Abstract
Background
Metabolic syndrome and/or its components have been demonstrated to be risk factors for several cancers. They are also found to influence survival in breast, colon and prostate cancer, but the prognostic value of metabolic syndrome in gastric cancer has not been investigated.
Methods
Clinical data and pre-treatment information of metabolic syndrome of 587 patients diagnosed with early stage gastric cancer were retrospectively collected. The associations of metabolic syndrome and/or its components with clinical characteristics and overall survival in early stage gastric cancer were analyzed.
Results
Metabolic syndrome was identified to be associated with a higher tumor cell differentiation (P = 0.036). Metabolic syndrome was also demonstrated to be a significant and independent predictor for better survival in patients aged >50 years old (P = 0.009 in multivariate analysis) or patients with proximal gastric cancer (P = 0.047 in multivariate analysis). No association was found between single metabolic syndrome component and overall survival in early stage gastric cancer. In addition, patients with hypertension might have a trend of better survival through a good control of blood pressure (P = 0.052 in univariate analysis).
Conclusions
Metabolic syndrome was associated with a better tumor cell differentiation in patients with early stage gastric cancer. Moreover, metabolic syndrome was a significant and independent predictor for better survival in patients with old age or proximal tumors.
Introduction
Gastric cancer (GC) is the fourth most common cancer in men and fifth in women, about 10% of annual deaths from cancer are attributed to GC. In 2011, 464,000 men and 273,000 women died from GC according to estimates [1]. The mortality of GC has declined steadily worldwide, especially cancer in the fundus and pylorus [2]. Compared with developed countries, the incidence and mortality is higher in developing countries. Over 70% of new cases and deaths occur in developing countries, and the highest incidence rates are in Eastern Asia, Eastern Europe, and South America [1].
Metabolic syndrome (MetS) was first described as syndrome X in 1988, the basis of MetS was insulin resistance [3]. Till now, a commonly agreed-upon standard of the definition has not been reached yet. But MetS has been similarly defined to be composed of a group of risk factors for cardiovascular disease, which including increased blood pressure, plasma glucose, triglycerides, and body mass index (BMI)/waist circumference, and decreased high density lipoprotein as well [4]. Interestingly, there have been plenty of studies investigating the association between MetS and cancers. MetS and/or its components have been found to be possible risk factors of several cancers, including endometrial cancer [5], [6], ovarian cancer [7], colorectal cancer [6], [8], [9], breast cancer [6], [10], lymphoma, multiple myeloma [11], thyroid cancer [12], cervical cancer [13], liver cancer [6], [14], [15], skin cancer [16], biliary tract cancer [17], pancreatic cancer [6], [18], [19], bladder cancer [6], [20] oesophageal cancer and GC [21], [22].
There also have been some researches about the influence of MetS and/or its components on survival in cancers. MetS was identified to be a risk factor for cancer related mortality as a whole in South Korea and America [23], [24]. Concerning the impacts on certain cancers, patients with MetS were more inclined to suffer from cancer related death in prostate cancer [25], [26], and MetS was associated with poor prognosis in breast cancer as well [26], [27]. In colon cancer, elevated glucose or diabetes mellitus and elevated hypertension were found to be associated with worse survival, while dyslipidemia was exactly the opposite, especially in patients with early stage disease [28]. But the prognostic value of MetS as a whole in the survival of colon cancer patients was controversial [28], [29]. In GC, a few studies showed that BMI had no association with survival, while decreased high-density lipoprotein was a predictor of worse survival [30], [31], [32]. To date, as far as we are concerned, no study concerning about the prognostic value of MetS in the survival of GC patients has been carried out. Thus we collected clinical and survival data of patients with early stage GC, who were diagnosed and received treatment in our hospital, to analyze whether pre-treatment status of MetS and/or its components have any impact on the overall survival (OS) in Chinese patients with early stage GC.
Materials and Methods
Ethics statement
All patients provided written informed consent for their information to be stored and used in the hospital database. Study approval was obtained from independent ethics committees at Cancer Center of Sun Yat-Sen University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Study population
Based on the discharge diagnosis, 587 patients who were diagnosed with early stage GC and received treatment at Sun Yat-sen University Cancer Center in Guangzhou, China from March 23, 1999 to December 7, 2012 were included in this research. All patients were staged from I–III by the American Joint Committee on Cancer (AJCC) gastric cancer (GC) tumor-node-metastasis (TNM) stage system. In addition, all patients received operation for GC. The age range of patients included in our study was 24–83 years old. Cases without complete information about pre-treatment metabolic syndrome (MetS) were excluded. The data of pre-treatment MetS status and clinical information were retrospectively collected. For survival data, patients were followed-up by the follow-up department or at the outpatient department after discharge from hospital.
Criteria for the definition of MetS adopted in our study
Through integrated consideration of different versions of the definition of MetS [4] and the availability of items in those versions of definition from our data, we defined MetS in this research according to the National Cholesterol Education Program's Adult Treatment Panel III (ATP III) (≧3 of 5 criteria necessary): 1). Impaired glucose regulation or diabetes mellitus, Fasting plasma ≧110 mg/dl (6.1 mmol/L). 2). Abdominal obesity, because we didn't find the records of abdomen circumference in clinical data, body mass index (BMI) ≧25 Kg/m2 was applied to be the substitute. 3). Triglycerides ≧150 mg/dl (1.7 mmol/L). 4). High density lipoprotein (HDL) ≦40 mg/dl (1.04 mmol/L) for male, ≦50 mg/dl (1.3 mmol/L) for female. 5). Hypertension, Systolic blood pressure ≧130/diastolic blood pressure ≧80 mm Hg. The data about each component of MetS was collected from clinical records before treatment.
Statistical analysis
The statistical analyses were performed with SPSS for Windows V.13.0. A two tailed p value<0.05 was considered statistically significant. Differences of baseline clinical parameters between MetS positive and negative group were evaluated by chi-square test or Kruskal-Walli H test based on the type of the data and comparisons. Overall survival (OS) was the time interval from the date of diagnosis to death from GC, for patients who remained alive, the data were censored at the date of the last contact. OS curves were plotted with the Kaplan-Meier method, and differences were compared with log-rank test. Variables significantly prognostic in the univariate analysis were included in the multivariate survival analysis. Hazard ratio (HR) and 95% confidence interval (95% CI) were computed with the Cox proportional-hazards model.
Results
The association between MetS and baseline clinical characteristics
There were 587 cases analyzed, detailed information were listed in table 1. Among them, 79 cases (13.46%) were identified to meet the criteria of MetS, the rest of 508 cases (86.54%) were classified to be without MetS. Baseline clinical characteristics were compared between patients with and without MetS. As showed in table 1, there were no significant differences between patients with MetS and without MetS in gender, age, T stage (AJCC, 7th), N stage (AJCC, 7th), tumor size, tumor location, type of operation. While compared with patients with MetS, patients without MetS were testified to have a significantly more aggressive tumor cell differentiation (P = 0.036).
Table 1. Comparison of baseline clinical characteristics According to Metabolic Syndrome Status.
| No. of Patients (%) | |||
| Characteristic | With MetS | Without MetS | P value |
| No. of patients | 79(13.46) | 508(86.54) | |
| Gender | 0.082 | ||
| Male | 48(60.76) | 358(70.47) | |
| Female | 31(39.24) | 150(29.53) | |
| Age (yr) | 0.182 | ||
| ≧50 | 64(81.01) | 376(74.02) | |
| <50 | 15(18.99) | 132(25.98) | |
| T stage(AJCC, 7th) | 0.605 | ||
| T1a+T1b | 11(13.92) | 43(8.46) | |
| T2 | 7(8.86) | 82(16.14) | |
| T3 | 1(1.27) | 23(4.53) | |
| T4a+T4b | 60(75.95) | 360(70.87) | |
| N stage(AJCC, 7th) | 0.205 | ||
| N0 | 39(49.37) | 177(34.84) | |
| N1 | 6(7.59) | 84(16.54) | |
| T2 | 12(15.19) | 118(23.23) | |
| N3a+N3b | 22(27.85) | 129(25.39) | |
| Tumor size (cm) | 0.533 | ||
| ≤5 | 54(68.35) | 329(65.31) | |
| >5 | 25(31.65) | 179(34.69) | |
| Tumor location | 0.111 | ||
| Proximal* | 52(65.82) | 286(56.30) | |
| Distal* | 27(34.18) | 222(43.70) | |
| Degree of differentiation | 0.036 | ||
| Poorly or not differentiated, mucinous or signet ring adenocarcinoma | 53(67.09) | 390(76.77) | |
| Moderately differentiated addenocarcinoma | 18(22.78) | 102(20.08) | |
| Well differentiated addenocarcinoma | 8(10.13) | 16(3.15) | |
| Type of operation | 0.083 | ||
| Radical | 71(89.87) | 481(94.69) | |
| Palliative | 8(10.13) | 27(5.31) | |
Abbreviations and explanations: AJCC: American Joint Committee on Cancer, MetS: metabolic syndrome, Proximal*: the fundus, cardia and body of stomach, Distal*: the pylorus and antrum of stomach.
The impacts of Mets and/or its components on overall survival
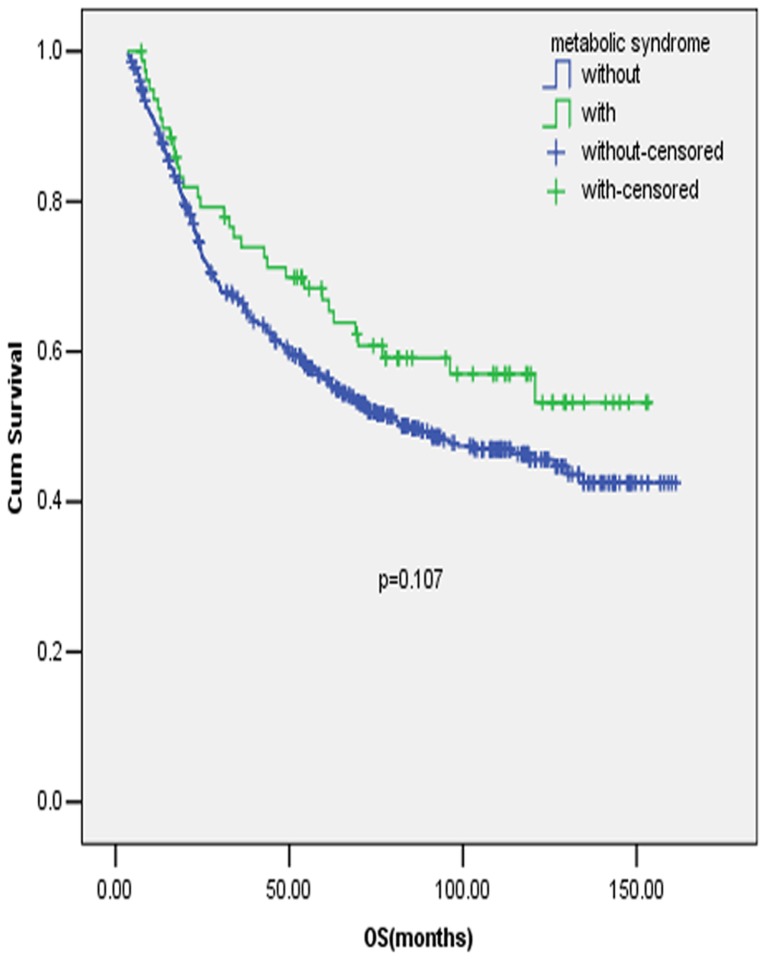
The survival analyses included 586 qualified cases with available survival information, and 1 case was excluded because of losing contact after discharge from our hospital. Judging from the survival curve by Kaplan-Meier method, there was a trend of better OS for patients with MetS compared with those without MetS (figure 1), but the difference was not statistically significant (P = 0.107). No apparent influence of the components of MetS on OS was found, either, nor did the number of items meeting the criteria of the definition of MetS have any important role in the OS of early stage GC.
Figure 1. Prognostic value of MetS in early stage gastric cancer patients.
Full legend: Patients with MetS had a trend of better survival compared with those without MetS in early stage gastric cancer (GC), but the difference was not statistically significant.
Because previous studies found that MetS and/or its components were associated with higher risk of cardiac rather than non-cardiac GC, in addition, the association was not found in early adulthood [21], [22], [33], we further performed the survival analysis stratified by age (<50 y/≧50 y) and tumor location (Proximal/Distal).
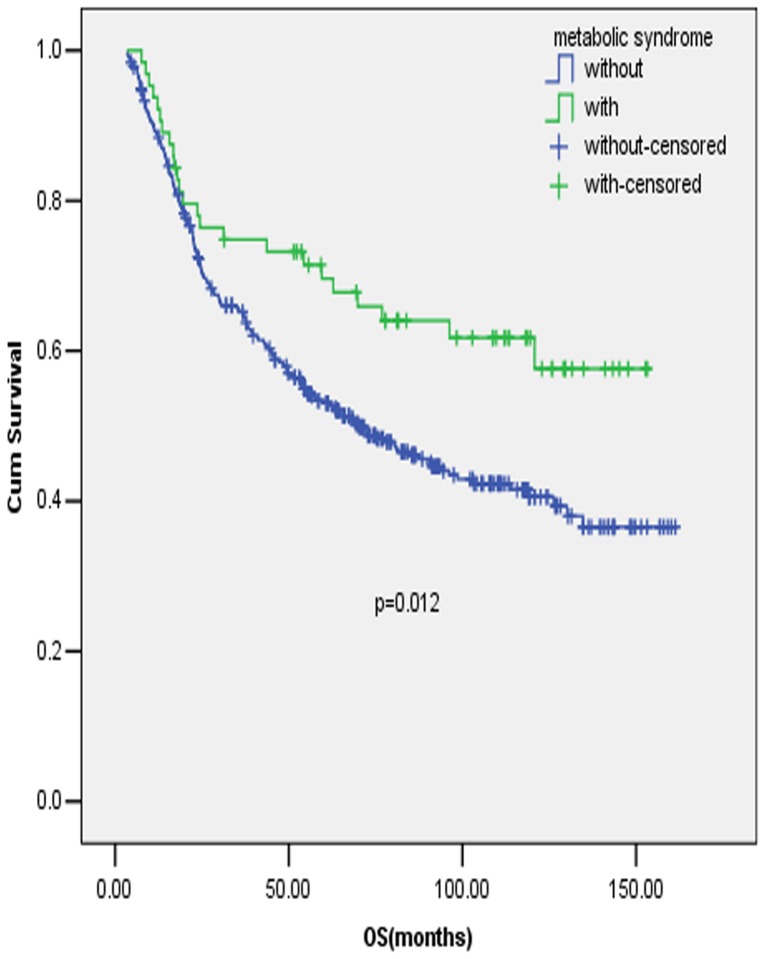
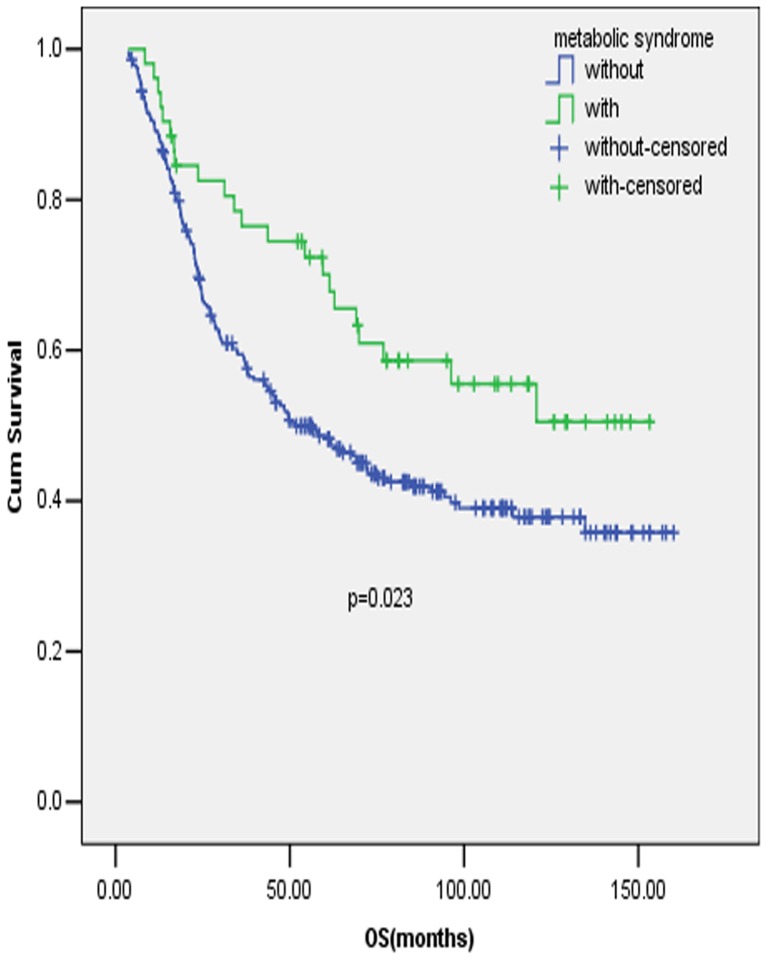
In patients aged <50 years old, MetS or its components were found to have no significant influence on OS in early stage GC. While in patients aged >50 years old, patients with MetS were found to have a significant better survival compared with patients without MetS (figure 2, P = 0.012), after adjusted by items significant prognostic in the univariate analysis, including tumor location, tumor size, T stage, N stage and surgery type, MetS was identified to be an independent prognostic factor for early stage GC in the multivariate analysis. (Table 2, P = 0.009) Similarly, MetS was found to be a significant and independent prognostic factor in proximal early stage GC (figure 3, P = 0.023 and table 3, P = 0.047), but the association was not identified in distal early stage GC.
Figure 2. Prognostic value of MetS in early stage gastric cancer patients with old age.
Full legend: Survival curves of early stage GC patients with and without MetS in patients aged >50 years old.
Table 2. Association between metabolic syndrome (MetS) and its components and overall survival in old patients (age≧50 y) in a univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | ||||
| Factors | Number | Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value |
| Gender (Male/Female) | 320/119 | 0.786(0.575–1.075) | 0.131 | ||
| Tumor location(Proximal/Distal) | 266/173 | 0.638(0.480–0.847) | 0.002 | 0.634(0.476–0.845) | 0.002 |
| Tumor size (≤5 cm/>5 cm) | 283/156 | 1.842(1.413–2.401) | <0.001 | 1.528(1.169–1.999) | 0.002 |
| Degree of differentiation (not differentiated/Poorly differentiated, mucinous or signet ring adenocarcinoma/Moderate differentiated/Well differentiated) | 4/311/103/21 | 0.874(0.694–1.100) | 0.250 | ||
| T stage(AJCC, 7th)(T1a/T1b/T2/T3/T4a/T4b) | 30/7/62/20/273/47 | 1.019(1.010–1.028) | <0.001 | 1.014(1.006–1.023) | 0.001 |
| N stage(AJCC, 7th)(N0/N1/N2/N3a/N3b) | 162/67/103/78/29 | 1.037(1.027–1.046) | <0.001 | 1.035(1.025–1.045) | <0.001 |
| Surgery type(radical/palliative) | 408/31 | 1.625(1.044–2.529) | 0.031 | 1.573(0.998–2.479) | 0.051 |
| Angiolymphatic invasion(No/Yes) | 413/26 | 1.388(0.807–2.386) | 0.236 | ||
| Chemotherapy (No/Yes) | 266/173 | 0.844(0.643–1.107) | 0.219 | ||
| MetS (No/Yes) | 375/64 | 0.584(0.382–0.893) | 0.012 | 0.565(0.368–0.868) | 0.009 |
| Number of MetS Components | 0.166 | ||||
| 0 | 115 | 1(Reference) | |||
| 1 | 152 | 1.007(0.720–1.408) | 0.969 | ||
| 2 | 108 | 1.074(0.749–1.538) | 0.698 | ||
| 3 | 50 | 0.630(0.380–1.046) | 0.074 | ||
| 4 | 14 | 0.476(0.173–1.321) | 0.151 | ||
| Single component meeting the criteria of MetS | |||||
| BMI (No/Yes) | 360/79 | 0.893(0.636–1.253) | 0.512 | ||
| Fasting plasma glucose or diabetes (No/Yes) | 387/52 | 0.888(0.581–1.357) | 0.582 | ||
| Blood pressure (No/Yes) | 244/195 | 0.862(0.660–1.125) | 0.274 | ||
| Triglycerides (No/Yes) | 368/71 | 0.839(0.577–1.221) | 0.359 | ||
| HDL (No/Yes) | 262/177 | 0.851(0.648–1.118) | 0.247 | ||
Abbreviations: AJCC: American Joint Committee on Cancer, MetS: metabolic syndrome, BMI: body mass index, HDL: high density lipoprotein.
Figure 3. Prognostic value of MetS in early stage gastric cancer patients with proximal tumors.
Full legend: Survival curves of early stage GC patients with and without MetS in patients with tumors proximally located.
Table 3. Association between metabolic syndrome (MetS) and overall survival in patients with proximal tumor in a univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | ||||
| Factors | Number | Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value |
| Gender (Male/Female) | 247/91 | 0.711(0.501–1.010) | 0.057 | ||
| age(<50/≧50) | 72/266 | 1.371(0.932–2.018) | 0.109 | ||
| Tumor size (≤5 cm/>5 cm) | 204/134 | 1.785(1.335–2.388) | <0.001 | 1.707(1.272–2.291) | <0.001 |
| Degree of differentiation (Poorly differentiated, mucinous or signet ring adenocarcinoma/Moderate differentiated/Well differentiated) | 233/88/17 | 0.781(0.596–1.024) | 0.074 | ||
| T stage(AJCC, 7th)(T1a/T1b/T2/T3/T4a/T4b) | 21/4/46/17/213/37 | 1.009(1.000–1.018) | 0.041 | 1.006(0.997–1.015) | 0.222 |
| N stage(AJCC, 7th)(N0/N1/N2/N3a/N3b) | 126/52/78/62/20 | 1.036(1.025–1.046) | <0.001 | 1.035(1.025–1.046) | <0.001 |
| Surgery type(radical/palliative) | 312/26 | 1.380(0.857–2.221) | 0.186 | ||
| Angiolymphatic invasion(No/Yes) | 322/16 | 1.805(0.980–3.323) | 0.058 | ||
| Chemotherapy (No/Yes) | 211/127 | 0.879(0.651–1.188) | 0.402 | ||
| MetS (No/Yes) | 286/52 | 0.595(0.381–0.930) | 0.023 | 0.635(0.406–0.993) | 0.047 |
| Number of MetS Components | 0.177 | ||||
| 0 | 93 | 1(reference) | |||
| 1 | 119 | 1.034(0.715–1.495) | 0.860 | ||
| 2 | 74 | 1.173(0.788–1.748) | 0.432 | ||
| 3 | 41 | 0.589(0.340–1.022) | 0.060 | ||
| 4 | 11 | 0.758(0.274–2.101) | 0.595 | ||
| Single component meeting the criteria of MetS | |||||
| BMI (No/Yes) | 282/56 | 0.901(0.615–1.320) | 0.591 | ||
| Fasting plasma glucose or diabetes (No/Yes) | 298/40 | 0.906(0.569–1.442) | 0.677 | ||
| Blood pressure (No/Yes) | 202/136 | 0.951(0.708–1.278) | 0.738 | ||
| Triglycerides (No/Yes) | 279/59 | 0.807(0.539–1.207) | 0.296 | ||
| HDL (No/Yes) | 195/143 | 0.812(0.603–1.093) | 0.169 | ||
Abbreviations: AJCC: American Joint Committee on Cancer, MetS: metabolic syndrome, BMI: body mass index, HDL: high density lipoprotein.
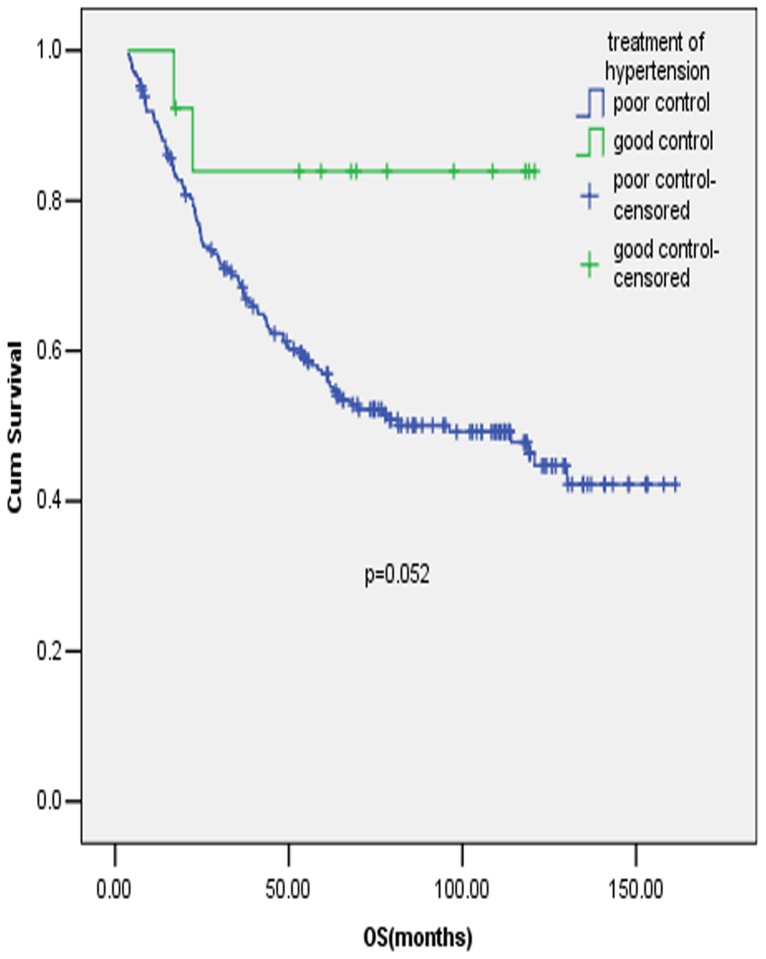
We further explored the effects of the control of MetS disorders on OS in early stage GC. From the medical records, we could only retrospectively find the information about the history of hypertension and diabetes mellitus, thus we couldn't study the effect of treatment for lipid metabolism disturbance on OS. 64 cases were identified to have a history of diabetes mellitus, with 6 (9.38%) cases having the blood glucose controlled to the normal range during the period of GC diagnosis and 58 cases (90.62%) out of control, and whether the blood glucose was controlled or not had no impact on OS in early stage GC. As for the control of blood pressure, among 224 cases with the history of hypertension, 13 (5.80%) cases were classified into the group of good control, and 211 (94.20%) cases in the group of poor control. There was a trend of better survival in the group of good control compared with the group of poor control, but the association was not statistically significant (figure 4, P = 0.052).
Figure 4. Prognostic value of antihypertension in early stage gastric cancer patients.
Full legend: Survival curves of early stage GC patients who had the history of hypertension with blood pressure in good and poor control.
Discussion
Our research found that in early stage (stage I–III) gastric cancer (GC), metabolic syndrome (MetS) was associated with an apparently better differentiation. In addition, MetS predicted a trend of better overall survival (OS) in early stage GC as a whole, but the association was not statistically significant. Further analyses splitting the data by age (<50 y/≧50 y) and tumor location (Proximal/Distal) drew conclusions that in early stage GC, MetS significantly and independently predicted a better survival both in old aged (≧50 y) patients and in patients with tumors proximally located.
There have been a number of studies investigating the association between MetS and/or its components and cancer risks, including GC. It was found that MetS and/or its components were associated with a higher risk of cardiac rather than non-cardiac GC, and the association was not found in early adulthood [21], [22], [33]. The impacts of MetS and/or its components on cancer survivals have been investigated in some other cancers. In prostate and breast cancers, MetS and/or its components were predictors of worse survival [25], [26], [27]. However, analogous studies in another cancer of the digestive system-colon cancer came to controversial conclusions about the importance of MetS in survival. Yang et al. found that elevated glucose or diabetes mellitus and elevated hypertension were predictors of worse survival, in contrast, dyslipidemia was a predictor of better survival, especially in patients with early stage disease in colon cancer [28], [29]. The importance of MetS and/or its components in GC has not been studied so far, hence we focused this study on early stage GC, and the survival analyses were performed in depth through splitting the data by age and tumor location.
Interestingly, our discoveries about the association of MetS and GC were not agreed with some previous conclusions in other cancers. Firstly, we found MetS to be associated with a better differentiation of the GC cells, while some previous studies found MetS and/or its components to be bound up with a more aggressive tumor type in colon and prostate cancer, despite the relevant result was controversial in breast cancer. [25], [34], [35], [36]. Secondly, we identified MetS to be a positive prognostic factor, but a large proportion of studies found MetS negatively predicted the survival in prostate and breast cancers, though the conclusion was inconsistent in colon cancer [25], [27], [28], [29]. Thirdly, when it came to the role of each component, we didn't identify any significant association with OS. In contrast, several previous studies identified elevated blood glucose to be associated with worse prognosis in breast cancer and colon cancer, and elevated blood pressure was associated with worse survival in colon cancer, while dyslipidemia was associated with improved survival in colon cancer [26], [28].
As far as we were concerned, our study was the first to investigate the association between MetS and the malignant grade as well as survival in GC. A previous study by Otani et al. found that lower adipose tissue volume was related to undifferentiated GC rather than differentiated GC [37], it might be a support point for the association of MetS with a better differentiation of the GC cells discovered in our research, owing to MetS was frequently associated with obesity and abnormal lipid metabolism, which indicating a higher adipose tissue volume. In addition, the difference between GC and some other cancers in the relation to the impact of MetS on survival might be attributed to the different tumor properties. Compared with breast, prostate and colon cancers, GC was more aggressive. What's more, GC was located in upper gastrointestinal tract, which meant GC had more influence on patients' nutrition condition. Thus more patients might suffer from malnutrition in GC compared with cancers mentioned above, in turn, a better nutrition status might have more important role to maintain life in GC. More than that, it was found that malnutrition increased the risk of stomach cancer mortality [38]. Compared with patients without MetS, those with MetS were less likely to suffer from Nutrition deficiency. This partly explained why MetS was associated with more favorable OS in GC, which was different from some other cancers. Another explanation for the superior survival in early GC patients with MetS was adiponectin, the level of which was lower in obese compared with lean subjects [39], and obese patients were more frequently associated with MetS. Shin et al. from South Korea found that adiponectin receptor was related to GC development, progression and worse survival [39], although the survival predicting effect was inconsistent with some previous smaller-size studies in Japan and Italy [40], [41]. For the role of each component in GC survival, our finding was in consistent with previous studies about the irrelevance of BMI [30], [32], but concerning about decreased high density lipoprotein, our data didn't show the predictive effect for a worse survival raised by a previous retrospective study with 184 cases [31]. Nevertheless, further investigations are necessary for a better and more accurate understanding of the role of MetS and/or its components on survival in GC.
In the analyses of the influences of the control of MetS disorders on OS in early stage GC, we only studied the associations in hypertension and diabetes mellitus. Due to the limited number of cases included, our conclusions had only limited importance. Although statins have been considered to have anti-tumor effect for a long time, a randomized phase II clinical trial showed no benefit for survival of additional use of pravastatin except for chemotherapy in advanced GC [42]. Despite diabetes was found to be a risk factor for cancer related mortality in GC, insulin use did not affect the outcomes [43], [44]. In addition, as far as we are concerned, there is no evidence for the role of the antihypertensive therapy in GC survival so far. In conclusion, there's plenty of scope to push investigations in this area further.
It was inevitable that there were some limitations in our study. To start with, it was a retrospective research, although all patients enrolled had received radical or palliative operation for GC, we didn't include disease free survival or progress free survival in analysis, because the relevant information might not be credible enough. In addition, the numbers of cases for the analysis of antihypertensive and hypoglycemic therapy were insufficient for dependable conclusions, but we supplied a clue and direction for future researches in this area.
Conclusions
Metabolic syndrome (MetS) was associated with a more moderate tumor cell differentiation in early stage gastric cancer (GC). Besides, MetS was a significant and independent predictor for better survival in certain groups of early stage GC, including patients with old age (age≧50 y) or patients with proximal GC.
Acknowledgments
We gratefully thank Professor Ying Guo in the epidemiology department for their suggestion in the statistical analysis. Besides, we thank the staff members in the Department of Medical Oncology at Sun Yat-sen University Cancer Center for their assistance and suggestion.
Funding Statement
This work was supported by the National High Technology Research and Development Program of China (863 Program), China (NO. 2012AA02A506), the National Natural Science Foundation of China (No. 81372570), The Science and Technology Department of Guangdong Province, China (No. 2012B031800088), and the Medical Scientific Research Foundation of Guangdong Province, China (No. C2011019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, et al. (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125: 666–673. [DOI] [PubMed] [Google Scholar]
- 3. Reaven GM (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 4. Cowey S, Hardy RW (2006) The metabolic syndrome: A high-risk state for cancer? Am J Pathol 169: 1505–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedenreich CM, Biel RK, Lau DC, Csizmadi I, Courneya KS, et al. (2011) Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 20: 2384–2395. [DOI] [PubMed] [Google Scholar]
- 6. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D (2012) Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35: 2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjorge T, Lukanova A, Tretli S, Manjer J, Ulmer H, et al. (2011) Metabolic risk factors and ovarian cancer in the Metabolic Syndrome and Cancer project. Int J Epidemiol 40: 1667–1677. [DOI] [PubMed] [Google Scholar]
- 8. Ulaganathan V, Kandiah M, Zalilah MS, Faizal JA, Fijeraid H, et al. (2012) Colorectal cancer and its association with the metabolic syndrome: a Malaysian multi-centric case-control study. Asian Pac J Cancer Prev 13: 3873–3877. [DOI] [PubMed] [Google Scholar]
- 9. Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S (2013) The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol 47: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reeves KW, McLaughlin V, Fredman L, Ensrud K, Cauley JA (2012) Components of metabolic syndrome and risk of breast cancer by prognostic features in the study of osteoporotic fractures cohort. Cancer Causes Control 23: 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagel G, Stocks T, Spath D, Hjartaker A, Lindkvist B, et al. (2012) Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can). Ann Hematol 91: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 12. Almquist M, Johansen D, Bjorge T, Ulmer H, Lindkvist B, et al. (2011) Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control 22: 743–751. [DOI] [PubMed] [Google Scholar]
- 13. Ulmer H, Bjorge T, Concin H, Lukanova A, Manjer J, et al. (2012) Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me-Can). Gynecol Oncol 125: 330–335. [DOI] [PubMed] [Google Scholar]
- 14. Borena W, Strohmaier S, Lukanova A, Bjorge T, Lindkvist B, et al. (2012) Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer 131: 193–200. [DOI] [PubMed] [Google Scholar]
- 15. Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, et al. (2011) Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 54: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagel G, Bjorge T, Stocks T, Manjer J, Hallmans G, et al. (2012) Metabolic risk factors and skin cancer in the Metabolic Syndrome and Cancer Project (Me-Can). Br J Dermatol 167: 59–67. [DOI] [PubMed] [Google Scholar]
- 17. Shebl FM, Andreotti G, Meyer TE, Gao YT, Rashid A, et al. (2011) Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer 105: 1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosato V, Tavani A, Bosetti C, Pelucchi C, Talamini R, et al. (2011) Metabolic syndrome and pancreatic cancer risk: a case-control study in Italy and meta-analysis. Metabolism 60: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 19. Wu Q, Chen G, Wu WM, Zhou L, You L, et al. (2012) Metabolic syndrome components and risk factors for pancreatic adenocarcinoma: a case-control study in China. Digestion 86: 294–301. [DOI] [PubMed] [Google Scholar]
- 20. Haggstrom C, Stocks T, Rapp K, Bjorge T, Lindkvist B, et al. (2011) Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 128: 1890–1898. [DOI] [PubMed] [Google Scholar]
- 21. O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC (2012) A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 61: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacInnis RJ, English DR, Hopper JL, Giles GG (2006) Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer 118: 2628–2631. [DOI] [PubMed] [Google Scholar]
- 23. Lee JS, Cho SI, Park HS (2010) Metabolic syndrome and cancer-related mortality among Korean men and women. Ann Oncol 21: 640–645. [DOI] [PubMed] [Google Scholar]
- 24. Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, et al. (2009) Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer 45: 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiang YZ, Xiong H, Cui ZL, Jiang SB, Xia QH, et al. (2013) The association between metabolic syndrome and the risk of prostate cancer, high-grade prostate cancer, advanced prostate cancer, prostate cancer-specific mortality and biochemical recurrence. J Exp Clin Cancer Res 32: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stebbing J, Sharma A, North B, Athersuch TJ, Zebrowski A, et al. (2012) A metabolic phenotyping approach to understanding relationships between metabolic syndrome and breast tumour responses to chemotherapy. Ann Oncol 23: 860–866. [DOI] [PubMed] [Google Scholar]
- 27. Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, et al. (2006) Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer 119: 236–238. [DOI] [PubMed] [Google Scholar]
- 28. Yang Y, Mauldin PD, Ebeling M, Hulsey TC, Liu B, et al. (2013) Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer 119: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 29. Shen Z, Ye Y, Bin L, Yin M, Yang X, et al. (2010) Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am J Surg 200: 59–63. [DOI] [PubMed] [Google Scholar]
- 30. Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, et al. (2012) Analysis of the impact of the body mass index in patients with gastric carcinoma. Surg Today 42: 945–949. [DOI] [PubMed] [Google Scholar]
- 31. Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N (2012) Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol 27: 1635–1640. [DOI] [PubMed] [Google Scholar]
- 32. Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, et al. (2013) Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol 20: 780–787. [DOI] [PubMed] [Google Scholar]
- 33. Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA (2007) Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 56: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colonna SV, Douglas CL, Lawrence JA (2012) A retrospective review of the metabolic syndrome in women diagnosed with breast cancer and correlation with estrogen receptor. Breast Cancer Res Treat 131: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Healy LA, Howard JM, Ryan AM, Beddy P, Mehigan B, et al. (2012) Metabolic syndrome and leptin are associated with adverse pathological features in male colorectal cancer patients. Colorectal Dis 14: 157–165. [DOI] [PubMed] [Google Scholar]
- 36. Healy LA, Ryan AM, Carroll P, Ennis D, Crowley V, et al. (2010) Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol) 22: 281–288. [DOI] [PubMed] [Google Scholar]
- 37. Otani K, Kitayama J, Kaisaki S, Ishigami H, Hidemura A, et al. (2008) Early gastric cancer shows different associations with adipose tissue volume depending on histological type. Gastric Cancer 11: 86–95. [DOI] [PubMed] [Google Scholar]
- 38. Li QD, Li H, Li FJ, Wang MS, Li ZJ, et al. (2012) Nutrition deficiency increases the risk of stomach cancer mortality. BMC Cancer 12: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shin E, Park DJ, Kim HH, Won NH, Choe G, et al. (2013) Adiponectin receptor expression in gastric carcinoma: implications in tumor development and progression. J Cancer Res Clin Oncol 139: 709–718. [DOI] [PubMed] [Google Scholar]
- 40. Barresi V, Grosso M, Giuffre G, Tuccari G, Barresi G (2009) The expression of adiponectin receptors Adipo-R1 and Adipo-R2 is associated with an intestinal histotype and longer survival in gastric carcinoma. J Clin Pathol 62: 705–709. [DOI] [PubMed] [Google Scholar]
- 41. Tsukada T, Fushida S, Harada S, Terai S, Yagi Y, et al. (2011) Adiponectin receptor-1 expression is associated with good prognosis in gastric cancer. J Exp Clin Cancer Res 30: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konings IR, van der Gaast A, van der Wijk LJ, de Jongh FE, Eskens FA, et al. (2010) The addition of pravastatin to chemotherapy in advanced gastric carcinoma: a randomised phase II trial. Eur J Cancer 46: 3200–3204. [DOI] [PubMed] [Google Scholar]
- 43. Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J (2012) Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes 120: 217–223. [DOI] [PubMed] [Google Scholar]
- 44. Tseng CH (2011) Diabetes conveys a higher risk of gastric cancer mortality despite an age-standardised decreasing trend in the general population in Taiwan. Gut 60: 774–779. [DOI] [PubMed] [Google Scholar]