Abstract
Sirtuins such as SIRT1 are conserved protein NAD+-dependent deacylases and thus their function is intrinsically linked to cellular metabolism. Over the past two decades, accumulating evidence has indicated that sirtuins are not only important energy status sensors but also protect cells against metabolic stresses. Sirtuins regulate the aging process and are themselves regulated by diet and environmental stress. The versatile functions of sirtuins and more specifically SIRT1 are supported by their diverse cellular location allowing cells to sense changes in energy levels in the nucleus, cytoplasm and mitochondrion. SIRT1 plays a critical role in metabolic health by deacetylating many target proteins in numerous tissues, including liver, muscle, adipose tissue, heart and endothelium. This sirtuin also exerts important systemic effects via the hypothalamus. This review will cover these topics and suggest that strategies to maintain sirtuin activity may be on the horizon to forestall diseases of aging.
Keywords: Sirtuins, SIRT1, calorie restriction, aging, metabolism, CR mimetics, NAD+
Sirtuins: indispensable energy sensors
Sirtuins are class III histone deacylases that consume one molecule of NAD+ during each deacylation cycle [1]. The first identified sirtuin protein is silent information regulator 2 (SIR2) from Saccharomyces cerevisiae. SIR2 was originally characterized as a chromatin silencing component which repressed gene transcription at selected loci [2]. Soon after the discovery that SIR2 extended yeast replicative lifespan [3,4], the orthologs of SIR2 were proposed to carry out same lifespan prolonging effects in Caenorhabditis elegans [5,6], and in Drosophila melanogaster [7], and to mediate beneficial effects of calorie restriction (CR) on health and longevity[7–10]. These findings were challenged in 2011 by a study suggesting that SIR2 orthologs in worms and flies did not mediate increases in lifespan [11]. As discussed in the next section below, more recent studies in many organisms have now confirmed the original hypothesis that sirtuins are conserved diet-sensitive, anti-aging proteins.
In mammals, the anti-aging functions of sirtuins are conserved [12,13]. There are seven mammalian sirtuins, SIRT1–7, which function to regulate metabolism in non-redundant ways in many tissues. Because sirtuins are located in distinct cellular compartments, they are able to coordinate cellular responses to CR throughout the organism. SIRT1, SIRT6 and SIRT7 are localized in the nucleus, where they function to deacetylate histones thereby influencing gene expression epigenetically [14]. SIRT1 also deacetylates specific transcription factors and enzymes to influence their activities, as described below. SIRT2 was originally described as a cytosolic sirtuin, however, recent data show that SIRT2 is also found in the nucleus where it functions to modulates cell cycle control [15–17]. SIRT3, SIRT4 and SIRT5 are localized in mitochondria, and regulate the activities of metabolic enzymes and moderate oxidative stress in this organelle [18]. In general, sirtuins 3–5 respond to CR by switching cells to favor mitochondrial oxidative metabolism, along with induction of accompanying stress tolerance.
In this review, we focus our attention on SIRT1, the most studied sirtuin, but also touch briefly on other mammalian paralogs of SIRT1. We focus on the metabolic functions of SIRT1 and other sirtuins in critical tissues to mediate physiological adaptability to diets. We also discuss briefly some of the challenges and controversies that have emerged about the role of sirtuins in CR, and critically assess new findings that have begun to resolve these differences. While we will not cover the large body of data on sirtuins and diabetes and neurodegenerative diseases, but will address the relationship between sirtuins and cancer. Finally, we will consider emerging findings on the importance of the sirtuin co-substrate NAD+ in aging and diseases.
The evolving role of sirtuins in CR and aging
The finding that sirtuins are NAD+ dependent deacetylases [1] prompted the suggestion that they helped mediate the effects of CR in an active process. This idea contrasted with earlier proposals that CR extended life span by passive mechanisms, such as lowering the production of reactive oxygen species. In model organisms, nutrient limitation was shown to extend the life span via sirtuins in yeast, Drosophila, and C. elegans [19]. However, some laboratories observed life span extension by nutrient limitation that was independent of SIR2 orthologs [11,20,21]. Part of the difficulty in interpreting these data is that laboratories may use a variety of protocols to limit nutrients. Another potential problem is differences in strain backgrounds among laboratories. Since several other nutrient sensors besides sirtuins exist, such as insulin signaling [22], target of rapamycin (TOR) [23], and AMP-activated protein kinase (AMPK) [24], varied experimental conditions between different laboratories may activate different nutrient sensing pathways. In the lower organisms, it therefore seems extremely likely that multiple pathways, including sirtuins, can elicit the benefits of nutrient limitation. In mice, the same murine strains are used under the same limitation of food of roughly the same composition. The lines of evidence that sirtuins mediate effects of CR in mammals are numerous and are outlined below.
First, the non-histone proteins targeted for deacetylation by sirtuins closely define those pathways involved in metabolic adaptation to CR, for example oxidative metabolism in mitochondria (Figure 1) [14]. Second, CR induces the expression of the sirtuins, SIRT1 [25], SIRT3 [26], and SIRT5 [27] in mice and SIRT1 in humans [28]. Conversely, a high fat diet can trigger the loss of SIRT1 in mice via proteolysis [29], and obesity can reduce the expression of SIRT1 in humans [30,31]. Third, loss of function mutations in specific sirtuin genes can reduce specific outputs of CR. For example, SIRT1 knockout mice do not show the usual increase in physical activity induced by this diet [32]. In addition, brain-specific knockout of SIRT1 in mice do not show the characteristic changes in the somatotropic axis (growth hormone/IGF-1) induced by CR[33]. Most revealingly, SIRT1 knockout mice do not live longer on a CR diet [34,35]. As for other sirtuins, knocking out the mitochondrial SIRT3 prevents the protective effect of CR against neuronal degeneration, leading to hearing loss [36]. In this case, SIRT3 is required to reduce oxidative damage in crucial hair cell neurons of the cochlea. Deletion of the mitochondrial SIRT5 prevents the up-regulation of the urea cycle, which is required to reduce blood ammonia when amino acids serve as energy sources [27]. SIRT3 null mice also show a defect in regulation of the urea cycle [37].
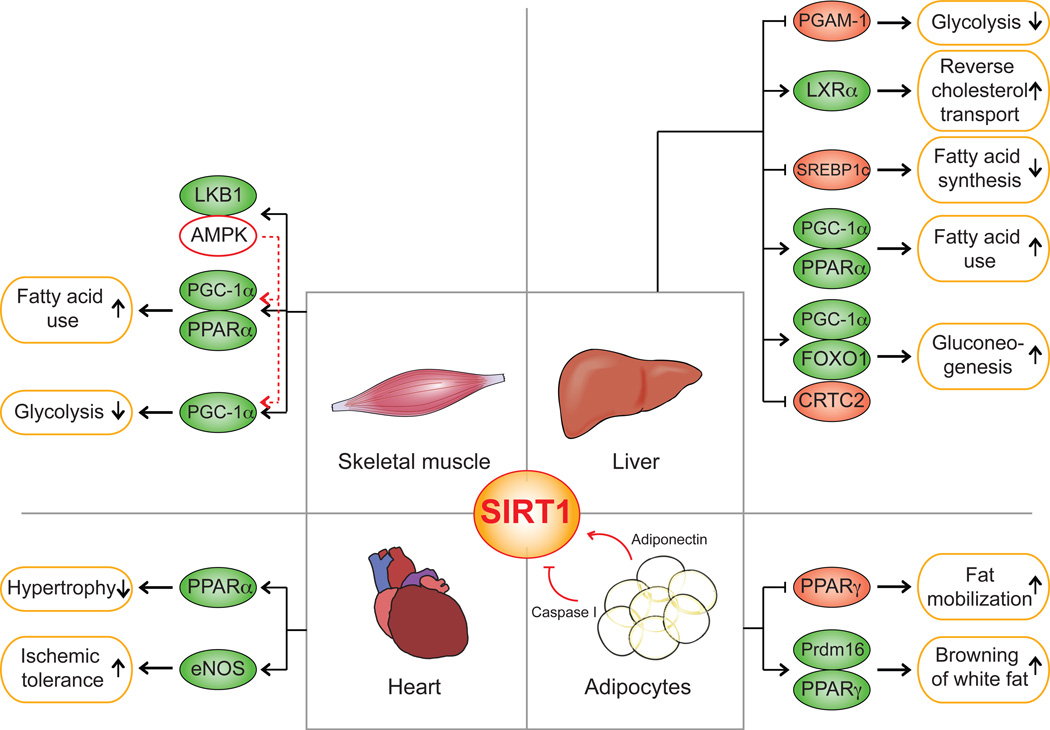
Figure 1. SIRT1 mediates metabolic benefits in various tissues.
Major metabolic tissues, such as liver, heart, white adipose tissue (WAT) and skeletal muscle are depicted to illustrate SIRT1 functions. In the liver, SIRT1 supports gluconeogenesis via PGC-1α and FOXO1[57], and facilitates CRCT2 degradation upon prolonged fasting [56]. SIRT1 inhibits glycolysis by repressing glycolytic enzyme PGAM-1 [59]. In the liver, SIRT1 responds to fasting and promotes fatty acid oxidation by activating PPARα [58] and inhibits fatty acid synthesis by targeting SREBP1c for degradation [63]. SIRT1 is a positive regulator of LXR and thus regulates whole-body cholesterol homeostasis [64]. In the skeletal muscle, SIRT1 exerts similar actions on increasing fatty acid utilization, and reduces glycolysis as described above [75]. Here SIRT1 and AMPK comprise a reciprocal positive regulating loop. AMPK activates SIRT1 by up-regulating the gene encoding the NAD+ synthetic enzyme, NAMPT [78,79]. Reciprocally SIRT1 activates AMPK by deacetylating LKB1 [80]. In WAT, SIRT1 mobilizes fat from WAT via PPARγ to drive lipid utilization in liver and muscle [83]. In addition, by deacetylating PPARγ to facilitate Prdm16 binding, SIRT1 drives a white fat browning to enhance energy expenditure [84]. SIRT1 protein is degraded post high fat diet challenge by activated caspase I [29] and can be up-regulated by adiponectin [82]. SIRT1 also benefits the heart by increasing ischemic tolerance via an activation of eNOS [85]. SIRT1 additionally protects against cardiac hypertrophy through PPARα activation [87]. Targets that are directly activated by SIRT1 are shown in green. Those are repressed or inhibited by SIRT1 are in pink.
A number of reports have also demonstrated that over-expression of SIRT1 in transgenic mice can mitigate disease syndromes much like CR, including diabetes, neurodegenerative diseases, liver steatosis, bone loss and inflammation [38–42]. Tissues responsible for these effects are discussed in detail below (Figure 1). Conversely, compromised sirtuin activity contributes to metabolic syndrome and diabetes and exacerbates the effects of a high fat diet in mice and humans [29,43]. In addition, SIRT1 activators like resveratrol [44] and newer sirtuin activating compounds (STACs) [45], exert effects that are similar to those of CR as revealed by measures of whole animal physiology [46] or by transcriptional profiling [47]. Importantly, a recent study strongly suggests that the effects of resveratrol and all 117 described STACs are direct, and not an artifact of assays using fluorophore-containing peptides. STACs target an allosteric site in SIRT1, which is separate from the catalytic domain, and thus activate the deacetylation of substrates containing lysines with nearby hydrophobic residues [48]. In toto these data comprise a compelling set of evidence that sirtuins are fundamentally involved in mediating effects of CR.
Although a paper by Burnett et al. [11] did not find life span extension in SIR2 ortholog transgenic lines of C. elegans or Drosophila, many other studies have established such a connection [5–7,10,49–51]. In the past year, new studies were reported confirming the importance of SIR2 orthologs in slowing aging and extending life span in yeast [52], C. elegans [53,54] and Drosophila [55]. Extension of murine life span has also been reported for transgenic lines of SIRT6 [12] or SIRT1 [13]. Thus, there is also compelling evidence that sirtuins regulate aging, which is consistent with their important role in CR.
Metabolic regulation in the liver
Whole body glucose homeostasis is critically regulated by the liver. When blood glucose levels are low, due to fasting or CR, hepatic metabolism immediately shifts to glycogen breakdown and then gluconeogenesis to ensure glucose supply, and ketone body production to bridge energy deficits. Fasting also activates muscle and liver oxidation of fatty acids produced by lipolysis in white adipose tissue. Several transcription factors are involved in a sophisticated switch to adapt to energy deprivation, and SIRT1 mediates the metabolic switch during fasting (Figure 1) [56]. During the initial (post glycogen breakdown) phase of fasting, pancreatic alpha cells produce glucagon to systemically activate gluconeogenesis in the liver via the cyclic AMP response element binding protein (CREB) and its coactivator, CREB-regulated transcription coactivator 2 (CRTC2). During more prolonged fasting, however, this effect is cancelled by SIRT1-mediated CRTC2 deacetylation, which targets the coactivator for ubiquitin/proteasome -mediated destruction [56]. SIRT1 then triggers the next stage of gluconeogenesis by deacetylating and activating the peroxisome proliferator-activated receptor (PPAR) γ coactivator 1α (PGC-1α), a coactivator for forkhead box O1 (FOXO1) [57]. Besides supporting gluconeogenesis, PGC-1α is also important for mitochondrial biogenesis, which assists the liver in accommodating the reduced energy status. Meanwhile, to increase energy production, SIRT1 stimulates fatty acid oxidation by deacetylating and activating the nuclear receptor, PPARα [58]. SIRT1 can also shut down the production of energy via glycolysis by deacetylating and repressing glycolytic enzymes, for example, phosphoglycerate mutase-1 (PGAM-1)[59]. Interestingly, another nuclear sirtuin, SIRT6, has also been reported to repress glycolysis by serving as a corepressor for hypoxia-inducible factor 1α (HIF-1α) [60]. As SIRT6 itself is transcriptionally activated by SIRT1, sirtuins might regulate cellular physiology in a coordinated way to determine the duration of each phase of fasting [61].
In addition to glucose homeostasis, the liver plays important roles in controlling lipid and cholesterol homeostasis. During fasting, fat and cholesterol synthesis in the liver is turned off, and lipolysis in white adipose tissue (WAT) is favored. The major hepatic transcription factors for lipogenesis and cholesterol synthesis are proteins belonging to sterol regulatory element binding protein (SREBP) family [62]. Upon fasting, SIRT1 deacetylates SREBP1 and targets the protein for destruction through the ubiquitin/proteasome system (Figure 1) [63]. The result is repression of fat and cholesterol synthesis, consistent with the finding that SIRT1 liver-specific knockout mice develop hepatic steatosis [58]. For regulating cholesterol homeostasis, SIRT1 also regulates the oxysterol receptor (LXRα) to assist in reverse cholesterol transport from peripheral tissues by up-regulating the LXRα target gene ATP-binding cassette transporter A1 (ABCA1) [64]. The cholesterol regulatory loop can be further modulated through the bile acid receptor, e.g., farnesoid X receptor (FXR), which is important for biosynthesis of bile acids and cholesterol catabolic pathways. SIRT1 deacetylates and activates FXR [65], and FXR can also up-regulate SIRT1 by repressing the SIRT1 targeting microRNA, mir-34a [66]. Similarly, SIRT6 appears to regulate cholesterol levels by repressing SREBP1/2, both at the level of their expression and their post-translational cleavage into the active form [67,68]. These results again point out collaborative roles of nuclear sirtuins, in this case in hepatic lipid metabolism.
Finally, SIRT1 plays an important role in maintaining circadian regulation of metabolic processes in the liver by regulating the cell-autonomous, circadian clock in that tissue [69,70]. This regulation involves the deacetylation of two central components of the clock, BMAL1 and PER2, in the liver.
Besides nuclear sirtuins, mitochondrial SIRT3 is critical in fatty acid oxidation in the mitochondria. Upon fasting or calorie restriction, SIRT3 protein level and activity are up-regulated in mitochondria [26] to promote fatty acid oxidation via deacetylating long-chain-specific acyl-coenzyme A dehydrogenase (LCAD) [71]. This sirtuin also activates the urea cycle [37] and. ketogenesis in liver [72]. Interestingly, mitochondrial SIRT4 shows opposite activity to SIRT1 and SIRT3. SIRT4 depletion prevents steatosis upon high fat diet feeding [73]. In addition, SIRT4 represses PPARα to inhibit fatty acid oxidation, meanwhile also represses malonyl CoA decarboxylase 1 (MCD1) to supports lipid synthesis [74]. Thus multiple sirtuins play many important roles in tuning liver metabolism to nutrient availability.
Metabolic regulation in the muscle and WAT
The switch from carbohydrate to lipid use for energy production is induced in skeletal muscle by exercise or fasting. When SIRT1 levels are elevated upon fasting, PGC-1α is deacetylated by this sirtuin to activate genes for fat oxidation (Figure 1) [75]. AMPK is also activated by energy depletion (resulting in higher AMP levels in cells) and drives the expression of the PGC-1α gene under these conditions [76]. The combined result is increased mitochondrial biogenesis and fatty acid oxidation in the muscle [75,77]. The effect of SIRT1 and AMPK can also be amplified through a reciprocal positive regulatory loop. AMPK can increase NAD+ levels by up-regulating NAMPT (nicotinamide phosphoribosyltransferase), one of the crucial enzymes for NAD biosynthesis [78,79]. Reciprocally, SIRT1 can deacetylate the serine/threonine kinase liver kinase B1 (LKB1) to activate AMPK [80]. Muscle thus mediates an essential anti-diabetic function by oxidizing fatty acids. The mitochondrial SIRT3 also drives oxidative metabolism in skeletal muscle as well as liver. SIRT3 deacetylates and activates pyruvate dehydrogenase (PDH), and loss of SIRT3 results in impaired oxidative metabolism [81].
WAT also regulates physiology systemically through secretion of adipokines, such as leptin and adiponectin. Adiponectin combats obesity and diabetes, enhances insulin sensitivity, and promotes proper glucose homeostasis. During exercise, the muscle adiponectin receptor is activated and induces expression of SIRT1, AMPK and PGC-1α in a Ca++ dependent manner, which in turn drive fatty acid oxidation and mitochondrial biogenesis [82]. A number of SIRT1 benefits have been reported to occur via WAT. During fasting, SIRT1 can promote fat mobilization from WAT to support lipid oxidation in liver and muscle [83]. Further, SIRT1 can induce white fat to switch into metabolically active brown fat by deacetylating two critical lysine residues on PPARγ (Figure 1) [84]. Conversely, excess energy, which can be induced by a high fat diet, causes activation of caspase I as a part of the inflammasome, which cleaves SIRT1 in WAT [29]. This reduction in adipose SIRT1 contributes to the metabolic dysfunction induced by this diet. In summary, sirtuins also play critical roles in adapting the physiology of muscle and WAT to nutrient availability.
Metabolic regulation in vascular endothelium and the heart
Another major age-associated disease is atherosclerosis, which is caused in part by chronic inflammation in the blood vessel. With aging, the lack of regeneration capacity together with senescence and cell death strongly compromise the function of blood vessels. Nitric oxide is crucial in maintaining a functioning vascular endothelium. Nitric oxide can promote angiogenesis and smooth muscle proliferation, and reduces the accumulation of atherosclerotic plaques. Moreover, the production of nitric oxide from the endothelial nitric oxide synthase (eNOS) is important for muscle relaxation, lowering of blood pressure, and general endothelium health. Interestingly, SIRT1 and eNOS form a positive regulatory loop: upon CR, eNOS induces SIRT1 expression and, SIRT1 further promotes eNOS activity through deacetylation [85].
In the heart, eNOS plays a role in responding to CR by promoting SIRT1 entry into the nucleus, which increases myocardial ischemic tolerance [86]. Additionally, SIRT1 protects against hypertrophy through PPARα activation and increased fat oxidation (Figure 1) [87]. However, high levels of cardiac-specific expression of SIRT1 can be detrimental [88]. It is noteworthy that exogenous NAD + supplementation via NAD precursors can block hypertrophy by a mechanism that appears to require SIRT3 [89]. Besides SIRT1, other nuclear sirtuins were also demonstrated to be vital in maintaining cardiac health. Both SIRT6 and SIRT7 deficient mice displayed cardiac hypertrophy phenotype due to up-regulated IGF signaling and p53 activity, respectively [90,91]. These findings indicate that sirtuins exert beneficial effects on cardiac health by coordinating nuclear and mitochondrial programs.
Metabolic regulation in the hypothalamus
The hypothalamus is an area in the brain important for coordinating systemic mammalian physiology (Figure 2). Diurnal activities, including feeding, body temperature, energy expenditure and other metabolic functions are all governed by specific neurons within the hypothalamus. SIRT1 levels in the hypothalamus change in response to diet, and appear to mediate several aspects of hypothalamic control (Figure 2) [92]. For example, the response of the somatotropic axis to CR is blocked in the SIRT1 brain specific knockouts [33]. Further, during CR, SIRT1 level increases in the dorsomedial hypothalamus (DMH) and lateral hypothalamus (LH), and SIRT1 transgenic over-expression in these neurons promotes higher physical activity and increased body temperature through up-regulation of the orexin type 2 receptor, a receptor involved in the central feedback mechanisms that regulate feeding behavior [93]. In the anorexigenic pro-opiomelanocortin (POMC) neurons, SIRT1 is essential in maintaining normal energy expenditure. Mice devoid of SIRT1 specifically in POMC neurons are susceptible to diet induced obesity [94]. SIRT1 in the SF1 neurons is also protective against obesity and diabetes [95]. In addition, SIRT1 can influence feeding by repressing the FOXO1 dependent release of the orexigenic agouti-related peptide [96] and controlling the signaling output in the ventromedial hypothalamic neurons (VMH) [97]. However, pan-neuronal deletion of SIRT1 was surprisingly associated with insulin sensitization in hypothalamic neurons and systemically [98]. In the suprachiasmatic nucleus (SCN), which mediates circadian control centrally in mammals, SIRT1 can influence the circadian amplitude via up-regulation of the core transcription factor, BMAL1 and other components of the clock machinery (Figure 2) [99]. It is important to note that SIRT1 level declines with aging in the SCN, and indeed, over-expressing SIRT1 can delay the age-related decline in central circadian function [99]. Moreover, transgenic over-expression of SIRT1 in the DMH and LH slows aging and extends life span in both males and females [13]. In conclusion, it is becoming clear that the hypothalamus may be a central regulator of aging and the weight of evidence suggests that hypothalamic SIRT1 plays an important role in this brain region in aging-related function.
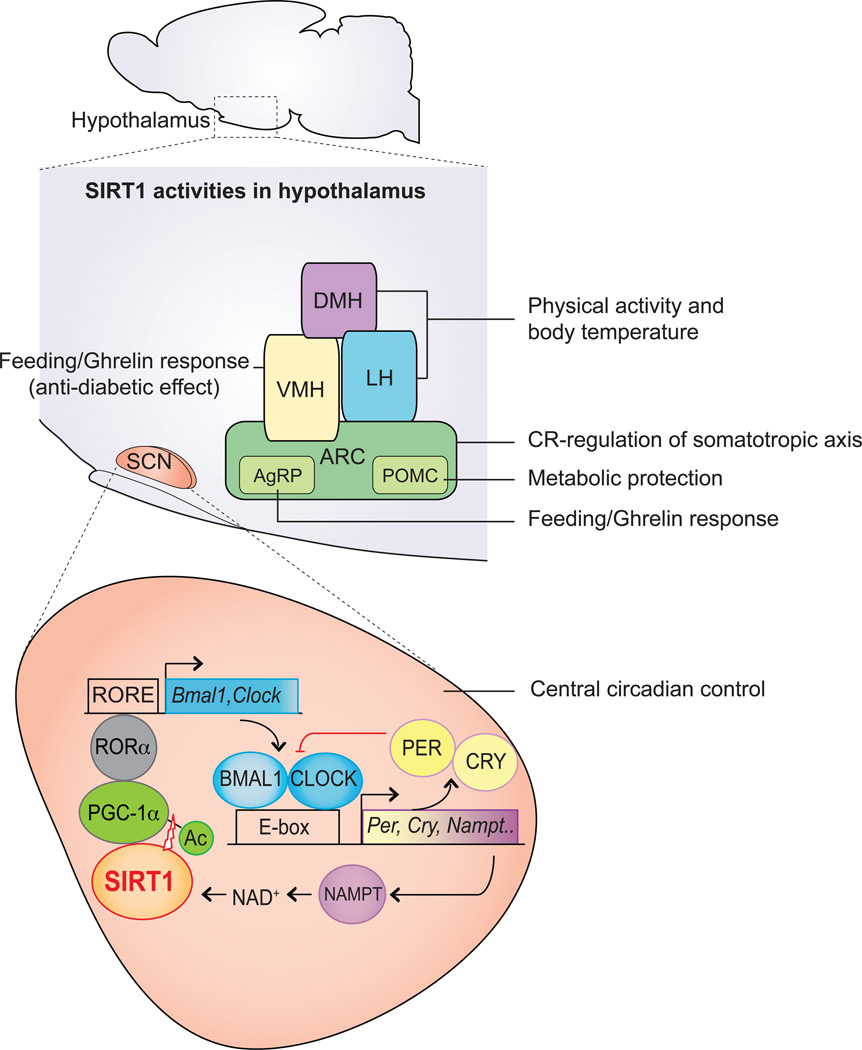
Figure 2. SIRT1 regulates metabolic functions centrally through the hypothalamus.
This figure indicates major regions in the mouse hypothalamus that are influenced by SIRT1: dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), lateral hypothalamus (LH), arcuate nucleus (ARC), agouti related peptide producing neurons(AgRP), proopiomelanocortin neurons(POMC) and suprachiasmatic nucleus (SCN). High levels of SIRT1 in DMH and LH promote physical activity and increased body temperature [93]. In POMC neurons, loss of SIRT1 causes susceptibility to diet induced obesity [94]. In addition, SIRT1 regulates feeding and ghrelin response through the AgRP neurons and VMH to counter diabetes [95–97]. In the SCN, SIRT1 assures robust central circadian control by increasing the amplitude of the indicated machinery of the clock [99]
Sirtuins and cancer
Numerous experimental evidences exist that supports a relationship between sirtuins and cancer. Significantly, several sirtuins have been reported to have tumor suppressing activities: SIRT1 overexpression is sufficient to suppress colon cancer growth in the APCmin/+ model [100]. Further, mice heterozygous for both SIRT1 and p53 developed spontaneous tumors, indicating that SIRT1 might function as a haplo-insufficient tumor suppressor [101]. However, it should be noted that numerous reports also show that SIRT1 expression can positively correlate with malignancy, in certain human cancers [14]. These findings led to the idea that SIRT1 expression may suppress tumor formation, but its expression may actually aid the growth of certain, already established tumors. SIRT2 regulates cell cycle, and mice deficient for SIRT2 develop mammary tumors in females and hepatocellular carcinoma in males, due to an altered anaphase-promoting complex/cyclosome activity [102].
Mitochondrial SIRT3 and nuclear SIRT6 repress the use of carbon from carbohydrates through glycolysis, by inhibiting the function of HIF-1α [60,103]. Similarly, mitochondrial SIRT4 regulates the use carbon from amino acids by repressing glutamate dehydrogenase [104]. Thus, SIRT3, 4, and 6 all repress the Warburg effect, in which deregulation of glycolysis and glutaminolysis occur in many human tumors and support tumor growth. Accordingly, the loss of SIRT3 [103] or SIRT6 [105] induces glycolysis, and loss of SIRT4 induces glutaminolysis [104]. Finally, loss of each of these three sirtuins has been found in many human tumors, with loss of up to 40% in breast and ovarian cancer for SIRT3, and 20% of all cancers for SIRT6 [104–106].
The emerging role of NAD+ in aging
SIRT1 activity can be regulated post-transcriptionally by several mechanisms, including phosphorylation [107,108], interactions with other proteins such as DBC1 [109,110], or changes in NAD+ levels [111]. Importantly, AMPK activates expression of the NAD biosynthetic enzyme NAMPT, linking the activity of these two crucial energy-sensing pathways [79]. Moreover, it appears likely that NAD+ levels decline with aging, which would lead to a reduction in sirtuin activity and also blunt the effects of resveratrol and other STACs. It is not clear whether this decline in NAD+ is severe enough to affect metabolic enzymes, which bind it tightly as a cofactor. The decline in NAD+ was first noticed in transgenic mice over-expressing SIRT1 in pancreatic beta cells [112]. These mice show enhanced glucose stimulated insulin secretion when they are young, but lose this phenotype when they become old (18–24 months). Importantly, administration of the NAD+ precursor, nicotinamide mono-nucleotide (NMN) can restore the metabolic phenotype in old transgenic mice. This finding suggests that a decrease in NAD+ with aging was responsible for the blunting of the phenotype in pancreatic beta cells of SIRT1 transgenic mice. More recently, supplementation with NAD precursors has been shown to restore NAD+ levels and prevent diet- or aging-induced diabetes in wild type mice [113,114]. Another recent paper suggests that a metabolite derived from the NAD+ precursor nicotinamide, 1-methyl nicotinamide, may have beneficial effects in worms [115].
An aging-induced decline in NAD+ levels has also been linked to an aging dependent activation of poly-ADP-ribose polymerase (PARP)[54]. This enzyme responds to DNA damage and ADP-ribosylates proteins at sites of DNA breaks by cleaving NAD+. This finding is complementary to an earlier study showing that PARP-1 inhibition in mice increased NAD+ content and up-regulated SIRT1 [116]. Thus, one can imagine that aging is associated with chronic DNA damage leading to NAD+ depletion and sirtuin inactivation. Indeed, a decrease in the activity of SIR-2.1 in worms or SIRT1 in mammals also leads to mitochondrial dysfunction, possibly related to increased acetylation of FOXO and PGC-1α [54]. Thus, one can trace an aging-dependent mechanism connecting the nucleus and mitochondria resulting from NAD+ deficiency.
Lastly, the circadian clock also regulates NAD+ levels via the enzyme NAMPT [117,118]. This connection was recently shown to lead to circadian cycles of SIRT3 activation, mitochondrial protein deacetylation, and oxidative metabolism [119]. In mice mutant for the circadian clock, SIRT3 function is thus defective and oxidative metabolism becomes dysfunctional. Importantly, NAD+ supplementation of the mutant mice via NMN can help correcting this metabolic defect.
Concluding remarks and future perspectives
Almost 14 years ago, yeast Sir2 and its mammalian ortholog SIRT1 were recognized as NAD+-dependent deacetylases, which immediately inspired research into the roles of sirtuins in metabolic regulation. Now it is well accepted that sirtuins play important roles in a broad spectrum of biological processes. Sirtuins function to slow aging and various disorders associated with aging, including metabolic diseases, cancer, and neurodegenerative conditions. Sirtuins respond to the energy availability provided by the diet to determine the acetylation status of histones, key transcription factors and metabolic enzymes. This coordinated response helps deliver the benefits of CR on health and physiology. Indeed, STACs have been shown to directly target SIRT1 [48,120], and present a promising strategy to ameliorate age-related diseases. Novel drugs for other sirtuins may also become possible and offer additional benefits. And finally, NAD+ supplementation in combination with STACs may offer a synergetic strategy to promote healthy aging.
Outstanding questions.
Several pathways, including sirtuins, insulin signaling, TOR, and AMP kinase are proposed to mediated CR. Do they work in a synergistic manner, or do they act independently? How extensively do they crosstalk?
Will STACs and NAD+ supplementation provide a broad, new strategy to forestall aging and diseases? Would these synergize with drugs affecting other pathways above?
How do sirtuins communicate, besides sensing NAD+ levels, for collaborative actions among cellular compartments? Are there additional feedback loops to program communication between nuclear and mitochondrial sirtuins?
Overexpression of SIRT1 in the brain extended lifespan. Are the hypothalamic nuclei, i.e. DMH and LH, the only regions important for the longevity effect? Can SIRT1 overexpression in the periphery extend lifespan?
While other sirtuins are tumor suppressors, SIRT1, seem to play both tumor suppressor and oncogenic roles. Is SIRT1 generally preventative for establishment of cancer? Under what conditions does SIRT1 foster growth of established tumors?
Highlights.
Sirtuins respond to energy level changes and execute salutary effects resemble calorie restriction.
Sirtuins mediate CR effects in various cellular compartments and are crucial metabolic regulators in multiple tissues.
Small molecules that enhance sirtuin activities, including CR mimetics and NAD+ derivatives, are promising strategies to ameliorate age-related diseases.
Acknowledgments
We apologize to researchers whose work was not cited due to space limitations. This work was supported by grants from the NIH and the Glenn Foundation for Medical Research to L. Guarente. H.-C. Chang is an Ellison Medical Foundation Fellow of the Life Science Research Foundation. LG consults for GSK, Chronos, Elysiumhealth, and Inside Tracker.
Glossary
- Adiponectin
a hormone that is mainly secreted from the adipose tissue. Adiponectin is important to maintain glucose level and fatty acid oxidation. When it binds to its G protein-coupled receptors, adiponectin can up-regulate SIRT1 and AMP kinase and promote metabolic fitness.
- Calorie restriction (CR)
a dietary regiment in mice and rats that provides ~70% of the calories of an ad libitum diet. It has been modeled with discrepant results in lower organisms.
- CREB regulated transcription coactivator 2 (CRTC2)
a transcriptional coactivator for the transcription factor cAMP response element-binding protein (CREB). CRTC2 responds to glucagon upon fasting and up-regulates gluconeogenic gene expressions.
- Endothelial nitric oxide synthase (eNOS)
an enzyme that produces nitric oxide. Nitric oxide is a key to suppress inflammation and promote blood vessel relaxation, thus high eNOS activity is protective for cardiovascular health. eNOS is deacetylated and activated by SIRT1.
- Liver X receptor (LXR)
a nuclear receptor closely related to PPARs, and FXR. LXR is a major regulator for whole-body cholesterol and lipid homeostasis. SIRT1 deacetylates LXR and activates downstream target ABCA1, an ATP-binding cassette (ABC) transporter to promote cholesterol efflux.
- Nicotinamide adenine dinucleotide (NAD+)
a coenzyme that is involved in redox reactions in living cells and thus crucial for many metabolism enzymes. It is also a co-substrate for sirtuins and PARP-1.
- Peroxisome proliferator-activated receptor alpha (PPARα)
a nuclear receptor that regulates lipid metabolism in the liver. PPARα targets include HMG-CoA synthase (a rate limiting enzyme of ketogenesis), medium-chain acyl-CoA dehydrogenase (involved in β-oxidation) and carnitine palmitoyltransferase I (CPT I, involved in the transport of long-chain fatty acyl groups into the mitochondria). SIRT1 activates PGC-1α, to further up-regulate PPARα gene targets.
- Peroxisome proliferator-activated receptor gamma (PPARγ)
a nuclear receptor that promotes fat storage in white adipose tissue. SIRT1 can repress PPARγ to trigger lipolysis, or promote a white fat “browning” program via PPARγ deacetylation.
- Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)
a transcriptional coactivator that interacts with a number of transcription factors to facilitate gluconeogenesis, fatty acid oxidation, and mitochondrial biogenesis. PGC-1 α is deacetylated and activated by SIRT1.
- Poly [ADP-ribose] polymerase 1 (PARP1)
an enzyme that plays important role in single strand DNA break repair. It can also mediate double strand DNA break repair when BRCA1/2 activities are low. PARP1 consumes NAD+ and thus its activation can reduce the activity of SIRT1.
- Sirtuin-activating compounds (STACs)
firstly identified as polyphenols, e.g. resveratrol, that activate SIRT1. Synthetic small molecules have also been identified, which impede diseases such as diabetes in rodents.
- Sterol regulatory element binding protein (SREBP)
a main transcription factor for lipid and cholesterol synthesis in the liver. SREBP repression can ameliorate non-alcoholic fatty liver disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein sir2 is an nad-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 2.Klar AJ, Fogel S, Macleod K. Mar1-a regulator of the hma and hmalpha loci in saccharomyces cerevisiae. Genetics. 1979;93(1):37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair DA, Guarente L. Extrachromosomal rdna circles--a cause of aging in yeast. Cell. 1997;91(7):1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M, McVey M, Guarente L. The sir2/3/4 complex and sir2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan M, Guarente L. Regulation of caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477(7365):E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- 7.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SJ, Defossez PA, Guarente L. Requirement of nad and sir2 for life-span extension by calorie restriction in saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 9.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418(6895):344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA. Yeast life-span extension by calorie restriction is independent of nad fluctuation. Science. 2003;302(5653):2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, et al. Absence of effects of sir2 overexpression on lifespan in c. Elegans and drosophila. Nature. 2011;477(7365):482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin sirt6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 13.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of nk2 homeobox 1 in the dmh and lh. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27(19):2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human sirt2 nad-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23(9):3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. Sirt2 is a histone deacetylase with preference for histone h4 lys 16 during mitosis. Genes Dev. 2006;20(10):1256–1261. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano L, Martinez-Redondo P, Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB, Kane-Goldsmith N, Tong Q, Rabanal RM, Fondevila D, et al. The tumor suppressor sirt2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of h4k20 methylation. Genes Dev. 2013;27(6):639–653. doi: 10.1101/gad.211342.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35(12):669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 2012;8(5):287–296. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- 20.Kaeberlein M, Andalis AA, Liszt GB, Fink GR, Guarente L. Saccharomyces cerevisiae ssd1-v confers longevity by a sir2p-independent mechanism. Genetics. 2004;166(4):1661–1672. doi: 10.1534/genetics.166.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in c. Elegans. Nature. 2007;447(7144):545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 22.Kenyon C. A pathway that links reproductive status to lifespan in caenorhabditis elegans. Ann N Y Acad Sci. 2010;1204:156–162. doi: 10.1111/j.1749-6632.2010.05640.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SC, Rabinovitch PS, Kaeberlein M. Mtor is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn BB, Alquier T, Carling D, Hardie DG. Amp-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the sirt1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 26.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, et al. Mammalian sir2 homolog sirt3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. Sirt5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137(3):560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of sirt1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16(2):180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen SB, Ølholm J, Paulsen SK, Bennetzen MF, Richelsen B. Low sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int J Obes (Lond) 2008;32(8):1250–1255. doi: 10.1038/ijo.2008.78. [DOI] [PubMed] [Google Scholar]
- 31.Costa Cdos S, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, Mottin CC, Guaragna RM. Sirt1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20(5):633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires sirt1. Science. 2005;310(5754):1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 33.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal sirt1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23(24):2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, et al. Sirt1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3(3):e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. Sirt1 but not its increased expression is essential for lifespan extension in caloric restricted mice. Aging Cell. 2013 doi: 10.1111/acel.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41(2):139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, et al. Sirt1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 41.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1(3) doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biason-Lauber A, Boni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C, Keller C, Meyer-Boni M, Meier DT, Brorsson C, Timper K, et al. Identification of a sirt1 mutation in a family with type 1 diabetes. Cell Metab. 2013;17(3):448–455. doi: 10.1016/j.cmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 45.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, et al. Small molecule activators of sirt1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam YY, Peterson CM, Ravussin E. Resveratrol vs. Calorie restriction: Data from rodents to humans. Exp Gerontol. 2013;48(10):1018–1024. doi: 10.1016/j.exger.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, et al. Evidence for a common mechanism of sirt1 regulation by allosteric activators. Science. 2013;339(6124):1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 50.Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. Elegans sir-2.1 interacts with 14-3-3 proteins to activate daf-16 and extend life span. Cell. 2006;125(6):1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Rizki G, Iwata TN, Li J, Riedel CG, Picard CL, Jan M, Murphy CT, Lee SS. The evolutionarily conserved longevity determinants hcf-1 and sir-2.1/sirt1 collaborate to regulate daf-16/foxo. PLoS Genet. 2011;7(9):e1002235. doi: 10.1371/journal.pgen.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stumpferl SW, Brand SE, Jiang JC, Korona B, Tiwari A, Dai J, Seo JG, Jazwinski SM. Natural genetic variation in yeast longevity. Genome Res. 2012;22(10):1963–1973. doi: 10.1101/gr.136549.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludewig AH, Izrayelit Y, Park D, Malik RU, Zimmermann A, Mahanti P, Fox BW, Bethke A, Doering F, Riddle DL, Schroeder FC. Pheromone sensing regulates caenorhabditis elegans lifespan and stress resistance via the deacetylase sir-2.1. Proc Natl Acad Sci U S A. 2013;110(14):5522–5527. doi: 10.1073/pnas.1214467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, et al. The nad(+)/sirtuin pathway modulates longevity through activation of mitochondrial upr and foxo signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. Dsir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2(6):1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, Olefsky J, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of pgc-1alpha and sirt1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 58.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of sirt1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hallows WC, Yu W, Denu JM. Regulation of glycolytic enzyme phosphoglycerate mutase-1 by sirt1 protein-mediated deacetylation. J Biol Chem. 2012;287(6):3850–3858. doi: 10.1074/jbc.M111.317404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, et al. The histone deacetylase sirt6 regulates glucose homeostasis via hif1alpha. Cell. 2010;140(2):280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, et al. Hepatic-specific disruption of sirt6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12(3):224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horton JD, Goldstein JL, Brown MS. Srebps: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, Israelian K, et al. Conserved role of sirt1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator srebp. Genes Dev. 2010;24(13):1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. Sirt1 deacetylates and positively regulates the nuclear receptor lxr. Mol Cell. 2007;28(1):91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. Fxr acetylation is normally dynamically regulated by p300 and sirt1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10(5):392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, Kemper JK. A pathway involving farnesoid x receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microrna-34a inhibition. J Biol Chem. 2010;285(17):12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao R, Xiong X, Depinho RA, Deng CX, Dong XC. Hepatic srebp-2 and cholesterol biosynthesis are regulated by foxo3 and sirt6. J Lipid Res. 2013;54(10):2745–2753. doi: 10.1194/jlr.M039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elhanati S, Kanfi Y, Varvak A, Roichman A, Carmel-Gross I, Barth S, Gibor G, Cohen HY. Multiple regulatory layers of srebp1/2 by sirt6. Cell Rep. 2013;4(5):905–912. doi: 10.1016/j.celrep.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. Sirt1 regulates circadian clock gene expression through per2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 70.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The nad+-dependent deacetylase sirt1 modulates clock-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, et al. Sirt3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, et al. Sirt3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl coa synthase 2 and regulates ketone body production. Cell Metab. 2010;12(6):654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. Sirt4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285(42):31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laurent G, de Boer VC, Finley LW, Sweeney M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA, Haigis MC. Sirt4 represses pparalpha activity to suppress hepatic fat oxidation. Mol Cell Biol. 2013 doi: 10.1128/MCB.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through sirt1/pgc-1alpha. EMBO J. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardie DG, Ross FA, Hawley SA. Ampk: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jager S, Handschin C, St-Pierre J, Spiegelman BM. Amp-activated protein kinase (ampk) action in skeletal muscle via direct phosphorylation of pgc-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating sirt1 through ampk-mediated regulation of nampt. Dev Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. Ampk regulates energy expenditure by modulating nad+ metabolism and sirt1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan F, Cacicedo JM, Ruderman N, Ido Y. Sirt1 modulation of the acetylation status, cytosolic localization, and activity of lkb1. Possible role in amp-activated protein kinase activation. J Biol Chem. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jing E, O'Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62(10):3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, et al. Adiponectin and adipor1 regulate pgc-1alpha and mitochondria by ca(2+) and ampk/sirt1. Nature. 2010;464(7293):1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 83.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing ppargamma. Nature. 2004;429(6993):771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by sirt1-dependent deacetylation of ppargamma. Cell. 2012;150(3):620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104(37):14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: Possible involvement of nitric oxide-dependent increase in nuclear sirt1. Am J Physiol Heart Circ Physiol. 2008;295(6):H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with pparalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2011;90(2):276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 88.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100(10):1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 89.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous nad blocks cardiac hypertrophic response via activation of the sirt3-lkb1-amp-activated kinase pathway. J Biol Chem. 2010;285(5):3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, et al. The sirtuin sirt6 blocks igf-akt signaling and development of cardiac hypertrophy by targeting c-jun. Nat Med. 2012;18(11):1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102(6):703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 92.Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain sirt1: Anatomical distribution and regulation by energy availability. J Neurosci. 2008;28(40):9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. Sirt1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30(30):10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, et al. Sirt1 deacetylase in pomc neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. Sirt1 deacetylase in sf1 neurons protects against metabolic imbalance. Cell Metab. 2011;14(3):301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasaki T, Kim HJ, Kobayashi M, Kitamura YI, Yokota-Hashimoto H, Shiuchi T, Minokoshi Y, Kitamura T. Induction of hypothalamic sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151(6):2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- 97.Velasquez DA, Martinez G, Romero A, Vazquez MJ, Boit KD, Dopeso-Reyes IG, Lopez M, Vidal A, Nogueiras R, Dieguez C. The central sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes. 2011;60(4):1177–1185. doi: 10.2337/db10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu M, Sarruf DA, Li P, Osborn O, Sanchez-Alavez M, Talukdar S, Chen A, Bandyopadhyay G, Xu J, Morinaga H, Dines K, et al. Neuronal sirt1 deficiency increases insulin sensitivity in both brain and peripheral tissues. J Biol Chem. 2013;288(15):10722–10735. doi: 10.1074/jbc.M112.443606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang HC, Guarente L. Sirt1 mediates central circadian control in the scn by a mechanism that decays with aging. Cell. 2013;153(7):1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, et al. The sirt1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3(4):e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, et al. Impaired DNA damage response, genome instability, and tumorigenesis in sirt1 mutant mice. Cancer Cell. 2008;14(4):312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, Ji J, et al. Sirt2 maintains genome integrity and suppresses tumorigenesis through regulating apc/c activity. Cancer Cell. 2011;20(4):487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, et al. Sirt3 opposes reprogramming of cancer cell metabolism through hif1alpha destabilization. Cancer Cell. 2011;19(3):416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, Xu X, et al. Sirt4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23(4):450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, et al. The histone deacetylase sirt6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151(6):1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, et al. Sirt3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo X, Williams JG, Schug TT, Li X. Dyrk1a and dyrk3 promote cell survival through phosphorylation and activation of sirt1. J Biol Chem. 2010;285(17):13223–13232. doi: 10.1074/jbc.M110.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The camp/pka pathway rapidly activates sirt1 to promote fatty acid oxidation independently of changes in nad(+) Mol Cell. 2011;44(6):851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JE, Chen J, Lou Z. Dbc1 is a negative regulator of sirt1. Nature. 2008;451(7178):583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 110.Escande C, Chini CC, Nin V, Dykhouse KM, Novak CM, Levine J, van Deursen J, Gores GJ, Chen J, Lou Z, Chini EN. Deleted in breast cancer-1 regulates sirt1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120(2):545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of nadh. Genes Dev. 2004;18(1):12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific sirt1-overexpressing (besto) mice. Aging Cell. 2008;7(1):78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key nad(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, et al. The nad(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, et al. Parp-1 inhibition increases mitochondrial metabolism through sirt1 activation. Cell Metab. 2011;13(4):461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, et al. Circadian clock feedback cycle through nampt-mediated nad+ biosynthesis. Science. 2009;324(5927):651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the nad+ salvage pathway by clock-sirt1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, et al. Circadian clock nad+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013 doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. Sirt1 activation by small molecules: Kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285(43):32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]