Abstract
Aims
Exposure to hyperbaric oxygen (HBO2) causes an antinociceptive response in mice. However, breathing oxygen (O2) at an elevated pressure can potentially cause oxygen toxicity. The aim of this study was to identify the determinants of HBO2 antinociception and the toxicity profile of HBO2.
Main methods
Male NIH Swiss mice were assessed for acute antinociceptive responsiveness under room air or 100% O2 at 1.0 or 3.5 atmospheres absolute (ATA), using the acetic acid-induced abdominal constriction test. For the oxygen toxicity test, mice were exposed to 3.5 ATA oxygen for 11 min, 60 min, 60 min daily for 2 days (120 min) or 60 min daily for 4 days (240 min), then assessed by analyzing the levels of two oxidative stress markers, MDA (malondialdehyde) and protein carbonyl in brain, spinal cord and lung.
Key Findings
Only the combination of 100% O2 and 3.5 ATA caused significant antinociception. The antinociceptive effect of 100% O2 was pressure-dependent up to 3.5 ATA. In the oxygen toxicity test, mice exposed to HBO2 for different time intervals had levels of brain, spinal cord and lung MDA and protein carbonyl that were comparable to that of control animals exposed to room air.
Significance
Treatment with 100% O2 evokes a pressure-dependent antinociceptive effect. Since there was no significant increase in levels of the oxidative stress markers in the tested tissues, it is concluded HBO2 at 3.5 ATA produces antinociception in the absence of oxidative stress in mice.
Keywords: hyperbaric oxygen, antinociception, oxidative stress, malondialdehyde, protein carbonyl
Introduction
Hyperbaric oxygen (HBO2) therapy has been approved by the FDA for a limited set of clinical indications [Gesell, 2008. However, there are a number of clinical reports whereby HBO2 therapy appears to be effective in a broader range of conditions, including several examples of chronic pain [Peach, 1995; Kiralp et al., 2004; Yildiz et al., 2004; Handschel et al., 2007]. There is clinical evidence for an analgesic effect of HBO2; however, the mechanism of this analgesic action is poorly understood.
There have been few animal studies exploring the efficacy of HBO2 in the relief of pain, and these have focused largely on the anti-inflammatory rather than antinociceptive effects of HBO2 [Warren et al., 1979; Sümen et al., 2001]. Other studies measured both antinociception and anti-inflammatory effects [Wilson et al., 2006]. However, the antinociception effect in this animal model did not manifest itself until nearly two hours after the HBO2 treatment.
We have reported that treatment of mice with HBO2 @ 3.5 atmospheres absolute (ATA) resulted in a very rapid-onset antinociceptive effect (i.e., within 5 min). However, whether this biological response is the effect of the 100% oxygen (O2) or both is unclear. It is equally uncertain whether the antinociceptive effect is associated with an oxidative stress induced by reactive species of oxygen and nitrogen (N2) as a result of HBO2 treatment [Thom, 2009].
The present study was conducted to determine the optimal conditions for expression of the antinociceptive response to acute exposure to HBO2, ascertain whether the antinociceptive effect was pressure-dependent, and assess the possible oxidative injury caused by our hyperbaric oxygen exposure.
Materials and Methods
Animals
Male NIH Swiss mice, weighing 18–22 g, were purchased from Harlan Laboratories (Indianapolis, IN) and used in these experiments, which were approved by an institutional animal care and use committee and carried out in accordance with The Guide for the Care and Use of Laboratory Animals, 8th Edition (National Academies Press, Washington, DC, 2010).
Mice were housed five per cage in the AAALAC-accredited Wegner Hall Vivarium with access to food and water ad libitum. The facility was maintained on a 12-h light:dark cycle (lights on 0700–1900 h) under standard conditions (22°C room temperature, 33% humidity). Mice were kept in the holding room for at least four days following arrival in the facility and prior to experimentation. All measures to minimize pain or discomfort were taken by the investigators.
Exposure to Hyperbaric Oxygen
Five groups of eight mice each were treated with 0.6% glacial acetic acid, placed in the hyperbaric chamber, exposed to 100% O2 at 1.0, 2.0, 2.5, 3.0 or 3.5 ATA for 5 min, and assessed for antinociceptive responsiveness for 6 min for a total of 11 min of HBO2 treatment. Mice were placed in a B-11 research hyperbaric chamber (Reimers Systems, Inc., Lorton, VA) as previously described [Zelinski et al., 2009]. The chamber was ventilated with 100% O2, U.S.P. (A-L Compressed Gases Inc., Spokane, WA) at a flow rate of 20 L/min to minimize nitrogen and carbon dioxide accumulation. The pressure within the cylindrical clear acrylic chamber (27.9 cm diameter × 55.9 cm L) was increased at a rate of 1.0 ATA/min to the desired pressure and maintained for 11 min (5 min for onset of nociception plus 6 min antinociceptive testing). The mice were allowed to breathe spontaneously during HBO2 treatment. After completion of the HBO2 exposure, mice were then decompressed at a rate of 1.0 ATA/min. Control groups of mice were exposed to room air, and experimental groups of mice were exposed to either compressed air (A-L Compressed Gases) or 100% O2 circulated through the chamber at either 1.0 or 3.5 ATA and maintained for 11 min. Decompression occurred as described above.
In determining whether the antinociceptive effect of 100% O2 was pressure-related, the pressure of the HBO2 was varied at 1.0, 1.5, 2.0, 2.5, 3.0 and 3.5 ATA. In groups of control animals, the pressure of compressed air was set at 1.0, 2.0 or 3.5 ATA.
Antinociceptive Testing
Antinociceptive responsiveness was assessed using the abdominal constriction test as previously described [Zelinski et al., 2009]. Mice were treated i.p. with 0.1 ml per 10 g body weight of 0.6% glacial acetic acid and placed into the hyperbaric chamber. Exactly 5 min later, the number of abdominal constrictions—lengthwise stretches of the torso with concave arching of the back—in each animal was counted for 6 min while under HBO2. Multiple raters were used for some but not all experiments; at least one of the raters was blinded to the drug treatment. All experiments were consistently conducted between 1300 and 1700 h. The control reference group was exposed to room air. The degree of antinociception (inhibition of abdominal constrictions) produced in various treatment groups of mice was calculated as:
Measurement of Lipid Peroxidation and Protein Carbonyl
Mice were exposed to HBO2 @ 3.5 ATA for 11 min, 60 min, 120 min (60 min daily for 2 days), 240 min (60 min daily for 4 days) or room air (eight mice were used for each treatment), then ketamine/xylazine anesthetized immediately after the final HBO2 exposure. Then the mice were perfused with phosphate buffered saline (PBS) containing heparin to remove the blood. The tissue was collected and resuspended with PBS containing 1 mM EDTA and homogenized on ice at 50 mg/mL. After spinning at 10,000 × g for 5 min at 4°C, the supernatant was collected for assay. Levels of malondialdehyde (MDA) were determined, using a commercially available colorimetric assay for lipid peroxidation (TBARS Assay Kit, Cayman Chemical Company, Ann Arbor, MI). The absorbance at 530–540 nm was measured to obtain the concentration of MDA. Protein carbonyls were measured using a commercially available quantitative protein C carbonyl assay kit (Cayman Chemical, Ann Arbor, MI). The sample to be assayed was homogenized in 50 mM phosphate buffer containing 1 mM EDTA, pH 6.7, and then centrifuged at 10,000 × g for 5 min under 4°C. The supernatant was collected for assay. The protein concentration was determined using a BCA protein assay kit. The MDA content and protein carbonyl content were normalized to protein concentration for each sample. The purpose of the EDTA was to inhibit further production of these oxidative markers during the measurement process. Therefore, the oxidative marker levels in Tables 1 and 2 are tissues levels at the time of death.
Table 1.
Influence of HBO2 Treatment on Tissue Levels of Oxidative Stress Markers
| Influence of HBO2 on Tissue MDA Levels (nmol/mg protein) | ||||||
|---|---|---|---|---|---|---|
| Tissue | NBA | 11 min HBO2 | 60 min HBO2 | 120 min HBO2 | 240 min HBO2 | Positive Control |
| Brain | 9.16 ± 0.85 | 7.30 ± 1.06 | 7.81 ± 1.77 | 8.23 ± 2.19 | 8.62 ± 2.47 | 19.88 ± 3.88 a |
| Spinal Cord | 7.01 ± 1.74 | 8.33 ± 1.12 | 7.15 ± 1.21 | 7.75 ± 1.24 | 7.18 ± 1.78 | 17.51 ± 5.21 a |
| Lung | 6.23 ± 1.54 | 7.15 ± 2.89 | 6.73 ± 1.76 | 7.48 ± 2.38 | 7.00 ± 1.99 | 29.52 ± 8.89 a |
| Influence of HBO2 on Tissue Protein Carbonyl Levels (nmol/mg protein) | ||||||
|---|---|---|---|---|---|---|
| Tissue | NBA | 11 min HBO2 | 60 min HBO2 | 120 min HBO2 | 240 min HBO2 | Positive Control |
| Brain | 8.68 ± 3.64 | 9.95 ± 3.24 | 8.68 ± 2.28 | 8.16 ± 1.25 | 8.34 ± 2.44 | 24.54 ± 3.21 a |
| Spinal Cord | 8.42 ± 0.83 | 8.66 ± 3.48 | 9.44 ± 2.99 | 8.01 ± 2.20 | 8.13 ± 2.60 | 20.98 ± 2.63 a |
| Lung | 6.64 ± 1.98 | 7.55 ± 2.07 | 8.40 ± 1.65 | 6.85 ± 3.10 | 9.35 ± 2.41 | 36.94 ± 18.13 a |
Significance of difference:
p < 0.01, compared to the Room Air control group (one-way ANOVA and post-hoc Bonferroni’s multiple comparison test).
For the positive control, each sample from room air group were homogenized (with buffer without EDTA), centrifuged, and then incubated with H2O2 (5 mM), similar to Sewerynek et al. [1995]. Briefly, the homogenates were incubated with H2O2 for 60 min at 37°C, then placed into ice for 10 min to stop the reaction. Then the homogenates were centrifuged at 13,200 × g for 5 min at 4°C. The supernatants were collected for assay.
Statistical Analysis of Data
One-way ANOVA with a post-hoc Bonferroni multiple comparison test was used to compare the antinociception data and levels of MDA and protein carbonyl in groups exposed to different HBO2 regimens. Unpaired t-test was used to determine the difference between antinociceptive effects of HBO2 and compressed air at the same pressure.
Results
Basic Characterization of HBO2-Induced Acute Antinociception
Mice were randomly assigned to four different treatment groups: normobaric air (NBA; room air or compressed air @ 1.0 ATA), normobaric oxygen (NBO2; room air replaced by 100% O2 @ 1.0 ATA), hyperbaric air (HBA; compressed air @ 3.5 ATA) and hyperbaric oxygen (HBO2; 100% O2 @ 3.5 ATA). Fig. 1 shows that NBA, NBO2 and HBA treatment groups had no demonstrable antinociceptive response. While in the HBO2 treatment group, increasing the pressure to 3.5 ATA resulted in a 93.8 ± 2.2% antinociceptive effect. This was significantly different from all three control groups.
Fig. 1.
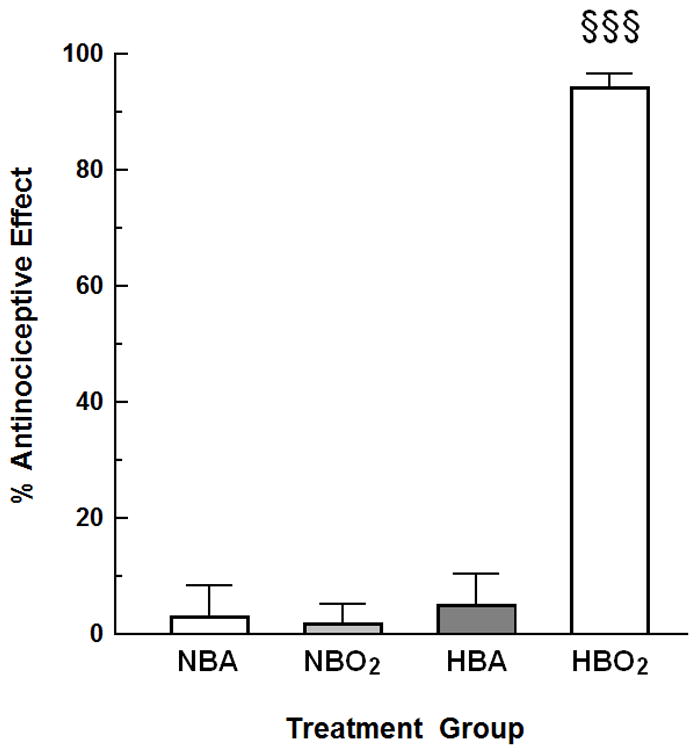
The effect of oxygen and/or hyperbaric pressure on nociception in mice. Groups: NBA, normobaric (room) air; NBO2, normobaric (100%) oxygen; HBA, hyperbaric (room) air (3.5 ATA); and HBO2, hyperbaric oxygen (100% oxygen at 3.5 ATA). The data are expressed as the mean ± S.E.M. of 8–13 mice per group. Significance of difference: §§§, P < 0.001, compared to NBA, NBO2 and HBA treatment groups (one-way ANOVA and post-hoc Bonferroni’s multiple comparison test). There were no significant differences among NBA, NBO2 or HBA.
HBO2-Induced Antinociception Is Pressure-Dependent
Four groups of eight mice each were treated with 0.6% glacial acetic acid, placed in the hyperbaric chamber, exposed to 100% O2 at 1.0, 2.0, 2.5, 3.0 or 3.5 ATA for 5 min, and assessed for antinociceptive responsiveness for 6 min for a total of 11 min of HBO2 treatment. Fig. 2 shows a linear relationship between the pressure of the O2 exposure and the percent antinociceptive effect.
Fig. 2.
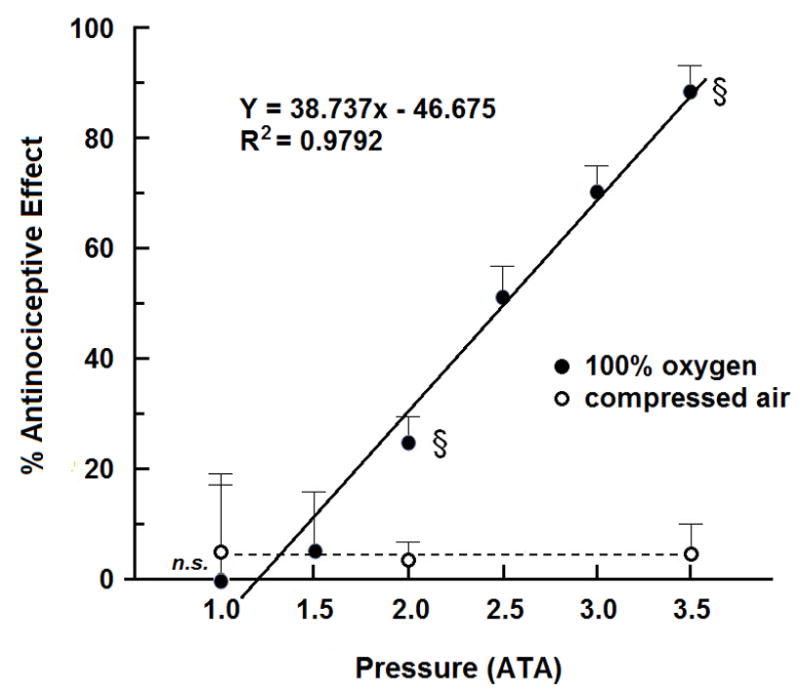
The influence of increasing pressure on oxygen-induced antinociceptive effect in mice. Each symbol (solid square for compressed air and solid diamond for 100% oxygen) represents the mean antinociceptive response of 8 mice at each pressure. The solid line shows the linear relationship between percent antinociceptive effect to 100% O2 and pressure; the dotted line shows that pressure alone fails to evoke an antinociceptive effect in mice exposed to compressed air. Significance of difference: §, P < 0.05, n.s., not significant, compared to compressed air at the same pressure (unpaired t-test).
Effect of HBO2 on Levels of Oxidative Stress Markers in Brain, Spinal Cord and Lung
To determine whether the designed hyperbaric oxygen exposure caused oxidative injury in mice, the oxidative injury was assessed by analyzing the levels of two oxidative stress markers, MDA (malondialdehyde) and protein carbonyl in the brain, spinal cord and lung after HBO2 treatment @ 3.5 ATA for 11, 60, 120 or 240 min. There were no significant differences in MDA and protein carbonyl levels between mice exposed to HBO2 and NBA mice in brain, lung and spinal cord (Table 1). The positive controls, on the other hand, showed a significant increase in levels of MDA and protein carbonyl.
Discussion
HBO2 therapy has been approved by the American Medical Association (AMA) and the Food and Drug Administration (FDA) for the treatment of the following fourteen conditions: air or gas embolism; carbon monoxide poisoning and smoke inhalation; carbon monoxide poisoning complicated by cyanide poisoning; clostridial myonecrosis (gas gangrene); crush injury, compartment syndrome and other acute traumatic ischemias; decompression sickness (the “bends”); enhancement of healing in selected problem wounds; exceptional blood loss (anemia); necrotizing soft tissue infections; refractory osteomyelitis; radiation tissue damage (osteoradionecrosis); compromised skin grafts and flaps; thermal burns; and idiopathic sudden sensorineural hearing loss [Gesell, 2008; Murphy-Lavoie et al., 2012]. But there are also clinical reports and anecdotal evidence of HBO2 efficacy in a much broader range of clinical conditions [Helms et al., 2011].
Although HBO2 treatment is not currently indicated in treatment of pain per se, HBO2 therapy is reported to induce an analgesic effect in relief of various types of pain in human subjects, including complex regional pain syndrome [Peach, 1995; Kiralp et al., 2004], fibromyalgia [Yildiz et al., 2004], headache [Wilson et al., 1998; Di Sabato et al., 1997], rheumatoid arthritis [Rui-Chang, 1994], chronic osteomyelitis [Handschel et al., 2007] and radiotherapy of cancer [Jones et al., 2006; Dall’Era et al., 2006]. The mechanism of this clinical analgesic effect of HBO2 treatment is not known, although some have attributed this effect of HBO2 to its anti-inflammatory properties [Weisz et al., 1997; Yang et al., 2006].
Animal studies related to HBO2 treatment of pain have been limited. Earlier studies focused largely on the anti-inflammatory rather than the analgesic effect of HBO2 [Warren et al., 1979; Sümen et al., 2001]. More recently, inflammatory pain induced by carrageenan in rats was evaluated after HBO2 exposure at 2.4 ATA for 90 min [Wilson et al., 2006, 2007]. HBO2 treatment was found to reduce inflammation (as determined by reduced paw swelling) as well as mechanical hypersensitivity (as determined by increased threshold for paw withdrawal) [Wilson et al., 2006]. The ability of HBO2 treatment to reduce inflammation and pain was comparable to that of acetylsalicylic acid treatment [Wilson et al., 2007].
In humans breathing air normally at sea level the alveolar pO2 (pAO2) is approximately 102 mm Hg and breathing 100% oxygen it increases to 673 mm Hg. During HBO2 therapy the patient breathes 100% O2 and the alveolar pO2 increases rapidly as the pressure in the hyperbaric chamber increases, so that at 2.0 ATA and at 2.5 ATA the pAO2 increases to 1433 mmHg and to about 1813 mmHg respectively, the latter a 17-fold increase as compared to breathing air at 1.0 ATA [Jain, 1999]. These increases are attained within minutes, thus, the tissue O2 concentrations correspondingly increase in a rapid manner.
The onset of the antinociception in this study occurred within 5 minutes of the exposure of the mice to HBO2 and continued for another 6 minutes during the abdominal constriction test. It is possible that full antinociception may not have occurred within the 5 minutes of HBO2 exposure prior to the testing period, especially during the lower pressures applied during the pressure-response experiments. However, there did not appear to be any noticeable time difference between periods of abdominal constrictions in the animals, and the pressure-response curve was linear.
Oxygen toxicity is the main physiologic limitation on the use of HBO2-enriched breathing mixtures for medical applications and scientific, technical and military diving [Hampson and Atik, 2003]. It has been argued that oxidative stress is fundamental to HBO2 therapy [Thom, 2009]. Metabolic activation of molecular oxygen, as well as the chemical and physical interaction of cells with their environment, frequently give rise to reactive oxygen species (ROS), such as superoxide, hydrogen peroxide or singlet oxygen [Halliwell and Gutteridge, 1984]. Low levels of ROS result in an adaptation response by increasing cellular antioxidant activity. Higher levels that overwhelm the antioxidative capacity of the cells cause oxidative damage manifesting as protein oxidation, lipid peroxidation, DNA injury and membrane damage, eventually culminating in disease and death.
As our previous studies have routinely treated mice with HBO2 @ 3.5 ATA [Zelinski et al., 2009; Ohgami et al., 2009; Chung et al., 2010; Quock et al., 2011], we have to ascertain whether the HBO2 treatment induced oxidative stress. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds, which include reactive carbonyl compounds, such as MDA [Esterbauer, 1985]. MDA is a naturally occurring product of lipid peroxidation. Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals and is used as an indicator of oxidative stress in cells and tissues and the measurement of thiobarbituric acid reactive substances (TBARS) is a well-established method for screening and monitoring lipid peroxidation [Yagi, 1998; Armstrong and Browne, 1994]. The most general indicator and, by far, the most commonly used marker of protein oxidation is protein carbonyl content [Stadtman and Oliver, 1991]. The most convenient procedure is the reaction between DNPH and protein carbonyl. DNPH reacts with protein carbonyls, forming a Schiff base to produce the corresponding hydrazone, which can be analyzed spectrophotometrically.
Our results showed that levels of both lipid peroxidation and protein carbonyl were not significantly increased after the HBO2 exposures, even following HBO2 @ 3.5 ATA for 60 min daily for 4 days. It is known that higher pressures and longer exposure times of HBO2 treatment can lead to oxygen toxicity to various organs, such as the brain [Liu et al., 2012] and the lung [Sun et al., 2011], but whether our exposure procedure produced oxygen toxicity was not previously known. HBO2 treatment can activate antioxidant as well as oxidant systems. Whether oxygen toxicity is induced depends on the balance of these antioxidant/oxidant systems. It would appear that our 11-min and 60-min HBO2 exposures didn’t disrupt this balance. It is more interesting that even the daily 60-min HBO2 treatments for 2 days or 4 days also failed to produce oxygen toxicity. Recently the preconditioning effect of HBO2 has been widely explored. It was found that HBO2 pretreatment could prevent oxidative injuries to organs [Yan et al., 2013; Liu et al., 2011]. Speit and Bonzheim [2003] implicated heme oxygenase-1 in HBO2-induced protection of A549 cells against oxygen toxicity induced by a second HBO2 treatment 24 h later. Recently it was reported that daily exposure to 90 min HBO2 @ 2.8 ATA for as many as 40 days failed to significantly elevated levels of MDA, protein carbonyl and glutathionine peroxidase in the cerebral cortex, inner white matter and cerebellum [Simsek et al., 2012]. These findings, albeit at a lower atmospheric pressure than that applied in the present study, as well as our results are consistent in demonstrating a lack of oxygen toxicity. It is possible that in the present study, the 24-h interval between each HBO2 exposure prevent the accumulation of oxygen toxicity, but the adaptive and preconditioning actions induced by earlier HBO2 exposure(s) may contribute to these results.
We acknowledge that DNA damage is also an important marker of oxidative stress, and DNA strand breaks could be a site of oxidative damage from HBO2 exposure. Endogenous antioxidant levels are other important indicators of oxidative injury. These indictors should be measured when we further explore the oxidative/antioxidative status and the measurement of NO and other ROS would also accumulate our knowledge on this aspect. Also, this study was conducted in rodents and the results need to be reassessed when applied to humans.
Conclusions
The antinociceptive effect of 100% O2 was pressure-dependent up to 3.5 ATA. Mice exposed to HBO2 @ 3.5 ATA for 11 min, 60 min, 60 min daily for 2 days or 60 min daily for 4 days failed to show a significant increase in either protein carbonyl or MDA in the brain, spinal cord or lung. It is concluded that HBO2 at 3.5 ATA is able to produce significant antinociception without causing oxygen toxicity in mice. This also suggests that the HBO2-induced antinociception is not associated with oxidative stress.
Acknowledgments
This research was supported by NIH Grants GM-77153 and AT-007222 and funds from the WSU College of Pharmacy and the Chico Hyperbaric Center (Chico, California).
Footnotes
This work was presented in part at Experimental Biology 2013, Boston, Massachusetts, April 20–24, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- Chung E, Zielinski LM, Ohgami Y, Shirachi DY, Quock RM. Hyperbaric oxygen treatment induces a two-phase antinociceptive response of unusually long duration in mice. J Pain. 2010;11:847–53. doi: 10.1016/j.jpain.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era MA, Hampson NB, His RA, Madsen B, Corman JM. Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87–90. doi: 10.1016/S0022-5347(06)00491-5. [DOI] [PubMed] [Google Scholar]
- Di Sabato F, Rocco M, Martelletti P, Giacovazzo M. Hyperbaric oxygen in chronic cluster headaches: influence on serotonergic pathways. Undersea Hyperb Med. 1997;24:117–22. [PubMed] [Google Scholar]
- Esterbauer H. Lipid peroxidation products: formation, chemical properties and biological activities. In: Poli G, Cheeseman KH, Dianzani MU, Slater TF, editors. Free Radicals in Liver Injury. IRI Press; Oxford: 1985. pp. 29–47. [Google Scholar]
- Gesell LB, editor. The hyperbaric oxygen therapy committee report: indications and results. 12. Durham: Undersea and Hyperbaric Medical Society; 2008. [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbarix oxygen therapy. Undersea Hyperb Med. 2003;30:147–53. [PubMed] [Google Scholar]
- Handschel J, Brüssermann S, Depprich R, Ommerborn M, Naujoks C, Kübler NR, Meyer U. Evaluation of hyperbaric oxygen therapy in treatment of patients with osteomyelitis of the mandible. Mund Kiefer Gesichtschir. 2007;11:285–90. doi: 10.1007/s10006-007-0073-5. [DOI] [PubMed] [Google Scholar]
- Helms A, Evans AW, Chu J, Sahgal A, Ostrowski R, Sosiak T, Wolf G, Gillett J, Whelan H. Hyperbaric oxygen for neurologic indications—action plan for multicenter trials in: stroke, traumatic brain injury, radiation encephalopathy & status migrainosus. Undersea Hyperb Med. 2011;38:309–19. [PubMed] [Google Scholar]
- Jain KK. Physical, physiological, and biochemical aspects of hyperbaric oxygenation. In: Jain KK, editor. Textbook of hyperbaric medicine. Seattle: Hogrefe and Huber Publishers; 1999. pp. 11–27. [Google Scholar]
- Jones K, Evans AW, Bristow RG, Levin W. Treatment of radiation proctitis with hyperbaric oxygen. Radiother Oncol. 2006;78:91–4. doi: 10.1016/j.radonc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kiralp MZ, Yildiz S, Vural D, Keskin I, Ay H, Dursun H. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J Int Med Res. 2004;32:258–62. doi: 10.1177/147323000403200304. [DOI] [PubMed] [Google Scholar]
- Liu S, Li R, Ni X, Cai Z, Zhang R, Sun X, Quock RM, Xu W. Perfluorocarbon-facilitated CNS oxygen toxicity in rats: reversal by edaravone. Brain Res. 2012;1471:56–65. doi: 10.1016/j.brainres.2012.06.051. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun XJ, Liu J, Kang ZM, Deng XM. Heme oxygenase-1 could mediate the protective effects of hyperbaric oxygen preconditioning against hepatic ischemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2011;38:675–82. doi: 10.1111/j.1440-1681.2011.05560.x. [DOI] [PubMed] [Google Scholar]
- Murphy-Lavoie H, Piper S, Moon RE, Legros T. Hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss. Undersea Hyperb Med. 2012;39:777–92. [PubMed] [Google Scholar]
- Ohgami Y, Zylstra CC, Quock LP, Chung E, Shirachi DY, Quock RM. Nitric oxide in hyperbaric oxygen-induced acute antinociception in mice. NeuroReport. 2009;20:1325–9. doi: 10.1097/WNR.0b013e3283305a49. [DOI] [PubMed] [Google Scholar]
- Peach G. Hyperbaric oxygen and the reflex sympathetic dystrophy syndrome: a case report. Undersea Hyperb Med. 1995;22:407–8. [PubMed] [Google Scholar]
- Quock LP, Zhang Y, Chung E, Ohgami Y, Shirachi DY, Quock RM. The acute antinociceptive effect of HBO2 is mediated by a NO–cyclic GMP–PKG–KATP channel pathway in mice. Brain Res. 2011;1368:102–7. doi: 10.1016/j.brainres.2010.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui-Chang Y. Rheumatoid arthritis treated with HBO. Proc 11th Int Congr Hyperb Med. San Pedro: Best Publishing; 1994. [Google Scholar]
- Sewerynek E, Melchiorri D, Ortiz GG, Poeggeler B, Reiter RJ. Melatonin reduces H2O2-induced lipid peroxidation in homogenates of different rat brain regions. J Pineal Res. 1995;19:51–6. doi: 10.1111/j.1600-079x.1995.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Simsek K, Ozler M, Yildirim AO, Sadir S, Demirbas S, Oztosun M, Korkmaz A, Ay H, Oter S, Yildiz S. Evaluation of the oxidative effect of long-term repetitive hyperbaric oxygen exposures on different brain regions of rats. Scientific World Journal. 2012;2012:849183. doi: 10.1100/2012/849183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speit G, Bonzheim I. Genotoxic and protective effects of hyperbaric oxygen in A549 lung cells. Mutagenesis. 2003;18:545–8. doi: 10.1093/mutage/geg028. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Oliver CN. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Sümen G, Çimşit M, Eroğlu L. Hyperbaric oxygen treatment reduces carrageenan-induced acute inflammation in rats. Eur J Pharmacol. 2001;431:265–8. doi: 10.1016/s0014-2999(01)01446-7. [DOI] [PubMed] [Google Scholar]
- Sun Q, Cai J, Liu S, Liu Y, Xu W, Tao H, Sun X. Hydrogen-rich saline provides protection against hyperoxic lung injury. J Surg Res. 2011;165:e43–9. doi: 10.1016/j.jss.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–95. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J, Sacksteder MR, Thuning CA. Therapeutic effect of prolonged hyperbaric oxygen in adjuvant arthritis of the rat. Arthritis Rheum. 1979;22:334–9. doi: 10.1002/art.1780220404. [DOI] [PubMed] [Google Scholar]
- Weisz G, Lavy A, Adir Y, Melamed Y, Rubin D, Eidelman S, Pollack S. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J Clin Immunol. 1997;17:154–9. doi: 10.1023/a:1027378532003. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Toepfer VE, Senapati AK, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment is comparable to acetylsalicylic acid treatment in an animal model of arthritis. J Pain. 2007;8:924–30. doi: 10.1016/j.jpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res. 2006;1098:126–8. doi: 10.1016/j.brainres.2006.04.088. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Foresman BH, Gamber RG, Wright T. Hyperbaric oxygen in the treatment of migraine with aura. Headache. 1998;38:112–5. doi: 10.1046/j.1526-4610.1998.3802112.x. [DOI] [PubMed] [Google Scholar]
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Meth Mol Biol. 1998;108:101–6. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J Cereb Blood Flow Metab. 2013;33:396–406. doi: 10.1038/jcbfm.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Nandi J, Wang J, Bosco G, Gregory M, Chung C, Xie Y, Yang X, Camporesi EM. Hyperbaric oxygenation ameliorates indomethacin-induced enteropathy in rats by modulating TNF-α and IL-1β production. Dig Dis Sci. 2006;51:1426–33. doi: 10.1007/s10620-006-9088-2. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Kiralp MZ, Akin A, Keskin I, Ay H, Dursun H, Cimsit M. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J Int Med Res. 2004;32:263–7. doi: 10.1177/147323000403200305. [DOI] [PubMed] [Google Scholar]
- Zelinski LM, Ohgami Y, Chung E, Shirachi DY, Quock RM. A prolonged nitric oxide-dependent, opioid-mediated antinociceptive effect of hyperbaric oxygen in mice. J Pain. 2009;10:167–72. doi: 10.1016/j.jpain.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


