Abstract
Background
More people are presenting with mild cognitive impairment (MCI), frequently a precursor to dementia but we do not know how to reduce deterioration.
Aims
To systematically review Randomised Controlled Trials (RCTs) evaluating effects of any intervention for MCI on cognitive, neuropsychiatric, functional, global outcomes, life quality, or incident dementia.
Methods
We reviewed the 41 studies fitting predetermined criteria, assessed validity using a checklist, calculated standardised outcomes, and prioritised primary outcome findings in placebo-controlled studies.
Results
The strongest evidence was that cholinesterase inhibitors did not reduce incident dementia. Cognition improved in single trials of: a heterogeneous psychological group intervention over 6 months; piribedil, a dopamine agonist over 3 months; and donepezil over 48 weeks. Nicotine improved attention over 6 months. There was equivocal evidence that Huannao Yicong improved cognition and social functioning.
Conclusions
There was no replicated evidence that any intervention was effective. Cholinesterase inhibitors and rofecoxib are ineffective in preventing dementia. Further good quality RCTs are necessary and preliminary evidence suggests these should include trials of psychological group interventions and piribedil.
INTRODUCTION
Mild cognitive impairment (MCI) is a heterogeneous state between normal aging and early dementia. It has been referred to as objective cognitive complaint for age, in a person with essentially normal functional activities, who does not have dementia1. It affects 19% of people aged 65 and over2. Around 46% of people with MCI develop dementia within 3 years compared to 3% of the population of the same age3. Petersen distinguishes subtypes, depending on whether single or multiple cognitive domains are affected, and whether there is a predominant memory complaint. Amnestic MCI (aMCI), in which memory is affected more often progresses to Alzheimer’s disease (AD), while MCI affecting a single non-memory domain may herald Frontotemporal or Lewy Body dementia. A vascular aetiology is more likely in multiple domain MCI1. Thus a group of people with MCI may differ from each other, clinically and neuropathologically.
The number of individuals diagnosed with MCI is growing in Western countries as people are encouraged to present early with memory problems to avoid crisis, but we know little about how to treat it. The UK National Institute for Health and Clinical Excellence (NICE) recommends follow-up to ensure dementia is diagnosed and care planned at an early stage, but no specific treatments4. Jorm et al5 calculated that the dementia prevalence would be halved if its onset were delayed for five years. Neuroprotection, treating vascular risk factors or increasing cognitive reserves could theoretically delay dementia, and could be targeted at people with MCI who are at a particularly high risk of developing it. Previous reviews have focussed on specific treatments for MCI. Systematic reviews of Randomised Controlled Trials (RCTs) of all cholinesterase inhibitors6, donepezil7 and galantamine8 concluded there are marginal beneficial effects which are outweighed by the risks of adverse events, including an unexplained increased mortality rate with galantamine. A 2009 Cochrane review found that memory training (specific neuropsychological exercises to improve memory) improved immediate and delayed verbal recall in people with MCI compared to a no-treatment, but not an active control9. More recent reviews have included RCT and non-RCT studies and suggested that cognitive interventions may improve memory for specific information, with less evidence that these effects can generalise10–13.
We aimed to carry out the first systematic review of all types of intervention for MCI, to identify the best current treatment evidence.
METHODS
Search strategy and selection criteria
We searched PubMed (1946-), Web of Science (1900-), Cochrane Systematic Reviews Database (c.1993-), PsycINFO (1880-), CINAHL (1937-) and AMED (1985-) through 10 July 2012 (and updated it 27 January 2013), using the words: “mild cognitive”, “cognitive impairment”, “benign senescent forgetfulness” OR “age associated cognitive decline” AND treatment AND (controlled trial OR RCT). No limits were applied for language or time published. We searched references of included papers and systematic reviews identified in the search and contacted experts.
We included RCTs evaluating any treatment for MCI which reported as an outcome: cognition (specific domains or global), conversion to dementia; functional, behavioural, quality of life or global measures. We included studies where all participants or a separately analysed subgroup had MCI.
Data extraction
One author (CC) extracted study characteristics (see Tables 1–2). We contacted two authors to request unreported data; and obtained this for one14 but not the second study15.
Table 1.
Characteristics and validity of studies specifying one or two primary efficacy outcomes
| Study | Support sources | Inclusion criteria | Treatment | n | Control | n | Duration | Primary outcome | Validity*** | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||
| Barnes18 | Posit Science, Gov (USA) |
cognitive complaint in >1 domain and no dementia | Computerised, cognitive training | 22 | Audio books, online news, computer game | 25 | 6 weeks | RBANS total score | y | y | y | y | n |
| Buschert19 | None declared | Age 50+, aMCI* | 20 × 2 hour group memory training & cognitive stimulation | 10 | Monthly groups 1-hour sessions doing paper and pencil exercises | 12 | 6 months | ADAS Cog | y | y | n | y | n |
| MMSE | |||||||||||||
| Chiu29 | Gov (Taiwan) | aMCI* | 1080mg EPA and 720mg DHA | 14 | Olive oil | 9 | 6 months | ADAS Cog | y | y | n | y | n |
| CIBIC | |||||||||||||
| DeKosky35 | NCCAM Schwabe Pharmaceuticals |
Age 75+ ; MCI** | Ginkgo biloba 120mg bd | 256 | Placebo | 226 | 6 years | Incident dementia | y | y | y | y | y |
| Doody15 | Eisai Pfizer |
Age 45–90, MCI*, daily contact with informant | Donepezil | 379 | Placebo | 378 | 48 weeks | ADAS Cog | y | y | y | y | y |
| CDR-SB | |||||||||||||
| Feldman31 | Novartis | CDR= 0·5,< 9 New York University delayed paragraph recall score, no dementia | Rivastigmine | 508 | Placebo | 510 | 2 years | Cognitive test battery | y | y | y | y | y |
| Incident AD | |||||||||||||
| Gomez-Isla44 | J. Uriach y Companıa S.A. | SuMC, MMSE >23, recall test > 1.5 SD below expected & no dementia | Triflusal | 129 | Placebo | 128 | 13 months | ADAS-Cog | n | y | y | y | n |
| Jean53 | Gov (Canada) & Alzheimer society | aMCI* | 2 × weekly individual errorless learning sessions | 11 | Errorful learning and psycho-education | 11 | 3 weeks | Episodic & semantic recall | n | y | n | y | y |
| Kinsella20 | Alzheimer’s Australia, Hospital & University grant | aMCI* | Five, weekly 1.5-hour family memory strategy interventions | 22 | Waitlist | 22 | 5 weeks | Prospective memory | n | y | n | y | n |
| Li28 | Not stated | MCI*; aged 60–80 SuMC, GDS level 2–3 or CDR 0·5; no ADL imp; MMSE 24–27 | 3 Huannao Yicong capsules 3× daily | 31 | Placebo (hydergin) | 31 | 2 months | Cognitive effect Index | n | n | n | n | n |
| Nagaraja23 | Gov (India) Serdia Pharmaceuticals |
SuMC, no dementia, aged 60+ and MMSE 21–25 | Piribedil 50mg od | 30 | Placebo | 30 | 3 months | MMSE (responder) | n | y | y | y | y |
| Newhouse21 | Gov (USA), Pfizer | aMCI*, non-smoker | 15mg/day nicotine patch | 39 | Placebo | 35 | 6 months | CPT | y | y | y | y | y |
| MCI-CGIC | |||||||||||||
| Petersen45 | Eisai, Pfizer Gov body (USA) |
age 55–90; aMCI*, CDR 0·5; MMSE 24–30; memory recall 1·5–2·0 sd below norm | donepezil titrated to 10mg | 257 | Placebo | 259 | 3 years | Incident AD | y | y | y | y | n |
| 2000IU vitamin E | 253 | ||||||||||||
| Salloway48 | Eisai, Pfizer | Aged 55–90, SuMC, MMSE 24+, CDR 0·5 | Donepezil titrated to 10mg | 133 | Placebo | 137 | 24 weeks | NYPDRT | n | y | y | y | y |
| MCI-CGIC | |||||||||||||
| Thal49 | Merck Research Laboratories | age 65+, SuMC, MMSE>23, CDR=0·5, BDRS<3.5, AVLT<38 | Rofecoxib 25mg od | 129 | Placebo | 128 | <4 years | Incident AD | y | y | y | y | y |
| Unverzagt14 | Gov (USA) | AVLT 1.5 SD below predicted score, no dementia | 10 × 1 hour group: memory training | 49 | No treatment | 52 | 6 weeks, booster at 11 months | Memory, reasoning and speed composite Measures1 |
n | y | n | y | n |
| Reasoning training | 39 | ||||||||||||
| Speed training | 53 | ||||||||||||
| van Uffelen38;54 | (Viatris; Gov, (Netherlands) | MCI* | folic acid (5mg), vitamins B12 (0·4mg) & B6 (50mg) | 78 | Placebo | 74 | 1 year | AVLT 1–5 words | y | y | y | y | y |
| 2×weekly, group walking | 77 | activities | 75 | AVLT 6 words | |||||||||
| Winblad51 | Janssen-Cilag Johnson&Johnson | CDR=0·5, memory score > 0·5; age 50+ | Galantamine 16–24mg od | 494 | Placebo | 496 | 2 years | Incident dementia | y | y | y | y | y |
| 532 | 526 | ||||||||||||
| Zhao25 | Gov (China) | 60–85, SMC, aMCI * | Ginkgo biloba | 60 | Usual treatment | 60 | 6 months | Memory test | y | n | n | y | y |
| NPR | |||||||||||||
Composite outcome measures tested: memory [mean Rey Auditory Verbal Learning Test, Hopkins Verbal learning Test, and Rivermead Behavioral Memory Test z-scores], reasoning [mean Letter Series, Letter Sets, and Word Series tests], and processing speed [mean Useful Field of View task scores].
diagnosed according to Petersen criteria;
defined by International working group guidelines;
see method – numbers refer to questions asked about validity;
AD= Alzheimer’s disease; ADASCog =Alzheimer’s Disease Assessment Scale-cognitive subscale; aMCI=amnestic mild cognitive impairment; AVLT = Rey Auditory Verbal Learning Test; BDRS=Blessed dementia rating score ; CDR-SB= clinical dementia rating stage (sum of boxes); ChEI= cholinesterase inhibitors; CIBIC= Clinician’s Interview-Based Impression of Change; CPT=Connors continuous performance test (reaction time standard error change/interstimulus intervals) ; DHA= docosahexaenoic acid; EPA=eicosapentanoic acid; GDS= Global deterioration scale; Gov = National government supported funding source; MCI= Mild Cognitive impairment; MMSE=Mini Mental State Examination;NCCAM= National Center for Complementary and Alternative Medicine; NPR=nonsense picture recognition; NYPDRT=New York University Paragraph Delayed Recall test ; RBANS= Repeatable Battery for Assessment of Cognitive Status; SD=Standard deviation; SuMC=Subjective memory complaint
Table 2.
Characteristics and validity of studies that do not specify one or two primary efficacy outcomes meeting inclusion criteria for this review, with number of outcomes and significant results
| Study (support source) | Inclusion criteria | Treatment | n | Control | n | Duration | Measures on which control and intervention groups differed significantly; post-intervention mean (sd) and statistical comparisons | Validity (answers to questions 1–5 in method) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | 1 | 2 | 3 | 4 | 5 | |||||||||
| Baker17 (Gov, USA) | aMCI*; sedentary adults | 1 hour, individual, high intensity aerobic exercise, 4 days a week | 23 | Stretching control | 19 | 6 months | DSST | n/a | n/a | F=4·18; P=·05 | n | y | n | y | n |
| Verbal fluency | n/a | n/a | F=4·87; P=·04 | ||||||||||||
| Trail making test B | n/a | n/a | F=4.58; P=.04 | ||||||||||||
| Busse26 (not stated) | MCI*; sedentary adults | 1 hour, twice a week of resistance training | 14 | No treatment | 17 | 9 months | Rivermead memory behavioural test | 18.9 | 15.33 | ANOVA (group/time): p=0.021 | y | n | y | y | n |
| $De Jager30(Gov, UK; Charities) | Aged 70+; MCI* | 0·8mg folic acid, 0·5mg vitamin B12 and 20mg vitamin B6 | 110 | Placebo | 113 | 2 years | CDT correctly performed | Low H1: 13.5(1.7) High H: 12.6(1.9) |
Low H: 13.0(1.7) High H: 12.3(2.4) |
OR=1·3, p=0·015 | y | y | y | y | n |
| Ferris52 (Forest research institute) | Aged 50–79; MMSE 27+; AAMI diagnosis** | Memantine 20mg | 30 | placebo | 30 | 3 months | Cogscreen symbol digit accuracy | n/a | n/a | F=2.86, 0.05 | y | y | y | y | y |
| Finn55 (LumosityInc.) | Aged 60+; MCI diagnosis, MMSE<24 | 30 computerised cognitive training sessions, each with 4–5 exercises | 12 | Waitlist | 13 | 8 weeks | Visual sustained attention (CANTAB) | 0.90 (0.05) | 0.79 (0.13) | F=11·95, p=0·004 | y | y | n | y | n |
| Forlenza59(Gov, Brazil; Charities) | aged 60+; Mayo clinic aMCI criteria | Lithium | 21 | Placebo | 20 | 1 year | n | y | n | y | n | ||||
| Fu36 (Peking University; Gov, China) | MCI*, MMSE 24–27; no ChEI | Wuzi Yanzong granules 4·5g sachet as a drink, twice a day | 18 | Placebo | 18 | 3 months | Memory quotient | 15·83 (17·54) | 5·17 (13·5) | p<0·05 | n | y | y | y | n |
| Koontz22(Janssen) | MCI*, MMSE >25 | Galantamine titrated to 12mg bd | 8 | Placebo | 11 | 16 weeks | Problem solving (CANTAB) | 8·3 (1·89) | 7·0 (1·41) | p=0·023 | n | y | n | y | n |
| Pattern recognition memory | 2449 (807) | 2239(690) | p=0·001 | ||||||||||||
| Krikorian32(Welch food) | SuMC & CDR “mild” | grape juice 444–621ml/d | 5 | Placebo | 7 | 12 weeks | CVLT | 38.6 | 33.2 | F= 5·55; P=0·04 | n | y | y | y | n |
| Luijpen39(Gov, Netherland) | MCI*, living in care home | TENS 30 minutes a day 5 days a week for 6 weeks | 17 | Placebo | 17 | 6 weeks | GDS | 7.35 (3.22) | 11.82 (6.79) | F= 4·35 p= 0·02 | y | y | y | y | n |
| GARS | 39.35 (10.14) | 45.41 (13.71) | F=3·90, p=0·03 | ||||||||||||
| Mowla43(none stated) | MCI* and HDS <10 | Fluoxetine titrated to 20mg | 23 | Placebo | 21 | 8 weeks | MMSE | 27.0 (1.5) | 24.1 ±1.5 | MWU, p=0.003 | n | y | n | y | n |
| Immediate memory (WMS) | 10.8(4.9) | 7.62 (4.7) | MWU p=0.015 | ||||||||||||
| Delayed memory (WMS) | 9.28(5.1) | 5.95(4.3) | MWU p=0.008 | ||||||||||||
| Park33 (LG) | aged 40–75; GLDS 2/3; MMSE 21–26; SuMC | green tea extract and L-theanine | 45 | Placebo | 46 | 16 weeks | n | y | y | y | n | ||||
| Pelton27 | Aged 50+, depression or memory clinic out-patients; SuMC, NPT >1SD below normal; MMSE>19; no ADL impairment | Antidepressant + donepezil | 12 | Antidepressant + placebo | 9 | 12 weeks | Buschke Selective Reminding Test immediate recall | 37.8 (10.9) | 39.0 (12.0) | ANOVA: F=3.0, p=0.05 | y | y | y | y | n |
| Rapp46(none stated) | MCI* | 6, weekly 2 hour groups, psycho-education, memory skills & homework | 9 | Given manual | 10 | 6 weeks | n | y | n | y | n | ||||
| Rondanelli40(none stated) | MCI*, MMSE 24+ | Nutritional supplement (see text) | 11 | Placebo | 14 | 12 weeks | MMSE | −2·07c | 1·01c | p=0·0011 | y | y | y | y | n |
| Rozzini47 (not stated) | Aged 63–78, MCI*, living independently, GDS<5 | Neuropsychological training + ChEI | 15 | ChEI | 22 | 9 months | Short story recall | 11.0 (3.5) | 8.3 (3.5) | t=2·3, p=0·03 | n | y | n | y | n |
| ChEI | 22 | No treatment | |||||||||||||
| Scherder41 (Gov, Netherlands) | MCI* and abbreviated MMSE score of 7+ | Half hour assisted walk, 3× a day/6 weeks | 15 | Half had additional social visit | 15 | 6 weeks | category fluency | 24.80 (11.37) | 20.27 (9.51) | F=5·02; p=0·02 | n | y | n | y | n |
| hand and face exercises, same duration | 13 | category fluency | 25.69 (8.14) | 20.27 (9.51) | F=3·27; p=0·04 | ||||||||||
| Trail making | 273.15 (139.85) | 253.73 (150.03) | F=5·03; p=0·02 | ||||||||||||
| Sinn34 (Gov, Australia; Novasel) | MMSE 22+, SuMC, normal daily functioning | EPA-rich fish oil | 17 | Vegetable oil | 15 | 6 months | GDS | n/a | n/a | LMM: t=2·2, p=0·04 | n | y | y | y | n |
| DHA-rich fish oil | 18 | n/a | n/a | LMM: t=2·6, p<0·01 | |||||||||||
| Letter fluency | n/a | n/a | LMM: t=2·1, p=0·04 | ||||||||||||
| Suzuki42 (Gov, Japan) | Aged 65+, aMCI* | multicomponent exercise group 90mins, 2days/week | 25 | Educational group | 25 | 1 year | MMSE | n/a | n/a | LMM: F=3.4, p=0.04 | y | n | y | y | n |
| WMS-LM I | n/a | n/a | LMM: F=3.9, p=0.03 | ||||||||||||
| Verbal fluency | n/a | n/a | LMM: F=4.1, p=0.02 | ||||||||||||
| Tian37 (Gov, China) | MCI*, SuMC, age 45–70 CDR <1; MMSE 25+ | Ginseng | 30 | Placebo | 15 | 12 weeks | Cognitive score | 5.3c | 6.2c | p<0·05 | n | n | n | n | n |
| Verbal subscale | 18·6c | −6·0c | p<0·05 | ||||||||||||
| Troyer24 ** | aMCI* | 10× 2 hour relaxation and memory strategy groups | 24 | Waitlist | 24 | 6 months | n | n | y | n | n | ||||
| Pelton27 (Gov, USA; Pfizer, Inc. & Esai, Inc.) | aMCI* | 10× 2 hour relaxation and memory strategy groups | 24 | Waitlist | 24 | 6 months | n | n | y | n | n | ||||
| Zhou50 (Gov, China) | MCI*; SuMC, MMSE 24–27, aged < 80, HDS<18 | Shenyin oral liquid 10ml bd | 42 | Placebo | 37 | 1 year | CDT | 1·25 (1·13) | 0·26 (1·1) | p<0·001 | n | n | n | y | n |
| Picture recognition | 5.15 (4·7) | 0·63 (5·0) | p<0·05 | ||||||||||||
| vitamin E tablets 500mg | 38 | 3·68 (6·51) | 0·63(4·99) | p<0·05 | |||||||||||
Summary statistics are given by median split of homocysteine levels; high H indicates value for those scoring above this cut point, Low H for those scoring below
diagnosed according to Petersen criteria 1 ;
Charity, Dejardins Financial & Richter Usher & Vineberg
AAMI diagnosed using Crook criteria;
aMCI=amnestic mild cognitive impairment; AVLT = Rey Auditory Verbal Learning Test; C=mean change rather than mean post-intervention displayed; CANTAB=Cambridge Automated Neuropsychiatric Test Assessment Battery; CDT=Clock drawing test; ChEI= cholinesterase inhibitor; DSST=Digit Symbol Substitution Test EL= eligible for inclusion in this review; CVLT=California verbal learning test; F= MANOVA statistic; GDS=Geriatric Depression Scale; GLDS: Global deterioration scale; GARS=Groninger Activity Restriction Scale; Gov = National government supported funding source; HDS=Hamilton depression score; LG=LG Household & Health Care, Ltd LMM=Linear Mixed Model; M=Mean; MC=Mean change, baseline to follow-up; MD= Mean difference between groups at follow-up; MCI= mild cognitive impairment; MMSE=Minim mental state examination; n/a= not available; NPT= Neuropsychological testing battery; SD=Standard deviation; SuMC=Subjective memory complaint; TCPR=time to correct pattern recognition; OR = odds ratio; WMS=Wechsler Memory Scale III; WMS-LM; Logical Memory subtest of the Wechsler memory scale-revised
To assess risk of bias, two authors (CC, RL) independently evaluated study validity using questions adapted from the Critical Appraisal Skills Programme (CASP) (http://www.sph.nhs.uk/sph-files/casp-appraisal-tools/rct%20appraisal%20tool.pdf; accessed 24/07/12)
Were participants appropriately allocated to intervention and control groups? (Was randomisation independent?)
Were patients and clinicians, as far as possible, “masked” to treatment allocation?
Were all patients who entered the trial accounted for and an intention to treat analysis used?
Were all participants followed up and data collected in the same way?
Was a power calculation carried out, based on one of our outcomes of interest?
Disagreements were resolved by consensus between authors.
Analysis
We compared control and intervention groups post-intervention. We prioritised results from placebo-controlled studies that identified one or two primary outcomes, as these were less likely to have reported significant chance findings. For primary outcome results we displayed standardised outcomes in Forest plots (standardised mean differences (SMD), standardised mean change (SMC), hazard ratios (HR) or odds ratio (OR)) for primary outcomes using statsdirect statistical software version 2.7.9 16; for some studies where these results were unavailable we calculated SMD or SMC from mean (or mean change), appropriate standard deviations and n for intervention and control groups post-intervention. Our calculations sometimes indicated a significant between group difference where the authors’ multivariate calculations did not, or vice versa, and we indicated in the text where this occurred. For all other results we tabulated statistical comparisons between groups, and for the few studies where groups were not directly compared calculated SMD as above. We planned to meta-analyse results where three or more studies with comparable interventions reported comparable outcomes.
Role of funding source
This study was completed by the authors in their capacities as employees of University College London and Johns Hopkins Medicine. These institutions had no role in the study design, collection, analysis and interpretation of data, writing the report, or the decision to submit it for publication.
RESULTS
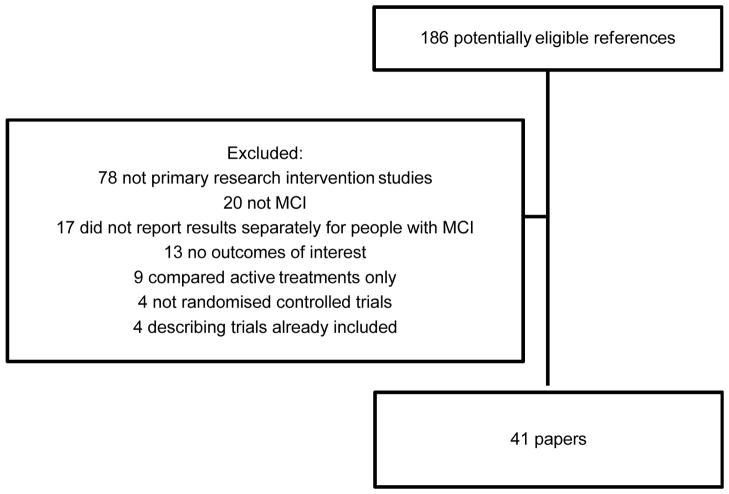
Figure 1 shows a PRISMA diagram describing the results of our search strategy. We included 41 unique studies and listed excluded studies in Appendix A. Five Chinese studies were translated by RL; the remainder were published in English.
Figure 1.
Details of search strategy
Validity
Table 1 describes the 20 (49%) studies that identified one or two primary outcomes: 16 were placebo-controlled and 13 used intention to treat analyses. Other studies are displayed in Table 2. We rated the overall validity of studies (see methods). 11/20 studies that identified primary outcomes and 5/23 studies that did not had Validity Scores (VS) of 4 or 5, the highest levels of evidence.
Description of studies
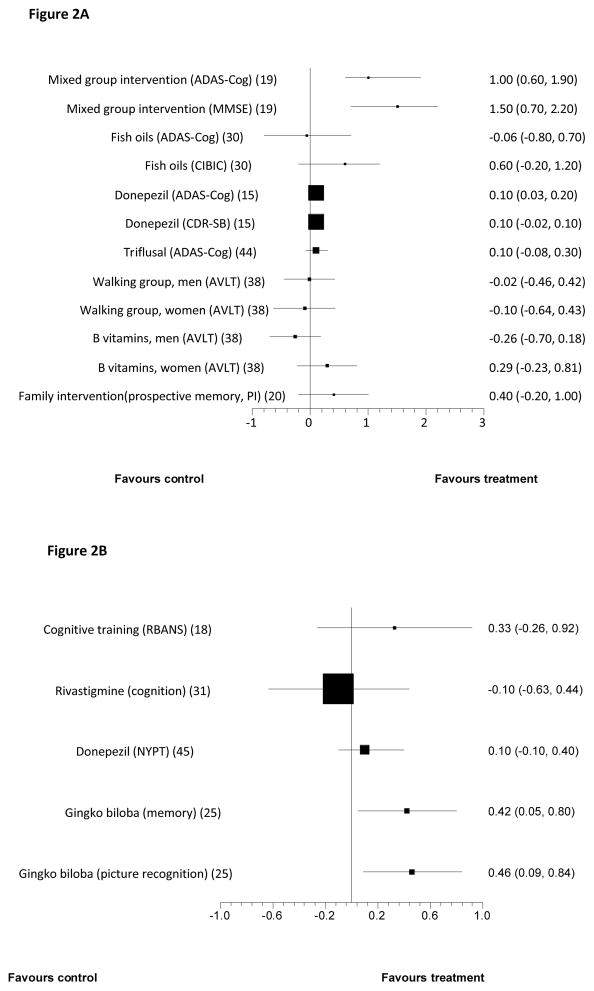
Included studies recruited people with MCI via clinics or clinician referrals17–28, advertisements24;29–34, screening older populations21;35–38, care homes23;39–41, the local Alzheimer’s society34, pre-existing research registers31;42, a rehabilitation center43 or a welfare institution.25 Several did not report the source of participants11;15;44–52. Tables 1 and 2 describe funding sources, inclusion criteria, sample sizes, comparators and duration of studies. Figure 2 reports results on all primary outcomes for which standardised outcomes could be calculated; this was not possible for four studies14;21;51;53 because data was not available or did not approximate the normal distribution, so these results are described in the text. In Table 2, we report statistically significant between group differences from studies without primary outcomes. Non-significant findings for all studies are in Tables 1b and 2b (appendices). The only intervention evaluated in more than two studies was donepezil; three donepezil trials included the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) at 6 months, but as data were not available from one, we could not meta-analyse these findings.
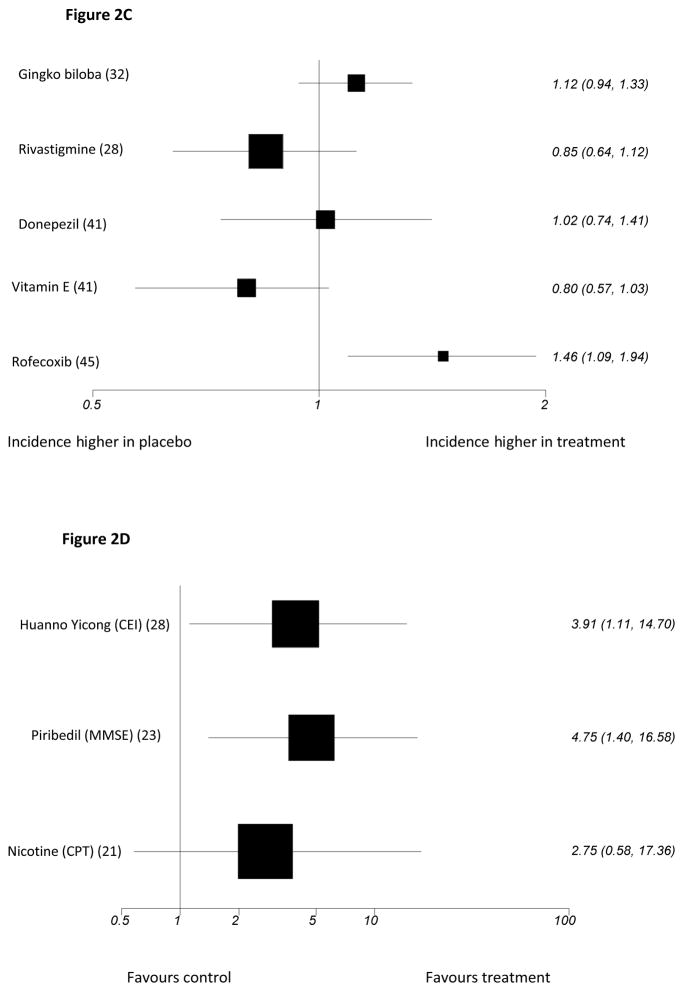
Figure 2.
Forest plots showing between group comparisons for standardised primary outcomes post-intervention for all studies citing one or two primary outcomes(see text for study duration)1
Figure 2A: Studies with outcomes expressed as Standardised Mean Difference (with 95% confidence intervals)
Figure 2B: Studies with outcomes expressed as Standardised Mean Change from baseline (with 95% confidence intervals)
Figure 2C: Studies reporting Hazard Ratios (95% confidence intervals) for incident dementia or Alzheimer’s disease (log scale)
Figure 2D: Studies for which outcomes expressed as odds ratio for response (95% Confidence intervals) (log scale)
See Table 1 for key to abbreviations
Findings on primary outcomes in placebo-controlled studies
Cognition improved in small studies of group memory training, cognitive stimulation and reminiscence over 6 months; and piribedil, a dopamine receptor agonist over 3 months23. In a large, adequately powered study, donepezil improved cognition compared to placebo with 48 weeks’ treatment15. Nicotine patches improved attention in a small study of non-smokers over 6 months which was adequately powered to detect a difference on this outcome21. Huannao Yicong, a Chinese herbal preparation containing ginseng, demonstrated efficacy on a measure of cognition and social functioning over 8 weeks in a small per protocol, responder analysis, but not when mean scores were compared between groups28.
The only finding replicated on primary outcome measures involved galantamine51 (in two studies) and other cholinesterase inhibitors (in two studies31; 45) that did not prevent conversion to dementia. In adequately powered trials, conversion to dementia was also not reduced by: vitamin E45 or Gingko biloba35. Rofecoxib, in an adequately powered study increased dementia incidence49. Cognitive score was not improved by: 2 years of rivastigmine, or 13 months of the non-steroidal anti-inflammatory drug (NSAID) triflusal 44, or, in underpowered studies, by 6 weeks of computerised cognitive training18 or DHA (docosahexaenoic acid) and EPA (eicosapentanoic acid) taken for 6 months29; or a moderate-intensity walking programme compared to low intensity relaxation, balance and flexibility exercises54.
Non-pharmacological interventions
Computer-assisted cognitive training (3 studies)
All three studies were probably under-powered so while results were not promising there was insufficient evidence to draw conclusions about its efficacy. Only Barnes et al18 specified primary outcomes (Validity score (VS)=4). They evaluated a programme that involved distinguishing between similar sounding words and matching sentences with pictures, for 100 minutes daily, 5 days a week. The control group listened to audio books, read an online newspaper and played a computer game. There was no significant difference between groups on the primary outcome, the Repeatable Battery for Assessment of Cognitive Status (RBANS) after 6 weeks (Figure 2). The only significant difference, favouring the intervention was on the delayed memory RBANS subscale (SMD = 0·53, 0·05 to 1·10).
Two studies tested computer-assisted training, but did not specify primary outcomes. Finn et al55 evaluated a 2 month programme in a higher quality study (VS=4), and Rozzini et al47 a 9 months programme (VS=3). In both studies, most results were not significant; only results for the visual attention CANTAB (Cambridge Automated Neuropsychiatric Test Assessment Battery) subscale55 and short story recall47 favoured the interventions (Table 2).
Summary
Global cognition did not improve with cognitive training in two trials, in one of which it was a primary outcome, and there were no consistently significant findings on other secondary outcomes. Studies were all underpowered.
Longer term group psychological interventions (2 studies)
Two lower quality studies tested 6 month group interventions. Results were conflicting. Buschert et al19 (VS=3 and identifying primary outcomes) tested a manualised memory training and cognitive stimulation programme, consisting of 20, 2-hour weekly group sessions comparing 10 people in the intervention group with 12 in the control group. The memory training used mnemonics, calendars, notes and prompts; face-name association and errorless learning. In errorless learning, information was repeated frequently to avoid recall mistakes, with repetitions becoming further apart with successful recalls (spaced retrieval). The programme also included reminiscence, psychomotor and recreational tasks (e.g. playing with balloons), multisensory stimulation and social interaction. Participants did homework, which carers were encouraged to support. The control group met monthly and did paper and pencil exercises. Only participants who attended at least half of sessions were included in analyses. Global cognition, the primary outcome improved in the intervention group in our univariate (Table 1) and the authors’ analyses that controlled for baseline score, age and educational status on both primary outcome measures. Montgomery-Asberg Depression rating scale scores were also lower in the intervention compared with control group adjusting for these factors (mean (sd) 0.7 (1.3) for treatment group and 3.8 (6.1) for controls, F(1,18) = 8.8, p < 0.01).
Troyer et al24 in a small study (VS=1) evaluated ten, 2-hour sessions, including psychoeducation, recreation, memory training and strategies, relaxation and directing to community resources. The authors found no significant differences between groups post-intervention on several measures of recall (Table 2b).
Summary
Twenty sessions of memory training, reminiscence, cognitive stimulation, psychomotor recreation and social interaction improved global cognition on a primary outcome in a single, very small, 6-month placebo-controlled trial which did not carry out an intention to treat analysis.
Ten sessions of memory training, psychoeducation and relaxation did not improve recall on secondary outcomes in one small 6-month trial.
Short term (6 week) psychological group interventions (two studies)
Both studies were lower quality (VS=2) and were underpowered so while neither improved memory there was insufficient evidence to reject this intervention type. Unverzagt et al14 specified primary outcomes and compared three types of group, 10-session interventions teaching specific cognitive strategies with no treatment. These were: memory training strategies; reasoning training, and processing speed training. Booster training was provided to 60% of participants, approximately 11 months later. Memory training participants did not improve post-intervention, or 1 or 2 years later on the composite memory measure versus those receiving no treatment (SMD = −0·01, −0·18 to −0·10; p>0.05). Participants receiving the processing speed intervention improved on processing speed (SMD = −1.4, −1·1 to −0·8; p<0·001) and the reasoning group participants improved on reasoning post-intervention (SMD=0.57;p<0·001, and 2 years (SMD=0·28;p<0·05), but not 1 year later (SMD=0·21;p>0·05), compared to those receiving no treatment.
Rapp et al46 did not specify primary outcomes. They evaluated six, weekly 2-hour groups, of psychoeducation, relaxation, memory strategies (cueing, categorization, chunking, method of loci) and homework. Participants received a manual, which was also sent to the control group who otherwise had no treatment. There were no significant differences between groups on several memory measures, post-intervention or 6 months later.
Summary
Memory did not improve over 6 weeks in two short-term, underpowered group intervention trials teaching memory strategies, which were not placebo controlled.
Specific interventions to improve reasoning and processing speed respectively significantly improved these primary outcomes, in an underpowered single, non-placebo controlled trial.
Family psychological interventions (one study)
This lower quality study indicated that a family psychological intervention might improve prospective memory. Kinsella et al20 (VS=2) compared a course of five, weekly 1.5-hour family intervention groups to a waitlist control. Groups involved problem-solving everyday memory difficulties and practicing possible strategies. Written session material was provided. Results on the primary outcome, an unvalidated (to our knowledge) prospective memory test favoured the intervention 2 weeks and 4 months post-intervention, controlling for baseline scores and age; the SMD for this comparison was not significant in our univariate analysis (Figure 2).
Summary
Prospective memory improved up to 4 months later in this underpowered trial that was not placebo-controlled, on a non-validated measure but only when baseline memory scores were taken into account.
Individual psychological interventions (one study)
This lower quality study (VS=3) found that an individual psychological intervention did not improve memory. Jean et al53 evaluated six individual, 45-minute sessions over 3 weeks, focussing on errorless learning of picture-name associations with spaced retrieval (see earlier). In the control condition, the pictures were presented without spaced retrieval. Participants were given written information about memory. The unvalidated primary outcomes evaluated the number of unknown faces (episodic memory) and famous names (semantic memory) matched correctly. There was no significant treatment effects on these measures in mixed linear models (F(2, 35) = 49·390, F(2, 35) = 11·569), or on MMSE.
Summary
Six individual sessions of errorless learning and spaced retrieval did not improve prospective memory in one placebo-controlled, underpowered study where this was a primary outcome.
Exercise
Exercise has been associated with favourable effects on neuronal survivability and function, neuroinflammation, vascularization, neuroendocrine response to stress, and brain amyloid burden. It also improves cardiovascular health, which is associated with cognitive health17.
Group exercise programmes (two studies)
Results from two studies comparing year-long, twice-weekly, group based exercise programmes to active control conditions were mixed. In a very high quality study (VS=5), van Uffeln54 compared a moderate-intensity walking programme to low intensity relaxation, balance and flexibility exercises, and found no significant effect in any cognitive domain, including the primary outcome of immediate word recall (Figure 2A) or in quality of life. In a lower quality study (VS=3; no primary outcomes specified), Suzuki et al42 compared groups involving circuit training and some outdoor walking to a control group who attended three health promotion classes. The intervention group improved in terms of MMSE score, immediate memory and verbal fluency (Table 2).
Individual exercise programmes (three studies)
None of these lower quality studies specified primary outcomes. Busse et al26 (VS=3) evaluated 9 months of resistance exercises, for one hour twice a week. Scores on a test of every day memory (Rivermead behaviour memory test) improved in the intervention group relative to the no treatment controls, but CAMCog (Cambridge cognition examination) scores and digit span did not.
The remaining two studies had VSs of 2. Baker et al17 compared cognition in adults exercising less than 90 minutes weekly participating in a 6-month high-intensity aerobic exercise intervention or a stretching and balance exercise control. Each intervention was for 1 hour, 4 days a week. The first 8 sessions, and thereafter one session a week were supervised. Adherence was monitored. Significant between-group effects, favouring intervention, were reported on the Digit Symbol Substitution Test (DSST) (attention and processing speed), trail making test B and verbal fluency (Table 2). For verbal fluency, effects were more apparent for category than letter fluency (letter, P=·20; category, P=·03). Scherder et al41 compared: assisted walking for 30 minutes, three days a week for 6 weeks; hand and face exercises for the same duration; and a control group, half of whom received additional social visits. The only significant between group differences, all in favour of the interventions, were on the category fluency and trail making tests (Table 2).
Summary
A very high quality study found that memory, the primary outcome, did not improve with a year long aerobic exercise group compared to a relaxation, balance and flexibility exercise active control group. A lower quality study found that participants in a similar intervention improved on fluency, memory and global cognition relative to a health promotion control.
The studies of individual exercise studies were low quality and their results were inconsistent. Category fluency and trail-making test scores improved with individual aerobic exercise on secondary outcomes in two studies, of 6 weeks and 6 months duration, but no other cognitive outcomes improved in more than one study.
Pharmacological interventions
Cholinesterase inhibitors (9 studies)
Three large studies compared donepezil 10mg daily to placebo, and results were inconsistent. The highest quality (VS=5) by Doody et al15 was a 48-week study that included two primary outcome measures: ADAS-Cog, on which results favoured donepezil, and the CDR (Clinical Dementia Rating) on which there was no significant between-group difference (Figure 2). On secondary outcomes, only patient global assessment differed significantly between groups, in favour of donepezil. The two other large studies were also higher quality (VS=4). Salloway et al48 carried out a 24-week, adequately powered study there were no significant differences between donepezil and placebo on the primary outcomes, the New York University Paragraph Delayed Recall test or the CGIC (Clinician Global Impression of Change), or on any secondary outcomes except for ADAS-Cog. Petersen et al45 found no significant difference between groups on conversion to AD, the primary outcome (Figure 2) or any other measures over 3 years.
One small, lower quality study (VS=1) which did not identify primary outcomes found that donepezil and antidepressant treatment improved immediate memory but not other cognitive outcomes, compared with antidepressants alone27.
Galantamine was investigated in three trials, and results on primary outcomes in the highest quality trials were not significant. Winblad et al51 evaluated galantamine, titrated to 12mg twice daily in two large, high quality, 24 month, placebo-controlled RCTs (VSs=5). Neither reported a significant effect on the primary outcome, incident dementia (22·9% vs 22·6%, p = 0·15; 25·4% vs 31·2%, p = 0·62). On secondary measures, statistical comparisons favoured galantamine in one of the two studies for global functioning (measured on the CDR) and attention (DSST). In a small, lower quality trial, Koontz et al22 only reported significant between-group differences on two subscales of the CANTAB, both measuring executive functioning (Table 2).
One high quality study (VS=5), by Feldman et al31 compared 3–12mg daily of rivastigmine and placebo. There were no significant differences between participants on any measures over 2 years, including the primary outcome, progression to AD (Figure 2).
Finally, Rozzini et al47 compared people receiving any cholinesterase inhibitor to those taking placebo after 1 year in a lower quality study (VS=2). There were no significant between group differences on any measures (Table 2b - appendix).
Summary
Incident AD was not reduced in four, higher quality trials where this was the primary outcome – two evaluated galantamine, one donepezil, and one rivastigmine.
Donepezil improved global cognition in one high quality trial where it was a primary outcome measure, and a second where it was a secondary outcome, but global cognition did not improve in the five other large, high quality trials of cholinesterase inhibitors.
Donepezil did not improve global functioning in one trial where this was a primary outcome. Galantamine improved global functioning in one trial on a secondary outcome measure.
Galantamine improved executive functioning and attention on secondary outcome measures in a single trial.
Donepezil as an adjunct to antidepressants improved immediate memory, also on a secondary outcome.
Piribedil (one study)
Piribedil is a dopamine receptor agonist. Animal models have suggested acetylcholine release in hippocampi and the frontal cortex as a putative mechanism of action. Nagaraja et al23 evaluated this over 3 months in a higher quality trial (VS=4) with 30 in each group, all with MMSE of 21–25; the primary outcome, response on MMSE (predefined as a score of 26+), favoured piribedil (Figure 2). Mean MMSE change from baseline also favoured the intervention group (t=2·83, p<0·01). It was well tolerated.
Summary
Pirbidel improved cognition over 3 months on a primary outcome in one small placebo-controlled study.
Nicotine(one study)
Brain nicotinic receptors are important for cognitive function21. Newhouse et al21 compared transdermal nicotine (titrated to a 15mg patch/day) to placebo in a very high quality study (VS=5 with identified primary outcomes). Attention, measured on the Cognitive Performance Test improved (F=4·89, p =0·031; effect size 0·78) but global functioning on the CGIC did not, on mixed models repeated-measures, analyses of variance. On secondary outcome measures, the treatment group showed less forgetting in between immediate and delayed recall than placebo (F =4·42, p= 0·04), better delayed word recall (F= 5·92, p=0·018) and less anxiety on the older Adult Self Report worries and anxiety subscales (F= 3·48, p =0·04; F=3·14, p=0·05).
Summary
Nicotine patches improved attention, but not global functioning, over 6 months on primary outcomes in one, high quality study. Delayed recall and self-reported anxiety improved on secondary outcomes.
Huannao Yicong (one study)
Li et al28 evaluated this Chinese medicinal compound, which includes ginseng in a study that identified primary outcomes but was low quality (VS=0). Increases or changes in hippocampal mitochondria have been proposed as mechanisms of action. Over 2 months, comparisons favoured the intervention on the primary outcome, response (improvement of 6+ points) on the Cognitive Effect Index (CEI), which comprised the MMSE, Cognitive Capacity Screening Examination (CCSE) and Social Functioning scale (SF-36). We found that the mean difference in CEI scores between groups post-treatment was not significant (Table 1). The analyses excluded people who did not take their medication.
Summary
Results in one, low quality trial were equivocal: more participants taking Huannao Yicong than placebo responded on a cognition and social functioning measure, but the mean difference between groups on this measure was not significant.
Gingko biloba (two studies)
Results from these studies were inconsistent, but the highest quality trials suggested it is ineffective. Proposed mechanisms of action of Gingko biloba include increasing brain blood supply, reducing blood viscosity, modifying neurotransmitter systems, and reducing oxygen free radical density56. In a very high quality study (VS=5), deKosky et al35;57 found that 240mg daily, taken for a median of 6·1 years, did not reduce incident dementia or AD. In a lower quality (VS=3), 6-month study which was not placebo controlled, Zhao et al25 reported that participants prescribed 56·7mg daily Gingko biloba performed better than those receiving no treatment on nonsense picture recognition and logical memory tests (Figure 2).
Summary
On primary outcomes, 240mg daily Gingko biloba did not reduce incident dementia in a very high quality trial over 6 years; while 56·7mg daily improved cognition in a second trial compared with usual treatment.
NSAIDs (two studies)
NSAIDs reduce brain neurotoxic inflammatory responses, so could improve cognition58. Thal et al49, in a very high quality large study (VS=5) found significantly more incident cases of AD over 4 years in participants randomised to 25mg daily of rofecoxib (a COX-2 inhibitor) than those taking placebo (Figure 2). There was no significant difference between groups on secondary outcomes. In 2003, rofecoxib was withdrawn due to cerebrovascular and cardiovascular side effects.
Gomez-Isla et al44 evaluated 900mg a day of triflusal, a COX-1 and COX-2 inhibitor in a lower quality study (VS=3), which was terminated early. It found no significant difference between groups on the primary cognitive outcome, ADAS-Cog (Figure 2). The only significant finding on secondary outcomes was a lower rate of conversion to AD in the intervention group (HR = 2·10; 1·10 to 4·01; P=0·024).
Summary
Rofecoxib increased incident cases of AD in one very high quality study on a primary outcome.
One trial of triflusal reported no significant effect on cognition on a primary outcome measure, although it was associated with a reduced risk of conversion to AD on a secondary outcome.
B vitamins (two studies)
Higher homocysteine plasma concentrations are associated with cognitive impairment; levels are decreased by B vitamins. Two placebo-controlled trials investigated the effectiveness of B vitamins (folic acid, B12 and B6). In a very high quality study (VS=5 and primary outcome identified), van Uffelen38 found no significant difference on the primary outcome of immediate memory over 6 months. On secondary outcomes, the group taking vitamins performed better than placebo group on the DSST (attention and processing speed; longitudinal regression, coefficient not given, p=0.02). De Jager et al30 found in a lower quality (VS=3), 2 year study that executive functioning improved relative to placebo (Table 2).
Summary
Immediate memory did not improve in a high quality study in which this was primary outcome. Out of numerous secondary measures, attention improved in one trial and executive functioning in another, so results were inconsistent.
Vitamin E (two studies)
One large, higher quality trial (VS=4) reported by Petersen et al45 found no significant treatment effect of vitamin E (2000IU) on the primary outcome measure, progression to AD or on a range of secondary outcomes over 3 years. Zhou et al50 reported in a lower quality study (VS=1) that participants receiving vitamin E 500mg daily improved versus placebo on picture recognition (Table 2).
Summary
Vitamin E did not reduce incident dementia in one high quality study on a primary outcome.
In a lower quality study 500mg daily was associated with improvement in picture recognition, a secondary outcome.
Omega-3 Polyunsaturated fatty acids (PUFA) (two studies)
DHA (docosahexaenoic acid) and EPA (eicosapentanoic acid) are dietary PUFA, which have structural and functional roles in the brain. Both these studies had VS of 3. Chiu et al29 found that, as primary outcomes, ADAS-Cog improved over 6 months in people taking 1080mg EPA and 720mg DHA versus placebo after adjusting for age, gender, and education, but no differences were reported on the CIBIC-plus (global functioning). When we calculated SMD for ADAS-Cog at follow-up between groups, there were no significant differences (Table 1).
Sinn et al34 in a small study compared groups receiving EPA-rich fish oil (1670mg EPA and 160mg DHA), and DHA-rich fish oil (1550mg DHA and 400mg EPA) to placebo. Using a linear mixed model analysis, letter fluency scores significantly improved over 6 months in the DHA group versus placebo, and depressive symptoms, measured using the Geriatric Depression Score were reduced in both groups (Table 2).
Summary
Cognition improved on a primary outcome in one study, but only after adjusting for age, gender and education.
Verbal fluency improved with DHA-rich fish oil and depressive symptoms were reduced by DHA and EPA-rich oil after 6 months in a single small study on secondary outcome measures.
Interventions evaluated in single trials without primary outcomes (Table 2)
Ten different interventions have been evaluated in single trials, not specifying one or two primary outcomes. Three were higher quality trials (VSs 4+). These found that: Transcutaneous Electrical Nerve Stimulation (TENS) treatment reduced ADL impairment and depression over 6 weeks in the only trial we reviewed that did not measure cognition39; and that in 3 month trials, memantine improved information processing speed but not cognition52; and a nutritional supplement composed of: DHA 720mg, EPA 286mg, vitamin E 16 mg, soy phospholipids 160mg, tryptophan 95mg and melatonin 5mg40 improved cognition. Fluoxetine43, Shenyin oral liquid50, Ginseng37, Wuzi Yanzong36, grape juice32 Green tea33 and lithium59 were ineffective in single, lower quality trials.
Discussion
Our most striking finding is the lack of good quality evidence except in the pharmacological trials. These enable us to more confidently reject cholinesterase inhibitors as useful in preventing conversion of MCI to dementia, and confirm NICE guidance that cholinesterase inhibitors should not be prescribed clinically for MCI4. The only non-pharmacological intervention for which we found preliminary evidence, in a single, placebo-controlled trial on co-primary outcomes was a heterogeneous group programme of memory training, reminiscence and cognitive stimulation, recreation and social interaction, which improved cognition over 6 months. There was equivocal evidence that a group intervention for families might improve prospective memory from a trial that was not placebo-controlled. We also found replicated evidence on secondary outcomes that category fluency improved with individual aerobic exercise programmes, of 6 weeks and 6 months duration, and delayed recall improved in two studies evaluating computerised cognitive training programmes. These latter studies had multiple secondary outcomes, thus increasing the possibility of a chance finding and the clinical benefit of isolated improvements in these domains is unclear. Most studies were underpowered and lack of evidence of efficacy is not evidence of lack of efficacy.
In pharmacological studies, donepezil improved cognition over a year in two trials, one on a primary outcome, but in general the evidence from seven studies of cholinesterase inhibitors was not promising. The strongest evidence we found was that cholinesterase inhibitors31;51;45 did not reduce the incidence of dementia. Given the safety concerns around the use of cholinesterase inhibitors in MCI8, we think that trials of alternative therapeutic agents are now needed.
Piribedil, a dopamine agonist was effective on a cognitive primary outcome in one study. However, the criteria for MCI were not strict and the authors acknowledge that some of the participants may have had dementia. Nicotine patches improved attention on a primary outcome over 6 months, and also verbal recall on a secondary outcome; and we found equivocal evidence that Huannao Yicong, a Chinese herbal preparation, may improve cognition and social functioning.
It is disappointing that we did not find stronger evidence of efficacy, but nonetheless some of the interventions included warrant further investigation. It is unclear why there have been no further trials of Piribedil in MCI since the positive trial reported in 2001; a trial of Piribedil in people with Parkinson’s disease has not yet reported (NCT01007864). The effectiveness of Huannao Yicong in one trial, albeit of low validity, could indicate that further exploration of Chinese Medicine treatments of MCI may be fruitful. There was limited evidence that exercise therapies improved executive functioning. Resistance training, walking and aerobic exercises may well differ in their effects, and given the positive impact of exercise on general health this would also be an interesting area for future study.
Limitations
This is one of the first comprehensive reviews of all treatments evaluated for MCI. Methodological challenges for MCI trial include deciding inclusion criteria. Nearly two-thirds of studies used Petersen criteria. Some of the studies only included participants with aMCI while others included other subtypes so even within those using Petersen criteria, target groups were heterogeneous. Some people with MCI have prodromal Alzheimer’s Disease or will progress to vascular or other subtypes of dementia. Only two- thirds of people with MCI progress to dementia in their lifetime60, limiting the power of secondary prevention studies that recruit MCI populations. The heterogeneity and instability of the MCI diagnosis militate against finding positive results in MCI trials. It is interesting that while in the trial reported by Petersen et al45, vitamin E did not prevent AD in people with aMCI overall, it was effective at doing so among carriers of one or more apolipoprotein Eε4 alleles, perhaps because this is a more homogenous group, more likely to have prodromal AD. Availability of biomarkers may enable future trials to recruit participants according to disease process rather than clinical deficits. For example, trials of pharmacological agents targeting people with early AD may recruit people with aMCI and probable AD61. Biomarkers may also allow participants to be recruited earlier in the disease process, at the stage of subjective memory impairment which usually precedes MCI. By the time MCI develops the pathological process may be too advanced for treatments to be preventative, perhaps because the brain is by this point very vulnerable to other comorbidities which lead to a dementia, even though progression of the original pathology is halted. A second challenge is deciding on a primary outcome. “Conversion” trials are difficult to power adequately as only 10% of people with MCI convert every year to dementia, and this rate seems to be lower in RCTs31. Incident dementia is often the primary outcome as dementia prevention is a clear goal, but Schneider has suggested it is a problematic endpoint because many participants would be on the cusp of dementia and dementia onset is influenced by numerous biological and environmental factors61. We prioritised placebo-controlled trials, because this evidence is most directly applicable to current practice. There are no evidence-based interventions for MCI and most people with it receive no active treatment. We included a broad range of clinical outcomes, but excluded studies evaluating subjective experiences of memory or biological markers. We included papers in all languages, but only searched English language databases. We planned to meta-analyse findings from three or more studies, but in practice only donepezil was evaluated in more than two studies, and this could not be meta-analysed as required data was unavailable from one study.
Conclusions
There is no evidence, replicated on primary outcomes that any intervention is effective for MCI on the outcomes studied. Results for cholinesterase inhibitors in MCI, the most widely studied intervention, are unpromising. More high quality randomised controlled trials are urgently needed. This review would support further trials of a heterogeneous group psychological intervention, and a dopamine agonist as interventions targeting cognition.
Footnotes
All other authors report no conflicts of interest.
Authors’ contributions
CC and RL searched for studies and rated study validity; RL translated the Chinese papers; CC drafted the paper; all authors critically revised the draft for important intellectual content and approved the final version for submission.
Declarations of interest: CL has received grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation Inc.; he has been a Consultant/Advisor to Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel, BMS; and received Honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor.
Contributor Information
Claudia Cooper, Mental Health Sciences Unit, University College London, Holborn Union Building, Highgate Hill, London N19 5LW, United Kingdom.
Ryan Li, Mental Health Sciences Unit, University College London, Holborn Union Building, Highgate Hill, London N19 5LW, United Kingdom.
Constantine Lyketsos, Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine and Johns Hopkins Bayview, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Gill Livingston, Mental Health Sciences Unit, University College London, Holborn Union Building, Highgate Hill, London N19 5LW, United Kingdom.
Reference List
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Lopez OL, Kuller LH, Becker JT, Dulberg C, Sweet RA, Gach HM, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 2007;64(3):416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 3.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, et al. Conversion to dementia from mild cognitive disorder - The Cache County Study. Neurology. 2006;67(2):229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Clinical Excellence (NICE) Dementia CG42. 2006. [Google Scholar]
- 5.Jorm AF, Korten AE, Henderson AS. The Prevalence of Dementia - A Quantitative Integration of the Literature. Acta Psychiatrica Scandinavica. 1987;76(5):465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 6.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3):CD006104. doi: 10.1002/14651858.CD006104. [DOI] [PubMed] [Google Scholar]
- 8.Loy C, Schneider L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD001747. doi: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011;(1):CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr. 2011;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–296. doi: 10.1097/JGP.0b013e3181c37ce9. [DOI] [PubMed] [Google Scholar]
- 12.Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev. 2012;36(4):1163–1178. doi: 10.1016/j.neubiorev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Stott J, Spector A. A review of the effectiveness of memory interventions in mild cognitive impairment (MCI) Int Psychogeriatr. 2011;23(4):526–538. doi: 10.1017/S1041610210001973. [DOI] [PubMed] [Google Scholar]
- 14.Unverzagt FW, Kasten L, Johnson KE, Rebok GW, Marsiske M, Koepke KM, et al. Effect of memory impairment on training outcomes in ACTIVE. Journal of the International Neuropsychological Society. 2007;13(6):953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 16.Stats direct statistical software [ 2012.
- 17.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer’s disease: a pilot study. J Alzheimers Dis. 2011;25(4):679–694. doi: 10.3233/JAD-2011-100999. [DOI] [PubMed] [Google Scholar]
- 20.Kinsella GJ, Mullaly E, Rand E, Ong B, Burton C, Price S, et al. Early intervention for mild cognitive impairment: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2009;80(7):730–736. doi: 10.1136/jnnp.2008.148346. [DOI] [PubMed] [Google Scholar]
- 21.Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology. 2012;78(2):91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koontz J, Baskys A. Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen. 2005;20(5):295–302. doi: 10.1177/153331750502000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaraja D, Jayashree S. Randomized study of the dopamine receptor agonist piribedil in the treatment of mild cognitive impairment. Am J Psychiatry. 2001;158(9):1517–1519. doi: 10.1176/appi.ajp.158.9.1517. [DOI] [PubMed] [Google Scholar]
- 24.Troyer AK, Murphy KJ, Anderson ND, Moscovitch M, Craik FI. Changing everyday memory behaviour in amnestic mild cognitive impairment: a randomised controlled trial. Neuropsychol Rehabil. 2008;18(1):65–88. doi: 10.1080/09602010701409684. [DOI] [PubMed] [Google Scholar]
- 25.Zhao MX, Dong ZH, Yu ZH, Xiao SY, Li YM. Effects of Ginkgo biloba extract in improving episodic memory of patients with mild cognitive impairment: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2012;10(6):628–634. doi: 10.3736/jcim20120605. [DOI] [PubMed] [Google Scholar]
- 26.Busse AL, Filho JW, Magaldi RM, Coelho VA, Melo AC, Betoni RA, et al. Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment:results of a controlled trial. Einstein. 2008;6(4):402–407. [Google Scholar]
- 27.Pelton GH, Harper OL, Tabert MH, Sackeim HA, Scarmeas N, Roose SP, et al. Randomized double-blind placebo-controlled donepezil augmentation in antidepressant-treated elderly patients with depression and cognitive impairment: a pilot study. International Journal of Geriatric Psychiatry. 2008;23(7):670–676. doi: 10.1002/gps.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Yao MJ, Zhao WM, Guan J, Cai LL, Cui L. A randomized, controlled, double-blind trial of Huannao Yicong capsule in senile patients with mild cognitive impairment. Zhong Xi Yi Jie He Xue Bao. 2008;6(1):25–31. doi: 10.3736/jcim20080106. [DOI] [PubMed] [Google Scholar]
- 29.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 30.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 31.Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 32.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103(5):730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 33.Park SK, Jung IC, Lee WK, Lee YS, Park HK, Go HJ, et al. A combination of green tea extract and l-theanine improves memory and attention in subjects with mild cognitive impairment: a double-blind placebo-controlled study. J Med Food. 2011;14(4):334–343. doi: 10.1089/jmf.2009.1374. [DOI] [PubMed] [Google Scholar]
- 34.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n−3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107(11):1682–1693. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- 35.DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu H, Wang XM, Liu GX. Effects of modified Wuzi Yanzong Granule on memory ability and volume of hippocampus measured by MRI in patients with mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(12):1066–1069. [PubMed] [Google Scholar]
- 37.Tian JZ, Zhu AH, Zhong J. A follow-up study on a randomized, single-blind control of King’s Brain pills in treatment of memory disorder in elderly people with MCI in a Beijing community. Zhongguo Zhong Yao Za Zhi. 2003;28(10):987–991. [PubMed] [Google Scholar]
- 38.van Uffelen JG, Chinapaw MJ, van MW, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42(5):344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 39.Luijpen MW, Swaab DF, Sergeant JA, Scherder EJ. Effects of transcutaneous electrical nerve stimulation (TENS) on self-efficacy and mood in elderly with mild cognitive impairment. Neurorehabil Neural Repair. 2004;18(3):166–175. doi: 10.1177/0888439004268785. [DOI] [PubMed] [Google Scholar]
- 40.Rondanelli M, Opizzi A, Faliva M, Mozzoni M, Antoniello N, Cazzola R, et al. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr Neurosci. 2012;15(2):46–54. doi: 10.1179/1476830511Y.0000000032. [DOI] [PubMed] [Google Scholar]
- 41.Scherder EJ, Van PJ, Deijen JB, Van Der Knokke S, Orlebeke JF, Burgers I, et al. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health. 2005;9(3):272–280. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, et al. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurology. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mowla A, Mosavinasab M, Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled, clinical trial. J Clin Psychopharmacol. 2007;27(1):67–70. doi: 10.1097/JCP.0b013e31802e0002. [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Isla T, Blesa R, Boada M, Clarimon J, Del ST, Domenech G, et al. A randomized, double-blind, placebo controlled-trial of triflusal in mild cognitive impairment: the TRIMCI study. Alzheimer Dis Assoc Disord. 2008;22(1):21–29. doi: 10.1097/WAD.0b013e3181611024. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 46.Rapp S, Brenes G, Marsh AP. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging Ment Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- 47.Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry. 2007;22(4):356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- 48.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 49.Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 50.Zhou RQ, Lin SM, Yuan Q. Clinical study on effect of Shenyin Oral Liquid in treating mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(9):793–795. [PubMed] [Google Scholar]
- 51.Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 52.Ferris S, Schneider L, Farmer M, Kay G, Crook T. A double-blind, placebo-controlled trial of memantine in age-associated memory impairment (memantine in AAMI) International Journal of Geriatric Psychiatry. 2007;22(5):448–455. doi: 10.1002/gps.1711. [DOI] [PubMed] [Google Scholar]
- 53.Jean L, Simard M, Wiederkehr S, Bergeron ME, Turgeon Y, Hudon C, et al. Efficacy of a cognitive training programme for mild cognitive impairment: results of a randomised controlled study. Neuropsychol Rehabil. 2010;20(3):377–405. doi: 10.1080/09602010903343012. [DOI] [PubMed] [Google Scholar]
- 54.van Uffelen JG, Chin APM, Hopman-Rock M, van MW. The effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: a randomized, controlled trial. Qual Life Res. 2007;16(7):1137–1146. doi: 10.1007/s11136-007-9219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn M, McDonald S. Computerised Cognitive Training for Older Persons With Mild Cognitive Impairment: A Pilot Study Using a Randomised Controlled Trial Design. Brain Impairment. 2011;12(3):187–199. [Google Scholar]
- 56.Birks J, Evans JG. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD003120.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Snitz BE, O’Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 59.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351–356. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 60.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. British Journal of Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 61.Schneider LS. The Potential and Limits for Clinical Trials for Early Alzheimer’S Disease and Some Recommendations. Journal of Nutrition Health & Aging. 2010;14(4):295–298. doi: 10.1007/s12603-010-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]