Abstract
Adolescents with a family history of alcoholism (FHP) are at heightened risk for developing alcohol use disorders (AUDs). The nucleus accumbens (NAcc), a key brain region for reward processing, is implicated in the development of AUDs. Thus, functional connectivity of the NAcc may be an important marker of risk in FHP youth.
Resting state functional magnetic resonance imaging (rs-fcMRI) was used to examine the intrinsic connectivity of the NAcc in 47 FHP and 50 family history negative (FHN) youth, ages 10–16 years old.
FHP and FHN adolescents showed significant group differences in resting state synchrony between the left NAcc and bilateral inferior frontal gyri and the left postcentral gyrus (PG). Additionally, FHP youth differed from FHN youth in right NAcc functional connectivity with the left orbitofrontal cortex (OFC), left superior temporal gyrus, right cerebellum, left PG, and right occipital cortex. These results indicate that FHP youth have less segregation between the NAcc and executive functioning brain regions, and less integration with reward-related brain areas, such as the OFC. The findings of the current study highlight that premorbid atypical connectivity of appetitive systems, in the absence of heavy alcohol use, may be a risk marker in FHP adolescents.
Keywords: Alcohol, Ventral Striatum, Adolescence, Familial, Risk
1. Introduction
1.1. Family History of Alcoholism
Adolescents with a family history of alcoholism (FHP) have elevated risk for developing alcohol use disorders (AUDs) compared to youth without a family history (FHN) of AUDs (Dawson et al., 1992; Goodwin et al., 1974; Sher et al., 1991). In an effort to understand psychological and neurobiological factors that contribute to increased vulnerability for alcohol abuse, behavioral and neuroimaging research has focused on identifying markers of risk in FHP youth. As there are various cognitive (Corral et al., 2003; Harden and Pihl, 1995; Poon et al., 2000), neurobiological (Cservenka et al., 2012; Cservenka and Nagel, 2012; Mackiewicz Seghete et al., 2013; Schweinsburg et al., 2004; Silveri et al., 2011), and psychological (Sher, 1991) abnormalities in FHP youth in the absence of heavy alcohol consumption, there is likely some heritable risk factor associated with developing an AUD. Given that adolescence is a time of heightened risk for the emergence of alcohol abuse, it is critical to examine functional brain differences between FHP and FHN youth prior to the initiation of any heavy alcohol use. By doing so, FHP-related neurobiological markers can be identified in the absence of alcohol-induced neurotoxicity, and examined during a period of active brain development, when cognitive, affective, and reward-driven systems (Casey and Jones, 2010; Ernst et al., 2006b; Galvan et al., 2006; Somerville and Casey, 2010) may contribute to addiction vulnerability.
1.2. Nucleus Accumbens and Alcohol Use Disorders
Alcoholism is a disorder associated with dysfunctional mesolimbic reward circuitry (see Noble (1996) for review). However, there is a debate as to whether abnormalities in alcoholics’ reward-related brain circuitry are a consequence of long-term alcohol abuse, or if these regions show premorbid atypical structure and/or function that contributed to the development of AUDs. The nucleus accumbens (NAcc) is a primary region of reward processing and response (Knutson et al., 2001). Structural changes, functional alterations, and neuroadapations of this region are implicated in heavy alcohol use, across human (Claus et al., 2011; Wu et al., 2010) and animal studies (Alaux-Cantin et al., 2013; Szumlinski et al., 2007). The NAcc, which is part of the mesoscorticolimbic dopamine pathway (Berendse et al., 1992; Nauta et al., 1978), has extensive functional connections to other reward-related regions, including orbitofrontal cortex (OFC), basal ganglia, and the amygdala (Cauda et al., 2011). Thus, understanding functional connectivity of the NAcc is important for identifying atypical reward-related connectivity patterns that may contribute to risk for addiction.
In alcoholics and heavy drinkers, structural studies have found reduced NAcc volume (Makris et al., 2008), while functional magnetic resonance imaging (fMRI) suggests increased blood oxygen level-dependent (BOLD) response in this region in the presence of alcohol-related cues (Claus et al., 2011; Kareken et al., 2004; Myrick et al., 2004; Wrase et al., 2007). Further, also seen in alcoholics (Beck et al., 2009; Wrase et al., 2007), adults and youth with a family history of alcoholism show blunted NAcc response during monetary reward anticipation compared to their FHN peers (Andrews et al., 2011; Yau et al., 2012). Additionally, FHP youth show increased functional connectivity of the NAcc with precuneus, somatosensory, and sensorimotor regions during reward anticipation (Weiland et al., 2013). This suggests that familial risk for alcoholism may be associated with abnormal reward processing, even in the absence of alcohol abuse. However, to our knowledge, there have been no studies published in FHP youth examining functional connectivity of the NAcc, in the absence of task-related demands (i.e., during rest).
1.3. Resting State Functional Connectivity of the Nucleus Accumbens
Resting state functional connectivity magnetic resonance imaging (rs-fcMRI) is a technique that characterizes the synchronous fluctuation of the BOLD timecourse at rest (Biswal et al., 1995). This technique has been used to identify distinct brain networks and the development of their functional connections (Dosenbach et al., 2007; Fair et al., 2007; Fox et al., 2005). Studies of resting state synchrony in healthy individuals indicate that the NAcc shows positive functional connectivity, or integration, with other reward and affect-related regions, such as the OFC, striatum, and amygdala (Barnes et al., 2010; Di Martino et al., 2008). In contrast, the NAcc shows negative functional connectivity, or segregation, with regions implicated in cognitive control, such as dorsolateral prefrontal and inferior parietal cortices (Barnes et al., 2010; Di Martino et al., 2008). Recently, studies in long-term abstinent alcoholics have shown that the NAcc has decreased functional connectivity with limbic regions, including the caudate and the medial dorsal and anterior nuclei of the thalamus, and increased connectivity with executive functioning brain areas, such as the dorsolateral prefrontal cortex and inferior parietal lobule (Camchong et al., 2013a; Camchong et al., 2013b). The authors interpreted these patterns of connectivity as compensatory mechanisms that allowed for continued abstinence, as they were associated with improved neuropsychological performance.
Despite these alterations in resting state synchrony observed in abstinent alcoholics, it is yet unknown whether FHP and FHN youth differ in resting state connectivity of the NAcc. This analysis is critical to differentiating consequences of long-term alcohol use from underlying risk for the development of AUDs. Thus, the goal of the current study was to use rs-fcMRI in a sample of 10–16 year old FHP and FHN youth, who had not used alcohol or other substances heavily. While healthy adults exhibit segregation of ventral striatal and fronto-parietal brain regions (Barnes et al., 2010; Di Martino et al., 2008), FHP youth and young adults have reduced functional response in these same regions (Andrews et al., 2011; Cservenka et al., 2012; Cservenka and Nagel, 2012; Mackiewicz Seghete et al., 2013; Yau et al., 2012). Interestingly, weaker NAcc response in FHP individuals during reward anticipation is related to behavioral impulsivity (Andrews et al., 2011), which could suggest interference between reward and cognitive control brain activity. Thus, we predicted that at-risk youth would have less segregation between these regions, which would suggest less distinct dissociation of reward and cognitive control brain areas. Additionally, the NAcc is typically positively functionally connected to other reward-related brain regions, such as the OFC (Barnes et al., 2010; Di Martino et al., 2008), which is essential for reward learning (Tanabe et al., 2013), and has smaller volume and increased activity to alcohol cues in those with addiction (Claus et al., 2011; Tanabe et al., 2009). Furthermore, individuals at risk for alcoholism show decreased laterality of OFC volume between right and left hemispheres (Hill et al., 2009). Given these OFC abnormalities in substance dependence and risk for alcoholism, we also hypothesized that FHP youth would have less integration between the NAcc and other reward-related brain regions, such as the OFC, suggestive of poorer within-network integration of appetitive brain regions.
2. Materials and Methods
2.1. Participants
Participants, ages 10–16 years, were recruited from the local community. FHP youth were part of an ongoing longitudinal study and matched for demographic characteristics to FHN participants. To determine eligibility, structured interviews were conducted by telephone with the youth and one of their parents [Structured Clinical Interview; (Brown et al., 1994)]. Exclusionary criteria included: lack of information on family history, family history of psychotic disorders, diagnosis of a DSM-IV psychiatric disorder [Diagnostic Interview Schedule for Children, Predictive Scales (Lucas et al., 2001)], significant lifetime alcohol or substance use (> 10 lifetime alcoholic drinks or > 2 drinks on any single occasion, > 5 uses of marijuana, > 4 cigarettes per day, any other drug use) [Customary Drinking and Drug Use Record; (Brown et al., 1998)], neurological illness, significant head trauma (loss of consciousness > 2 minutes), serious medical conditions, mental retardation or learning disability, prenatal exposure to drugs or alcohol, left-handedness [Edinburgh Handedness Inventory; (Oldfield, 1971)], MRI contraindications, and pregnancy. Written consent and assent were obtained from all youth and their parents in accordance with the Oregon Health & Science University (OHSU) Institutional Review Board.
129 adolescents (62 FHN, 67 FHP), underwent magnetic resonance imaging scanning. After excluding participants with poor nucleus accumbens delineation (n = 5), excessive head movement (n = 24), or data preprocessing errors (n = 3), the final sample included 97 participants (see Table 1 for demographic information).
Table 1.
Participant Demographics. Means and standard deviations unless otherwise noted.
| FHP1 | FHN2 | Statistic | P value | |
|---|---|---|---|---|
| N | 47 | 50 | - | - |
| Female/male | 22/25 | 21/29 | X2 = 0.23 | 0.63 |
| Age | 14.57 (1.31) | 14.34 (1.28) | t = 0.87 | 0.39 |
| Puberty3 | 3.66 (0.98) | 3.48 (0.89) | U = 1005, Z = −1.32 | 0.19 |
| IQ4 | 111.19 (9.90) | 113.28 (11.32) | t = −0.97 | 0.34 |
| GPA5,† | 3.41 (0.53) | 3.63 (0.39) | U = 747, Z = −2.11 | 0.035 |
| SES6,† | 34.60 (14.29) | 28.56 (12.36) | U = 843, Z = −2.41 | 0.016 |
| Sensation seeking7† | 47.51 (20.67) | 36.26 (17.18) | t = 2.77 | 0.007 |
| Delay discounting log[k]8 | −4.90 (2.11) | −5.60 (2.50) | t = −1.32 | 0.19 |
| Family history density9 | 0.57 (0.26) | 0 | - | - |
| Alcohol use10 | N = 2 | N = 3 | - | - |
| Marijuana use11 | N = 6 | N = 2 | - | - |
| Cigarette use12 | N = 2 | N = 1 | - | - |
Family history positive for alcoholism.
Family history negative for alcoholism.
Pubertal Development Scale (Petersen et al., 1988); scores translated to Crockett stages, range 1 to 5, with higher scores reflecting greater maturity (Crockett and Petersen, 1987).
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
Grade Point Average; values range from 0 to 4, with higher scores reflecting higher grades. Missing: FHP: N = 6, FHN: N = 1.
Hollingshead Index of Social Position; higher scores indicate lower socioeconomic status (Hollingshead, 1957).
Zuckerman-Kuhlman Impulsive Sensation Seeking Questionnaire (Zuckerman et al., 1993); scores range from 0 to 90, with higher scores reflecting greater sensation seeking. FHP: N = 47, FHN: N = 42.
Mitchell, 1999. Values closer to 0 indicate greater preference of small immediate reward over larger delayed reward. FHP: N = 46; FHN: N = 31.
Family history density of AUDs (Zucker et al., 1994); total possible values range from 0 to 2 and include AUDs in parents, aunts and uncles, and grandparents. Higher values represent more familial AUDs.
No more than 2 drinks on any single occasion. Maximum alcohol occasions ≤ 3 for FHP, and ≤ 2 for FHN youth.
Maximum marijuana occasions ≤ 5 for FHP, and ≤ 3 for FHN youth.
Maximum cigarette use occasions ≤ 1 for FHP, and ≤ 1 for FHN youth.
Significant difference between groups (P < 0.05).
AUD, alcohol use disorder.
2.2. Family History of Alcohol Use Disorders
The Family History Assessment Module (Rice et al., 1995) was used with at least one biological parent and the participating youth, to assess the presence of AUDs, as defined by DSM-IV criteria, in first (biological parents) and second degree relatives (biological aunts, uncles, and grandparents). Youth were categorized as FHN or FHP based on this information. FHN youth had no relatives with a history of AUDs. FHP youth had at least one parent or two or more second-degree relatives on the same side of the family with a history of AUDs. For FHP youth, a Family History Density (FHD) score was calculated indicating the degree of familial AUDs: parents contributed 0.5, grandparents 0.25, and aunts and uncles a weighted ratio of 0.25 divided by the number of their siblings. In the FHP group, scores ranged from 0.04 to 1.50. The presence of first or second-degree relatives with AUD is a robust measure of vulnerability to AUDs or other substance abuse (Stoltenberg et al., 1998).
2.3. Participant Characteristics
Intellectual functioning (IQ) was estimated with the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), and academic achievement was estimated with self-reported grade point average (GPA). Socioeconomic status was estimated with the Hollingshead Index of Social Position (Hollingshead, 1957), administered to parents. Pubertal development was evaluated with the self-report Pubertal Development Scale (Petersen et al., 1988) and scores were translated into Tanner Stages (Carskadon and Acebo, 1993). All demographic variables were inspected for normal distribution. Sex and race were analyzed with chi-square tests. Two-sample t-tests or Mann-Whitney U-tests were used to compare groups on normally and non-normally distributed demographic variables, respectively. Statistical analyses were performed in IBM SPSS statistics for Windows (Corp., Released 2011).
2.4. Imaging Procedures
Participants were scanned in a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil, located at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1-weighted MPRAGE scanning sequence (TI = 900ms, flip angle = 10°, TE = 3.58 ms, TR = 2300 ms, acquisition matrix 256 × 240, resolution 1 × 1 × 1.1 mm). Whole brain resting state functional images were acquired over two runs (TR = 2500 ms, TE = 30 ms, flip angle = 90°, resolution = 3.75 × 3.75 × 3.8 mm, FOV = 240 mm2, 36 slices, 100 TRs, time of acquisition for each run 4:17). During the resting state scan, participants viewed a white fixation cross, centered on a black background, projected on a mirror mounted on the head coil. They were instructed to fixate on the cross and remain still.
2.5. Image Processing and Analyses
Previously published, standard image preprocessing steps were used (Costa Dias et al., 2013; Fair et al., 2007; Fair et al., 2012; Mills et al., 2012) to reduce non-neuronal artifacts (Miezin et al., 2000). Preprocessing of images included correction of slice timing from the interleaved acquisition, removal of MR signal offset central spike, and signal normalization to a mode value of 1000. A temporal band-pass filter was applied to remove high frequency (0.009Hz < f < 0.08Hz) signal noise from heart rate and respiration. Additional preprocessing steps included regressing the white matter and ventricular signal from the pre-defined regions of interest (ROIs), regressing the global signal from the whole brain, and regressing the derivatives of the white matter, ventricular, and whole-brain signals. Importantly, no spatial smoothing was applied due to the interest in examining signal in small sub-cortical structures. Anatomical images were transformed to 3 mm3 Talairach space (Talairach and Tournoux, 1988); functional images were also transformed and then co-registered to the anatomical images. Alignment of the functional and anatomical data was visually inspected.
Because rs-fcMRI is sensitive to head movement, additional preprocessing steps were applied to reduce motion artifact. Specifically, regression of the three translational and three rotational shift directions was first applied. Additionally, variance of signal change from the average signal (DVAR), excluding frames with signal intensities greater than absolutes value of 8, was used to censor frames due to excessive head movement (Fair et al., 2012; Shannon et al., 2011). While the identification of movement frames in the image is similar, this value is higher than previously proposed (Fair et al., 2012; Power et al., 2012) due to the absence of spatial smoothing. The threshold for acceptable frames removed was set at 40%, leaving at least 5 minutes of resting state data for each participant. Youth with greater than 40% of frames removed (9 FHN, 15 FHP) were excluded from further analyses. Furthermore, frame-to-frame displacement (FD) was calculated for each participant’s remaining frames (FDi = |Δdix|+|Δdiy|+|Δdiz|+|Δαi|+|Δβi|+|Δγi|), where Δdix = d(i−1)x−dix) (Power et al., 2012). There were no significant group differences in the average percent of frames removed due to motion (FHP = 12.52, SD = 10.57; FHN = 10.97, SD = 10.04; t = −0.74, P = 0.49), nor in FD (FHP = 0.11, SD = 0.03; FHN = 0.11, SD = 0.03; t = 0.15, P = 0.88) or DVAR (FHP = 5.91, SD = 0.67; FHN = 5.89, SD = 0.64; t = 0.15, P = 0.88) remaining mean, after censoring.
Whole-brain seed-based connectivity analysis was used to examine functional connectivity of the NAcc with other areas of the brain. The NAcc was defined for each participant using FMRIB Software Library’s FMRIB Integrated Registration and Segmentation Tool, an automated segmentation tool that determines grey and white matter boundaries, using the youth’s T1-weighted anatomical image (Patenaude et al., 2011). NAcc ROIs were converted to 3 mm3 voxels in Talairach space. Each of the ROIs was visually inspected for precision of definition. Four FHP and one FHN youth had poorly anatomically placed ROIs, and were excluded from further analyses. Rs-fcMRI was analyzed by correlating the timecourse of the NAcc BOLD response with all other voxels in the brain, generating a resting state functional connectivity map of the left and right NAcc for each participant. Correlation coefficients (r values) were transformed to Fisher Z scores to improve normality. In-house software developed at Washington University in St. Louis, was used to generate the following statistical maps. One-sample t-tests were performed on the Fisher’s Z transformed correlation coefficients to examine the functional connectivity patterns of the left and right NAcc for FHP and FHN youth. Next, two-sample t-tests (assuming unequal variance, P < 0.05) were performed to examine whole-brain functional connectivity differences between the groups. Monte Carlo simulation was used to avoid Type I error at a voxel threshold of P < 0.05, Z ≥ 2.25, and a cluster threshold ≥ 53 contiguous voxels. Peak Z coordinates for the whole-brain group differences in connectivity were extracted using the following procedure. Twenty mm radius sphere were created around peak Z scores ≥ 20 mm apart. These clusters were masked by the Monte Carlo corrected maps.producing three clusters of significant group differences for the left NAcc and five clusters for the right NAcc (Table 2). Thus, voxels that did not pass multiple comparisons correction were eliminated. Correlation coefficients (r values) for each individual were extracted for correlations between left and right nucleus accumbens and the clusters of significant group differences. These were used in hierarchical regressions to control for nuisance variables (section 3.2.3.), as well as to examine family history density relationships with functional connectivity (section 3.2.4.). Finally, results of group differences were surface-mapped onto the PALS-B12 atlas, using Caret software (Van Essen et al., 2001), except for cerebellar group differences, which were displayed in volumetric space and overlaid on a template brain.
Table 2.
Significant Group Differences in Resting State Nucleus Accumbens Functional Connectivity
| Group Differences | FHP | FHN | Z score | Number of Voxels | Peak Talairach | |||
|---|---|---|---|---|---|---|---|---|
| N | 47 | 50 | x | y | z | |||
| L NAcc | ||||||||
| L PG | FHP>FHN | + | − | 3.83 | 103 | −26 | −43 | 60 |
| R IFG | FHP>FHN | − | − | 3.71 | 84 | 44 | 25 | 9 |
| L IFG | FHP>FHN | − | − | 3.83 | 80 | −47 | 13 | 17 |
| R NAcc | ||||||||
| R CER | FHP<FHN | − | + | −3.80 | 224 | 44 | −62 | −36 |
| L STG | FHP>FHN | − | − | 3.76 | 104 | −59 | −51 | 10 |
| L OFC | FHP<FHN | − | + | −3.32 | 70 | −3 | 8 | −20 |
| R OCC | FHP>FHN | − | − | 2.71 | 59 | 15 | −62 | −7 |
| L PG | FHP>FHN | + | − | 3.62 | 53 | −64 | −10 | 19 |
CER = cerebellum, FHN = family history negative, FHP = family history positive, IFG = inferior frontal gyrus, L = left, NAcc = nucleus accumbens, OCC = occipital cortex, OFC = orbitofrontal cortex, PG = postcentral gyrus, R = right, STG = superior temporal gyrus, + = positive functional connectivity, − = negative functional connectivity, FHP > FHN = more positive functional connectivity or less negative functional connectivity in FHP compared with FHN youth, FHP < FHN = less positive functional connectivity in FHP compared with FHN youth.
3. Results
3.1. Demographics
97 subjects were included in the final analyses of resting state functional connectivity (47 FHP, 50 FHN). Demographic characteristics of the samples are described in Table 1. Participants were well matched on age, sex ratio, race, pubertal stage, and IQ (all p > 0.05). However, they differed on SES (FHN = 28.56, SD = 12.37, FHP = 34.60, SD = 14.29, U = 842, Z = −2.41, P = 0.016) and GPA (FHN = 3.62, SD = 0.39, FHP = 3.41, SD = 0.53, U = 746, Z = −2.11, P = 0.035), both of which were higher in the FHN group. For all demographic values, all subjects were within three SD of the mean, except one FHN subject with low SES. Due to the exclusionary criteria put in place, only 7 FHP and 3 FHN youth had ever used alcohol or substances in this sample (Table 1).
3.2. Resting State Functional Connectivity
3.2.1. Nucleus Accumbens Functional Connectivity in FHP and FHN Youth
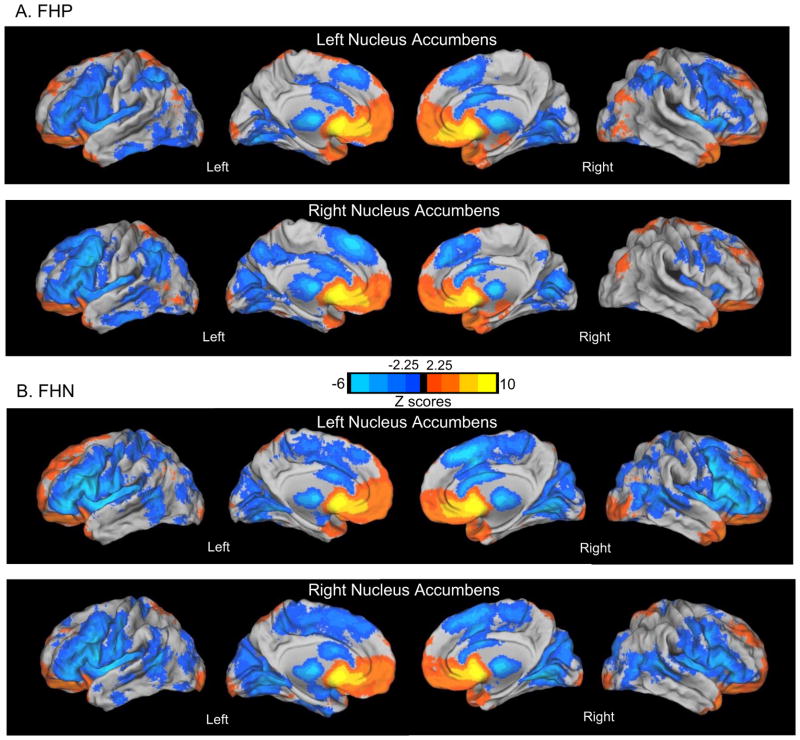
At a gross level, one-sample t-test maps of each group showed that both FHP and FHN youth had positive functional connectivity (integration) between the NAcc and the basal ganglia, including the caudate and anterior portions of the putamen. Additionally, all youth showed positive functional connectivity between the NAcc and the anterior temporal lobes. Both groups had mostly positive functional connectivity with medial orbitofrontal cortex (except for an area of group differences below, section 3.2.2). FHP and FHN youth showed negative functional connectivity (segregation) from dorsal regions of the prefrontal cortex, as well as from the superior and inferior parietal cortices, regions implicated in higher order executive functioning (Figure 1, Monte Carlo corrected (Z = 2.25, P < 0.05)).
Figure 1.
Left and Right Nucleus Accumbens Functional Connectivity in FHP and FHN Youth
3.2.2. Group Differences in Nucleus Accumbens Functional Connectivity
Significant group differences in left NAcc functional connectivity were found with the left inferior frontal gyrus (IFG), right IFG, and left postcentral gyrus (PG) (Figure 2 and Table 2). In both the left and right IFG, FHP and FHN youth had negative functional connectivity (segregation) with the left NAcc; however, in FHP youth, there was significantly weaker segregation between these regions. FHN youth showed segregation between the left PG and left NAcc; however, FHP youth showed positive functional connectivity (integration) between these regions.
Figure 2.
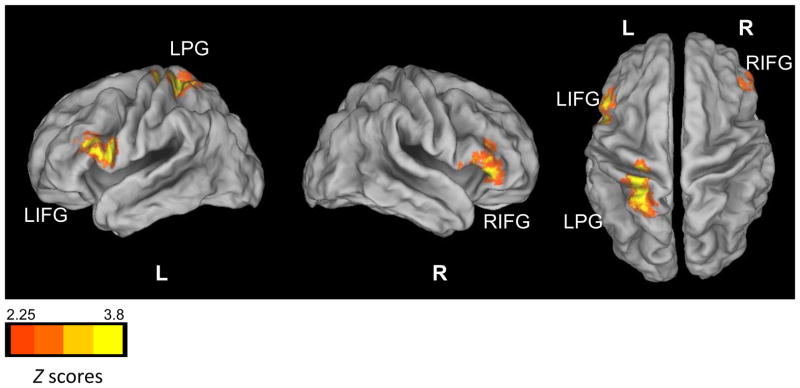
Group Differences in Left Nucleus Accumbens Resting State Functional Connectivity
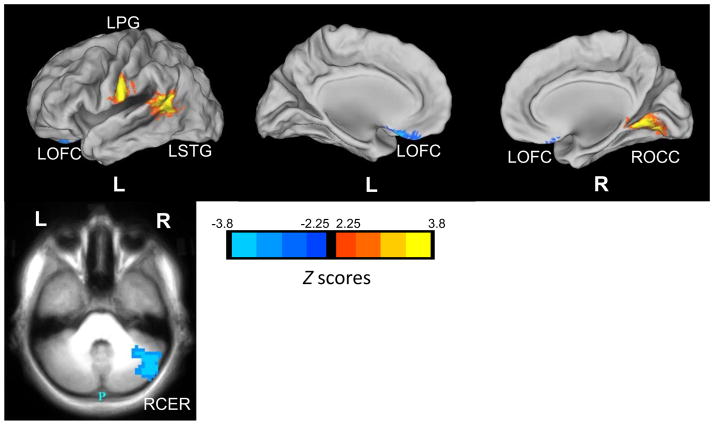
Significant group differences in right NAcc functional connectivity were found with the left OFC, the left superior temporal gyrus (STG), the left PG, the right occipital cortex, and the right cerebellum (Figure 3 and Table 2). As expected, FHN youth had positive functional connectivity (integration) between the right NAcc and left OFC, but FHP youth had negative functional connectivity (segregation) between these regions. The right occipital cortex and left STG both showed weaker segregation with the right NAcc in FHP than FHN youth. With the left PG, FHP youth again had an opposite pattern of functional connectivity from FHN youth. This was also true for the right cerebellum, which showed integration with the right NAcc in FHN youth, but segregation in the FHP group. No family history × sex interactions were present in the pairs of ROIs that showed significant group differences in connectivity.
Figure 3.
Group Differences in Right Nucleus Accumbens Resting State Functional Connectivity
3.2.3. Hierarchical Regressions with GPA and SES
Since GPA and SES were significantly different between the groups, hierarchical regression was performed to see if family history status was still a significant predictor of group differences in resting state functional connectivity, after controlling for these nuisance covariates. SES did not contribute significantly to functional connectivity in any of the areas of left and right NAcc group functional connectivity differences. However, GPA was a significant predictor of functional connectivity between the left NAcc and left IFG (F(1,88) = 6.52, β = −0.26, t = −2.15, P = 0.03, R2 change = 0.07), as well as the right NAcc and left STG (F(1,88) = 4.61, β = −0.22, t =−2.55, P = 0.01, R2 change = 0.05). When family history status was added to the model, it accounted for significantly more variance in connectivity in these two areas of group differences (left NAcc-left IFG: F(1,88) = 23.65, β = 0.55, t = 6.62, P < 0.001, R2 change = 0.28; right NAcc-left STG: F(1,88) = 14.51, β = 0.46, t = 4.82, P < 0.001, R2 change = 0.20). Thus, while GPA explained some of the variance in connectivity, family history status was a significantly better predictor (as seen by the significant F change of the P-values reported above) of resting state functional connectivity in these regions.
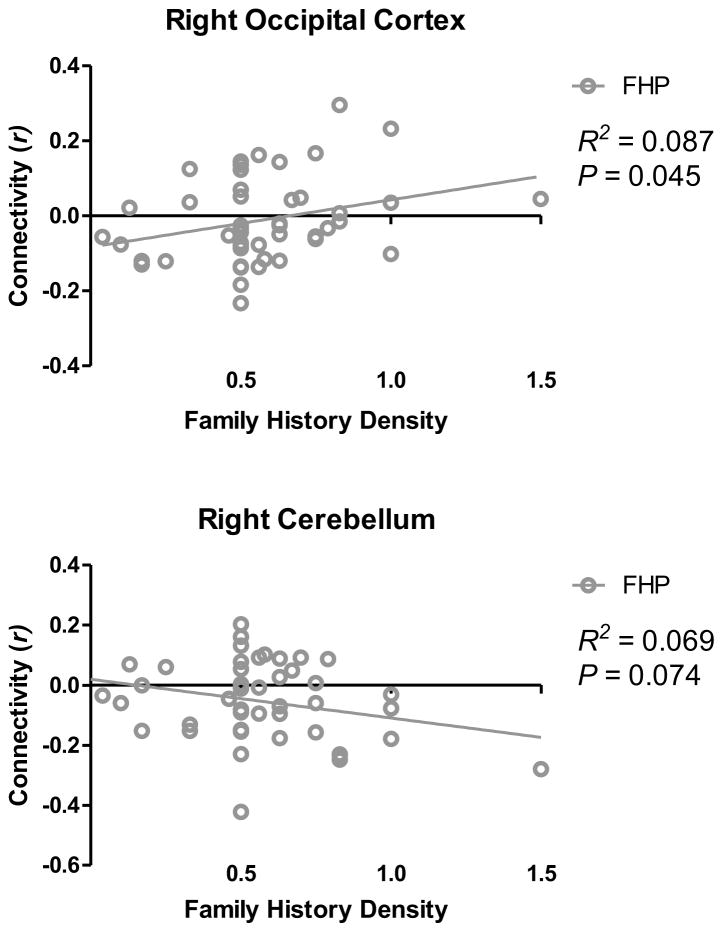
3.2.4. Nucleus Accumbens Functional Connectivity and Family History Density of Alcoholism
In FHP youth, FHD was significantly related to functional connectivity between the right NAcc and the cluster in the right occipital cortex in which significant group differences in connectivity were observed (R2 = 0.09, β = 0.29, t = 2.01, P = 0.045; Figure 4). Here, greater FHD was associated with less segregation between the right NAcc and right occipital cortex. Additionally, there was a trend-level association between FHD and connectivity between the right NAcc and right cerebellum (R2 = 0.07, β = −0.13, t = −1.83, P = 0.07; Figure 4). Specifically, in this cluster, higher FHD was related to less positive functional connectivity (less integration) between the right NAcc and right cerebellum.
Figure 4.
Family History Density and Functional Connectivity Relationships.
3.2.5. Nucleus Accumbens Functional Connectivity and Relationships with Sensation Seeking and Impulsivity
In addition to familial history of alcoholism, personality features, such as sensation seeking and impulsivity, may be important risk markers for adolescent alcohol use. Thus, we examined whether there were significant group differences on the Zuckerman-Kuhlman Impulsive Sensation Seeking questionnaire (Zuckerman et al., 1993), as well as behavior on a computerized delay discounting task (Mitchell, 1999). Next, we examined whether scores from these measures correlated with group differences in connectivity. Eighty-nine of the 97 participants had sensation seeking scores in our sample. Eight FHN participants, included from another study, were not administered this questionnaire. FHP youth had significantly higher sensation seeking levels than FHN youth, t = 2.78, p = 0.007 (Table 1). Higher sensation seekers had less segregation between the left NAcc and left IFG at trend-level (R2 = 0.04, β = 0.20, t = 1.86, p = 0.067).
Seventy-eight participants in our sample were administered the delay discounting task. We chose to examine log [k] as our measure of impulsivity, as it reflects the preference of smaller immediate rewards over larger delayed rewards. There were no significant group differences in mean log [k]: FHP (n = 46) = −4.65, FHN (n = 31) = −5.60, t = 1.32, p = 0.19 (one outlier excluded from analysis). There was one trend level relationship between log [k] and right NAcc-right PG connectivity (R2 = 0.05, β = −0.21, t = −1.88, p = 0.065), such that greater discounting was associated with more negative functional connectivity between these regions.
4. Discussion
The goal of this study was to investigate differences in resting state synchrony of the NAcc between FHP and FHN youth, since intrinsic connectivity of this brain region may be an important neurobiological marker of AUD risk. As hypothesized, there was significantly less segregation between the NAcc and regions of the fronto-parietal network in FHP youth, which could suggest poorer dissociation of cognitive control and reward networks. There was also less integration of the right NAcc and left OFC in FHP youth, which was consistent with our prediction that at-risk youth would have reduced synchrony between the NAcc and other reward-related structures, suggesting poorer integration of appetitive brain regions. In addition, FHP youth showed opposite patterns of functional connectivity with bilateral NAcc and the left PG, and had less segregation between the right NAcc and left STG and right OCC. Interestingly, FHP youth showed segregation between the right NAcc and right cerebellum, while these two regions were positively connected in FHN youth.
4.1. Nucleus Accumbens Resting State Connectivity and Risk for Alcoholism
The findings in the current study present a novel profile of familial history risk associated with atypical patterns of reward network connectivity. As adult studies of ventral striatal resting state functional connectivity have shown (Barnes et al., 2010; Di Martino et al., 2008), the typical pattern of NAcc positive functional connectivity is integration with other limbic structures, such as the OFC or ventromedial prefrontal cortex (PFC), which have extensive structural (Nakano et al., 1999) and functional (Cauda et al., 2011) connections with the NAcc. Thus, the segregated pattern of connectivity between these regions in FHP youth appears to be atypical and may increase vulnerability towards alcohol abuse.
Additionally, it is important to examine parallels between rs-fcMRI and task-related fMRI, since resting state connectivity closely corresponds to task-related fMRI BOLD signal and behavioral performance (Fox et al., 2007; Smith et al., 2009). Parallel findings from these two techniques may provide important and complementary information about these brain networks. For example, research in alcoholics has shown that poor reward-related decision-making is linked to deficits in ventromedial PFC functioning (Bechara et al., 2001), which could reflect dissociation of subcortical and prefrontal pathways needed for adaptive decision-making that leads to heightened reward-driven behavior. Furthermore, the OFC is a critical brain region for reward-related valuation (Elliott et al., 2008; Peters and Buchel, 2009), and learning (Galvan et al., 2005). Neuroimaging studies suggest that both the NAcc and OFC show heightened activity during the processing of rewards (Spicer et al., 2007). Thus, improper feedback between the OFC to the NAcc, due to weak functional integration between these structures, could lead to atypical reward valuation, possibly increasing the likelihood for bottom-up driven and potentially risky behavior in FHP youth. Recently, substance-dependent individuals performing the Iowa Gambling Task, had reduced prediction error BOLD response in both the OFC and ventral striatum compared with healthy controls. The authors argued that disrupted trial-to-trial prediction error signaling from the NAcc to the OFC could account for these findings (Tanabe et al., 2013). Thus, if FHP youth have preexisting weaker resting state synchrony between the NAcc and OFC than FHN adolescents, they could be more vulnerable to poor reward-related decisions.
FHP youth also showed weaker segregation between the left NAcc and bilateral IFG, a brain area implicated in executive functioning (Somerville et al., 2011; Tamm et al., 2002) that is negatively correlated with NAcc BOLD activity in healthy adults (Cauda et al., 2011). Thus, reduced negative functional connectivity between reward and cognitive processing brain regions in FHP youth may be indicative of atypical dissociation of brain networks, since healthy brain development is associated with both within-network integration and between-network segregation (Fair et al., 2007). Response inhibition, which is a key function of inferior frontal gyri (Tamm et al., 2002), could be less effective during heated situations, if it is more weakly dissociated from reward-related brain areas. Tasks that recruit both executive and reward networks, such as those that require reward-related decision-making, are needed to examine behavioral relationships with NAcc and frontal lobe connectivity in these youth.
While not originally hypothesized, it is also noteworthy that FHP and FHN adolescents differed in right NAcc and right cerebellum connectivity, with FHN youth showing positive functional connectivity between these structures, and FHP youth showing anti-correlated synchrony. Atypical cerebellar structure is implicated in both alcoholism (De Bellis et al., 2005) and risk for AUDs (Hill et al., 2007; Hill et al., 2011). Additionally, we have previously found atypical cerebellar activity during risky decision-making in FHP youth (Cservenka and Nagel, 2012), and a negative association between FHD of alcoholism and cerebellar response during spatial working memory (Mackiewicz Seghete et al., 2013). Further, FHP adolescents have reduced fronto-cerebellar connectivity compared with their peers (Herting et al., 2011), which is also present in adults with AUDs (Rogers et al., 2012), suggesting that there may be a heritable component to alterations in cerebellar functioning and connectivity. Based on the current findings, other cerebellar functional connections may also be aberrant in FHP youth. Even though direct structural connections between the NAcc and cerebellum are unknown, it is possible that third party brain regions are responsible for the coactivation patterns observed (Cauda et al., 2011). Additionally, FHD showed a trend-level relationship between right NAcc and right cerebellum connectivity, suggesting that familial loading of alcoholism could affect intrinsic connectivity profiles. Further work is needed to understand how particular cerebellar lobules may differ in resting state connectivity with the NAcc in at-risk youth to understand of the specificity of these findings.
Interestingly, the patterns of negative functional connectivity in FHN youth between the right NAcc and left STG, right NAcc and left OCC, and bilateral NAcc and left PG, correspond to what has been seen in healthy adults (Di Martino et al., 2008). Thus, reduced segregation between brain structures with widespread functionality also represents an atypical connectivity profile in FHP youth. This could indicate that reward network dissociation from the rest of the brain is aberrant and may lead to abnormal signaling among functionally distinct brain regions, suggesting not one, but multiple pathways of risk. A similar finding was reported by Weiland et al. (2013), who found that during reward anticipation in task-related fMRI, FHP youth had positive functional connectivity with the PG, opposite from what was observed in FHN youth. The PG is implicated in orientation of visual attention (Corbetta, 1998), and while speculative, weaker dissociation of reward-related systems and the PG could increase attention to appetitive stimuli in FHP youth. Future work should investigate similarities between task-related and resting state functional connectivity findings in FHP youth.
Finally, while not significant, there were two trend-level relationships between sensation seeking and left NAcc connectivity, as well as impulsivity and right NAcc connectivity across the entire participant sample. FHP youth who had less segregation between the left NAcc and left IFG compared with their peers were also higher sensation seekers. Thus, this correlation may be partly driven by both higher sensation seeking and reduced segregation of these brain regions in FHP compared with FHN youth. Nonetheless, sensation seeking may be another critical endophenotype for alcoholism-related risk and an important correlate of resting state synchrony. Further, greater preference for smaller immediate vs. larger delayed rewards was related to more negative connectivity between the right NAcc and left PG, the connectivity pattern present in FHN youth. Thus, some patterns of intrinsic connectivity may confer protection or resilience in FHP youth who had positive connectivity between these regions, a pattern that related to less discounting behavior.
4.2. Limitations
While this is the first study examining resting state functional connectivity of the NAcc in FHP youth, there are some limitations that should be noted. First, the brain regions that showed significant group differences in left and right NAcc connectivity were not identical between the two seed regions. The right NAcc differences were more varied, and included the OFC, somatosensory, visual, temporal, and cerebellar brain regions. There is some literature to suggest that the right NAcc and right hemisphere is more affected in AUDs (Makris et al., 2008; Oscar-Berman and Bowirrat, 2005), and thus could be a region in FHP adolescents that shows more altered connectivity than the left NAcc. However, future work is needed to replicate these differences.
Second, since this study was a cross-sectional examination of NAcc functional connectivity, cause-and-effect relationships between connectivity and alcohol use cannot be inferred. Longitudinal investigations will be important to understand if any of the connectivity differences seen in the current analysis are predictive of future alcohol abuse, as well as determine the effect of alcohol-induced neurotoxicity on reward network connectivity.
Third, due to the sensitivity of rs-fcMRI to excessive head movement, 15 FHP and 9 FHN youth were excluded from the sample. Exclusion of these participants may have resulted in removal of variance in youth of interest, particularly given that more participants were excluded from the FHP sample. For example, head movement may relate to symptoms of inattentiveness or impulsive personality, as it is often a significant challenge for fMRI studies in children with attention-deficit hyperactivity disorder (Epstein et al., 2007; Fair et al., 2012). Since those characteristics further confer risk for alcohol use during adolescence (Ernst et al., 2006a; Jester et al., 2008), it is likely that some adolescents at high risk were not included in this dataset.
Finally, it should be noted that since youth with psychiatric disorders were excluded from this study, not all alcoholism-related risk factors, including clinically diagnosed externalizing or internalizing disorders, were present in this sample, and thus the level of risk in our participants was likely reduced. However, by examining familial alcoholism in the absence of psychopathology, our findings may help guide prevention strategies aimed specifically at intergenerational transmission of AUDs.
4.3. Conclusions
Compared to FHN youth, FHP youth had significantly weaker segregation of the NAcc and cortical executive control regions, suggesting poorer dissociation of appetitive and cognitive systems. FHP youth also had weaker synchrony between the NAcc and OFC, indicating poorer integration of the reward network. The aberrant pattern of NAcc connectivity seen in FHP youth may represent a risk factor for developing AUDs, as it could lead to improper reward learning, due to weaker integration with the OFC, and interference with cognitive control, as a function of its reduced segregation from brain areas associated with executive processes. Future work should characterize resting state synchrony of other brain regions implicated in the development of AUDs to have a better understanding of intrinsic connectivity patterns that could be heritable risk factors in FHP individuals, and thereby increase vulnerability for alcohol abuse.
Acknowledgments
Grant support for the authors of this study was provided by R01 AA017664 (Nagel), U01 AA021691 (Nagel), a pilot grant to Nagel from the Portland Alcohol Research Center (P60 AA010760 (Crabbe)), R01 MH096773 (Fair), R00 MH091238 (Fair), and the Oregon Clinical & Translational Research Institute (Fair).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biological Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM, Petersen SE, Schlaggar BL. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Frontiers in Systems Neuroscience. 2010;4:18. doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. Journal of Comparative Neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol and Drugs. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied and Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 2013a;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, Fein G. Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug and Alcohol Dependence. 2013b;131:56–65. doi: 10.1016/j.drugalcdep.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: A combined functional connectivity and structure-based meta-analysis. Journal of Cognitive Neuroscience. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp., I. Released 2011. IBM SPSS Statistics for Windows. Version 20.0. IBM Corp; Armonk, NY: [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: A three-year follow-up. Journal of Studies on Alcohol and Drugs. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug and Alcohol Dependence. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: An fMRI study of youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Medial orbitofrontal cortex codes relative rather than absolute value of financial rewards in humans. Eur J Neurosci. 2008;27:2213–2218. doi: 10.1111/j.1460-9568.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson M, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Vitolo A, Kotler LA, Jarrett MA, Spicer J. Assessment and prevention of head motion during imaging of patients with attention deficit hyperactivity disorder. Psychiatry Research. 2007;155:75–82. doi: 10.1016/j.pscychresns.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006a;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006b;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Moller N, Hermansen L, Winokur G, Guze SB. Drinking problems in adopted and nonadopted sons of alcoholics. Archives of General Psychiatry. 1974;31:164–169. doi: 10.1001/archpsyc.1974.01760140022003. [DOI] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. Journal of Abnormal Psychology. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. NeuroImage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: Association with allelic variation in GABRA2 and BDNF. Psychiatry Research. 2011;194:304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: 1957. [Google Scholar]
- Jester JM, Nigg JT, Buu A, Puttler LI, Glass JM, Heitzeg MM, Fitzgerald HE, Zucker RA. Trajectories of childhood aggression and inattention/hyperactivity: Differential effects on substance abuse in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1158–1165. doi: 10.1097/CHI.0b013e3181825a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: Preliminary findings. Alcoholism: Clinical and Experimental Research. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2013;37:390–398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Frontiers in Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Chiba T. Afferent connections to the ventral striatum from the medial prefrontal cortex (area 25) and the thalamic nuclei in the macaque monkey. Annals of the New York Academy of Sciences. 1999;877:667–670. doi: 10.1111/j.1749-6632.1999.tb09297.x. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Noble EP. Alcoholism and the dopaminergic system: a review. Addiction Biology. 1996;1:333–348. doi: 10.1080/1355621961000124956. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Disease and Treatment. 2005;1:211–229. [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29:15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: Differences related to subtypes of familial alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1020–1027. [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcoholism: Clinical and Experimental Research. 2012;36:294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Developments in Alcoholism. 1991;9:301–326. [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcoholism: Clinical and Experimental Research. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer J, Galvan A, Hare TA, Voss H, Glover G, Casey B. Sensitivity of the nucleus accumbens to violations in expectation of reward. Neuroimage. 2007;34:455–461. doi: 10.1016/j.neuroimage.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; New York: 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, Banich MT. Reduced Neural Tracking of Prediction Error in Substance-Dependent Individuals. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association: JAMIA. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wu HM, Wang XL, Chang CW, Li N, Gao L, Geng N, Ma JH, Zhao W, Gao GD. Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neuroscience Letters. 2010;473:77–81. doi: 10.1016/j.neulet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime alcohol use. Journal of Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. J Pers Soc Psychol. 1993;65:757–768. [Google Scholar]