Summary
Hexokinase-II (HK-II) catalyzes the first step of glycolysis and also functions as a protective molecule, however, its role in protective autophagy has not been determined. Results showed that inhibition of HK-II diminished, while overexpression of HK-II potentiated, autophagy induced by glucose deprivation in cardiomyocyte and non-cardiomyocyte cells. Immunoprecipitation studies revealed that HK-II binds to and inhibits the autophagy suppressor, mTOR complex 1 (TORC1), and this binding was increased by glucose deprivation. The TOS motif, a scaffold sequence responsible for binding TORC1 substrates, is present in HK-II and mutating it blocked its ability to bind to TORC1 and regulate protective autophagy. The transition from glycolysis to autophagy appears to be regulated by a decrease in glucose-6 phosphate. We suggest that HK-II binds TORC1 as a decoy substrate and provides a previously unrecognized mechanism for switching cells from a metabolic economy based on plentiful energy, to one of conservation, under starvation.
Introduction
In response to stress, apoptotic (type I) and necrotic (type III) cell death programs are activated, while survival signaling is also elicited to salvage the heart (Galluzzi et al., 2007; Whelan et al., 2010). Although autophagy was initially discovered as a type II programmed cell death mechanism, in which cells undergo “self-digestion” of intracellular contents and organelles, recent evidence reveals that autophagy can serve as a protective mechanism (Colell et al., 2007; Deter and De Duve, 1967; Hamacher-Brady et al., 2006; Levine and Kroemer, 2008; Lum et al., 2005; Matsui et al., 2007; Rabinowitz and White, 2010; Whelan et al., 2010). The limited ability of adult cardiomyocytes to proliferate means that irreversible loss by cell death plays a crucial role in heart diseases, including ischemic injury (Adams et al., 1998; Baines et al., 2005; Whelan et al., 2012). In the heart, excessive autophagy has been shown to be maladaptive in pressure-overload induced heart failure and reperfusion injury (Matsui et al., 2007; Zhu et al., 2007), while an increase in autophagy confers cardioprotection against energy depletion induced by starvation or ischemia (Levine and Kroemer, 2008; Lum et al., 2005; Matsui et al., 2007; Nakai et al., 2007; Rabinowitz and White, 2010).
Mechanistic (mammalian) target of rapamycin (mTOR) complex 1 (TORC1) and AMPK have been established to be the key negative and positive regulators of autophagy (Sengupta et al., 2010; Wullschleger et al., 2006; Yuan et al., 2013). TORC1 is a signaling complex containing mTOR, raptor (the defining component of TORC1) and mammalian LST8/G-protein β-subunit like protein (mLST8/GβL) (Kim et al., 2002; Kim et al., 2003). Under nutrient-rich conditions, TORC1 activity is increased in response to amino acid increases or upstream kinases including Akt, supporting cellular growth (Inoki et al., 2002; Sengupta et al., 2010). Raptor is an essential scaffold for TORC1 mediated phosphorylation of downstream target molecules such as p70S6K and 4E-BP1 (Kim et al., 2002). These TORC1 substrates bind to raptor through their TOS motif (mTOR signaling motif) in order to be phosphorylated by mTOR (Nojima et al., 2003; Schalm and Blenis, 2002). TORC1 serves as a brake on autophagy, as initially noted by the observations that inhibition of TORC1 by rapamycin induces autophagy (Schmelzle and Hall, 2000). Recent seminal studies have identified the serine/threonine kinase, ULK1, a mammalian homolog of yeast Atg1, as an mTOR substrate: phosphorylation of ULK-1 at Ser757/758 (mouse/human) by mTOR inhibits its activity and thereby autophagy (Kim et al., 2011; Shang et al., 2011). AMP-activated protein kinase (AMPK) is a sensor for metabolic state activated during starvation. Activation of AMPK inhibits the TORC1 pathway, activates ULK-1 and positively regulates autophagy (Egan et al., 2011b; Gwinn et al., 2008; Inoki et al., 2003; Kim et al., 2011; Shang et al., 2011; Takagi et al., 2007).
Hexokinase (HK) catalyzes the first step of glycolysis, phosphorylating glucose to glucose-6-phosphate. Hexokinase-II (HK-II) is a predominant isoform in the heart, as well as adipose and skeletal muscle, and is upregulated in many types of tumor. In contrast, hexokinase-I (HK-I) is ubiquitously expressed (Pastorino and Hoek, 2003; Wilson, 2003). There is increasing recognition that energy metabolism and cellular protection utilize common signaling pathways and it has been suggested that HK-II plays an important role, not only in glycolysis, but also in cell survival. We have previously demonstrated that HK-II plays a significant role in Akt mediated mitochondrial protection against opening of the mitochondrial permeability transition pore in cardiomyocytes (Miyamoto et al., 2008; Sun et al., 2008) and others have demonstrated that HK-II competes with apoptotic Bcl-2 family proteins, such as Bax and t-Bid, to prevent outer mitochondrial membrane rupture (Pastorino et al., 2002; Robey and Hay, 2006).
Although it is established that glucose deprivation activates autophagy through TORC1 inhibition, the mechanism of signal integration between glycolytic and TORC1/autophagy pathways has not been elucidated. Here we demonstrate for the first time that, in response to glucose deprivation, HK-II binds to TORC1 through its TOS motif, decreasing TORC1 activity, and thereby positively regulates protective autophagy.
Results
Inhibition of HK-II by 2-Deoxy-D-glucose decreases glucose deprivation induced autophagy
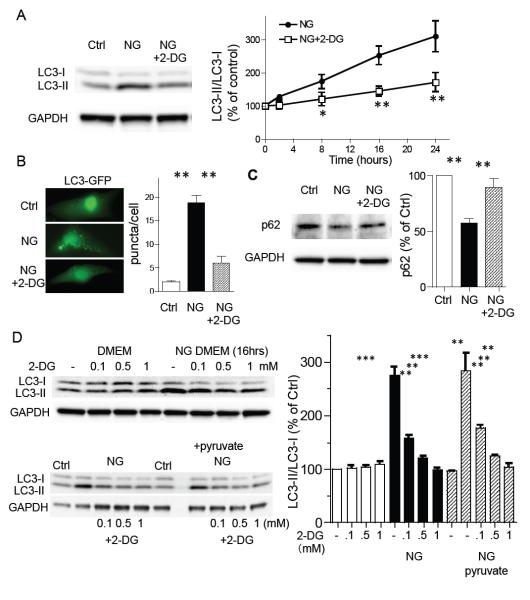
Neonatal rat ventricular myocytes (NRVMs) were cultured in the absence of glucose (glucose free DMEM) for 16 hrs and whole cell lysates were subjected to Western blotting for changes in LC3-I and II, a hallmark of autophagy. Glucose deprivation lead to a time dependent increase in the LC3-II/I ratio (fig. 1A, B). 2-deoxy-D-glucose (2-DG) is an analogue of glucose which is phosphorylated by hexokinases, but not metabolized further to produce ATP, and has been used as a hexokinase inhibitor by competing with glucose. Remarkably, 2-DG (0.5 mM) treatment prevented the increase in LC3-II/I induced by glucose deprivation for up to 24 hrs (fig. 1A), but not that induced by staurosporine (Chu et al., 2013), (fig. S1A). Treatment with 0.5 mM 2-DG also decreased the formation of GFP-LC3 puncta (fig. 1B). As LC3-II/I can also accumulate under conditions in which autophagic flux is inhibited, we examined the levels of p62, a protein incorporated into autophagosomes and degraded in autolysosomes. Glucose deprivation significantly decreased levels of p62, an effect significantly attenuated by 2-DG (fig. 1C), confirming functional autophagy and previous observations in cardiomyocytes (Gottlieb and Mentzer, 2010; Hariharan et al., 2010). The inhibition of LC3-II/I by 2-DG in glucose free medium was concentration dependent (0.1 mM to 1 mM) but without effect in the presence of glucose (fig. 1D). It has been reported that 2-DG activates, rather than inhibits, autophagy (DiPaola et al., 2008; Wang et al., 2011; Xi et al., 2011). Similarly, we confirmed that the high concentrations of 2-DG used in other studies (≥5 mM) in the presence of 25 mM glucose increased the LC3-II/I ratio in our system (fig. S1B). The inhibition of glucose deprivation induced autophagy mediated by 0.5 mM 2-DG was also evident when mitochondria were supplemented with 5 mM pyruvate, a downstream product of glycolysis, to glucose free medium indicating that the effect of 2-DG is not due to a reduction in respiration (fig. 1D). Together, these data implicate HK-II in the induction of autophagy by glucose deprivation.
Figure 1. Glucose deprivation induced autophagy is inhibited by 2-deoxy-D-glucose (2-DG) in neonatal rat ventricular myocytes (NRVMs).
(A) Cardiomyocytes were cultured in DMEM or no-glucose (NG) DMEM in the presence or absence of 2-deoxy-D-glucose (2-DG; 0.5 mM) for 16 hrs and subjected to Western blotting for LC3 (left panels). The time-course of the LC3-II/LC3-I ratio induced by glucose deprivation, plus or minus 2-DG (0.5 mM), assessed by Western blotting (right panel; n=8-10). *P<0.05, **P<0.01 vs each time point after glucose deprivation. (B) To visualize formation of autophagy, NRVMs were infected with LC3-GFP adenovirus. After 24 hrs, cells were subjected to glucose deprivation (NG) in the presence or absence of 2-DG (0.5 mM) for 16 hrs. (C) Western blotting for p62. Cells were cultured in DMEM or no-glucose (NG) DMEM in the presence or absence of 2-deoxy-D-glucose (2-DG; 0.5 mM) for 16 hrs. **P<0.01 (n=6). (D) Cardiomyocytes were cultured in DMEM or NG-DMEM in the presence or absence of 2-DG (0.1, 0.5 and 1 mM). To energize mitochondria, 5 mM pyruvate was added (lower blots). **P<0.01, ***P<0.001 n=7. Data are mean ± SEM.
Knockdown of HK-II attenuates, but overexpression of HK-II potentiates, autophagy induced by glucose deprivation
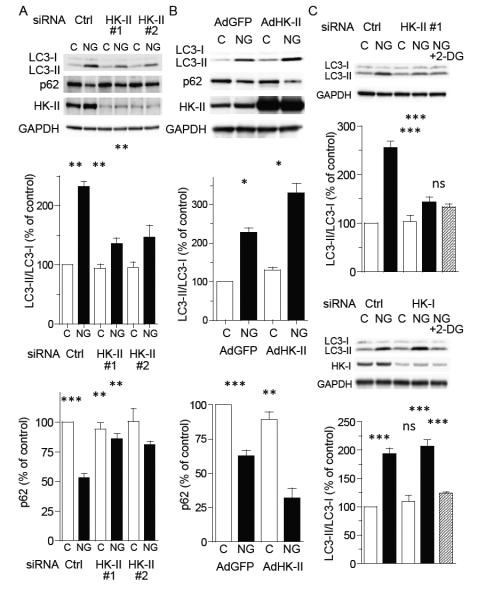
To more directly examine the role of HK-II in autophagy induced by glucose deprivation, HK-II expression was decreased using two different small interfering RNAs (siRNA). While there was no effect in the presence of glucose (basal), HK-II knockdown (by ~70%) in NRVMs significantly inhibited the increase in the LC3-II/I ratio and attenuated the decrease in p62 expression in response to glucose deprivation (fig 2A). Adenoviral overexpression of wild-type HK-II slightly increased basal and markedly potentiated glucose deprivation induced LC3-II formation as well as the decrease in p62 expression (fig. 2B), further supporting a role for HK-II in autophagy induced by glucose withdrawal. Adding pyruvate (5 mM) to glucose free media did not alter the effects of HK-II knockdown or overexpression on LC3-II formation (data not shown). In cells treated with HK-II siRNA, 2-DG treatment did not decrease the LC3-II/I ratio (fig. 2C). Unlike HK-II, knockdown of hexokinase-I (HK-I) did not inhibit the increase in LC3-II/I ratio induced by glucose deprivation, while treatment with 2-DG still inhibited the LC3-II/I ratio (fig. 2C), suggesting a specific role for HK-II.
Figure 2. Hexokinase-II (HK-II) regulates glucose deprivation induced autophagy in cardiomyocytes.
(A) NRVMs transfected with control siRNA (si-Ctrl) or two different HK-II siRNAs (si-HK-II #1 and #2) were cultured in DMEM or no-glucose (NG) DMEM for 16 hrs, (n=9). (B) Cells were infected with GFP (Ad-GFP; control) or wild-type HK-II (AdHK-II) adenoviruses and subjected to 16 hrs glucose deprivation 24 hrs (n=9). (C) Cells were transfected with control siRNA (si-Ctrl), HK-II siRNA (si-HK-II-#1) or HK-I siRNA and cultured in DMEM or no-glucose (NG) DMEM in the presence or absence of 0.5 mM 2-DG for 16 hrs (n=6-8). *P<0.05, **P<0.01, ***P<0.001. ns; not significant. Data are mean ± SEM.
Knockdown of HK-II increases, but overexpression of HK-II decreases, cell death induced by glucose deprivation
A protective role for autophagy under conditions of starvation has previously been demonstrated (Levine and Kroemer, 2008; Lum et al., 2005; Matsui et al., 2007; Nakai et al., 2007; Rabinowitz and White, 2010). NRVMs were subjected to glucose deprivation for 24 hours and apoptosis assessed by DNA fragmentation. As expected, inhibition of autophagy with 3-Methyladenine (3-MA; 10 mM) increased glucose deprivation-induced apoptosis, supporting a protective role of autophagy (fig. 3A). We therefore examined the effects of HK-II inhibition with 2-DG or HK-II knockdown and demonstrated that these interventions also significantly increased apoptotic cell death (fig. 3A and B). However, overexpression of HK-II largely attenuated glucose deprivation induced apoptosis (fig. 3C). These results show that HK-II stimulated autophagy confers protection to myocytes against the effects of glucose deprivation.
Figure 3. HK-II contributes to cardiomyocyte protection against glucose deprivation induced apoptosis.
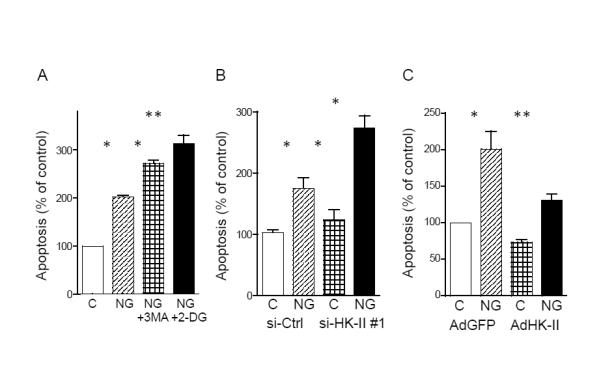
(A) NRVMs were cultured in DMEM or no-glucose (NG) DMEM for 24 hrs ± 10 mM 3-Methyladenine (3-MA) or 0.5 mM 2-DG. DNA fragmentation was determined by ELISA-based assay (n=5). (B) NRVMs transfected with control siRNA (si-Ctrl) or HK-II siRNA (si-HK-II) were subjected to glucose deprivation for 24 hrs (n=6). (C) NRVMs expressing GFP or HK-II were subjected to glucose deprivation for 24 hrs (n=8). *P<0.05, **P<0.01. Data are mean ± SEM. See also Figure S1.
A critical role for AMPK in inhibition of TORC1 and stimulation of autophagy under glucose starvation has been widely accepted (Sengupta et al., 2010; Wullschleger et al., 2006; Yuan et al., 2013). To determine whether AMPK signaling is modulated by HK-II during glucose deprivation, AMPK activity was assessed by Western blotting for phosphorylation of AMPK (P-AMPK) at Thr172, which has been shown to be required for AMPK activation. Consistent with previous studies, P-AMPK increased in response to glucose deprivation, but the response was unaffected by HK-II knockdown or overexpression (fig. S2A). The phosphorylation status of acetyl-coA carboxylase and raptor, established downstream targets of AMPK (Yuan et al., 2013), was also unchanged, suggesting that HK-II does not modify the activation status of AMPK in the presence or absence of glucose (fig S2B, C). Akt can lead to TORC1 activation, opposing the effect of AMPK on autophagy. However, glucose deprivation did not affect phosphorylation of Akt in NRVMs, nor was it affected by HK-II knockdown nor overexpression (fig. S2D). Additionally, we examined the effect of HK-II on autophagy induced by rapamycin, a TORC1 inhibitor. HK-II knockdown did not decrease the increase in LC3-II/I ratio induced by rapamycin (50 nM for 18 hrs) and rapamycin induced autophagy was not affected by addition of 0.5 mM 2-DG in control or HK-II siRNA treated cells (fig. S2E). These results suggest that HK-II positively regulates autophagy at the level of TORC1.
HK-II contributes to suppression of TORC1 activity under glucose deprivation
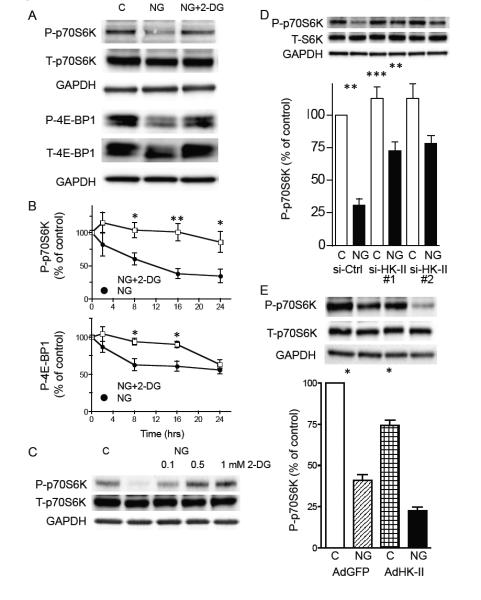
To examine TORC1 activity, phosphorylation of TORC1 substrates, p70S6K and 4E-BP1 was assessed by Western blotting. Phosphorylation of p70S6K at Thr389 and 4E-BP1 at Thr37/46 was significantly decreased by glucose deprivation and this inhibition was prevented by treatment with 2-DG (0.5 mM; fig. 4A and B). The ability of 2-DG to prevent decreases in P-p70S6K was dose-dependent (fig. 4C), similar to that previously observed for the inhibition of LC3-II formation in figure 1D. Furthermore, siRNA-mediated HK-II knockdown also significantly attenuated glucose deprivation induced decreases in P-p70S6K, while overexpression of HK-II enhanced this response (fig. 4D and E). In contrast, as observed with the LC3-II/I ratio (fig. 2C), HK-I knockdown failed to affect the decrease in P-p70S6K induced by glucose deprivation, while the response was still reversed by 2-DG treatment (fig. S3). These results suggest that HK-II, but not HK-I, negatively regulates TORC1 activity during glucose deprivation.
Figure 4. HK-II contributes to decrease in TORC1 activity under glucose deprivation.
(A) Representative Western blotting for phosphorylated p70S6K at Thr389 and phosphorylated 4E-BP1 at Thr37/46. NRVMs were cultured in DMEM (control) or in no-glucose (NG) DMEM in the presence or absence of 2-DG (0.5 mM) for 16 hrs. (B) Time-course of changes in P-p70S6K and P-4E-BP1 after glucose deprivation ± 2-DG (0.5 mM; n=6-8). *P<0.05, **P<0.01 vs. each time point after glucose deprivation. (C) Dose-dependent effect of 2-DG on inhibition of decrease in P-p70S6K induced by 16 hrs glucose deprivation. (D) Knockdown of HK-II attenuated the decrease in P-p70S6K induced by glucose deprivation. NRVMs transfected with control siRNA (si-Ctrl) or HK-II siRNAs (si-HK-II #1 and #2) were subjected to 16hrs glucose deprivation (n=7). (E) Overexpression of WT HK-II enhanced the inhibitory effect of glucose deprivation on P-p70S6K (n=8). *P<0.05, **P<0.01. Data are mean ± SEM.
Role of HK-II catalytic activity in regulation of autophagy
We then determined the effects of a kinase-dead (KD) mutant HK-II (D209A/D657A) on the LC3-II/I ratio and p62 under glucose deprivation. As observed for wild-type HK-II, expression of the kinase-dead mutant potentiated the increase in the LC3-II/I ratio, and decrease in p62 levels following glucose deprivation, and further decreased P-p70S6K (fig. 5A and B). These findings indicate that the enzymatic activity of HK-II is not necessary for its ability to inhibit TORC1 activity under glucose deprivation and suggest that HK-II serves as a binding protein to negatively regulate TORC1 in response to glucose deprivation.
Figure 5. Role of kinase activity of HK-II on regulation of autophagy under glucose deprivation.
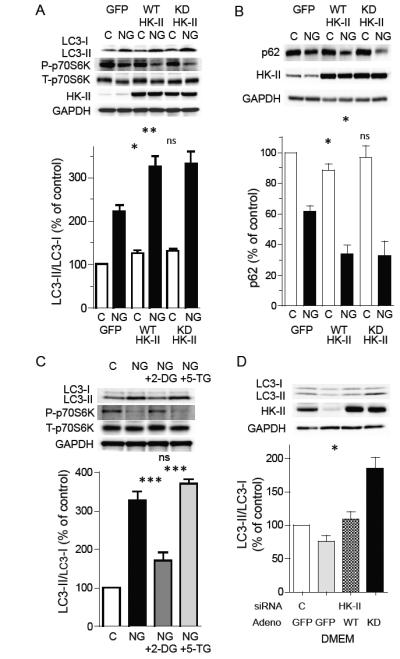
(A) NRVMs expressing GFP (control), HK-II (WT HK-II) or kinase-dead HK-II (KD HK-II) were cultured in the presence or absence of glucose for 16 hrs (n=8). (B) Representative Western blots and quantitative analysis of effects of WT and KD HK-II on p62 are shown (n=6). (C) Glucose deprivation induced increase in LC3-II and decrease in P-p70S6K are inhibited by 2-deoxy-D-glucose (2-DG), but not by 5-thio-glucose (5-TG). Cells were cultured in DMEM or NG DMEM in the presence or absence of 0.5 mM 2-DG or 0.5 mM 5-TG for 16 hrs. (D) Effect of KD HK-II in HK-II knocked down cells cultured in DMEM. HK-II was knocked down by siRNA (#1) followed by adenoviral infection with GFP, WT HK-II or KD HK-II (n=7). *P<0.05, **P<0.01, ***P<0.001, ns; not significant. Data are mean ± SEM.
Although it is clear that HK-II kinase activity is not required for its effect on autophagy, kinase activity is nevertheless a requirement for its role in glycolysis and so might act instead to suppress its effect on autophagy. Thus, we hypothesized that HK-II uses the presence of G-6P (control condition) or 2-DG-6P (NG+2-DG condition) to switch between glycolytic and autophagic functions. We tested this possibility by comparing 2-DG to 5-thio-glucose (5-TG), which binds to hexokinase but cannot be phosphorylated (Wilson and Chung, 1989). NRVMs were cultured in the absence of glucose with or without 2-DG (0.5 mM) or 5-TG (0.5 mM) for 16 hrs. As shown in figure 5C, the glucose deprivation-induced increase in the ratio of LC3-II/I was significantly reduced by 2-DG, however, this response was not mimicked by 5-TG. Likewise the decrease in P-p70S6K induced by glucose deprivation was inhibited by 2-DG, but not by 5-TG. Concentrations of 5-TG, up to 5 mM, also failed to alter the increase in the LC3-II/I ratio caused by glucose deprivation (fig. S4A). To further demonstrate that G-6P, produced by HK-II catalytic activity, is an important factor suppressing HK-II mediated autophagy, endogenous HK-II was decreased by siRNA treatment and WT or kinase-dead HK-II were adenovirally reintroduced, cultured in the presence of glucose, and LC3-II/I ratio was assessed (fig. 5D). Introduction of kinase-dead HK-II, but not WT HK-II, into HK-II depleted cells increased the LC3-II/I ratio in the presence of glucose, suggesting that the autophagic function of HK-II is inhibited by its glycolytic activity.
We and others have shown that mitochondrial HK-II confers cellular protection against acute stress (Miyamoto et al., 2008; Pastorino et al., 2002; Roberts et al., 2013; Robey and Hay, 2006; Sun et al., 2008). To determine whether mitochondrially-bound HK-II plays a regulatory role in protective autophagy under glucose deprivation, we examined the effect of expression of the N-terminus deletion mutant of HK-II (ΔN HK-II) which has been shown to lack the ability to bind to mitochondria (Roberts et al., 2013; Sui and Wilson, 1997). Interestingly, the expression of ΔN HK-II still retains the ability to potentiate the increase in LC3-II/I ratio and decrease in p62 induced by glucose deprivation (fig. S4B), suggesting that the autophagic effect of HK-II is distinct from the established role of HK-II at mitochondria.
HK-II directly binds to TORC1 and association is increased by glucose deprivation
To determine whether HK-II interacts with TORC1 directly to inhibit its activity in response to glucose deprivation, immunoprecipitation studies were carried out (fig. 6). Cells were cultured in the presence or absence of glucose for 16 hrs and mTOR was immunoprecipitated followed by Western blotting for HK-II. It is reported that the mTOR-raptor complex (TORC1) is readily dissociated using non-ionic detergents and CHAPS is commonly used for immunoprecipitation of TORC1 (Kim et al., 2002). Consistent with previous observations obtained in non-cardiomyocyte cells, an association between mTOR and raptor was preserved in immunoprecipitations carried out using 0.3 % CHAPS. However, only by replacing CHAPS with 0.02 % digitonin could HK-II pull-out also be observed, suggesting that HK-II only weakly binds to mTOR (fig. 6A). When raptor was knocked-down using siRNA, binding of GβL to mTOR was not disrupted as shown previously (Kim et al., 2003; Wang et al., 2007), however, the mTOR-HK-II association was largely diminished (fig. 6B), suggesting that HK-II associates with TORC1. On the contrary, HK-I in the mTOR immunocomplex was not evident and the observed faint signal was insensitive to raptor knockdown.
Figure 6. HK-II associates with TORC1 and this is increased by glucose deprivation.
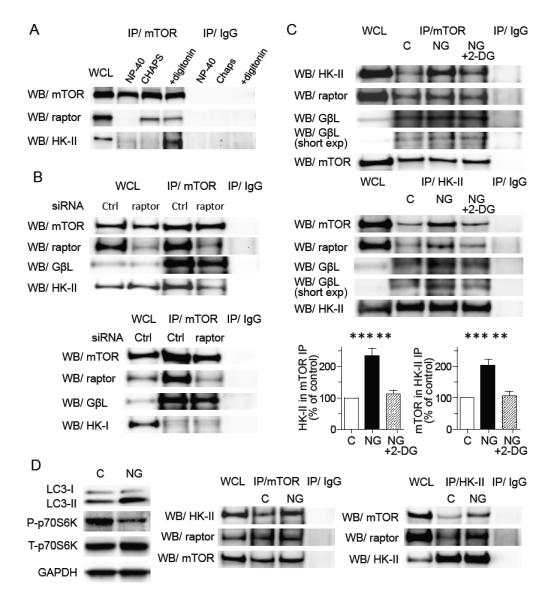
(A) Association of HK-II with mTOR is sensitive to the detergent. Cells were harvested in 0.5 % NP-40 lysis buffer, 0.3% Chaps lysis buffer or 0.02% digitonin lysis buffer, subjected to mTOR immunoprecipitation and Western blotting for HK-II and raptor. (B) Knockdown of raptor decreases the association of mTOR with HK-II but not with GβL (upper panels). HK-I does not associate with mTOR (lower panels). NRVMs transfected with control siRNA or raptor siRNA were subjected to glucose deprivation for 16 hrs and mTOR immunoprecipitation was carried out. (C) Glucose deprivation increases association between HK-II and TORC1 which is inhibited by 0.5 mM 2-DG. NRVMs were subjected to glucose deprivation ± 2-DG for 16 hrs and mTOR or HK-II were immunoprecipitated. Right panels show quantitative analysis of the association of HK-II with mTOR (n=5), **P<0.01, ***P<0.01. (D) Adult mouse hearts perfused with NG DMEM show an increase in LC3-II, decrease in P-p70S6K, and increase in association between HK-II and mTOR. Adult mouse hearts were perfused in the Langendorff mode. After 1 hr perfusion with DMEM (control) or NG DMEM, hearts were homogenized in digitonin containing buffer and subjected to Western blotting or immunoprecipitation. Data are mean ± SEM.
We next determined whether the interaction between TORC1 and HK-II is affected by glucose deprivation (fig. 6C). Glucose deprivation significantly increased the association of mTOR with HK-II and addition of 2-DG to glucose free media decreased this association in NRVMs. Additional studies carried out using a HK-II antibody for immunoprecipitation also showed that association of HK-II with mTOR was increased following glucose deprivation and disrupted by treatment with 2-DG. Immunofluorescence studies also showed that co-localization between HK-II and mTOR was increased by glucose deprivation and this was reversed by addition of 2-DG (fig. S5). Glucose deprivation did not dissociate raptor nor GβL from mTOR immunocomplex (fig. 6C upper panels), suggesting that TORC1 integrity is preserved during glucose deprivation as previously demonstrated (Kim et al., 2003; Wang et al., 2007). TORC1 integrity was also preserved after addition of 2-DG. Importantly, glucose deprivation increased the levels of raptor and GβL in the HK-II immunocomplex which was reversed by addition of 2-DG (fig. 6C lower panels) supporting that HK-II binding to TORC1 is increased by glucose withdrawal and reversed by 2-DG. To confirm these results in vivo, adult mouse hearts were isolated and perfused in the Langendorff mode (fig. 6D). Whole tissue homogenates prepared from hearts subjected to 60 min glucose free perfusion showed increased LC3-II/LC3-I ratio and decreased P-p70S6K. Immunoprecipitation studies revealed that the association between HK-II and mTOR, and that between HK-II and raptor, were increased in the heart in response to glucose deprivation. Similar to the observations obtained in NRVMs, the raptor-mTOR association was preserved and the association of HK-II with raptor was increased under glucose deprivation.
The TOS motif in HK-II mediates its binding to TORC1 and effects on autophagy
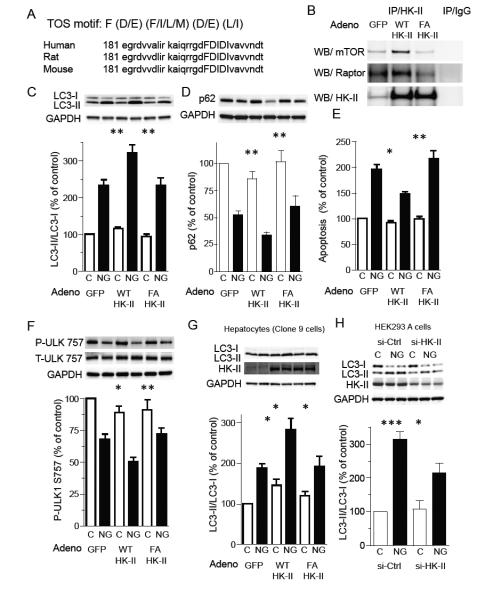
A TOS motif (mTOR signaling motif) is critical for binding to raptor and subsequent phosphorylation of TORC1 substrates, such as p70S6K and 4E-BP, and the first phenylalanine residue of the TOS motif has been shown to be essential for its function (Nojima et al., 2003; Schalm and Blenis, 2002). The NIH database revealed that HK-II contains a potential TOS motif (FDIDI) which is conserved in mouse, rat and human (fig. 7A). To determine whether this sequence is critical for HK-II binding to TORC1, we introduced a point mutation of the phenylalanine residue at position 199 to alanine (F199A). The mutant was subcloned into adenovirus and GFP (control), HK-II (wild-type; WT) and HK-II F199A were expressed in NRVMs, subjected to glucose deprivation, and HK-II immunoprecipitated. Overexpressed WT HK-II associated with mTOR and raptor, but HK-II F199A did not show association above control levels (endogenous HK-II), despite similar levels of WT and F199A in the immunocomplex (fig. 7B). We then examined the effect of HK-II F199A expression on the glucose deprivation induced increase in the LC3-II/I ratio and decrease in p62 expression. Unlike WT HK-II, HK-II F199A failed to potentiate the increase in LC3-II/I or decrease in p62 induced by glucose deprivation (fig. 7C and D). HK-II F199A also failed to provide protection against apoptotic cell death (fig. 7E). Glucose deprivation decreased phosphorylation of ULK-1 at Ser757, the TORC1 site, in control (GFP) cardiomyocytes, and this decrease was further enhanced by expression of HK-II WT but not by HK-II F199A (fig. 7F). It has been reported that, in response to the release of mTOR-mediated inhibition of ULK-1, phosphorylation of ULK-1 at Ser555 mediated by AMPK is increased to stimulate autophagy (Egan et al., 2011a; Kim et al., 2011). We also observed that glucose deprivation increased P-ULK-1 at Ser555 and this response was enhanced by WT HK-II but not by F199A mutant (fig. S6A).
Figure 7. The TOS motif in HK-II is responsible for HK-II binding to TORC1 and HK-II mediated regulation of autophagy under glucose deprivation.
(A) HK-II contains an mTOR signaling motif (TOS motif) which is conserved in mouse, rat and human (FDIDI). (B) The association of HK-II with mTOR is increased by overexpression of wild-type (WT) HK-II, but not by the TOS motif mutant HK-II (F199A; FA). NRVMs expressing GFP (control), WT HK-II or F199A mutant HK-II were subjected to glucose deprivation for 16 hrs and HK-II was immunoprecipitated. (C) WT HK-II, but not the FA mutant, enhances the increase in LC3-II/LC3-I ratio induced by glucose deprivation (16 hrs; n=10). (D) WT HK-II, but not the FA mutant, enhances the decrease in p62 induced by glucose deprivation (16 hrs; n=8). (E) WT HK-II, but not F199A mutant (FA), provides cardiomyocyte protection against glucose deprivation. Cells were cultured in no-glucose DMEM for 24 hrs and DNA fragmentation was examined (n=7). (F) Glucose deprivation (16 hrs) decreases phosphorylation of ULK-1 at Ser757 and this is enhanced by WT HK-II, but not by F199A mutant (FA; n=10). (G) Clone 9 cells (hepatocyte cell line) were infected with AdGFP, AdHK-II or AdF199A HK-II (FA) and subjected to glucose deprivation for 18 hrs (n=5). (H) HEK293A cells were transfected with control siRNA (si-Ctrl) or HK-II siRNA (si-HK-II #1) and subjected to 8 hrs glucose deprivation (n=4-8). *P<0.05, **P<0.01, ***P<0.001. Data are mean ± SEM.
To further demonstrate the role of HK-II and its TOS motif in glucose deprivation induced autophagy, endogenous HK-II was knocked down by siRNA treatment and WT or F199A HK-II were adenovirally introduced (fig. S6B). HK-II knockdown significantly decreased development of autophagy induced by glucose deprivation, as observed in figure 2, and this was rescued by add-back of WT HK-II, but not by F199A HK-II. These data support the important role of the TOS motif in HK-II mediated regulation of autophagy under glucose starvation.
Expression of the HK-II gene is nearly silent in the liver thus hepatocytes have been used to determine the role of HK-II (Goel et al., 2003). When WT HK-II was exogenously expressed in clone 9 cells, the increase in the LC3-II/LC3-I ratio induced by glucose deprivation was significantly potentiated. In contrast, expression of F199A HK-II did not potentiate the LC3-II/LC3-I ratio (fig. 7G). Conversely, HEK293 cells have been shown to express HK-II and are widely used to obtain insights into mechanisms of autophagy. HEK293A cells were transfected with control- or HK-II siRNAs and subjected to glucose deprivation for 8 hrs. Glucose deprivation induced autophagy in control cells and this response was significantly attenuated by HK-II knockdown (fig. 7H).
Discussion
Hexokinase-II (HK-II) phosphorylates glucose at the first step in glycolysis and is therefore a critical mediator of cellular metabolism. A growing body of evidence also suggests that HK-II contributes to cellular protection against stress. Here we demonstrate that HK-II positively regulates protective autophagy in response to glucose withdrawal and that this is mediated via an interaction with TORC1 (fig. S6C).
Previous work has established (Kim et al., 2011; Matsui et al., 2007) that glucose deprivation induces time-dependent development of autophagy, as evidenced by increased LC3-II/ratio as well as a decrease in p62 expression (Gottlieb and Mentzer, 2010). Crosstalk between the glycolytic pathway and autophagy in mediating this effect remains largely unknown. In agreement with previous findings, glucose deprivation in cardiomyocytes produced a strong induction of autophagy, however, blocking HK-II using 2-DG inhibited it. Direct evidence for a regulatory role of HK-II in glucose deprivation induced autophagy was provided by observations that siRNA mediated HK-II knockdown inhibits autophagy, whereas adenoviral overexpression enhances it (fig. 2). 2-DG inhibits glycolysis, which can result in a decrease in oxidative respiration because it cannot be metabolized further after HK-II phosphorylation. However, supplementing mitochondria with pyruvate, to restore oxidative phosphorylation, does not reverse the inhibitory effect of 2-DG, indicating that downstream effects on mitochondrial energy production are not involved. The inhibitory effect of 0.5 mM 2-DG on glucose deprivation induced autophagy appears to be through regulation of HK-II, since this concentration of 2-DG did not change the development of autophagy in HK-II knocked down cells in the absence of glucose (fig. 2C) nor regulate staurosporine or rapamycin induced autophagy (fig. S1A and S3). Knockdown of HK-I also had no effect on, or 2-DG inhibition of, autophagy induced by glucose deprivation (fig. 2C), suggesting a specific regulatory role for HK-II, although a role for HK-I cannot be completely ruled out due to potential expression level differences ie lower HK-I expression levels. Strikingly, exogenous expression of HK-II in clone 9 cells, which lack HK-II gene expression, significantly potentiates basal and starvation induced autophagy, while knockdown of HK-II reduces autophagy in HEK293A cultured in glucose free media. Thus, regulation of autophagy by HK-II is not limited to cardiomyocytes, but appears to play a controlling role in a variety of cell types expressing HK-II. This is, to our knowledge, the first experimental evidence that HK-II functions as a positive regulator of autophagy in the absence of glucose.
2-DG has previously been recognized as a stimulator, not inhibitor, of autophagy. We reconcile these observations by demonstrating that 2-DG (up to 1 mM) did not induce autophagy in cardiomyocytes cultured in the presence of glucose (fig. 1D), but higher concentrations (>5 mM), such as those used by others demonstrating stimulation of autophagy (DiPaola et al., 2008; Wang et al., 2011; Xi et al., 2011), did increase the LC3-II/I ratio (fig. S1B). Thus it is likely that 0.5 mM 2-DG is enough to prevent autophagy in the absence of glucose but not enough to induce autophagy in the presence of glucose (25 mM in DMEM). The dose-dependent effect of 2-DG could be different in different cell types (dependence on glycolysis), existence of other stress signals and the amount of competing glucose influencing metabolic inhibition and subsequent activation of AMPK-autophagy pathway.
The TORC1 complex is a major signaling nexus acting to suppress autophagy under growth conditions (Sengupta et al., 2010) so we hypothesized that HK-II positively regulates autophagy by inhibiting TORC1 signaling. Indeed, HK-II overexpression enhances the glucose deprivation dependent dephosphorylation of downstream targets of TORC1, p70S6K and 4E-BP1, whereas 2-DG or HK-II knockdown inhibits this response. Mirroring these findings, phosphorylation at Ser2481 of mTOR, implicated to be an autophosphorylation site and indicative of TORC1 activity (Soliman et al., 2010), displays parallel changes to those in its substrates (data not shown). ULK-1, a positive regulator of autophagy, has been demonstrated to be inhibited when phosphorylated at Ser757/758 by mTOR (Kim et al., 2011; Shang et al., 2011). We also demonstrated that phosphorylation of Ser757 in ULK-1 is reduced by glucose deprivation in cardiomyocytes, and decreased further by WT HK-II overexpression. Together, these results support previous observations that TORC1 activity is decreased by glucose deprivation and demonstrate that HK-II is a mediator of this response. The inhibitory effect of 2-DG on decrease in P-4E-BP1 was diminished after 24hrs, although the effect on P-p70S6K was still evident. This difference could be a time-dependent increase in apoptotic signaling, which is enhanced by 2-DG. 4E-BP1 is cleaved by caspase under apoptotic conditions, resulting in loss of the RAIP motif in the N-terminus (Tee and Proud, 2002), which plays a supportive role in the association with TORC1. Thus the ability of TORC1 to regulate 4E-BP1 might be diminished by increased apoptotic signaling, leading to loss of 2-DG effect on P-4E-BP1 after 24hrs.
A direct interaction between HK-II and the TORC1 complex was investigated using immunoprecipitation and revealed that HK-II directly binds to TORC1 and that this association is regulated by glucose availability (fig. 6). This finding was also supported by immunofluorescence data showing that co-localization of HK-II with mTOR is increased by glucose withdrawal. Binding between raptor and its substrates has been shown to be mediated through a TOS motif (Nojima et al., 2003; Schalm and Blenis, 2002), which was discovered to exist at amino acid position 199 (FDIDI) in HK-II and was also conserved between rat, mouse and human. Mutation of the TOS motif (F199A) rendered HK-II unable to bind to mTOR, or to regulate autophagy, in response to glucose deprivation in either cardiomyocytes or hepatocytes. Furthermore, the F199A mutation failed to affect Ser757 ULK-1 phosphorylation. Interestingly, HK-I, which lacks a TOS motif, does not regulate TORC1 activity assessed by P-p70S6K (fig. S3B) nor autophagy formation induced by glucose deprivation in cardiomyocytes (fig. 2C). Together, these data provide evidence for the role of the TOS motif in the HK-II-TORC1 association and suggest a specific role of HK-II in regulation of TORC1-autophagy pathway under glucose deprivation.
AMPK is recognized as a key sensor of energy status and shown to be indispensable for ischemia induced protective autophagy. We also observed that AMPK is robustly activated by glucose deprivation, however, this response is not altered by manipulation of HK-II expression. These results indicate that HK-II does not promote autophagy via regulation of AMPK, but instead inhibits TORC1 activity and thereby facilitates the autophagic processes driven by AMPK under starvation, as observed in augmented P-ULK at Ser555 (fig. S6A).
Akt activates the TORC1 pathway to stimulate cellular growth and inhibit autophagy (Inoki et al., 2002; Sengupta et al., 2010). One of mechanisms by which Akt activates TORC1 is phosphorylation of PRAS40 (Sancak et al., 2007; Wang et al., 2007). PRAS40 is an inhibitory binding protein of TORC1 and the TOS motif in PRAS40 is responsible for its binding. Akt phosphorylates PRAS40, sequestering it from TORC1 and terminating PRAS40 mediated TORC1 inhibition. In this study, the activity of Akt is not altered by glucose deprivation nor by manipulation of HK-II expression in cardiomyocytes. These results suggest that PRAS40 serves as a negative regulator under basal conditions and the inhibitory effect is released by Akt mediated phosphorylation under growth conditions, while HK-II could contribute to suppression of TORC1 activity under starvation conditions. Interestingly, activation of the Akt/TORC1 pathway has been demonstrated to positively regulate HK-II expression (Bhaskar et al., 2009; Osawa et al., 1996). Thus, under growth conditions, TORC1 stimulates HK-II expression to metabolically support cellular growth and protection, while HK-II elicits an inhibitory effect on TORC1 activity upon starvation to enhance autophagy. This dual regulation for HK-II and mTOR would provide an adaptive defense mechanism to preserve cellular integrity dependent on metabolic status.
Although the mechanism by which glucose withdrawal switches the role of HK-II from glycolysis to autophagy induction is unclear, our results suggest that the transition is suppressed in the presence of G-6P. In other words, a reduction in cellular G-6P, resulting from glucose withdrawal, triggers increased binding of HK-II to TORC1, while addition of 2-DG to glucose free media, and subsequent accumulation of 2-DG-6P, inhibits this association. This is supported by our observations that 5-TG, a non-phosphorylatable glucose analogue had no effect on autophagy markers. Additionally, after endogenous HK-II was knocked down, introduction of a kinase-dead HK-II mutant increased autophagy in the presence of glucose (fig. 5D). The cellular distribution of HK-II changes in response to metabolic conditions and it has been well established that G-6P or 2-DG-6P dissociates HK-II from mitochondria, increasing the amount in the cytosol (John et al., 2011; Kabir and Wilson, 1993). Changes in intracellular localization of mTOR have also been reported in previous studies indicating that mTOR localizes to and is activated at ER/Golgi as well as lysosomes (Drenan et al., 2004; Kim et al., 2013). Thus, changes in intracellular localization of HK-II and mTOR might play a role in increased association of HK-II with mTOR by glucose deprivation. However, a mitochondrial binding deficient mutant of HK-II (ΔN HK-II) showed enhanced autophagy under glucose deprivation, comparable to WT HK-II (fig. S4B), strongly suggesting that mitochondria are not a target site for the mTOR/HK-II association. Much additional study will be required, however, to elucidate the intracellular localization of HK-II and mTOR and to determine the precise mechanism for transition from a glycolytic to pro-autophagic role.
Hexokinase-II has been demonstrated to confer cellular protection against acute cellular insults, such as oxidative stress in which its mitochondrial binding and kinase activity plays a role in inhibiting mitochondrial death pathways (Miyamoto et al., 2008; Pastorino et al., 2002; Roberts et al., 2013; Robey and Hay, 2006; Sun et al., 2008). Our results unveil a previously unrecognized, kinase activity independent, role of HK-II in TORC1 inhibition, and subsequent development of protective autophagy during starvation. This could provide a means to prolong survival against sustained metabolic stress such as ischemic heart condition. Conversely it has been well established that most tumor cells have high glycolytic activity (Warburg effect), accompanied by upregulation of HK-II (Pedersen, 2007). Coupled with recent studies suggesting that inhibition of autophagy could be a new therapeutic strategy, HK-II represents a multipronged target for prevention of cancer cell survival. Taken together, our data suggests that, in response to starvation, HK-II detects glucose depletion to adapt to metabolic suppression by facilitating autophagy.
Experimental Procedures
Cell Culture
Neonatal rat ventricular myocytes isolation, culture and infection was carried out as described previously (Miyamoto et al., 2008). Pre-designed HK-II ON-TARGETplus siRNA (siHK-II #1) for rat and control siRNA were purchased from Thermo Scientific. Pre-designed HK-II siRNA (siHK-II #2) was also purchased from Qiagen.
Western blotting and Immunoprecipitation
Preparation of whole cell lysates for Western blotting was performed as previously reported (Miyamoto et al., 2008). Information on antibodies can be found in the Supplemental. For immunoprecipitation studies, cells were washed with ice-cold PBS twice and lysed in hypotonic/digitonin buffer (20 mM PIPES [pH 7.2], 5 mM EDTA, 3 mM MgCl2, 10 mM glycerophosphate, 10 mM pyrophosphate, 0.02% digitonin plus protease and phosphatase inhibitors). In some experiments, 0.5% NP-40 alternative or 0.3% CHAPS were used instead of digitonin.
Statistical Analysis
Results are reported as averages ± SEM. Comparisons between two groups were accomplished using unpaired Student’s t test. Experiments with more than two groups were compared by ANOVA followed by the Tukey post-hoc test. A P-value of <0.05 was considered statistically significant.
Supplementary Material
Highlights.
In the absence of glucose, HK-II stimulates autophagy.
HK-II promotes autophagy by binding to, and inhibiting, TORC1.
The interaction with TORC1 is mediated by a TOS motif in HK-II.
Glucose-6 phosphate appears to suppress the autophagic role of HK-II.
Acknowledgements
The authors would like to thank Dr J.H. Brown for providing manuscript input. This work was supported by National Institutes of Health grant HL097037 to Shigeki Miyamoto.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For additional details regarding the methods used, see the Online Data Supplement.
References
- Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Nogueira V, Patra KC, Jeon SM, Park Y, Robey RB, Hay N. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition. Mol Cell Biol. 2009;29:5136–5147. doi: 10.1128/MCB.01946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Deter RL, De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967;33:437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola RS, Dvorzhinski D, Thalasila A, Garikapaty V, Doram D, May M, Bray K, Mathew R, Beaudoin B, Karp C, et al. Therapeutic starvation and autophagy in prostate cancer: a new paradigm for targeting metabolism in cancer therapy. Prostate. 2008;68:1743–1752. doi: 10.1002/pros.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Liu X, Bertram PG, Zheng XF. FKBP12-rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J Biol Chem. 2004;279:772–778. doi: 10.1074/jbc.M305912200. [DOI] [PubMed] [Google Scholar]
- Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011a;7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011b;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- Goel A, Mathupala SP, Pedersen PL. Glucose metabolism in cancer. Evidence that demethylation events play a role in activating type II hexokinase gene expression. J Biol Chem. 2003;278:15333–15340. doi: 10.1074/jbc.M300608200. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS One. 2011;6:e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir F, Wilson JE. Mitochondrial hexokinase in brain of various species: differences in sensitivity to solubilization by glucose 6-phosphate. Arch Biochem Biophys. 1993;300:641–650. doi: 10.1006/abbi.1993.1089. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Hoffman GR, Poulogiannis G, Buel GR, Jang YJ, Lee KW, Kim BY, Erikson RL, Cantley LC, Choo AY, Blenis J. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell. 2013;49:172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- Osawa H, Sutherland C, Robey RB, Printz RL, Granner DK. Analysis of the signaling pathway involved in the regulation of hexokinase II gene transcription by insulin. J Biol Chem. 1996;271:16690–16694. doi: 10.1074/jbc.271.28.16690. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB. Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem. 2013;288:23798–23806. doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Chen S, Du F, Li S, Zhao L, Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci U S A. 2011;108:4788–4793. doi: 10.1073/pnas.1100844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui D, Wilson JE. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch Biochem Biophys. 1997;345:111–125. doi: 10.1006/abbi.1997.0241. [DOI] [PubMed] [Google Scholar]
- Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- Tee AR, Proud CG. Caspase cleavage of initiation factor 4E-binding protein 1 yields a dominant inhibitor of cap-dependent translation and reveals a novel regulatory motif. Mol Cell Biol. 2002;22:1674–1683. doi: 10.1128/MCB.22.6.1674-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence JC., Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Chung V. Rat brain hexokinase: further studies on the specificity of the hexose and hexose 6-phosphate binding sites. Arch Biochem Biophys. 1989;269:517–525. doi: 10.1016/0003-9861(89)90135-5. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol. 2011;67:899–910. doi: 10.1007/s00280-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.