Abstract
Background
Cerebellar hypoplasia is common problem for preterm infants, and infants that suffer intraventricular hemorrhage (IVH). To evaluate the effects of IVH on cerebellar growth and development, we used a neonatal rabbit model of systemic glycerol to produce IVH.
Methods
New Zealand White rabbit kits were surgically delivered 2 d preterm, and treated with i.p. glycerol (3.25 to 6.5 g/kg). Controls were born at term. IVH was documented by ultrasound. Brain MRI volumes, cerebellar foliation, proliferation (Ki-67) and Purkinje cell density were done at two weeks of life. Tissue glycerol and glutathione concentrations were measured.
Results
Glycerol increased IVH, subarachnoid hemorrhages and mortality in a dose-dependent manner. Total cerebellar volumes, cerebellar foliation and cerebellar proliferation were decreased in a dose-dependent manner. Glycerol accumulated rapidly in blood, brain and liver and was associated with increased glutathione concentration. All of these results were independent of IVH status.
Conclusions
Cerebellar hypoplasia was induced after glycerol administration in a dose-dependent manner. Given rapid tissue accumulation of glycerol, dose dependent decreased brain growth and lack of IVH effect on measured outcomes we question the validity of this model as glycerol toxicity cannot be ruled out. A more physiologic model of IVH is needed.
Introduction
While mortality of preterm infants weighing less than 1000 g is decreasing, up to 50 % of preterm survivors have cognitive, learning, social, behavioral and motor deficits (1–2). Cerebellar hypoplasia has recently been documented in preterm infants with poor neurological outcomes (3–5). The cerebellum increases in size by almost 5-fold between 24 and 40 weeks post conceptual age, making it vulnerable to both developmental disruption and injury (6). Risk factors associated with cerebellar hypoplasia in preterm infants include intraventricular hemorrhage (IVH), hemosiderin deposition, periventricular leukomalacia, hypoperfusion from patent ductus arteriosus, low pH in first 5 days of life, low bicarbonate levels and chorioamnionitis (6–7). The mechanisms of injury from these insults are not known.
IVH increases the risk of poor outcome in the absence of other injuries (8). One hypothesis of cerebellar hypoplasia following IVH is that blood mixes with the cerebral spinal fluid and coats the cerebellum (9–10). Subsequent breakdown of blood may disturb communication between the proliferative external granular layer (EGL) of the cerebellum and the overlying meningeal tissues resulting in disruption of normal cerebellar lamination (9, 11). Evidence in support of this hypothesis comes from MRI studies showing siderosis of hypoplastic cerebellum in infants with history of IVH (12–13).
To examine the effects of IVH on cerebellar development, we employed the previously described rabbit model of systemic glycerol-induced IVH (14–15). In this model, the proposed mechanism of brain injury begins when systemic glycerol produces a decrease in intracranial pressure that is followed by a reperfusion that produces germinal matrix hemorrhage with extension into the lateral ventricles (16). Because the effect of IVH on cerebellar development has not been characterized, we adopted this model to confirm its usefulness for understanding cerebellar hypoplasia following IVH in preterm infants. We hypothesized that glycerol-induced IVH would decrease EGL proliferation and produce cerebellar hypoplasia.
Results
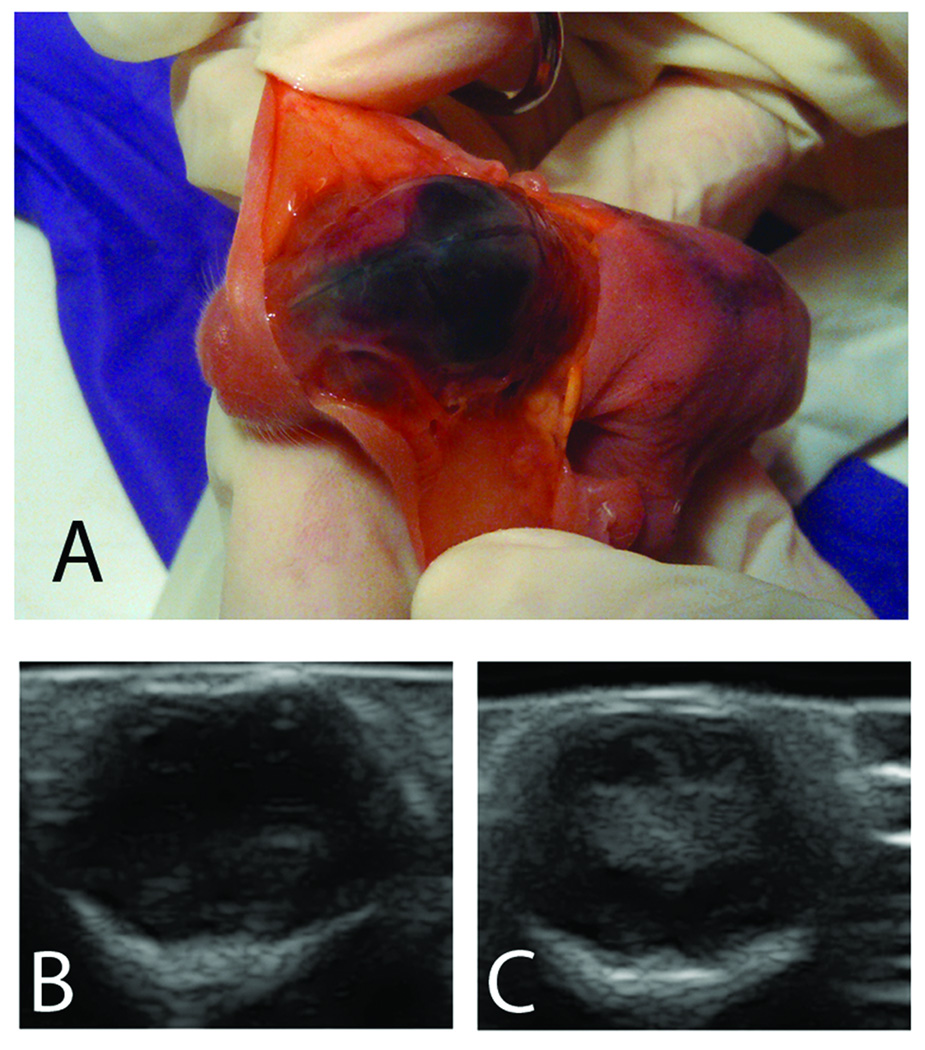
100 percent of neonatal rabbits injected with i.p. glycerol developed subarachnoid hemorrhages (SAH) within 2h of injection. We observed these SAHs through the skin overlaying the skull. In Figure 1, Panel A, we have incised and retracted the skin to show the extent of a SAH visible through the skull. Figure 1 also shows examples of ultrasound images from a glycerol-treated animal with no detectable IVH (Panel B) and a glycerol-treated animal with IVH (Panel C). Data for prevalence of SAH, IVH and mortality are listed in Table 1. Mortality was defined as death before two weeks of age. Mortality and IVH rates increased with increasing glycerol dose. Postmortem examination did not reveal pneumonia or other signs of infection, but we did observe cases of dilated intestines and discolored organs. No seizures or evidence of increased intracranial pressures were noted. SAH did not predict subsequent IVH as only a fraction of animals exhibited IVH when examined with ultrasound 24 h after glycerol injection. The size of IVHs at 24 h varied from small (ventricle only) to large (ventricle and parenchyma). In all 3 glycerol-treated animals with IVH that survived two weeks, posthemorrhagic hydrocephalus was present at necropsy.
Figure 1.
In Panel A, a euthanized neonatal rabbit kit is held with skin retracted to expose the skull and show subarachnoid hemorrhage which is always present after glycerol injection. At 24 hours after glycerol injection, coronal ultrasound scans were collected to document the presence of intraventricular hemorrhage (IVH). Panel B shows an ultrasound image from an animal with no indication of IVH, and Panel C shows an ultrasound image from an animal with large IVH present 24 h after glycerol injection.
Table 1.
Prevalence of subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH) and mortality for neonatal rabbits treated with different doses of systemic glycerol.
| Glycerol dose (g/kg) | 0 | 3.25 | 4.87 | 6.5 |
|---|---|---|---|---|
| Number | 34 | 18 | 4 | 28 |
| SAH at 2 h % (N) | 0% | 100% (18)† | 100% (4)† | 100% (28)† |
| IVH at 24 h % (N) | 0% | 22% (4) | 50% (2) | 46% (13) |
| Mortality % (N) | 18% (6) | 61% (11)* | 25% (1) | 82% (23)† |
Kruskal-Wallis test with Dunn's multiple comparison test
P ≤ 0.01 or 0.001 respectively compared to control animals (0 g/kg dose).
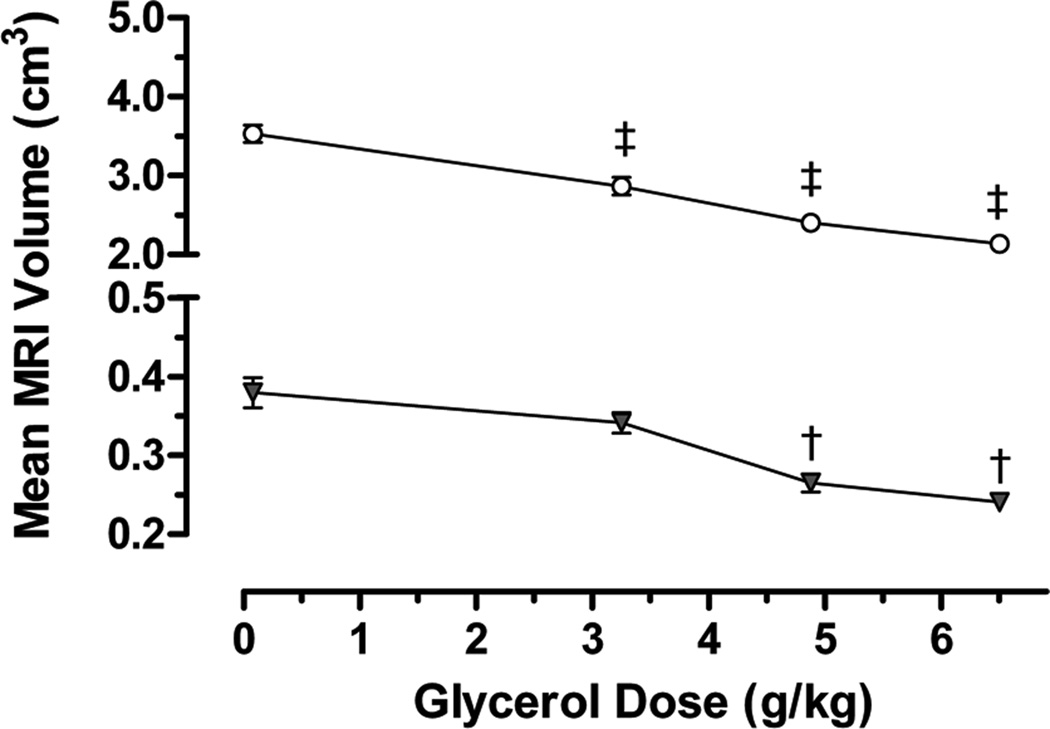
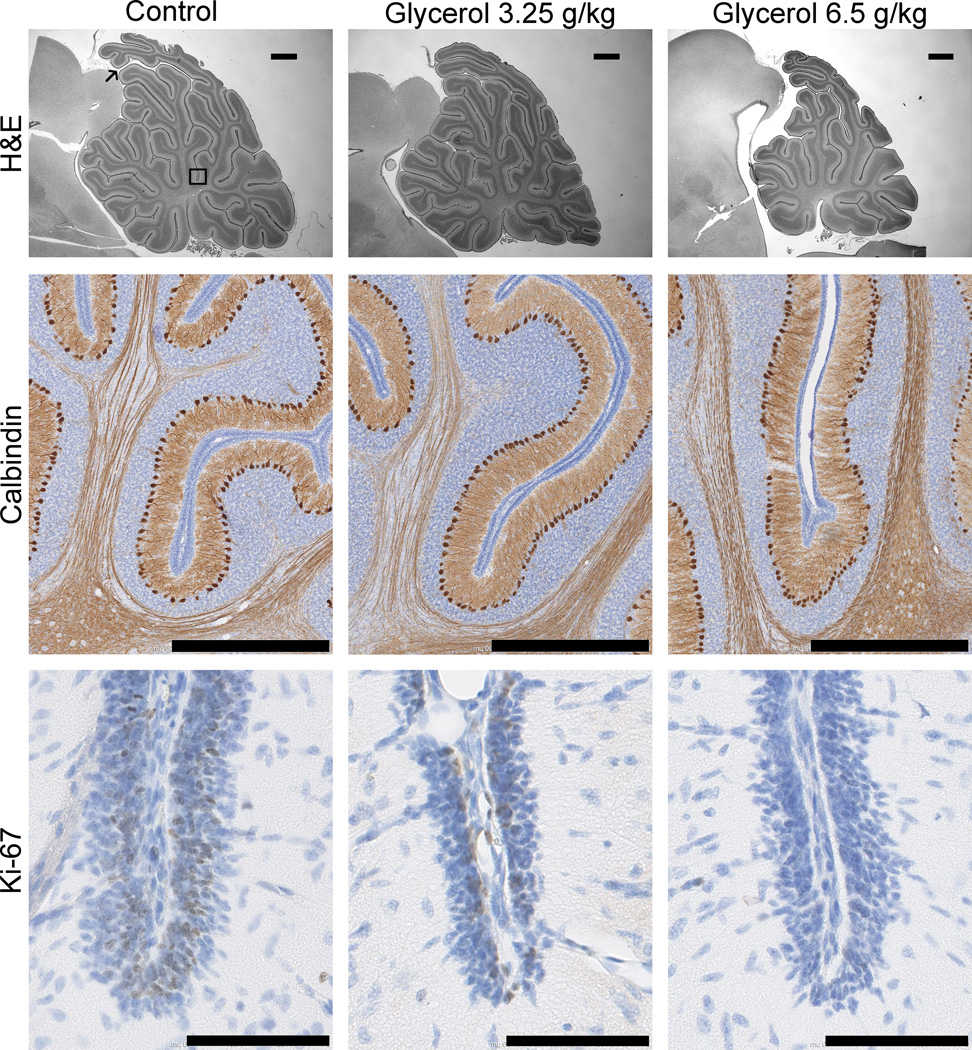
Brain volume was evaluated by ex vivo MRI (N = 18). Interrater reliability of brain segmentation was determined by Dice similarity coefficients. The Dice similarity coefficients between the three tracers ranged from 0.931 to 0.998. Glycerol reduced total brain and cerebellar volumes in a dose-dependent manner (Figure 2) independent of presence of IVH. To evaluate cerebellar growth, we examined the histology shown in Figure 3 which includes lamination of the cerebellum, proliferation of the EGL and Purkinje cell density in a subset of animals (N = 11). Table 2 quantifies cerebellar indices such as foliation (lobes I–III perimeter), primary fissure length (distance to first lobule of lobe V), internal granular, molecular and EGL thicknesses, as well as Ki-67-immunopositive proliferation and Purkinje cell density. In the EGL, glycerol treatment decreased cerebellar foliation complexity, size, EGL thickness and reduced cell proliferation (indicated by decreased Ki-67 immunolabeling). Note that the increase in Purkinje cell density in the 6.5 g/kg glycerol group is likely a secondary to decreased cerebellar growth. All of these effects of glycerol were dose related but unrelated to IVH status.
Figure 2.
Line plots showing dose-dependent decreases in rabbit MRI volumes (means ± SEM) for total brain (unfilled circle) and cerebellum (filled triangle). Premature rabbit kits were delivered by C-section and treated with glycerol at the doses indicated then, two weeks after treatment, kits were killed and brains were perfusion fixed and removed for subsequent MRI measurements (N = 3–5 per dose). ANOVA differences compared to 0-dose control are indicated as ‡ = P ≤ 0.001 and † = P ≤ 0.05 (Dunnett’s post-hoc tests). MRI volumes did not correlate with the presence of IVH.
Figure 3.
Photomicrographs showing the dose-dependent effects of systemic glycerol treatment on cerebellar development. Brain sections from control (left column) and glycerol-treated (right columns) rabbit kits were stained for H&E (top row, scale bar 1 mm), calbindin-D28K (middle row, scale bar 800 µm) and Ki-67 (bottom row, scale bar 90 µm). Measurements were made along the primary fissure of the vermis, as denoted in the upper left panel by the thin black arrow marking the start, and the black box marking the end of the region of interest. Cerebellar growth was compromised because the density of calbindin-immunopositive Purkinje cells was increased, and the proportion of Ki-67-immunopositive proliferating external granular layer cells (thick black arrows) was decreased in glycerol-treated kits. Morphological measurements using H&E-stained images indicated that the foliation is less complex, and the thickness of the external granular layer is decreased at the base of the primary fissure in glycerol-treated kits.
Table 2.
Histological indices describing cerebellar development in neonatal rabbits treated with/without systemic glycerol.
| Dose of glycerol (g/kg) | |||
|---|---|---|---|
| 0 | 3.25 | 6.5 | |
| Number | 4 | 4 | 3 |
| Lobes I–III perimeter (mm) | 29.1 ± 2.7 | 24.2 ± 1.6 | 19.8 ± 1.1* |
| Primary fissure length (µm) | 1345 ± 71 | 1031 ± 46† | 810 ± 35‡ |
| Internal granular layer thickness (µm) | 69.5 ± 2.0 | 64 ± 5.6 | 74.1 ± 2.6 |
| Molecular layer thickness (µm) | 171 ± 4 | 143 ± 9 | 166 ± 8 |
| External granular layer thickness (µm) | 35.5 ± 4.4 | 18.9 ± 2.8‡ | 26.2 ± 3.1* |
| Purkinje cell density (% total area) | 0.287 ± 0.003 | 0.285 ± 0.013 | 0.319 ± 0.008* |
| Ki-67 immunolabeling (% total area) | 0.627 ± 0.122 | 0.452 ± 0.121 | 0.125 ± 0.060* |
Data are presented as means ± SEM. Kruskal-Wallis test with Dunn's multiple comparison test were performed on the Purkinje density and Ki-67 positivity. ANOVA with Dunnett's post hoc comparisons to control (0) was used for the rest:
P ≤ 0.05, 0.01, or 0.001 respectively.
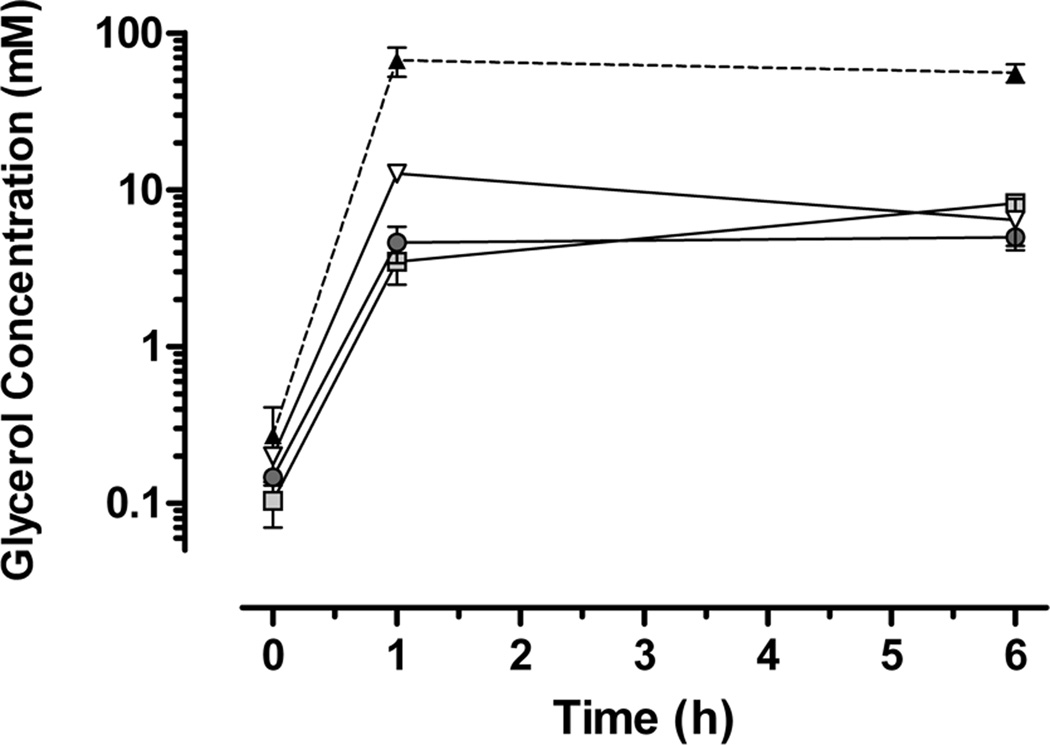
Because glycerol treatment affected cerebellar development and brain growth without producing IVH, we investigated post hoc the possibility that glycerol may accumulate in tissues to produce direct toxic effects. Figure 4 illustrates that one hour after systemic injection (6.5 g/kg), glycerol concentrations increased more than 240 fold in plasma, more than 30 fold in hippocampus and cerebellum, and more than 60 fold in liver, and glycerol remained elevated in tissues for at least 6 hours. Lastly, the total glutathione concentration in cortical brain tissue was elevated by 13% at one hour, and by 27% at six hours post glycerol injection.
Figure 4.
Line plots showing that glycerol is elevated more than 240 fold in blood plasma, and more than 30 to 60 fold in tissues at one and six hours after glycerol administration (data ± SEM). Glycerol concentrations are plotted on a logarithmic scale for plasma (filled triangle), hippocampus (light gray square), cerebellum (dark gray circle) and liver (unfilled triangle). Newborn rabbits, untreated (time 0) or glycerol treated (6.5 g/kg i.p.), were killed at one or six hours post treatment and samples of brain and liver tissue were collected and snap frozen, then homogenized and assayed for glycerol content by ELISA (N = 3 per time point). Subarachnoid hemorrhages were evident shortly after injection, but no intraventricular hemorrhages were detected after brain removal.
Discussion
The key findings of our study are that: 1) there is a glycerol dose-dependent increase in IVH 2) there is a glycerol dose-dependent decrease in total brain and cerebellar volumes which were independent of IVH, 3) post-hemorrhagic hydrocephalus is caused by glycerol injection though the mechanism has not been elucidated 4) cerebellar proliferation and foliation were decreased and 5) glycerol rapidly accumulates in tissues including brain. We also found this model to be difficult to work with due to high mortality rates, and inconsistent rates and severity of IVH.
Our findings may be contrasted with those from prior reports that used systemic glycerol in neonatal rabbits. For example, our neonatal rabbit mortality was double the 30% rate reported previously (14) and, in many cases, the cause of death was uncertain as thriving kits were simply found dead with no evidence of aspiration or IVH at necropsy. One potential cause of death would be acute renal failure because systemic injection of 50% glycerol is known to produce a nephrotoxicity similar to rhabdomyolysis (17–18). Although prior reports found that glycerol produced similar rates of prevalence for SAH (75%) and IVH (70–80%) (14–15), we found that these two effects did not correspond. When present, we observed considerable variation in the size of IVH identified by ultrasound. Thus it appears that IVH produced by systemic glycerol is infrequent, inconsistent and variable, and together these findings confirm the observation that the frailty of newborn rabbits and inconsistency of IVH diminish the value of this model (19). One limitation of our study is that we did not measure intracranial pressures. Prior studies found that glycerol lowered intracranial pressure in a dose-dependent manner (16, 20).
Cerebral growth was decreased after glycerol injection independent of IVH status. MRI measures of forebrain and cerebellar volumes, and measurements of cerebellar morphometry identified dose-dependent effects of glycerol on brain growth. A number of proliferative processes may be affected by glycerol treatment (21–22). For example, glycerol disrupts maturation of oligodendrocyte precursors and this may reduce overall brain growth (14). Although we did not measure cerebral perfusion, it is unlikely that the global impairment of brain growth was due to glycerol-induced hypoperfusion (16). Given the universal presence of SAH, it is possible that SAH, but not IVH, is a contributing factor in the retardation of brain growth. In hamsters, SAH interferes with meninges and related proliferation signals and reduces proliferation in the EGL (11). Similarly, glycerol acts directly to disrupt membrane and cytoskeletal proteins that thereby interfere with cellular interactions that mediate normal brain growth (23).
Toxins that disturb cell proliferation during development are particularly detrimental. Cerebellar development is a prolonged process during which progenitor cells of the EGL proliferate, migrate, and differentiate continuously for up to 2 postnatal months in rabbit, or 7 postnatal months in humans (24–26). Thus there is a large window of vulnerability during which this developmental sequence may be disrupted. In this study, a single injection of glycerol decreased cerebellar MRI volume and decreased foliation of the primary fissure. Both cell proliferation (Ki-67) and laminar width were decreased in the EGL. The decreased proliferation seen 14 days post-injection is likely the principal mechanism underlying reduced cerebellar growth and may also indicate that glycerol-mediated toxicity is enduring. The coincident increase in Purkinje cell density we observed is likely secondary to decreased foliation and reduced cerebellar area with relative preservation of Purkinje neurons. Alternatively, increased Purkinje density could occur as an artifact of histological processing if brain edema were present in vivo. Recently, it was determined that cerebellar neuronal granule cells actually derive from astroglia that generate granule cell precursors (27). Given the proximity of the EGL to the meninges and the prolonged period of postnatal cerebellar development, it is possible that cerebellum is particularly susceptible to toxic effects of glycerol and/or SAH. Our data certainly support this hypothesis.
It is apparent that glycerol may produce brain toxicity directly in the absence of IVH. Systemic glycerol (6.5 g/kg) rapidly accumulated in blood, liver and brain tissues. We acknowledge that this effect was not detected when the dose of glycerol was 1.15 g/kg (28). Notably, the progressive accumulation of total glutathione content in brain is an indication that a single injection of systemic glycerol alters cerebral biochemistry independent of IVH. This is problematic because it appears that IVH cannot be reliably produced unless a high dose of glycerol is administered and, at high doses, direct toxic effects of glycerol cannot be disqualified as an alternative explanation for any resulting brain injury.
For infants born prematurely, neurological complications arise when the capillaries of the the immature germinal matrix lose their integrity and produce IVH and subsequent ventricular dilatation (29). Glycerol is highly lipophilic and can be used in vitro to lyse cells (30) and, when used as a cryoprotectant, glycerol is known to disrupt the integrity of the membrane and the actin cytoskeleton of spermatozoa (31–32). Since our study indicates that glycerol readily penetrates brain tissues, then perhaps glycerol produces cerebral hemorrhage by directly lysing susceptible endothelial membranes including those in the germinal matrix.
In conclusion, the validity of the use of systemic glycerol to model neonatal IVH is questionable for several reasons. First, the mortality due to this treatment is high, possibly due to known renal toxicity. Second, although this treatment reliably produces SAH, IVH is inconsistent and variable. Third, the design cannot disqualify the alternative interpretation that brain injury resulting from systemic injection of glycerol is mediated by direct toxic action. A better model of spontaneous IVH still needs to be found given that organotypical brain slices exposed to whole blood exhibited pathophysiological changes in the absence of glycerol (33).
Methods
Animals
All experimental protocols were approved by the Animal Care and Use Committees at the University of Washington in accordance with U.S. NIH guidelines. Timed-pregnant New Zealand White does (Western Oregon Rabbit Co, Philomath, OR) were purchased (N = 13). On embryologic day 29, the doe was sedated with isoflurane for a non-survival Caesarean section (N = 9). The preterm kits (N = 63) were extracted from the uterus, dried and placed in an incubator pre-warmed to a temperature of 35°C. Control kits (N = 34) were born vaginally at term.
Brain injury
Preterm kits were injected with 3.25, 4.87, or 6.5 g/kg of glycerol i.p. (Sigma Chemical Co., St. Louis, MO) given as a 50% solution (by volume) at 2h of age to induce IVH (15).
Feeding
Kits were hand fed twice daily with a commercial rabbit milk substitute formulated to support neonatal rabbit growth (Fox Valley Animal Nutrition, Inc., Sun City, AZ), supplemented with 60 gm/L lyophilized colostrum (Naturade, Orange, CA). Commercial nipples (Chris's Squirrels and More, LLC, Somers, CT) were used. For the first 2 days, kits were fed 100mL/kg/day, and thereafter feeding volumes increased gradually to 280 mL/kg/day.
Ultrasound
Cranial ultrasound was performed at 24h of age to document presence of IVH using a SonaScape S8 with 10-5 MHz L743 linear array probe (SonoScape Co., Ldt., Shenzhen, China).
MRI acquisition
At 12 days post-term, surviving kits were euthanized. Rabbit brains were extracted after perfusion fixation and placed in Multihance (Bracco Diagnostics Inc., Princeton, NJ) 1:100 in PBS for 28 days. Brains were then placed in Fomblin (Solvay Solexis, West Deptford, NJ) for ex vivo imaging (N=17) on Bruker Avance III 14 Tesla (600) Ultrashield high resolution 89mm vertical bore magnet with ParaVision version 5.1 software. The TurboRARE T2 sequence with a scan time of 7.5 to 8h was conducted with the following settings: Field of view (FOV) 2.82 cm, slice thickness 0.11 mm, interslice distance 0.11 mm, slices 256, axial slice orientation matrix P1 256, repetition time 27807 ms, echoes 1, TE effective 1 33 ms, TE effective 2 33 ms, Averages 30, FOV read 2.82 cm, FOV P1 2.82 cm, matrix read 256, matrix P1 256, anti-alias read 1, anti-alias P1 1, resolution read 0.011 cm/pixel, resolution read P1 0.011 cm/pixel, iso distance −1.6 mm, pack extent 28.16 mm, scheme interlaced, readout L-R, repetition time 27807 ms, echoes 1, TE effective 1 33 ms, TE effective 2 33 ms, averages 30, bandwidth 1 39682.5, flipback on, fat suppression on, rare partitions 8.
Volumetric analyses
RView software (34) was used for volume rendering of T2 weighted images. Structures were isolated using voxel-based intensity thresholds with manual limits. Tracings were performed by C.T. and two students (H.S. and O.J.) blinded to treatment group. Specific boundaries for total brain and cerebellum were defined. Total brain volume included the cerebral hemispheres, diencephalon, brainstem, and cerebellum. Ventricular cerebrospinal fluid was excluded, the brainstem was truncated posterior to the area postrema and the optic bulbs were excluded anterior to the fissura rhinalis (35). Final images were rendered in 3D and inspected for accuracy before structural volumes were computed.
Morphometric measurements of cerebellum
After MRI, the brains were imbedded in paraffin and sagittal sections 10 µm thick were made. Using ImageJ software (NIH), the perimeter of lobes I–III was outlined on H&E-stained images of sagittal slices through the vermis. The distance from the primary fissure sulcus to the first branch point of lobe V was measured along the internal granule layer. The thickness of the internal/external granule and molecular layers was measured at the primary fissure sulcus.
Immunohistochemistry
All staining procedures were run on the Leica Bond Automated Immunostainer with Leica bond kits (Leica Microsystems Inc., Buffalo Grove, IL). Slides were baked for 30 min at 60°C and deparaffinized. Antigen retrieval comprised of citrate or EDTA for 20m at 100°C. Slides were blocked in normal donkey serum (10% in TBS) for 10 min at room temperature. Primary antibodies mouse anti-human Ki-67 clone MIB-1 (1:1000, Dako North America, Inc., CA) or mouse anti-chicken Calbindin (1:10,000, Swant, Marly, Switzerland) were applied at room temperature for 30 min. Leica goat anti mouse HRP polymer was applied for 30 min at room temperature and blocked with peroxide for 10 min. Leica bond mixed refine (DAB) detection applied twice for 10 min at room temperature. Slides were counterstained with hematoxylin.
Slides were scanned using a Nanozoomer Digital Pathology slide scanner (Olympus America; Center Valley, Pennsylvania). The digital images were then imported into Visiopharm software (Hoersholm, Denmark) for analysis. Using the Visiomorph Digital Pathology module, regions of interest were applied around relevant areas using a tissue detect protocol and manual cleanup. The software was then programmed to label positively stained areas versus normal tissue areas, using project-specific configurations created for each staining set in a blinded manner. Images were processed in batch using these configurations to generate the desired output calculations.
Glycerol accumulation in the brain
Tissue glycerol concentrations were measured in a subset of kits killed at 0, 1 or 6h after injection of 6.5 mg/kg of glycerol. The kits were euthanized and plasma was obtained by heart puncture prior to exsanguination by heparinized saline. The brain was maintained on ice while the hippocampus and cerebellum were excised. The hippocampus, cerebellum and liver samples were then frozen in liquid nitrogen. The tissues were homogenized in a 50/50 mixture of chloroform and methanol. Tissue supernatant and plasma were extracted and processed for glycerol concentration using a glycerol assay (BioAssay Systems, Hayward, CA) per assay instructions.
Antioxidant status
The total glutathione content was measured using a commercial competitive assay (OxiSelect Total Glutathione Assay Kit, Cell Biolabs, Inc) on cortex tissue samples at 0, 1 and 6h after glycerol injection. Samples were homogenized per assay instructions. After centrifugation, the supernatant was diluted 1:100 and the assay performed. Results were normalized to the 0h time point and expressed as a percentage change.
Statistics
Results are given as mean ± SEM. Mortality was defined as death prior to 12 days post-term. Kruskal-Wallis testing with Dunn’s multiple comparison test and ANOVA with Dunnett's post hoc comparisons were performed using SPSS. All glycerol animals were randomized in a blinded fashion. All data were analyzed in a blinded fashion.
Acknowledgements
We would like to thank Sarah Rammeli, Olivia Janson and Henry Smelser for animal care work and data gathering and MRI tracing (O.J. and H.S.).
Statement of financial support:
National Institutes for Health 5K12HD043376-10, USA
University of Washington Neonatology: Neonatal Bioresearch Fund, Seattle, WA
University of Washington Neonatology Divisional funds, Seattle, WA.
Footnotes
Disclosure statement:
The authors do not have any financial or conflicts of interest to report.
References
- 1.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126:342–351. doi: 10.1542/peds.2010-0710. [DOI] [PubMed] [Google Scholar]
- 2.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 3.Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and Nature of Cerebellar Injury in the Extremely Premature Survivor with Cerebral Palsy. J Child Neurol. 2005;20:60–64. doi: 10.1177/08830738050200011001. [DOI] [PubMed] [Google Scholar]
- 4.Limperopoulos C, Benson CB, Bassan H, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116:717–724. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 5.Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252:190–199. doi: 10.1148/radiol.2521081525. [DOI] [PubMed] [Google Scholar]
- 6.Limperopoulos C, Soul JS, Gauvreau K, et al. Late Gestation Cerebellar Growth Is Rapid and Impeded by Premature Birth. Pediatrics. 2005;115:688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 7.Messerschmidt A, Prayer D, Brugger PC, et al. Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol. 2008;12:455–460. doi: 10.1016/j.ejpn.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97:329–338. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam EWY, Miller SP, Studholme C, et al. Differential Effects of Intraventricular Hemorrhage and White Matter Injury on Preterm Cerebellar Growth. J Pediatr. 2011;158:366–371. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inage YW, Itoh M, Wada K, Hoshika A, Takashima S. Glutamate transporters in neonatal cerebellar subarachnoid hemorrhage. Pediatr Neurol. 2000;23:42–48. doi: 10.1016/s0887-8994(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 11.von Knebel Doeberitz C, Sievers J, Sadler M, Pehlemann FW, Berry M, Halliwell P. Destruction of meningeal cells over the newborn hamster cerebellum with 6-hydroxydopamine prevents foliation and lamination in the rostral cerebellum. Neuroscience. 1986;17:409–426. doi: 10.1016/0306-4522(86)90256-3. [DOI] [PubMed] [Google Scholar]
- 12.Castro Conde JR, Martínez ED, Rodríguez RC, Rodríguez De Hoyos AL. CNS Siderosis and Dandy-Walker Variant After Neonatal Alloimmune Thrombocytopenia. Pediatr Neurol. 2005;32:346–349. doi: 10.1016/j.pediatrneurol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Messerschmidt A, Brugger PC, Boltshauser E, et al. Disruption of Cerebellar Development: Potential Complication of Extreme Prematurity. Am J Neuroradiol. 2005;26:1659–1667. [PMC free article] [PubMed] [Google Scholar]
- 14.Chua CO, Chahboune H, Braun A, et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40:3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiadis P, Xu H, Chua C, et al. Characterization of Acute Brain Injuries and Neurobehavioral Profiles in a Rabbit Model of Germinal Matrix Hemorrhage. Stroke. 2008;39:3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- 16.Conner ES, Lorenzo AV, Welch K, Dorval B. The role of intracranial hypotension in neonatal intraventricular hemorrhage. J Neurosurg. 1983;58:204–209. doi: 10.3171/jns.1983.58.2.0204. [DOI] [PubMed] [Google Scholar]
- 17.Korrapati MC, Shaner BE, Schnellmann RG. Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J Pharmacol Exp Ther. 2012;341:126–136. doi: 10.1124/jpet.111.190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger E, Rech VC, Feksa LR, Wannmacher CM. Intraperitoneal glycerol induces oxidative stress in rat kidney. Clin Exp Pharmacol Physiol. 2008;35:928–933. doi: 10.1111/j.1440-1681.2008.04942.x. [DOI] [PubMed] [Google Scholar]
- 19.Coulter DM, LaPine T, Gooch WM., 3rd Intraventricular hemorrhage in the premature rabbit pup. Limitations of this animal model. J Neurosurg. 1984;60:1243–1245. doi: 10.3171/jns.1984.60.6.1243. [DOI] [PubMed] [Google Scholar]
- 20.Cantore G, Guidetti B, Virno M. Oral Glycerol for the Reduction of Intracranial Pressure. J Neurosurg. 1964;21:278–283. doi: 10.3171/jns.1964.21.4.0278. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe JP, Dinsdale CJ. Inhibition of cell proliferation by glycerol. Life Sci. 1991;48:1511–1517. doi: 10.1016/0024-3205(91)90275-g. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama N, Mizuguchi T, Aoki T, et al. Glycerol suppresses proliferation of rat hepatocytes and human HepG2 cells. J Surg Res. 2002;103:236–242. doi: 10.1006/jsre.2002.6367. [DOI] [PubMed] [Google Scholar]
- 23.Dinsdale CJ, Mirza FM, Wiebe JP. Glycerol alters cytoskeleton and cell adhesion while inhibiting cell proliferation. Cell Biol Int Rep. 1992;16:591–602. doi: 10.1016/s0309-1651(06)80001-9. [DOI] [PubMed] [Google Scholar]
- 24.Lossi L, Ghidella S, Marroni P, Merighi A. The neurochemical maturation of the rabbit cerebellum. J Anat. 1995;187(Pt 3):709–722. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KR., Jr The Cerebellar Cortex of the Rabbit. An Electron Microscopic Study. J Comp Neurol. 1963;121:459–483. doi: 10.1002/cne.901210312. [DOI] [PubMed] [Google Scholar]
- 26.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 27.Silbereis J, Heintz T, Taylor MM, et al. Astroglial cells in the external granular layer are precursors of cerebellar granule neurons in neonates. Mol Cell Neurosci. 2010;44:362–373. doi: 10.1016/j.mcn.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse JM, Coxon RV. The entry of glycerol into brain tissue. J Neurol Sci. 1970;10:305–311. doi: 10.1016/0022-510x(70)90157-7. [DOI] [PubMed] [Google Scholar]
- 29.Whitelaw A. Periventricular hemorrhage: a problem still today. Early Hum Dev. 2012;88:965–969. doi: 10.1016/j.earlhumdev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Bautista ML, Altaf W, Lall R, Wapnir RA. Cord blood red cell osmotic fragility: a comparison between preterm and full-term newborn infants. Early Hum Dev. 2003;72:37–46. doi: 10.1016/s0378-3782(03)00009-4. [DOI] [PubMed] [Google Scholar]
- 31.Macias Garcia B, Ortega Ferrusola C, Aparicio IM, et al. Toxicity of glycerol for the stallion spermatozoa: effects on membrane integrity and cytoskeleton, lipid peroxidation and mitochondrial membrane potential. Theriogenology. 2012;77:1280–1289. doi: 10.1016/j.theriogenology.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 32.Check ML, Check JH, Long R. Detrimental effects of cryopreservation on the structural and functional integrity of the sperm membrane. Arch Androl. 1991;27:155–160. doi: 10.3109/01485019108987666. [DOI] [PubMed] [Google Scholar]
- 33.Vinukonda G, Hu F, Upreti C, et al. Novel organotypic in vitro slice culture model for intraventricular hemorrhage of premature infants. J Neurosci Res. 2012;90:2173–2182. doi: 10.1002/jnr.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob FD, Habas PA, Kim K, et al. Fetal hippocampal development: analysis by magnetic resonance imaging volumetry. Pediatr Res. 2011;69:425–429. doi: 10.1203/PDR.0b013e318211dd7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shek JW, Wen GY, Wisniewski HM. Atlas of the rabbit brain and spinal cord. 1st edn. Basel, NY: Karger; 1986. pp. 1–139. [Google Scholar]