Abstract
Purpose
Reduced stress and reduced risk of cancer recurrence are among the many benefits of physical activity (PA) for cancer survivors. Exercise behaviors are linked to motivational factors. We investigated the associations between motivational profile, self-reported levels of PA and stress and mental functioning in 94 post-treatment breast cancer survivors who voluntarily enrolled in an exercise program.
Methods
Participants completed Apter Motivational Style Profile (AMSP), Life Time of Physical Activity (LTPA) Questionnaire, International Physical Activity Questionnaire (IPAQ), Medical Outcomes Short Form SF-36® (SF-36), Perceived Stress Scale (PSS) and provided 10 saliva specimens (to measure cortisol levels). PA levels were calculated in metabolic equivalent hours per week (MET-hrs/wk).
Results
Participants reported high levels of current and historical PA (M = 39.2 MET-hrs/wk, SD = 39.7; M = 14.2 MET-hrs/wk, SD = 15.4, respectively). They also reported high levels of stress (M = 33.6, SD = 4.5) coupled with low mental functionality as measured by SF-36 Mental Components Scale (MCS) (M = 44.4, SD = 8.8). PSS was negatively associated with MCS (r = −0.27, p = 0.009). Salivary cortisol was not associated with any measure. Participants had a conformist (“follow rules”) and alloic (“about others”) motivational profile. No motivational, exercise history or stress variables were associated with current PA.
Conclusions
As expected, participants reported higher levels of stress and lower mental functioning. Participants presented a unique motivational profile relative to the general population. Further research into the associations of motivation, exercise behaviors and stress are warranted.
Keywords: stress, breast cancer, exercise, motivation
INTRODUCTION
Overwhelming evidence continues to accumulate demonstrating the benefits of exercise in reducing morbidity and mortality while improving individual quality of life (QoL) and overall health [1–3]. These benefits also apply to cancer survivors and [3–5] include reducing their risk of cardiovascular disease and recurrent cancers [3]. However, cancer survivors tend to decrease their level of PA after diagnosis, and most never regain their former levels after treatment [3, 6, 7]. Approximately four of every five breast cancer survivors do not meet national exercise recommendations at 10 years post diagnosis [7]. Breast cancer survivors who are more obese and less physically active have a higher risk for cancer recurrence and mortality [8, 9]. Studies show that many cancer survivors report significantly lower levels of mental, emotional, physical and social functioning than gender and age-matched peers [10, 11] thus engaging in PA is important behavior for cancer survivors [3, 12].
In 1987 the National Institute for Mental Health (NIMH) published a consensus statement on the influence of exercise on mental health. The statement concluded that exercise is positively associated with mental health and well-being; appropriate exercise results in reductions in various stress indices including stress hormones; and exercise has beneficial emotional effects across all ages and in both sexes [13]. Moreover, many studies including meta-analyses have validated the benefits of exercise and PA on anxiety, perceived stress and release of stress hormones [14–16]. Chronic stress has been associated with many negative health outcomes [17, 18] including primary and secondary cancer risk [19–21]. Coupled with common daily stressors, cancer survivors have the additional stressful life event experience of cancer including stressful treatments [22] and an often reported fear of recurrence. Consequently, cancer survivors often report higher levels of stress than those without the experience of cancer [23–26].
Given the physical and mental benefits of PA for breast cancer survivors, focusing in on the associations between exercise, stress and understanding the motivation to engage in exercise is an important area of research. There are many factors that influence the decision to exercise or to adopt other cancer prevention behaviors (screening, smoking cessation, etc.). One key factor in deciding to engage or not engage in health behaviors is a person’s individual motivation at the time of the behavior.
An encompassing theory that can be employed to understand motivation and human behavior is Reversal Theory (RT) [27]. RT is based on a phenomenological approach to understanding human motivation. The theory posits that individuals alternate (“reverse”) between four pairs of mutually exclusive motivational states (telic vs. paratelic, conformist vs. negativistic, mastery vs. sympathy, autic vs. alloic). In the telic state, the end-goal is what motivates; in the paratelic state, enjoying the process is more important. In the conformist state, one is motivated by a desire to abide by rules or norms; in the negativistic state, motivation comes from the need to break free of rules. The mastery state is characterized by the need to win and the desire for power; the sympathy state is about caring and affection. The autic-alloic pair differs in terms of for whom the individual seeks primary benefit: self (autic) or others (alloic).
Reversal Theory (RT) helps explain how motivational states drive proximal behavior [27]. Certain states correspond to improved increased felt enjoyment in the moment of doing the behavior. The perception of a positive experience is often a function of state consistency with what RT describes as “dominances”- a measure of an individual’s preference to be in a certain motivational state [27]. We are not aware of any published research that has investigated exercise and cancer survivorship using RT as its theoretical framework. In this study we investigated the associations between motivational profile, self-reported levels of PA and stress and mental functioning in 94 post-treatment breast cancer survivors who voluntarily enrolled in an exercise program.
METHODS
Recruitment
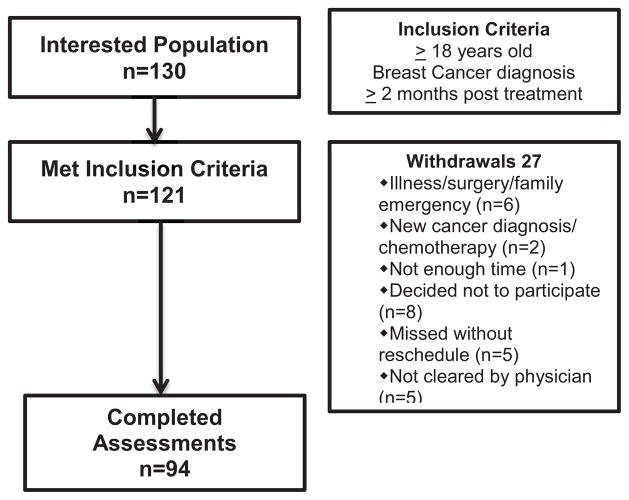
After obtaining institutional review board (IRB) approval, participants were recruited with assistance from the ThriveWell™ Cancer Foundation’s DIVA (Deriving Inspiration and Vitality through Activity) self-referral program in San Antonio, Texas. The DIVA program offers support services at no cost for breast cancer survivors. Flyers advertising the study were distributed to local oncology clinics and relayed via local radio and television announcements. Participants responding to these notices and/or wanting to register for the DIVA program called the DIVA center to express interest and were screened for eligibility. Inclusion criteria consisted of being 18 years of age or older; history of invasive or ductal-in-situ breast cancer and completion of treatment (chemotherapy, radiation, surgery or combination) for at least 2 months prior to enrollment; able to read/write in English; and able to provide informed consent. A total of 130 potential participants expressed interest in the study and 121 met the inclusion criteria. Of the ones who were eligible, 94 provided informed consent and completed the assessments (see Figure 1).
Fig. 1.
Flow diagram of IMPACT study accrual
Procedures
After providing informed consent, participants were asked to complete an interviewer-administered Lifetime of Physical Activity (LTPA) Questionnaire [28]. After the LTPA participants then completed a questionnaire packet that included demographic information, medical history, International Physical Activity Questionnaire (IPAQ)-Short Form [29], Medical Outcomes Short Form SF-36® (SF-36) [30], Perceived Stress Scale (PSS) [31], and the Apter Motivational Style Profile (AMSP) [32]. After completing the packet, participants were given a packet containing 10 vials to collect saliva for cortisol sampling. Participants received a $25 gift card as compensation for their time.
Measures
We chose to use these specific questionnaires for several reasons. The LTPA provides an indication of an individual’s level of activity over their entire lifetime, while the IPAQ provides a 7-day snapshot of current activity (previous week). Using both a lifetime and a current PA measure we aimed for a better perspective of the participant’s overall engagement of PA. We also expected that overall engagement of PA would be reflected in the scales scores of the SF-36, particularly the composite scores- physical component scales (PCS) and mental component scale (MCS). We anticipated those with higher engagement of PA (past or present) would have higher PCS and MCS scores. Although the SF-36 gives a general indication of mental functioning, we also wanted to assess overall (“global”) perceived stress as well as a biological marker of stress, (i.e., cortisol). Taken together with the motivational profile as scored by the AMSP, we reasoned that these measures would give us valid instruments to investigate the association between motivation, PA levels and stress (mental functioning, perceived stress and physiological). Each instrument is detailed further below.
Lifetime Total Physical Activity (LTPA) Questionnaire [28]
The LTPA was used to assess PA patterns over one’s lifetime to include exercise/sports activities. Engaged PA for at least 2 hours per week for at least 4 months of the year, or at least 10 times during one’s lifetime, is included in the operational definition. We asked participants to specify activity beginning at the earliest recalled memory (e.g., P.E. in school) through to the present day; the particular type of activity (e.g. volleyball); age started/finished; number of months out of the year; times per week; activity duration in hours/min; and activity intensity (“1- activities that are done sitting, 2- activities that require minimal effort, 3- activities that are not exhausting, that increase the heart rate slightly and may cause slight perspiration, and 4- activities that increase the heart rate and cause perspiration”). Activity-specific (e.g., volleyball) energy cost values expressed in metabolic equivalents (METs) were then calculated by consulting the published Compendium of PA [33] (MET is the metabolic equivalent of the level of energy consumption for a body at rest; the higher a MET value, the higher the energy requirement). MET values of activities can range from 0.9 (sleeping) to 18 (running at a 17.5 km/hour pace) [33].
International Physical Activity Questionnaire (IPAQ) (Short Form) [29]
The IPAQ is used for measuring PA among diverse groups of populations [29]. The IPAQ Short Form asks specific types of PA undertaken: walking, moderate-intensity, and vigorous-intensity. The total score requires summation in minutes and frequency in days, computed by weighting each type of activity by its energy requirements, (defined in METs), to yield a score in MET-minutes of activity per week [29]. For example: “During the last 7 days, on how many days did you do vigorous physical activities like heavy lifting, digging, aerobics, or fast bicycling?” Reliability for this instrument is high, with Spearman’s ρ of 0.8 [29].
Medical Outcomes Short Form (SF-36) [30]
The SF-36 is used to assess health-related quality of life (QoL) physical and mental functioning. The SF-36 is comprised of 36 items, encompassing 8 health concept subscales: 1) Physical Functioning (PF): 2) Role-Physical (RP); 3) Bodily Pain (BP); 4) General Health (GH); 5) Vitality (VT); 6) Social Functioning (SF); 7) Role-Emotional (RE); and 8) Mental Health (MH). Sample question: “During the past 4 weeks, have you had any of the following problems with your work or other regular daily activities as a result of your emotional problems (such as feeling depressed or anxious) accomplished less than you would like?” with possible responses of “1- Yes” or “2- No”. Factor analyses of the subscales have identified two aggregate factors interpreted as the ‘physical component scale’ (PCS) and the ‘mental component scale’ (MCS). These measures can be treated separately statistically with reliability estimates for the summary scores exceeding 0.90. The higher scores indicate higher physical and mental functioning (‘well-being”) of the individual, respectively. The scores for the SF-36 have been normed for the general population and specific sub-populations [30]. For our analyses we focused on the MCS scores.
Perceived Stress Scale (PSS) [31]
The PSS is used as a measure of global perceived stress according to the degree that life circumstances are appraised as stressful over the course of the previous 4 weeks. The scale ranges from 0–4 (“0= never”, “1= almost never”, “2= sometimes”, “3= fairly often” and “4= very often”). A sample question is: “In the last 4 weeks, how often have you felt that you were unable to control the important things in your life?” Higher scores have been associated with increased susceptibility to illness and greater vulnerability to depressive symptoms or negative affect in response to stressful life events [31].
Salivary Cortisol
Saliva specimens were collected using Salivette kits (Sarstedt, Inc. Newton, NC) consisting of a cotton swab in a polypropylene vial. To collect the specimen, the participant placed the swab in her mouth until it became saturated, then returned the cotton roll into the vial. Each participant provided five specimens per day for two consecutive days. Samples were collected upon waking, 45 minutes later, 8 hours after waking, 12 hours after waking, and right before going to bed. Participants mailed the specimens back to the research staff in pre-addressed, pre-paid mailers. Upon receipt of the samples, research staff logged their arrival and stored them in a −80°C freezer until all participants’ specimens were collected. The specimens were then shipped to Kirschbaum Laboratories in Dresden, Germany. Salivary cortisol concentrations were determined by immunoassay using an in-house DELFIA system (Pharmacia, Uppsala, Sweden) [34]. Results were reported in nmol/liter and e-mailed to the research staff. Salivary cortisol concentrations were log-transformed, plotted against time-points 2–5 for each day, and a linear regression analysis was created to determine slope. Slopes were averaged for each day and an overall mean slope over the two days was obtained. This methodology of assessing diurnal rhythm slope has been previously used in breast cancer survivors where flatter slopes were predictive of poorer outcomes [35].
Motivational Profile [32]
The Apter Motivational Styles Profile (AMSP) was used to assess motivational profiles [32]. The AMSP is a 40-item questionnaire representing each of the 8 subscale motivational states: 1) Telic (serious), 2) Paratelic (playful), 3) Conforming (compliant to rules), 4) Negativistic (rebellious), 5) Mastery (powerful), 6) Sympathy (relational), 7) Autic (self-oriented), and 8) Alloic (other-oriented). A sample question from the AMSP is as follows: “I do things that I consider important,” with 6 possible responses: “0= never”, “1= seldom”, “2= sometimes”, “3= often”, “4= very often”, “5 = always”.
An individual’s preferred state or “dominance” is calculated by subtracting the scores for each of the pairs: “telic-paratelic”, “conforming-negativistic”, “mastery-sympathy”, and “autic-alloic”. A resultant positive score (≥ 0) within each combination indicates a dominance of either “telic”, “conformist”, “mastery”, or “autic”, respectively; whereas a negative score (< 0) indicates a dominance of “paratelic”, “negativistic”, “sympathy” or “alloic”, respectively. Test-retest correlations of the AMSP over a 12-week period found external reliability to range from 0.61 to 0.92 for the subscales [36].
Treatment of Data
Data were entered into a study-specific comprehensive information management system maintained by the Department of Epidemiology and Biostatistics at the University of Texas Health Science Center at San Antonio (UTHSCSA). Reports were generated for the AMSP, PSS, and SF-36, and then uploaded to an SPSS© Statistics (version 19, 2011, IBM Corp, Somers, NY) database. Descriptive statistics were performed (variable mean for central tendency and standard deviation for variability), followed by bivariate correlation analyses to test for associations between variables. Regression analysis was run with both the IPAQ score and MCS score as dependent variables, PSS score and salivary cortisol slope as predictor variables. To determine the influence of motivational dominance on current PA, regression with IPAQ score as the dependent variable and motivational dominances scores as predictor variables were also run.
Missing Values
Sixteen of the AMSP, SF-36 and PSS had one or several items missing. For the AMSP the average item value for the particular subscale corresponding to the missing item was used [32]. The same procedure was followed for the SF-36 with the missing item recoded using the average value and the scores recalculated [30]. For the PSS, the total score obtained was divided by the number of items filled out. This average value was used for the missing item and added to the score originally obtained. Out of 94 administrations of the IPAQ, only 10 were not filled out enough to use, or were missing the entire scale.
RESULTS
Participant descriptive characteristics are shown in Table 1. Participants averaged 56.2 years of age. As a group, participant motivational profile included being goal-oriented (telic dominance= 4.3), highly conformist (conformist dominance= 11.4), sympathetic (mastery dominance= −1.0), and focused on others or alloic, (autic dominance= −6.6). Participants reported being historically fairly active (M = 14.2 MET hrs/week, SD = 15.4) and currently very active (M = 39.2, SD = 39.7). The SD indicated a high variability in self-reported history of PA and especially in current level of PA. Participants self-reported high levels of overall stress (M = 33.6, SD = 4.5), a value higher than published norms [37]. This high level of stress was coupled with lower MCS scores (M = 44.4, SD = 8.8), which were lower than published norms [30].
Table 1.
Participant Characteristics, n=94.
| M | SD | Min | Max | |
|---|---|---|---|---|
| Age | 56.2 | 7.9 | 42 | 78 |
| Lifetime of Physical Activity | 14.2 | 15.4 | 1.0 | 120.5 |
| IPAQ Score | 39.2 | 39.7 | 0 | 190.2 |
| Perceived Stress Scale Score | 33.5 | 4.5 | 22.0 | 45.0 |
| Mental Component Score | 44.4 | 8.8 | 11.8 | 59.9 |
| Physical Component Score | 43.8 | 6.3 | 30.3 | 61.0 |
| Cortisol (Mean Slope) | −.61 | .23 | −1.21 | −.11 |
| Telic Score | 4.3 | 4.0 | −5 | 15.0 |
| Conformist Score | 11.4 | 4.9 | 0 | 24.0 |
| Mastery Score | −1.0 | 2.6 | −8.0 | 5.5 |
| Autic Score | −6.6 | 3.3 | −14.5 | 0.0 |
Note: LTPA= Lifetime of Physical Activity (MET-hrs/week); IPAQ = International Physical Activity Questionnaire (current activity in MET-hrs/week).
As expected, PSS was negatively associated with SF-36 MCS score, (r = −.27, p = .009). (The more stress, the less mental functioning.) PSS score was associated with mastery dominance (r = .20, p = .05); SF-36 MCS score was associated with conformist dominance (r = .21, p = .04) and negatively associated with autic dominance (r=−.24, p = .02) (i.e., higher MCS scores were associated with more alloic or “other” focus). Cortisol slope was not associated with any variable. PA was not associated with any of the stress or motivational variables (see Table 2). Regression analyses showed no variable as a predictor of current PA (data not shown).
Table 2.
Correlational Stress, Motivational Profiles and Physical Activity
| PSS | MCS | Cortisol | Telic | Conf | Mast | Autic | LTPA | IPAQ | |
|---|---|---|---|---|---|---|---|---|---|
| PSS | X |
−.27* p = 0.009 |
NS | NS | NS |
.20* p = 0.048 |
NS | NS | NS |
| MCS | X | NS | NS |
.21* p = 0.043 |
NS |
−.24* p = 0.022 |
NS | NS | |
| Cortisol | X | NS | NS | NS | NS | NS | NS | ||
| Telic | X |
.51** p < 0.001 |
NS |
−.30* p = 0.044 |
NS | NS | |||
| Conf | X | NS |
−.33* p = 0.001 |
NS | NS | ||||
| Mast | X |
−.21* p = 0.044 |
NS | NS | |||||
| Autic | X | NS | NS | ||||||
| LTPA | X | NS | |||||||
| IPAQ | X |
Note: NS = Not Significant; PSS = Perceived Stress Scale; MCS = Mental Component Scale; Cortisol = Average salivary cortisol slope (2 days); Conf = Conformist Dominance; Mast = Mastery Dominance; LTPA= Lifetime of Physical Activity (MET-hrs/week); IPAQ = International Physical Activity Questionnaire (current activity in MET-hrs/week);
p <.05;
p <.001.
DISCUSSION
Despite the benefits of an active lifestyle, only 25% of the adult U.S. population engages in at least moderate intensity PA [38], and for cancer survivors that proportion is lower[3, 7]. PA is important for cancer survivors’ QoL, physical functioning, mental affect and long-term health outcomes [3–5]. Cancer survivors understandably report higher levels of stress than age-matched peers with no history of cancer. Thus, understanding the motivational variables associated with engaging in PA and the effects of PA on stress and mental QoL aspects specific for cancer survivors are important areas of research. Here we investigated these associations in 94 post-treatment breast cancer survivors who consented to participate in an exercise intervention.
Our group of survivors reported high levels of both historical and current PA. However, as is often seen in self-report, there was high variability in both past and current activity indicating a bias toward over-estimation. Although we used PA in our analyses, the accuracy of information is admittedly unreliable. This variability likely contributed to the failure to find any significant predictors of current PA with the variables we investigated. Results might have been different if we had used objective measures in addition to self-reports, such as accelerometry data for current level of PA.
As expected, our breast cancer survivors reported higher stress levels coupled with lower mental functioning when compared with the general population. Cancer survivors experience the stressors of daily living; but also, the often acute distress associated with the cancer experience. The very high PSS score (M = 33.6, SD = 4.5), much higher than comparative norms [22] suggests such stressors exist in our sample of breast cancer survivors. This high level of stress was coupled with lower MCS scores (M = 44.4, SD = 8.8) relative to published norms. These results suggest that cancer survivors can greatly benefit from the QoL enhancing benefits of PA [3].
We collected salivary cortisol and analyzed the average daily slope over a consecutive two-day period. Psychosocial effects on cancer progression may be measured, and possibly mediated, by disruption of circadian function [19]. A blunted slope indicates less favorable outcomes for breast cancer survivors [35]. Our population’s mean cortisol results of −0.61 log (nmol/L) suggest a more ‘healthy’ cortisol rhythm. Surprisingly, cortisol slope was not associated with any other variable, even PSS or SF-36 MCS. One explanation might be that salivary cortisol is an “immediate” indicator for stress i.e., cortisol levels in the saliva change in as few as 3 to 4 minutes, whereas the PSS & SF-36 ask participants to consider the previous 4 weeks. Given the same time frames, SF-36 MCS and PSS were negatively associated (i.e., the more perceived stress, the lower the mental well-being). To better determine association of these variables with cortisol, sampling should match the same time period, i.e., completed over a similar 4-week period). This is an opportunity for future research.
An intriguing result of our study was the unique motivation profile of our breast cancer survivor population. This profile differs from published norms [36]. Moreover, this unique profile matches very closely with breast cancer survivors [39] and endometrial cancer survivors [40] that have engaged in our previous exercise studies (see Table 3). It appears that the women who have been enrolling in our studies have motivation profiles that are unusually conformist and alloic. This may not be totally surprising when one considers that our exercise interventions require conforming to what are new exercise regimens for many of our participants while filling out many self-report forms, which also require “conforming”. Moreover, we often hear from our participants that one of the main reasons they are enrolling in the research trial is “for the next generation” or for “others to not have to go through the experience of cancer”. We would expect that this may be common dialogue in other research settings as well. We did find associations between these motivational dominances and stress variables that seem to support this population’s unique motivation profile. For example, it is not surprising to find MCS scores associated with conformist and alloic (“about others”) dominances for this specific population. This is an area that warrants further study, e.g., expanding sampling to other cancer populations to determine if similar profiles are seen.
Table 3.
Dominance Scores for Female Cancer Survivors Participating in Exercise Studies who Completed the Apter Motivational Style Profile, Means and (Standard Deviation).
| Dominance | “Steps to Health” Endometrial Cancer Survivors n=100 | “IMPACT” Breast cancer survivors n=94 | “Viva” Breast cancer survivors n=150 | Norms n=1248 |
|---|---|---|---|---|
| Telic-Paratelic | 4.4 (4.4) | 4.3 (4.0) | 2.4 (4.8) | 1.0 |
| Conformist-Rebellious | 12.1 (5.8) | 11.4 (4.9) | 11.2 (6.1) | 4.4 |
| Mastery-Sympathy | −1.5 (3.4) | −0.98 (2.6) | −0.32 (3.6) | −1.2 |
| Autic-Alloic | −6.1 (4.1) | −6.6 (3.3) | −6.9 (3.9) | −1.2 |
Note: Total participants in studies n=344.
Limitations
Our results need to be interpreted in light of some limitations. First, our self-report measures of past and current PA had high variability and thus became an issue in terms of validity. Secondly, although we showed some expected associations between stress and mental functioning, we did not show any associations between our biological marker for stress, cortisol, and self-report measures, probably due to incongruent sampling time periods.
Conclusion
Our post-treatment breast cancer participants reported high levels of perceived stress and low mental functioning. Through examination of our participants’ motivational profiles, we identified a characteristic profile that closely matches other cancer survivors that have enrolled in our exercise-related studies and seems to be unique relative to the general population. If we discover a consistent profile in cancer survivors enrolling in clinical research trials, then the intervention could be designed and tailored to individual profiles and could be applied to the whole spectrum of cancer prevention behaviors, such as energy balance, smoking cessation and screening to name a few. Further research into the associations of motivation, health promoting behaviors and stress are warranted.
Acknowledgments
The project described was supported by Susan G. Komen Foundation Award # SAB08-00005. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Susan G. Komen Foundation. The project described was supported by Award Number K22 CA154626 (D.C.H.), U54 CA153511 from the National Cancer Institute (NCI) (A.G.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Work in R.L.’s lab is supported by an NIH grant (CA161349) and Cancer Prevention Research Institute of Texas (CPRIT) grant (RP110524). The authors gratefully acknowledge the support of the Cancer Therapy and Research Center at The University of Texas Health Science Center at San Antonio, an NCI-designated Cancer Center (P30CA054174) and Patient-Reported Outcomes, Survey, and Population Research (PROSPR) Shared Resource, NCI funded CA16672. The authors gratefully acknowledge the support of Dr. Amy Lang, the ThriveWell™ Cancer Foundation, and the START Center for Cancer Care.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this study was performed using the highest ethical standards complying with all United States laws and declare that they have no conflict of interest. The authors have full control of all primary data and will allow the Journal of Supportive Care in Cancer to review the data upon request.
References
- 1.United States Department of Health and Human Services. [Accessed 29 December 2012.];Physical activity guidelines for Americans. 2008 www.health.gov/PAGuidelines.
- 2.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 4.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–52. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 6.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215–26. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 7.Mason C, Alfano CM, Smith AW, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomark Prev. 2013 Apr 10; doi: 10.1158/1055-9965.EPI-13-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res. 2011;4(4):522–9. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson RE, Cadmus LA, Emond JA, et al. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Basen-Engquist K, Scruggs S, Jhingran A, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200(3):288 e1–8. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SM, Alfano C, Wilder Smith A, et al. Sedentary Behavior, Health-Related Quality of Life and Fatigue among Breast Cancer Survivors. J Phys Act Health. 2012 Mar;10(3):350–8. doi: 10.1123/jpah.10.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courneya KS, Mackey JR, Jones LW. Coping with cancer: can exercise help? The Physician SportsMed, 2000. 2000;28(5):49–73. doi: 10.3810/psm.2000.05.896. [DOI] [PubMed] [Google Scholar]
- 13.Morgan WP, O’Connor PJ. Exercise and mental health. In: Dishman RK, editor. Exercise adherence. Human Kinetics Publishers, Inc; Champaign, IL: 1988. pp. 91–119. [Google Scholar]
- 14.Hughes DC, Leung P, Naus MJ. Using single-system analyses to assess the effectiveness of an exercise intervention on quality of life for Hispanic breast cancer survivors: a pilot study. Soc Work Health Care. 2008;47(1):73–91. doi: 10.1080/00981380801970871. [DOI] [PubMed] [Google Scholar]
- 15.Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: to crack the 40-year-old nut, replace the 40-year-old nutcracker! Ann Behav Med. 2008;35(2):136–49. doi: 10.1007/s12160-008-9025-z. [DOI] [PubMed] [Google Scholar]
- 16.Milani RV, Lavie CJ. Reducing psychosocial stress: a novel mechanism of improving survival from exercise training. Am J Med. 2009;122(10):931–8. doi: 10.1016/j.amjmed.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 18.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 19.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–8. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 20.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood AK, Bhatty R, Kammat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12(2):369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Ashing-Giwa KT, Padilla G, Tejero J, et al. Understanding the breast cancer experience of women: a qualitative study of African American, Asian, American, Latina and Caucasian cancer survivors. Psycho-Oncology. 2004;13(2004):408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benyamini Y, McClain CS, Leventhal EA, et al. Living with the worry of cancer: health perceptions and behaviors of elderly people with self, vicarious, or no history of cancer. Psycho-Oncology. 2003;12(2):161–72. doi: 10.1002/pon.637. [DOI] [PubMed] [Google Scholar]
- 25.Stabiner K. To dance with the devil the new war on breast cancer. New York: 1997. [Google Scholar]
- 26.Andersen BL, Kiecolt-Glaser JK, Glaser R. Biobehavioral model of cancer: stress and disease course. Am Psychol. 1994;49(5):385–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apter M. An introduction to Reversal Theory. In: Apter J, editor. Motivational styles in everyday life: a guide to Reversal Theory. American Psychological Association; Washington, DC: 2001. pp. 3–37. [Google Scholar]
- 28.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–74. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Snow KK, Kosinski M, et al. SF-36® Health survey manual and interpretation guide. Quality Metric Incorporated; Lincoln, RI: 2000. [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 32.Apter International. Research manual for the Apter Motivational Style Profile (AMSP) Apter International; Ltd Manassas, VA: 2004. [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 34.Dressendorfer RA, Kirschbaum C, Rohde W, et al. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43(7):683–92. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 35.Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 36.Apter M, Mallows R, Williams S. The development of the Motivational Style Profile. Personal Individ Differ. 1998;24:7–18. [Google Scholar]
- 37.Cohen S, Williamson GM. In: Perceived stress in a probability sample of the United States. Spacapan S, Oskamp S, editors. The social psychology of health Sage; Newbury Park, CA: 1988. [Google Scholar]
- 38.Center for Disease ControlPrevention . Trends in leisure-time physical inactivity by age, sex, and race/ethnicity--United States, 1994–2004. MMWR Morb Mortal Wkly Rep. 2005;54(39):991–4. [PubMed] [Google Scholar]
- 39.Hughes DC, Tirado-Gomez M, Vallejo L, et al. Comparing determinants of physical activity in Puerto Rican, Mexican-American, and non-Hispanic white breast cancer survivors. Fourth American Association for Cancer Research Conference on The Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved; Washington, D.C: 2011. [Google Scholar]
- 40.Hughes DC, Baum GP, Perkins HY, et al. Metamotivational States and Exercise Behaviors in Previously Sedentary Endometrial Cancer Survivors. 14th International Reversal Theory Conference; New Orleans, LA. 2009. [Google Scholar]