Abstract
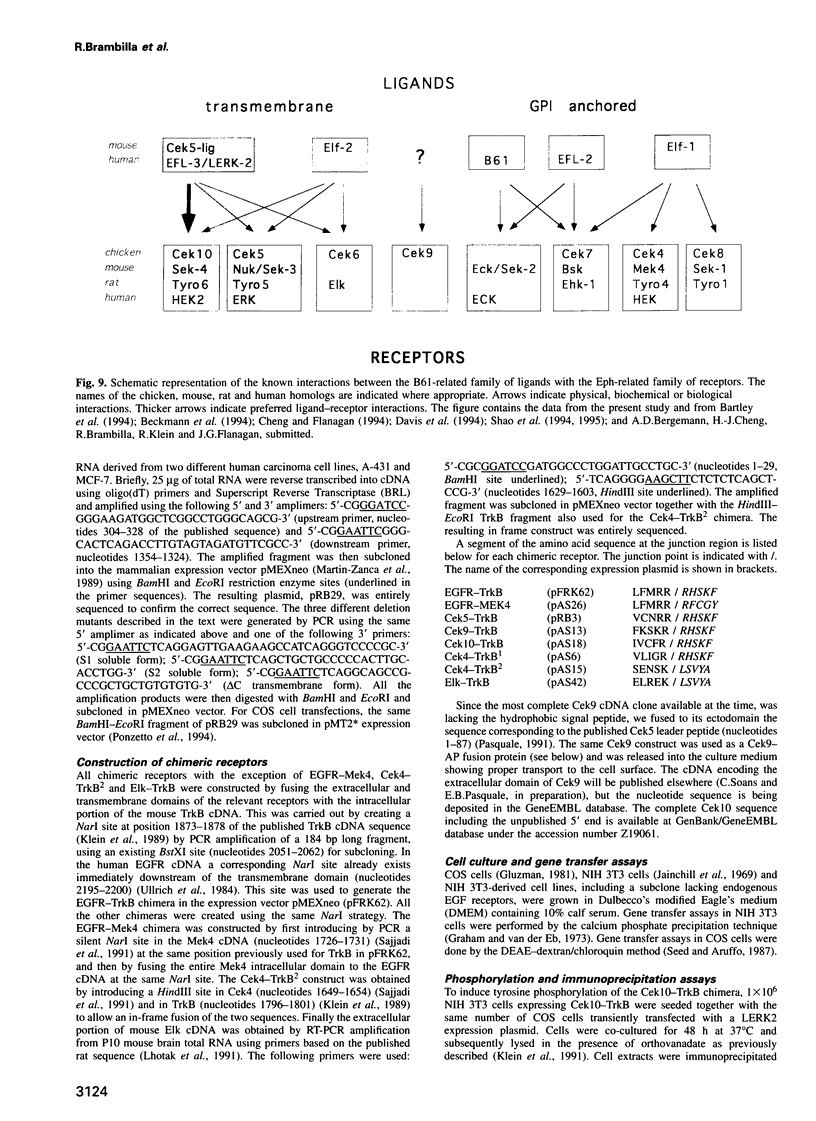
The Eph-related family of receptor tyrosine kinases consists of at least 13 members, several of which display distinctive expression patterns in the developing and adult nervous system. Recently, a small family of ligands, structurally related to the B61 protein, was identified. Binding of these ligands to Eph-related receptors did not, however, elicit measurable biological signals in cultured cells. In order to study functional interactions between B61-related ligands and Eph-related receptors, we constructed chimeric receptors, containing an Eph-related ectodomain and the cytoplasmic domain of the TrkB neurotrophin receptor. Expression and activation of such chimeric receptors in NIH 3T3 cells induced transformation in focus formation assays. Membrane-bound LERK2 ligand is shown to signal through three different Eph-related receptors, namely Cek5, Cek10 and Elk. LERK2, however, fails to interact functionally with the Cek9 receptor. Quantitative analysis including binding assays indicates that Cek10 is the preferred LERK2 receptor. Preliminary mutagenesis of the LERK2 protein suggests a negative regulatory role for its cytoplasmic domain in LERK2 signaling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. Nerve growth factor: a tale of two receptors. Oncogene. 1993 Aug;8(8):2033–2042. [PubMed] [Google Scholar]
- Bartley T. D., Hunt R. W., Welcher A. A., Boyle W. J., Parker V. P., Lindberg R. A., Lu H. S., Colombero A. M., Elliott R. L., Guthrie B. A. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994 Apr 7;368(6471):558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Becker N., Seitanidou T., Murphy P., Mattéi M. G., Topilko P., Nieto M. A., Wilkinson D. G., Charnay P., Gilardi-Hebenstreit P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech Dev. 1994 Jul;47(1):3–17. doi: 10.1016/0925-4773(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Beckmann M. P., Cerretti D. P., Baum P., Vanden Bos T., James L., Farrah T., Kozlosky C., Hollingsworth T., Shilling H., Maraskovsky E. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994 Aug 15;13(16):3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme B., Holtrich U., Wolf G., Luzius H., Grzeschik K. H., Strebhardt K., Rübsamen-Waigmann H. PCR mediated detection of a new human receptor-tyrosine-kinase, HEK 2. Oncogene. 1993 Oct;8(10):2857–2862. [PubMed] [Google Scholar]
- Cagan R. L., Krämer H., Hart A. C., Zipursky S. L. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992 May 1;69(3):393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- Cao H., Bangalore L., Bormann B. J., Stern D. F. A subdomain in the transmembrane domain is necessary for p185neu* activation. EMBO J. 1992 Mar;11(3):923–932. doi: 10.1002/j.1460-2075.1992.tb05131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. J., Flanagan J. G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994 Oct 7;79(1):157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Gale N. W., Aldrich T. H., Maisonpierre P. C., Lhotak V., Pawson T., Goldfarb M., Yancopoulos G. D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994 Nov 4;266(5186):816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Segatto O., Taylor W. G., Aaronson S. A., Pierce J. H. EGF receptor and erbB-2 tyrosine kinase domains confer cell specificity for mitogenic signaling. Science. 1990 Apr 6;248(4951):79–83. doi: 10.1126/science.2181668. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Heterologous transmembrane signaling by a human insulin receptor-v-ros hybrid in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5101–5105. doi: 10.1073/pnas.84.15.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Marengere L. E., McGlade J., Olivier J. P., Conlon R. A., Holmyard D. P., Letwin K., Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994 Apr;9(4):1001–1014. [PubMed] [Google Scholar]
- Holzman L. B., Marks R. M., Dixit V. M. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol. 1990 Nov;10(11):5830–5838. doi: 10.1128/mcb.10.11.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Klein R., Parada L. F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989 Dec 1;8(12):3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Tapley P., Barbacid M. trkC encodes multiple neurotrophin-3 receptors with distinct biological properties and substrate specificities. EMBO J. 1993 Aug;12(8):3083–3094. doi: 10.1002/j.1460-2075.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Dull T. J., Lax I., Schlessinger J., Ullrich A. HER2 cytoplasmic domain generates normal mitogenic and transforming signals in a chimeric receptor. EMBO J. 1989 Jan;8(1):167–173. doi: 10.1002/j.1460-2075.1989.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehväslaiho H., Lehtola L., Sistonen L., Alitalo K. A chimeric EGF-R-neu proto-oncogene allows EGF to regulate neu tyrosine kinase and cell transformation. EMBO J. 1989 Jan;8(1):159–166. doi: 10.1002/j.1460-2075.1989.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S., Givol D., Yarden Y. A specific combination of substrates is involved in signal transduction by the kit-encoded receptor. EMBO J. 1991 Mar;10(3):647–654. doi: 10.1002/j.1460-2075.1991.tb07993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoták V., Greer P., Letwin K., Pawson T. Characterization of elk, a brain-specific receptor tyrosine kinase. Mol Cell Biol. 1991 May;11(5):2496–2502. doi: 10.1128/mcb.11.5.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoták V., Pawson T. Biological and biochemical activities of a chimeric epidermal growth factor-Elk receptor tyrosine kinase. Mol Cell Biol. 1993 Nov;13(11):7071–7079. doi: 10.1128/mcb.13.11.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y., Hirai H., Takaku F. Overexpression confers an oncogenic potential upon the eph gene. Oncogene. 1990 Mar;5(3):445–447. [PubMed] [Google Scholar]
- Pasquale E. B., Connor R. J., Rocholl D., Schnürch H., Risau W. Cek5, a tyrosine kinase of the Eph subclass, is activated during neural retina differentiation. Dev Biol. 1994 Jun;163(2):491–502. doi: 10.1006/dbio.1994.1165. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Deerinck T. J., Singer S. J., Ellisman M. H. Cek5, a membrane receptor-type tyrosine kinase, is in neurons of the embryonic and postnatal avian brain. J Neurosci. 1992 Oct;12(10):3956–3967. doi: 10.1523/JNEUROSCI.12-10-03956.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E. B. Identification of chicken embryo kinase 5, a developmentally regulated receptor-type tyrosine kinase of the Eph family. Cell Regul. 1991 Jul;2(7):523–534. doi: 10.1091/mbc.2.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P. M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994 Apr 22;77(2):261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Honegger A. M., Schlessinger J., Ullrich A. Cytoplasmic domains determine signal specificity, cellular routing characteristics and influence ligand binding of epidermal growth factor and insulin receptors. EMBO J. 1989 Oct;8(10):2943–2954. doi: 10.1002/j.1460-2075.1989.tb08444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene. 1993 Jul;8(7):1807–1813. [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B., Subramani S. Identification of a new eph-related receptor tyrosine kinase gene from mouse and chicken that is developmentally regulated and encodes at least two forms of the receptor. New Biol. 1991 Aug;3(8):769–778. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Lou L., Pandey A., Pasquale E. B., Dixit V. M. cDNA cloning and characterization of a ligand for the Cek5 receptor protein-tyrosine kinase. J Biol Chem. 1994 Oct 28;269(43):26606–26609. [PubMed] [Google Scholar]
- Shao H., Lou L., Pandey A., Verderame M. F., Siever D. A., Dixit V. M. cDNA cloning and characterization of a Cek7 receptor protein-tyrosine kinase ligand that is identical to the ligand (ELF-1) for the Mek-4 and Sek receptor protein-tyrosine kinases. J Biol Chem. 1995 Feb 24;270(8):3467–3470. doi: 10.1074/jbc.270.8.3467. [DOI] [PubMed] [Google Scholar]
- Tuzi N. L., Gullick W. J. eph, the largest known family of putative growth factor receptors. Br J Cancer. 1994 Mar;69(3):417–421. doi: 10.1038/bjc.1994.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]