require the resources and infrastructure to provide closer monitoring during the first two to three months of TB and HIV co-treatment, as this is a particularly vulnerable time for treatment-related complications. The risk for TB IRIS was greatest in the first month after ART initiation, with median times to TB IRIS presentation of 18 and 15 days in earlier and later ART initiation arms, respectively. Further research is needed to define optimal monitoring and management strategies for TB IRIS in resource constrained settings.
Two randomized trials examining when to initiate ART in HIV-TB co-infection had similar findings to the current study, but with several notable differences. Both the Cambodian-based CAMELIA[14, 15] and South African-based SAPIT[16] studies found a significantly higher rate of TB IRIS with earlier ART (36% vs. 16%, p<0.001, and 20.1% vs. 8.4%, p<0.001, respectively). However, both studies had a higher overall TB IRIS rate than the current study (26.0% and 12.5%, respectively). This increased rate is attributable in part to the enrollment requirement for AFB smear positive specimens, increasing the likelihood of culture positive TB in the TB suspects; indeed, among STRIDE enrollees, the TB IRIS rate was higher in AFB smear or culture positive participants (12.6%). CAMELIA's high TB IRIS rate of 25% was also derived from the lower median CD4+ cell count of participants (25 vs. 77 cells/mm3 in STRIDE). It is possible that there were additional factors, including racial differences in the study populations. Of note, STRIDE's TB IRIS rate in South African participants (14.9%) is quite similar to the 14.2% reported in the South African SAPIT study, suggesting that racial background may contribute to TB IRIS, in addition to local provider focus on recognition of TB IRIS and access to radiographic tools for IRIS evaluation. CAMELIA reported 154 TB IRIS cases with 7 (4.5%) deaths attributed to TB IRIS, 6 of which occurred in the earlier arm, and SAPIT reported 2 (2.5%) deaths out of 80 TB IRIS events, both in the earlier ART arm. The mortality benefit of earlier ART initiation in both of these studies outweighed the mortality attributed to TB IRIS; however, TB patients with advanced AIDS need to be monitored closely in the first months of ART for complications of TB IRIS, which can be severe and, in rare cases, fatal. Limitations TB IRIS is a well-characterized clinical syndrome[8, 17]; however, diagnosis can be challenging as there is no confirmatory laboratory test, and other AIDS- and TB-related processes need to be excluded. Presence of TB IRIS was determined by study investigator evaluation during required study visits every four weeks as well as any unscheduled visits for toxicity evaluations. It is possible that participants with more advanced immunosuppression and those in the earlier ART arm may have been seen more frequently at unscheduled visits due to AIDS complications or medication toxicity, respectively, and thus assessed more often for TB IRIS creating a diagnostic bias. However, an increase in TB IRIS has consistently been reported in other series in association with earlier ART as well as low CD4+ cell counts[6, 17].
Conclusion
TB IRIS was more common in participants with earlier ART initiation and CD4+ cell counts < 50 cells/mm3. Overall TB IRIS occurrence was infrequent at 7.6%. When TB IRIS occurred, the majority (69%) of cases were mild to moderate in severity; however 31% were hospitalized with TB-IRIS and over half received corticosteroids. As ART is increasingly implemented within 2 weeks after TB treatment initiation to reduce AIDS-related complications and mortality, HIV-TB programs will need the diagnostic capabilities, clinical resources and provider training necessary to diagnose and manage tuberculosis-related immune reconstitution inflammatory syndrome.
Acknowledgments
We thank the study participants, the site principal investigators and staff for their exceptional efforts to conduct the study, coordinate efforts with the in-country tuberculosis-control programs, and to help build the capacity of integrated HIV-tuberculosis services; the data managers, Carol Suckow, B.S.N., and Lynne Jones, B.S.; the clinical trials specialist Evelyn Hogg; the DAIDS protocol pharmacist, Ana Martinez, R.Ph.; the field representative, Janet Nicotera, R.N.,B.S.N.; the study's laboratory technologist, Patty Anthony, B.S., C.L.S; the laboratory-data coordinator, Travis Behm, B.S.; and the community representative, Martha Tholanah Mensah-King.
Funding Source: This work was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases and the Statistical and Data Management Center (SDMC) UM1 AI068634 funded by the National Institute of Allergy and Infectious Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Antiretroviral medications donated by Gilead Sciences and Merck Pharmaceuticals.
A.L., M.K., X.W., J.A., P.I., S.S, C.B., D.H, I.S., and J.K. have received research grant support to their institutions to support this work and to support travel to study-related meetings. A.L. has received research grant support to her institution from Cepheid. I.S. is on the advisory board for Mylan Pharmaceuticals.
Footnotes
Authors Contributions: Contribution to authorship: A.L., S.S., I.S., P.I., M.K., X.W., J.A., D.H., and J.K. are members of the protocol study team and were involved in study design and conduct. M.N. is a protocol team member, and oversaw participant enrollment and management. C.B. participated in study monitoring and provided ACTG leadership. M.K., X.W., and J.A. were responsible for statistical analysis. All authors contributed to manuscript preparation.
Potential conflicts of interest: All other authors have reported no potential conflict of interest.
All Authors have submitted the JAIDS form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Michelle A. Kendall, Center for Biostatistics in AIDS Research, Harvard School of Public Health
Mulinda Nyirenda, College of Medicine, Blantyre, Malawi.
Xingye Wu, Center for Biostatistics in AIDS Research, Harvard School of Public Health
Prudence Ive, Faculty of Heath Sciences, University of the Witwatersrand.
Constance A. Benson, Antiviral Research Center, University of California, San Diego.
Janet W. Andersen, Center for Biostatistics in AIDS Research, Harvard School of Public Health
Susan Swindells, Division of Infectious Diseases, University of Nebraska Medical Center, Omaha, NE.
Ian M. Sanne, Faculty of Health Sciences, University of the Witwatersrand.
Diane V. Havlir, HIV/AIDS Division, San Francisco General Hospital, University of California, San Francisco.
Johnstone Kumwenda, College of Medicine, Blantyre, Malawi.
References
- 1.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. The New England journal of medicine. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. The New England journal of medicine. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet infectious diseases. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. Aids. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 7.Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One. 2012;7:e40623. doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. Journal of American Statistical Assocation. 1958;53:457–481. [Google Scholar]
- 10.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Sociey. 1972;34:187–220. [Google Scholar]
- 11.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 12.Emdin CA, Millson P. A systematic review evaluating the impact of task shifting on access to antiretroviral therapy in sub-Saharan Africa. African health sciences. 2012;12:318–324. doi: 10.4314/ahs.v12i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meintjes G, Rangaka MX, Maartens G, Rebe K, Morroni C, Pepper DJ, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48:667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. The New England journal of medicine. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laureillard D, Marcy O, Madec Y, Chea S, Chan S, Borand L, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in the camelia randomized. AIDS. 2013 doi: 10.1097/01.aids.0000432456.14099.c7. [DOI] [PubMed] [Google Scholar]
- 16.Naidoo K, Yende-Zuma N, Padayatchi N, Jithoo N, Nair G, Bamber S, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Annals of Internal Medicine. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clinics in chest medicine. 2009;30:797–810. x. doi: 10.1016/j.ccm.2009.08.013. [DOI] [PubMed] [Google Scholar]