Abstract
Background
Early repolarization pattern in the ECG has been associated with increased risk for ventricular tachycardia/fibrillation (VT/VF), particularly when manifest in inferior leads. This study examines the mechanisms underlying VT/VF in the early repolarization syndrome (ERS).
Method
Transmembrane action potentials (AP) were simultaneously recorded from 2 epicardial and 1 endocardial site of coronary-perfused canine left-ventricular (LV) wedge preparations, together with a pseudo-ECG. Transient outward current (Ito) was recorded from epicardial myocytes isolated from inferior and lateral LV of the same heart.
Results
J wave area (pseudo-ECG), epicardial AP notch magnitude and index were larger in inferior vs. lateral wall preparations at baseline and after exposure to provocative agents (NS5806+verapamil+acetylcholine (ACh)). Ito density was greater in myocytes from inferior vs. lateral wall (18.4±2.3pA/pF vs. 11.6±2.0pA/pF;p<0.05). A combination of NS5806 (7μM) and verapamil (3μM) or pinacidil (4μM), used to pharmacologically model the genetic defects responsible for ERS, resulted in prominent J-point and ST-segment elevation. ACh (3μM), simulating increased vagal tone, precipitated phase-2-reentry-induced polymorphic VT/VF. Using identical protocols, inducibility of arrhythmias was 3-fold higher in inferior vs. lateral wedges. Quinidine (10μM) or isoproterenol (1μM) restored homogeneity and suppressed VT/VF.
Conclusion
Our data support the hypothesis that 1) ERS is caused by a preferential accentuation of the AP notch in LV epicardium; 2) this repolarization defect is accentuated by elevated vagal tone; 3) higher intrinsic levels of Ito account for the greater sensitivity of the inferior LV wall to development of VT/VF; 4) quinidine and isoproterenol exert ameliorative effects by reversing the repolarization abnormality.
Keywords: J wave syndrome, ventricular fibrillation, transient outward potassium current, parasympathetic tone, electrophysiology
1. Introduction
Early repolarization (ER)1 pattern in the ECG is characterized by a J point and ST segment elevation, sometimes manifest as a notch or slur on the QRS (J wave). The ER pattern, long thought to be benign, was recently proposed to have a malignant component on the basis of the association of ER with development of ventricular tachycardia and fibrillation (VT/VF) in an experimental model consisting of canine ventricular wedge preparations, thus identifying the ER syndrome (ERS) [1,2]. Validation of this hypothesis came several years later with the demonstration that patients with ER, particularly in the inferior or infero-lateral ECG leads are at a higher risk for VT/VF [3–5]. The co-occurrence of a J point elevation in the right precordial leads (V1–V3) has been linked to a more severe phenotype often associated with the development of electrical storms [4,6].
Recent studies have demonstrated that gain of function mutations in KCNJ8, the gene responsible for the pore forming subunit of the ATP-sensitive potassium channel (KATP), is associated with ERS [7–9]. Loss of function mutations in the α1, β2 and α2δ subunits of the cardiac L-type calcium channel (CACNA1C, CACNB2, and CACNA2D1) have also been identified as causative in patients with ERS [10]. Watanabe et al. described [11] loss of function mutations in SCN5A in patients with idiopathic ventricular fibrillation associated with early repolarization. Sodium channel blocker challenge resulted in an accentuation of early repolarization and development of VT/VF.
Vagal activity has long been implicated in the development of an ER pattern in the ECG [12,13] and recent clinical observations suggest that parasympathetic tone contributes to the electrocardiographic and arrhythmic manifestations of ERS [14,15]. Sleep is commonly associated with spontaneous VF in patients with ERS [16]. Indeed heart rate spectral analysis has identified a sudden rise in vagal activity just before the development of VF in patients diagnosed with cases of idiopathic VF (IVF) [17].
A number of studies have shown that an ER pattern in the inferior ECG leads (II, III and aVF) is associated with a much higher risk for the development of VT/VF [18,19]. The electrophysiological basis for this distinction is not known and presents a critical gap in our knowledge.
The present study was designed to probe the mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of ERS, to elucidate the role of vagal influences, and to better understand the basis for the greater susceptibility to VT/VF when the ER substrate resides in the inferior region of the ventricular myocardium. The study was specifically designed to test the hypothesis that an outward shift in the balance of current contributing to the early phase of the left ventricular (LV) epicardial action potential (AP) either via an increase in IK-ATP or transient outward current (Ito) or via a decrease in calcium inward current (ICa) in LV wedge preparations can recapitulate the ECG and arrhythmic manifestations of ERS. Thus, we sought to test the hypothesis that pharmacologic modeling of the genetic defects associated with ERS, using pinacidil and NS5806 to increase IK-ATP and Ito and verapamil and acetylcholine (ACh) to reduce ICa or pilsicainide to reduce INa, could give rise to a substrate capable of inducing phase 2 reentry and polymorphic VT/VF. Finally, we test the hypothesis that increased levels of Ito sensitize the ventricular epicardium to development of ER and ERS and those higher levels of Ito in the inferior wall can explain why an ER pattern in the inferior ECG leads is associated with a higher risk for sudden cardiac arrest.
2. Methods
All experiments were carried out in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85-23, Revised 1996) and approved by the Institutional Animal Care and Use Committee. Adult dogs (20–35 kg) of either sex were used in this study.
2.1. Wedge Preparations
Detailed methods for isolation, perfusion, and recording of action potentials from coronary-perfused canine ventricular wedge preparations have been reported previously [20,21] and are described in the Online Supplement. All arrhythmias developed spontaneously following exposure to the provocative agents during steady state pacing at cycle length of 1000 to 2000 msec.
2.2. Voltage-clamp studies using enzymatically-dissociated canine cardiomyocytes
Cardiomyocytes were isolated as previously described [22]. Ito was measured in isolated left ventricular lateral and inferior epicardial myocytes at 37°C using the whole-cell patch clamp technique [23]. Details of the protocol are presented in the Online Supplement.
2.3. Statistics
Results are expressed as mean ± S.E.M. Statistical comparisons were made using Student's t-test for Table 1 and Figure 7 B. Kruskal-Wallis one-way analysis of variance followed by Student-Newman-Keuls post-hoc test was used for Figure 7 C and D.
Table 1.
Effects of provocative agents on transmural and epicardial (Epi) dispersion of repolarization and on action potential (AP) parameters in perfused left ventricular wedge preparations.
| Endo APD90 (ms) | Epi1 APD90 (ms) | Epi2 APD90 (ms) | Endo APD50 (ms) | Epi1 APD50 (ms) | Epi2 APD50 (ms) | Notch Magnitude (% ofPh0)* | Notch Index† | TDR (ms) | EDR (ms) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 223.2 ± 2.1 | 199.7 ± 5.9 | 201.8 ± 3.9 | 177.8 ± 3.9 | 169.5 ± 7.6 | 170.7 ± 4.2 | 14.0 ± 3.1 | 255.2 ± 54.3 | 17.0 ± 3.5 | 14.7 ± 2.8 |
| NS 5806 (7 μM) | 226.2 ± 5.1 | 203.7 ± 6.5 | 207.2 ± 2.6 | 176.5 ± 7.4 | 169.3 ± 8.0 | 176.1 ± 2.5 | 31.7 ± 4.0 | 780.8 ± 116.0 | 17.7 ± 5.3 | 13.3 ± 2.8 |
| Verapamil (3 μM) | 227.0 ± 7.9 | 225.9 ± 8.3a | 222.5 ± 4.6a | 167.9 ± 6.9 | 188.8 ± 7.9 | 186.5 ± 3.8a | 38.9 ± 5.0b | 2551.9 ± 182.8b | 21.3 ± 4.4 | 14.6 ± 6.4 |
| Acetylcholine (3 μM)‡ | 217.3 ± 3.1 | 129.9c ± 17.2 | 246.3 ± 26.6 | 157.1 ± 2.1 | 69.1c ± 17.8 | 202.7 ± 26.4 | 42.2 ± 5.1 | 4654.5 ± 894.0 | 80.8c ± 15.5 | 111.2c ± 24.1 |
Epicardial AP notch data (notch magnitude and notch index) measured before loss of the AP dome at epicardial sites.
Notch index = Notch Magnitude × (Ph 0 to Ph 2 interval) which approximates the area of the notch.
Epicardial dispersion of repolarization (EDR) and transmural dispersion of repolarization (TDR) reported here were measured in cases at which the addition of Acetylcholine caused loss of the AP dome at EPI 1 site but no EPI 2, with the development of Phase 2 Reentry.
APD90; APD50= action potential durations at 90%and 50% repolarization. Results are mean ± S.E.M. a=p < 0.05, b=p < 0.01 vs control, c=p < 0.05 vs NS5806 + verapamil combination. Basic cycle length =1000 ms; n=5.
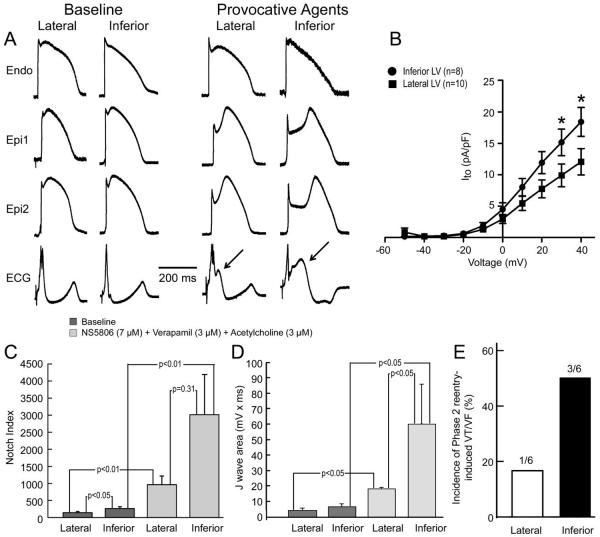
Figure 7.
Basis for greater vulnerability of inferior wall to early repolarization syndrome-mediated arrhythmia. Results shown are from left ventricular (LV) wedge preparations isolated from the inferior and lateral regions of the same hearts. A: Under baseline conditions, the action potential (AP) notch is more prominent in the epicardium (Epi) of the inferior wall as compared with the lateral wall and the response (accentuation of AP notch, increase in J wave) to a combination of NS5806 (7 μM) + Verapamil (3 μM) + Acetylcholine (ACh) (3 μM) is much more pronounced in the Epi of the inferior wall. APs shown are recorded from the lateral and the inferior part of the LV of the same dog. Arrows show increased J wave upon application of provocative agents. Endo = endocardial. B: Transient outward current density is more pronounced in Epi myocytes isolated from inferior vs. lateral wall. C: Notch index of APs recorded from the Epi surface of paired inferior vs. lateral wedge preparations isolated from the same heart under baseline conditions and after exposure to the same combination of provocative agents [NS5806 (7 μM) + Verapamil (3 μM) + ACh (3 μM)]. Results are mean ± S.E.M. D: J wave area recorded from paired inferior vs. lateral wedge preparations isolated from the same heart under baseline conditions and after exposure to the same combination of provocative agents [NS5806 (7 μM) + Verapamil (3 μM) + ACh (3 μM)]. Results are mean ± S.E.M. E: Incidence of phase 2 reentry –induced VT/VF after exposure of the lateral and inferior wedge preparations isolated from the same heart to the same combination of provocative agents.
3. Results
3.1. Early Repolarization ECG pattern is caused by a preferential accentuation of the AP notch in LV epicardium
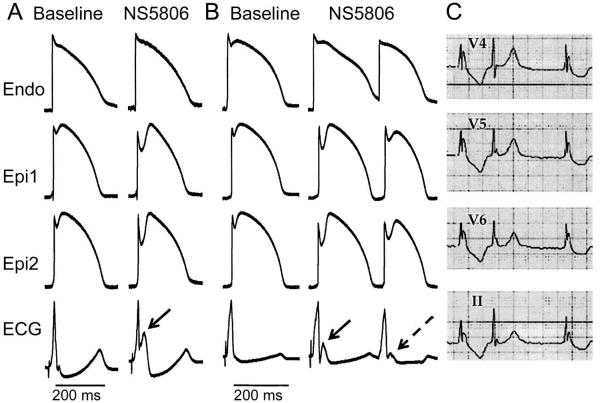
In the first series of experiments, we sought to examine the effects of Ito augmentation on AP and ECG characteristics of coronary-perfused wedge preparations isolated from the inferior and lateral regions of canine LV. Figure 1 shows two examples of the effect of the Ito agonist NS5806 (7 μM) to accentuate the epicardial AP notch and to induce J-point elevation, thus generating a prominent ER pattern. Qualitatively similar accentuation of the epicardial AP notch and induction of J-point elevation were obtained in 8 experiments. Because Ito is relatively slow to recovery from inactivation, availability of the current is reduced in premature beats, resulting in a diminished phase 1 AP notch. Figure 1B illustrates an example of a reduced epicardial AP notch and J wave associated with a premature beat in another inferior LV wedge preparation. Figure 1C shows a clinical example of this phenomenon recorded from a 16 y/o female with type 3 ERS. A pronounced ER pattern was apparent in the left precordial ECG leads. At the time of the recording the patient was in atrial fibrillation, which was associated with large fluctuations in RR interval. The beat following the abbreviated RR interval exhibited a much reduced J wave and ER pattern, closely mirroring the activity observed in the LV wedge.
Figure 1.
Augmentation of the transient outward current (Ito) promotes early repolarization and J wave manifestation in left ventricle (LV) wedge preparations from the inferior wall of the LV of the canine heart. Each panel shows simultaneous recordings from one endocardial (Endo) and two epicardial (Epi) sites together with a pseudo-ECG. A: Recorded under control conditions and 35 min following addition of NS5806 (7 μM); Basic Cycle Length (BCL)=1000 ms. B: Recorded from another wedge preparation under control conditions and 30 min after addition of NS5806 (7 μM); BCL=2000 ms. The Ito agonist gives rise to prominent J waves (solid arrows) secondary to accentuation of the Epi action potential (AP) notch. A much diminished Epi AP notch and ECG J wave (dashed arrow) is associated with a closely coupled premature beat. C: Clinical example of accentuated J wave in a patient with early repolarization syndrome and atrial fibrillation. Note the marked attenuation of the J wave attending the abbreviated RR interval.
3.2. Pharmacologic modeling of genetic mutations associated with early repolarization syndrome
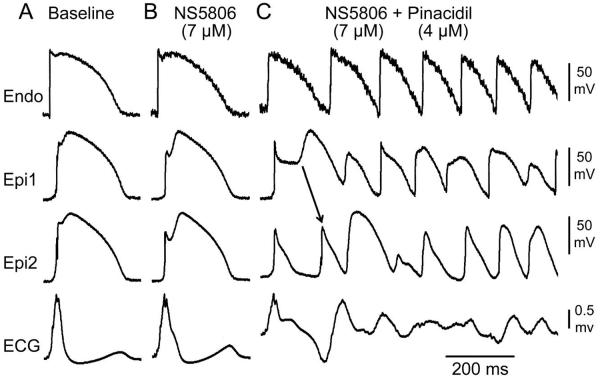
Pharmacologic modeling of the genetic defects known to underlie ERS was evaluated in another series of experiments. A gain of function in IK-ATP was modeled using the IK-ATP opener pinacidil. Figure 2 illustrates the electrocardiographic and arrhythmic manifestations resulting from addition of 4 μM pinacidil together with 7 μM NS5806. The combination produced an outward shift in the balance of current in the early phases of the epicardial AP, leading to marked accentuation of epicardial AP notch, giving rise to a prominent ER, heterogeneous loss of the AP dome, and the development of phase 2 reentry-induced VT/VF. Similar changes in transmembrane and ECG activity was recorded in 3 of 6 wedge preparations isolated from the LV wall of the canine heart.
Figure 2.
Augmentation of the increase in ATP-sensitive potassium current (IK-ATP) leads to development of phase 2 reentry and polymorphic ventricular tachycardia (VT). Each panel shows simultaneous recordings from one endocardial (Endo) and two epicardial (Epi) sites together with a pseudo-ECG of a canine left ventricular wedge preparation. A: Control; B and C: The IK-ATP agonist pinacidil (4 μM) and Ito agonist NS5806 (7 μM) were used to pharmacologically model a gain of function mutation in IK-ATP in the setting of a high level of Ito. Phase 2 reentry and polymorphic VT/ ventricular fibrillaton developed 20 min after addition of pinacidil. Basic Cycle Length=1000 ms.
Loss of function of ICa mutations were pharmacologically modeled using the calcium channel blocker verapamil and INa mutations were modeled using the sodium channel blockers pilsicainide (5 μM, n=2) or procainamide (50 μM-200 μM, n=3). Figures 3–5 illustrate examples of the effect of a combination of verapamil, pilsicainide and NS5806 to produce a net outward shift in the balance of current in the early phases of the epicardial AP, causing marked accentuation of epicardial AP notch, thus giving rise to a prominent ER, heterogeneous loss of the AP dome, and the development of phase 2 reentry-induced VT/VF. Similar heterogeneous loss of the AP dome leading to development of phase 2 reentry-induced VT/VF was recorded in 5 of 10 wedge preparations isolated from the LV wall of the canine heart. Figure 5B illustrates an example in which the addition of pilsicainide induced VT/VF when a combination of NS5806 + verapamil + ACh failed to do to so.
Figure 3.
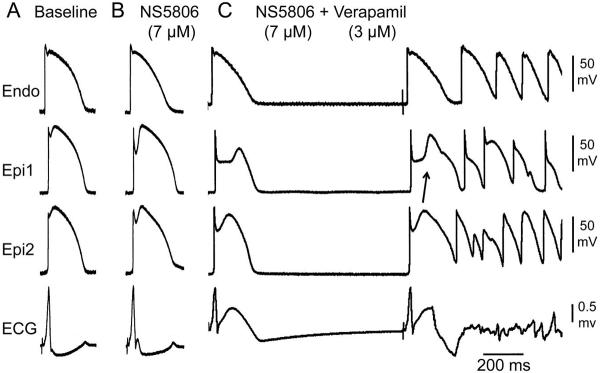
Calcium channel inhibition leads to development of phase 2 reentry and polymorphic ventricular tachycardia (VT) in a left ventricular wedge preparation. Each panel shows simultaneous recordings from one endocardial (Endo) and two epicardial (Epi) sites together with a pseudo-ECG. A: Control; B: NS5806 (7 μM) accentuated the Epi action potential (AP) notch causing the appearance of a distinct J wave. C: Addition of verapamil (3 μM) to pharmacologically model loss of function calcium channel mutations further accentuated the epicardial AP notch, thus greatly amplifying the J wave, causing it to appear as an ST segment elevation. Phase 2 reentry and polymorphic VT/ ventricular fibrillation developed 15 min after addition of verapamil. Basic Cycle Length=1000 ms.
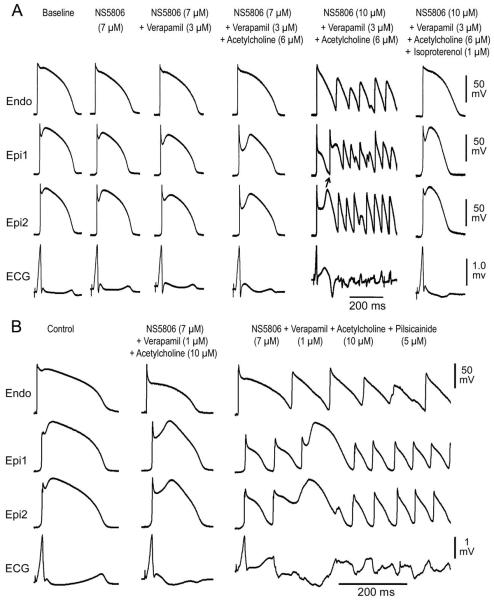
Figure 5.
A: Isoproterenol normalizes early repolarization (ER) pattern and prevents the development of ventricular tachycardia/ventricular fibrillation (VT/VF) in a left ventricular wedge model of ER syndrome. Each panel illustrates transmembrane action potentials (APs) simultaneously recorded from 1 endocardial (Endo) and 2 epicardial (Epi) sites, together with a pseudo-ECG. Exposure to NS5806 (7 μM) accentuated the epicardial AP notch, thus augmenting the J wave. Addition of verapamil (3 μM) further accentuated manifestation of the J wave. Addition of acetylcholine (6 μM) further enlarged the Epi AP notch and J wave. Increasing NS5806 to 10 μM caused heterogeneous loss of the AP dome in the Epi, giving rise to Phase 2 Reentry and VT/VF. F: Isoproterenol (1 μM) restored the Epi AP dome, normalized the ECG and abolished all arrhythmic activity. Basic Cycle Length=1000 ms.
B: When a combination of NS5806+verapamil+ACh failed to induce VT/VF, the addition of the sodium channel blocker pilsicainide caused accentuation of the ER phenotype leading to the development of VT/VF. Basic Cycle Length=500 ms.
3.3. Mechanism underlying arrhythmogenic influence of parasympathetic tone in ERS
Vagal influences are known to accentuate the ECG and arrhythmic manifestations in patients with ERS. In a third series of experiments, we examined the basis for these effects by exposing wedge preparations sensitized with a combination of 7 μM NS5806 and 3 μM verapamil to ACh. Figure 4 shows an example in which the combination of NS5806 and verapamil caused marked accentuation of the epicardial AP notch, thus inducing J-point and ST segment elevation, but no arrhythmia developed. Addition of ACh (3 μM), to mimic increased vagal tone, accentuated epicardial AP notch magnitude, increased notch index, facilitated loss of the epicardial AP dome (Table 1), enhanced ST segment elevation and precipitated repeated episodes of phase 2 reentry and polymorphic VT/VF. Similar exacerbation of arrhythmic activity by ACh was observed in 5 out of 11 wedge preparations isolated from LV wall of the canine heart that failed to exhibit arrhythmic activity when exposed to NS5806 (7 μM) and verapamil (3 μM) alone.
Figure 4.
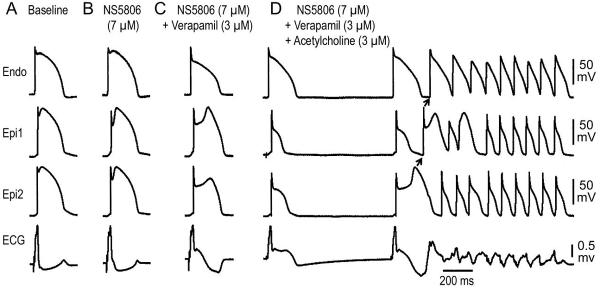
Acetylcholine (ACh) leads to development of phase 2 reentry and polymorphic ventricular tachycardia (VT) in an experimental model of early repolarization syndrome. Each panel shows simultaneous recordings from one endocardial (Endo) and two epicardial (Epi) sites together with a pseudo-ECG. A: Control; B and C: The combination of verapamil (3 μM) and transient outward current agonist (NS5806, 7 μM) caused an early repolarization pattern to develop in the left ventricular wedge preparation, but no arrhythmogenic substrate. D: The addition of ACh, used to mimic increased vagal tone (ACh, 3 μM), led to the development of phase 2 reentry and polymorphic VT/ ventricular fibrillation. Basic Cycle Length=1000 ms.
3.4. Quinidine and isoproterenol suppress arrhythmogenesis in experimental models of ERS
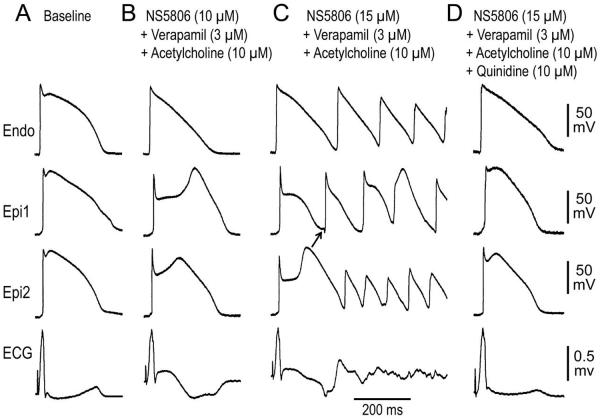
Quinidine and isoproterenol have been shown to exert ameliorative effects in the J wave syndromes. In another series of experiments, we probed the cellular basis for these effects in our ERS model. We induced the ERS phenotype using a combination of NS5806 (7–15 μM) + verapamil (3 μM) + acetylcholine (3–10 μM). Quinidine (10 μM) and isoproterenol (1 μM) each reversed the repolarization abnormality responsible for ERS by restoring the epicardial AP dome, thus reducing ST segment elevation and suppressing VT/VF (Figures 5 and 6). Quinidine restored electrical homogeneity by restoring the epicardial action potential dome, and thus prevented or suppressed VT/VF in 4 of 4 preparations and isoproterenol in 5 of 6 preparations.
Figure 6.
Quinidine normalizes early repolarization (ER) pattern and suppresses ventricular tachycardia/ventricular fibrillation (VT/VF) in a left ventricular wedge model of ER syndrome. Each panel shows transmembrane action potentials (APs) simultaneously recorded from 1 endocardial (Endo) and 2 epicardial (Epi) sites, together with a pseudo-ECG. A: Control; B: ER phenotype was induced by NS5806 (10 μM) + Verapamil (3 μM) + Acetylcholine (10 μM); C: Increasing NS5806 concentration to 15 μM, led to the development of Phase 2 Reentry, D: Recorded 20 min after addition of 10 μM quinidine. Quinidine restored the epicardial AP dome, abolished ER and ST segment elevation and suppressed VT/VF. Basic Cycle Length=1000 ms.
3.5 Cellular and ionic basis for the greater susceptibility of the inferior LV wall to development of VT/VF
A number of clinical studies report that ER in the inferior ECG leads is associated with a higher level of risk of arrhythmic events and sudden cardiac death. In a final series of experiments we probed the cellular and ionic basis for the higher susceptibility of the inferior wall to arrhythmias in the setting of ER. In inferior and lateral wedge preparations isolated from the same dogs, epicardial AP notch magnitude recorded from the inferior wall was greater than that recorded from the lateral wall. At baseline Phase 1 magnitude in inferior and lateral wall preparations was 14.1±1.7 % vs. 8.4±1.2 % of Phase 0 amplitude, p<0.05; after application of provocative agents (7 μM NS5806 + 3 μM verapamil + 3 μM ACh) it was 34.4±6.0 % vs. 23.1±4.3 % of Phase 0 amplitude (p<0.05, n=6). Notch index at baseline was 270.3±48.3 vs. 149.7±26.3, p<0.05, n=5 in inferior and lateral preparations and after application of provocative agents increased to 3015.5±1178.1 vs. 969.4±246.2, respectively (Figure 7C, n=5; p=0.31).
J wave area recorded in wedge preparations from the inferior wall was slightly although not significantly greater than that recorded from the lateral wall (Figure 7D, n=4) under baseline conditions (6.5±2.0 mV × ms vs. 4.4±1.1 mV × ms) and significantly greater after application of provocative agents (7 μM NS5806 + 3 μM verapamil + 3 μM ACh) (60.3±25.6 mV × ms vs. 18.4±0.5 mV × ms; p<0.05).
To test the hypothesis that differences in Ito contribute to these distinctions in action potential characteristics and pharmacologic responsiveness, we measured Ito in epicardial myocytes isolated from the inferior vs. lateral LV wall. Ito density was significantly larger in cells from the inferior vs. lateral wall (18.4 ± 2.3 vs. 11.6 ± 2.0 pA/pF at +40 mV, respectively; p<0.05, Figure 7B).
Finally, using identical protocols (7 μM NS5806 + 3 μM verapamil + 3 μM ACh) we found that incidence of phase 2 reentry and VT/VF was 3-fold higher in inferior vs. lateral wedges isolated from the same dogs (Figure 7E).
4. Discussion
4.1. Summary of main findings
The present study advances our understanding of the cellular and ionic basis for the electrocardiographic and arrhythmic manifestations of the early repolarization syndrome, providing data in support of the hypothesis that ERS is caused by a preferential accentuation of the AP notch in LV epicardium; that this repolarization defect is accentuated by cholinergic agonists and that higher intrinsic levels of Ito account for the greater vulnerability of the inferior LV wall to VT/VF. Finally, we provide a mechanistic understanding of the ameliorative effects of quinidine and isoproterenol, showing their ability to reverse the repolarization abnormalities responsible for ERS.
4.2. Accentuation of the J wave
Under physiological conditions, the presence of an AP notch in epicardium but not endocardium creates a transmural voltage gradient that can cause the inscription of the J wave of the ECG [24]. Accentuation of this transmural gradient across the right ventricular (RV) wall has been shown to lead to repolarization defects that underlie the ECG and arrhythmic manifestations of Brugada syndrome (BrS) [1]. The present study provides evidence in support of the hypothesis that a similar accentuation of transmural gradients across the LV wall underlies the repolarization abnormalities responsible for ERS, giving rise to J point elevation, distinct J waves, or slurring of the terminal part of the QRS.
4.3. Induction of Phase 2 Reentry
ERS has been associated with gain of function mutations in KCNJ8 giving rise to an increase in IK-ATP [7–9] and loss of function mutations in the α1, β2 and α2δ subunits of the cardiac L-type calcium channel (CACNA1C, CACNB2, and CACNA2D1) leading to a reduction in ICa [10,25] as well as loss of function mutations in SCN5A leading to a decrease in INa. [11] We pharmacologically modeled these genetic defects using the IK-ATP agonist pinacidil, the ICa antagonist verapamil and the INa antagonists pilsicainide or procainamide. In the setting of a prominent Ito, induced using NS5806, both pinacidil and verapamil were found to cause all-or-none repolarization, leading to loss of the AP dome at some epicardial sites but not others, resulting in an epicardial dispersion of repolarization (EDR). Propagation of the AP dome from sites at which it was maintained to sites at which it was lost caused local re-excitation via a phase 2 reentry mechanism within the epicardium. Loss of the dome in the epicardium also created a transmural dispersion of repolarization (TDR) giving rise to a vulnerable window across the ventricular wall which, when captured by a closely coupled extrasystole generated in the epicardium, induced VT/VF (Figures 2 and 3). When a combination of NS+verapamil+ACh failed to induce VT/VF, the addition of the sodium channel blocker pilsicainide (Figure 5, panel B) or procainamide caused accentuation of the ER phenotype leading to the development of VT/VF. Consistent with the observation of Watanabe et al, [11] we found that the sodium channel block is capable of accentuating the ER phenotype leading to the induction of VT/VF.
4.4. Cholinergic influence accentuates the repolarization defect
Vagal influences are thought to play a prominent role in the development of life threatening arrhythmias in patients with the early repolarization syndrome [26]. The parasympathetic nervous system has been shown to modulate the manifestation of the J wave and ST segment elevation in patients with ERS [13,14]. Acetylcholine has been shown to exert direct effects on the electrical response of canine ventricular myocardium, preferentially in epicardium [27]. ACh was shown to depress the AP plateau in canine right ventricular epicardium but not endocardium, resulting in an ST-segment elevation and to accentuate the AP notch, thus facilitating loss of the RV action potential dome in the presence of pinacidil or flecainide [1].
In the present study, we mimic vagal activity using ACh and demonstrate its ability to facilitate heterogeneous loss of the dome in LV epicardium in the presence of NS5806 and verapamil, leading to an ST segment elevation and development of phase 2 reentry in the free wall of the LV (Figures 4–7 and Table 1).
Vagal innervation has recently been shown to be quite extensive in the ventricles [28]. Takahashi et al. demonstrated in dogs that vagal efferent fibers cross to the ventricles in the superficial epicardium at the atrioventricular groove in a base to apex direction and, within 1–2 cm of the atrioventricular groove, penetrate the myocardium en route to transmurally innervating the rest of the LV free wall and part of the inter-ventricular septum [29]. Efferent vagal stimulation has also been reported to exert potent negative inotropic effects in dog ventricular myocardium [30] and to exert direct inhibition of ICa [31]. ICa inhibition is likely also a consequence of “accentuated antagonism” wherein vagal activity antagonizes the effects of sympathetic stimulation to increase ICa [32]. Interestingly, cardiomyocytes have been shown to actively secrete ACh and thus amplify vagal influences [33,34]. Adrenergic stimulation was also shown to up-regulate expression levels of cholinergic components [34]. Thus, cholinergic influences on the electrical activity of ventricular myocardium are robust.
4.5. Higher intrinsic levels of Ito account for the greater sensitivity of the inferior LV wall to development of VT/VF
Numerous studies have reported a higher risk for the development of arrhythmias and sudden cardiac arrest in patients with an ER pattern in the inferior or infero-lateral leads of the ECG [16,18,35–41]. We investigated the cellular and ionic basis for the greater vulnerability of the inferior region of the LV for development of VT/VF in the setting of ERS. Our findings support the hypothesis that cells in the inferior region of ventricular epicardium possess a higher level of Ito than cells in the lateral LV and that this predisposes the inferior region to develop phase 2 reentry and VT/VF when provocative agents are introduced to pharmacologically model the genetic defects known to underlie ERS. Our results are in agreement with those reported by Szentandrassy and co-workers [42] showing AP with a more prominent phase1 in cells isolated from the apical vs. basal regions of the canine and human LV. From the description provided, the apical preparations studied overlapped with what we consider to be the inferior wall of the LV and their basal samples overlap with what we consider to be the lateral wall. Consistent with these findings, Ito density as well as expression of genes that encode Ito, including KChIP2 and Kv1.4, were significantly more abundant in apical vs. basal myocytes. Kv4.3 expression was also higher in apical tissues, although it did not reach statistical significance.
Of note is the fact that Ito density at +40 mV in the inferior wall of the LV (18.4±2.3pA/pF) was intermediate between that found in the lateral wall of the LV (11.6±2.0pA/pF;p<0.05) and the RV (24.2 ± 0.5pA/pF), the latter known to predispose to the development of the electrocardiographic and arrhythmic manifestations of BrS [43].
It is noteworthy, that apico-basal differences in adrenergic innervation have been reported, with a 10-fold higher concentration of norepinephrine in tissues in the base vs. apex of the LV [44]. It is tempting to speculate that reduced sympathetic activity in the apical regions of the LV contributes to the greater vulnerability of the inferior wall to arrhythmogenesis. This would also be consistent with the well-known ameliorative effect of β adrenergic activity in the setting of ERS [45,46]. Interestingly, Roten et al. [46] reported that in response to β adrenergic stimulation with isoproterenol, J waves may persist in a subset of patients with precordial and inferior J waves, but never in lateral location. This heterogeneous response to isoproterenol may indicate distinctive autonomic and/or cellular and ionic mechanisms in the anterior, lateral and inferior regions of ventricular myocardium.
4.6. Pharmacologic approach to therapy of ERS
Our study demonstrates the ability of quinidine and isoproterenol to reverse the repolarization abnormalities responsible for ERS, thus exerting an ameliorative effect. Both agents act to restore the epicardial AP dome by causing an inward shift in the balance of current; quinidine via its inhibition of Ito and isoproterenol by its augmentation of ICa. These actions restore homogeneity across the left ventricular wall, thus suppressing all arrhythmic activity and eliminating the substrate for the development of phase 2 reentry and VT/VF.
These effects of quinidine and isoproterenol in the LV wedge preparation provide a mechanistic understanding of their beneficial effects in the clinic. A recent multicenter study examined the effectiveness of quinidine and isoproterenol in normalizing the ECG pattern and in suppressing arrhythmia recurrence in patients with ERS [45]. Isoproterenol infusion was reported to immediately suppress electrical storms in 7 of 7 patients. Quinidine was effective in 9 of 9 patients, decreasing recurrent VF from 33±35 episodes to 0 for 25±18 months. Moreover, quinidine normalized the ECG in all cases.
Our experimental findings provide strong rationale for developing agents possessing Ito blocking, anticholinergic, as well as IK-ATP antagonistic properties as possible alternatives to oral quinidine for chronic treatment of symptomatic ERS in cases in which the drug is not tolerated or produces unacceptable adverse effect.
4.7. Study limitations
As with all data derived from experimental animal models, extrapolation from in vitro models to the clinic must be done with great care. It is noteworthy that the RV wedge preparation was the first to identify quinidine and isoproterenol for the treatment of BrS in 1999 [1]. Both agents are used today for primary and secondary prevention and as adjunct therapy for BrS. Thus, the ventricular wedge preparation has been highly predictive of the clinical efficacy of all pharmacologic agents thus far tested. ERS thus far has been associated with loss of function mutations in ICa and INa and gain of function mutations in Ito and IK-ATP. Our study demonstrates that a loss of function in ICa or INa and gain of function in IK-ATP can contribute to manifestation of the ER phenotype in the setting of an enhanced Ito. Additional studies are also clearly needed to probe the mechanism underlying the regional differences in Ito, which are responsible for the arrhythmic vulnerability of the inferior wall in the LV as well for disparate response of closely apposed regions to the provocative agents, making phase 2 reentry possible. It is noteworthy that the concentration of ACh needed to precipitate arrhythmic activity in vivo is expected to be much lower than that needed in our models because the bradycardia-effect of parasympathetic influence on sinus rate is lacking.
Supplementary Material
Highlights
We examine the mechanisms underlying ventricular tachycardia/ventricular fibrillation in early repolarization syndrome.
Preferential accentuation of action potential notch in left ventricle epicardium leads to early repolarization syndrome.
This repolarization defect is accentuated by elevated vagal tone.
Higher levels of the transient outward current (Ito) contribute to arrhythmic vulnerability of inferior left ventricle wall.
Quinidine & isoproterenol prevent V early repolarization syndrome by reversing the repolarization abnormality.
Acknowledgments
This study was supported by a postdoctoral fellowship grant from the Heart Rhythm Society to IK (supported by an educational grant from St. Jude Medical), grant HL47678 from NHLBI (CA), NYSTEM grant #C026424 (CA) and the Masons of New York, Florida, Massachusetts, Connecticut, Maryland and Rhode Island.
We are grateful to José M. Di Diego, M.D. for continuous support and personal guidance and to Serge Sicouri, M.D. and Vladislav V. Nesterenko, Ph.D. for helpful discussions and support and to Timothy K. Knilans M.D. for providing us with the ECG recordings. We also gratefully acknowledge the technical assistance of Judy Hefferon, Rebecca Warren and Robert Goodrow Jr.
Abbreviations
- ACh
acetylcholine
- AP
action potential
- BrS
Brugada syndrome
- EDR
epicardial dispersion of repolarization
- ER
early repolarization
- ERS
early repolarization syndrome
- ICa
calcium inward current
- Ito
transient outward current
- IVF
idiopathic ventricular fibrillation
- KATP
ATP-sensitive potassium channel
- LV
left ventricle
- RV
right ventricle
- TDR
transmural dispersion of repolarization
- VT/VF
ventricular tachycardia/ventricular fibrillation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- [1].Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- [2].Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- [3].Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, De Roy L, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–23. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- [4].Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–9. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–8. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- [6].Nam GB, Ko KH, Kim J, Park KM, Rhee KS, Choi KJ, et al. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs. Brugada syndrome. Eur Heart J. 2010;31:330–9. doi: 10.1093/eurheartj/ehp423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–8. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- [8].Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–71. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barajas-Martinez H, Hu D, Ferrer T, Onetti CG, Wu Y, Burashnikov E, et al. Molecular genetic and functional association of Bugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm. 2012;9:548–55. doi: 10.1016/j.hrthm.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, et al. Mutations in the cardiac L-type calcium channel associated J wave sydnrome and sudden cardiac death. Heart Rhythm. 2010;7:1872–82. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Watanabe H, Nogami A, Ohkubo K, Kawata H, Hayashi Y, Ishikawa T, et al. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol. 2011;4:874–81. doi: 10.1161/CIRCEP.111.963983. [DOI] [PubMed] [Google Scholar]
- [12].Wilhelm M, Brem MH, Rost C, Klinghammer L, Hennig FF, Daniel WG, et al. Early repolarization, left ventricular diastolic function, and left atrial size in professional soccer players. Am J Cardiol. 2010;106:569–74. doi: 10.1016/j.amjcard.2010.03.072. [DOI] [PubMed] [Google Scholar]
- [13].Marcus RR, Kalisetti D, Raxwal V, Kiratli BJ, Myers J, Perkash I, et al. Early repolarization in patients with spinal cord injury: prevalence and clinical significance. J Spinal Cord Med. 2002;25:33–8. doi: 10.1080/10790268.2002.11753599. [DOI] [PubMed] [Google Scholar]
- [14].Mizumaki K, Nishida K, Iwamoto J, Nakatani Y, Yamaguchi Y, Sakamoto T, et al. Vagal activity modulates spontaneous augmentation of J-wave elevation in patients with idiopathic ventricular fibrillation. Heart Rhythm. 2012;9:249–55. doi: 10.1016/j.hrthm.2011.09.055. [DOI] [PubMed] [Google Scholar]
- [15].Koutbi L, Roussel M, Haissaguerre M, Deharo JC. Hyperpnea test triggering malignant ventricular arrhythmia in a child with early repolarization. Heart Rhythm. 2012;9:1153–6. doi: 10.1016/j.hrthm.2012.02.022. [DOI] [PubMed] [Google Scholar]
- [16].Kawata H, Noda T, Yamada Y, Okamura H, Satomi K, Aiba T, et al. Effect of sodium-channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm. 2012;9:77–83. doi: 10.1016/j.hrthm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- [17].Kasanuki H, Ohnishi S, Ohtuka M, Matsuda N, Nirei T, Isogai R, et al. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation. 1997;95:2277–85. doi: 10.1161/01.cir.95.9.2277. [DOI] [PubMed] [Google Scholar]
- [18].Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–37. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- [19].Junttila MJ, Sager SJ, Tikkanen JT, Anttonen O, Huikuri HV, Myerburg RJ. Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur Heart J. 2012;33:2639–43. doi: 10.1093/eurheartj/ehs110. [DOI] [PubMed] [Google Scholar]
- [20].Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53–64. doi: 10.1016/j.yjmcc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zygmunt AC, Goodrow RJ, Antzelevitch C. Sodium effects on 4-aminopyridine-sensitive transient outward current in canine ventricular cells. Am J Physiol. 1997;272:H1–H11. doi: 10.1152/ajpheart.1997.272.1.H1. [DOI] [PubMed] [Google Scholar]
- [23].Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- [24].Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–9. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- [25].Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gross GJ. Early repolarization and ventricular fibrillation: vagally familiar? Heart Rhythm. 2010;7:653–4. doi: 10.1016/j.hrthm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- [27].Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–27. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- [28].Ulphani JS, Cain JH, Inderyas F, Gordon D, Gikas PV, Shade G, et al. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm. 2010;7:1113–9. doi: 10.1016/j.hrthm.2010.03.043. [DOI] [PubMed] [Google Scholar]
- [29].Takahashi N, Barber MJ, Zipes DP. Efferent vagal innervation of canine ventricle. Am J Physiol. 1985;248:H89–H97. doi: 10.1152/ajpheart.1985.248.1.H89. [DOI] [PubMed] [Google Scholar]
- [30].DeGeest H, Levy MN, Zieske H, LIPMAN RI. Depression of ventricular contractility by stimulation of the vagus nerves. Circ Res. 1965;17:222–35. doi: 10.1161/01.res.17.3.222. [DOI] [PubMed] [Google Scholar]
- [31].Yang ZK, Boyett MR, Janvier NC, McMorn SO, Shui Z, Karim F. Regional differences in the negative inotropic effect of acetylcholine within the canine ventricle. J Physiol (Lond) 1996;492(Pt 3):789–806. doi: 10.1113/jphysiol.1996.sp021346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–45. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- [33].Kakinuma Y, Akiyama T, Sato T. Cholinoceptive and cholinergic properties of cardiomyocytes involving an amplification mechanism for vagal efferent effects in sparsely innervated ventricular myocardium. FEBS J. 2009;276:5111–25. doi: 10.1111/j.1742-4658.2009.07208.x. [DOI] [PubMed] [Google Scholar]
- [34].Rocha-Resende C, Roy A, Resende R, Ladeira MS, Lara A, de Morais Gomes ER, et al. Non-neuronal cholinergic machinery present in cardiomyocytes offsets hypertrophic signals. J Mol Cell Cardiol. 2012;53:206–16. doi: 10.1016/j.yjmcc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Junttila MJ, Sager SJ, Tikkanen JT, Anttonen O, Huikuri HV, Myerburg RJ. Use {15361} Eur Heart J. 2012;33:2639–43. doi: 10.1093/eurheartj/ehs110. [DOI] [PubMed] [Google Scholar]
- [36].Noseworthy PA, Tikkanen JT, Porthan K, Oikarinen L, Pietila A, Harald K, et al. The early repolarization pattern in the general population clinical correlates and heritability. J Am Coll Cardiol. 2011;57:2284–9. doi: 10.1016/j.jacc.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011;123:2666–73. doi: 10.1161/CIRCULATIONAHA.110.014068. [DOI] [PubMed] [Google Scholar]
- [38].Rosso R, Adler A, Halkin A, Viskin S. Risk of sudden death among young individuals with J waves and early repolarization: putting the evidence into perspective. Heart Rhythm. 2011;8:923–9. doi: 10.1016/j.hrthm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- [39].Rosso R, Glikson E, Belhassen B, Katz A, Halkin A, Steinvil A, et al. Distinguishing “benign” from “malignant early repolarization”: The value of the ST-segment morphology. Heart Rhythm. 2012;9:225–9. doi: 10.1016/j.hrthm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- [40].Rosso R, Halkin A, Viskin S. J waves and early repolarization: do not confuse me with the facts! Heart Rhythm. 2012;9:1603–4. doi: 10.1016/j.hrthm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [41].Viskin S, Rosso R, Halkin A. Making sense of early repolarization. Heart Rhythm. 2012;9:566–9. doi: 10.1016/j.hrthm.2011.11.042. [DOI] [PubMed] [Google Scholar]
- [42].Szentandrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J, et al. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res. 2005;65:851–60. doi: 10.1016/j.cardiores.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [43].Di Diego JM, Sun ZQ, Antzelevitch C. Ito and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–H561. doi: 10.1152/ajpheart.1996.271.2.H548. [DOI] [PubMed] [Google Scholar]
- [44].Pierpont GL, DeMaster EG, Cohn JN. Regional differences in adrenergic function within the left ventricle. Am J Physiol. 1984;246:H824–H829. doi: 10.1152/ajpheart.1984.246.6.H824. [DOI] [PubMed] [Google Scholar]
- [45].Haissaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, et al. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–9. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- [46].Roten L, Derval N, Sacher F, Pascale P, Scherr D, Komatsu Y, et al. Heterogeneous response of J wave syndromes to beta-adrenergic stimulation. Heart Rhythm. 2012;9:1970–6. doi: 10.1016/j.hrthm.2012.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.