Abstract
The stratified squamous epithelium of the esophagus forms a tight protective barrier. Defects of the barrier function contribute to gastroesophageal reflux disease (GERD), which is manifested as damage to the esophageal epithelium due to exposure to the gastrointestinal refluxate. In this review, we discuss the involvement of NFkB and Nrf2 in esophageal epithelial barrier function. Understanding these molecular pathways in the esophagus may help us develop therapeutic strategies to improve clinical outcomes in patients with GERD.
Keywords: Nrf2, NFkB, GERD, esophageal epithelium, esophageal barrier, esophageal epithelial barrier, tight junction, reflux disease
Gastresophageal reflux disease (GERD) causes heartburn, which leads to substantial impairment of quality of life and work productivity. The prevalence of GERD is increasing worldwide, especially in the developed world. It has become the most commonly seen gastrointestinal disorder. Additionally, some patients with GERD may develop further complications like strictures and Barrett’s esophagus, the latter predisposing to esophageal adenocarcinoma.
GERD is mainly treated with lifestyle modifications, pharmacotherapy, and occasionally anti-reflux surgery. Anti-acid therapy, especially proton pump inhibitors (PPI), remains the mainstay in the management of GERD.1 The long-term use of PPIs has a great efficacy and safety profile.2 However, as many as 10–40% of patients find their symptoms inadequately controlled, especially at night. Weakly acidic reflux, bile reflux, mechanical stimulation, and esophageal hypersensitivity may all contribute to PPI failures.3 In addition, long-term side effects of PPI therapy are still of concern in clinical practice and in public media (New York Times, June 26, 2012): (1) potential cancer promotion due to hypergastrinemia4 or Cyp1A1 induction;5 (2) nutritional malabsorption and subsequent conditions due to hypochlorhydria; (3) interference with the antiplatelet effect of clopidogrel; (4) increased risk of bacterial infections and alterations of gastric microbiota6; (5) increased risk of bone fracture.7 Therefore, it is important to study the mechanism of GERD and develop additional treatment modalities.
Esophageal Defense and GERD
The esophageal defense mechanism is made up of pre-epithelial defense, epithelial defense and post-epithelial defense. The stratum corneum comprises the uppermost seven to eight cell layers and these are primarily designed as a mechanical barrier for protection against luminal contents. The combination of apical junction complexes (AJCs) and apical cell membranes within the stratum corneum is largely what accounts for the esophageal epithelium being ‘electrically tight’.8 The tight junction (TJ), adherens junction and desmosome comprising the AJC are highly complex organelles. For TJ in esophageal epithelium, occludin and claudins (Cldns) are the major proteins bridging the intercellular space, with Cldn1 and Cldn4 being the most highly expressed.9 Overexpression of Cldn4 increases transepithelial electrical resistance (TEER) and lowers paracellular permeability for cations in MDCK II cells,10 suggesting that Cldn4 is potentially a major TJ protein contributing to barrier function in esophageal squamous epithelium. Cldn1 deficiency causes postnatal death due to skin barrier defect to the water loss.11 Cldn4 deficiency causes urothelial hyperplasia and progressive hydronephrosis.12 However, esophageal phenotypes of Cldn1 or Cldn4 deficient mice have not yet been studied. In the adherens junction, the major transmembrane adhesive protein is E-cadherin, and conditional knockout of E-cadherin in mouse esophageal epithelium significantly increased its permeability.13 In desmosomes, the transmembrane adhesive proteins are the desmogleins and desmocollin.
During gastresophageal reflux, luminal acid causes injury of the esophageal epithelium by altering the AJC and causes an early increase in paracellular permeability and dilated intercellular space (DIS).14 These alterations include changes in the TJ proteins (occludin, Cldn1, Cldn3, Cldn4), the number of desmosomes, and cleavage of the adherens junction protein E-cadherin.13 Following reflux-induced injury, oxidative stress and inflammation take place in the esophageal epithelium.15 Using surgical models with rats, we have shown that gastresophageal reflux produces oxidative damage in esophageal epithelium, and antioxidants have chemopreventive effects on adenocarcinoma.16 Oxidative stress may also induce DIS and impair the barrier function as well.17,18 Inflammation is triggered by the release of chemokines and cytokines when cells are stimulated by reflux; this promotes and perpetuates injury directly by attenuating epithelial barrier function, and indirectly by altering neuromuscular transmission of esophageal smooth muscle. Consequently, inflammation can reduce lower esophageal sphincter pressure, impair peristaltic contractility, and contract longitudinal muscle, promoting creation of a hiatal hernia. In effect, inflammation can both augment reflux and delay clearance, producing a vicious cycle.
NFkB, Epithelial Defense and GERD
During gastresophageal reflux, NFκB positively regulates the expression of IL1β, IL6 and IL8 in immune cells and esophageal epithelial cells, which in turn directly or indirectly activates NFκB pathway. Many studies have clearly shown that levels of IL8 and IL1β were upregulated and NFκB was activated in the esophageal epithelium of GERD patients.19 In vitro experiments showed that exposure of human esophageal epithelial cells to acid or bile acid was able to activate NFκB pathway.20,21
NFkB pathway is known to regulate expression of TJ proteins. TNFα has been shown to remove Cldn1 from TJ and increase Cldn2 expression in colon cancer cells, and increase paracellular permeability of the cell monolayer.22 In endothelial cells, TNFα downregulated expression of Cldn5, Cldn1 and ZO1.23 In the esophageal epithelium, ChIP assay demonstrated binding of NFkBp50 subunit to the promoter of murine Cldn4 (unpublished data). It has been shown that deficiency of TJ proteins (e.g., Cldn7, occludin) or an adherens junction protein (p120 catenin) produced chronic inflammation, in which NFkB activation plays a critical role.24-26
Our recent study on mouse models and previous studies on the rabbit model and human patients suggest that the impairment of esophageal barrier function during gastresophagael reflux may result from synergistic actions of chemical injury and NFκB-mediated inflammation. With surgically induced duodenal and mixed reflux models in mice, our study showed that NFκB pathway plays an integral role in gastresophageal reflux-induced impairment of esophageal barrier function. Treatment with an NFκB inhibitor suppressed NFκB-regulated cytokines and inhibited damage to esophageal barrier function in vivo.27 It should be noted that gastric acid reflux in mouse esophagus does not produce evident changes of esophageal barrier function and inflammation, unlike gastric acid exposure in the rabbit model and human patients. The rodent esophagus (e.g., rat, mouse) is lined by a fully keratinized epithelium that is more sensitive to duodenal refluxate than to gastric refluxate. On the contrary, rabbit esophagus with a partially keratinized epithelium and human esophagus with a non-keratinized epithelium are sensitive to both refluxates.
Nrf2, Epithelial Defense and GERD
As a major cellular defense pathway, the Nrf2 pathway is known to regulate expression of enzymes involved in detoxification and anti-oxidative stress response. Nrf2 forms heterodimers with small Maf proteins and binds to the antioxidant response elements of target genes when cells are exposed to oxidative stress or electrophiles. Keap1 (Kelch-like ECH-associated protein 1) inhibits the function of Nrf2 by retaining Nrf2 in the cytoplasm under normal physiological conditions, and by allowing nuclear translocation of Nrf2 under stress conditions.28
The developmental process of mouse esophageal epithelium is divided into three phases based on morphological changes: (a) The specification phase is defined as the phase during which the definitive endoderm differentiates into the esophagus. (b) The metaplasia phase is defined as the phase during which the simple columnar epithelium in the esophagus undergoes metaplastic changes (stratification, squamation and keratinization) into a keratinized stratified squamous epithelium. (c) The maturation phase is defined as the phase during which the keratinized stratified squamous epithelium continues to thicken and finally forms the esophageal epithelium in adults. During esophageal development, the Nrf2/Keap1 pathway had a baseline activity in the metaplasia phase and was further activated in the maturation phase. Keap1 deficient mice developed esophageal hyperkeratosis probably due to activation of PPARβ/δ and the PI3K/Akt pathway. It is concluded that the activity of Nrf2/Keap1 pathway is required for the maturation of the esophagus.29 Since the keratinized layer is the major protective layer against physical stress and chemical injuries, and terminally differentiated keratinocytes express proteins which can provide protection by quenching reactive oxygen species, we further investigated the role of Nrf2 in esophageal epithelial barrier function, using both human samples and mouse surgical models.30 We found Nrf2 was activated in GERD as evidenced by its nuclear localization and overexpression. Consistent with our observations, previous studies on rat models of GERD also showed upregulation of Nrf2 target genes, such as HO1 and MT1.16 Using wild-type and knockout mice, we found that Nrf2 deficiency clearly reduced TEER in the absence of reflux. These data are consistent with previous work suggesting that gastresophageal reflux produced reactive oxygen species, induced DIS, and impaired the barrier function of esophageal epithelium.17,18 Interestingly, both immunostaining and western blotting showed significant downregulation of Cldn4 in the esophageal epithelium of Nrf2 deficient mice. ChIP analysis clearly showed binding of Nrf2 to the predicted sites in the promoter region of mouse Cldn4 gene. We then concluded that Nrf2 deficiency impairs esophageal barrier function through disrupting the integrity of energy-dependent tight junction. In vitro experiments have shown that acid, bile salts, and acidified bile salts modulated the barrier function of epithelial cells by altering the expression and localization of Cldn4.31,32 ChIP-seq experiments have shown that Nrf2 binds to Cldn4 promoter in mouse embryonic fibroblasts.33 Nrf2 activator, Quercetin, significantly enhanced intestinal barrier function through upregulation of Cldn4 in Caco-2 cells.34
A new question may be asked: Can Nrf2 activators regulate esophageal barrier function against gastresophageal reflux? Since the discovery of sulforaphane, a compound extracted from broccoli, many Nrf2 activators have been discovered and tested for their therapeutic efficacy in animal models and clinical trials. Triterpenoids (e.g., CDDO-Me) are among the most potent Nrf2 activators.35 The biochemical mechanism of these compounds has been well studied. These compounds act on Cys residues of Keap1 to activate Nrf2. Because of their good safety profiles, both sulforaphane and CDDO-Me have been used in clinical trials for human diseases associated with oxidative stress such as cancer and chronic kidney disease.36
It should be noted that Nrf2 is a double-edged sword. During cancer chemotherapy, Nrf2 activators actually help cancer cells survive chemotherapy-induced oxidative stress.37 K-Ras(G12D), B-Raf(V619E) and Myc(ERT2) each increased the transcription of Nrf2 to confer a more reduced intracellular environment. Genetic targeting of the Nrf2 pathway impairs K-Ras(G12D)-induced proliferation and tumorigenesis in vivo.38 This is probably why point mutations of Keap1 and Nrf2 have been frequently detected in human squamous cell carcinoma of the lung, head and neck, and the esophagus.
To reconcile the good and bad sides of Nrf2, Kensler and Wakabayashi proposed a concept of “inflection point” to explain why intermittent dosing with Nrf2 activators is unlikely to promote carcinogenesis.39 Indeed, systemic sulforaphane administration did not promote the growth of K-ras(G12D)-induced lung tumors and had no significant effect on the growth of established tumor xenografts in nude mice.40 The consensus in this field is that activation of Nrf2 pathway by small-molecule inducers does not replicate the magnitude and duration of genetic perturbation, and thus will not promote carcinogenesis especially when used in a short-term.
Interactions Between NFkB and Nrf2
The NFkB and Nrf2 pathways interface at several points to control the transcription or function of their downstream targets. Antagonism and synergy occur between members of these two pathways through direct effects on transcription factors, protein-protein interactions, or second-messenger effects on target genes. More and more evidence confirmed the crosstalk between Nrf2 and NFkB under pathological conditions.41 Recent data have suggested the Keap1/Cul3/Rbx1 E3-ubiquitin ligase complex as a commonly machinery regulating both the Nrf2 and the NFkB pathways. Genetic disruption of this complex has been shown to be a key mechanism of NFkB activation in human lung cancer.42 In fact, Keap1 functions as an IKKβ E3 ubiquitin ligase. Deletion of Keap1 led to the accumulation and stabilization of IKKβ and upregulation of NFkB-derived tumor angiogenic factors.43
On one hand, Nrf2 and NFkB can be functionally antagonistic. Absence of Nrf2 induces more aggressive inflammation through activation of NFkB and downstream proinflammatory cytokines in astrocytes.44 Activation of Nrf2-mediated antioxidative signaling attenuates NFkB-mediated inflammatory response in a colitis-associated colorectal cancer model.45 Nrf2−/− mice showed increased pulmonary NFkB activity and inflammatory response after traumatic brain injury.46 Keap1 physically associates with NFkB-p65 in vitro and in vivo, and NFkB signaling inhibits Nrf2 pathway through the interaction of p65 and Keap1.47 p65 also represses the Nrf2 pathway at the transcriptional level through HDAC3 and CBP.48
On the other hand, NFkB and Nrf2 both regulate a subset of target genes, including HO1, GCLC, Gαi2, and IL8. Recently, it has been reported that p65 even has a direct role in antioxidant homeostasis contributing to redox balance in renal cells. There seems to be a synergy between NFkB-p65 and Nrf2 in regulating antioxidative response in these cells.49 NFkB subunits p50 and p65 induce transcription of Nrf2 in AML cells at a specific promoter kB site, and thus encourage resistance to chemotherapy-induced cytotoxicity.50
Conclusion and Perspectives
In conclusion, our studies have shown that gastresophageal reflux exposure activates two key pathways in esophageal epithelium, the NFkB pathway and the Nrf2 pathway. Activation of NFκB pathway impairs esophageal barrier function and activation of Nrf2 pathway may play a protective role. Thus, targeting the NFκB pathway and activating the Nrf2 pathway may strengthen esophageal barrier function against gastresophageal reflux. Both genetic and pharmacological approaches may be used to further test in vivo and in vitro roles of these pathways in esophageal barrier function. For example, to determine whether NFkB and Nrf2 pathways impact barrier function through Cldn1 or Cldn4, gastresophageal reflux can be surgically produced in Cldn1 or Cldn4 conditional knockout mice, EDL2Cre;Cldn1loxP/loxP and EDL2Cre;Cldn4loxP/loxP, in which Cldn1 or Cldn4 is specifically deleted in esophageal squamous epithelium. After treating these mice with an NFkB inhibitor or an Nrf2 activator, we can examine esophageal barrier function, morphology and molecular alterations in these mice. These studies will help us better understand how Nrf2 and NFκB interact with each other in regulating esophageal epithelial defense, and may potentially develop novel GERD therapy (Fig. 1).
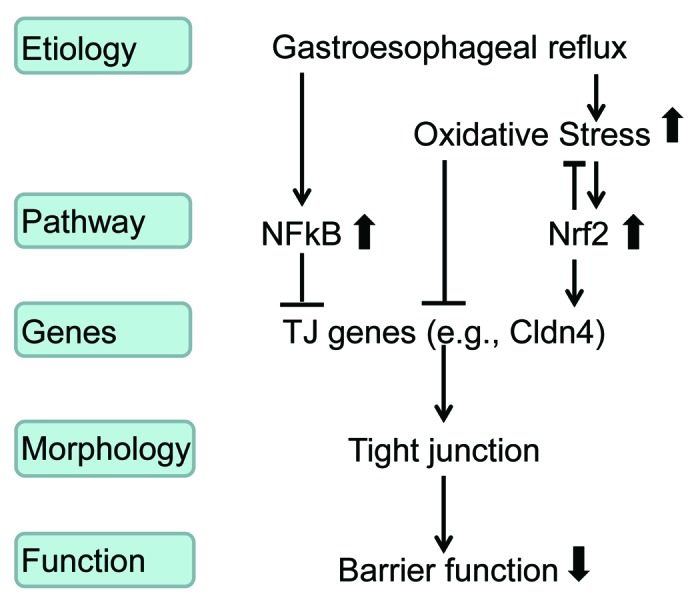
Figure 1. NFkB and Nrf2 regulate esophageal barrier function during gastresophageal reflux. This mechanistic model implies that NFkB inhibitor and Nrf2 activator can be potentially used for GERD therapy.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by research grants from NIH/NCI (U54 CA156735), Takeda Pharmaceutical Company Ltd. (MA-NC-D-156), and North Carolina Biotechnology Center (2011-MRG-1101).
Note
Commentary review with reference to our recent paper: Chen H, Hu Y, Fang Y, Tevebaugh W, Djukic Z, Yamamoto M, Shaheen NJ, Orlando RC, Chen X (2013). Nrf2 deficiency impairs the barrier function of mouse esophageal epithelium. Gut doi:10.1136/gutjnl-2012-303731.
Glossary
Abbreviations:
- AJCs
apical junction complexes
- Cldn
claudin
- DIS
dilated intercellular space
- GERD
gastroesophageal reflux disease
- PPI
proton pump inhibitors
- TJ
tight junction
- TEER
transepithelial electrical resistance
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/27463
References
- 1.Armstrong D, Sifrim D. New pharmacologic approaches in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2010;39:393–418. doi: 10.1016/j.gtc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Bateman DN, Colin-Jones D, Hartz S, Langman M, Logan RF, Mant J, Murphy M, Paterson KR, Rowsell R, Thomas S, et al. SURVEIL (Study of Undetected Reactions, Vigilance Enquiry into Links) Group Mortality study of 18 000 patients treated with omeprazole. Gut. 2003;52:942–6. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershcovici T, Fass R. Management of gastroesophageal reflux disease that does not respond well to proton pump inhibitors. Curr Opin Gastroenterol. 2010;26:367–78. doi: 10.1097/MOG.0b013e32833ae2be. [DOI] [PubMed] [Google Scholar]
- 4.Haigh CR, Attwood SE, Thompson DG, Jankowski JA, Kirton CM, Pritchard DM, Varro A, Dimaline R. Gastrin induces proliferation in Barrett’s metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615–25. doi: 10.1053/gast.2003.50091. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi H, Shimamoto K, Taniai E, Ishii Y, Morita R, Suzuki K, Shibutani M, Mitsumori K. Liver tumor promoting effect of omeprazole in rats and its possible mechanism of action. J Toxicol Sci. 2012;37:491–501. doi: 10.2131/jts.37.491. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Yuan YC, Leontiadis GI, Howden CW. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93–114. doi: 10.1097/MCG.0b013e3182333820. [DOI] [PubMed] [Google Scholar]
- 7.Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873–82. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ Microbiol. 2013 doi: 10.1111/1462-2920.12285. [DOI] [PubMed] [Google Scholar]
- 9.Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 10.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 11.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7:e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovov B, Que J, Tobey NA, Djukic Z, Hogan BL, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039–47. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodland P, Sifrim D. Oesophageal mucosal barrier: a key factor in the pathophysiology of non-erosive reflux disease (NERD) and a potential target for treatment. Gut. 2013 doi: 10.1136/gutjnl-2013-305101. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40:13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–29. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- 17.Farré R, Fornari F, Blondeau K, Vieth M, De Vos R, Bisschops R, Mertens V, Pauwels A, Tack J, Sifrim D. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut. 2010;59:164–9. doi: 10.1136/gut.2009.194191. [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Iijima K, Ara N, Asanuma K, Endo H, Asano N, Koike T, Abe Y, Imatani A, Shimosegawa T. Reactive nitrogen oxide species induce dilatation of the intercellular space of rat esophagus. Scand J Gastroenterol. 2010;45:282–91. doi: 10.3109/00365520903469956. [DOI] [PubMed] [Google Scholar]
- 19.O’Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–64. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldman A, Chen HD, Roesly HB, Hill KA, Tome ME, Dvorak B, Bernstein H, Dvorak K. Characterization of squamous esophageal cells resistant to bile acids at acidic pH: implication for Barrett’s esophagus pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G292–302. doi: 10.1152/ajpgi.00461.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG, Shaker R. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2009;296:G388–98. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- 22.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–55. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 23.Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFκB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2012;57:269–75. doi: 10.1016/j.cyto.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R, Cao J, Chen YH. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142:305–15. doi: 10.1053/j.gastro.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci U S A. 2008;105:15399–404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y, Chen H, Hu Y, Djukic Z, Tevebaugh W, Shaheen NJ, Orlando RC, Hu J, Chen X. Gastroesophageal reflux activates the NF-κB pathway and impairs esophageal barrier function in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G58–65. doi: 10.1152/ajpgi.00438.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Li J, Li H, Hu Y, Tevebaugh W, Yamamoto M, Que J, Chen X. Transcript profiling identifies dynamic gene expression patterns and an important role for Nrf2/Keap1 pathway in the developing mouse esophagus. PLoS One. 2012;7:e36504. doi: 10.1371/journal.pone.0036504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Hu Y, Fang Y, Djukic Z, Yamamoto M, Shaheen NJ, Orlando RC, Chen X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2013 doi: 10.1136/gutjnl-2012-303731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshima T, Koseki J, Chen X, Matsumoto T, Miwa H. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab Invest. 2012;92:22–31. doi: 10.1038/labinvest.2011.139. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Oshima T, Shan J, Fukui H, Watari J, Miwa H. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2012;303:G199–208. doi: 10.1152/ajpgi.00454.2011. [DOI] [PubMed] [Google Scholar]
- 33.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–29. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–74. doi: 10.3945/jn.108.100867. [DOI] [PubMed] [Google Scholar]
- 35.Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev. 2012;64:1055–81. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, Zhu J, Zhang YH, Chen YS, Friesen MD, et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 2011;4:384–95. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623–6. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 38.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–9. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kombairaju P, Ma J, Thimmulappa RK, Yan SG, Gabrielson E, Singh A, Biswal S. Prolonged sulforaphane treatment does not enhance tumorigenesis in oncogenic K-ras and xenograft mouse models of lung cancer. J Carcinog. 2012;11:8. doi: 10.4103/1477-3163.98459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buelna-Chontal M, Zazueta C. Redox activation of Nrf2 & NF-κB: a double end sword? Cell Signal. 2013;25:2548–57. doi: 10.1016/j.cellsig.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Thu KL, Pikor LA, Chari R, Wilson IM, Macaulay CE, English JC, Tsao MS, Gazdar AF, Lam S, Lam WL, et al. Genetic disruption of KEAP1/CUL3 E3 ubiquitin ligase complex components is a key mechanism of NF-kappaB pathway activation in lung cancer. J Thorac Oncol. 2011;6:1521–9. doi: 10.1097/JTO.0b013e3182289479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36:131–40. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm. 2012;2012:217580. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–9. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin W, Zhu L, Guan Q, Chen G, Wang QF, Yin HX, Hang CH, Shi JX, Wang HD. Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Ann Clin Lab Sci. 2008;38:221–7. [PubMed] [Google Scholar]
- 47.Yu M, Li H, Liu Q, Liu F, Tang L, Li C, Yuan Y, Zhan Y, Xu W, Li W, et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011;23:883–92. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–27. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.George LE, Lokhandwala MF, Asghar M. Novel role of NF-κB-p65 in antioxidant homeostasis in human kidney-2 cells. Am J Physiol Renal Physiol. 2012;302:F1440–6. doi: 10.1152/ajprenal.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rushworth SA, Zaitseva L, Murray MY, Shah NM, Bowles KM, MacEwan DJ. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–98. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]


