Abstract
The existence of a barrier between the central nervous system (CNS) and the systemic circulation has been described over one hundred years ago. Since the discovery that this barrier was instigated by the barrier properties of the brain endothelial cells, research has focused on the identification of pathways how the brain endothelial cells are instructed to form the highly specialized blood-brain barrier (BBB). Even though our current understanding of BBB development is far from complete, recent literature shows a rise in knowledge of CNS-specific cues that can drive BBB development.
In this commentary, we will provide a brief overview of brain selective factors that are critical in the development of barrier properties in the brain endothelium; in particular the role of retinoic acid will be discussed.
Keywords: blood-brain barrier development, radial glial cells, retinoic acid, neurovascular unit
The Blood-Brain Barrier
The vasculature of the brain functions as a specialized barrier to protect the central nervous system (CNS) from the systemic circulation by restricting entry of unwanted molecules and immune cells into the brain, by active removal of cytotoxic compounds. Importantly, the endothelial cells that line the lumen of the cerebral vessels are also equipped to actively supply the brain with essential nutrients and oxygen through specific transport mechanisms. The blood-brain barrier (BBB) is not a rigid barrier but a dynamic structure that receives continuous input from the CNS cells it protects. This allows for a thorough response to the local demands for oxygen, nutrients, and buffering which is crucial for the maintenance of a CNS homeostasis that favors optimal neuronal function.1-4
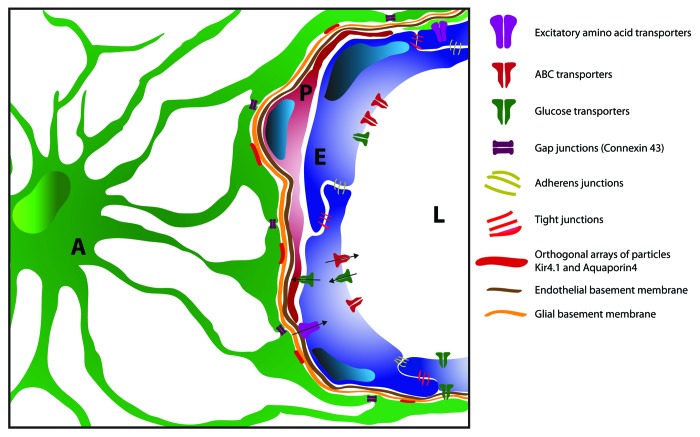
The blood-brain barrier (BBB) is composed of highly specialized brain endothelial cells (ECs) that line the vascular wall of the brain capillaries. Brain ECs form a tight structural barrier by the polarized assembly of tight junction (TJ) proteins at the luminal side of the endothelium and a metabolic barrier by the expression of membrane efflux pumps. Brain ECs are enclosed together with pericytes within the basement membrane onto which astrocytes firmly project their endfeet. Combined with the input of neurons and microglia this structure forms the neurovascular unit (NVU), which ensures optimal protection of the CNS from harmful compounds and the close regulation of CNS homeostasis.1-4 A schematic overview of the NVU in the healthy CNS is depicted in Figure 1.
Figure 1. Schematic overview of the blood-brain barrier in the healthy CNS. The endothelial cells (E) of the BBB are interconnected via tight and adherens junctional complexes and express various uptake [GLUT, expressed at the luminal site (L)] and efflux (ABC-transporters, EEAT) transporters to regulate the flow of nutrients, metabolites, and harmful serum-components. Pericytes (P) tightly interact with the endothelial cells and both are encapsulated by the endothelial basement membrane. Astrocytes (A) project their processes to the endothelial basement membrane that form endfeet structures. These endfeet form the glial basement membrane and completely envelop the CNS microvasculature. Astrocyte endfeet form orthogonal arrays of particles at the glial basement membrane which incorporate high levels of ion (Kir 4.1) and water channels (Aquaporin-4), thereby regulating local osmotic homeostasis. Astrocyte endfeet form gap junctions (connexin 43) with neighboring endfeet which allows for rapid communication and exchange of metabolites.
The BBB limits both transcellular and paracellular passage of cells and molecules from the systemic circulation into the CNS and vice versa. Transcellular passage of hydrophilic molecules is limited due to a low rate of transcytotic vesicles, an extremely low pinocytotic activity, expression of active efflux membrane pumps of the ATP-binding cassette (ABC) family such as P-glycoprotein, and high metabolic activity (cytosolic enzymes and transporters).5-7 To buffer excess amounts of neurotransmitters like glutamate from the CNS, brain ECs possess excitatory amino acid transporters (EAAT) 1–3, to limit neurotoxicity. In order to closely regulate the influx of only those components that are necessary in the CNS, brain ECs harbor specific transporters that actively transport nutrients like glucose into the CNS by glucose transporters (GLUT1–3).1-4
Paracellular diffusion of hydrophilic molecules and trafficking of immune cells is restricted by a network of TJ complexes which allow firm adhesion of brain ECs to each other and sealing of the inter-endothelial space. Adjacent brain ECs express continuous rows of transmembrane proteins that make homophilic contact in the intercellular space and form TJs. Claudins and occludin are the most important membranous components of TJs, but the participation of junctional adhesion molecules (JAMs) and adherens junctions (Cadherins) are important as well. The C-terminal cytoplasmic domain of occludin, the first described TJ protein, is associated with the zona occludens (ZO)-1 and ZO-2 proteins, which link occludin to the cytoskeleton. Claudins make up a family of proteins that consist of at least 23 closely related members. At the BBB, the presence of claudin-1,-3-, 5 and recently -12 has been reported.8-11
The endothelium of the CNS microvasculature shows a high degree of specialization to form the BBB, and the regulatory process behind this specialization is still largely unknown. However, in the past decade, a number of pathways involved in the development and maintenance of the brain ECs have been put forward.
Cells Involved in BBB Functioning
CNS cells surrounding the endothelial layer are thought to provide angiogenic ECs with the appropriate signals for BBB maturation, as well as with the signals required for maintenance of the mature BBB-phenotype. The different cell types involved in the differentiation of the endothelial cells into their barrier phenotype as observed in the BBB are described below.
Astrocytes
Astrocytes are strongly represented within the neurovascular unit, ensheathing over 95% of the abluminal microvascular surface. It was this observation that gave rise to the idea that astrocytic processes formed the BBB, until electron microscopic studies showed that brain ECs were responsible for barrier function in brain microvasculature.
Astrocytes are able to influence a number of features of the brain ECs, leading to increased integrity of the BBB. TJ expression and TJ complex formation and maturation, expression and localization of brain EC transporters, and specialized enzyme systems have been shown to be upregulated under astrocyte influence. The notion that astrocytes can induce and maintain BBB properties in brain ECs through physical interaction and secreted agents has been widely accepted. Astrocyte processes extending toward CNS microvessels terminate in specialized (perivascular) endfeet structures onto the basal lamina surrounding the brain ECs. Astrocyte endfeet associated with brain ECs show a high density of orthogonal arrays of particles (OAPs), organized arrays of ion- and volume-regulating membrane particles identified by freeze fracture, containing channels like the water channel aquaporin-4 (AQP4) and the potassium ion channel Kir 4.1.12 Membrane proteins in OAPs represent a strong polarization of perivascular astrocyte function and correlate with the expression of the basement membrane molecule agrin, an important proteoglycan for BBB integrity,13 responsible for the correct localization of AQP4 (see Figure 1). The distribution of these channels in OAPs is most likely important in the regulation of BBB homeostasis, as disruption of this distribution is associated with microvascular damage in neuropathologies such as Alzheimers disease.14
The observation of astrocyte conditioned medium inducing junction formation in brain ECs in vitro15,16 gave rise to the idea that astrocyte-derived secreted factors were able to influence the BBB properties of brain ECs. Numerous astrocyte-derived agents have since then been described, mainly by in vitro studies, as modulators of brain EC barrier function. Transforming growth factor-β (TGFβ) secreted by astrocytes has been shown to mediate the regulation of tissue plasminogen activator and the anticoagulant thrombomodulin.17 Glial-derived neurotrophic factor (GDNF) has been found to enhance barrier function in brain ECs through the induction of TJ expression.18 Fibroblast growth factor (FGF) was found to decrease BBB permeability19 consistent with the observation that FGF knockout mice show decreased levels of TJ proteins and BBB integrity loss and was shown to be an astrocyte secreted factor that increases TJ expression of brain ECs with an important effect on BBB permeability.19,20 Recently, sonic hedgehog (Shh), a member of the Hh pathway, was shown to be produced and secreted by perivascular astrocytes in the human and mouse adult brain and that microvascular brain ECs expressed the receptors and the intracellular machinery to respond to Hh ligands Pharmacological neutralization of Hh receptors or genetic deletion of Hh receptors lead to enhanced permeability of the BBB and loosening of the TJs.21 Together, these observations confirm the important role of perivascular astrocytes in the regulation of the BBB in the adult CNS.
Pericytes
Pericytes are perivascular, contractile cells that closely associate with capillary walls, and directly contact the brain EC membrane.22 Pericytes are thought to exert influences on the brain EC, through their specialized junctions, involving gap junctions, TJs, and AJs.23,24 Although the molecular mechanism by which pericytes mediate vascular integrity is not yet understood, perivascular pericytes are known to release growth factors and angiogenic molecules which are able to regulate microvascular permeability and angiogenesis. Besides influencing BEC function, pericytes also contribute to the stability of microvessels and cover a large part of the abluminal BEC surface, further influencing BBB permeability.23,24 The regulation of blood flow in CNS capillaries by pericytes has been shown to result from pericytes contracting and relaxing in a regulated manner.
Neurons
Due to the high metabolic need of neurons and the dynamic pattern of neural activity, the CNS requires a tight regulation of the microcirculation which provides the necessary nutrients and means of waste transport. The coupling of brain activity and CNS blood flow is therefore crucial for normal neuronal functioning.25 Although the cellular aspect of this coupling is not fully understood, the involvement of all components of the neurovascular unit seems to be necessary for the regulation of CNS blood flow by neurons.26 Besides the indirect regulation of blood flow, neurons are also found to directly innervate brain EC or brain EC-associated astrocytes functioning as a liaison for neuronal-endothelial coupling. Because disruption of BBB integrity is often found to accompany pathological changes in CNS blood flow, it was suggested that the observed BBB permeability changes were due to active involvement of neurons in BBB integrity. Indeed, noradrenergic, serotonergic, cholinergic, and GABA-ergic neurons have been found to directly contact the microvascular endothelium.27-30 Although the mechanism of action is unknown, neurons innervating the neurovascular unit are thought to regulate BBB permeability. An example of this regulation is shown by the loss of cholinergic innervation of the CNS microvasculature, resulting in impaired cerebrovascular functioning in AD. In short, neurons in the NVU do not only play an active part in the regulation of CNS blood flow, but also seem able to directly influence BBB permeability, through direct innervations of BEC.
Development of the Blood-Brain Barrier
The vascular system of the CNS arises early in embryogenesis through the invasion of vascular plexus-forming angioblasts into the head region, followed by invasion of the CNS by vascular sprouts from the peri-neural vascular plexus, extending toward the ventricles.31 Peripheral vascular system development has been described in detail and various signaling systems taking part in vasculogenesis, angiogenesis, and differentiation have been uncovered. However, few reports exist on developmental CNS-specific cues for the induction of the specialized EC phenotype found at the BBB.
Pericytes
Pericytes have recently emerged as a major contributor to the development of the BBB during embryogenesis, showing increased permeability of the BBB and dysregulation of junction protein localization in the microvasculature of pericyte-deficient mice. Although contradictory evidence exists regarding the induction of brain EC-specific gene expression patterns by pericytes, the presence of these perivascular cells, thought to originate from neural crest cells, is crucial in the very early stage of BBB development. Since many developmental events occur in parallel at the immature BBB, it seems likely that the interplay of radial glia, pericytes, and neural progenitors is needed for the correct patterning and subsequent maturation of the BBB. Interestingly, pericyte-deficiency during development also leads to mislocalization of astrocyte-endfeet on the endothelial basal lamina and a disturbed polarization of endfeet-specific proteins.32,33
Radial glia
During CNS development, radial glial cells provide structural and trophic cues and in the later stages of development differentiated astrocyte endfeet projections provide an almost complete enveloping of the brain microvasculature in adult vertebrates.34 The search for CNS-specific signals which affect the BBB phenotype in brain ECs has implicated astrocytes and glial progenitors as inducers of a specific BBB-phenotype in brain EC. Interestingly, the developmental window in which the vasculature invades the developing CNS and matures into the BBB overlaps with the induction of neuronal differentiation and outgrowth and both systems share guidance cues and differentiation-inducing signaling pathways.35 Radial glial cells have a prominent role in neuronal differentiation and outgrowth, leading to the hypothesis that these cells also function as BBB-inducing cells during development. This is illustrated by the fact that until recently, sonic hedgehog (Shh)-signaling in the CNS has been mostly associated with neuronal patterning and differentiation. However, Shh was shown to be released by fetal astrocytes, and able to induce BBB-properties in ECs during embryonic CNS development.21
Wnt-signaling
CNS-specific Wnt/β-catenin signaling has been firmly implicated in normal BBB-development. Animal models in which β-catenin activation was ablated showed decreased BBB-maturation and increased permeability, whereas no effects were reported on the non-CNS vasculature. Furthermore, the expression of Wnt-ligands associated with canonical β-catenin activation in ECs where shown to be region dependant and only detectable in neural progenitors.36-38
Morphogens and BBB onset: role for retinoic acid
The recent finding that a morphogen like Shh induces BBB properties, paved the way for the investigation of other well-known neuronal differentiation signals, in view of BBB development. Considering the overlap in guidance and differentiation cues of both the neuronal and vascular systems during CNS development, and the involvement of radial glial cells in both processes, we hypothesized that the morphogen retinoic acid (RA) may have a role in BBB onset. Retinoic acid (RA) is a powerful vitamin A-derived morphogen in early CNS development and radial glia-derived RA is crucial in normal neurogenesis.
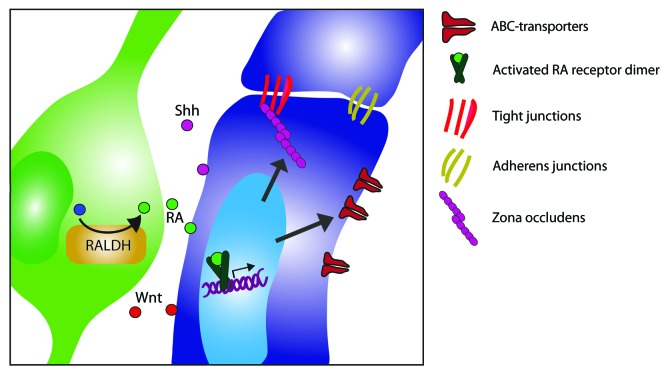
Upon our recent publication,39 the involvement of RA in BBB development during CNS embryogenesis had not been investigated. Using our in vitro models for the human BBB, we first described that astrocyte-conditioned medium (ACM) derived from fetal astrocyte cultures affects barrier properties. We furthermore showed that the enzyme responsible for RA-production, retinaldehyde dehydrogenase (RALDH), is expressed by radial glial cells in the developing human CNS. The release of RA by radial glial cells has also been shown by others, as well as the dependence of neural progenitors on radial glia-derived RA as a differentiation factor. However, our group was the first to report on the effect of radial glia-derived RA on brain ECs and BBB development, which is summarized in Figure 2.
Figure 2. Schematic overview of CNS-specific cues critical in blood-brain barrier development. During development, radial glial cells express the rate-limiting enzyme in the synthesis of retinoic acid, retinaldehyde dehydrogenase (RALDH) and secrete the bioactive form of vitamin A, retinoic acid (RA). In early development, brain endothelial cells express nuclear RA receptors. Upon binding of RA to its receptors, in particular RA receptor β (RAR-β), an activated RA receptor dimer is formed which induces down-stream signaling, leading to increased expression of junction molecules and ABC transporters. Other secreted factors from radial glial cells or surrounding pericytes release soluble factors, such as sonic hedge hog (Shh) and Wnt. The interplay of such molecules induces proper development of the barrier properties within the endothelium and further ensures normal brain endothelial cell function.
Combined with the recent report of sonic hedgehog (Shh)-induced barrier formation,21 these findings reflect the importance of astrocyte precursors in the induction of the BBB during CNS development. Another important consideration is the difference between human and mouse studies, when investigating developmental mechanisms. Most developmental studies incorporate the detailed investigation of the murine CNS, which does not always extrapolate well to the human situation, as exemplified by species-differences in CNS drug discovery. We therefore also investigated the developing human CNS, in which microvascular responsiveness to RA was shown by the presence of endothelial RA receptor-β. Even though this is not final proof, it does indirectly show the involvement of RA signaling of BBB induction in the human CNS.
Further investigation of the effects of RA on human brain ECs in vitro showed a positive regulation of BBB-characteristics like high electrical barrier resistance combined with an increased expression of genes associated with high paracellular and transcellular resistance. However, in vitro studies using brain ECs and varying culture conditions like cell-conditioned media, astrocyte and pericyte co-culture, or endogenous and exogenous ligands have led to a plethora of soluble molecules that are reported to increase brain EC-function or barrier-related gene expression. Whether all described soluble factors are involved in either development or maintenance of the BBB however, cannot be based on in vitro studies alone.39 Importantly, the interplay of the identified pathways in the distinct time frames during embryogenesis and the down stream effects on the BBB development need to be elucidated.
Perspective
A better understanding of all developmental systems converging on brain ECs during BBB development does not merely answer fundamental scientific questions. New insights in BBB development might also provide new avenues of research in neurological disorders with a known involvement of BBB disruption. Regeneration of the barrier with existing developmental pathways or the yet unidentified interplay thereof could prove to be an interesting field for future therapeutic strategies. Continued efforts to understand the fundamental mechanisms behind BBB development and maintenance will widen our view on the possibilities to redefine or restore the barrier upon disruption, and is therefore a crucial part of disease-related BBB research.
Acknowledgments
This work was supported by a grant from the Dutch foundation of MS Research (MS 07–615).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/26882
References
- 1.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 5.Löscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–54. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58:140–61. doi: 10.1124/pr.58.2.3. [Review] [DOI] [PubMed] [Google Scholar]
- 8.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. doi: 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 9.Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol. 2000;79:707–17. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- 10.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–8. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 12.Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–13. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 13.Noell S, Fallier-Becker P, Beyer C, Kröger S, Mack AF, Wolburg H. Effects of agrin on the expression and distribution of the water channel protein aquaporin-4 and volume regulation in cultured astrocytes. Eur J Neurosci. 2007;26:2109–18. doi: 10.1111/j.1460-9568.2007.05850.x. [DOI] [PubMed] [Google Scholar]
- 14.Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging. 2000;21:349–55. doi: 10.1016/S0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 15.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–9. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 16.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke. 1999;30:1671–8. doi: 10.1161/01.STR.30.8.1671. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, et al. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun. 1999;261:108–12. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- 19.el Hafny B, Bourre JM, Roux F. Synergistic stimulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities by retinoic acid and astroglial factors in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1996;167:451–60. doi: 10.1002/(SICI)1097-4652(199606)167:3<451::AID-JCP9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–6. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 22.Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev. 2005;50:258–65. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu F, Sano Y, Maeda T, Abe MA, Nakayama H, Takahashi R, Ueda M, Ohtsuki S, Terasaki T, Obinata M, et al. Peripheral nerve pericytes originating from the blood-nerve barrier expresses tight junctional molecules and transporters as barrier-forming cells. J Cell Physiol. 2008;217:388–99. doi: 10.1002/jcp.21508. [DOI] [PubMed] [Google Scholar]
- 25.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lee EJ, Hung YC, Lee MY. Early alterations in cerebral hemodynamics, brain metabolism, and blood-brain barrier permeability in experimental intracerebral hemorrhage. J Neurosurg. 1999;91:1013–9. doi: 10.3171/jns.1999.91.6.1013. [DOI] [PubMed] [Google Scholar]
- 27.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–62. doi: 10.1016/S0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 28.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab. 1997;17:894–904. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92:163–75. doi: 10.1016/S0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 30.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–71. doi: 10.1002/(SICI)1096-9861(20000529)421:2<161::AID-CNE3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 31.Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–8. doi: 10.1016/0166-2236(90)90043-A. [DOI] [PubMed] [Google Scholar]
- 32.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 33.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chanas-Sacre G, Rogister B, Moonen G, Leprince P. Radial glia phenotype: origin, regulation, and transdifferentiation. J Neurosci Res. 2000;61:357–63. doi: 10.1002/1097-4547(20000815)61:4<357::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- 36.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–17. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–50. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 39.Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, et al. Retinoic acid induces blood-brain barrier development. J Neurosci. 2013;33:1660–71. doi: 10.1523/JNEUROSCI.1338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]