Abstract
Background
Controlled somatosensory stimulation strategies have demonstrated merit in developing oral feeding skills in premature infants who lack a functional suck, however, the effects of orosensory entrainment stimulation on electrocortical dynamics is unknown.
Objective
To determine the effects of servo-controlled pneumatic orocutaneous stimulation presented during gavage feedings on the modulation of aEEG and rEEG activity.
Methods
Two-channel EEG recordings were collected during 180 sessions that included orocutaneous stimulation and non-stimulation epochs among 22 preterm infants (mean gestational age = 28.56 weeks) who were randomized to treatment and control ‘sham’ conditions. The study was initiated at around 32 weeks post-menstrual age (PMA). The raw EEG was transformed into amplitude-integrated EEG (aEEG) margins, and range-EEG (rEEG) amplitude bands measured at 1-minute intervals and subjected to a mixed models statistical analysis.
Results
Multiple significant effects were observed in the processed EEG during and immediately following 3-minute periods of orocutaneous stimulation, including modulation of the upper and lower margins of the aEEG, and a reorganization of rEEG with an apparent shift from amplitude bands D and E to band C throughout the 23-minute recording period that followed the first stimulus block when compared to the sham condition. Cortical asymmetry also was apparent in both EEG measures.
Conclusions
Orocutaneous stimulation represents a salient trigeminal input which has both short- and long-term effects in modulating electrocortical activity, and thus, is hypothesized to represent a form of neural adaptation or plasticity that may benefit the preterm infant during this critical period of brain maturation.
Keywords: somatosensory, orofacial, brain, prematurity, electroencephalography, experience-dependent
Introduction
Controlled somatosensory stimulation strategies have merit in developing oral feeding skills in premature infants who lack a functional suck.1,2 In our recent work, a pressure-modulated pacifier, programmed to mimic the temporal dynamics of a non-nutritive suck (NNS), was shown to be highly effective in promoting ororhythmic pattern formation and NNS in preterm infants with respiratory distress syndrome,3 and those with chronic lung disease.4 Establishing NNS improves nipple feeding performance, facilitates the transition from gavage to full nipple feeds,5 and decreases the length of hospital stay in preterm infants.6 Overall, there do not appear to be any short-term negative effects as a result of somatosensory interventions designed to promote NNS and feeding.
Given the evidence supporting the use of somatosensory stimulation to promote suck development, a logical question follows concerning the potential benefit of such stimulation on brain development. The infant brain is a developing organ of enormous complexity, whose initial form is specified through genetic instruction, with pathway formation and network tuning continuously refined by experience and activity-dependent mechanisms.7 Somatosensory interventions that promote oromotor behavior presumably play a significant role in providing the preterm brain with a rich stream of synchronous neural activity along trigeminal pathways which presumably enhance thalamocortical development. Mapping the effects of oral somatosensory stimulation on the developing brain should be possible with reduced-montage electroencephalography which is currently used to monitor and map brain maturation, and assess neurological status in preterm infants.8 The dual-channel amplitude-integrated electroencephalogram (aEEG) and the range electroencephalogram (rEEG), reflect two signal processing methods designed to provide integrated brain activity and time-compressed, continuous bedside electrocortical monitoring.9
The aEEG has provided important normative data on brain maturation in preterm infants at different gestational (GA) and post-menstrual age (PMA).8,10–16 Several aEEG characteristics, including voltage, continuity, and sleep-wake cycling, mature with increasing GA and PMA. For example, with greater GA the relative amount of continuous activity (aEEG > 5µV and maximal amplitude between 20 and 40µV) tends to increase while discontinuous patterns decrease. The number of bursts per hour tends to decrease with advancing GA. Sleep state differentiation appears in neurologically normal infants at 27–29 weeks PMA,14,17 and is strongly associated with good long-term prognosis.11 Long-term outcome can be predicted by aEEG and EEG with 75–80% accuracy at 24 postnatal hours in very preterm infants (28 to 32 weeks GA), and in infants with no early indication of brain injury.18
Compared to aEEG, the rEEG represents a less conservative estimate of peak-to-peak amplitude derived from raw EEG. The rEEG provides a more precise estimate of peak-to-peak amplitude based on the raw EEG tracing when compared with aEEG, correlates strongly with PMA14, and may serve as a biomarker for brain maturation and quantification of EEG suppression in brain injury. In our view, use of the rEEG will permit a better understanding of the effects of repeated somatosensory stimulation on electrocortical activity. Studies incorporating measures of aEEG and rEEG during somatosensory interventions offer exciting opportunities to advance our understanding of stimulation-dependent brain activity and its effects on brain maturation in health and disease among extremely premature infants.
To date, nearly all studies of preterm brain cortical activity using aEEG and rEEG have been designed to map developmental features of maturation (continuity, amplitude margins, amplitude bands, etc.) and/or pathologic brain activity (e.g., seizures, discontinuity) during resting or quiescent states. However, stimulation of the nervous system also plays an important role in brain development and neurodevelopmental outcome.19 Studies aimed at mapping the relations between sensory stimulation and modulation of the aEEG and EEG are rare in preterm infants.
The primary aim of this investigation was to determine the effects of servo controlled pulsed orocutaneous stimulation presented during gavage feedings begun at around 32 weeks PMA on the modulation of aEEG and rEEG activity in the amplitude domain among medically stable preterm infants monitored in the neonatal intensive care unit (NICU).
Methods
Patients
Twenty-two (22) healthy preterm infants (16M/6F), with a mean GA of 28.6 wks (SD=2.1), birthweight of 1229.8 gms (SD=338.40), and PMA of 32.17 wks (SD=1.1) at the time of testing. Parents were consented in accordance with the Santa Clara Valley Medical Center Human Subjects Institutional Review Board approval. Inclusion Criteria: GA of 24–32 weeks, and at least 28 weeks PMA at the time of enrollment. Exclusion Criteria: Chromosomal abnormalities, multiple congenital anomalies or any major congenital anomalies. Infants with history of severe IVH, necrotizing enterocolitis (≥ stage III), vocal cord paralysis, seizures, and meningitis, or nippling all feeds at the time of enrollment.
Experimental Design
Study infants were randomly assigned to two groups, including those who received pulsed orocutaneous stimulation (Treatment), and those who did not (Control). The stimulation was delivered by a servo-controlled pneumatic amplifier (NTrainer System, Innara Health, Inc., Shawnee, KS USA) specially designed to transmit repeating pneumatic pulse trains to the infant’s mouth through a regular (green) Soothie™ silicone pacifier.3 Three-minute pneumatic orocutaneous stimulation periods were interleaved with 5.5 minute pause periods, where the pacifier was removed from the infant’s mouth (see Table 1). The control infants received a sham stimulation program in which infants were offered the same type of Soothie pacifier without patterned stimulation (blind pacifier). The staging of a single stimulation session was given concurrently with gavage. Infants had up to three daily sessions at routine feedings scheduled, every three hours. Infants were swaddled with limbs at midline, and in a quiet-awake to drowsy state during stimulation.
Table 1.
Stimulation schedule. Nine sequential data blocks are indicated by P1 through P9. The pulsed or blind ‘sham’ pacifier is presented to the infant during P3, P5, and P7. No stimulation is presented during P1, P2, P4, P6, P8, and P9. (1) NT-On: Experimental pacifier with pneumatic pulsatile stimulation, (2) NT-Off: Experimental pacifier without pneumatic pulsatile stimulation (out of infant’s mouth), (3) sham PAC-On: Controls with blind (non-pulsatile) pacifier stimulation, and (4) sham PAC-Off: Controls without blind (non-pulsatile) pacifier stimulation (out of infant’s mouth).
| Periods | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Base | Base | NT-On | NT-Off | NT-On | NT-Off | NT-On | Post | Post |
| Control | Base | Base | PAC-On | PAC-Off | PAC-On | PAC-Off | PAC-On | Post | Post |
| Duration (m) | 3 | 3 | 3 | 5.5 | 3 | 5.5 | 3 | 3 | 3 |
EEG recording and signal processing
Four neonatal hydrogel sensors (Natus Medical Incorporated, San Carlos, California) were placed in the C3, C4, P3, and P4 positions according to the international 10–20 system for EEG monitoring. EEG signals were recorded on a BRM3 monitor (Brainz, Natus Medical Incorporated, San Carlos, California USA) for up to 4-days beginning at approximately 32 weeks PMA (see Figure 1). The right- and left-side EEG signals were amplified 5000 times and bandpass-filtered [1 Hz – 50 Hz], and digitized at 256 Hz. Brainz Analyze Research (v1.5) software was used to derive the aEEG maxima/mean/minima, and rEEG amplitude bands (A [0–10µV], B [10–25µV], C [25–50µV], D [50–100µV], and E [>100µV]) at 1-min intervals. These EEG measures were derived from nine sequential epochs (data blocks), spanning 32 minutes each, and centered over the pneumatic orocutaneous or the blind pacifier ‘sham’ stimulus conditions. A total of 1620 EEG blocks were analyzed among the 22 infants. The average number of orosensory EEG sessions sampled per infant was 8.18 (SE=1.09). Portions of recordings were excluded from analysis if electrode impedance exceeded 10 kΩ, or if there was the presence of movement, electrical noise artifact, or asymmetry of voltage in one channel.
Figure 1.
Preterm infant with aEEG and pneumatically pulsed stimulation through a regular Philips AVENT BPA-free Soothie silicone pacifier coupled to the digitally-controlled handpiece of the NTrainer System. EEG signals derived from hydrogel electrodes placed at C3-P3, and C4-P4 were recorded on a bedside aEEG monitor (BRM3; Natus Medical Incorporated, San Carlos, California USA).
Statistical Analyses
Mixed models for repeated measures were used to compare the aEEG and rEEG amplitude measures between four stimulus conditions (Table 1), including (1) NT-On: Experimental pacifier with pneumatic pulse stimulation, (2) NT-Off: Experimental pacifier removed from the infant’s mouth, (3) PAC-On: Controls with blind (non-pulsatile) pacifier stimulation, and (4) PAC-Off: Blind (non-pulsatile) pacifier removed from the infant’s mouth. Adjusting for the infants’ gestational ages and birth weights, mixed models estimated the stimulus effect on each outcome via the use of restricted maximum likelihood estimator and compound symmetric error covariance structure. When the stimulus effect was significant at 0.05 alpha level, pair-wise comparisons of adjusted means were peformed using a Bonferroni-corrected p-value. All analyses were conducted using SAS 9.3.
Results
aEEG amplitude
The presence of the patterned pneumatic orocutaneous stimulation, and its aftereffects produced a significant reorganization of the EEG recorded from the left and right hemisphere in preterm infants as reflected in aEEG and rEEG amplitude parameters. An example of the bi-hemispheric aEEG sampled from C3-P3 and C4-P4 on a preterm infant (32 weeks PMA) is shown in Figure 2. Indexed events at 56, 58, and 60 represent the onset of 3-minute pulsed orocutaneous stimulation periods interleaved with 5.5 minute no-stimulus periods. Note the presence of aEEG modulation of lower and upper amplitude margins in the electrophysiological record during pulsed somatosensory stimulation.
Figure 2.
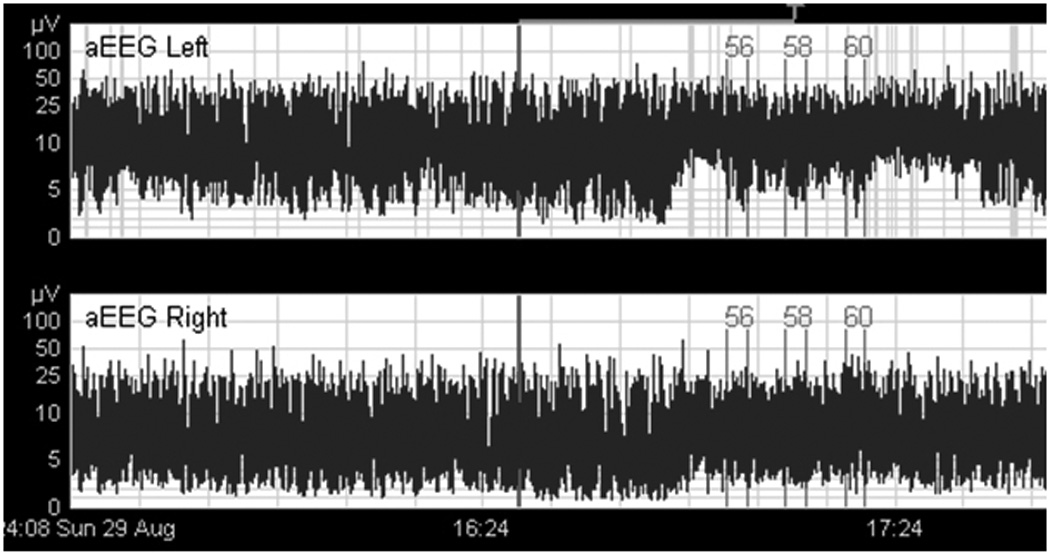
Bi-hemispheric aEEG (C3-P3, and C4-P4) on a preterm infant (32 wks PMA). Indexed events (#56, 58, and 60) represent the onset of 3-minute pulsed orocutaneous stimulation periods interleaved with 5.5 minute no-stimulus periods. Note the presence of aEEG amplitude modulation in the electrophysiological record during somatosensory stimulation.
In the aEEG domain, stimulus condition yielded significant main effects for aEEG maxima, mean, and minima in the left hemisphere (p<.0001), and significant main effects for aEEG maxima and mean in the right hemisphere (Table 2). Stimulus condition was also a significant main effect for the crosshead measures of aEEG maxima and mean. The crosshead measure results from linking or summing the cortical potentials between the left (C3-P3) and right (C4-P4) electrode montages. Cortical response asymmetry during patterned orocutaneous stimulation was apparent, with the largest changes in aEEG amplitude measures occurring in the left hemisphere. Plots for the left, right, and crosshead aEEG amplitude margins with significant Bonferroni pairwise comparisons are shown in Figure 3. For example, the blind pacifier condition yielded an average aEEG maxima in left and right hemisphere of 12.89 µV and 12.81 µV, respectively, whereas the addition of the patterned orocutaneous stimulation yielded an average aEEG maxima of 11.68 µV and 13.38 µV, respectively (p<.001). Based on the individual hemispheric measures, the presence of the pulsatile oral somatosensory stimulation, distinct from a blind pacifier alone, alters the balance in excitation with significant attenuation of the aEEG in the left hemisphere and slight facilitation in the right hemisphere. This translates to an interhemispheric difference of 1.7 µV during pulsatile oral somatosensory stimulation and only 0.08 µV in the presence of a blind pacifier (p<.001). The crosshead measure did not detect the cortical asymmetry. Behaviorally, the orocutaneous stimulation had a calming effect for preterm infants who began the stimulation period in the quiet-alert state and often transitioned to a drowsy-sleep state.
Table 2.
Mixed model adjusted means.
| NT-On Levels 3,5,7 |
NT-Off Levels 1,2,4,6,8,9 |
PAC-On Levels 3,5,7 |
PAC-Off Levels 1,2,4,6,8,9 |
Group Effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SE | M | SE | M | SE | M | SE | F | df | p |
| aEEG max left | 11.68 | 0.96 | 11.91 | 0.96 | 12.89 | 0.96 | 13.27 | 0.96 | 59.36 | 3, 30 | 0.000 |
| aEEG mean left | 7.64 | 0.44 | 7.62 | 0.44 | 8.25 | 0.44 | 8.37 | 0.44 | 52.09 | 3, 30 | 0.000 |
| aEEG min left | 4.56 | 0.25 | 4.51 | 0.25 | 4.95 | 0.25 | 4.98 | 0.25 | 27.95 | 3, 30 | 0.000 |
| aEEG max right | 13.38 | 0.61 | 13.64 | 0.60 | 12.81 | 0.62 | 13.89 | 0.60 | 11.11 | 3, 30 | 0.000 |
| aEEG mean right | 8.38 | 0.26 | 8.42 | 0.26 | 8.21 | 0.27 | 8.55 | 0.26 | 4.10 | 3, 30 | 0.015 |
| aEEG min right | 4.99 | 0.17 | 4.93 | 0.17 | 4.96 | 0.17 | 5.02 | 0.17 | 0.95 | 3, 30 | 0.430 |
| aEEG max ×head | 18.91 | 1.49 | 19.43 | 1.49 | 19.32 | 1.50 | 20.69 | 1.49 | 28.94 | 3, 30 | 0.000 |
| aEEG mean ×head | 11.57 | 0.62 | 11.74 | 0.62 | 11.49 | 0.62 | 12.01 | 0.62 | 10.73 | 3, 30 | 0.000 |
| aEEG min ×head | 7.21 | 0.26 | 7.16 | 0.26 | 7.02 | 0.26 | 7.08 | 0.26 | 2.08 | 3, 30 | 0.123 |
| BAND A left | 0.77 | 0.28 | 1.06 | 0.27 | 0.96 | 0.29 | 1.13 | 0.28 | 3.05 | 3, 30 | 0.044 |
| BAND B left | 10.54 | 1.10 | 9.44 | 1.08 | 8.82 | 1.13 | 8.40 | 1.09 | 7.66 | 3, 30 | 0.001 |
| BAND C left | 35.83 | 2.74 | 32.12 | 2.72 | 27.08 | 2.76 | 24.30 | 2.73 | 127.61 | 3, 30 | 0.000 |
| BAND D left | 37.78 | 1.95 | 38.67 | 1.93 | 43.17 | 1.98 | 43.05 | 1.94 | 37.18 | 3, 30 | 0.000 |
| BAND E left | 15.04 | 3.72 | 18.67 | 3.70 | 19.94 | 3.74 | 23.10 | 3.71 | 48.19 | 3, 30 | 0.000 |
| BAND A right | 1.06 | 0.30 | 1.21 | 0.28 | 0.87 | 0.32 | 0.89 | 0.29 | 1.60 | 3, 30 | 0.210 |
| BAND B right | 9.15 | 1.09 | 8.79 | 1.06 | 8.38 | 1.12 | 7.42 | 1.07 | 5.56 | 3, 30 | 0.004 |
| BAND C right | 31.88 | 2.50 | 27.88 | 2.48 | 26.67 | 2.52 | 22.99 | 2.48 | 69.54 | 3, 30 | 0.000 |
| BAND D right | 38.24 | 2.20 | 37.82 | 2.18 | 42.48 | 2.23 | 42.24 | 2.19 | 29.02 | 3, 30 | 0.000 |
| BAND E right | 19.65 | 3.62 | 24.26 | 3.59 | 21.60 | 3.64 | 26.46 | 3.60 | 32.44 | 3, 30 | 0.000 |
| BAND A total | 0.15 | 0.10 | 0.32 | 0.10 | 0.29 | 0.11 | 0.36 | 0.10 | 4.45 | 3, 30 | 0.011 |
| BAND B total | 4.11 | 0.76 | 4.05 | 0.74 | 4.18 | 0.78 | 4.03 | 0.75 | 0.09 | 3, 30 | 0.967 |
| BAND C total | 24.86 | 2.41 | 20.92 | 2.39 | 17.13 | 2.43 | 13.93 | 2.40 | 121.19 | 3, 30 | 0.000 |
| BAND D total | 44.49 | 3.11 | 42.06 | 3.09 | 46.23 | 3.13 | 43.82 | 3.10 | 17.32 | 3, 30 | 0.000 |
| BAND E total | 26.36 | 4.63 | 32.62 | 4.60 | 32.17 | 4.65 | 37.86 | 4.62 | 61.52 | 3, 30 | 0.000 |
Figure 3.
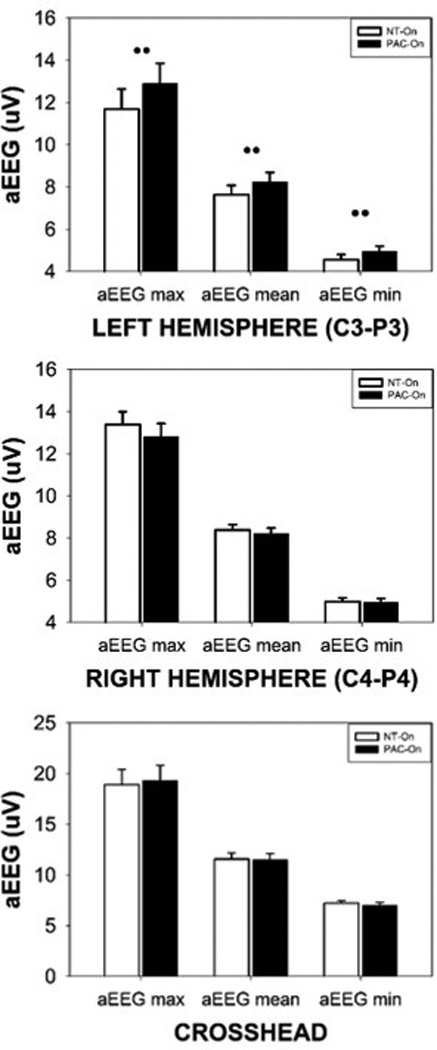
Mean aEEG measures (maxima, mean, and minima) sampled from the left hemisphere (C3-P3), right hemisphere (C4-P4), and the crosshead montage pooled among all preterm infants during pulsed orocutaneous (NT-On) and sham pacifier stimulation conditions (PAC-On) (significant Bonferroni pairwise comparisons indicated, •• p<.0001).
rEEG amplitude bands
The presence of the patterned pneumatic orocutaneous stimulation produced a significant reorganization of rEEG amplitude bands in both hemispheres (Figures 4 and 5). Overall, significant proportions of the rEEG shifted from the E and D bands to the lower amplitude C band. Considerably less or no change was observed among bands A and B which are at the low end of rEEG voltage range. Asymmetry was also observed with the degree of amplitude band reorganization (shifting from D and E, to C band) greater in the left hemisphere compared to the right hemisphere (Figure 4). As shown in Figure 6, stimulus condition yielded significant main effects for crosshead amplitude bands A (0–10 µV, p=.011), C (25–50 µV, p<.0001), D (50–100 µV, p<.0001), and E (>100 µV, p<.0001). The proportion of rEEG adjusted means in the E and C bands for the sham blind pacifier condition was 32.17% and 17.13%, respectively. Preterm infants who received the pulsatile orocutaneous stimulation manifest a significant shift in rEEG power from the E band (−26.36%) to the C band (+24.86%). A persistence or ‘after-effect’ in the reorganization of the rEEG power banding was observed during the 5.5-minute no-stimulus epochs that followed each of the 3-minute orocutaneous stimulation periods (Table 2). This after-effect was also significantly different between the sham blind pacifier and pulsatile oral somatosensory stimulation condition (p<.0001). Thus, the 3-minute pulsed somatosensory stimulation epochs served to enhance rEEG band C activity while suppressing higher voltage in rEEG bands D and E. Preterm infants exposed to the pulsed orocutaneous stimulation also yielded a greater proportion of band C activity throughout the 23-minute recording period that followed the first stimulus block when compared to the blind pacifier condition.
Figure 4.
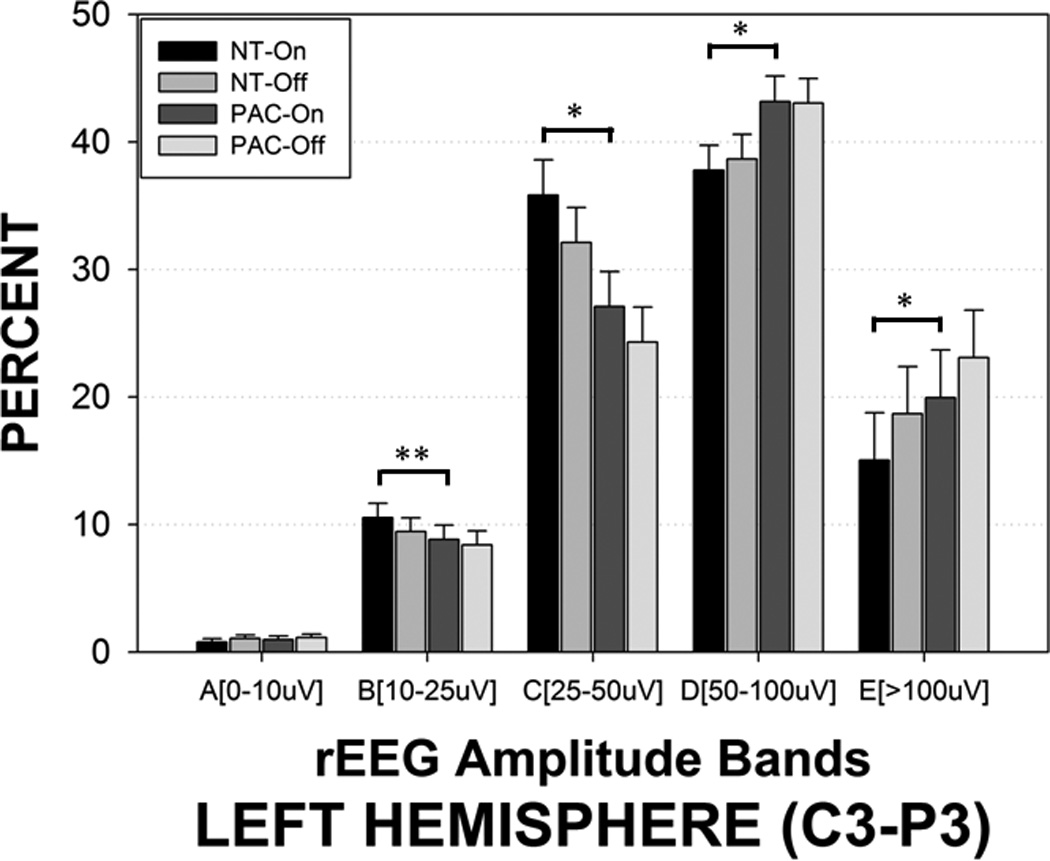
rEEG amplitude bands sampled from the left hemisphere (C3-P3) in preterm infants during pulsed orocutaneous and blind ‘sham’ pacifier stimulation conditions. NT-On indicates the pneumatically charged pacifier is in the mouth, NT-Off indicates the charged pacifier is out of the mouth, sham PAC-On indicates the regular Soothie pacifier is in the mouth, and sham PAC-Off indicates the regular Soothie pacifier is out of the baby’s mouth. Significant Bonferroni pairwise contrasts between orosensory entrainment and sham pacifier stimulation conditions indicated by horizontal bars (* p<.001, ** p=.020).
Figure 5.
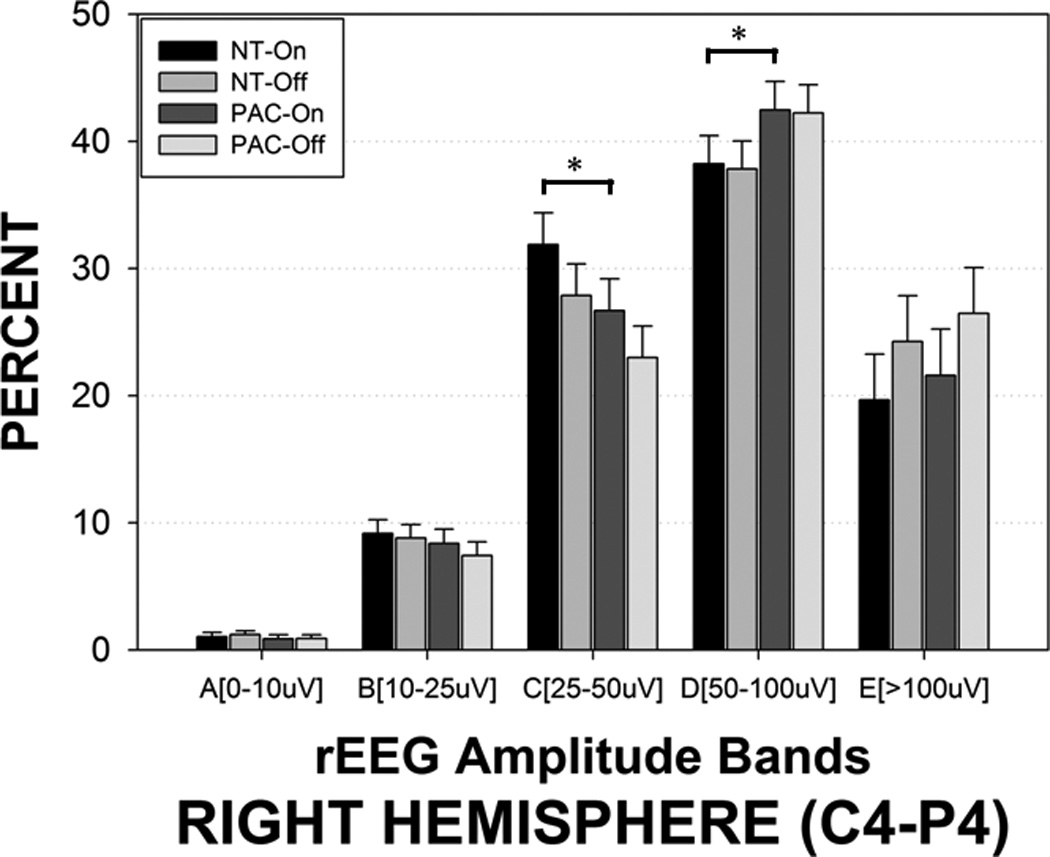
rEEG amplitude bands sampled from the right hemisphere (C4-P4) in preterm infants during oral pulsed orocutaneous and blind ‘sham’ pacifier stimulation conditions. NT-On indicates the pneumatically charged pacifier is in the mouth, NT-Off indicates the charged pacifier is out of the mouth, sham PAC-On indicates the regular Soothie pacifier is in the mouth, and sham PAC-Off indicates the regular Soothie pacifier is out of the baby’s mouth. Significant Bonferroni pairwise contrasts between orosensory entrainment and sham pacifier stimulation conditions indicated by horizontal bars (* p<.001).
Figure 6.
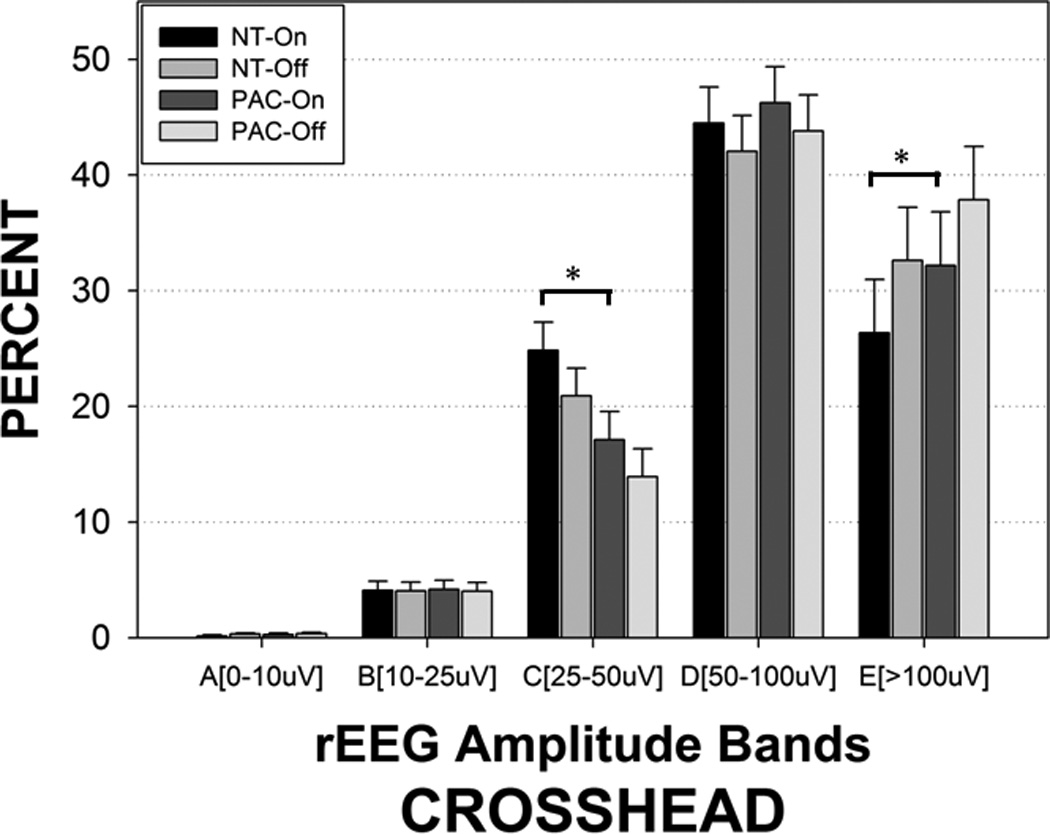
rEEG amplitude bands sampled from the crosshead montage (P3–P4) in preterm infants during pulsed orocutaneous and blind ‘sham’ pacifier stimulation conditions. NT-On indicates the pneumatically charged pacifier is in the mouth, NT-Off indicates the pneumatically-charged pacifier is out of the mouth, sham PAC-On indicates the regular Soothie pacifier is in the mouth, and sham PAC-Off indicates the regular Soothie pacifier is out of the baby’s mouth. Significant Bonferroni pairwise contrasts between orosensory entrainment and sham pacifier stimulation conditions indicated by horizontal bars (* p<.001).
Discussion
The EEG measures described for the newborn reflect complex processes related to cerebral and subcortical maturation, state, and stimulus-related activity. Subcortical inputs from brainstem and thalamus provide an essential source of neural activity to the developing neocortex. Preterm delivery disrupts specific aspects of cerebral development, such as the thalamocortical system20,21 and is correlated to EEG progression. The fetal subplate zone is the origin of thalamocortical and corticocortical afferents and probably contributes to EEG activity directly and indirectly via its cortical connections.22 Diffusion tensor MRI reveals that connections between the thalamus and the frontal cortices, supplementary motor areas, occipital lobe and temporal gyri are significantly diminished in preterm infants (mean GA 283/7 weeks scanned at term-equivalent age).23
The patterned pneumatic orocutaneous stimulation used in the present study achieves salience presumably due to the synchronous activation of large populations of primary trigeminal orocutaneous afferents in the preterm infant. These mechanosensitive afferents project along the trigeminal lemniscus to the ventroposteromedial nucleus of the thalamus, and onto corresponding thalamocortical pathways to facilitate development and stabilization of ororhythmic pattern-generating circuits in the preterm brain. The formation of precise neural connections is thought to involve two distinct mechanisms: those that are activity-independent and those that require neuronal activity.7 Activity-independent mechanisms occur early in fetal life and involve ‘molecular sensing' for axon outgrowth, pathfinding, and target selection. In contrast, the refinement of initially diffuse connections within targets almost always requires neuronal activity. For the orofacial system, this process of refinement spans a protracted period of development that begins in utero around 7.5 weeks PMA as evidenced by reflex sensitivity to touch stimulation24 which shows local sign during infancy and childhood.25 Evoked neural activity affords the postnatal organism a mechanism for adaptation such that experience itself drives brain maturation. The development and stability of synaptic connections in the nervous system are influenced by the pattern of electrical activity and competitive interaction between adjacent nerve terminals. Activity-dependent neuronal selection is essential for normal development, and conceivably could be utilized as a neurotherapeutic intervention to assist preterm infants at risk for neurodevelopmental sequelae.
Results from the present study have shown numerous short- and longer-term effects of orocutaneous stimulation on aEEG amplitude margins and rEEG amplitude bands. Short-term changes in the aEEG and rEEG were found during the 3-minute stimulation periods, while longer-term changes were noted by the persistence or ‘after-effects’ in the EEG waveform in the minutes following stimulus cessation. This is indirect evidence of adaptation or plasticity among thalamocortical circuits. The 3-minute pulsed orocutaneous stimulation epochs significantly enhanced band C (25–50 µV) rEEG activity while suppressing higher voltage band E (> 100 µV) rEEG activity. In fact, preterm infants exposed to the pulsed orocutaneous stimulation yielded a greater proportion of band C activity throughout the 23-minute recording period that followed the first stimulus block when compared to infants in the sham pacifier condition. O’Reilly and colleagues14 reported that the percentages of the high voltage band (band E), and low voltage band (band A), decreased with advancing PMA, while the percentage of the middle band voltage (band C) increased. These changes were correlated with the increase in the lower margin and decrease in the upper margin of the rEEG, and bandwidth narrowing. The distribution of the rEEG values becomes less variable and concentrates around band C (25–50 µV), a characteristic of the maturing infant. Thus, the low-dose pulsed orocutaneous stimulation as used in the present study appears to promote a more ‘mature’ state of electrocortical dynamics in preterm infants which also persists after the somatosensory stimulus is removed.
The significant asymmetry in aEEG margin amplitudes among preterm infants recorded at 32 weeks PMA reported in the present study is consistent with a number of studies documenting anatomic and functional hemispheric asymmetry from the fetal period through infancy and into childhood.26,27 Post-mortem studies have shown that some cortical gyri, including the superior frontal gyrus, the superior temporal gyrus and Heschl’s gyrus appear in the right hemisphere 1 or 2 weeks earlier than in the left,28 and a recent neuroimaging study in preterm newborns has revealed a right temporal sulcus larger than the left.29 By contrast, the planum temporale and Heschl’s gyrus are larger on the left side in fetuses and infants.28 Gray and white matter volumes in neonates are larger in the left hemisphere which is opposite in adults.30 Using magnetic resonance imaging (MRI) and quantitative tools to measure cortical folding and development of the in vivo neonatal brain, Dubois et al.29 discovered an early rightward morphological asymmetry during the 3rd trimester of gestational life. Hemispheric asymmetry in the progression of myelination was observed in infants (age 3 to 11 months)31 with myelination occurring earlier in the left compared to the right cerebral hemisphere. However, the pattern of myelination is reversed in the cerebellum. Using diffusion tensor imaging and spatial localization methods, Dubois and colleagues27 demonstrated early leftward symmetries in the arcuate fasciculus and corticospinal tract. These results suggest that the early macroscopic geometry, microscopic organization, and maturation of these white matter bundles are correlated to functional lateralization (speech-language, handedness, etc).
Collectively, these observations raise the following intriguing question. Does somatosensory stimulation delivered to preterm infants during late gestation offer neuroprotective qualities? Extrauterine life is a pathological condition for the premature infant who must endure and adapt to dramatic changes in the sensory milieu. Certainly, the possibility that low-dose somatosensory stimulation has neuroprotective qualities seems likely given the exciting findings from a recent study in an animal model of perinatal hypoxia in which environmental enrichment stimulation was found to be highly neuroprotective.32 Movement-related afference and exogenous stimulation play an important role in brain function and psychomotor development of children, and is hypothesized to minimize the risk of developmental disorders among preterm infants. For example, massage of the chest, upper limbs, abdomen, legs, back, and face resulted in increased aEEG amplitudes and significantly increased the dominant frequency of δ, α, θ, and β waves in the EEG.33 Recently, the role of individualized newborn developmental care (gentle approach to care, light dimming, rest periods, flexed position with appropriate support, and skin-to-skin contact), was examined for its effect on neurobehavioral, electrophysiological and neurostructural development of 30 preterm infants with severe intrauterine growth restriction (IUGR) randomized to control and experimental care.19 Experimental infants were healthier, showed significantly improved brain development (i.e., more cortical gray matter) and better neuronal tract development in the internal capsule, corpus callosum, and occipital lobe. The positive diffusion MRI anatomical findings were consistent with enhanced association cortex connectivities as reflected in EEG coherence analyses, and better neurobehavioral outcomes.
The present study illustrates how neonatology practitioners can apply a new functional somatosensory stimulation regimen with aEEG and rEEG for monitoring electrocortical activity and brain maturation in the NICU. Several features of this approach are parsimonious with this form of brain monitoring in preterm infants. First, human infants are precocial for trigeminal orofacial cutaneous sensitivity34,35 to support sucking, feeding, airway protection, and state control. Second, the high innervation density and representation of rapidly conducting mechanoreceptors in the lips, tongue, oral mucosa, and mandible are associated with high cortical magnification factors which is defined as the ratio between the area of representation in the primary somatosensory cortex (S1) to the area of the skin.36 Serendipitously, the dual-channel EEG recording montage used routinely by many NICU’s world-wide is situated over the infant’s lateral cerebral convexity (e.g., proximal to the face cortex) to sample brain activity correlated with trigeminal mechanosensory events. Third, the pneumatic orocutaneous stimulation system used in the present study delivers a midline input to the infant’s mouth and anterior tongue, two highly sensitive cutaneous surfaces,37 rivaled only by the glabrous skin of the finger tips.
Application of patterned, low-dose pneumatic orocutaneous stimulation to the mouth of the preterm infant at around 32 weeks PMA achieves synchronous activation of trigeminal mechanosensitive afferents which drive thalamocortical afferents to modulate the activity of the orofacial sensorimotor cortex. Multiple significant effects were observed in the 2-channel electroencephalogram, including modulation of the upper and lower margins of the aEEG, and a robust reorganization of rEEG with shifts in the proportion of voltages from amplitude bands D and E to band C. Cortical asymmetry also was apparent in both aEEG and rEEG amplitude measures. This is the first study to map the effects of a highly controlled oral somatosensory input on the amplitude features of electrocortical activity in preterm infants. Future longitudinal studies will focus on the relation between low-dose somatosensory stimulation, electrocortical activation and brain maturation, and its neuroprotective qualities over an extended sampling period in the NICU.
Supplementary Material
Acknowledgments
Implementation of this program would not have been possible without extreme dedication of SCVMC NICU staff and families, Santa Clara County First Five and Valley Medical Center Foundation. This study was supported in part by grants NIH R01 DC003311 (SM Barlow), NIH P30 HD02528, and the Sutherland Family Foundation.
Footnotes
Financial Disclosure
None of the authors have a direct financial relation with the manufacturers of the Soothie pacifier, Brainz EEG monitor and Analyze software, nor with the SAS statistical software. Dr. Barlow is the inventor of the NTrainer System which is registered and licensed by the University of Kansas to Innara Health, Incorporated (Olathe, Kansas USA).
References
- 1.Fucile S, Gisel EG, McFarland DH, Lau C. Oral and non-oral sensorimotor interventions enhance oral feeding performance in preterm infants. Dev Med Child Neurol. 2011;53:829–835. doi: 10.1111/j.1469-8749.2011.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha AD, Moreira MEL, Pimenta HP, Ramos JRM, Lucena SL. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birth weight infant. Early Hum Dev. 2007;83:385–388. doi: 10.1016/j.earlhumdev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SM, Finan DS, Chu S, Lee J. Patterns for the premature brain: Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatol. 2008;28:541–548. doi: 10.1038/jp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow SM, Lee J, Wang J, Oder A, Hall S, Knox K, Weatherstone K, Thompson D. Frequency-modulated orocutaneous stimulation promotes non-nutritive suck development in preterm infants with respiratory distress syndrome or chronic lung disease, and preterm infants of diabetic mothers. J Perinatology. doi: 10.1038/jp.2013.149. acceptable pending revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingham P, Ashikaga T, Abbasi S. Prospective study of non-nutritive sucking and feeding skills in premature infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F194–F200. doi: 10.1136/adc.2009.164186. [DOI] [PubMed] [Google Scholar]
- 6.Pinelli J, Symington AJ. The Cochrane Collaboration. Issue 6. John Wiley & Sons, Ltd. The Cochrane Library; 2010. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants; pp. 1–34. 2010. [Google Scholar]
- 7.Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr Res. 1999;45:447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Griesmaier E, Enot DP, Bachmann M, Neubauer V, Hellström-Westas L, Kiechl-Kohlendorfer U, Keller M. Systematic characterization of amplitude-integrated EEG signals for monitoring the preterm brain. Pediatr Res. 2013;73:226–235. doi: 10.1038/pr.2012.171. [DOI] [PubMed] [Google Scholar]
- 9.Maynard DE. EEG analysis using an analogue frequency analyser and a digital computer. Electroencephalogr Clin Neurophysiol. 1967;23:487. [PubMed] [Google Scholar]
- 10.Hellström-Westas L, de Vries LS, Rosén I. Atlas of Amplitude-Integrated EEGs in the Newborn. 2nd edition. United Kingdom: Informa Healthcare; 2008. pp. 1–187. [Google Scholar]
- 11.Hellström-Westas L, Rosén I, Svenningsen NW. Cerebral function monitoring during the first week of life in extremely small low birthweight (ESLBW) infants. Neuropediatrics. 1991;22:27–32. doi: 10.1055/s-2008-1071411. [DOI] [PubMed] [Google Scholar]
- 12.Niemarkt HJ, Andriessen P, Peters CHL, Pasman JW, Blanco CE, Zimmerman LJ, Bambang Oetomor S. Quantitative analysis of amplitude-integrated electroencephalogram patterns in stable preterm infants, with normal neurological development at one year. Neonatology. 2010;97:175–182. doi: 10.1159/000252969. [DOI] [PubMed] [Google Scholar]
- 13.Olischar M, Klebermass K, Kuhle S, Hulek M, Kohlhauser C, Rücklinger E, Pollak A, Weninger M. Reference values for amplitude integrated electroencephalographic activity in preterm infants younger than 30 weeks’ gestational age. Pediatrics. 2004;113:e61–e66. doi: 10.1542/peds.113.1.e61. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly D, Navakatikyan MA, Filip M, Greene D, Van Marter LJ. Peak-to-peak amplitude in neonatal brain monitoring of premature infants. Clinical Neurophysiol. 2012;123:2139–2153. doi: 10.1016/j.clinph.2012.02.087. [DOI] [PubMed] [Google Scholar]
- 15.Zhang D, Liu Y, Hou X, Shou C, Luo Y, Ye D, Ding H. Reference values for amplitude-integrated EEGs in infants from preterm to 3.5 months of age. Pediatrics. 2011:e1280–e1287. doi: 10.1542/peds.2010-2833. [DOI] [PubMed] [Google Scholar]
- 16.Sisman J, Campbell DE, Brion LP. Amplitude-integrated EEG in preterm infants: maturation of background pattern and amplitude voltage with postmenstrual age and gestational age. J Perinatol. 2005;25:391–396. doi: 10.1038/sj.jp.7211291. [DOI] [PubMed] [Google Scholar]
- 17.Curzi-Dascalova L, Figueroa JM, Eiselt M, Christova E, Virassamy A, d'Allest AM, Guimarâes H, Gaultier C, Dehan M. Sleep state organization in premature infants of less than 35 weeks’ gestational age. Pediatr Res. 1993;34:624–628. doi: 10.1203/00006450-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Wikström S, Pupp IH, Rosén I, Norman E, Fellman V, Ley D, Hellström-Westas L. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr. 2012;101:719–726. doi: 10.1111/j.1651-2227.2012.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Als H, Duffy FH, McAnulty G, Butler SC, Lightbody L, Kosta S, Weisenfeld NI, Robertson R, Parad RB, Ringer SA, Blickman JG, Zurakowski D, Warfield SK. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol. 2012;32:797–803. doi: 10.1038/jp.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. The effect of preterm birth on thalamic and cortical development. Cortex. 2012;22:1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostovic I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatrica. 2010;99:e1119–e1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 22.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2012 doi: 10.1016/j.cortex.2012.07.006. http://dx.doi.org/10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald GE, Windle WF. Some observations on early human fetal movements. J Comp Neurol. 1942;76:159–167. [Google Scholar]
- 25.Barlow SM, Finan D, Bradford PT, Andreatta R. Transitional properties of the mechanically evoked perioral reflex from infancy through adulthood. Brain Res. 1993;623:181–188. doi: 10.1016/0006-8993(93)91425-r. [DOI] [PubMed] [Google Scholar]
- 26.Mento G, Suppiej A, Altoè G, Bisiacchi PS. Functional hemispheric asymmetries in humans: electrophysiological evidence from preterm infants. Eur J Neurosci. 2010;31:565–574. doi: 10.1111/j.1460-9568.2010.07076.x. [DOI] [PubMed] [Google Scholar]
- 27.Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19:414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- 28.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 29.Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Hüppi PS. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2007;18:1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Guido G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SCR, Murphy DGM. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmaso N, Silbereis J, Komitaova M, Mitchell P, Chapman K, Ment LR, Schwartz ML, Vaccarino FM. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J Neurosci. 2012;32(26):8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudnicki J, Boberski M, Butrymowicz E, Niedbalski P, Ogniewski P, Niedbalski M, Niedbalski Z, Podraza W, Podraza H. Recording of amplitude-integrated electroencephalography, oxygen saturation, pulse rate, and cerebral blood flow during massage of premature infants. Am J Perinatol. 2012 doi: 10.1055/s-0032-1310529. DOI http://dxdoi.org/10.1055/s-0032-1310529. [DOI] [PubMed] [Google Scholar]
- 34.Barlow SM, Dusick A, Finan DS, Coltart S, Biswas A. Mechanically evoked perioral reflexes in premature and term human infants. Brain Res. 2001;899:251–254. doi: 10.1016/s0006-8993(01)02239-9. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey T. Some correlations between the appearance of human fetal reflexes and the development of the nervous system, growth, and maturation of the brain. Prog Brain Res. 1964;4:93–135. [Google Scholar]
- 36.Toda T, Taoka M. Converging patterns of inputs from oral structures in the postcentral somatosensory cortex of conscious macaque monkeys. Exp Brain Res. 2004;58:43–49. doi: 10.1007/s00221-004-1869-2. [DOI] [PubMed] [Google Scholar]
- 37.Barlow SM. Mechanical frequency detection thresholds in the human face. Exp Neurology. 1987;96:253–261. doi: 10.1016/0014-4886(87)90044-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



