Abstract
Rho GTPases are cytoskeleton-regulating proteins that mediate the formation of intercellular junctions. Their localized activation by Rho GEFs (guanine-nucleotide exchange factors) and the selective activation of downstream effectors have emerged as areas of active research in the cell adhesion field. We reported recently that the Rho-specific GEFs Syx (Synectin-binding RhoA exchange factor) and TEM4 (Tumor Endothelial Marker 4) are both essential for endothelial junction maturation and barrier function. Syx is recruited to cell contacts via its C-terminal PDZ binding motif and it’s interaction with Mupp1 and the Crumbs polarity complex, while the junctional localization of TEM4 requires it’s N-terminal domain and interaction with the cadherin-catenin complex. Our findings support multiple roles for RhoA in junction formation and maintenance. They also suggest that selective coupling of RhoA activation to Dia1 and/or ROCK signaling is critical for determining endothelial junction integrity.
Keywords: endothelial, epithelial, cell junctions, GEFs, Rho GTPases, cadherin, adhesion
Cell junctions act as gatekeepers to maintain the adhesion and barrier functions of epithelial and endothelial monolayers. The proper regulation of cell contacts is important for physiological events such as vascular development, tissue regeneration, and organ morphogenesis. Under circumstances where cell-cell adhesion is impaired, pathological conditions such as infection, inflammation, edema, ischemia, and cancer can arise, leading to severe and potentially life-threatening consequences. The adherens junctions (AJs) and tight junctions (TJs) are two specialized adhesive structures that regulate the barrier function of epithelial and endothelial monolayers. The main adhesion-related proteins at AJs include cadherins, nectins, and catenins, whereas TJs are defined and regulated by the claudins, occludins, junctional adhesion molecules (JAMs), and zonula occludens (ZOs) proteins.1 Although these AJ and TJ-associated proteins provide some level of adhesiveness to maintain nascent cell-cell contacts, stable cell-cell cohesion requires adhesion receptor clustering and re-organization of the underlying actin cytoskeleton, processes regulated by Rho GTPases.
The Rho GTPases form a subfamily of the Ras superfamily and exist in an active, GTP-bound state and an inactive, GDP-bound state. It is well documented that Rho GTPases regulate cell adhesion by manipulating the dynamics and reorganization of the actin cytoskeleton.2 Rho proteins are themselves controlled by three classes of molecules: the guanine-nucleotide exchange factors (GEFs), which activate Rho proteins at specific sub-cellular locations; the GTPase activating proteins (GAPs), which inactivate Rho GTPases; and the guanine nucleotide-dissociation inhibitors (GDIs), which interact with and sequester inactive Rho proteins in the cytosol. As the molecular “on” switches, the GEFs have become interests of study in the field of cell-cell adhesion.
The Rho GEF Syx in intercellular junctions
We recently published that the Rho-specific synectin-binding exchange factor (Syx; also known as PLEKHG5 or TECH) is essential for the maintenance of intercellular junctions and the barrier function of endothelial cells (ECs).3 Previous studies had established that Syx selectively activates RhoA,4-6 is involved in EC migration,7 and regulates angiogenesis in both the zebrafish and mouse.8 Syx binds to and co-localizes with multi-PDZ domain protein 1 (Mupp1) and protein associated with Lin7 (Pals1),3,9,10 members of the Crumbs polarity complex whose function in ECs is largely unclear. shRNA-mediated downregulation of Syx in human umbilical vein endothelial cells (HUVECs) produced a pronounced reduction in trans-endothelial impedance, a measure of barrier integrity and function, which was coupled to disrupted junctional localization of cortical actin, VE-cadherin and ZO1.3 Consistent with these observations, when compared with syx+/+ mice, syx knockout mice exhibited increased capillary leakage, as evidenced by a higher rate of microsphere extravasation from tracheal venules and increased Evan blue dye leakage from vessels in the skin. Collectively, the data strongly suggest that Syx plays a key role in determining junction stability and vessel permeability both in vitro and in vivo.
Our data also indicated that the opposing effects of Vascular Endothelial Growth Factor (VEGF) and Angiopoietin-1 (Ang1) exert on cell junctions are mediated, at least in part, by junctional Syx. VEGF caused translocation of Syx from cell junctions, resulting in junction disassembly, whereas Ang1 maintained Syx at the junctions, promoting junction stabilization.3 In agreement with the observation that Syx is required for Ang1-mediated stabilization of EC junctions, Ang1 is known to regulate vascular leakiness, vascularization, inflammation, as well as tumor cell intra- and extravasation.11-14 On the other hand, the VEGF-induced translocation of Syx from EC junctions was caused by protein kinase D1 (PKD1)-mediated phosphorylation of Syx at Ser806, which reduced Syx association to its junctional anchor, Mupp1. Removal of junctional Syx resulted in the activation of Src, which can destabilize both AJ15,16 and TJ complexes,17,18 inducing junction disassembly.
RhoA and Src signaling are thought to be involved in a number of conditions that depend on endothelial junction disassembly, including angiogenesis, leukocyte transendothelial migration, viral hemorrhagic fever, sepsis, and acute respiratory distress syndrome (ARDS).16,19-21 The ability of Ang1 to antagonize VEGF-induced junction disassembly by regulating Syx’s subcellular localization, potentially implicates Syx in these pathological conditions. Interestingly, we found that in addition to endothelial cells, Syx regulates junction stability and monolayer integrity of MDCK cells (unpublished observations), a prototypical epithelial cell model. Disruption of monolayer integrity results in inflammation.22 Consistent with this premise, Syx resides in the highly unstable 1p36.3 region, which is commonly altered in developmental and inflammatory diseases,4,23 as well as in cancer.24 Therefore, it is possible that Syx regulates inflammatory responses and/or acts as a tumor suppressor by promoting cell junction integrity. Alternatively, Syx may affect tumor cell invasion and metastasis by regulating tumor cell migration or intra/extravasation, as is the case with Ang1. Syx is already known to affect endothelial cell migration,7,10 and the Syx-associated proteins Pals1 and Pals1-associated TJ protein (PATJ) are required for the directional migration of epithelial cells.25 Importantly, of 303 Syx polymorphisms identified to date, 13 are missense mutations that could affect Syx activity (NCBI and Applied Biosystems SNP databases). It is intriguing to speculate that some of these SNPs may be markers of compromised vascular integrity and elevated susceptibility of individual patients to pathological conditions such as viral hemorrhagic fever, sepsis, ARDS, or tumor metastasis.
Importantly, we identified Diaphanous 1 (Dia1) as a Syx effector that modulates junction stability, which was opposed by ROCK (Rho kinase) activity in HUVECs. Dia and ROCK, two known effectors of Rho signaling, are involved in regulating cytoskeleton dynamics. They are important candidates proposed to impact cell adhesion and junction integrity, through mechanisms that are still largely unclear. The remaining of this article will review the findings of Dia and ROCK in cell junction remodeling, and discuss the possibility that balanced signaling of these two effectors downstream of specific Rho GEFs is required for proper cell junction formation.
Dia and ROCK: friends or foes?
Dia1 and ROCK are crucial regulators of the actin cytoskeleton: the former drives de novo nucleation and elongation of actin filaments,26 the latter stabilizes existing actin filaments and facilitates actomyosin contraction,27 and the two work together to modulate the actin cytoskeleton and regulate cellular behavior.28
Our work demonstrates the importance of Syx-mediated Dia1 signaling in promoting EC adhesion.3 In agreement, downregulation of Dia1 results in junctional defects in HUVECs (Fig. 1). In contrast, activated Dia1 preserves junction stability by sequestering Src, a known mediator of junction disassembly, to prevent β-arrestin-mediated VE-cadherin endocytosis.16,29 In epithelial cells, expression of Dia1 reinforces the adhesion zone, whereas Dia1 knockdown results in decreased junctional E-cadherin.30 Formin-1, a Dia1 related protein, is required at the intercellular junctions to direct actin cable formation and to support cell-cell contact in mouse keratinocytes in vivo.31 Furthermore, Dia1 knockout mice exhibit severe defects in neuroepithelial junction formation and polarization, supporting its indispensable role in regulating cell-cell adhesion and monolayer integrity.32 The observation that Syx is coupled to a polarity complex and acts through Dia1 to stabilize cell-cell adhesion suggests that it acts as a critical checkpoint for maintaining normal cell monolayer physiology.
Figure 1. Effect of silencing endogenous Syx or Dia1 on VE-cadherin and ZO1 localization in HUVECs. Western blot analysis shows the silencing efficacy of the syx or dia1 shRNA constructs in cells.
ROCK, on the other hand, appears to play a negative role in affecting EC junction stability, especially in the absence of Syx.3 Indeed, ROCK can disrupt AJ and TJ integrity by increasing actomyosin contractility.33-36 Inhibition of ROCK activity is directly correlated with an increase in junction stability and monolayer barrier function, suggesting that ROCK plays a role in the induction of monolayer leakiness.37,38 Consistent with this, ROCK is required for VEGF, histamine, thrombin, and bradykinin-induced permeability, indicating that it is one of the key regulators of endothelial junction disassembly.39-41 Interestingly, evidence has also emerged to support a role for ROCK in promoting junction formation and stabilization. Depletion of endogenous ROCK was shown to affect endothelial barrier function and junctional VE-cadherin in ECs,42 as well as cell-cell adhesion in epithelial cells.43 Inhibition of ROCK activity compromised the organization of the actin cytoskeleton and the proper distribution of AJ components, thus preventing normal junction formation.44,45 These effects likely involve ROCK-mediated Myosin II activation at areas of cell-cell contact, which is thought to promote cadherin clustering and stabilization at the AJs.46 It is important to note that the two isoforms of ROCK, ROCK1 and 2, have both overlapping and unique functions in endothelial cells. Both ROCK isoforms regulate stress fiber formation. However, ROCK2 is a more potent driver of endothelial cell migration and in vitro angiogenesis,47,48 perhaps attributed to its selective ability to induce myosin phosphatase and cofilin phosphorylation.49 In contrast, ROCK1 is enriched at areas of endothelial cell-cell contact and physically associates with the cadherin complex via p120 catenin, suggesting that it is more relevant in regulating cell-cell adhesion.43,49 Differences in experimental design, cellular background, and isoform expression could possibly explain the contradicting findings of ROCK in junction regulation; however, the phenotypes observed may represent the outcome of highly regulated signaling, where the function of both Dia1 and ROCK and their crosstalk should be taken into consideration.
Working in synergy: selective and balanced signaling by Dia and ROCK
The following propositions, which take into consideration recent findings, may clarify how Dia1 and ROCK can work together to modulate cell-cell cohesion.
First, the recruitment to specific subcellular locations and the strength of Dia1 and ROCK activities are critical determinants for cellular outcome. Dia1 is proposed to have a housekeeping function in stabilizing cell junctions.30 Even though the mechanism of Dia1 recruitment to cell-cell contacts is unclear, its basal activity is likely maintained by upstream RhoA signaling at the junctions. ROCK binds several junctional proteins such as p120, Shroom 3, and p114RhoGEF,43,50,51 suggesting that its recruitment to the cell membrane is highly regulated and that its activation is dependent on its immediate associated protein complex. In a quiescent cell monolayer, ROCK and Dia1 may function together28 to stabilize the junctions through: a) the clustering of adhesion receptors and the suppression of cadherin endocytosis, and b) by promoting the formation and alignment of actomyosin bundles parallel to the cell border at the mature AJs of the Zonula Adherens (ZA) (Fig. 2). In the context of a sealed, patent monolayer, the parallel orientation of the actomyosin bundles could minimize contractile forces directly impacting the cell-cell junctions, allowing adhesive forces to prevail. We postulate that when junction integrity is altered due to depletion of essential junctional proteins (e.g., in cancer), suppression of Dia1, or presence of extracellular signaling inducing further ROCK activation, the balanced act between Dia1 and ROCK is compromised, causing either unopposed or increased actomyosin contractility that induces junction disassembly and promotes cell migration52 (Fig. 3). While not tested directly, it is possible that these conditions induce the transition of the belt-like ZA to the recently reported spot-like (or punctate) AJ morphology (pAJs),53 and the change from parallel circumferential actomyosin bundles to actin fibers laterally attached to the pAJs (Fig. 3). Our data are consistent with such a transition, which could lead to increased contractile forces exerted at the pAJs, leading to ROCK-mediated junction disassembly.3 Indeed, we showed that ROCK inhibition partially reverses junctional defects in Syx downregulated cells,3 supporting the hypothesis that the combined effect of Dia and ROCK determines EC junction stability.
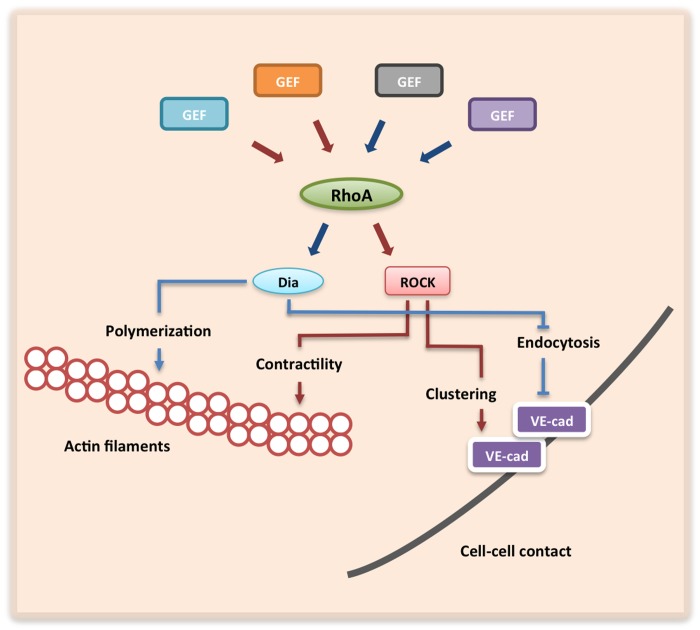
Figure 2. Effects of Dia1 and ROCK signaling on endothelial junctions and their underlying actin cytoskeleton. Junctionally localized RhoGEFs selectively activate Dia1 (blue arrows) or ROCK (red arrows) via RhoA. Dia1 promotes actin polymerization while ROCK induces actomyosin-induced contractility. Furthermore, Dia1 and ROCK promote junction integrity independently by inhibiting VE-cadherin endocytosis and promoting VE-cadherin clustering, respectively, at sites of cell-cell contact.
Figure 3. Proposed model of balanced Dia1 and ROCK activities in cell-cell cohesion. Under normal physiological conditions, the two Rho effectors work in synergy to align and maintain a circumferential actomyosin bundle, and to promote the formation of mature belt-like endothelial AJs. However, loss of Dia1 signaling, and/or increased signaling via ROCK promotes the transition of belt-like AJs to punctate AJs. This transition is accompanied by a shift from the parallel, circumferential actomyosin bundles into unorganized, radially attached actin stress fibers that exert contractile forces able to disrupt junction stability.
Additionally, the direct crosstalk between Dia and ROCK may be an integral mechanism for modulating cell-cell cohesion. Formin homology domain protein 1 (FHOD1), an endothelial enriched protein closely related to Dia1, is activated via phosphorylation by ROCK.54 FHOD1 also physically associates with ROCK in a Src-dependent manner and facilitates cytoskeleton rearrangement.55 Furthermore, phosphorylation by ROCK increases the ability of mDia2, another Dia family member, to polymerize actin filaments.56 Interestingly, Dia1 can function upstream of Rho/ROCK signaling in mediating neutrophil chemotaxis and regulating cancer cell morphology.57,58 Although additional studies are needed to elucidate the specific roles of these two Rho effectors in the context of the intercellular junctions, it is likely that junctional Dia and ROCK corroborate with each other to promote the formation and stabilization of intercellular junctions, while Dia1 or ROCK misregulation leading to unopposed contractility at the pAJs induces junction disassembly.
An important question for further study is how junctional Rho activation is coupled to either Dia or ROCK signaling. A potential mechanistic explanation is the existence of different junctional RhoGEF-Rho modules that determine selective effector signaling. For example, p114RhoGEF-associated ROCK II activation51 may cooperate with Syx/Dia1 in mediating junction stability. The close proximity of the two Rho GEF complexes at the junctions could facilitate the synergistic effect of Dia and ROCK, thus positively influencing intercellular junctions. A similar condition may exist between the Rho GEFs TEM4 and Syx. As with Syx, TEM4 is essential for EC junction integrity and barrier function. Unlike Syx, which utilizes a C-terminal PDZ binding motif to interact with a polarity complex and localize to the junctions, TEM4 utilizes its N-terminal domain to associate with the cadherin-catenin complex at the AJs.59 While the downstream effector of TEM4-mediated RhoA activation is currently unknown, the data indicate that at least two Rho-specific exchange factors, TEM4 and Syx, are essential for EC junction integrity and barrier function. The inability of these Rho GEFs to complement each other’s function at the junctions strongly suggests that RhoA activation is highly regulated and is critical at multiple steps in junction formation and maintenance.
In conclusion, the integration of fine-tuned Rho GTPase activation by junctional Rho GEFs coupled with selective effector signaling represents a highly versatile mechanism for the precise spatiotemporal regulation of cell-cell adhesion. Despite a recent surge of studies showing how selective Rho GEFs influence junction integrity and plasticity,3,51,59,60 uncovering additional Rho regulators (GEFs, GAPs, or GDIs) that affect the formation and maintenance of intercellular junctions is essential for understanding the function of Rho GTPases and their effectors at intercellular junctions and their misregulation in human disease, including inflammation and cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/27132
References
- 1.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 3.Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol. 2012;199:1103–15. doi: 10.1083/jcb.201207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Toledo M, Coulon V, Schmidt S, Fort P, Blangy A. The gene for a new brain specific RhoA exchange factor maps to the highly unstable chromosomal region 1p36.2-1p36.3. Oncogene. 2001;20:7307–17. doi: 10.1038/sj.onc.1204921. [DOI] [PubMed] [Google Scholar]
- 5.Marx R, Henderson J, Wang J, Baraban JM. Tech: a RhoA GEF selectively expressed in hippocampal and cortical neurons. J Neurochem. 2005;92:850–8. doi: 10.1111/j.1471-4159.2004.02930.x. [DOI] [PubMed] [Google Scholar]
- 6.Ngok SP, Geyer R, Kourtidis A, Storz P, Anastasiadis PZ. Phosphorylation-mediated 14-3-3 protein binding regulates the function of the rho-specific guanine nucleotide exchange factor (RhoGEF) Syx. J Biol Chem. 2013;288:6640–50. doi: 10.1074/jbc.M112.432682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Horowitz A. A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol Biol Cell. 2006;17:1880–7. doi: 10.1091/mbc.E06-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnaas MK, Moodie KL, Liu ML, Samant GV, Li K, Marx R, Baraban JM, Horowitz A, Ramchandran R. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in Vivo. Circ Res. 2008;103:710–6. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estévez MA, Henderson JA, Ahn D, Zhu XR, Poschmann G, Lübbert H, Marx R, Baraban JM. The neuronal RhoA GEF, Tech, interacts with the synaptic multi-PDZ-domain-containing protein, MUPP1. J Neurochem. 2008;106:1287–97. doi: 10.1111/j.1471-4159.2008.05472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–53. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 12.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/S0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 13.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 14.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 15.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–65. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 16.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–7. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 18.Pedram A, Razandi M, Levin ER. Deciphering vascular endothelial cell growth factor/vascular permeability factor signaling to vascular permeability. Inhibition by atrial natriuretic peptide. J Biol Chem. 2002;277:44385–98. doi: 10.1074/jbc.M202391200. [DOI] [PubMed] [Google Scholar]
- 19.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102:1030–41. doi: 10.1160/TH09-09-0640. [DOI] [PubMed] [Google Scholar]
- 21.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 22.Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–35. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho JH, Nicolae DL, Ramos R, Fields CT, Rabenau K, Corradino S, Brant SR, Espinosa R, LeBeau M, Hanauer SB, et al. Linkage and linkage disequilibrium in chromosome band 1p36 in American Chaldeans with inflammatory bowel disease. Hum Mol Genet. 2000;9:1425–32. doi: 10.1093/hmg/9.9.1425. [DOI] [PubMed] [Google Scholar]
- 24.Marshall B, Isidro G, Martins AG, Boavida MG. Loss of heterozygosity at chromosome 9p21 in primary neuroblastomas: evidence for two deleted regions. Cancer Genet Cytogenet. 1997;96:134–9. doi: 10.1016/S0165-4608(96)00300-7. [DOI] [PubMed] [Google Scholar]
- 25.Shin K, Wang Q, Margolis B. PATJ regulates directional migration of mammalian epithelial cells. EMBO Rep. 2007;8:158–64. doi: 10.1038/sj.embor.7400890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci. 2013;126:1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–8. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 29.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 30.Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–82. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- 31.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thumkeo D, Shinohara R, Watanabe K, Takebayashi H, Toyoda Y, Tohyama K, Ishizaki T, Furuyashiki T, Narumiya S. Deficiency of mDia, an actin nucleator, disrupts integrity of neuroepithelium and causes periventricular dysplasia. PLoS One. 2011;6:e25465. doi: 10.1371/journal.pone.0025465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie H, Xue YX, Liu LB, Liu YH, Wang P. Role of RhoA/ROCK signaling in endothelial-monocyte-activating polypeptide II opening of the blood-tumor barrier: role of RhoA/ROCK signaling in EMAP II opening of the BTB. J Mol Neurosci. 2012;46:666–76. doi: 10.1007/s12031-011-9564-9. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 35.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci U S A. 2007;104:17686–91. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–15. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 37.Chang YW, Marlin JW, Chance TW, Jakobi R. RhoA mediates cyclooxygenase-2 signaling to disrupt the formation of adherens junctions and increase cell motility. Cancer Res. 2006;66:11700–8. doi: 10.1158/0008-5472.CAN-06-1818. [DOI] [PubMed] [Google Scholar]
- 38.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 39.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/S1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 40.Ma T, Liu L, Wang P, Xue Y. Evidence for involvement of ROCK signaling in bradykinin-induced increase in murine blood-tumor barrier permeability. J Neurooncol. 2012;106:291–301. doi: 10.1007/s11060-011-0685-3. [DOI] [PubMed] [Google Scholar]
- 41.Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem. 2008;283:29888–96. doi: 10.1074/jbc.M803880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27:2332–9. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 43.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121:566–79. doi: 10.1053/gast.2001.27060. [DOI] [PubMed] [Google Scholar]
- 45.Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312:1637–50. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–42. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–42. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D’Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–95. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, Arumugam A, Ongusaha PP, Lakshmanaswamy R, Liao JK, et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013;13:205–19. doi: 10.2174/156652413804486296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- 51.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–66. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol. 2011;194:643–56. doi: 10.1083/jcb.201104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27:618–28. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hannemann S, Madrid R, Stastna J, Kitzing T, Gasteier J, Schönichen A, Bouchet J, Jimenez A, Geyer M, Grosse R, et al. The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. J Biol Chem. 2008;283:27891–903. doi: 10.1074/jbc.M801800200. [DOI] [PubMed] [Google Scholar]
- 56.Staus DP, Taylor JM, Mack CP. Enhancement of mDia2 activity by Rho-kinase-dependent phosphorylation of the diaphanous autoregulatory domain. Biochem J. 2011;439:57–65. doi: 10.1042/BJ20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182:3837–45. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 58.Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–83. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ngok SP, Geyer R, Kourtidis A, Mitin N, Feathers R, Der C, Anastasiadis PZ. TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J Cell Sci. 2013;126:3271–7. doi: 10.1242/jcs.123869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–28. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]