VATER association was first described in the early 1970s as the non-random co-occurrence of congenital malformations including: Vertebral defects, Anal atresia, Tracheo-Esophageal fistula (TEF) with or without esophageal atresia (EA), and Radial and Renal dysplasia.1 Following initial reports, it was suggested that “V” should include Vascular anomalies (including single umbilical artery). Cardiac malformations (“C”) and Limb (“L”) anomalies other than radial anomalies were also added, such that the term “VACTERL” became the most common descriptor, despite variable evidence for the inclusion of features such as cardiac or renal anomalies.2–6 The presence of VACTERL association, which usually requires at least 3 component features and the absence of evidence for an overlapping condition, is estimated to occur in approximately 1/10,000–1/40,000 live births.7, 8
Just as there are challenges in defining the condition, there is no standard approach for the initial diagnostic work-up of a neonate with identified or suspected VACTERL association. This can be problematic: missed manifestations may obfuscate the etiological work-up8; delay medical interventions, potentially contributing to higher morbidity and mortality9; result in less informed and effective counseling.
To attempt to address these issues, we assembled a multi-disciplinary group of clinicians and researchers whose expertise focuses on VACTERL association and/or its individual component features. Following review of the literature, and based upon our collective experience, we offer suggestions for the evaluation of individuals identified or suspected to have VACTERL association.
Literature Search
We conducted a PubMed-based literature search for case reports and collections of patients identified or suspected of having VACTERL association and/or associated component features. Search terms included the following: Anal atresia; Anorectal malformations; Cardiac anomalies; Cardiac malformations; Cardiovascular anomalies; Cardiovascular malformations; Esophageal atresia; Genitourinary anomalies; Genitourinary malformations; Imperforate anus; Limb anomalies; Limb malformations; Radial anomalies; Radial dysplasia; Renal anomalies; Renal malformations; TEF; Tracheo-esophageal fistula; VACTERL; VATER; Vertebral anomalies; Vertebral malformations. Only articles describing human patients were considered, and articles were excluded if they did not pertain to component features specifically seen in VACTERL association.
SUGGESTED DIAGNOSTIC APPROACH
Patient criteria: to whom does the suggested diagnostic approach apply?
Individuals with any two component features of VACTERL association or either TEF/EA or ARM are frequently found to have additional VACTERL-type anomalies.10–12 These additional anomalies may be difficult to detect without a high index of suspicion and/or specific testing. We suggest that our approach be applied to all individuals with at least 2 component features of VACTERL association, and all individuals in whom TEF/EA or ARM is diagnosed. Following the diagnostic work-up suggested here, the presence of VACTERL association is typically considered when at least three component features are present and there is no alternate causal explanation. Another view weights the component features differently, requiring the presence of at least one “core” feature: anorectal malformations (ARM) and/or TEF/EA in addition to at least two other component features.7, 8
Additional considerations
We discuss each feature individually, though many tests may be relevant to more than one organ system. After the identification of component features, further work-up and/or treatment will largely depend on the type and severity of the identified malformation, and may therefore vary widely. We also offer diagnostic suggestions based on current etiologic knowledge (; available at www.jpeds.com).
It is important to emphasize several points at the outset. First, the suggested approach should be performed in concert with routine newborn screening (i.e., hearing and blood-based screening). In addition to its general benefits, newborn screening can detect clinically significant findings that may occur in conditions in the differential diagnosis, such as hearing impairment in Townes-Brocks syndrome.8 Second, although a range of practitioners may perform the suggested work-up, the involvement of a clinical geneticist is optimal. Finally, though the suggested work-up is intended for the neonatal period, the approach might also be applied to an individual who is first recognized as having features of VACTERL association at an older age (which is not uncommon).9
Vertebral anomalies
Description
Vertebral anomalies, described in 60–90% of affected individuals, can affect any of the vertebrae, may involve single or multiple vertebrae, and may vary in severity.7, 12, 13 Characteristic vertebral anomalies include segmentation defects and may be accompanied by rib anomalies and/or abnormal spinal curvatures.12, 13 The presence of congenital costovertebral anomalies may require orthopedic tracking, as well as medical/surgical interventions. Surgical interventions are indicated at the earliest age-appropriate time related to the specific procedure.9, 14–17
In addition to frank vertebral anomalies, tethered spinal cord may occur, especially in those with caudal features such as ARM and/or urogenital anomalies.18–21 Tethered spinal cord may co-occur with anomalies such as terminal filum or conus medullaris lipomas, intramedullary ependymal cysts, meningomyelocele, and syringohydromyelia.18, 20 A tethered spinal cord can cause significant morbidity by impacting neurological function, including related to continence. Early detection of tethered cord, allowing timely surgical treatment, has shown a significant outcome benefit (though the benefit may differ in symptomatic vs. asymptomatic patients).22, 23
Initial work-up
The initial X-rays to detect vertebral anomalies should include dedicated sacral views to allow inference of the degree of caudal regression (through sacral ratio calculation), which can inform continence-related prognosis.24 Further imaging, such as with magnetic resonance imaging (MRI) or three-dimensional computed tomography (CT) may be necessary to delineate complex malformations, though issues related to radiation exposure and sedation risk should be considered.25
A peripheral neurological/muscle examination, including assessment of reflexes (deep tendon and primitive), assessment of muscle tone (including rectal), and response to tactile stimulation can elucidate signs of spinal cord pathology related to tethered cord and/or other vertebral anomalies.26 Imaging to detect the presence of a tethered spinal cord should also be part of the initial work-up. MRI is the most sensitive modality, but ultrasound is considered an adequate screening tool and has the advantage of being readily available, efficient, affordable, and avoids sedation. Because of the advantage of ultrasound, MRI may be reserved for patients with X-ray identified lower spinal anomalies.18 The choice between MRI and ultrasound may also be influenced by availability, cost, and the timing of testing. In general, ultrasound to evaluate tethered cord may be performed up to approximately three months of age.18
Anorectal Malformations
Description
ARM, which are frequently accompanied by genitourinary (GU) anomalies (including complex cloacal malformations), are reported in 55–90% of affected individuals.7, 13, 24, 27
The timing and surgical approach can differ dramatically depending on the ARM type and related anomalies such as GU malformations. Early detection of ARM and GU anomalies is also critical in preventing GU infections and preserving renal function.24, 27
Initial work-up
Individuals should undergo careful physical examination and observation for signs of ARM and accompanying GU anomalies (e.g., abdominal mass in hydrocolpos). This examination should attempt to delineate the type of ARM/GU anomaly, including signs of recto-urinary fistulas, which may warrant further studies. In unclear circumstances, a cross-table lateral X-ray may help assess distal rectal anatomy, including for signs of ARM (e.g., an air column in the distal rectum).24 In the case of a known ARM, a prone cross-table lateral pelvic X-ray (invertogram) may help judge the proximity of the distal rectum to the perineal skin, which can guide neonatal treatment in terms of the choice of anoplasty or colostomy.24, 27
An abdominal ultrasound should be performed to assess for accompanying GU anomalies.24, 27 Fluids and nutrition should be administered intravenously, and a nasogastric/orogastric (NG/OG) tube should be placed to keep the stomach decompressed to decrease the risk of vomiting and related aspiration.24
Cardiac malformations (and cardiovascular anomalies)
Description
Cardiac malformations have been reported in 40–80% of affected individuals, ranging from subtle anomalies, including vascular anomalies, that may not be clinically significant to complex malformations necessitating multiple surgical and long-term medical interventions.7, 13 In addition to medical/surgical interventions, cardiovascular anomalies may warrant specific inpatient cardiac monitoring and precautions, and may impact the overall care plan, including the timing and approach to other surgeries.28–30 The presence of cardiovascular malformations may affect the anesthetic plan.31 Patients with mild or severe congenital heart anomalies, such as occur in VACTERL association, may also be at risk of supraventricular and ventricular arrhythmias.32
Initial work-up
Trans-thoracic echocardiogram may detect and characterize cardiac malformations. An ECG may identify electrical disturbances not ascertained by echocardiogram.32
Depending on the results of the echocardiogram, additional techniques, such as cardiac MRI, may be necessary. If surgical interventions are planned, careful delineation of any accompanying vascular anomalies is indicated (e.g., with Doppler ultrasound of the affected area and/or echocardiogram; in complex cases, studies such as MR angiogram should be included).30, 33, 34
Tracheo-esophageal fistula with/without esophageal atresia
Description
A variety of TEF/EA types have overall been described in 50–80% of individuals with VACTERL association.7, 13 Early interventions, including those related to fluid and nutrition management, can decrease complications (eg, aspiration).35
Initial work-up
As with certain other component features, an important initial step is physical examination and observation, including an attempt to pass an NG/OG tube.35–37 Clinical findings (e.g., increased oral secretions/choking during feeding, failure to pass an NG/OG tube) can be supported by radiological diagnostic clues, such as NG tube coiling or an absent gastric bubble on X-ray.37 These X-rays should be performed with an NG/OG tube in place - even if it meets resistance on placement - to help show the defect’s location. Further imaging, such as contrast studies, is rarely required unless there is a clear clinical indication related to patient presentation or medical history. Reasons in the medical history might include gestational polyhydramnios because of fetal inability to swallow amniotic fluid.38 The echocardiogram can evaluate anomalies of the heart and large vessels in preparation for surgical repair.39
Of note, H-type TEF diagnosis can be difficult, as there is normal NG/OG placement and gastric bubble, and the infant may present only with findings such as choking/respiratory distress with feeds. In this instance, a swallow study or rigid bronchoscopy may be required.40 As with ARM, fluids and nutrition should be given intravenously.
Renal anomalies
Description
Renal anomalies, which may be accompanied by ureteral and GU anomalies, have been described in 50–80% of affected individuals.7, 13 With severe anomalies that compromise renal function, renal transplant may be eventually required.41 However, early diagnosis and management may preserve function via prevention of infection and management of vesico-ureteral reflux (VUR). Optimizing adequate bladder emptying is especially important in obstructive hydronephrosis.
Initial work-up
Further testing after renal ultrasound, such as a voiding cystourethrogram, serial ultrasound testing, and renal function studies (e.g., mercapto-acetyl-triglycine (MAG3)/furosemide scan), may be required in the presence of renal anomalies or if there is other evidence of manifestations such as VUR.42–44 In addition, individuals with VACTERL association frequently have GU anomalies.45 Physical examination may yield findings warranting further medical/surgical interventions.27, 45
Limb anomalies
Description
Radial anomalies were first described as a defining feature, but a wider variety of limb anomalies has been reported in 40–55% of affected individuals.7, 13 The identification of limb anomalies can help plan interventions, including early physical therapy and eventual surgery.46
Initial work-up
Physical examination to assess for limb anomalies affecting any of the limbs, in addition to isolated radial anomalies should be part of the overall assessment. If limb anomalies are suspected/detected, radiologic studies (initially with X-rays) can be performed.
Hydrocephalus
Description
VACTERL with hydrocephalus (VACTERL-H), often due to aqueductal stenosis, may be a distinct condition or may represent a continuum with “more general” VACTERL.7 The condition has been reported with X-linked inheritance in some families, though other inheritance modes have been described.47–49 Genetic causes are known in some instances; these include mutations in ZIC3, or as a manifestation of Fanconi anemia due to FANCB or FANCD1 mutations.50–52 Surgical interventions (including shunting) may be beneficial to treat hydrocephalus. Such interventions may improve outcomes, including related to neuro-developmental sequelae.
Initial work-up
A cranial ultrasound in infancy can be used to detect hydrocephalus.53
Additional work-up (including medical/family history, physical examination, other testing)
Description
The causes of VACTERL association remain largely unknown, though scattered case reports have identified etiologies, as well as non-genetic risk factors (e.g., maternal diabetes mellitus).7, 54–56 Although VACTERL association is usually sporadic, a relatively small number of familial cases have been described, and there may be an over-representation of VACTERL-type anomalies in relatives of probands.57, 58 A thorough family history can help with the differential diagnosis (e.g., the possibility of other conditions may be raised) and can help inform discussions regarding recurrence risks.8
In addition to VACTERL-type anomalies, malformations affecting diverse organ systems have been described.7 Even though none is common enough to warrant standard testing, clinicians should be aware that other congenital anomalies may co-exist, and have a low threshold for further related investigations.
Initial work-up
A medical and family history and physical examination by a clinical geneticist and genetic counselor may reveal clues to direct genetic testing as well as prompt further investigations related to specific findings.
As large-scale sequencing becomes increasingly available and accurate, techniques such as whole-exome/genome sequencing, or parallel sequencing of many genes, may be an efficient way to interrogate multiple genes and search for novel disease causes.55, 59
In addition to sequencing, analysis for causative copy-number variants (CNVs) (such as with high-density microarray) is warranted, as a small proportion of individuals can be identified with causal deletions or duplications and because such cytogenomic studies are generally indicated for individuals with multiple congenital anomalies. The frequency of de novo CNVs in individuals with VACTERL association is low (<5%), but CNVs that act as putative genetic susceptibility factors may be identified.60 If a high-density microarray is not available, a standard, high-resolution karyotype is still indicated, as some conditions that include features of VACTERL association, and which may be difficult to identify clinically especially in the prenatal/neonatal period, can be diagnosed by karyotype and/or related cytogenomic studies (e.g., some trisomies).
A small proportion (especially those with radial ray abnormalities) of individuals may have Fanconi anemia, and testing through chromosomal breakage studies, such as diepoxybutane (DEB) assay, is indicated in all individuals with VACTERL association.8, 61
A peripheral blood CBC may inform the differential diagnosis, such as related to thrombocytopenia-absent radius syndrome.62 Some hematologic anomalies (e.g., thrombocytopenia) may also affect management.
Finally, in addition to the other recommendations, early intervention services can be beneficial for many neonates VACTERL association-type anomalies. Patients may not be considered to be impaired at diagnosis but are at risk of disability and in the United States would qualify for services under the individuals with Disabilities Education Act (PL99–142). If available, a pediatric physiatrist should follow the infant every 3–6 months to monitor for signs of impairment and disability, oversee rehabilitation, and prescribe adaptive equipment.
Other diagnostic possibilities
A thorough clinical work-up may suggest the presence of a testable disorder, as many disorders overlap or include features of VACTERL association; in many of these conditions, the genetic causes are known and clinical testing is available8 Patients may also demonstrate features of VACTERL association due to relatively large genomic imbalances, such as may be detected by microarray, though many of these patients will demonstrate additional, distinct findings.60, 63 Finally, free web-based algorithms are available that can help generate a differential diagnosis based on specific patient features.64
DISCUSSION
The relative rarity and wide spectrum of VACTERL association challenges the ability to have a “gold standard” case definition. We nonetheless expect that this algorithm can be beneficial to a diverse group of medical practitioners. The suggested work-up may involve considerable expense, but applying this algorithm early in life may ultimately decrease morbidity as well as costs to the health care system. Further research is necessary to determine the optimal cost-benefit ratio. Though the phenotypic spectrum is becoming better understood, the causes of VACTERL association remain largely elusive. Our research groups, as well as others, are currently leveraging new genomic research methods in order to search for genetic explanations of VACTERL association. This can be a lengthy process, but when these causes have been unraveled, they may improve understanding about the disease process specifically, basic developmental processes in general, and allow more informed genetic counseling. Understanding the causes of VACTERL association may lead to more specific clinical algorithms. For example, individuals who are known to have a mutation in one gene may be at higher risk for severe cardiac malformations, and others, with a mutation in a different gene, may be more prone to genitourinary and renal anomalies. Being able to categorize patients genetically may allow the practice of genomic medicine by enabling more individually-tailored medical plans.
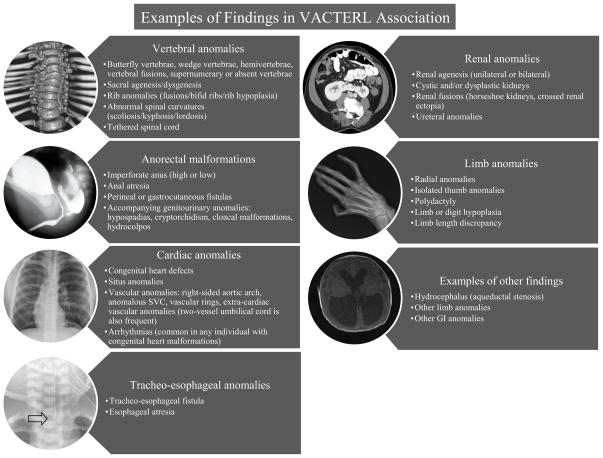
Figure 1.
Examples of findings in VACTERL association. The images in the circles represent radiological examples of findings. Vertebral anomalies: 3-D CT reconstruction showing thoracic segmentation anomalies; Anorectal malformations: contrast imaging showing a rectoprostatic urethral fistula in a patient with an anorectal malformation; Cardiac anomalies: chest x-ray showing a midline heart in a patient with situs anomalies as part of VACTERL association; Tracheo-esophageal anomalies: x-ray showing the position of feeding tube placement (arrow) due to a tracheal pouch; Renal anomalies: axial view from an abdominal CT showing a horseshoe kidney; Limb anomalies: skeletal anomalies affecting the right radius, wrist, and thumb; Examples of other findings: axial view from a brain MRI showing hydrocephalus (due to aqueductal stenosis) Abbreviations: 3-D: three-dimensional; CT: computed tomography; GI: Gastrointestinal
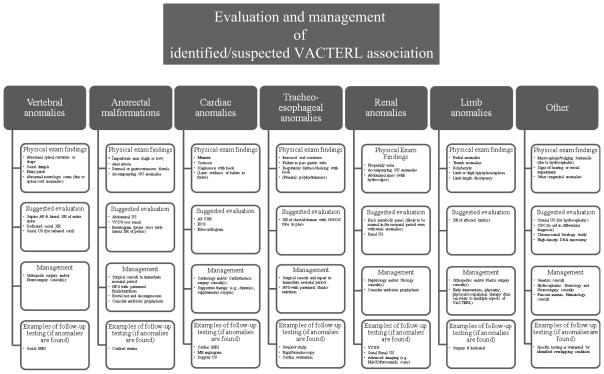
Figure 2.
Graphical representation of suggested algorithm for neonates affected with VACTERL association.
Acknowledgments
Supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, nor the US Government.
We thank Dr Max Muenke (Editor of Molecular Genetics and Genomic Medicine) for support and mentorship and Julia Fekecs for help with illustrations (both funded by National Human Genome Research Institute, National Institutes of Health). All authors express their deepest gratitude to the patients and families who participate in their research on VACTERL association and related disorders.
Abbreviations
- AP
Anterior-Posterior
- ARM
Anorectal Malformations
- AXR
Abdominal X-ray
- CBC
Complete Blood Count
- CNV
Copy Number Variant
- CT
Computed Tomography
- CXR
Chest X-ray
- DEB
Diepoxybutane
- EA
Esophageal Atresia
- ECG
Electrocadiogram
- GU
Genitourinary
- MAG3
technetium-99m mercaptoacetyltriglycine
- MR
Magnetic resosance
- MRI
Magnetic Resonance Imaging
- NG
Nasogastric
- NPO
nil per os
- OG
Orogastric
- TEF
Tracheo-esophageal Fistula
- US
ultrasound
- VACTERL
Vertebral defects, Anal atresia, Cardiovascular anomalies, Tracheo-esophageal fistula with or without Esophageal atresia, Renal anomalies, Limb anomalies
- VATER
Vertebral defects, Anal atresia, Tracheo-esophageal fistula with or without Esophageal atresia, Radial and Renal dysplasia
- VACTERL-H
VACTERL with Hydrocephalus
- VUR
Vesicoureteral reflux
- VCUG
Voiding Cystourethrogram
- XR
x-ray
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quan L, Smith DW. The VATER association. Vertebral defects, Anal atresia, T-E fistula with esophageal atresia, Radial and Renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–7. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- 2.Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–9. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- 3.Khoury MJ, Cordero JF, Greenberg F, James LM, Erickson JD. A population study of the VACTERL association: evidence for its etiologic heterogeneity. Pediatrics. 1983;71:815–20. [PubMed] [Google Scholar]
- 4.Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–36. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Kallen K, Mastroiacovo P, Castilla EE, Robert E, Kallen B. VATER non-random association of congenital malformations: study based on data from four malformation registers. Am J Med Genet. 2001;101:26–32. doi: 10.1002/ajmg.1201. [DOI] [PubMed] [Google Scholar]
- 6.Czeizel A, Ludanyi I. An aetiological study of the VACTERL-association. Eur J Pediatr. 1985;144:331–7. doi: 10.1007/BF00441773. [DOI] [PubMed] [Google Scholar]
- 7.Solomon BD. VACTERL/VATER Association. Orphanet J Rare Dis. 2011;6:56. doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon BD, Bear KA, Kimonis V, de Klein A, Scott DA, Shaw-Smith C, et al. Clinical geneticists’ views of VACTERL/VATER association. Am J Med Genet A. 2012;158A:3087–100. doi: 10.1002/ajmg.a.35638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raam MS, Pineda-Alvarez DE, Hadley DW, Solomon BD. Long-term outcomes of adults with features of VACTERL association. European Journal of Medical Genetics. 2011;54:34–41. doi: 10.1016/j.ejmg.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoll C, Alembik Y, Dott B, Roth MP. Associated malformations in patients with esophageal atresia. Eur J Med Genet. 2009;52:287–90. doi: 10.1016/j.ejmg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Keckler SJ, St Peter SD, Valusek PA, Tsao K, Snyder CL, Holcomb GW, 3rd, et al. VACTERL anomalies in patients with esophageal atresia: an updated delineation of the spectrum and review of the literature. Pediatr Surg Int. 2007;23:309–13. doi: 10.1007/s00383-007-1891-0. [DOI] [PubMed] [Google Scholar]
- 12.Solomon BD, Pineda-Alvarez DE, Raam MS, Bous SM, Keaton AA, Velez JI, et al. Analysis of component findings in 79 patients diagnosed with VACTERL association. Am J Med Genet A. 2010;152A:2236–44. doi: 10.1002/ajmg.a.33572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oral A, Caner I, Yigiter M, Kantarci M, Olgun H, Ceviz N, et al. Clinical characteristics of neonates with VACTERL association. Pediatr Int. 2012;54:361–4. doi: 10.1111/j.1442-200X.2012.03566.x. [DOI] [PubMed] [Google Scholar]
- 14.Winter RB. Posterior spinal fusion in scoliosis: indications, technique, and results. Orthop Clin North Am. 1979;10:787–800. [PubMed] [Google Scholar]
- 15.Weber TR, Smith W, Grosfeld JL. Surgical experience in infants with the VATER association. J Pediatr Surg. 1980;15:849–54. doi: 10.1016/s0022-3468(80)80291-0. [DOI] [PubMed] [Google Scholar]
- 16.Muraji T, Mahour GH. Surgical problems in patients with VATER-associated anomalies. J Pediatr Surg. 1984;19:550–4. doi: 10.1016/s0022-3468(84)80102-5. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham ME, Charles G, Boachie-Adje O. Posterior vertebral column resection for VATER/VACTERL associated spinal deformity: a case report. HSS J. 2007;3:71–6. doi: 10.1007/s11420-006-9021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill BR, Yu AK, Tyler-Kabara EC. Prevalence of tethered spinal cord in infants with VACTERL. J Neurosurg Pediatr. 2010;6:177–82. doi: 10.3171/2010.5.PEDS09428. [DOI] [PubMed] [Google Scholar]
- 19.Koo BN, Hong JY, Song HT, Kim JM, Kil HK. Ultrasonography reveals a high prevalence of lower spinal dysraphism in children with urogenital anomalies. Acta Anaesthesiol Scand. 2012;56:624–8. doi: 10.1111/j.1399-6576.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 20.Teo AT, Gan BK, Tung JS, Low Y, Seow WT. Low-lying spinal cord and tethered cord syndrome in children with anorectal malformations. Singapore Med J. 2012;53:570–6. [PubMed] [Google Scholar]
- 21.Levitt MA, Patel M, Rodriguez G, Gaylin DS, Pena A. The tethered spinal cord in patients with anorectal malformations. J Pediatr Surg. 1997;32:462–8. doi: 10.1016/s0022-3468(97)90607-2. [DOI] [PubMed] [Google Scholar]
- 22.Hajnovic L, Trnka J. Tethered spinal cord syndrome--the importance of time for outcomes. Eur J Pediatr Surg. 2007;17:190–3. doi: 10.1055/s-2007-965133. [DOI] [PubMed] [Google Scholar]
- 23.Tuuha SE, Aziz D, Drake J, Wales P, Kim PC. Is surgery necessary for asymptomatic tethered cord in anorectal malformation patients? J Pediatr Surg. 2004;39:773–7. doi: 10.1016/j.jpedsurg.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Levitt MA, Pena A. Anorectal malformations. Orphanet J Rare Dis. 2007;2:33. doi: 10.1186/1750-1172-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rufener S, Ibrahim M, Parmar HA. Imaging of congenital spine and spinal cord malformations. Neuroimaging Clin N Am. 2011;21:659–76. viii. doi: 10.1016/j.nic.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43:236–48. doi: 10.1159/000098836. [DOI] [PubMed] [Google Scholar]
- 27.Levitt MA, Pena A. Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg. 2010;19:128–38. doi: 10.1053/j.sempedsurg.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Allen SR, Ignacio R, Falcone RA, Alonso MH, Brown RL, Garcia VF, et al. The effect of a right-sided aortic arch on outcome in children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2006;41:479–83. doi: 10.1016/j.jpedsurg.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 29.Olgun H, Karacan M, Caner I, Oral A, Ceviz N. Congenital cardiac malformations in neonates with apparently isolated gastrointestinal malformations. Pediatr Int. 2009;51:260–2. doi: 10.1111/j.1442-200X.2008.02711.x. [DOI] [PubMed] [Google Scholar]
- 30.Arbell D, Golender J, Khalaileh A, Gross E. Search for the azygos: a lesson learnt from a case with left superior vena cava, esophageal atresia and tracheo-esophageal fistula. Pediatr Surg Int. 2009;25:121–2. doi: 10.1007/s00383-008-2291-9. [DOI] [PubMed] [Google Scholar]
- 31.Chin C. ABCs of Neonatal Cardiac Anesthesia. Artif Organs. 2013;37:100–2. doi: 10.1111/j.1525-1594.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 32.Ashburn DA, Harris L, Downar EH, Siu S, Webb GD, Williams WG. Electrophysiologic surgery in patients with congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003;6:51–8. doi: 10.1053/pcsu.2003.50013. [DOI] [PubMed] [Google Scholar]
- 33.Bicakci U, Tander B, Ariturk E, Rizalar R, Ayyildiz SH, Bernay F. The right-sided aortic arch in children with esophageal atresia and tracheo-esophageal fistula: a repair through the right thoracotomy. Pediatr Surg Int. 2009;25:423–5. doi: 10.1007/s00383-009-2354-6. [DOI] [PubMed] [Google Scholar]
- 34.Wood JA, Carachi R. The right-sided aortic arch in children with oesophageal atresia and tracheo-oesophageal fistula. Eur J Pediatr Surg. 2012;22:3–7. doi: 10.1055/s-0031-1285906. [DOI] [PubMed] [Google Scholar]
- 35.Morgan RD, O’Callaghan JM, Wagener S, Grant HW, Lakhoo K. Surgical correction of tracheo-oesophageal fistula and oesophageal atresia in infants with VACTERL association: a retrospective case-control study. Pediatr Surg Int. 2012;28:967–70. doi: 10.1007/s00383-012-3165-8. [DOI] [PubMed] [Google Scholar]
- 36.Spitz L. Oesophageal atresia treatment: a 21st-century perspective. J Pediatr Gastroenterol Nutr. 2011;52 (Suppl 1):S12. doi: 10.1097/MPG.0b013e3182116082. [DOI] [PubMed] [Google Scholar]
- 37.Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24. doi: 10.1186/1750-1172-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houben CH, Curry JI. Current status of prenatal diagnosis, operative management and outcome of esophageal atresia/tracheo-esophageal fistula. Prenat Diagn. 2008;28:667–75. doi: 10.1002/pd.1938. [DOI] [PubMed] [Google Scholar]
- 39.Alberti D, Boroni G, Corasaniti L, Torri F. Esophageal atresia: pre and post-operative management. J Matern Fetal Neonatal Med. 2011;24 (Suppl 1):4–6. doi: 10.3109/14767058.2011.607558. [DOI] [PubMed] [Google Scholar]
- 40.Karnak I, Senocak ME, Hicsonmez A, Buyukpamukcu N. The diagnosis and treatment of H-type tracheoesophageal fistula. J Pediatr Surg. 1997;32:1670–4. doi: 10.1016/s0022-3468(97)90503-0. [DOI] [PubMed] [Google Scholar]
- 41.Telkes G, Reusz G, Szabo AJ, Langer RM. A single-center experience with kidney transplantation in the verteberal, anal, cardiac, tracheoesophageal, renal, and limb birth detects (VACTERL) association. Transplant Proc. 2011;43:1250–1. doi: 10.1016/j.transproceed.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 42.Sfakianakis GN, Sfakianaki E, Georgiou M, Serafini A, Ezuddin S, Kuker R, et al. A renal protocol for all ages and all indications: mercapto-acetyl-triglycine (MAG3) with simultaneous injection of furosemide (MAG3-F0): a 17-year experience. Semin Nucl Med. 2009;39:156–73. doi: 10.1053/j.semnuclmed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Aktas GE, Inanir S. Relative renal function with MAG-3 and DMSA in children with unilateral hydronephrosis. Ann Nucl Med. 2010;24:691–5. doi: 10.1007/s12149-010-0397-3. [DOI] [PubMed] [Google Scholar]
- 44.Burgu B, Aydogdu O, Soygur T, Baker L, Snodgrass W, Wilcox D. When is it necessary to perform nuclear renogram in patients with a unilateral neonatal hydronephrosis? World J Urol. 2012;30:347–52. doi: 10.1007/s00345-011-0744-6. [DOI] [PubMed] [Google Scholar]
- 45.Solomon BD, Raam MS, Pineda-Alvarez DE. Analysis of genitourinary anomalies in patients with VACTERL (Vertebral anomalies, Anal atresia, Cardiac malformations, Tracheo-Esophageal fistula, Renal anomalies, Limb abnormalities) association. Congenit Anom (Kyoto) 2011;51:87–91. doi: 10.1111/j.1741-4520.2010.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maschke SD, Seitz W, Lawton J. Radial longitudinal deficiency. J Am Acad Orthop Surg. 2007;15:41–52. doi: 10.5435/00124635-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Iafolla AK, McConkie-Rosell A, Chen YT. VATER and hydrocephalus: distinct syndrome? Am J Med Genet. 1991;38:46–51. doi: 10.1002/ajmg.1320380112. [DOI] [PubMed] [Google Scholar]
- 48.Genuardi M, Chiurazzi P, Capelli A, Neri G. X-linked VACTERL with hydrocephalus: the VACTERL-H syndrome. Birth Defects Orig Artic Ser. 1993;29:235–41. [PubMed] [Google Scholar]
- 49.Corsello G, Giuffre L. VACTERL with hydrocephalus: a further case with probable autosomal recessive inheritance. Am J Med Genet. 1994;49:137–8. doi: 10.1002/ajmg.1320490133. [DOI] [PubMed] [Google Scholar]
- 50.Chung B, Shaffer LG, Keating S, Johnson J, Casey B, Chitayat D. From VACTERL-H to heterotaxy: variable expressivity of ZIC3-related disorders. Am J Med Genet A. 2011;155A:1123–8. doi: 10.1002/ajmg.a.33859. [DOI] [PubMed] [Google Scholar]
- 51.McCauley J, Masand N, McGowan R, Rajagopalan S, Hunter A, Michaud JL, et al. X-linked VACTERL with hydrocephalus syndrome: further delineation of the phenotype caused by FANCB mutations. Am J Med Genet A. 2011;155A:2370–80. doi: 10.1002/ajmg.a.33913. [DOI] [PubMed] [Google Scholar]
- 52.Kopic S, Eirich K, Schuster B, Hanenberg H, Varon-Mateeva R, Rittinger O, et al. Hepatoblastoma in a 4-year-old girl with Fanconi anaemia. Acta Paediatr. 2011;100:780–3. doi: 10.1111/j.1651-2227.2010.02116.x. [DOI] [PubMed] [Google Scholar]
- 53.Guillerman RP. Infant craniospinal ultrasonography: beyond hemorrhage and hydrocephalus. Semin Ultrasound CT MR. 2010;31:71–85. doi: 10.1053/j.sult.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Castori M. Diabetic embryopathy: a developmental perspective from fertilization to adulthood. Mol Syndromol. 2013;4:74–86. doi: 10.1159/000345205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reutter H, Ludwig M. VATER/VACTERL Association: Evidence for the Role of Genetic Factors. Mol Syndromol. 2013;4:16–9. doi: 10.1159/000345300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siebel S, Solomon BD. Mitochondrial Factors and VACTERL Association-Related Congenital Malformations. Mol Syndromol. 2013;4:63–73. doi: 10.1159/000346301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DA. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010;127:731–3. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartels E, Jenetzky E, Solomon BD, Ludwig M, Schmiedeke E, Grasshoff-Derr S, et al. Inheritance of the VATER/VACTERL association. Pediatr Surg Int. 2012;28:681–5. doi: 10.1007/s00383-012-3100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon BD, Pineda-Alvarez DE, Hadley DW, Hansen NF, Kamat A, Donovan FX, et al. Exome Sequencing and High-Density Microarray Testing in Monozygotic Twin Pairs Discordant for Features of VACTERL Association. Mol Syndromol. 2013;4:27–31. doi: 10.1159/000345406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brosens E, Eussen H, van Bever Y, van der Helm RM, Ijsselstijn H, Zaveri HP, et al. VACTERL Association Etiology: The Impact of de novo and Rare Copy Number Variations. Mol Syndromol. 2013;4:20–6. doi: 10.1159/000345577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alter BP, Rosenberg PS. VACTERL-H Association and Fanconi Anemia. Mol Syndromol. 2013;4:87–93. doi: 10.1159/000346035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albers CA, Paul DS, Schulze H, Freson K, Stephens JC, Smethurst PA, et al. Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nat Genet. 2012;44:435–9. S1–2. doi: 10.1038/ng.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon BD, Pineda-Alvarez DE, Hadley DW, Keaton AA, Agochukwu NB, Raam MS, et al. De novo deletion of chromosome 20q13.33 in a patient with tracheo-esophageal fistula, cardiac defects and genitourinary anomalies implicates GTPBP5 as a candidate gene. Birth Defects Res A Clin Mol Teratol. 2011;91:862–5. doi: 10.1002/bdra.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohler S, Schulz MH, Krawitz P, Bauer S, Dolken S, Ott CE, et al. Clinical diagnostics in human genetics with semantic similarity searches in ontologies. Am J Hum Genet. 2009;85:457–64. doi: 10.1016/j.ajhg.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]