Abstract
Nonsense suppression therapy is a therapeutic approach aimed at treating genetic diseases caused by in-frame premature termination codons (PTCs; also commonly known as nonsense mutations). This approach utilizes compounds that suppress translation termination at PTCs, which allows translation to continue and partial levels of deficient protein function to be restored. We hypothesize that suppression therapy can attenuate the lysosomal storage disease mucopolysaccharidosis type I-Hurler (MPS I-H), the severe form of α-L-iduronidase deficiency. α-L-iduronidase participates in glycosaminoglycan (GAG) catabolism and its insufficiency causes progressive GAG accumulation and onset of the MPS I-H phenotype, which consists of multiple somatic and neurological defects. 60-80% of MPS I-H patients carry a nonsense mutation in the IDUA gene. We previously showed that 2-week treatment with the designer aminoglycoside NB84 restored enough α-L-iduronidase function via PTC suppression to reduce tissue GAG accumulation in the Iduatm1Kmke MPS I-H mouse model, which carries a PTC homologous to the human IDUA-W402X nonsense mutation. Here we report that long-term NB84 administration maintains α-L-iduronidase activity and GAG reduction in Iduatm1Kmke mice throughout a 28-week treatment period. Examination of more complex MPS I-H phenotypes in Iduatm1Kmke mice following 28-week NB84 treatment revealed significant moderation of the disease in multiple tissues, including the brain, heart and bone, that are resistant to current MPS I-H therapies. This study represents the first demonstration that long-term nonsense suppression therapy can moderate progression of a genetic disease.
Keywords: nonsense suppression, readthrough, premature termination codons, nonsense mutations, mucopolysaccharidosis I-Hurler, lysosomal storage disease
INTRODUCTION
Nonsense suppression therapy is a therapeutic approach aimed at treating diseases caused by nonsense mutations. Suppression of translation termination at nonsense mutations occurs when an aminoacyl-tRNA base pairs with a premature termination codon (PTC) and the amino acid carried by the aminoacyl-tRNA is incorporated into the nascent polypeptide at the site of the PTC. This allows translation elongation to continue in the original ribosomal reading frame and results in the production of full-length polypeptide. A number of drugs have been found to stimulate nonsense suppression and restore partial function of deficient proteins associated with a variety of genetic diseases [1].
In this study, we examined whether nonsense suppression therapy can moderate the progression of the lysosomal storage disease mucopolysaccharidosis I-Hurler (MPS I-H). MPS I-H is caused by a severe deficiency of the enzyme α-L-iduronidase, which is encoded by the IDUA gene. Loss of α-L-iduronidase function results in an inability to degrade the glycosaminoglycans (GAGs) dermatan sulfate and heparan sulfate. This leads to progressive accumulation of these GAGs and onset of the MPS I-H phenotype that consists of multiple somatic and neurological defects [2]. MPS I-H patients are born without symptoms; however, presentation of the disease manifests during infancy with frequent respiratory and/or ear infections, hernia development, restricted joint movement, altered facial features and skeletal deformities. Developmental delay usually becomes apparent by 12 to 24 months of age followed by a progressive cognitive decline and onset of multiple neurological abnormalities. Progressive joint and skeletal disease leads to significant disability. Furthermore, MPS I-H patients develop progressive valvular and cardiac disease. Without therapeutic intervention, most MPS I-H patients succumb to the disease in their first decade due to cardiorespiratory failure and neurologic disease.
MPS I-H is an excellent candidate disease for nonsense suppression therapy. First, genotype/phenotype correlation studies indicate that MPS I-H has a low threshold for correction since as little as 0.3-1% of normal α-L-iduronidase function significantly alleviates the MPS I-H phenotype [3, 4]. Second, nonsense mutations are prevalent among MPS I-H patients, where it is estimated that 60-80% of MPS I-H patients carry a nonsense mutation [5].
We previously found that the aminoglycoside gentamicin restored enough α-L-iduronidase via PTC suppression to normalize GAG accumulation and lysosomal morphology in cultured primary MPS I-H patient fibroblasts [6]. However, current clinical aminoglycosides are prohibited from long-term use for suppression therapy due to their toxicity [7, 8]. Recently, a novel rational drug design strategy was devised to generate new aminoglycosides that are more effective in mediating PTC suppression and less toxic than conventional aminoglycosides [9]. One of the aminoglycoside derivatives created by this drug design strategy, NB84, restored enough α-L-iduronidase activity to reduce GAG accumulation by 15-65% in Iduatm1Kmke mouse tissues after two weeks of administration [10, 11]. Fibroblasts from patients with attenuated forms of MPS I (Scheie; Hurler-Scheie) have been reported to retain 20–70% fewer GAGs than cells from MPS I-H patients [4]. Based on these values, the level of GAG reduction observed in Iduatm1Kmke mice treated with NB84 may be sufficient to attenuate the severe MPS I-H phenotype in multiple tissues.
In the current study, we examined whether long-term NB84 treatment can reduce or prevent progression of morphological and functional defects associated with MPS I-H in Iduatm1Kmke mice. We found that the restoration of α-L-iduronidase function and the corresponding decrease in GAG accumulation associated with NB84 treatment could be maintained for 28 weeks in Iduatm1Kmke mice. Furthermore, Iduatm1Kmke mice treated with NB84 for 28 weeks exhibited moderation of the MPS I-H phenotype in multiple tissues including the CNS, heart and bone, which are resistant to current MPS I therapies. This suggests that nonsense suppression therapy, alone or in combination with other MPS I therapies, may be effective in treating MPS I-H in patients that carry nonsense mutations.
MATERIALS AND METHODS
Animal Treatment
The Baasov lab (Technion-Israel Institute of Technology) synthesized NB84 [9]. NB84 was dissolved in sterilized PBS and administered at a dose of 30 mg/kg via daily subcutaneous injections to 10-week-old mice daily for 2 weeks and to 3-week-old mice three times weekly (M/W/F) for either 9 or 28 weeks. Age-matched untreated mutant and wild-type control cohorts were included for each treatment group. Clinical chemistry analysis of blood and urine samples was conducted by Antech Diagnostics. All animal work was conducted according to relevant national and international guidelines. All animal protocols used in this study were reviewed and approved by the UAB IACUC (APN#120109344).
Biochemical Assays
Assays to determine α-L-iduronidase, β-hexosaminidase, and β-glucuronidase activities and sulfated GAG levels were performed as previously described [10]. Enzyme specific activities were calculated as picomoles of released substrate per mg of total protein per hour. Enzyme activities remained linear over the incubation times. Tissue sulfated GAG levels were calculated as μg of GAGs per mg of defatted, dried tissue using a dye-binding assay (Blyscan), where chondroitin 6-sulfate was used as a reference.
Elevated Zero Maze
The apparatus consists of a circular maze, 70 centimeters in diameter, raised 40 cm above the platform, and divided into four equal sections. Two opposite sections have 15 cm high sides of non-transparent material, and the other two have only a 0.5 cm high wall. The animal is put in the arena at the juncture of a high wall and a low wall, and observed for 4 minutes, with a camera driven tracker system, (Ethovision, Noldus, The Netherlands). The system records the position of the animal in the arena at 5 frames/second. The test was performed once per animal to prevent habituation. After each testing day and in between animals the apparatus is wiped down with chlorhexidine and 70% ethanol and allowed to air-dry.
Western blotting
Fifty micrograms of total protein lysate was subjected to SDS-PAGE and transferred to Immoboline-P membrane (Millipore). Blots were incubated with antibodies to GFAP (EMD Millipore), IBA-1 (Wako Chemicals), LAMP-1 (DSHB), or tubulin (Abcam) according to manufacturer instructions. 125I-Protein A (GE)-bound proteins were detected and quantified using a Storm PhosphoImager and ImageQuant software (GE).
Immunohistochemistry
The right brain hemisphere was collected in 4% paraformaldehyde (PFA) at sacrifice after 5 minutes of perfusion with PBS. After 18-24 hours in PFA the tissue was rinsed twice for 1 hour each in 70% ethanol and then maintained in 70% ethanol. Tissue was subsequently processed, paraffin-embedded, and sectioned on a microtome at 9μm thickness. Sections were then stained using fluorescent antibodies to GFAP (EMD Millipore), IBA-1 (Wako Chemicals), LAMP-1 (DSHB), then amplified using HRP-conjugated secondary antibodies (VectorLabs ImmPress anti-mouse and Invitrogen Superpic anti-rabbit) and TSA amplification (Perkin Elmer Cy3+ and Fit-C+); bis-benzamide DNA. Sections were imaged on a Nikon A1 Confocal Scanning Laser microscope and images generated using version 4.13 Nikon Elements Software at UAB’s High Resolution Imaging Facility.
Ultrasound Echocardiography
Doppler transthoracic echocardiography was performed by an experienced sonographer using a Visual Sonics Vevo 2100 Imaging System and 30MHz MS400 MicroScan transducer (FUJIFILM VisualSonics, Inc., Toronto). Mice were anesthetized initially with 5% isoflurane and then maintained with 1% to 2% isoflurane. Core temperatures and heart rate were continuously monitored and maintained at ~37 degrees and ~500 beats per minute, respectively. The chests of the mice were treated with a chemical hair remover to reduce ultrasound attenuation. B-mode guided M-mode measurements of left ventricular wall thickness of the septum and posterior walls were made at the papillary muscle level. An apical four-chamber B-mode color Doppler view was used to position pulse wave Doppler measurement of flow at the tips of the mitral valve leaflets. M-mode was also used to measure ascending aorta lumen diameter and wall thickness at the midpoint between the aortic sinus and the innominate artery. Flow was measured at the aortic root using M-mode Doppler and evaluation of flow turbulence in the aorta was evaluated with color Doppler. When possible, measurements were made on three consecutive beats.
Micro-computed tomography
Excised mouse femurs were scanned using the Scanco uCT40 desktop cone-beam micro-CT scanner. Bones were placed vertically into 12mm diameter holders and scanned at 12 μm resolution, 70kVp, 114μA with an integration time of 200ms. Cortical bone was scanned at the midshaft and consisted for 25 slices, 12μm in thickness. Fewer scans are needed for cortical bone due to its uniformity. Scans were reconstructed and the region of interest was drawn at the outside of the cortical bone such that all of the cortical bone and marrow were included. No trabecular bone was included. The threshold for cortical bone was set at 282 and the 3-D reconstruction was performed on all 25 slices. Data was obtained on bone volume (BV), total volume (TV), BV/TV, and cortical thickness. The trabecular bone was scanned from the growth plate consisted of 200 slices, with each slice 12μM in thickness. Scans were reconstructed into 2-D slices and 100 slices were analyzed using the μCT Evaluation Program (v5.0A, Sanco Medical). The region of interest was drawn on each of the 100 slices just inside the cortical bone to include only trabecular bone and marrow. The threshold for trabecular bone was set at 212 to distinguish it from the marrow. The 3-D reconstruction was performed using all the outlined slices. No cortical bone was included in the analysis. Data was obtained on bone volume (BV), total volume (TV), BV/TV, trabecular number (number of intersections between bone and non-bone components), trabecular thickness, trabecular separation (space between trabeculae), connectivity density (density of trabecular connections), and structural model index (trabecular bone structure characterization: plate-like (SMI=0); rod-like (SMI=3); sphere-like (SMI=4).
Osteoclast TRAP staining
Mouse femurs were excised and fixed in 70% ethanol, decalcified, embedded in paraffin, and longitudinally sectioned into 5μm sections. Osteoclasts within femur sections were stained for tartrate-resistant acid phosphatase (TRAP) as previously described [12]. Areas of bone and TRAP staining were measured in red and green channel images, respectively, using ImagePro Plus v6.2 software (Media Cybermetics, Rockville, MD). TRAP-stained material was quantitated per area (mm2) of bone examined.
Statistics
All statistics were calculated with two-tailed unpaired t-tests using InStat or GraphPad Prism software.
RESULTS
Long-term NB84 treatment attenuates MPS I-H CNS phenotypes
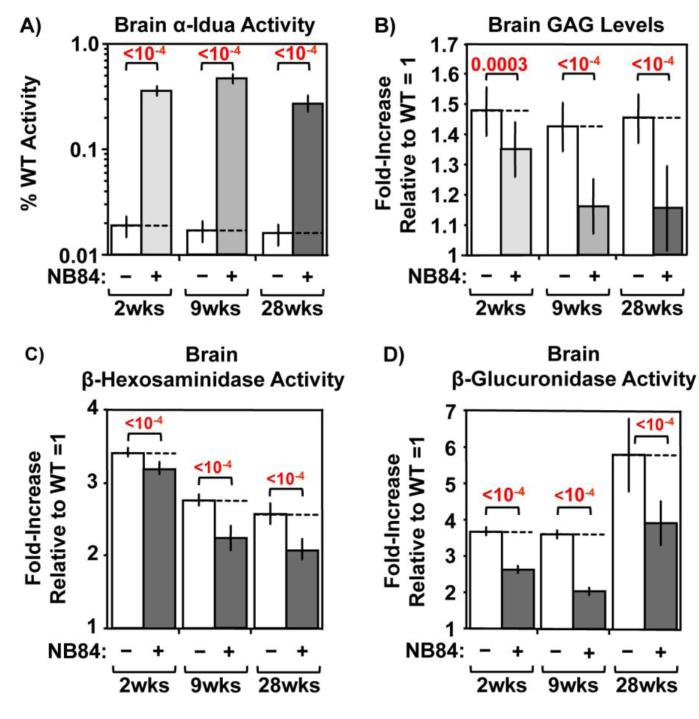
MPS I-H patients develop intellectual disability; however, patients with attenuated forms of MPS I (Scheie; Hurler-Scheie) have normal intellect. Genotype-phenotype correlations among MPS I patients indicate that only 0.3-1% of normal α-L-iduronidase activity can preclude neurodegeneration and normalize lifespan [3, 4]. Systemic enzyme replacement therapy (ERT) cannot prevent neurodegeneration in MPS I-H patients because the recombinant enzyme cannot enter the central nervous system (CNS) [13]. In contrast, aminoglycosides enter the CNS at ~10-20% of their serum concentration [14, 15]. We therefore evaluated whether long-term NB84 administration alleviated CNS phenotypes in Iduatm1Kmke mice. Compared to wild-type controls, less than 0.02% of residual α-L-iduronidase activity is present in whole brain lysates from untreated Iduatm1Kmke mice (Figure 1A). Similar to the level of α-L-iduronidase function restored after 2-week or 9-week NB84 treatment [11], we found ~0.4% of normal α-L-iduronidase function was restored in Iduatm1Kmke mouse whole brain lysates after 28 weeks of NB84 treatment. We next determined whether this enzyme level reduced GAG accumulation (Figure 1B). Remarkably, we found that GAG levels were reduced ~60% in whole brain lysates from Iduatm1Kmke mice treated with NB84 for 9 or 28 weeks compared to untreated controls, a ~2-fold greater GAG reduction than observed after 2-week NB84 treatment [10, 11]. This enhancement in GAG reduction may be the result of longer administration and/or the earlier initiation of NB84 treatment for the long-term studies (begun in 3-week-old mice for the long-term studies as opposed to 10-week-old mice for the 2-week study).
Figure 1. NB84 treatment alleviates MPS I-H biochemical endpoints in mouse brain.
A) α-L-iduronidase activity, B) sulfated GAG levels, C) β-hexosaminidase activity, and D) β-glucuronidase activity were quantified in whole brain lysates. Data shown are values for untreated (−) and treated (+) MPS I-H mice that have been normalized to WT levels (=1). Dashed lines indicate the average values obtained from untreated mice. n=5 mice per group. p values are indicated above brackets.
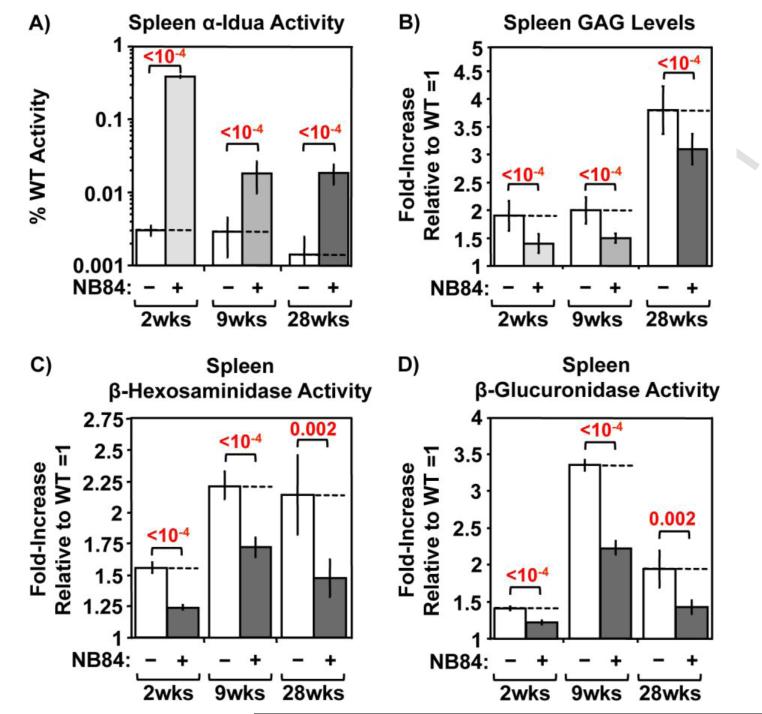
In addition, excessive GAG accumulation has been shown to be associated with an increase in lysosomal proliferation for many MPS diseases [6, 10, 11, 16]. Consistent with this observation, increased lysosomal proliferation was also found in Iduatm1Kmke mice as indicated by an elevation in the activity of multiple lysosomal enzymes, including β-hexosaminidase (Figure 1C) and β-glucuronidase (Figure 1D). However, a significant reduction in the activity of these lysosomal enzymes was observed in brain lysates of NB84-treated Iduatm1Kmke mice, indicating that the GAG reduction mediated by NB84 treatment is able to reduce secondary lysosomal proliferation. Together, these results indicate that NB84 enters the CNS, restores enough α-L-iduronidase activity to reduce GAG storage, and can maintain the improvements observed in MPS I-H biochemical endpoints for >6 months. Similar results were also observed for MPS I-H biochemical endpoints in other tissues, including the spleen (Figure 2) and heart (Figure 4A).
Figure 2. NB84 treatment alleviates MPS I-H biochemical endpoints in mouse spleen.
A) α-L-iduronidase activity, B) sulfated GAG levels, C) β-hexosaminidase activity, and D) β-glucuronidase were quantified in spleen lysates. Data shown are values for untreated (−) and treated (+) MPS I-H mice that have been normalized to WT levels (=1). Dashed lines indicate the average values obtained from untreated mice. n=5 mice per group. p values are indicated above brackets.
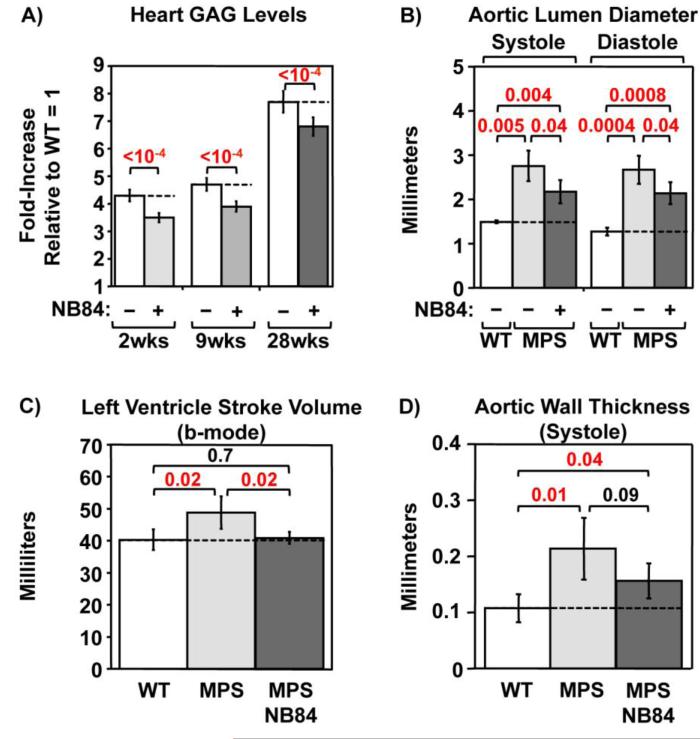
Figure 4. NB84 treatment moderates progression of heart defects in MPS I-H mice.
A) Sulfated GAGs were quantified in whole heart lysates. Data shown are values for untreated (−) and treated (+) MPS I-H mice that have been normalized to WT levels (=1). Dashed lines indicate average values obtained from untreated mice. n=5 mice per group. Doppler echocardiography measurements of: B) aortic lumen diameter at systole (contraction) and diastole (relaxation), C) ventricle stroke volume, and D) aortic wall thickness in WT mice, untreated MPS I-H mice, and MPS I-H mice treated with NB84 for 28 weeks. Dashed lines indicate average values obtained from WT mice. n= 3-4 WT mice; n= 4-5 untreated or treated MPS mice. p values are indicated above brackets.
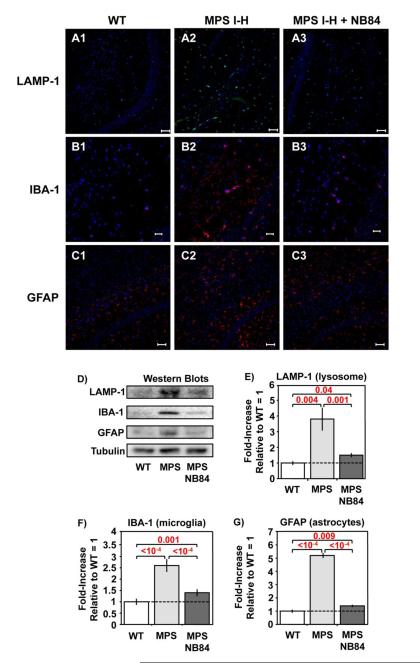
Evidence of neuropathology was previously reported in MPS I and MPS III knockout mice [17, 18]. We evaluated neuropathology in Iduatm1Kmke mice by examining the following CNS markers using immunohistochemistry (IHC) and western blotting: LAMP-1, a lysosomal marker increased during lysosomal proliferation; IBA-1, an activated macrophage/microglia marker indicative of neuroinflammation; and GFAP, an activated astrocyte marker upregulated in response to CNS distress/disturbance. IHC staining of brain sections indicated elevations in the abundance of all three markers in untreated 31-week-old MPS I-H mice compared to age-matched WT controls. This upregulation was partially relieved in MPS I-H mice treated with NB84 treatment for 28 weeks (Figure 3A-C). Quantification of the three CNS markers in whole brain lysates using western blotting indicated their levels were increased 2.5 to 5 fold in untreated MPS I-H mice relative to wild-type controls (Figure 3D-G). However, the abundance of all three CNS markers decreased ~80% in Iduatm1Kmke mice treated with NB84 for 28 weeks relative to untreated controls, suggesting that NB84 treatment significantly moderated the progression of neuropathology associated with MPS I-H.
Figure 3. NB84 treatment moderates neuropathology in MPS I-H mice.
A-C) Representative images of immunohistochemistry staining for CNS markers: A1-3) LAMP-1 and B1-3) IBA-1 in the hippocampus, and C1-3) GFAP in the cerebral cortex of 31-week-old untreated WT and MPS I-H male controls and MPS I-H mice treated with NB84 for 28 weeks. All samples were counter-stained with bis-benzamide nuclear stain (blue). LAMP-1 and GFAP are shown at 20X magnification with a scale bar = 50μm; IBA-1 is shown at 40X magnification with a scale bar = 20μm. D) Representative western blots of whole brain lysates from WT mice, untreated MPS I-H mice, or MPS I-H mice treated with NB84 for 28 weeks. Western quantitation data for: E) LAMP-1; F) IBA-1; G) GFAP. The data shown is normalized to tubulin levels (=1). Dashed lines indicate average values obtained from WT mice. n=3 WT mice; n=6 untreated MPS mice; n=5 treated MPS mice. p values are indicated above brackets.
Long-term NB84 treatment attenuates MPS I-H cardiac phenotypes
GAG accumulation in the heart tissues of MPS I patients leads to cardiomyopathy, thickened cardiac valves, progressive narrowing and occlusion of epicardial coronary arteries, and eventual congestive heart failure [19]. ERT and hematopoietic stem cell transplantation (HSCT) alleviate much of the MPS I cardiac phenotype, but cannot prevent valvular disease [13, 20]. Whole heart lysates from untreated Iduatm1Kmke mice revealed a 4 to 8 fold increase in GAG accumulation (Figure 4A, which leads to significant thickening of the aorta, aortic valve, and atrioventricular valves [21]. Doppler echocardiography assessments of 31-week-old Iduatm1kmke mice recapitulated previous reports evaluating MPS I animal models and MPS I patients [19, 22, 23]. Abnormalities were observed only in male Iduatm1Kmke mice and included dilation of the ascending aorta with concomitant turbulent blood flow. Morphological and functional measures revealed increases in aortic lumen diameter (Figure 4B), left ventricular stroke volume (Figure 4C), and aortic wall thickness (Figure 4D). Treatment with NB84 for 2, 9 or 28 weeks reduced GAG storage in whole heart lysates by ~15-20% relative to untreated controls (Figure 4A). In male Iduatm1Kmke mice treated with NB84 for 28 weeks, the modest GAG reduction contributed to a 38-46% reduction in aortic lumen diameter, a 93% decrease in left ventricular stroke volume, and a 64% decrease in aortic wall thickness relative to male Iduatm1Kmke controls (Figure 4B-D). Together, these results demonstrate that suppression therapy reduced MPS I-H progression in the hearts of male Iduatm1Kmke mice.
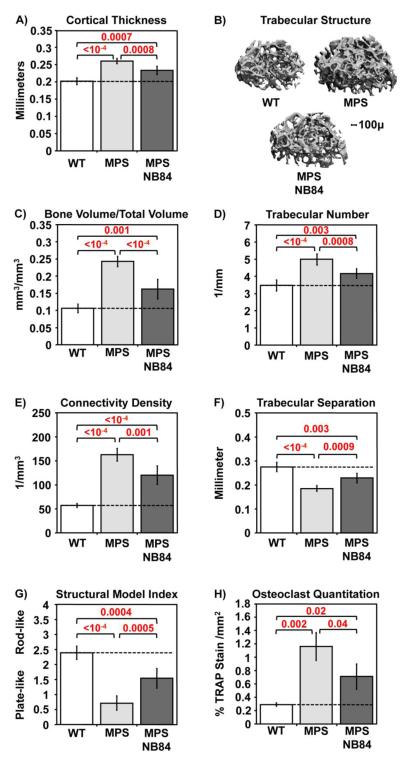
Long-term NB84 treatment attenuates MPS I-H bone phenotypes
Musculoskeletal defects are a major cause of morbidity among the various MPS diseases. MPS I patients develop severe abnormalities in bone structure as well as articular cartilage and joints [24] that cannot be prevented by ERT or HSCT [13, 20]. Skeletal abnormalities that develop in MPS I-H patients include short stature, spinal misalignment, long bone deformation, macrocephaly, hip dysplasia, chest deformity, and facial dysmorphism. Iduatm1Kmke mice recapitulate many of the clinical skeletal defects observed in MPS I-H patients, including facial dysmorphism, thickening of their digits, and thoracolumbar kyphosis as well as thickening of the zygotic arch, broadening of the ribs, and shortening and thickening of the long bones [21]. Micro-computed tomography (micro-CT) analysis of the three-dimensional architecture of femurs from 31-week-old male Iduatm1Kmke mice (relative to wild-type controls) indicated abnormalities in both cortical and trabecular bone (Figure 5A-G) that are consistent with defects reported in other MPS animal models [25] and in MPS patients [24]. In agreement with findings in MPS I-H knockout mice [12, 26], a 4-fold elevation in osteoclast number was observed in the femoral growth plates of Iduatm1Kmke mice relative to wild-type controls (Figure 5H). Consistent with the accumulation of aminoglycosides in bone-forming tissues at 10-20% of their serum concentration [27], we found significant improvements in multiple bone parameters in Iduatm1Kmke mice treated with NB84 for 28 weeks compared to untreated controls. Micro-CT analyses indicate a ~40-60% improvement in cortical and trabecular bone parameters (Figure 5A-G). Osteoclast abundance was also reduced by ~40% (Figure 5H). These data indicate that NB84 treatment attenuates progression of MPS I-H bone abnormalities.
Figure 5. NB84 treatment moderates progress of bone defects in MPS I-H mice.
Micro-CT measurements of femurs from WT mice, untreated MPS I-H mice, or MPS I-H mice treated with NB84 for 28 weeks: A) cortical bone thickness, B) representative trabecular bone images, C) trabecular bone volume/total volume, D) trabecular number; E) trabecular connectivity density, F) trabecular separation; G) trabecular structural model index. H) Osteoclast quantification in femoral growth plates using TRAP staining. p values are indicated above brackets. Dashed lines indicate average values obtained from WT mice. For panels A and C-G, n= 5-6 WT mice and n=6 untreated and treated MPS mice. For panel H, n= 3 mice per group. p values are indicated above brackets. See the Methods for detailed descriptions of the micro-CT parameters.
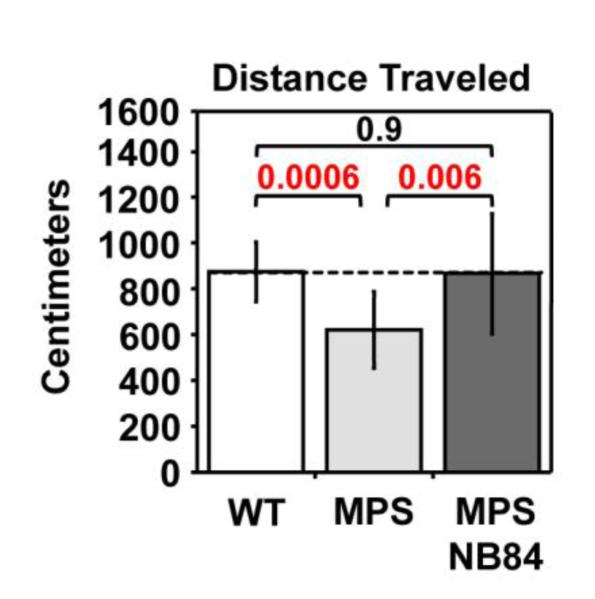
Long-term NB84 treatment improves MPS I-H mouse activity
Behavioral defects have been reported in MPS I and MPS III knockout mice [28-30]. Using the elevated zero maze assay, we found a significant decrease in activity (distance traveled) in 31-week-old Iduatm1Kmke mice relative to age-matched wild-type controls (Figure 6). However, in Iduatm1Kmke mice treated with NB84 for 28 weeks, distance traveled was restored to wild-type levels, indicating a general improvement in phenotype. This activity assay might be considered analogous to the “six minute walk test”, an endpoint used in clinical trials to assess functional improvement [31].
Figure 6. NB84 treatment improves activity levels in MPS I-H mice.
The distance traveled was measured in 31-week-old WT and MPS I-H mice using the elevated zero maze assay. The dashed line indicates the average value obtained from WT mice. n=9 WT mice; n=17 untreated MPS mice; n=10 NB84-treated MPS mice. p values are indicated above brackets. Additional assay details can be found in the Methods.
Long-term NB84 treatment did not induce toxicity in mice
Traditional aminoglycosides are associated with nephrotoxicity and ototoxicity [7, 8]. To determine whether long-term NB84 treatment induced toxicity in mice, sera from mice treated with NB84 for 28 weeks and untreated controls were subjected to clinical chemistry analyses that included multiple endpoints for liver and kidney function (Antech Diagnostics). Of the 18 parameters evaluated, only three (alkaline phosphatase, bilirubin, creatinine) changed significantly in NB84-treated mice relative to untreated controls (Table 1). However, comparison of these endpoint values relative to averages reported for C57BL/6 mice (the genetic background of Iduatm1Kmke mice) in the Jackson Laboratories Mouse Phenome Database [32] indicated that these differences are within normal parameters. No anomalies attributable to NB84 treatment were found during histological analysis of liver, kidney, lung, brain, or heart. Also, no significant changes in weight or food consumption were observed. These results indicate that long-term NB84 administration did not induce significant toxicity in mice.
Table 1.
Comprehensive Serum Clinical Chemistry Analysis to Evaluate NB84 Toxicity
| Endpoint | Untreated controls (n=12) |
NB84-treated mice (n=6) |
p values (treated versus untreated) |
Average values for C57BL/6 mice |
|---|---|---|---|---|
| (ALT) alanine aminotransferase (U/L) |
59 ± 36 | 44 ± 21 | 0.36 | 43 |
| albumin (g/dL) | 2.7 ± 0.2 | 2.6 ± 0.2 | 0.32 | 3 |
| alkaline phosphatase (U/L) |
74 ± 17 | 103 ± 18 | 0.004 | 96 |
| (AST) aspartate aminotransferase (U/L) |
136 ± 99 | 112 ± 39 | 0.58 | 157 |
| bilirubin, total (mg/dL) | 0.27 ± 0.05 | 0.20 ± 0.05 | 0.01 | 0.45 |
| calcium (mg/dL) | 8.6 ± 0.2 | 8.5 ± 0.2 | 0.33 | 9.4 |
| chloride (mEq/L) | 112 ± 2 | 110 ± 3 | 0.11 | 115 |
| cholesterol (mg/dL) | 95 ± 21 | 80 ± 25 | 0.2 | 84 |
| (CPK) creatine phosphokinase (U/L) |
744 ± 640 | 702 ± 429 | 0.89 | 558 |
| creatinine (mg/dL) | 0.16 ± 0.05 | 0.10 ± 0.05 | 0.03 | 0.23 |
| globulin (g/dL) | 2.5 ± 0.2 | 2.6 ± 0.2 | 0.33 | |
| glucose (mg/dL) | 210 ± 41 | 195 ± 24 | 0.42 | 184 |
| phosphorous (mg/dL) | 6.8 ± 0.7 | 7.1 +0.3 | 0.34 | 7.2 |
| potassium (mEq/L) | 5.7 ± 0.6 | 6.1 ± 0.3 | 0.15 | 6.3 |
| protein, total (g/dL) | 5.2 ± 0.2 | 5.2 ± 0.3 | 0.99 | 5.8 |
| sodium (mEq/L) | 150 ± 6 | 149 ± 4 | 0.72 | 150 |
| sodium/potassium ratio |
27 ± 3 | 30 ± 11 | 0.38 | NA |
| urea nitrogen (mg/dL) | 28 ± 6 | 31+3 | 0.27 | 28 |
DISCUSSION
Current treatments for MPS I include enzyme replacement therapy (ERT) and hematopoietic stem cell transplantation (HSCT). ERT, the intravenous administration of recombinant α-L-iduronidase protein (aldurazyme™), treats many somatic defects associated with MPS I, but cannot prevent development of bone, heart valve and ocular abnormalities [2]. In addition, aldurazyme is unable to penetrate the CNS, and therefore does not prevent onset of neurological abnormalities associated with MPS I-H. HSCT treats many features of MPS I, including neurological defects, but only if treatment is initiated at an early age (≤ 2yrs) [2]. However, heart valve, bone and ocular abnormalities persist. The limitations, cost and potential risks associated with current MPS I treatments indicate that more effective treatment options are needed. In this report, we investigate the potential of nonsense suppression therapy to treat MPS I-H. We found that long-term administration of the nonsense suppression drug NB84 restored enough α-L-iduronidase function to reduce GAG accumulation and ameliorate MPS I-H progression in multiple tissues of the Iduatm1Kmke MPS I-H mouse, including the CNS, heart and bone. Consistent with previous studies that have shown early intervention increases the therapeutic benefit of ERT or HSCT for treating MPS I-H [33-37], we also found that more robust improvements in MPS I-H biochemical endpoints were observed in the CNS when suppression therapy was administered to younger mice. This may be due to the ability of drugs to penetrate the blood-brain barrier at a greater level in younger mice [38] and/or the prevention of GAG accumulation during early ages. Overall, these results suggest suppression therapy may be a viable treatment for MPS I-H, either alone or in combination with other MPS I treatments.
The concept of PTC suppression as a treatment for diseases caused by nonsense mutations was introduced almost two decades ago [39]. Subsequently, ~100 studies have shown that nonsense suppression can partially restore expression of deficient proteins associated with ~40 different diseases [1]. However, no study to date has demonstrated that suppression therapy can prevent or alleviate progression of a disease phenotype. The meager progress in the development of suppression therapy may be attributed to the lack of: 1) safe, effective drugs; 2) relevant animal models; and 3) adequate disease endpoints to effectively assess therapeutic efficacy. The current study addresses each of these limitations and makes significant contributions to the advancement of nonsense suppression therapy. First, MPS I-H represents an excellent candidate disease for suppression therapy due to its low threshold for correction and the high frequency of nonsense mutations in MPS I-H patients. Furthermore, the Iduatm1Kmke MPS I-H mouse is a superb animal model to test suppression therapy because it closely recapitulates the human MPS I-H phenotype and has abundant quantifiable disease endpoints. In addition, NB84 represents a major step forward in the development of safer, more effective nonsense suppression drugs. The toxicity associated with aminoglycosides is unrelated to their ability to suppress PTCs associated with cytoplasmic ribosomes, but rather is due to off-target binding of aminoglycosides to membrane lipids [40] and their affinity for mitochondrial ribosomes [41]. NB84 was designed using a rational drug design to better target eukaryotic cytoplasmic ribosomes in order to increase the effectiveness of PTC suppression and to decrease toxicity compared to conventional aminoglycosides [9]. In support of this design rationale, not only was NB84 found to suppress PTCs more effectively than traditional aminoglycosides [9-11], it was also shown to be significantly less toxic (~5 fold) in mammalian cells [9]. In addition, we found no evidence of toxicity in mice treated with NB84 for 28 weeks.
The data generated from this study using the MPS I-H model represents a critical proof of concept that demonstrates the feasibility of using suppression therapy to treat genetic diseases attributable to nonsense mutations. Based on information from the National Institutes of Health (NIH) Office of Rare Disease Research (http://rarediseases.info.nih.gov) and the National Organization for Rare Disorders (http://www.rarediseases.org), over 7,000 distinct genetic diseases have been identified that affect roughly 300 million people worldwide. Approximately 11% of the gene lesions that caused inherited human disease are nonsense mutations [42]. This indicates that millions of individuals could potentially benefit from nonsense suppression therapy. We anticipate that the advancement of personalized medicine and the continued development of safe, effective drugs will allow suppression therapy to progress into a treatment for many genetic diseases.
Research Highlights.
Long-term nonsense suppression therapy using the drug NB84 moderated MPS I-H disease progression in the Iduatm1Kmke mouse model.
MPS I-H moderation was observed in the CNS, heart and bone.
Activity levels improved in NB84-treated MPS I-H mice.
This is the first study to show long-term nonsense suppression therapy can moderate progression of a disease.
ACKNOWLEDGEMENTS
We thank the following UAB core facilities for assistance in phenotypic analyses: Terry Lewis, UAB Neuroscience Molecular Detection Core, P30 NS47466; Thomas Van Groen, UAB Neuroscience Behavioral Assessment Core, P30 NS47466; Shawn Williams, UAB High Resolution Imaging Facility, P30 CA013148; Maria Johnson, UAB Small Animal Phenotyping Core, P60 DK079626; Wayne Bradley, UAB Center for Heart Failure Research; and the UAB Animal Resources Program Comparative Pathology Laboratory. This research was supported by grants from the University of Pennsylvania “Improved Therapies for MPS I Grant Program” (MPS I-11-001-01 for KMK and DMB), NIH 1R01GM094792 (TB & DMB), and the US-Israel Binational Science Foundation (2006/301 for TB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee HL, Dougherty JP. Pharmaceutical therapies to recode nonsense mutations in inherited diseases. Pharmacol. Ther. 2012;136:227–266. doi: 10.1016/j.pharmthera.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Muenzer J, Wraith JE, Clarke LA. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 3.Ashton LJ, Brooks DA, McCourt PA, Muller VJ, Clements PR, Hopwood JJ. Immunoquantification and enzyme kinetics of alpha-L-iduronidase in cultured fibroblasts from normal controls and mucopolysaccharidosis type I patients. Am J Hum Genet. 1992;50:787–794. [PMC free article] [PubMed] [Google Scholar]
- 4.Bunge S, Clements PR, Byers S, Kleijer WJ, Brooks DA, Hopwood JJ. Genotype-phenotype correlations in mucopolysaccharidosis type I using enzyme kinetics, immunoquantification and in vitro turnover studies. Biochim. Biophys. Acta. 1998;1407:249–256. doi: 10.1016/s0925-4439(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DA, Muller VJ, Hopwood JJ. Stop-codon read-through for patients affected by a lysosomal storage disorder. Trends in molecular medicine. 2006;12:367–373. doi: 10.1016/j.molmed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Keeling KM, Brooks DA, Hopwood JJ, Li P, Thompson JN, Bedwell DM. Gentamicin-mediated suppression of Hurler syndrome stop mutations restores a low level of alpha-L-iduronidase activity and reduces lysosomal glycosaminoglycan accumulation. Hum. Mol. Genet. 2001;10:291–299. doi: 10.1093/hmg/10.3.291. [DOI] [PubMed] [Google Scholar]
- 7.Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity Current opinion in otolaryngology & head and neck. surgery. 2010;18:454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 9.Nudelman I, Glikin D, Smolkin B, Hainrichson M, Belakhov V, Baasov T. Repairing faulty genes by aminoglycosides: development of new derivatives of geneticin (G418) with enhanced suppression of diseases-causing nonsense mutations. Bioorg. Med. Chem. 2010;18:3735–3746. doi: 10.1016/j.bmc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Belakhov V, Kandasamy J, Baasov T, Li SC, Li YT, Bedwell DM, Keeling KM. The designer aminoglycoside NB84 significantly reduces glycosaminoglycan accumulation associated with MPS I-H in the Idua-W392X mouse. Mol. Genet. Metab. 2012;105:116–125. doi: 10.1016/j.ymgme.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keeling KM, Wang D, Dai Y, Murugesan S, Chenna B, Clark J, Belakhov V, Kandasamy J, Velu SE, Baasov T, Bedwell DM. Attenuation of nonsense-mediated mRNA decay enhances in vivo nonsense suppression. PloS one. 2013;8:e60478. doi: 10.1371/journal.pone.0060478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonaro CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCracken GH, Jr., Chrane DF, Thomas ML. Pharmacologic evaluation of gentamicin in newborn infants. J. Infect. Dis. 1971;124(Suppl):214–223. doi: 10.1093/infdis/124.supplement_1.s214. [DOI] [PubMed] [Google Scholar]
- 15.Smith AL, Daum RS, Siber GR, Scheifele DW, Syriopoulou VP. Gentamicin penetration into cerebrospinal fluid in experimental Haemophilus influenzae meningitis. Antimicrob. Agents Chemother. 1988;32:1034–1039. doi: 10.1128/aac.32.7.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson FL, Holley RJ, Langford-Smith KJ, Badrinath S, Liao A, Langford-Smith A, Cooper JD, Jones SA, Wraith JE, Wynn RF, Merry CL, Bigger BW. Neuropathology in mouse models of mucopolysaccharidosis type I, IIIA and IIIB. PloS one. 2012;7:e35787. doi: 10.1371/journal.pone.0035787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, Sun LY, Clarke LA, Tolar J, Blazar BR. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in hurler syndrome. Pediatr. Res. 2006;59:27–32. doi: 10.1203/01.pdr.0000190579.24054.39. [DOI] [PubMed] [Google Scholar]
- 20.Aldenhoven M, Boelens JJ, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol. Blood Marrow Transplant. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Shukla C, Liu X, Schoeb TR, Clarke LA, Bedwell DM, Keeling KM. Characterization of an MPS I-H knock-in mouse that carries a nonsense mutation analogous to the human IDUA-W402X mutation. Mol. Genet. Metab. 2010;99:62–71. doi: 10.1016/j.ymgme.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemes A, Timmermans RG, Wilson JH, Soliman OI, Krenning BJ, ten Cate FJ, Geleijnse ML. The mild form of mucopolysaccharidosis type I (Scheie syndrome) is associated with increased ascending aortic stiffness. Heart Vessels. 2008;23:108–111. doi: 10.1007/s00380-007-1013-x. [DOI] [PubMed] [Google Scholar]
- 23.Tolar J, Braunlin E, Riddle M, Peacock B, McElmurry RT, Orchard PJ, Blazar BR. Gender-related dimorphism in aortic insufficiency in murine mucopolysaccharidosis type I. J. Heart Valve Dis. 2009;18:524–529. [PMC free article] [PubMed] [Google Scholar]
- 24.Simonaro CM. Cartilage and chondrocyte pathology in the mucopolysaccharidoses: The role of glycosaminoglycan-mediated inflammation. Journal of pediatric rehabilitation medicine. 2010;3:85–88. doi: 10.3233/PRM-2010-0120. [DOI] [PubMed] [Google Scholar]
- 25.Rowan DJ, Tomatsu S, Grubb JH, Montano AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013;36:235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson S, Hashamiyan S, Clarke L, Saftig P, Mort J, Dejica VM, Bromme D. Glycosaminoglycan-mediated loss of cathepsin K collagenolytic activity in MPS I contributes to osteoclast and growth plate abnormalities. Am. J. Pathol. 2009;175:2053–2062. doi: 10.2353/ajpath.2009.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sorgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 2009;48:89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- 28.Pan D, Sciascia A, 2nd, Vorhees CV, Williams MT. Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau AA, Crawley AC, Hopwood JJ, Hemsley KM. Open field locomotor activity and anxiety-related behaviors in mucopolysaccharidosis type IIIA mice. Behav. Brain Res. 2008;191:130–136. doi: 10.1016/j.bbr.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Heldermon CD, Hennig AK, Ohlemiller KK, Ogilvie JM, Herzog ED, Breidenbach A, Vogler C, Wozniak DF, Sands MS. Development of sensory, motor and behavioral deficits in the murine model of Sanfilippo syndrome type B. PloS one. 2007;2:e772. doi: 10.1371/journal.pone.0000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald CM, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, Glanzman AM, Spiegel R, Barth J, Elfring G, Reha A, Peltz S. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013:1–14. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddatu TP, Grubb SC, Bult CJ, Bogue MA. Mouse Phenome Database (MPD) Nucleic Acids Res. 2012;40:D887–894. doi: 10.1093/nar/gkr1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laraway S, Breen C, Mercer J, Jones S, Wraith JE. Does early use of enzyme replacement therapy alter the natural history of mucopolysaccharidosis I? Experience in three siblings. Mol. Genet. Metab. 2013;109:315–316. doi: 10.1016/j.ymgme.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 34.de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, Mengel E, Offringa M, O'Meara A, Parini R, Rovelli A, Sykora KW, Valayannopoulos V, Vellodi A, Wynn RF, Wijburg FA. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet journal of rare diseases. 2011;6:55. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupont C, El Hachem C, Harchaoui S, Ribault V, Amiour M, Guillot M, Maire I, Froissart R, Guffon-Fouilhoux N. Hurler syndrome. Early diagnosis and successful enzyme replacement therapy: a new therapeutic approach. Case report. Arch. Pediatr. 2008;15:45–49. doi: 10.1016/j.arcped.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, Chen AH, Ponder KP, Haskins ME, Tippin BL, Le SQ, Passage MB, Guerra C, Dierenfeld A, Jens J, Snella E, Kan SH, Ellinwood NM. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol. Genet. Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Science translational medicine. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010;23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat. Med. 1996;2:467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 40.Laurent G, Carlier MB, Rollman B, Van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem. Pharmacol. 1982;31:3861–3870. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 41.Qian Y, Guan MX. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob. Agents Chemother. 2009;53:4612–4618. doi: 10.1128/AAC.00965-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mort M, Ivanov D, Cooper DN, Chuzhanova NA. A meta-analysis of nonsense mutations causing human genetic disease. Hum. Mutat. 2008;29:1037–1047. doi: 10.1002/humu.20763. [DOI] [PubMed] [Google Scholar]