Abstract
Toll-like receptors (TLRs) are fundamental sensor molecules of the host innate immune system, which detect conserved molecular signatures of a wide range of microbial pathogens and initiate innate immune responses via distinct signaling pathways. Various TLRs are implicated in the early interplay of host cells with invading viruses, which regulates viral replication and/or host responses, ultimately impacting on viral pathogenesis. To survive the host innate defense mechanisms, many viruses have developed strategies to evade or counteract signaling through the TLR pathways, creating an advantageous environment for their propagation. Here we review the current knowledge of the roles TLRs play in antiviral innate immune responses, discuss examples of TLR-mediated viral recognition, and describe strategies used by viruses to antagonize the host antiviral innate immune responses.
Abbreviations: TLR, Toll-like receptor; PRR, pattern recognition receptor; PAMP, pathogen-associated molecular pattern; RLR, RIG-I-like receptor; dsRNA, double-stranded RNA; ssRNA, single-stranded RNA; MyD88, myeloid differentiation primary response 88; VSV, vesicular stomatitis virus; IRF, IFN regulatory factor; NF-κB, nuclear factor-kappa B; pDC, plasmacytoid dendritic cell; IKK, IκB kinase; HCV, hepatitis C virus; DC, dendritic cell; MCMV, mouse cytomegalovirus; HSV, herpes simplex virus; CNS, central nervous system; EMCV, encephalomyocarditis virus; HIV, human immunodeficiency virus; WNV, West Nile virus; MMTV, mouse mammary tumor virus; KSHV, Kaposi's sarcoma-associated herpesvirus; ssDNA, single-stranded DNA; AAV, adeno-associated virus; EV71, enterovirus 71; HAV, hepatitis A virus; FMDV, foot-and-mouth disease virus
Keywords: virus, interferon, cytokine, interferon regulatory factor, nuclear factor-kappa B
Graphical abstract
Highlights
-
•
TLRs are membrane-bound sensors that activate innate immune responses to viruses.
-
•
TLRs recognize viral proteins on cell surface or viral nucleic acids in endosomes.
-
•
TLRs employ distinct pathways to induce interferon (IFN) antiviral and/or inflammatory responses.
-
•
Viruses have evolved elaborate tactics to circumvent TLR-mediated innate immunity.
-
•
TLRs regulate viral pathogenesis and are amenable to therapeutic purposes.
Introduction
As a mechanism of intrinsic defense against invading pathogens, the host innate immune system is equipped with germline-encoded pattern recognition receptors (PRRs). These evolutionary conserved receptors are fundamental in the recognition of microbial pathogens including bacteria, fungi, viruses, and parasites. They distinguish host components from pathogens by detecting moieties that are conserved within a class of pathogens known as pathogen-associated molecular patterns (PAMPs) and initiate signaling cascades that culminate in the expression of antimicrobial products and inflammatory cytokines and chemokines [1], [2], [3]. These antigen-independent innate immune responses act within hours and are at the frontline of the battle against invaders. No less important, they help instruct the development of a more time-consuming, antigen-specific adaptive immunity that often is pivotal for pathogen clearance and long-term immune memory.
Although vertebrate hosts encode several other classes of PRRs such as the RIG-I-like receptors (RLRs), NOD-like receptors, C-type lectin receptors, and sequestosome 1/p62-like receptors [4], [5], Toll-like receptors (TLRs) were the first to be identified and have been most thoroughly studied. First acknowledged in Drosophila, the Toll receptor was shown to be important for host defense against fungal infection [6]. Subsequently, 10 human (TLR1–TLR10) and 12 mouse (TLR1–TLR9 and TLR11–TLR13) homologs to the Toll receptor in Drosophila were characterized hence named TLRs [2], [7]. All of the TLRs are type I transmembrane proteins that are composed of an amino-terminal leucine-rich repeat-containing ectodomain responsible for PAMP recognition, a transmembrane domain, and a cytoplasmic carboxy-terminal Toll-interleukin-1 receptor (IL-1R) homology (TIR) domain that activates downstream signal transduction [8], [9]. Based on sequence homology, the vertebrate TLRs are classified into six major families, that is, TLR1, TLR3, TLR4, TLR5, TLR7, and TLR11 [10]. The TLR1 family encompasses TLR1, TLR2, TLR6, and TLR10. These reside on plasma membranes and recognize components of microbial cell walls and membranes such as lipoproteins and peptidoglycans. They function as a heterodimeric receptor, with TLR2 paired with one of the rest of the TLR1 family members. TLR4 and TLR5 also localize to plasma membrane and engage bacterial lipopolysaccharide (LPS) and flagellin, respectively [2]. On the contrary, members of the TLR3, TLR7, and TLR11 families are intracellular TLRs expressed in endosomes and lysosomes. Initially localizing to the endoplasmic reticulum after their synthesis, these TLRs depend on UNC93B1, a polytopic membrane protein for transport to endolysosomal compartments where they are processed by proteases to become functional receptors [11]. TLR3 recognizes double-stranded RNA (dsRNA) [12]. TLR7, TLR8, and TLR9 make up the TLR7 family, with TLR7 and TLR8 detecting single-stranded RNA (ssRNA) while TLR9 engaging unmethylated CpG DNA [2]. In the TLR11 family, TLR11 and TLR12 operate as a heterodimer for sensing profilin from the parasite Toxoplasma gondii [13], while TLR13 detects bacterial 23S ribosomal RNA [14], [15].
The TLRs differ in their expression among different cell types. Their signal transduction pathways also vary, being either myeloid differentiation primary response 88 (MyD88) dependent or TIR-domain-containing adaptor inducing interferon (IFN)-β (TRIF, also known as TICAM1) dependent based on adaptor usage [16], [17]. The MyD88-dependent pathway is activated by all TLRs except TLR3, which only signals through TRIF [18]. Interestingly, TLR4 activates both MyD88-dependent and TRIF-dependent pathways [17], [18], [19].
Of the TLRs characterized to date, several have been linked to antiviral immunity. Among these, TLR3, TLR7, TLR8, and TLR9 detect distinct forms of viral nucleic acids and are critical in the recognition of viral genetic materials in endolysosomal compartments and initiate antiviral responses. TLR2 and TLR4 are two additional TLR family members that have been implicated in the recognition of viral structural and nonstructural proteins leading to inflammatory cytokine production [20], [21], [22], [23]. There is also evidence that TLR13 may recognize viral infection such as that by vesicular stomatitis virus (VSV), although the exact PAMP sensed by TLR13 in this case remains unknown [24]. In this review, we summarize recent advances in the roles of TLRs and their pathways in innate antiviral immunity. We discuss examples of TLR-mediated viral recognition and describe strategies evolved by viruses to circumvent host antiviral innate immune responses triggered by TLRs.
Overview of Innate Immune Responses to Viruses and Their Induction Pathways Downstream of the TLRs
In response to viral infection, the host rapidly launches an innate immune response characterized by the production of IFNs and inflammatory cytokines and chemokines in efforts to prevent virus replication and eliminate the invader. IFNs act in a paracrine/autocrine fashion to activate the JAK-STAT pathways, upregulating the transcription of hundreds of IFN-stimulated genes, many of which possess broad antiviral activities. This reins in viral multiplication in infected cells and establishes an antiviral state in uninfected neighboring cells. In addition, IFNs activate various innate immune cells and immune effector cells, facilitating the development of adaptive immune responses [25]. Although most do not have direct antiviral effects, inflammatory cytokines and chemokines orchestrate the maturation of innate and adaptive immune cells and play a key role in their recruitment to the site of infection. Based on receptor usage, IFNs are classified into three types, type I (IFN-β and IFN-α), type II (IFN-γ), and type III [IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B)] IFNs [25]. Gene knockout studies in mice have established a predominant role for type I IFNs in protecting against many different viruses in vivo [26]. They have also revealed that type III IFNs play a crucial part in antiviral defenses of epithelial surface of respiratory and gastrointestinal tracts [27], [28]. Infants with homozygous mutations in the STAT1 allele producing a STAT1 deficiency died of a lethal virus-induced disease, illustrating the importance of the IFN system in control of viral infections in humans [29].
The initial engagement of TLRs and other classes of PRRs with viral PAMPs such as viral nucleic acids and viral proteins triggers the activation of distinct intracellular signaling pathways that are essential for the induction of the IFN antiviral and inflammatory cytokine responses [30]. The induction of these antiviral responses shares overlapping regulatory mechanisms and is dependent upon coordinated activation of the latent cytosolic transcriptional factors such as the IFN regulatory factors (IRFs), mainly IRF3 and IRF7, and nuclear factor-kappa B (NF-κB). Among these, NF-κB and IRF3 are constitutively expressed. In contrast, IRF7 expression is initially weak except in plasmacytoid dendritic cells (pDCs) but enhanced significantly upon stimulation by viruses or other stimuli such as type I IFNs. In resting cells, NF-κB is sequestered in the cytoplasm by a member of the IκB family of inhibitory proteins. Upon a variety of stimuli, such as viruses, TNFα, and IL-1β, the classical IκB kinase (IKK) complex composed of IKKα, IKKβ, and IKKγ (also known as NEMO) is activated, which in turn phosphorylates IκB, leading to IκB polyubiquitination and proteasomal degradation. As a result, NF-κB is liberated and migrates into the nucleus whereby it binds and activates target gene promoters such as those of IFN-β and numerous cytokines [31], [32]. Although NF-κB contributes to IFN induction, its main role is in the induction of proinflammatory cytokines. Viral activation of the IRFs, however, requires specific phosphorylations within their C-terminal parts by the IKK-related kinases, TBK1 or IKKε, in a complex containing IKKγ and TANK [33], [34], [35], [36]. This results in homodimerization or heterodimerization of IRF3 and/or IRF7 and subsequent nuclear translocation to bind to type I and type III IFN promoters. In general, IFN-β and IFN-λ1 promoters are predominantly activated by IRF3, while transcription of the IFN-α and IFN-λ2/3 relies more upon IRF7 [37], [38], [39]. Collectively, the IRFs and NF-κB transcription factors are major regulators of antiviral and inflammatory gene expression.
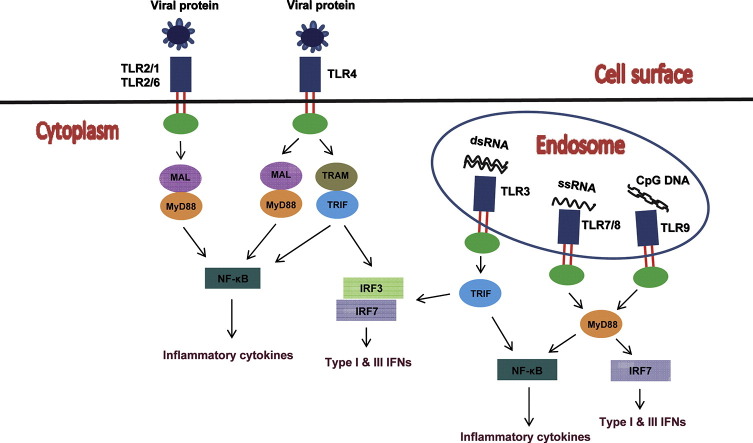
Upstream, several TLR signaling pathways mediate cell-type-specific regulation of IFN and/or inflammatory cytokine production in response to viral infections through activation of these essential transcription factors (Fig. 1 ). These include pathways initiated via TLR3, TLR7/TLR8/TLR9, TLR4, and TLR2. The specific response following engagement of the individual TLR, however, is determined by the subcellular compartment where the signal is initiated and by the TIR-domain-containing adaptor protein that is recruited. While activation of TLR signaling in endosomes can lead to either IFN or inflammatory cytokine induction, signaling from cell-surface-localized TLRs only results in inflammatory responses but not IFN expression. This may be explained by the proposal that TRAF3, a cytosolic adaptor essential for IFN induction via TLR and RLR signaling pathways [40], [41], does not have ready access to plasma membrane signaling complexes [42]. Based on usage of the two major TIR-domain-containing adaptors, the TLR signaling pathways are classified into MyD88-dependent and TRIF-dependent pathways. The former is used by all TLRs but TLR3, while the latter is triggered by TLR3 or TLR4. Of note, two additional TIR-domain-containing adaptors, MyD88-like (MAL, also known as TIRAP) and TRIF-related adaptor molecule (TRAM, also known as TICAM2), are required for bridging MyD88 to TLR2 and TLR4 and TRIF to TLR4, respectively. As described below, signaling through the TRIF-dependent pathways leads to the production of IFNs and inflammatory cytokines, while activation of the MyD88-dependent pathways culminates in induction of inflammatory cytokines, with the exception that, in specific immune cell types, it also results in IFN induction [2], [43], [44].
Fig. 1.
Recognition of viral PAMPs such as viral proteins, dsRNA, ssRNA, and CpG DNA, initiates an antiviral innate immune response mediated by TLRs. TLR2 and TLR4 are present on the cell surface and recognize viral proteins. TLR3, TLR7, TLR8, and TLR9 are intracellular viral nucleic-acid-sensing TLRs that are localized in endosomes. Viral dsRNA, ssRNA, and unmethylated CpG DNA are recognized by TLR3, TLR7/TLR8, and TLR9, respectively. Upon ligand recognition, TLR2 along with TLR6 or TLR1 and TLR4 recruits an additional adaptor protein, MAL, to link the TIR domain to MyD88. All TLRs except TLR3 recruit MyD88. TLR4 also recruits the adapter protein TRIF, as does TLR3. To activate the TRIF-dependent pathway, TLR4 requires the bridging adaptor TRAM and its trafficking into endosomes. The MyD88-dependent and TRIF-dependent signaling complexes through a cascade of signaling events leading to the activation of several transcription factors including NF-κB, IRF3, and IRF7. NF-κB transcriptionally regulates the expression of inflammatory cytokines and chemokines while IRF3 and IRF7 control the transcription of type I and type III IFN genes. Whereas TLR2 signaling only results in NF-κB activation in most cell types, TLR2 can traffic to endosomes in inflammatory monocytes upon engagement of specific viral ligands such as vaccinia virus or MCMV where it results in IRF3/IRF7 activation and type I IFN induction (not depicted).
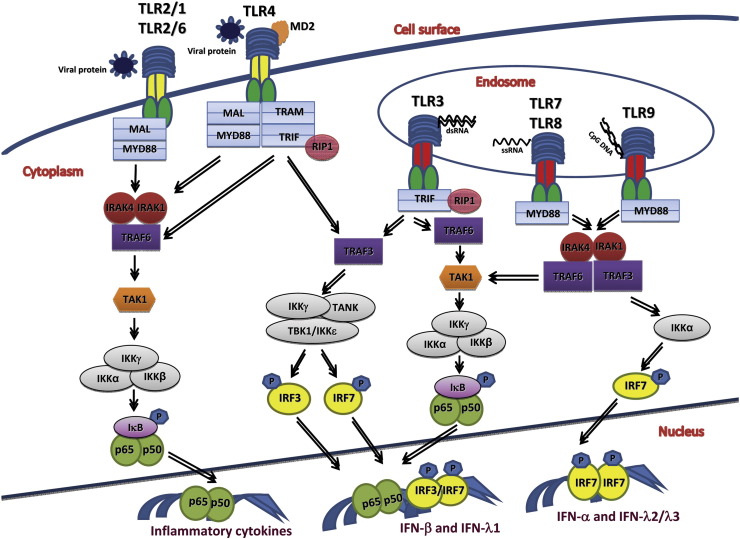
The TRIF-dependent pathway results in activation of both IRF3 and NF-κB arms of innate immunity. This pathway is engaged following stimulation by either TLR3 or TLR4 ligands. Of note, while TLR4 activates MAL-MyD88 signaling at the plasma membrane, its trafficking to endosomes is a prerequisite for activation of the TRAM-TRIF branch [42]. Although the endosomally localized TLR3 does not require the TRAM bridging adaptor for TRIF recruitment, it has a unique mandate—two tyrosine residues in the cytoplasmic domain of TLR3, Tyr858 and Tyr759, have to be phosphorylated by tyrosine kinases before the TLR3-TRIF association takes place. Of these, Tyr858 is phosphorylated by the epidermal growth factor receptor ErbB1, while Tyr759 is targeted by Bruton's tyrosine kinase and Src [45], [46]. Upon activation, TRIF associates with TRAF6 [47], [48] and RIP1 [49], activating the TAK1 kinase complex and subsequently the classical IKK complex, culminating in NF-κB activation and induction of proinflammatory cytokines and chemokines. In parallel, TRIF associates with TRAF3, relaying signaling to the noncanonical IKK kinases, TBK1 and/or IKKε in a complex containing IKKγ and TANK [33], [34], [35], [36], leading to the phosphorylation and activation of IRF3/IRF7 and the subsequent induction of type I and type III IFNs.
Engagement of the MyD88-dependent TLR pathways typically leads to production of proinflammatory cytokines and chemokines. Downstream signaling molecules such as kinases IRAK4, IRAK1, and ubiquitin ligase TRAF6 are recruited to MyD88 causing activation of a protein kinase complex consisting of TAK1. Upon activation, TAK1 phosphorylates IKKβ in the classical IKK complex ultimately resulting in the activation NF-κB and the subsequent expression of proinflammatory cytokines [2]. It should be noted that, however, in specific immune cell subsets such as the pDCs, which are known as professional IFN-producing cells, the MyD88-dependent pathways can lead to induction of copious amounts of type I IFNs upon TLR7 and TLR9 sensing of viral components in endolysosomal compartments. This requires recruitment of TRAF3 and IKKα to the MyD88–IRAK–TRAF6 complex and subsequent phosphorylation of IRF7 by IRAK1 and IKKα [50], [51]. As another example, inflammatory monocytes can recognize virion components of certain viruses such as vaccinia virus via a TLR2-MyD88-dependent pathway, resulting in expression of type I IFNs in addition to inflammatory cytokines. Although the detailed underlying mechanism remains elusive, TLR2-mediated IFN production but not TNF induction in this cell type depends on trafficking of this TLR to endosomes and on transcription factors IRF3 and IRF7 [43]. Interestingly, other IRF family members such as IRF1 and IRF8 have also been reported to participate in MyD88-dependent pathways leading to IFN induction in dendritic cell (DC) subsets [52], [53].
Roles of TLRs in Recognition of Viral PAMPs and Antiviral Immunity
As obligate intracellular parasites, viruses rely on their intimate interactions with host cells to complete their replication cycles. Not surprisingly, during viral infections, various types of viral PAMPs are sensed by different classes of host PRRs as non-self materials and trigger distinct signaling pathways culminating in induction of IFNs and/or proinflammatory cytokines. Viral nucleic acids with distinct features are recognized by different TLRs in endolysosomes and by various RLRs in cytoplasm, respectively. In addition, viral proteins released to extracellular milieu are detected by several TLRs on the plasma membrane. Since the characterization of the first human TLR about one and a half decades ago, numerous studies have been conducted to investigate the roles different TLRs play in recognizing viral PAMPs and in regulating antiviral immunity in vitro and in vivo. Some of the major findings are discussed here. It should be noted that the contribution of each TLR is different depending on the virus, cell type, and infection model examined.
Recognition of dsRNA by TLR3
As the first characterized nucleic-acid-sensing TLR, TLR3 recognizes dsRNA [12], a molecular signature of most viruses, either because it constitutes their genome or because it is generated as a replicative intermediate during their life cycle. As such, TLR3 has been shown to sense infections by dsRNA viruses, ssRNA viruses, and DNA viruses [2]. In addition, the TLR3 signaling pathway is stimulated by a synthetic dsRNA analog, polyriboinosinic:polyribocytidylic acid (poly I:C) [12]. Engagement of TLR3 with synthetic or viral dsRNAs activates the TRIF-dependent pathway, culminating in the induction of proinflammatory cytokines, chemokines, and type I and type III IFNs mediated by NF-κB and IRF3 activation [12], [54], [55], [56], [57], [58]. in vitro binding assays have revealed that the smallest dsRNA oligonucleotides capable of binding to a dimeric unit of TLR3 ectodomain are 40–50 bp long. However, only dsRNA ligands of 90 bp or longer activate TLR3 signaling in endosomes [59], the typical intracellular compartment where TLR3 resides in most cell types. Consistent with this, it has been found that dsRNA replicative intermediates of hepatitis C virus (HCV), a hepatotropic flavivirus, are required to have a minimal length of 80–100 bp to activate TLR3-dependent chemokine expression in hepatocytes [60].
TLR3 has a relatively wide tissue distribution, with its transcripts detected in many human and mouse tissues such as the placenta, lung, liver, heart, lymph node, spleen, and brain [12], [61], [62], [63], [64]. TLR3 protein is expressed by key sentinel cells of the innate immune system such as conventional dendritic cells and macrophages and non-immune cells including epithelial cells, natural killer cells, fibroblasts, astrocytes, hepatocytes, and endothelial cells [56], [57], [61], [63], [65], [66]. In contrast, TLR3 is absent from pDCs where TLR7 and TLR9 are present in high quantities. Likewise, TLR3 expression is absent in neutrophils and minimally expressed in T cells [67], [68]. This suggests that the versatility of antiviral immune responses may be mediated at least in part by the differential expressions of TLRs on specific cell types. With the exception that, in fibroblasts and some epithelial cells, it can be expressed on cell surface, TLR3 is localized in endosomes in most cell types [69] and signaling through this TLR is dependent on vaculolar acidification [60], [66], [69]. Unlike other intracellular TLRs whose localization is dictated by the transmembrane domain, TLR3 position in intracellular endosomes is dependent on a cytoplasmic linker region between the TIR domain and the transmembrane domain [70]. Interestingly, it has also been observed that TLR3 preferentially resides near phagosomes containing apoptotic cell particles, raising the possibility that the fusion of phagosomes with nearby endosomes may enable TLR3 recognition of dsRNA from apoptotic cells, such as those infected by viruses [70]. This mechanism could explain TLR3-mediated cross-priming of cytotoxic T lymphocytes by murine CD8alpha + DCs against viruses that do not directly infect these DCs [71]. Thus, in specific subsets of DCs, the role of TLR3 appears to shift from regulating proinflammatory and IFN antiviral responses to mediating the adaptive immune responses.
TLR3 has been demonstrated to serve as an essential PRR that detects and fends off some invading viral pathogens. However, the complexities of TLR3 signaling lies in the fact that TLR3's functional role can either mediate the establishment of an antiviral state in efforts to prevent virus replication and mitigate disease severity or facilitate an excessive and unregulated immune response to the infection that can be harmful to the host and may contribute to the severity of the disease. The importance of TLR3 in mediating the antiviral host defense has been exemplified in a number of in vivo and in vitro studies. Mice lacking TLR3 or bearing a lethal mutation in TRIF are hypersusceptible to mouse cytomegalovirus (MCMV), demonstrating a 1000-fold increase in spleen viral titers concomitant with diminished IFN levels in serum, when compared to wild-type mice [72], [73]. This hypersusceptible phenotype caused by TLR3 deficiency is also seen in mice infected by another DNA virus, herpes simplex virus (HSV) 2, with TLR3 knockout mice developing a more pronounced disease in the central nervous system (CNS) than wild-type mice or mice deficient in other TLRs [74]. In the absence of TLR3, astrocytes were unable to sense HSV-2 infection and produce IFN-β immediately after entry of the virus into the CNS, resulting in uncontrolled viral replication and spread. A protective role of TLR3 against HSV-1 has also been suggested in humans. Children born with deficiencies in the TLR3 pathway have a predisposition for HSV-1-induced encephalitis, a severe viral infection of the CNS [75], [76], [77], [78]. In addition, TLR3 has been found to mediate protection against various RNA virus infections. Following poliovirus infection, serum IFN production was abolished in TLR3 and TRIF knockout mice, and both the viral load in non-neural tissues and mortality rates were strikingly higher than those in wild-type mice [79]. TLR3 signaling also lessens the virulence of encephalomyocarditis virus (EMCV) and mediates the protection of the heart during virally induced injury [80]. In comparison to wild-type mice, TLR3-deficient mice were more susceptible to EMCV infection, having higher viral loads in the heart and liver, and impaired cytokine and chemokine responses in the heart were observed [80]. These mice also succumb to EMCV infection earlier than the wild-type mice [80]. Similar findings have been reported in TLR3 knockout mice infected with Coxsackievirus B3 or B4 [81], [82]. In a mouse model orally infected with rotavirus, a dsRNA virus responsible for viral diarrhea worldwide, it was shown that TLR3-mediated innate immunity contributed to restricting the virus replication in adult but not neonate animals. Specifically, TLR3 or TRIF deficiency in adult mice resulted in significantly increased viral shedding and decreased induction of antiviral and proinflammatory genes in intestinal epithelial cells [83]. The contribution of TLR3 to antiviral immunity has also been demonstrated in in vitro settings. TLR3 is capable of sensing HCV and dengue virus in cell culture, initiating an IFN response that restricts the replication of these flaviviruses [63], [84]. Knockdown of TLR3 in A549 cells abrogated pathogenic Hantaan virus-induced expression of MxA, a well-established marker for IFN production, concomitant with increased viral replication [85]. Furthermore, initiating antiviral immune responses with TLR3 agonists has been shown to provide protection from many different viruses including hepatitis B virus, influenza virus, certain human immunodeficiency virus (HIV) strains, and coronaviruses [86], [87], [88], [89], [90].
Although these aforementioned studies illustrate that TLR3-mediated antiviral response is beneficial in protecting the host against viruses, the activation of TLR3 signaling has also been observed to be deleterious in some other viral infections. Following the Phlebovirus Punta Toro virus infection, TLR3 knockout mice showed increased resistance to lethal infection and had a reduced liver disease associated with Punta Toro virus infection compared to wild-type mice. Evidence suggested that the phenotype was due to higher levels of inflammatory responses observed in wild-type mice than TLR3 null mice, signifying that an unregulated inflammatory response may mediate much of the damage demonstrated [91]. A detrimental role for TLR3-mediated inflammatory responses in influenza virus-induced acute pneumonia has also been reported. Despite harboring higher levels of viral replication in the lungs, TLR3 knockout mice survived influenza A virus infection better than wild-type mice because they produced significantly reduced levels of inflammatory mediators and had a lower frequency of CD8 + T cells in the bronchoalveolar airspace [92]. Likewise, TLR3-deficient mice infected with the DNA vaccinia virus exhibited lessen severity of disease and decreased mortality, concomitant with production of lower levels of inflammatory cytokines in serum and bronchoalveolar lavage fluid [93]. Furthermore, TLR3-mediated inflammatory responses facilitated West Nile virus (WNV) entry into CNS via destruction of the blood brain barrier resulting in lethal encephalitis [94]. However, the role of TLR3 in WNV infection appears to differ depending on infection model, being protective in another study [95]. Overall, TLR3-induced antiviral responses to viruses may be beneficial or harmful to the host. However, the host defense system is unique in that it has evolved to equip with other TLRs or other classes of PRRs that act in concert in mediating antiviral responses [96].
Recognition of viral ssRNA by TLR7/TLR8
Both TLR7 and TLR8 are members of the intracellular TLRs that primarily recognize viruses that enter the endosome through endocytosis [97], [98], [99]. Like TLR3 and TLR9 (see below), recognition of viral ligands in the endosome by TLR7/TLR8 is dependent on acidification of the endosomal vesicles [99]. Through this pathway, TLR7 and TLR8 are able to detect GU-rich and AU-rich ssRNA sequences of RNA viruses [97], [99], [100]. Upon engagement of ssRNAs in endosomes, these antiviral sensors initiate the MyD88-dependent pathway, culminating in synthesis of type I and type III IFNs and proinflammatory mediators via activation of IRF7 and NF-κB, respectively, depending on the cell type [101], [102], [103]. Based on evolutionary similarities, it has been understood that TLR9 forms a subfamily with TLR7 and TLR8, now known as the TLR7 subfamily [10], [104], [105]. However, because both TLR7 and TLR8 respond to single-stranded viral nucleic acids, they are commonly linked together; however, there are functional differences between the two [106], [107].
In humans and mice, TLR7 signaling is well known and critical for type I IFN production by pDCs in response to ssRNA viral stimulus [97]. The basis of this regulation lies in the fact that, like TLR9 (see below), TLR7 is predominately expressed in these specialized cell types, and to some extent, expression is detected in other immune cell types, such as B cells and monocytes/macrophages [44], [61], [108], [109], [110], [111]. On the other hand, TLR8 is known to primarily be expressed in monocytes/macrophages and myeloid dendritic cells [61], [110], [112]. In these cell types, TLR8 signaling mainly results in NF-κB activation and subsequent inflammatory cytokine expression [44]. Until recently, TLR8-mediated antiviral responses were only acknowledged in humans and, generally, murine TLR8 was thought to be incapable of mediating responses to TLR7/TLR8 agonist or TLR8 ligands [107], [113]. However, a recent report described murine TLR8 activation in response to viral stimuli [114]. Nevertheless, further studies are necessary to further clarify the functionality of mouse TLR8 in mediating the antiviral response.
Shortly after TLR7 and TLR8 were described as novel members of the TLR family, it was shown that both receptors respond to imidazoquinolines, low-molecular-weight synthetic compounds with potent antiviral activity [102], [108]. These synthetic molecules have been shown to mediate the TLR7 and TLR8 inflammatory responses differentially [106]. In human peripheral blood mononuclear cells, TLR7 and TLR8 responded to various imidazoquinoline molecules by producing different degrees of proinflammatory and IFN-induced cytokines responses [115]. Since then the roles TLR7 and TLR8 play in antiviral immunity have been characterized, they are now known to serve as endosomal PRRs for a number of ssRNA viruses including influenza, HIV-1, VSV, Sendai virus, Coxsackie B virus, coronaviruses (mouse hepatitis virus and severe acute respiratory syndrome coronavirus), and flaviviruses (HCV, dengue virus, and WNV) [97], [100], [116], [117], [118], [119], [120], [121], [122], [123]. While the canonical antiviral mechanism of TLR7 signaling involves IFN induction in pDCs, as demonstrated in studies conducted in mice infected with mouse hepatitis virus [122], pneumonia virus of mouse [124], and so on, pDC-independent and IFN-independent immune mechanisms downstream of the TLR7-MyD88 axis have recently surfaced. In one study, it was found that TLR7-deficient or MyD88-deficient mice were more susceptible to lethal West Nile encephalitis than wild-type controls. The increased viremia and elevated brain viral burden in the TLR7 or MyD88 knockout animals were attributed to reduced IL-23 response in resident tissue macrophages, which led to compromised immune cell homing to infected target cells [121]. Another study showed that TLR7 sensed two mouse retroviruses, mouse mammary tumor virus (MMTV) and Moloney murine leukemia virus upon their cellular entry, leading to MyD88-dependent neutralizing antibody production that was crucial for viral clearance [125].
Given the pivotal role for TLR7 and pDCs in sensing RNA viruses and initiating rapid IFN responses in the host, how viral ssRNAs are delivered to endosomal compartment where it engages TLR7/TLR8 has been under intensive investigation. Three different pathways have been proposed. The first one, also known as the exogenous pathway, depends on endocytosis and protease-mediated degradation of incoming virions [97], [99]. It is known to be utilized by viruses such as influenza and VSV. However, for viruses that enter host cells by direct membrane fusion, a second pathway exists. This mechanism operates through autophagy, which transports cytoplasmic viral ssRNAs to endolysosomes. Viruses that can be sensed via this mechanism include paramyxoviruses (Sendai virus and simian virus 5), VSV, and HIV [126], [127], [128], [129]. In the studies on simian virus 5 and HIV-1, siRNA technology and/or chemical inhibitors were employed to demonstrate the dependence on the autophagy pathway for TLR7-mediated induction of IFN-α and/or pDC maturation marker expression. In addition, although studying different viruses, both studies concluded that active viral replication is dispensable for activation of the autophagy-dependent TLR7 RNA sensing pathway in pDCs [128], [129]. Interestingly, in addition to contributing to viral RNA sensing, autophagy is also an important cellular antiviral mechanism downstream of TLR8 signaling. It was observed in human macrophages that TLR8 activation by ssRNA or imidazoquinolines stimulated a vitamin D and cathelicidin microbial peptide-dependent induction of autophagy, which resulted in inhibition of HIV-1 infection [130].
Recently, a third pathway was uncovered when a group of researchers studied how HCV-infected hepatoma cells or cells bearing self-replicating HCV RNA replicons (i.e., the donor cells) placed in co-culture with pDCs triggered production of type I IFNs in pDCs [123]. This response was found to be dependent on cell-to-cell contact and mediated by transfer of viral RNA-containing exosomes from the donor cells to pDCs in contact, followed by activation of the TLR7 pathway in pDCs [131]. Remarkably, this cell-to-cell RNA transfer to activate TLR7 signaling in pDCs is not confined to HCV, as also observed when the donor cells were replicating an alphavirus (Venezuelan equine encephalitis virus) replicon [123]. In addition, this innate sensing mechanism appears to operate in HIV infection. In a recent study, it was observed that HIV-infected lymphocytes were more potent IFN inducers than cell-free HIV virions when used to stimulate pDCs. Viral RNAs transmitted via cell-to-cell contacts activated IFN production in pDCs through TLR7 [132]. This study also demonstrated that replication-defective viruses in donor cells stimulated the response in pDCs as potently as wild-type virus, suggesting that this cell-to-cell viral RNA detection mechanism, like the exogenous and autophagy-dependent pathways, also does not require active viral replication. This is remarkably similar to what is known about viral DNA detection by TLR9 (see below). Taken together, the multi-faceted mechanisms of viral RNA sensing by TLR7 in pDCs are vivid demonstrations of the complexity of the host innate immune system in detecting and combating invading viruses.
Recognition of viral DNA by TLR9
TLR9 recognizes unmethylated CpG motifs that are commonly found in bacterial and viral DNA [133], [134]. TLR9 can also be stimulated by three different classes of synthetic CpG oligodeoxynucleotides (ODNs) mediating type I IFN production and B cell activation [135], [136]. Although frequently observed in bacterial and viral DNA, in most instances, mammalian DNA is methylated, this feature is one way that TLR9 discriminates between pathogen-derived DNA and host DNA. However, the discrimination between self and foreign nucleic acids not only is dependent on recognition of distinct features but also requires the ability of TLR9 to distinctively encounter foreign nucleic acids. Like the other intracellular TLRs, TLR9 localizes within the endoplasmic reticulum, endosomes, and lysosomes [137]. The compartmentalization of TLR9 facilitates the interaction with foreign DNA and decreases the risk of contact with self DNA that could ultimately lead to the development of autoimmune diseases. In experiments relocating TLR9 to the cell surface by altering the membrane spanning domain with that of TLR4 consequently, stimulated TLR9 mediated immune responses to self DNA [138]. As in the case of TLR3, TLR7, and TLR8, endosomal acidification is also fundamental for ligand recognition and TLR9-mediated antiviral responses [139].
Although expressed on several immune cell types including macrophages, B cells, myeloid dendritic cells, and conventional dendritic cells, TLR9 along with TLR7 is highly expressed in pDCs [44], [109], [110], [111], [140], which are known as professional IFN-producing cells in humans and mice following TLR7, TLR9 ligands, and viral stimuli [44], [111]. Recognition of viral DNA by TLR9 does not require active viral replication, as UV-inactivated virions also stimulate TLR9 responses. Ligation of TLR9 recruits the MyD88-dependent pathway, leading to IRF7-mediated IFN production or proinflammatory responses such as production of TNFα, IL-6, and IL-12 via the triggering of NF-κB. These differential responses are dependent on cell type. In pDCs, they are biased toward rapid, copious production of type I IFNs [2], [44].
Studies investigating the role of TLR9-mediated antiviral responses have observed TLR9 recognition of several double-stranded DNA viruses such as MCMV, HSV-1, HSV-2, and poxviruses [73], [133], [141], [142], [143]. Mutations in the receptor domain of TLR9 abolished its ability to recognize CpG oligonucleotides following MCMV infection [73]. Also, in response to MCMV infection, pDCs derived from TLR9 knockout mice were incapable of mounting a robust IFN-α response [73]. Likewise, following MCMV infection, mice with genetic alterations in their TLR9 gene lost the ability to secrete type I IFNs, activate natural killer cells, and were highly susceptible to the infection [73]. Varicella zoster virus and cytomegalovirus trigger IFN-α production via the TLR9-dependent pathway [144], [145]. Kaposi's sarcoma-associated herpesvirus (KSHV) infects pDCs, upregulating CD83 and CD86 expression and stimulating IFN-α production in these cells through TLR9 signaling [146]. TLR9 also recognizes adenovirus genomic DNA [147]. However, cooperative signaling by TLR2 and TLR9 is essential for antiviral responses against adenoviruses and EBV [148]. This dual cooperative signaling among TLR9 and TLR2 is also seen in HSV infection [149]. In contrast, nearly complete dependence on TLR9 signaling for antiviral protection was observed in mice infected a mouse poxvirus, ectromelia virus [142], [143]. In these studies, mice deficient for TLR9 were found to be > 100-fold more susceptible to lethal challenge by ectromelia virus than wild-type control animals.
While the TLR9-dependent recognition of double-stranded DNA viruses in pDCs is well established, whether this innate sensing mechanism contributes to detecting single-stranded DNA (ssDNA) viruses is less certain. Classified in the Parvoviridae family bearing ssDNA genomes, adeno-associated viruses (AAVs) have been widely used as vectors for in vivo gene therapy. Host immune responses, however, limit the efficacy of AAV vector-mediated gene transfer. Recently, it has been shown that AAV vectors activate pDCs via the TLR9-MyD88 pathway to produce type I IFNs, which drive up both CD8 + T cell and antibody responses leading to loss of transgene expression in vivo [150]. Two rodent parvoviruses, minute virus of mice and its rat homolog H-1PV, were also found to stimulate human peripheral blood mononuclear cells to produce IFN-β and IFN-α in a TLR9-dependent fashion, presumably by acting on pDCs [151]. These studies thus have suggested a role for TLR9 in sensing parvoviruses. However, another study found that pDCs failed to elicit an IFN response following stimulation by wild-type AAV type 2 or minute virus of mice, although they responded to purified viral genomic ssDNA [152].
Recognition of viral proteins by TLR2
Although they are best known for recognizing bacterial cell wall and membrane components, TLRs present on the cell surface such as TLR2 and TLR4 have also been found to mediate innate immune responses to certain viral pathogens. However, due to their cellular location, they mainly recognize viral envelope proteins or viral proteins released into extracellular milieu, unlike the intracellular TLRs that respond to viral nucleic acids. Although assumed to have a restricted cellular expression pattern, several types of the innate immune cells display TLR2 on their plasma membrane. Examples are DCs, macrophages, monocytes, B cells, and T cells including Tregs [153], [154]. Most lymphoid tissues express TLR2; however, TLR2 is found more abundantly in peripheral blood leukocytes [153]. Other cell types expressing TLR2 are microglia, endothelial, epithelial cells, and hepatocytes [155], [156], [157], [158].
TLR2 functions as a heterodimer with one of the other TLR1 family members, such as TLR1 or TLR6 located on the plasma membrane. Like all TLRs except TLR3, ligand recognition by TLR2 recruits the signaling adaptor molecule MyD88. An additional adaptor protein MAL is required for the interaction of TLR2 to MyD88 [159], acting as a linking adaptor via its TIR domain [9], [160]. In most cell types, engagement of TLR2 activates the classical MyD88-dependent pathway that relays signal through the IRAK family of proteins, TRAF6, and TAK1, leading to activation of the IKK complex and subsequent NF-κB activation. The outcome is the induction of proinflammatory cytokines and chemokines [161]. That said, TLR2 has long considered not to be linked to IFN production. Nonetheless, TLR2 was surprisingly found to contribute to IFN-α/IFN-β production in the spleens of mice infected with MCMV [162]. Data from a recent study helped explain this seemingly contradictory finding. It was found that a specific lineage of monocytes called inflammatory monocytes are able to sense vaccine virus and MCMV via TLR2, leading to type I IFN production. Although the detailed signaling mechanism is unclear, IFN induction requires TLR2 internalization and is dependent upon the MyD88 adaptor and the IRF3 and IRF7 transcription factors [43].
TLR2 is well known to be activated by an extended repertoire of bacterial lipoproteins, as well as some viral proteins. For the latter, TLR2 has been shown to recognize the glycoproteins B and H of human cytomegalovirus [163], [164], the glycoproteins gH/gL and gB of HSV [165], the UTPase of EBV [166], the hemagglutinin protein of measles virus [20], the nsp4 of rotavirus [21], and the core and NS3 proteins of HCV [167], mediating NF-κB activation and the subsequent induction of proinflammatory cytokines. In addition, TLR2 also has been implicated in host responses to infections by vaccinia virus [43], [168], lymphocytic choriomeningitis virus [169], varicella zoster virus [170], and RSV [171], although the exact viral PAMPs for TLR2 were not identified.
Studies conducted in mice have shown that TLR2 contributes to antiviral immunity that protects the host against infections by RSV, MCMV, and vaccinia virus. TLR2, working with TLR6, mediate innate immunity against RSV infection in mice by promoting leukocytes production of inflammatory mediators. This response was found to be crucial for suppressing viral replication in the lungs [171]. TLR2 knockout mice supported significantly higher levels of MCMV replication in spleen and liver than control mice due to decreased NK cell recruitment to both organs and reduced type I IFN expression in the spleen [162]. A protective role of TLR2 in vaccinia virus infection was demonstrated by the finding that TLR2 deficiency leads to elevated viral titers in the ovary [168]. Offering an explanation to the findings of the latter two studies, Barbalat et al. recently discovered that inflammatory monocytes recognize vaccinia virus and MCMV via TLR2, leading to IRF3/IRF7-dependent type I IFN production. Importantly, these authors showed that mice depleted of inflammatory monocytes prior to vaccinia virus infection had higher titers of virus in the livers and ovaries, demonstrating the critical role of this unusual TLR2 signaling pathway in this specific immune cell type in protecting against vaccinia virus infection [43]. In contrast to the findings with these aforementioned viruses, the picture of TLR2 in protective immunity against HSV is less clear. Two studies have reported that TLR2 acts in concert with TLR9 to stimulate innate antiviral responses, thereby protecting against brain infection of HSV-1 [172] and HSV-2 [149]. In contrast, others found a detrimental role of TLR2 in HSV-1 infection, showing that TLR2 mediates a deregulated inflammatory response to the virus that contributes to lethal encephalitis [173], [174]. Differences in virus strains, infection routes, and so on used in these studies could have led to the contradictory results observed.
Recognition of viral proteins by TLR4
Initially thought to be a sensor for only bacterial components, TLR4 was the first human Toll homolog identified [175]. It was also the first TLR shown to respond to viral pathogens. This study described a TLR4-mediated induction of IL-6 in response to respiratory syncytial membrane bound fusion (F) protein [22]. Unlike TLR2, TLR4 function is not dependent on forming heterodimers with additional TLRs. Instead, interactions with MD-2, an extracellular molecule, are required for TLR4-mediated host immune responses to its major ligand LPS [176], [177]. MD-2 deficiency in mice augments susceptibility to infection and diminishes their response to LPS [176]. Furthermore, cells lacking MD-2 limited TLR4 to the Golgi apparatus, whereas in wild-type cells, TLR4 localized on the cells plasma membrane, validating the importance of MD-2 in TLR4 localization and recognition of LPS [176].
TLR4 is expressed on many cell types, for example, antigen presenting cells, endothelial cells, and thyroid cells. Similar to TLR2, TLR4 signals from the cells surface to trigger the MyD88-dependent pathway via the bridging adaptor MAL, leading to early-phase NF-κB activation. However, unlike TLR2, TLR4 can also signal through the TRIF-dependent pathway after its endocytosis and trafficking to the endosome, where it recruits TRIF through TRAM [42]. The TLR4-TRAM-TRIF branch results in late-phase NF-κB activation, as well as IRF3 activation and subsequent type I IFN expression. Of note, full induction of proinflammatory mediators via the TLR4 pathway requires activation of both MyD88- and TRIF-dependent signaling [17].
Since the first report describing TLR4's role as a sensor for recognition of RSV F protein, several other studies have further expanded the list of viruses TLR4 can respond to. A number of viral glycoproteins have been shown to act as viral PAMPs that bind to and activate TLR4, leading to IFN-β and/or proinflammatory cytokine expression. Examples are VSV-G [178], Ebola virus GP [179], the envelope proteins of murine retroviruses MMTV, Moloney murine leukemia virus [180], and so on. Pretreatment with TLR4 ligands protects mice against infections by influenza virus [181], SARS coronavirus [90], and HSV-2 [182], suggesting that TLR4 signaling can induce protective antiviral immunity. However, data on whether TLR4 signaling in the context of viral infection is beneficial to the host or the virus are mixed, depending on the virus studied. A protective role of TLR4 was demonstrated in infections by KSHV, vaccinia virus, and RSV. It has been shown that mouse macrophages deficient in TLR4 expression were more susceptible to KSHV infection and that HIV patients bearing a mutant TLR4 allele were more likely to contract multicentric Castleman's disease, a lymphoproliferation due to enhanced KSHV replication [183]. Following pulmonary infection of vaccinia virus, mice lacking TLR4 or TRIF supported greater viral replication and had greater hypothermia and mortality than control animals [184]. Compared to wild-type controls, TLR4 knockout mice supported significantly higher levels of RSV replication in the lungs [171]. In contrast, TLR4 signaling appears to promote MMTV replication in mice by mediating the induction of IL-10 allowing the virus to persist indefinitely [185]. It also upregulates the expression of MMTV entry receptor on DCs [186]. Another study suggested that the TLR4-TRIF-TRAF6 axis contributes to oxidative stress and detrimental lung inflammation following challenge with inactivated H5N1 avian influenza virus. Accordingly, deletion of TLR4 or TRIF rendered mice resistant to H5N1 virus-induced acute lung injury [187]. In line with this report, others found that TLR4-deficient mice were protected from lethal infection by H1N1 influenza compared with wild-type controls [188].
Viral Evasion Strategies of TLR-Mediated Antiviral Immunity
During their co-evolution with hosts, many viruses have acquired elaborate strategies to circumvent host defense responses downstream of TLRs and other pathways. Almost every step of the signaling cascades is targeted by different viruses. Viral countermeasures include, but are not limited to, degrading TLR signaling components, disrupting the formation of signaling complexes, interfering with the activation and/or transcriptional activity of transcription factors, acting as molecular mimicry of cellular proteins, deubiquitinating signaling molecules, and so on. Herein, we describe a few examples in each category of the evasion mechanisms (Table 1 ).
Table 1.
Examples of viral countermeasures of TLR-mediated antiviral immunity.
| Viral countermeasures | Targets | Mechanism | Virus | Reference |
|---|---|---|---|---|
| Degradation of TLR signaling components | TRIF | 3C protease cleaves TRIF | Coxsackievirus B, EV71 | [189], [192] |
| 3CD precursor cleaves TRIF | HAV | [190] | ||
| NS3/4A serine protease cleaves TRIF | HCV | [191] | ||
| RTA targets TRIF for degradation through the ubiquitin-proteasome pathway | KSHV | [193] | ||
| MyD88 and MAL | ICP0 promotes proteasomal degradation of MyD88 and MAL | HSV-1 | [194] | |
| IKKγ | 3C protease cleaves IKKγ | FMDV | [195] | |
| IRF3 | Npro targets IRF3 for polyubiquitination and proteasomal degradation | BVDV, CSFV | [196], [197] | |
| NSP1 target IRF3 for polyubiquitination and proteasomal degradation | Rotavirus | [198] | ||
| IRF7 | 3C protease targets and hydrolyze IRF7 | EV71 | [199] | |
| RTA targets IRF7 for degradation by the ubiquitin-proteasome pathway | KSHV | [200] | ||
| Disruption of the formation of signaling complexes | TIR-containing adaptors | A46R associates with MyD88, TRIF, TRAM, and MAL via its TIR domain | Vaccinia virus | [201] |
| TRAF6 and IRAK2 | A52R interacts with TRAF6 and IRAK2 | Vaccinia virus | [202] | |
| TRAF3, TBK1, and IKKε | M protein associates with TRAF3, TBK1, and IKKε | SARS coronavirus | [203] | |
| Interference with the activation and/or transcriptional activity of transcription factors | IRF3 | NSP3 PLpro domain binds to IRF3 preventing its phosphorylation and nuclear translocation | SARS coronavirus | [204] |
| NS1 or ICP0 interacts with IRF3 and CBP sequestering the complex from binding to the IFN-β promoter | RSV, HSV-1 | [205], [206] | ||
| Molecular mimicry of cellular proteins | Mimics IκBα | A49 uses its IκBα-like motif to bind to and prevent the activity of β-TrCP. | Vaccinia virus | [207] |
| Mimics IRF7 | V protein associates with and acts as a decoy substrate for IKKα | Measles virus | [208] | |
| Mimics IRF3 | V proteins mimic IRF3 and act as alternative substrates for TBK1 and IKKε | Mumps virus, HPIV-2, and HPIV-5 | [209] | |
| Mimics IRFs | Encodes viral homologs of cellular IRFs | KSHV | [210] | |
| Deubiquitinating signaling molecules | TRAF3, TRAF6, and TBK1 | FMDV Lpro and SARS PLpro act as viral DUBs to remove ubiquitin chains from TLR signaling molecules |
FMDV SARS coronavirus |
[212] [213] |
A growing body of data suggest that degradation of signaling components is an effective means of viral counteraction of TLR signaling. Viruses accomplish this either by cleaving cellular substrates using virally encoded proteases or by usurping the host proteasome degradation pathway. Given its broad recognition of dsRNAs produced during infections by both RNA and DNA viruses, the TLR3 pathway is frequently targeted for viral inhibition. Its sole adaptor, TRIF, has emerged as a prime target for degradation by different viruses. The NS3/4A serine protease of HCV and the 3C proteases of several picornaviruses, Coxsackievirus B, enterovirus 71 (EV71), and hepatitis A virus (HAV), all recognize TRIF as a substrate for proteolytic cleavage, producing TRIF fragments that are unable to signal [189], [190], [191], [192]. Interestingly, in the case of HAV, it is the 3CD processing intermediate but not the mature 3C protease that is capable of TRIF cleavage. Notably, targeting TRIF for immune evasion is not confined to positive-strand RNA viruses; it has been observed that, during lytic infection with KSHV, a human γ-herpesvirus, TRIF protein levels were significantly downregulated. This was mediated by the KSHV immediate early transcription factor RTA (replication and transcription activator), which de-stabilized and targeted TRIF for degradation through the ubiquitin-proteasome pathway [193]. Since TRIF operates in the pathway before the signaling bifurcation, downregulation of TRIF enables viral inhibition of both IRF3 and NF-κB arms of the innate immune responses. MyD88 and MAL are also subject to viral destruction. The immediate early ICP0 protein of HSV-1 promotes proteasomal degradation of these two TLR adaptors, thereby inhibiting TLR2-dependent NF-κB activation and inflammatory cytokine production [194]. Unfortunately, signaling components downstream of the TLR adaptors and even the transcription factors are not spared from viral degradation. IKKγ (also known as NEMO), which is the regulatory subunit of the IKK complex bridging IRF and NF-κB activation pathways, is proteolytically cleaved and inactivated by the 3C protease of foot-and-mouth disease virus (FMDV) [195], a picornavirus. IRF3 is targeted for polyubiquitination and proteasomal degradation by the N-terminal proteases of bovine viral diarrhea virus and classical swine fever virus [196], [197], pestiviruses of the family Flaviviridae, and by the NSP1 of rotavirus, a dsRNA virus in the family Reoviridae [198]. IRF7 is hydrolyzed by EV71 3C protease [199]. This transcription factor is also known to be degraded via the ubiquitin-protease pathway by the KSHV RTA protein, which in this case acts as an E3 ubiquitin ligase [200].
Instead of promoting degradation of TLR signaling molecules, some viruses express proteins that physically associate with innate immune components, disrupting the formation of signaling complexes to impede downstream signal transduction. Poxviruses are well known for their multifaceted tactics of counteracting host defenses. Two viral encoded vaccinia virus proteins that play key roles in disrupting signaling from multiple TLRs are A46R and A52R. A46R contains a TIR domain, which enables this viral protein to associate with four of the five known TLR adaptor proteins, MyD88, TRIF, TRAM, and MAL, inhibiting the assembly of signaling complexes containing these adaptor molecules [201]. A52R, however, interacts with TRAF6 and IRAK2 and disrupts their incorporation into signaling complexes [202]. SARS coronavirus M protein physically associates with TRAF3, TBK1, and IKKε, thereby impeding the formation of TRAF3–TANK–TBK1–IKKε complex that is required for dsRNA-induced IRF3 phosphorylation and transcription from IFN-β and ISRE promoters [203]. This is an example of disrupting innate signaling complex assembly by an RNA virus.
The transcription factors controlling antiviral gene expression are also targeted by viral proteins for inhibition. For instance, the NSP3 of SARS coronavirus binds to IRF3 via its papain-like protease (PLpro) domain. This interaction prevents phosphorylation and nuclear translocation of IRF3 following engagement of TLR3 and RLR pathways [204]. RSV NS1 and HSV-1 ICP0 proteins also interact with IRF3, but they do not inhibit IRF3 phosphorylation. Instead, these form a complex with IRF3 and its transcriptional coactivator, CBP, sequestering the activated transcription factor complex from binding to the IFN-β promoter [205], [206].
Numerous viruses manipulate key cellular processes in antiviral innate immune responses by imitation of cellular proteins also known as molecular mimicry. Vaccinia virus protein A49 is a virulence factor that antagonizes type I IFN induction via TLR3/TLR4/TLR9 and RIG-I pathways by abolishing NF-κB activation. Mechanistically, A49 possesses an IκBα-like motif, through which it binds to and prevents the action of β-TrCP, an E3 ligase that targets phosphorylated IκBα for ubiquitination and degradation [207]. Measles virus V protein associates with and acts as a decoy substrate for IKKα, competing IRF7 for phosphorylation by this kinase. This effectively limits IFN induction in pDCs following engagement of TLR7/TLR9 [208]. The V proteins from mumps virus and human parainfluenza virus type 2 and type 5 mimic IRF3 and act as alternative substrates for the IRF3 kinases, TBK1 and IKKε, thereby inhibiting IRF3 phosphorylation and activation downstream of TLR3 and RLR signaling [209]. Interestingly, KHSV antagonizes the IFN antiviral responses by incorporating into its genome several viral homologs of the cellular IRFs, known as vIRFs, which suppress the activity of their cellular counterparts [210].
Ubiquitination, especially that promotes the formation of Lys63-linked ubiquitin chains, plays an important role in activating immune signaling pathways including those through the TLRs [211]. Emerging evidence suggests that deubiquitinating (DUB) enzymes are encoded by some viruses and are capable of acting on ubiquitin moieties attached to innate signaling components. A prime example is the leader proteinase (Lpro) of FMDV, which is a viral DUB enzyme that can remove ubiquitin chains from key TLR signaling molecules, such as TRAF6, TRAF3, and TBK1 [212]. Remarkably, the ability of Lpro to inhibit the induction of type I IFN response was found to correlate with its DUB activity. Recent data suggest that this immune evasion strategy is also employed by the PLpro of SARS coronavirus, which possesses DUB activity [213].
Targeting TLRs for Antiviral Therapeutic Interventions
There are many viruses that cause severe diseases in which there are neither vaccines nor effective antiviral therapies. As a result, millions of people lack treatment and preventative measures to these virally induced diseases that may lead to fatal outcomes. Hence, many viral diseases continue to be a challenging global health issue. Since the discovery of virus-sensing TLRs, a lot of research has been conducted to better understand the TLR-mediated antiviral responses during viral infections. Understanding TLR functions and their ligand recognition mechanisms has generated an intense interest in applying the knowledge to therapeutic applications. As such, much effort is being devoted to the development of TLR agonists for treating viral infections or as vaccine adjuvants. In addition, since under some circumstances TLRs can mediate host responses that enhance inflammation resulting in increased susceptibility to viral infection, the development of antagonists to TLRs for the treatment of certain virally induced diseases is also on the horizon. For the purpose of this review, only a concise summary of recent developments in TLR-targeted therapeutics is presented. However, readers are encouraged to read these excellent reviews for a more thorough description on promising agonists and antagonists of TLRs in discovery drug development phase or in clinical trials [214], [215], [216].
TLR3 agonists are efficient in eliciting antiviral responses such as type I IFNs and proinflammatory cytokines. Poly I:C is a very potent synthetic analog of viral dsRNA and is widely used in scientific research to study TLR3-mediated antiviral responses [217]. Although poly I:C can activate TLR3, it also can stimulate signaling via the cytoplasmic RLR sensors (RIG-I and MDA5) [218], [219]. TLR3 activation in innate immune cells can stimulate IL-12 and type I IFN and enhances major histocompatibility complex class II expression and antigen cross-presentation [71], [109], [220], [221], [222]. Poly I:C characteristics may make use of it as a vaccine adjuvant beneficial; however, the underlying principle of adjuvants is increased potency and decreased toxicity. Thus, derivatives of poly I:C were produced to lessen the toxicity of poly I:C given at high doses [223]. These include Ampligen [poly I:poly C (12)U; Hemispherx Biopharma] and polyinosinic:polycytidylic acid stabilized with poly-l-lysine and carboxymethylcellulose (poly-ICLC; Hiltonol) [224]. Currently, Ampligen is in development for treatment of HIV infection, influenza, hepatitis B and C infection, and chronic fatigue syndrome [214].
Therapeutically, TLR4 agonists stimulate the TLR4/MD-2 complex to induce potent Th1 immune responses. Currently, the TLR4 agonist monophosphoryl lipid A has been approved as an adjuvant component in a hepatitis B virus vaccine and a human papillomavirus vaccine [225], [226].
As the only approved TLR7 agonist for clinical use, the imidazoquinoline compound Imiquimod is used for treating genital warts caused by human papilloma virus infection [227]. Another TLR7 agonist, ANA-773, is developed for treating HCV infection. As an oral prodrug, ANA-773 induces IFN production and activates NK cells through its active metabolite. It has demonstrated efficacy in reducing serum HCV RNA levels in hepatitis C patients in clinical trials [214].
Synthetic ODNs containing unmethylated CpG dinucleotides are ligands for TLR9 and their stimulation triggers a robust production of type I IFNs [228]. IMO-2125 is a CpG ODN that is in phase I clinical trials for treating hepatitis C and has demonstrated promising antiviral activities [227]. CpG ODN is also being used as an adjuvant in the hepatitis B vaccine Hepislav, which is in phase 3 of clinical trials [229]. In a comparison study utilizing the traditional hepatitis B vaccine that requires 3 doses over a 6-month period, the authors observed that Hepislav stimulated a more robust production of antibodies against hepatitis B than the already approved vaccine [230].
Although recognition of viral pathogens by TLRs can initiate many intrinsic antiviral defense mechanisms to protect the host and eliminate the invader, stimulation of TLRs can enhance the severity of disease in certain viral infections, as exemplified by TLR3-mediated and TLR4-mediated detrimental inflammatory responses in influenza virus infection [92], [187]. This necessitates the development of therapeutics that mimics TLR antagonists in alleviating certain virally induced diseases. This concept was supported by a recent study conducted by Shirey et al., in which the authors showed that therapeutic applications of a TLR4 antagonist, Eritora, were able to block influenza virus-induced lethality in mice, which correlated with an inhibition on virus-induced lung pathology and pulmonary inflammatory cytokine expression [231].
Conclusion
In response to invading pathogens such as viruses, a powerful antiviral innate immune system is rapidly activated in the host. TLRs are important constituents of this system and recognize a wide variety of PAMPs that are conserved molecular signatures of bacteria and viruses. Of the TLRs that have been identified, six represent a subclass that recognizes viral ligands. TLRs are predominately expressed in immune cells but also found in a variety of cell types. TLR3, TLR7/TLR8, and TLR9 are intracellular receptors located in endosomal compartments in which they detect viral dsRNA, ssRNA, and unmethylated CpG DNA, respectively. TLR2 and TLR4 reside on the cell surface and are stimulated by viral glycoproteins and, in some cases, nonstructural proteins released to extracellular milieu. The signaling mechanisms leading to the induction of antiviral innate immune responses are dependent on the particular TLR activated, its stimulus, and cell type. Through MyD88-dependent and/or TRIF-dependent pathways, TLRs elicit the production of proinflammatory cytokines and/or type I and type III IFNs via activation of the essential transcription factors NF-κB and IRF family members, tailoring the innate immune responses and shaping the subsequent, antigen-specific adaptive immunity. These immune responses often contribute to viral clearance and disease resolution but sometimes can be harmful to the host. In the past decade and a half, much has been learned concerning TLR structures, ligand recognition, signaling mechanisms, and viral countermeasures of TLR signaling, but our knowledge of the precise roles TLRs play in antiviral immunity and viral disease pathogenesis in vivo falls short. Clearly, progresses in these areas using animal infection models that recapitulate viral diseases in humans are urgently needed and will unequivocally help develop novel therapeutic and preventive approaches against viral infections.
Acknowledgements
Research in the authors' laboratory was supported by National Institutes of Health grants AI069285 and AI101526. S.N.L. is supported by a National Institutes of Health T32 Training Grant (AI078906).
Edited by E. Freed and M. Gale
References
- 1.Kawai T., Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R., Janeway C.A. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 4.Palsson-McDermott E.M., O'Neill L.A. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- 5.Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol. 2012;24:21–31. doi: 10.1016/j.coi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemaitre B., Nicolas E., Michaut L., Reichhart J.M., Hoffmann J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 8.Akira S., Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Bowie A., O'Neill L.A. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 10.Roach J.C., Glusman G., Rowen L., Kaur A., Purcell M.K., Smith K.D. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi K., Iwasaki A. Different routes to the same destination. Elife. 2013;2:e00572. doi: 10.7554/eLife.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 13.Andrade W.A., Souza Mdo C., Ramos-Martinez E., Nagpal K., Dutra M.S., Melo M.B. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13:42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X.D., Chen Z.J. Sequence specific detection of bacterial 23S ribosomal RNA by TLR13. Elife. 2012;1:e00102. doi: 10.7554/eLife.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldenburg M., Kruger A., Ferstl R., Kaufmann A., Nees G., Sigmund A. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 16.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K., Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 20.Bieback K., Lien E., Klagge I.M., Avota E., Schneider-Schaulies J., Duprex W.P. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y., Mansell A., Ussher J.E., Brooks A.E., Manning K., Wang C. Rotavirus NSP4 triggers secretion of proinflammatory cytokines from macrophages via Toll-like receptor 2. J Virol. 2013;87:11160–11167. doi: 10.1128/JVI.03099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones E.A., Popova L., Kwinn L., Haynes L.M., Jones L.P., Tripp R.A. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 23.Mogensen T.H., Paludan S.R. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J Mol Med (Berl) 2005;83:180–192. doi: 10.1007/s00109-004-0620-6. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z., Cai Z., Sanchez A., Zhang T., Wen S., Wang J. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem. 2011;286:4517–4524. doi: 10.1074/jbc.M110.159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borden E.C., Sen G.C., Uze G., Silverman R.H., Ransohoff R.M., Foster G.R. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 27.Sommereyns C., Paul S., Staeheli P., Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mordstein M., Neugebauer E., Ditt V., Jessen B., Rieger T., Falcone V. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupuis S., Jouanguy E., Al-Hajjar S., Fieschi C., Al-Mohsen I.Z., Al-Jumaah S. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen T.H., Paludan S.R. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T., Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Stark G.R. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 34.Guo B., Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S., tenOever B.R., Grandvaux N., Zhou G.P., Lin R., Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 36.Zhao T., Yang L., Sun Q., Arguello M., Ballard D.W., Hiscott J. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 37.Honda K., Takaoka A., Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Onoguchi K., Yoneyama M., Takemura A., Akira S., Taniguchi T., Namiki H. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 39.Osterlund P.I., Pietila T.E., Veckman V., Kotenko S.V., Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- 40.Hacker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L.C., Wang G.G. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 41.Oganesyan G., Saha S.K., Guo B., He J.Q., Shahangian A., Zarnegar B. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 42.Kagan J.C., Su T., Horng T., Chow A., Akira S., Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbalat R., Lau L., Locksley R.M., Barton G.M. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y.J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 45.Lee K.G., Xu S., Kang Z.H., Huo J., Huang M., Liu D. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc Natl Acad Sci U S A. 2012;109:5791–5796. doi: 10.1073/pnas.1119238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita M., Chattopadhyay S., Fensterl V., Saikia P., Wetzel J.L., Sen G.C. Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci Signal. 2012;5:ra50. doi: 10.1126/scisignal.2002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Z., Mak T.W., Sen G., Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004;101:3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato S., Sugiyama M., Yamamoto M., Watanabe Y., Kawai T., Takeda K. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 49.Meylan E., Burns K., Hofmann K., Blancheteau V., Martinon F., Kelliher M. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 50.Uematsu S., Sato S., Yamamoto M., Hirotani T., Kato H., Takeshita F. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino K., Sugiyama T., Matsumoto M., Tanaka T., Saito M., Hemmi H. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 52.Negishi H., Fujita Y., Yanai H., Sakaguchi S., Ouyang X., Shinohara M. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci U S A. 2006;103:15136–15141. doi: 10.1073/pnas.0607181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tailor P., Tamura T., Ozato K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 2006;16:134–140. doi: 10.1038/sj.cr.7310018. [DOI] [PubMed] [Google Scholar]
- 54.Kim H., Yang E., Lee J., Kim S.H., Shin J.S., Park J.Y. Double-stranded RNA mediates interferon regulatory factor 3 activation and interleukin-6 production by engaging Toll-like receptor 3 in human brain astrocytes. Immunology. 2008;124:480–488. doi: 10.1111/j.1365-2567.2007.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsukura S., Kokubu F., Kurokawa M., Kawaguchi M., Ieki K., Kuga H. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells. Clin Exp Allergy. 2006;36:1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 56.Park C., Lee S., Cho I.H., Lee H.K., Kim D., Choi S.Y. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 57.Tissari J., Siren J., Meri S., Julkunen I., Matikainen S. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174:4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 58.Li K., Chen Z., Kato N., Gale M., Lemon S.M. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 2005;280:16739–16747. doi: 10.1074/jbc.M414139200. [DOI] [PubMed] [Google Scholar]
- 59.Leonard J.N., Ghirlando R., Askins J., Bell J.K., Margulies D.H., Davies D.R. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc Natl Acad Sci U S A. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li K., Li N.L., Wei D., Pfeffer S.R., Fan M., Pfeffer L.M. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666–675. doi: 10.1002/hep.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdorfer B., Giese T. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 62.Nishimura M., Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 63.Wang N., Liang Y., Devaraj S., Wang J., Lemon S.M., Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824–9834. doi: 10.1128/JVI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarember K.A., Godowski P.J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q., Nagarkar D.R., Bowman E.R., Schneider D., Gosangi B., Lei J. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto M., Kikkawa S., Kohase M., Miyake K., Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364–1369. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 67.Tamassia N., Le Moigne V., Rossato M., Donini M., McCartney S., Calzetti F. Activation of an immunoregulatory and antiviral gene expression program in poly(I:C)-transfected human neutrophils. J Immunol. 2008;181:6563–6573. doi: 10.4049/jimmunol.181.9.6563. [DOI] [PubMed] [Google Scholar]
- 68.Wesch D., Beetz S., Oberg H.H., Marget M., Krengel K., Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto M., Funami K., Tanabe M., Oshiumi H., Shingai M., Seto Y. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 70.Nishiya T., Kajita E., Miwa S., Defranco A.L. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem. 2005;280:37107–37117. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
- 71.Schulz O., Diebold S.S., Chen M., Naslund T.I., Nolte M.A., Alexopoulou L. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 72.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S.O. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 73.Tabeta K., Georgel P., Janssen E., Du X., Hoebe K., Crozat K. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reinert L.S., Harder L., Holm C.K., Iversen M.B., Horan K.A., Dagnaes-Hansen F. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Audry M., Ciancanelli M., Yang K., Cobat A., Chang H.H., Sancho-Shimizu V. NEMO is a key component of NF-kappaB- and IRF-3-dependent TLR3-mediated immunity to herpes simplex virus. J Allergy Clin Immunol. 2011;128:e1–e4. doi: 10.1016/j.jaci.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herman M., Ciancanelli M., Ou Y.H., Lorenzo L., Klaudel-Dreszler M., Pauwels E. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J Exp Med. 2012;209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 78.Casrouge A., Zhang S.Y., Eidenschenk C., Jouanguy E., Puel A., Yang K. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 79.Abe Y., Fujii K., Nagata N., Takeuchi O., Akira S., Oshiumi H. The toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. J Virol. 2012;86:185–194. doi: 10.1128/JVI.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hardarson H.S., Baker J.S., Yang Z., Purevjav E., Huang C.H., Alexopoulou L. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 81.Negishi H., Osawa T., Ogami K., Ouyang X., Sakaguchi S., Koshiba R. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci U S A. 2008;105:20446–20451. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richer M.J., Lavallee D.J., Shanina I., Horwitz M.S. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One. 2009;4:e4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pott J., Stockinger S., Torow N., Smoczek A., Lindner C., McInerney G. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012;8:e1002670. doi: 10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai Y.T., Chang S.Y., Lee C.N., Kao C.L. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell Microbiol. 2009;11:604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 85.Handke W., Oelschlegel R., Franke R., Kruger D.H., Rang A. Hantaan virus triggers TLR3-dependent innate immune responses. J Immunol. 2009;182:2849–2858. doi: 10.4049/jimmunol.0802893. [DOI] [PubMed] [Google Scholar]
- 86.Mazaleuskaya L., Veltrop R., Ikpeze N., Martin-Garcia J., Navas-Martin S. Protective role of Toll-like receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses. 2012;4:901–923. doi: 10.3390/v4050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Isogawa M., Robek M.D., Furuichi Y., Chisari F.V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou Y., Wang X., Liu M., Hu Q., Song L., Ye L. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology. 2010;131:40–49. doi: 10.1111/j.1365-2567.2010.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 90.Zhao J., Wohlford-Lenane C., Fleming E., Lane T.E., McCray P.B., Perlman S. Intranasal treatment with poly(I*C) protects aged mice from lethal respiratory virus infections. J Virol. 2012;86:11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gowen B.B., Hoopes J.D., Wong M.H., Jung K.H., Isakson K.C., Alexopoulou L. TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J Immunol. 2006;177:6301–6307. doi: 10.4049/jimmunol.177.9.6301. [DOI] [PubMed] [Google Scholar]
- 92.Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hutchens M., Luker K.E., Sottile P., Sonstein J., Lukacs N.W., Nunez G. TLR3 increases disease morbidity and mortality from vaccinia infection. J Immunol. 2008;180:483–491. doi: 10.4049/jimmunol.180.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 95.Daffis S., Samuel M.A., Suthar M.S., Gale M., Diamond M.S. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.So E.Y., Kim B.S. Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia. 2009;57:1216–1226. doi: 10.1002/glia.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 98.Diebold S.S. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008;60:813–823. doi: 10.1016/j.addr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 101.Kawai T., Sato S., Ishii K.J., Coban C., Hemmi H., Yamamoto M. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 102.Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 103.Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 104.Du X., Poltorak A., Wei Y., Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–371. [PubMed] [Google Scholar]
- 105.Chuang T.H., Ulevitch R.J. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 106.Gorden K.B., Gorski K.S., Gibson S.J., Kedl R.M., Kieper W.C., Qiu X. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 107.Jurk M., Heil F., Vollmer J., Schetter C., Krieg A.M., Wagner H. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 108.Ito T., Amakawa R., Fukuhara S. Roles of toll-like receptors in natural interferon-producing cells as sensors in immune surveillance. Hum Immunol. 2002;63:1120–1125. doi: 10.1016/s0198-8859(02)00750-4. [DOI] [PubMed] [Google Scholar]
- 109.Kadowaki N., Ho S., Antonenko S., Malefyt R.W., Kastelein R.A., Bazan F. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 111.Colonna M., Trinchieri G., Liu Y.J. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 112.Alexopoulou L., Desnues B., Demaria O. Toll-like receptor 8: the awkward TLR. Med Sci (Paris) 2012;28:96–102. doi: 10.1051/medsci/2012281023. [DOI] [PubMed] [Google Scholar]
- 113.Forsbach A., Nemorin J.G., Montino C., Muller C., Samulowitz U., Vicari A.P. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J Immunol. 2008;180:3729–3738. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- 114.Martinez J., Huang X., Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci U S A. 2010;107:6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gantier M.P., Tong S., Behlke M.A., Xu D., Phipps S., Foster P.S. TLR7 is involved in sequence-specific sensing of single-stranded RNAs in human macrophages. J Immunol. 2008;180:2117–2124. doi: 10.4049/jimmunol.180.4.2117. [DOI] [PubMed] [Google Scholar]
- 116.Melchjorsen J., Jensen S.B., Malmgaard L., Rasmussen S.B., Weber F., Bowie A.G. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79:12944–12951. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gringhuis S.I., van der Vlist M., van den Berg L.M., den Dunnen J., Litjens M., Geijtenbeek T.B. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 118.Beignon A.S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D.G. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Triantafilou K., Orthopoulos G., Vakakis E., Ahmed M.A., Golenbock D.T., Lepper P.M. Human cardiac inflammatory responses triggered by Coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell Microbiol. 2005;7:1117–1126. doi: 10.1111/j.1462-5822.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 120.Wang J.P., Liu P., Latz E., Golenbock D.T., Finberg R.W., Libraty D.H. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006;177:7114–7121. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- 121.Town T., Bai F., Wang T., Kaplan A.T., Qian F., Montgomery R.R. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takahashi K., Asabe S., Wieland S., Garaigorta U., Gastaminza P., Isogawa M. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davidson S., Kaiko G., Loh Z., Lalwani A., Zhang V., Spann K. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol. 2011;186:5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kane M., Case L.K., Wang C., Yurkovetskiy L., Dikiy S., Golovkina T.V. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–145. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee H.K., Lund J.M., Ramanathan B., Mizushima N., Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 127.Reis e Sousa C. Immunology. Eating in to avoid infection. Science. 2007;315:1376–1377. doi: 10.1126/science.1140002. [DOI] [PubMed] [Google Scholar]
- 128.Manuse M.J., Briggs C.M., Parks G.D. Replication-independent activation of human plasmacytoid dendritic cells by the paramyxovirus SV5 requires TLR7 and autophagy pathways. Virology. 2010;405:383–389. doi: 10.1016/j.virol.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou D., Kang K.H., Spector S.A. Production of interferon alpha by human immunodeficiency virus type 1 in human plasmacytoid dendritic cells is dependent on induction of autophagy. J Infect Dis. 2012;205:1258–1267. doi: 10.1093/infdis/jis187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Campbell G.R., Spector S.A. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8:e1003017. doi: 10.1371/journal.ppat.1003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dreux M., Garaigorta U., Boyd B., Decembre E., Chung J., Whitten-Bauer C. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lepelley A., Louis S., Sourisseau M., Law H.K., Pothlichet J., Schilte C. Innate sensing of HIV-infected cells. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lund J., Sato A., Akira S., Medzhitov R., Iwasaki A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 135.Honda K., Ohba Y., Yanai H., Negishi H., Mizutani T., Takaoka A. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 136.Klinman D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 137.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K.A., Monks B.G., Knetter C.F. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 138.Barton G.M., Kagan J.C., Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 139.Hacker H., Mischak H., Miethke T., Liptay S., Schmid R., Sparwasser T. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.An H., Xu H., Yu Y., Zhang M., Qi R., Yan X. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett. 2002;81:165–169. doi: 10.1016/s0165-2478(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 141.Krug A., Luker G.D., Barchet W., Leib D.A., Akira S., Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 142.Samuelsson C., Hausmann J., Lauterbach H., Schmidt M., Akira S., Wagner H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118:1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sutherland D.B., Ranasinghe C., Regner M., Phipps S., Matthaei K.I., Day S.L. Evaluating vaccinia virus cytokine co-expression in TLR GKO mice. Immunol Cell Biol. 2011;89:706–715. doi: 10.1038/icb.2010.157. [DOI] [PubMed] [Google Scholar]
- 144.Yu H.R., Huang H.C., Kuo H.C., Sheen J.M., Ou C.Y., Hsu T.Y. IFN-alpha production by human mononuclear cells infected with varicella-zoster virus through TLR9-dependent and -independent pathways. Cell Mol Immunol. 2011;8:181–188. doi: 10.1038/cmi.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Varani S., Cederarv M., Feld S., Tammik C., Frascaroli G., Landini M.P. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol. 2007;179:7767–7776. doi: 10.4049/jimmunol.179.11.7767. [DOI] [PubMed] [Google Scholar]
- 146.West J.A., Gregory S.M., Sivaraman V., Su L., Damania B. Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated herpesvirus. J Virol. 2011;85:895–904. doi: 10.1128/JVI.01007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basner-Tschakarjan E., Gaffal E., O'Keeffe M., Tormo D., Limmer A., Wagner H. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- 148.Fiola S., Gosselin D., Takada K., Gosselin J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J Immunol. 2010;185:3620–3631. doi: 10.4049/jimmunol.0903736. [DOI] [PubMed] [Google Scholar]
- 149.Sorensen L.N., Reinert L.S., Malmgaard L., Bartholdy C., Thomsen A.R., Paludan S.R. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 150.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Raykov Z., Grekova S.P., Horlein R., Leuchs B., Giese T., Giese N.A. TLR-9 contributes to the antiviral innate immune sensing of rodent parvoviruses MVMp and H-1PV by normal human immune cells. PLoS One. 2013;8:e55086. doi: 10.1371/journal.pone.0055086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mattei L.M., Cotmore S.F., Li L., Tattersall P., Iwasaki A. Toll-like receptor 9 in plasmacytoid dendritic cells fails to detect parvoviruses. J Virol. 2013;87:3605–3608. doi: 10.1128/JVI.03155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Flo T.H., Halaas O., Torp S., Ryan L., Lien E., Dybdahl B. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69:474–481. [PubMed] [Google Scholar]
- 154.Zanin-Zhorov A., Cahalon L., Tal G., Margalit R., Lider O., Cohen I.R. Heat shock protein 60 enhances CD4 + CD25 + regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Alard J.E., Gaillard F., Daridon C., Shoenfeld Y., Jamin C., Youinou P. TLR2 is one of the endothelial receptors for beta 2-glycoprotein I. J Immunol. 2010;185:1550–1557. doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]
- 156.Yao X.D., Rosenthal K.L. Herpes simplex virus type 2 virion host shutoff protein suppresses innate dsRNA antiviral pathways in human vaginal epithelial cells. J Gen Virol. 2011;92:1981–1993. doi: 10.1099/vir.0.030296-0. [DOI] [PubMed] [Google Scholar]
- 157.Zhou S., Halle A., Kurt-Jones E.A., Cerny A.M., Porpiglia E., Rogers M. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194:70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Preiss S., Thompson A., Chen X., Rodgers S., Markovska V., Desmond P. Characterization of the innate immune signalling pathways in hepatocyte cell lines. J Viral Hepat. 2008;15:888–900. doi: 10.1111/j.1365-2893.2008.01001.x. [DOI] [PubMed] [Google Scholar]
- 159.Horng T., Barton G.M., Flavell R.A., Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 160.O'Neill L.A., Bowie A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 161.Oliveira-Nascimento L., Massari P., Wetzler L.M. The role of TLR2 in infection and immunity. Front Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szomolanyi-Tsuda E., Liang X., Welsh R.M., Kurt-Jones E.A., Finberg R.W. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006;80:4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boehme K.W., Guerrero M., Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 164.Compton T., Kurt-Jones E.A., Boehme K.W., Belko J., Latz E., Golenbock D.T. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leoni V., Gianni T., Salvioli S., Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J Virol. 2012;86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ariza M.E., Glaser R., Kaumaya P.T., Jones C., Williams M.V. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol. 2009;182:851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- 167.Dolganiuc A., Oak S., Kodys K., Golenbock D.T., Finberg R.W., Kurt-Jones E. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 168.Zhu J., Martinez J., Huang X., Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou S., Kurt-Jones E.A., Mandell L., Cerny A., Chan M., Golenbock D.T. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 170.Wang J.P., Kurt-Jones E.A., Shin O.S., Manchak M.D., Levin M.J., Finberg R.W. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79:12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murawski M.R., Bowen G.N., Cerny A.M., Anderson L.J., Haynes L.M., Tripp R.A. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lima G.K., Zolini G.P., Mansur D.S., Freire Lima B.H., Wischhoff U., Astigarraga R.G. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am J Pathol. 2010;177:2433–2445. doi: 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kurt-Jones E.A., Chan M., Zhou S., Wang J., Reed G., Bronson R. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang J.P., Bowen G.N., Zhou S., Cerny A., Zacharia A., Knipe D.M. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–2281. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Medzhitov R., Preston-Hurlburt P., Janeway C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 176.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 177.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Georgel P., Jiang Z., Kunz S., Janssen E., Mols J., Hoebe K. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 179.Okumura A., Pitha P.M., Yoshimura A., Harty R.N. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol. 2010;84:27–33. doi: 10.1128/JVI.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rassa J.C., Meyers J.L., Zhang Y., Kudaravalli R., Ross S.R. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shinya K., Ito M., Makino A., Tanaka M., Miyake K., Eisfeld A.J. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012;86:19–24. doi: 10.1128/JVI.06168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ashkar A.A., Mossman K.L., Coombes B.K., Gyles C.L., Mackenzie R. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog. 2008;4:e1000233. doi: 10.1371/journal.ppat.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lagos D., Vart R.J., Gratrix F., Westrop S.J., Emuss V., Wong P.P. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe. 2008;4:470–483. doi: 10.1016/j.chom.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hutchens M.A., Luker K.E., Sonstein J., Nunez G., Curtis J.L., Luker G.D. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4:e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jude B.A., Pobezinskaya Y., Bishop J., Parke S., Medzhitov R.M., Chervonsky A.V. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003;4:573–578. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 186.Burzyn D., Rassa J.C., Kim D., Nepomnaschy I., Ross S.R., Piazzon I. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J Virol. 2004;78:576–584. doi: 10.1128/JVI.78.2.576-584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nhu Q.M., Shirey K., Teijaro J.R., Farber D.L., Netzel-Arnett S., Antalis T.M. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mukherjee A., Morosky S.A., Delorme-Axford E., Dybdahl-Sissoko N., Oberste M.S., Wang T. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011;7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Qu L., Feng Z., Yamane D., Liang Y., Lanford R.E., Li K. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 2011;7:e1002169. doi: 10.1371/journal.ppat.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li K., Foy E., Ferreon J.C., Nakamura M., Ferreon A.C., Ikeda M. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lei X., Sun Z., Liu X., Jin Q., He B., Wang J. Cleavage of the adaptor protein TRIF by enterovirus 71 3C inhibits antiviral responses mediated by Toll-like receptor 3. J Virol. 2011;85:8811–8818. doi: 10.1128/JVI.00447-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ahmad H., Gubbels R., Ehlers E., Meyer F., Waterbury T., Lin R. Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF) J Biol Chem. 2011;286:7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van Lint A.L., Murawski M.R., Goodbody R.E., Severa M., Fitzgerald K.A., Finberg R.W. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J Virol. 2010;84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang D., Fang L., Li K., Zhong H., Fan J., Ouyang C. Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling. J Virol. 2012;86:9311–9322. doi: 10.1128/JVI.00722-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bauhofer O., Summerfield A., Sakoda Y., Tratschin J.D., Hofmann M.A., Ruggli N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J Virol. 2007;81:3087–3096. doi: 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen Z., Rijnbrand R., Jangra R.K., Devaraj S.G., Qu L., Ma Y. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology. 2007;366:277–292. doi: 10.1016/j.virol.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barro M., Patton J.T. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc Natl Acad Sci U S A. 2005;102:4114–4119. doi: 10.1073/pnas.0408376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lei X., Xiao X., Xue Q., Jin Q., He B., Wang J. Cleavage of interferon regulatory factor 7 by enterovirus 71 3C suppresses cellular responses. J Virol. 2013;87:1690–1698. doi: 10.1128/JVI.01855-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yu Y., Wang S.E., Hayward G.S. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 201.Stack J., Haga I.R., Schroder M., Bartlett N.W., Maloney G., Reading P.C. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–1018. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Harte M.T., Haga I.R., Maloney G., Gray P., Reading P.C., Bartlett N.W. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003;197:343–351. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Siu K.L., Kok K.H., Ng M.H., Poon V.K., Yuen K.Y., Zheng B.J. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3·TANK·TBK1/IKKepsilon complex. J Biol Chem. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Devaraj S.G., Wang N., Chen Z., Chen Z., Tseng M., Barretto N. Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J Biol Chem. 2007;282:32208–32221. doi: 10.1074/jbc.M704870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ren J., Liu T., Pang L., Li K., Garofalo R.P., Casola A. A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J Gen Virol. 2011;92:2153–2159. doi: 10.1099/vir.0.032987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Melroe G.T., Silva L., Schaffer P.A., Knipe D.M. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology. 2007;360:305–321. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mansur D.S., Maluquer de Motes C., Unterholzner L., Sumner R.P., Ferguson B.J., Ren H. Poxvirus targeting of E3 ligase beta-TrCP by molecular mimicry: a mechanism to inhibit NF-kappaB activation and promote immune evasion and virulence. PLoS Pathog. 2013;9:e1003183. doi: 10.1371/journal.ppat.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pfaller C.K., Conzelmann K.K. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J Virol. 2008;82:12365–12373. doi: 10.1128/JVI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lu L.L., Puri M., Horvath C.M., Sen G.C. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for inhibitor of kappaB kinase epsilon (IKKe)/TBK1. J Biol Chem. 2008;283:14269–14276. doi: 10.1074/jbc.M710089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee H.R., Kim M.H., Lee J.S., Liang C., Jung J.U. Viral interferon regulatory factors. J Interferon Cytokine Res. 2009;29:621–627. doi: 10.1089/jir.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bhoj V.G., Chen Z.J. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 212.Wang D., Fang L., Li P., Sun L., Fan J., Zhang Q. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J Virol. 2011;85:3758–3766. doi: 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One. 2012;7:e30802. doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Connolly D.J., O'Neill L.A. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12:510–518. doi: 10.1016/j.coph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 215.Gosu V., Basith S., Kwon O.P., Choi S. Therapeutic applications of nucleic acids and their analogues in Toll-like receptor signaling. Molecules. 2012;17:13503–13529. doi: 10.3390/molecules171113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hennessy E.J., Parker A.E., O'Neill L.A. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 217.Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 218.Wang Y., Cella M., Gilfillan S., Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 219.Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Davey G.M., Wojtasiak M., Proietto A.I., Carbone F.R., Heath W.R., Bedoui S. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243–2246. doi: 10.4049/jimmunol.0903013. [DOI] [PubMed] [Google Scholar]
- 221.Poulin L.F., Salio M., Griessinger E., Anjos-Afonso F., Craciun L., Chen J.L. Characterization of human DNGR-1 + BDCA3 + leukocytes as putative equivalents of mouse CD8alpha + dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Le Bon A., Etchart N., Rossmann C., Ashton M., Hou S., Gewert D. Cross-priming of CD8 + T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 223.Steinhagen F., Kinjo T., Bode C., Klinman D.M. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gnjatic S., Sawhney N.B., Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16:382–391. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Casella C.R., Mitchell T.C. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Didierlaurent A.M., Morel S., Lockman L., Giannini S.L., Bisteau M., Carlsen H. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 227.Es-Saad S., Tremblay N., Baril M., Lamarre D. Regulators of innate immunity as novel targets for panviral therapeutics. Curr Opin Virol. 2012;2:622–628. doi: 10.1016/j.coviro.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hemmi H., Kaisho T., Takeda K., Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol. 2003;170:3059–3064. doi: 10.4049/jimmunol.170.6.3059. [DOI] [PubMed] [Google Scholar]
- 229.Cooper C., Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV safety and efficacy. Expert Rev Vaccines. 2011;10:417–427. doi: 10.1586/erv.10.162. [DOI] [PubMed] [Google Scholar]
- 230.Halperin S.A., Ward B., Cooper C., Predy G., Diaz-Mitoma F., Dionne M. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine. 2012;30:2556–2563. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 231.Shirey K.A., Lai W., Scott A.J., Lipsky M., Mistry P., Pletneva L.M. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]