Abstract
Pomalidomide is a second generation IMiD (immunomodulatory agent) that has recently been granted approval by the Food and Drug Administration for treatment of relapsed multiple myeloma after prior treatment with two antimyeloma agents, including lenalidomide and bortezomib. A simple and robust HPLC assay with fluorescence detection for pomalidomide over the range of 1–500 ng/mL has been developed for application to pharmacokinetic studies in ongoing clinical trials in various other malignancies. A liquid-liquid extraction from human plasma alone or pre-stabilized with 0.1% HCl was performed, using propyl paraben as the internal standard. From plasma either pre-stabilized with 0.1% HCl or not, the assay was shown to be selective, sensitive, accurate, precise, and have minimal matrix effects (<20%). Pomalidomide was stable in plasma through 4 freeze-thaw cycles (<12% change), in plasma at room temperature for up to 2 hr for samples not pre-stabilized with 0.1% HCl and up to 8 hr in samples pre-stabilized with 0.1% HCl, 24 hr post-preparation at 4 °C (<2% change), and showed excellent extraction recovery (~90%). This is the first reported description of the freeze/thaw and plasma stability of pomalidomide in plasma either pre-stabilized with 0.1% HCl or not. The information presented in this manuscript is important when performing pharmacokinetic analyses. The method was used to analyze clinical pharmacokinetics samples obtained after a 5 mg oral dose of pomalidomide. This relatively simple HPLC-FL assay allows a broader range of laboratories to measure pomalidomide for application to clinical pharmacokinetics.
Keywords: Pomalidomide, HPLC, Fluorescence, Pharmacokinetics
1. Introduction
Pomalidomide is an oral IMiD and, along with lenalidomide, is a second generation analog of thalidomide. All three compounds directly inhibit angiogenesis at roughly the same potency [1] and each also inhibits TNF-α, however pomalidomide has up to 50,000x more potency against TNF-α than thalidomide [2]. Furthermore, pomalidomide was recently shown to not induce the teratogenic effects in zebrafish and chicken embryos that thalidomide and lenalidomide are known for [3].
The IMiDs target cereblon, a component of the E3 ubiquitin ligase that down-regulates interferon regulatory factor 4, which is a crucial factor for myeloma cell survival, and when inhibited can lead to teratogenicity [4]. Cereblon is encoded by the CRBN gene and low cereblon expression correlates to lenalidomide and pomalidomide resistance in multiple myeloma (MM) cells [5]. Pomalidomide can stimulate apoptosis and cell cycle arrest in MM, as well as enhance natural killer cells and natural killer T lymphocytes [6]. Pomalidomide has activity in myeloma refractory to first line treatments lenalidomide and/or bortezomib [7]. Pomalidomide was subsequently tested clinically in a MM population [8–13], in combination with dexamethasone, and was granted accelerated approval by the FDA. Pomalidomide was approved in July of 2013, for relapsed MM following treatment with two antimyeloma agents, including lenalidomide and bortezomib, which has demonstrated disease progression on or within 60 days of completion of the last therapy. Thalidomide and lenalidomide previously received FDA approval in 2006 for treatment of MM when given in combination with dexamethasone.
Pomalidomide has also shown in vitro activity in other diseases, such as anemia, myelofibrosis [14], leukemia [15], lymphoma [16], pancreatic cancer [17], and prostate cancer [18]. Furthermore, thalidomide has shown activity in Kaposi sarcoma [19], suggesting that pomalidomide may have potential activity in this tumor as well. Numerous early-phase clinical trials have begun testing the safety and efficacy of pomalidomide in many of those cancer types [14, 17, 20–25]. There is much variation in pomalidomide doses and schedules amongst these clinical trials and thus a need to study pomalidomide pharmacokinetics in these disease models to ensure safe, efficacious dosing. The literature has only two references for a pharmacokinetics-oriented bioanalytical assay [26, 27], but one uses mouse plasma/tissue and the other (in human plasma) does not provide full details for required stability tests such as freeze/thaw, plasma stability, post-preparative stability, etc. Therefore, a more robust assay, with useful validation and stability data, to quantitatively measure pomalidomide in human plasma at clinically relevant concentrations is greatly needed. Although more stable than thalidomide, pomalidomide is still susceptible to a clinically significant rate of hydrolysis (both enzymatic and non-enzymatic) [28]. Hoffman et al demonstrated that some of the most predominant pomalidomide metabolites in human urine and plasma are hydrolysis products [26].
Described here is a simple, sensitive, and selective HPLC assay with fluorescence detection for pomalidomide in the clinically relevant plasma concentration range of 1–500 ng/mL following a 5 mg oral dose. As there is no literature on stability data of pomalidomide in human plasma, this study performed assay validations, according to the FDA [29], in both plasma stabilized with 0.1% HCl (to reduce hydrolysis) or plasma alone. While this method was successfully applied to a clinical trial with a pharmacokinetic endpoint, the intention of this manuscript is to describe the assay and is not meant to be a description of the pharmacokinetic profile of pomalidomide.
2. Experimental
2.1 Materials
Pomalidomide (>99% purity) was purchased from Selleck Chemicals (Houston, TX). N-propyl p-hydroxybenzoate (propyl paraben), formic acid, hydrochloric acid (HCl), optima-grade acetonitrile (ACN), and ethyl acetate were purchased from Sigma-Aldrich (St. Louis, MO). Optima-grade methanol was purchased from Fisher Scientific (Pittsburgh, PA). De-ionized water was generated by a Hydro-Reverse Osmosis system (Durham, NC) connected to a Milli-Q UV Plus purifying system (Billerica, MA). Human plasma was provided by the Clinical Center Blood Bank of the National Institutes of Health (Bethesda, MD).
2.2 Preparation of stock solution
Master stock solutions were prepared individually by dissolving pomalidomide in DMSO and propyl paraben (used as an internal standard) in methanol at concentrations of 1 mg/mL (Figure 1). Each stock solution was stored in amber glass vials at −80° C, after a brief vortex and 15 min sonication. Working stock solutions in acetonitrile were prepared serially from the master stock and stored at −80°C. The working stock solutions were used to prepare the calibration curve, quality control (QC) and lower limit of quantification (LLOQ) samples.
Figure 1.

Structures of Pomalidomide and Propyl Paraben (Internal Standard)
Calibration standards in drug-free human heparinized plasma were prepared fresh daily in duplicate at final plasma concentrations of 1, 5, 10, 50, 100, 250, and 500 ng/mL for every analytical run. QC samples were prepared at final concentrations of 1.0 (LLOQ), 3.0 (low-QC), 200 (mid-QC), 400 (high-QC), and 5,000 ng/mL (10-fold dilution QC) by adding the required amount of working stock to plasma. QC samples were vortexed, aliquotted, and stored at −80 °C until analysis.
2.3 Sample preparation
Clinical samples were collected in heparinized tubes containing 0.1% HCl as a precaution for degradation. Therefore, 0.1% HCl in plasma was used to validate the assay. Fifty microliters of 1% HCl (made fresh daily) was added to 500 μL of drug-free plasma, standard, or QC samples, resulting in a final HCl concentration of 0.1%. To demonstrate the necessity of acidifying plasma with 0.1% HCl, each experiment was performed again without adding HCl. Next, 2 mL of 200 ng/mL propyl paraben in ethyl acetate was added to each sample, then subsequently vortexed for 30 sec and centrifuged for 10 min at 2000 rpm. The organic layer was dried down before being reconstituted with 50 μL of acetonitrile.
2.4 Instrument conditions
Thirty microliters of each sample was injected into an Agilent 1100 series HPLC (Santa Clara, CA, USA), which contains a quaternary pump, a refrigerated autosampler at 4 °C, column compartment at ambient temperature, and fluorescence and ultraviolet detectors. A Waters Nova-Pak® C18, 4 μm, 3.9 × 300 mm column was used for chromatographic separation. The mobile phase consisted of 0.5% formic acid (aqueous) and 0.5% formic acid in ACN. A mobile phase gradient was used with a 1 mL/min flow rate, where initially 20% organic was increased linearly to 100% over 6 min, and finally decreased to 20% by 6.1 min, where it was held until the end of the 9 min run. The fluorescence detector was set for excitation at 235 nm and emission at 520 nm for detection of pomalidomide. Propyl paraben (internal standard) was detected using ultraviolet absorbance at 266 nm. The retention times for pomalidomide and propyl paraben were 4.6 min and 6.4 min, respectively.
2.5 Validation
This assay was validated according to FDA requirements [29] in human plasma. There are no published studies regarding pomalidomide stability in human plasma, therefore this assay was validated in plasma with or without 0.1% HCl to determine whether acidification enhances its stability in plasma.
2.5.1. Linearity
By plotting the ratio of the analyte:internal standard peak area versus the concentration ratio of analyte:internal standard, a seven point calibration curve was created (1–500 ng/mL). The correlation coefficient (r2) and accuracy (as calculated by percent deviation; % DEV) were compared for all calibrators to determine the optimal choice of regression analysis and weighting.
2.5.2 Accuracy and precision
Each daily validation run consisted of a drug-free plasma extract, internal standard only, and seven calibration standards in duplicate, in addition to QC and LLOQ samples in quintuplet. The LLOQ samples were prepared fresh daily in five different lots of plasma, while the QC samples were thawed daily from frozen aliquots (either with or without pre-stabilization with 0.1% HCl) prepared previously in batch. Accuracy and precision were assessed by calculating the observed concentrations for the calibration standards in duplicate (n=8) and the quality control samples in quintuplet over 4 days (n=20). Accuracy was calculated as the percent difference between the mean observed concentration and the theoretical concentration. Precision, or the repeatability of the assay, was determined by the within-run precision (WRP) and between-run (BRP), as calculated below.
GM represents the grand mean over the four days, MSwit represents the within-group mean squared, MSbet represents the between-group mean squared, and n represents the number of repetitions (n=20, with QCs analyzed in quintuplet over four days). FDA guidelines for bioanalytical accuracy and precision were followed, with ± 15% variability allowed for all concentration levels, except the LLOQ, which is allowed ± 20% [29].
2.5.3 Benchtop Stability
The stability of pomalidomide in plasma at room temperature was analyzed over a 24 hr period. Pomalidomide samples in plasma at two concentrations (3 and 400 ng/mL) were either extracted immediately or allowed to sit at room temperature for 2, 4, 8, or 24 hr (each in triplicate). The resulting concentrations over the 24 hr period were compared to the fresh samples extracted immediately after preparation.
Stock solution stability of pomalidomide in acetonitrile and propyl paraben in acetonitrile at room temperature was tested over 6 hr and compared to freshly prepared stock solutions.
2.5.3.1. Freeze/Thaw Stability
Freeze/thaw stability tests were performed to assess the potential degradation of pomalidomide in human plasma during numerous freeze/thaw cycles. Samples were tested in triplicate at two concentrations (3 and 400 ng/mL), and subjected to four freeze/thaw cycles, where the freeze portion (−80 °C) of each cycle persisted for at least 12 hr. Upon completion of 4 cycles, the freeze/thaw samples were extracted and their analyte concentration was measured and compared to freshly prepared samples in the same analytical run.
2.5.3.2. Post-Preparative Stability
The post-preparative stability of pomalidomide and propyl paraben in the autosampler was measured. Samples at both low and high concentrations were re-injected 24 hr after the initial analysis and compared to previous sample concentrations.
2.5.4. Matrix Effects and Extraction Recovery
Matrix effects, which measure the effect of plasma constituents on the detectable signal, were determined through comparison of the pomalidomide peak area spiked in drug-free human heparinized plasma to the peak area resulting from pomalidomide spiked into pure ACN (reconstitution solution) at both a low (5 ng/mL) and high (250 ng/mL) concentration. Although more of an issue for mass spectrometric and not fluorescent assays, this experiment was nonetheless performed.
The efficiency, or percent recovery, of the liquid-liquid extraction of pomalidomide was assessed by calculating the ratio of the analyte peak areas when adding pomalidomide to blank plasma both before and after the addition of liquid extraction solvent (200 ng/mL propyl paraben in ethyl acetate). The control in this experiment was pomalidomide spiked after addition of the extraction solvent to represent 100% extraction. This was performed at both a low (5 ng/mL) and high (250 ng/mL) concentration, each in quintuplet (five different lots of plasma).
2.6 Clinical Application
A Phase I/II clinical trial of pomalidomide in Kaposi sarcoma in patients with or without HIV was approved by the National Cancer Institute Institutional Review Board, and registered at clinicaltrials.gov (NCT01495598). All patients signed informed consent. Each patient received a 5 mg oral dose of pomalidomide (Celgene Corp, Summit, NJ) daily for 21 days of a 28 day cycle. Pharmacokinetic blood samples were drawn pre-dose, as well as 1, 2, 3, 4, 6, 8, and 24-hr after the initial dose. Per protocol, blood was drawn into chilled sodium heparin tubes containing 60 μL of 1% HCl, resulting in a 0.1% HCl concentration after a 6 mL blood draw. The blood tubes were immediately placed on wet ice and centrifuged to obtain plasma. The plasma was then frozen at −80 °C until subsequent extraction and analysis by HPLC-FL.
3. Results and Discussion
3.1 Selectivity and Sensitivity
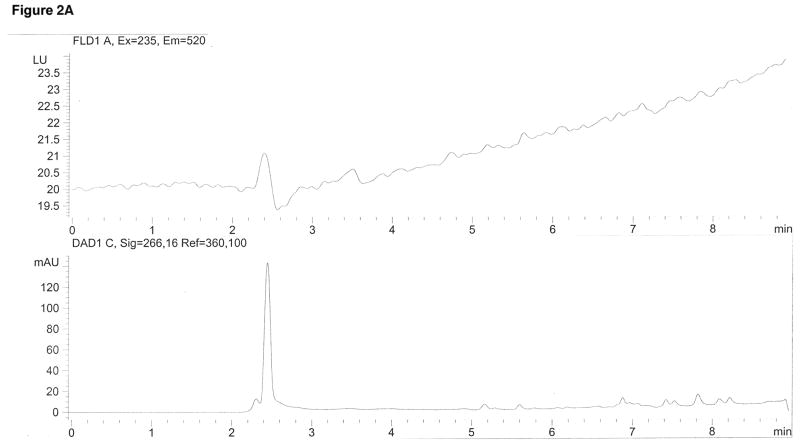
Pomalidomide and propyl paraben (internal standard) were selectively identified based on their unique fluorescent and ultraviolet absorbance signals, respectively, as well as their retention times with the established mobile phase and column based on a high purity (>99%) pomalidomide reference standard. Figure 2 demonstrates the ability of this assay to selectively identify pomalidomide and the internal standard. There were no interfering peaks from endogenous plasma components (Figure 2A), and the internal standard did not interfere with the pomalidomide peak (Figure 2B).
Figure 2.
HPLC Chromatograms
Pomalidomide was detected with fluorescence using 235 nm for excitation and 520 nm for emission at a retention time of 4.6 min (top pane). The internal standard propyl paraben was detected using ultraviolet absorbance at 266 nm at a retention time of 6.5 min (bottom pane). (A) is a drug-free plasma extract showing neither peak. (B) is an extract containing only the internal standard peak. (C) is a plasma extract spiked with the lower limit of quantification (LLOQ) of 5 ng/mL pomalidomide. (D) is a clinical plasma sample drawn 6 hr following oral administration of 5 mg pomalidomide.
This assay proved to be sufficiently sensitive for clinical pharmacokinetic studies, with the LLOQ at 1 ng/mL. Figure 2C demonstrates the LLOQ peak, possessing a signal/noise factor >5.
3.2 Validation
3.2.1 Calibration Linearity
The calibration standards, ranging from 1 to 500 ng/mL, were run in duplicate for four days for each validation set (with or without pre-stabilizing plasma with 0.1% HCl). Using a regression analysis weighting factor of 1/χ2, where χ is the concentration ratio of analyte:internal standard, the calibration standards proved to be linear, accurate and precise and were below the required 15% deviation (Table 1). Calibration curves without pre-stabilization averaged r2=0.9985 (n=4), and with pre-stabilization averaged r2=0.9943 (n=4).
Table 1.
Calibration Linearity in Plasma (A) or Plasma Pre-Stabilized with 0.1% HCl (B)
| A | |||||
|---|---|---|---|---|---|
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | DEV (%) | CV (%) | n |
| 1 | 1.00 | 0.06 | −0.09 | 5.97 | 8 |
| 5 | 4.88 | 0.23 | −2.40 | 4.71 | 8 |
| 10 | 10.18 | 0.40 | 1.76 | 3.96 | 8 |
| 50 | 50.57 | 1.99 | 1.14 | 3.94 | 8 |
| 100 | 102.90 | 3.69 | 2.90 | 3.58 | 8 |
| 250 | 249.33 | 12.61 | −0.27 | 5.06 | 8 |
| 500 | 479.79 | 34.27 | −4.04 | 7.14 | 8 |
| B | |||||
|---|---|---|---|---|---|
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | DEV (%) | CV (%) | n |
| 1 | 0.99 | 0.07 | −0.63 | 7.28 | 8 |
| 5 | 5.01 | 0.64 | 0.23 | 12.74 | 8 |
| 10 | 10.40 | 1.35 | 4.04 | 12.98 | 8 |
| 50 | 50.27 | 3.66 | 0.55 | 7.27 | 8 |
| 100 | 99.64 | 7.42 | −0.36 | 7.45 | 8 |
| 250 | 249.02 | 23.76 | −0.39 | 9.54 | 8 |
| 500 | 481.33 | 37.44 | −3.73 | 7.78 | 8 |
Abbreviations: GM, grand mean; SD, standard deviation; DEV (%), relative deviation from nominal value; CV (%), coefficient of variation; n, number of replicate observations within each validation run, i.e. two samples at each concentration were run on four separate occasions, for a total (n) of eight samples at each concentration.
3.2.2 Accuracy and Precision
The low (3 ng/mL), mid (200 ng/mL), high (400 ng/mL) and dilution (5,000 ng/mL) quality control samples and the lower limit of quantification samples (1 ng/mL) were run in quintuplet over 4 days. Both validation sets had quality control samples within the required 15% deviation and lower limit of quantification samples within the required 20% deviation (Table 2).
Table 2.
Accuracy and Precision in Plasma (A) or Plasma Pre-Stabilized with 0.1% HCl (B)
| A | ||||||
|---|---|---|---|---|---|---|
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | DEV (%) | WRP (%) | BRP (%) | n |
| 1.0 (LLOQ) | 1.10 | 0.12 | 10.0011.95 | 10.5010.29 | 4.296.61 | 1820 |
| 3.0 (LQC) | 2.79 | 0.26 | −7.506.84 | 6.126.06 | 7.638.43 | 2019 |
| 200 (MQC) | 194.96 | 12.26 | −2.52 | 2.97 | 6.24 | 20 |
| 400 (HQC) | 374.50 | 27.67 | −6.38 | 4.82 | 6.31 | 20 |
| 5,000 (DQC) | 4930.44 | 471.27 | −1.39 | 2.20 | 10.47 | 20 |
| B | ||||||
|---|---|---|---|---|---|---|
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | DEV (%) | WRP (%) | BRP (%) | n |
| 1.0 (LLOQ) | 1.07 | 0.10 | 10.807.33 | 10.0311.53 | −6.82 | 1820 |
| 3.0 (LQC) | 2.79 | 0.25 | −6.91–7.83 | 7.707.93 | 5.406.92 | 2019 |
| 200 (MQC) | 183.14 | 11.56 | −8.43 | 5.63 | 3.21 | 20 |
| 400 (HQC) | 406.26 | 60.43 | 1.56 | 13.95 | 5.80 | 20 |
| 5,000 (DQC) | 4538.70 | 407.55 | −9.23 | 5.73 | 7.78 | 20 |
Abbreviations: GM, grand mean; SD, standard deviation; DEV (%) relative deviation from nominal value; WRP, within-run precision; n, number of replicate observations within each validation run; BRP, between-run precision (A hyphen indicates MSwit>MSbet, thus BRP cannot be calculated and it was concluded that no additional variation was observed as a result of performing the assay in different runs)
3.3 Stability
Overall, samples pre-stabilized with 0.1% HCl experienced less degradation. Low and high concentration samples without 0.1% HCl were stable in plasma up to 2 hours at room temperature, while low and high concentration samples pre-stabilized with 0.1% HCl were stable up to 8 hours (Table 3). Furthermore, the 24 hour low concentration sample without 0.1% HCl experienced a 72.1% degradation as opposed to a 30.4 % degradation for the 24 hour low concentration sample pre-stabilized with 0.1% HCl (Table 3).
Table 3.
Benchtop Stability in Plasma (A) or Plasma Pre-Stabilized with 0.1% HCl (B)
| A | 3 ng/mL | 400 ng/mL | ||
|---|---|---|---|---|
| Time (hr) | GM (ng/mL) | DEV from fresh (%) | GM (ng/mL) | DEV from fresh (%) |
| 0 (Fresh) | 3.12 | - | 395.32 | - |
| 2 | 2.87 | −8.01 | 366.58 | −7.27 |
| 4 | 2.41 | −22.81 | 312.82 | −20.87 |
| 8 | 2.15 | −30.96 | 255.20 | −35.44 |
| 24 | 0.87 | −72.00 | 87.35 | −77.90 |
| B | 3 ng/mL | 400 ng/mL | ||
|---|---|---|---|---|
| Time (hr) | GM (ng/mL) | DEV from fresh (%) | GM (ng/mL) | DEV from fresh (%) |
| 0 (Fresh) | 3.40 | - | 426.78 | - |
| 2 | 2.86 | −15.80 | 432.73 | 1.40 |
| 4 | 2.99 | −11.94 | 417.52 | −2.17 |
| 8 | 2.98 | −12.39 | 407.11 | −4.61 |
| 24 | 2.36 | −30.41 | 311.47 | −27.02 |
Abbreviations: GM, grand mean; DEV (%) relative deviation from nominal value
Low and high concentration samples both with and without 0.1% HCl were stable through 4 freeze/thaw cycles (Table 4). A curious increase was observed during the third cycle in the low concentration sample set without 0.1% HCl stabilization. It seems likely that samples containing 0.1% HCl-stabilized plasma experienced less freeze/thaw degradation than QCs in non-stabilized plasma and are more consistent in general. The 24 hr post-preparative stability test revealed less than a 2% difference between samples analyzed immediately and those analyzed after 24 hr in the autosampler at 4 °C. A less than 5% difference in pomalidomide stock solution (acetonitrile) and propyl paraben stock solution (acetonitrile) was observed after 6 hr at room temperature.
Table 4.
Freeze/Thaw Stability in Plasma (A) or Plasma Pre-Stabilized with 0.1% HCl (B)
| A | 3 ng/mL | 400 ng/mL | ||
|---|---|---|---|---|
| Freeze/Thaw Cycles | GM (ng/mL) | DEV from fresh (%) | GM (ng/mL) | DEV from fresh (%) |
| 0 (Fresh) | 2.57 | - | 382.1 | - |
| 1 | 2.30 | −11.42 | 403.7 | 5.64 |
| 2 | 2.44 | −6.26 | 342.9 | −10.25 |
| 3 | 3.34 | 28.34 | 347.5 | −9.06 |
| 4 | 2.27 | −12.84 | 368.0 | −3.68 |
| B | 3 ng/mL | 400 ng/mL | ||
|---|---|---|---|---|
| Freeze/Thaw Cycles | GM (ng/mL) | DEV from fresh (%) | GM (ng/mL) | DEV from fresh (%) |
| 0 (Fresh) | 2.53 | - | 337.64 | - |
| 1 | 2.77 | 9.35 | 331.42 | −1.84 |
| 2 | 2.69 | 6.28 | 340.82 | 0.94 |
| 3 | 2.62 | 3.49 | 337.59 | −0.01 |
| 4 | 2.77 | 9.31 | 356.98 | 5.73 |
Abbreviations: GM, grand mean; DEV (%) relative deviation from nominal value
3.4 Extraction Recovery and Matrix Effects
Extraction recovery was 89.43% and 93.52% for the low (5 ng/mL) and high (250 ng/mL) concentration samples, respectively. This demonstrated that the liquid-liquid extraction employed in this study was capable of efficiently extracting pomalidomide from plasma. Matrix effects reduced pomalidomide fluorescent signal by 13.49% and 12.41% for the low and high concentrations, respectively, thus showing that plasma had little deleterious effects on the pomalidomide fluorescent signal, as expected.
3.5 Clinical application
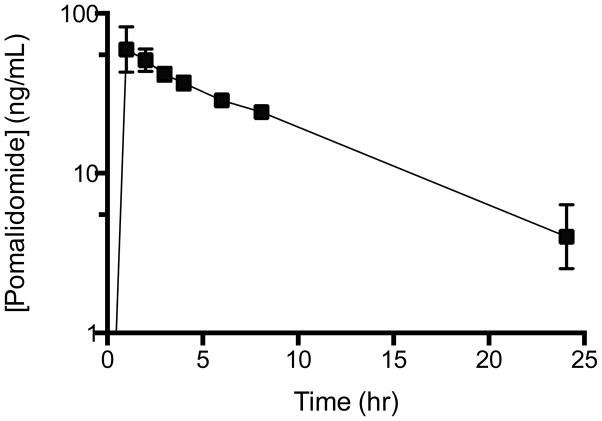
Pomalidomide plasma concentrations following an initial 5 mg oral dose were examined in four subjects to demonstrate clinical applicability. The data presented here was not intended to be a pharmacokinetic study, only to demonstrate applicability of the described method. Figure 2D demonstrates a typical HPLC chromatogram from a clinical study sample, with pomalidomide giving a strong fluorescent signal at 6 hr post dose. The average plasma concentration vs. time curve demonstrated good oral absorption and elimination of pomalidomide (Figure 3). Plasma levels remained above the LLOQ of 1 ng/mL through 24 hr post dose, indicating this assay is sensitive enough for pharmacokinetic studies.
Figure 3.
Plasma Concentration vs Time Curve
Pomalidomide plasma concentrations were measured at various time points in four subjects following their first oral dose of 5 mg pomalidomide. The LLOQ of 1 ng/mL is sufficient to measure pomalidomide levels 24 hr post dose.
3.6 Mass Spectrometric Data
A major reason HPLC with fluorescence detection was chosen over the more selective and sensitive mass spectrometric detection was due to the difficulty obtaining a stable mass spectrometric signal for pomalidomide. There are two published bioanalytical assays for pomalidomide, one in mouse plasma and brain using LC-APCI-MS/MS [27], and the other in human plasma using LC-ESI-MS/MS [26]. Both studies established the precursor ion (M+H+) at m/z 274, which upon tandem mass spectrometric (MS/MS) fragmentation produced a product ion mass spectrum that included ions at m/z 264, 229, 201, 163, and 84. When attempts at mass spectral analysis were undertaken as part of this study, the protonated molecular ion (M+H+) m/z 274 only produced one major product ion at m/z 195, which did not match up with any of the product ions from both previously published studies [26, 27], even though a pomalidomide reference standard of the highest commercial purity (>99%) was used for this study. Building off of the multiple reaction monitoring of m/z 274→195 did not allow for consistent, quantitative analyses, possibly from an incorrect product ion transition; thus the fluorescent detection was pursued.
Conclusions
In conclusion, this HPLC-FL assay was developed, validated, and optimized for routine application to clinical pharmacokinetic studies. This method has been shown to be accurate, precise, and selective. It shows pomalidomide is stable through 4 freeze/thaw cycles and up to 8 hr at room temperature in plasma pre-stabilized with 0.1% HCl. Pomalidomide in plasma not pre-stabilized was also stable through 4 freeze/thaw cycles, but demonstrated much less stability in plasma at room temperature (2 hr). The LLOQ of 1 ng/mL has been shown to be sufficiently sensitive for clinically relevant doses and resulting plasma concentrations of oral pomalidomide, with quantifiable levels still present after 24 hrs. The ability of our HPLC-FL/UV assay to accurately and precisely quantitate pomalidomide in human plasma up to 24 hr following a typical 5 mg oral dose increases the applicability of this assay, allowing a broader range of laboratories to perform pharmacokinetic studies on pomalidomide without the expenses and technical challenges associated with mass spectrometers.
Highlights.
HPLC assay with fluorescence detection for the quantification of pomalidomide
Simple liquid extraction procedure that recovers over 90% of drug from human plasma
Pomalidomide stability tested in both plasma and pre-stabilized plasma
This method was applied to a clinical trial to demonstrate applicability of assay
Acknowledgments
This study was funded in part the Intramural Research Program of the NIH, National Cancer Institute, and a CRADA between the National Cancer Institute and Celgene Corporation. This is a US Government work and there are no restrictions on its use. The views expressed within this paper do not necessarily reflect those of the US Government. We thank the medical research staf of the HIV and AIDS Malignancy Branch, the Center for Cancer Research, and the NIH Clinical Center who helped with the study. We also thank the nursing staff of the National Cancer Institute and the fellows of the Medical Oncology Branch at the National Cancer Institute for their care of our patients; Cynthia Graves for data management assistance; and Cancer Therapy and Evaluation Program for sponsoring the trial. Most importantly, we appreciate the patients with cancer who enroll in investigational trials to advance the knowledge of this disease.
Abbreviations
- HPLC
high performance liquid chromatography
- IMiD
Immunomodulatory drug
- QC
Quality control
- LLOQ
Lower limit of quantification
- IS
internal standard
- MM
multiple myeloma
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. The views in this manuscript are those of the authors and may not necessarily reflect NIH policy. No official endorsement is intended nor should be inferred.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 3.Mahony C, Erskine L, Niven J, Greig NH, Figg WD, Vargesson N. Pomalidomide is nonteratogenic in chicken and zebrafish embryos and nonneurotoxic in vitro. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1307684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683–687. doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, Gaidarova S, Chen R, Schafer PH, Handa H, Daniel TO, Evans JF, Chopra R. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, Mathiot C, Petillon MO, Macro M, Roussel M, Pegourie B, Kolb B, Stoppa AM, Hennache B, Brechignac S, Meuleman N, Thielemans B, Garderet L, Royer B, Hulin C, Benboubker L, Decaux O, Escoffre-Barbe M, Michallet M, Caillot D, Fermand JP, Avet-Loiseau H, Facon T. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–02. Blood. 2013;121:1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 8.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Stewart AK, Laumann K, Mandrekar SJ, Reeder C, Rajkumar SV, Mikhael JR. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Kyle RA, Fonseca R, Bergsagel PL, Roy V, Mikhael JR, Stewart AK, Laumann K, Allred JB, Mandrekar SJ, Rajkumar SV. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27:5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 10.Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Mikhael JR, Stewart AK, Laumann K, Allred JB, Mandrekar SJ, Rajkumar SV, Buadi F. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy DA, Macey MG, Streetly M, Schey SA, Brown KA. The neutropenia induced by the thalidomide analogue CC-4047 in patients with multiple myeloma is associated with an increased percentage of neutrophils bearing CD64. Int Immunopharmacol. 2006;6:1194–1203. doi: 10.1016/j.intimp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22:3269–3276. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol. 2008;141:41–51. doi: 10.1111/j.1365-2141.2008.07013.x. [DOI] [PubMed] [Google Scholar]
- 14.Lacy MQ, Rajkumar SV. Pomalidomide: a new IMiD with remarkable activity in both multiple myeloma and myelofibrosis. Am J Hematol. 2010;85:95–96. doi: 10.1002/ajh.21610. [DOI] [PubMed] [Google Scholar]
- 15.Shalapour S, Zelmer A, Pfau M, Moderegger E, Costa-Blechschmidt C, van Landeghem FK, Taube T, Fichtner I, Buhrer C, Henze G, Seeger K, Wellmann S. The thalidomide analogue, CC-4047, induces apoptosis signaling and growth arrest in childhood acute lymphoblastic leukemia cells in vitro and in vivo. Clin Cancer Res. 2006;12:5526–5532. doi: 10.1158/1078-0432.CCR-06-0719. [DOI] [PubMed] [Google Scholar]
- 16.Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, Muller GW, Worland PJ, Chan KW, Verhelle D. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69:7347–7356. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 17.Infante JR, Jones SF, Bendell JC, Spigel DR, Yardley DA, Weekes CD, Messersmith WA, Hainsworth JD, Burris HA., 3rd A phase I, dose-escalation study of pomalidomide (CC-4047) in combination with gemcitabine in metastatic pancreas cancer. Eur J Cancer. 2011;47:199–205. doi: 10.1016/j.ejca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little RF, Wyvill KM, Pluda JM, Welles L, Marshall V, Figg WD, Newcomb FM, Tosato G, Feigal E, Steinberg SM, Whitby D, Goedert JJ, Yarchoan R. Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2000;18:2593–2602. doi: 10.1200/JCO.2000.18.13.2593. [DOI] [PubMed] [Google Scholar]
- 20.Begna KH, Mesa RA, Pardanani A, Hogan WJ, Litzow MR, McClure RF, Tefferi A. A phase-2 trial of low-dose pomalidomide in myelofibrosis. Leukemia. 2011;25:301–304. doi: 10.1038/leu.2010.254. [DOI] [PubMed] [Google Scholar]
- 21.Cooney MM, Nock C, Bokar J, Krishnamurthi S, Gibbons J, Rodal MB, Ness A, Remick SC, Dreicer R, Dowlati A. Phase I trial of pomalidomide given for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;70:755–761. doi: 10.1007/s00280-012-1919-6. [DOI] [PubMed] [Google Scholar]
- 22.Dispenzieri A, Buadi F, Laumann K, LaPlant B, Hayman SR, Kumar SK, Dingli D, Zeldenrust SR, Mikhael JR, Hall R, Rajkumar SV, Reeder C, Fonseca R, Bergsagel PL, Stewart AK, Roy V, Witzig TE, Lust JA, Russell SJ, Gertz MA, Lacy MQ. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis PM, Jungnelius U, Zhang J, Fandi A, Beck R, Shepherd FA. A phase I study of pomalidomide (CC-4047) in combination with cisplatin and etoposide in patients with extensive-stage small-cell lung cancer. J Thorac Oncol. 2013;8:423–428. doi: 10.1097/JTO.0b013e318282707b. [DOI] [PubMed] [Google Scholar]
- 24.Mesa RA, Pardanani AD, Hussein K, Wu W, Schwager S, Litzow MR, Hogan WJ, Tefferi A. Phase1/-2 study of Pomalidomide in myelofibrosis. Am J Hematol. 2010;85:129–130. doi: 10.1002/ajh.21598. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A, Verstovsek S, Barosi G, Passamonti F, Roboz GJ, Gisslinger H, Paquette RL, Cervantes F, Rivera CE, Deeg HJ, Thiele J, Kvasnicka HM, Vardiman JW, Zhang Y, Bekele BN, Mesa RA, Gale RP, Kantarjian HM. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27:4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kasserra C, Reyes J, Schafer P, Kosek J, Capone L, Parton A, Kim-Kang H, Surapaneni S, Kumar G. Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration. Cancer Chemother Pharmacol. 2013;71:489–501. doi: 10.1007/s00280-012-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Wang J, Rozewski DM, Kolli S, Wu CH, Chen CS, Yang X, Hofmeister CC, Byrd JC, Johnson AJ, Phelps MA. Sensitive liquid chromatography/mass spectrometry methods for quantification of pomalidomide in mouse plasma and brain tissue. J Pharm Biomed Anal. 2014;88:262–268. doi: 10.1016/j.jpba.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepper ER, Smith NF, Cox MC, Scripture CD, Figg WD. Thalidomide metabolism and hydrolysis: mechanisms and implications. Curr Drug Metab. 2006;7:677–685. doi: 10.2174/138920006778017777. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2001. [Google Scholar]