Abstract
To cryopreserve cells, it is essential to avoid intracellular ice formation during cooling and warming. One way to do so is to subject them to procedures that convert cell water into a non-crystalline glass. Current belief is that to achieve this vitrification, cells must be suspended in very high concentrations of glass-inducing solutes (i.e., ≥ 6 molal) and cooled at very high rates (i.e., >> 1,000°C/min). We report here that both these beliefs are incorrect with respect to the vitrification of 8-cell mouse embryos. In this study, precompaction 8-cell embryos were vitrified in several dilutions of EAFS10/10 using various cooling rates and warming rates. Survival was based on morphology, osmotic functionality, and on the ability to develop to expanded blastocysts. With a warming rate of 117,500°C/min, the percentages of embryos vitrified in 1×, 0.75×, and 0.5× EAFS that developed to blastocysts were 93%, 92%, and 86%, respectively. And the percentages of morphological survivors that developed to expanded blastocysts were 100%, 92%, and 97%, respectively. Even when the solute concentration of the EAFS was reduced to 33% of normal, we obtained 40% functional survival of these 8-cell embryos.
Keywords: Embryos, oocytes, mouse, vitrification, solute concentration in media, cooling rates, warming rates, survival
Introduction
2×2×2×2–Interest in the cryopreservation of mammalian embryos and oocytes is accelerating in two major areas. One is in assisted reproduction for women; the other is as a cost-effective method of maintaining mutant lines of mice and other laboratory mammals. There are two methods of achieving cryopreservation: Slow equilibrium freezing and vitrification. In the former approach, ice forms first in the external medium. That causes the chemical potential of the water in the medium to drop below the chemical potential of the intracellular water. This differential creates an osmotic driving force for water to leave the cell and freeze externally. In slow equilibrium freezing, cells are cooled slowly enough so that the cell’s water content decreases sufficiently to keep its chemical potential in near equilibrium with the outside water and to fall to the equilibrium value before the cell has been cooled to a temperature that permits the ice nucleation of the remaining supercooled water.
The second approach, vitrification, is entirely different. It converts liquid water directly into a noncrystalline solid; i.e., a glass. Ice formation is blocked both in the medium and in the cells by suspending the cells in very high concentrations of solutes, including ones that permeate the cell, and by cooling them at high rates to temperatures below −100°C. There is increasing use of vitrification, again for two reasons. One is that freezing by slow cooling may be harmful. The other is that vitrification is a (superficially) simpler process. With respect to the former, some cell types are highly sensitive to chill injury; i.e., damage from low temperatures per se. Consequently, the slow cooling of such cell types translates into damage from long exposure times to low temperatures. Such chill-sensitive cells include some mammalian oocytes and embryos [14,22, 26], zebrafish oocytes and embryos [4,17,23], and Drosophila embryos [7].
There are two almost universally held beliefs about obtaining high survivals with vitrification. One is that the creation of the vitreous state requires the use of solutions with very high solute concentrations. The second is that it requires that cells be cooled at very high rates. With respect to the latter, we have subjected mouse oocytes to a wide range of cooling and warming rates, and have found that the latter is not true. The important factor is that the warming rate be very high [9,20,21].
More recently, we have demonstrated that the first of the two universally held beliefs also appears false. If one warms vitrified mouse oocytes exceedingly rapidly, one can obtain very high survivals even when the vitrification medium is diluted in half [20]. In our earlier papers [8], the criteria of survival were normality of the egg morphology and osmotic integrity of the cell membrane. In [21], we added a functional assay; namely, the ability of an oocyte to undergo in vitro fertilization (IVF) and develop into a 2-cell embryo. Because of zona hardening after vitrification [2], fertilization required partial dissection of the zona pellucida. For unknown reasons, we have not been able to induce the resulting 2-cell embryos to develop further in vitro. This limitation was one reason for the present paper in which we vitrified 8-cell embryos at various cooling rates, and after warming at various rates, used development from the 8-cell to the blastocyst as the functional assay. We see once again that if the warming rate is exceedingly high (117,500°C/min), morphological and functional survivals exceed 80% even with the vitrification solution diluted in half.
Materials and Methods
We have previously described the details of some of the methods [8,9]; consequently, here we only give details for those aspects that differed. The procedures for obtaining and manipulating the mouse embryos were carried out under the University of Tennessee Institutional Animal Care and Use Committee protocol 911-0710, approved 07 July 2010.
Collection of embryos
Female ICR mice (8–12 weeks old) were induced to superovulate with intraperitoneal injections of 5 IU of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG)(Sigma, St. Louis) given 48 h apart. Females were mated with mature males of the same strain. For the collection of 8-cell embryos, the oviducts of mated animals were flushed with modified phosphate buffered saline (PB1) medium at 68 h after injection of hCG. The 8-cell embryos (uncompacted) were washed and pooled in fresh PB1 medium in a culture dish under paraffin oil to await each suite of experiments.
Composition of the vitrification solutions
The vitrification solution (EAFS10/10) was developed by Pedro et al. [13] for cryopreservation of mouse oocytes at the metaphase stage of meiosis II. It consists of 10% (v/v) ethylene glycol (EG) and 10.7% (w/v) acetamide dissolved in a stock consisting of 30% (w/v) Ficoll 70 and 0.5 M sucrose in PB1 medium. The final concentration of sucrose and Ficoll are 0.4 M and 24% (w/v), respectively. The details of its mass composition were given in a previous report [18] and are summarized in the first row of Table 1. The concentrations are 3.23 molal EG and 3.27 molal acetamide as the highly permeating cryoprotectants [12], and 0.150 molal salts (as NaCl) and 0.720 molal sucrose as impermeable solutes, plus 20.68 wt% of Ficoll and 0.166 wt% of bovine serum albumin.
Table 1.
Derivative solute concentrations in various dilutions of the EAFS10/10 vitrification solution
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | (I) | (J) | (K) | (L) | (M) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative cncn. EAFS |
Total mass (g) |
Total volume (ml) |
Molality EG |
Molality acetamid |
Molality sucrose |
Molality NaCl |
Molar EG |
Molar acet. |
Molar sucrose |
Molar NaCl |
Osmol non-perm. |
Rel. Eq vol cell water |
| 1× | 11.52 | 10 | 3.23 | 3.27 | 0.72 | 0.15 | 2.283 | 2.283 | 0.503 | 0.105 | 0.996 | 0.277 |
| 0.75× | 11.14 | 10 | 2.01 | 2.04 | 0.45 | 0.15 | 1.589 | 1.613 | 0.356 | 0.119 | 0.726 | 0.380 |
| 0.5× | 10.76 | 10 | 1.15 | 1.16 | 0.26 | 0.15 | 0.999 | 1.008 | 0.226 | 0.130 | 0.533 | 0.518 |
| 0.33× | 10.51 | 10 | 0.7 | 0.71 | 0.16 | 0.15 | 0.640 | 0.649 | 0.146 | 0.137 | 0.436 | 0.633 |
Columns H–K: The molarities have been calculated from the molalities by the equations in an article titled “Molality” in Wikipedia, the free encyclopedia (http://en.wikipedia.org/wiki/Molality) for solutions with more than one solute. The contributions of Ficoll and BSA have been ignored. The molarity of the ith solute in moles/liter solution is Mi = Ww mi Mw, where Ww is the molecular weight of water in kg/mole (0.0018), mi is the molality of the ith solute in moles/kg of water, and Mw is the molarity of the water. Mw is equal to ρmw/(1 + Σ(mi Wi)), where ρ is the density of the solution, and mi Wi are the molalities of the ith solute times the molecular weight of that solute in kg/mole. (Note: The molarities listed in Columns H–K in Table 1 of [20] are erroneous. They were calculated incorrectly using a formula for solutions with single solutes.)
Column L: The nonpermeating solutes are sucrose and PBS (we ignore the very small contribution of the Ficoll and BSA.) Their combined osmolality is Column F + 0.276. We assume the osmolality of PBS to be equal to that of the same molality of NaCl. The osmolality of NaCl = 2φm where 2 is the number of species into which the molecule dissociates and φ is the osmotic coefficient.
Column M: The volume of water in the oocytes after equilibration with the external medium relative to the volume of water in an isotonic cell. It is 0.276/Column L.
Our study also made use of vitrification solutions that were diluted with PB1 to 0.75×, 0.50×, and 0.33× the molar concentrations of full strength EAFS. The molalities and molarities of the solutes in these three diluted solutions are also shown in Table 1. More details on the compositions are given in Table 2 of [21].
Table 2.
Protocol of each cooling procedures
| Protocol No. |
Cryotop covered with | Cooled by | Cooling ratea ± S.E. (°C/min) |
|---|---|---|---|
| 1 | Cap + glass tubeb | LN2 vaporc | 95±4 |
| 2 | Cap | LN2 vaporc | 876±11 |
| 3 | None | LN2 | 69,250±4,285 |
The cooling rates were determined by Kleinhans et al. [1]
Cryotop was covered with Cryotop cap and inserted into a 7 mm OD × 90 mm glass tube.
The cryotop was placed horizontally on a Styrofoam boat floated on the surface of LN2 in a Dewar flask for > 5 min before being immersed in LN2.
Vitrification procedure
Eight-cell embryos were transferred at 23°C into a 200µl drop of full-strength EAFS or one of the three dilutions. After 1 min 30 s, 5–6 embryos in a 0.1 µl drop of the medium were placed on a Cryotop (Kitazato BioBioPharma. Co., Ltd, Fuji, Japan). Then 30s later, cooling to −196°C was initiated. Thus, 2.0 min elapsed from the first exposure to the vitrification solution to the initiation of cooling. This is the exposure time that Pedro [11] found to yield the best survivals of ICR oocytes after vitrification. This relatively short time also reduced evaporation of the droplet prior to cooling [10]. We have depicted the Cryotop previously [1]. The thin plastic blade on which the droplet rests measures 20× 0.7× 0.1mm.
Achieving various cooling rates – physical set up and procedure
Samples were cooled at each of the three different rates shown in Table 2; namely, 95, 876, and 69, 250 °C/min. The fastest cooling was achieved by abruptly immersing the naked Cryotop leaf into liquid nitrogen (LN2) [−196°C]. To obtain the two lower rates, the Cryotop blade was surrounded by one or two insulating layers as indicated in Table 2 and cooled in LN2 vapor. The cooling rates (and warming rates) were measured on a dummy Cryotop with a 50 µm thermocouple cemented to the blade [1].
Achieving various warming rates
For each of the three cooling rates, samples were warmed at one of the three different rates shown in Table 3; namely, 610, 4050 and 117,500 °C/min. In some cases, the insulation present during cooling had to be removed at or near −196°C before initiating warming. For example, this was the case in samples cooled by Protocols 1 or 2 and warmed by Protocol 3. In Warming Protocol 1, the Cryotop was removed from LN2, held in room temperature air for 30 s, and then abruptly immersed into 2 ml of a solution of 0.5 M sucrose in PB1 at 23°C. In Warming Protocol 2, the insulated Cryotop was warmed at 4,050 °C/min by immersing it into water at 23°C. In Warming Protocol 3, the Cryotop was removed from LN2, and immediately immersed in the 23 °C sucrose/PB1 solution. The warming rate was 117,500 °C/min.
Table 3.
Protocol of each warming procedure
| Protocol No. |
Cryotop covered with |
Warmed by | Warming ratea ± S.E. (°C/min) |
|---|---|---|---|
| 1 | Cap | Air at 23 °C | 612±40 |
| 2 | Cap | Water at 23 °C | 4,050±328 |
| 5 | None | Sucrose solution 23 °C | 117,500±10,632 |
The warming rates were calculated by Kleinhans et al. [1].
Determination of Embryo Survival of Embryos on the Basis of Morphology and Membrane Intactness
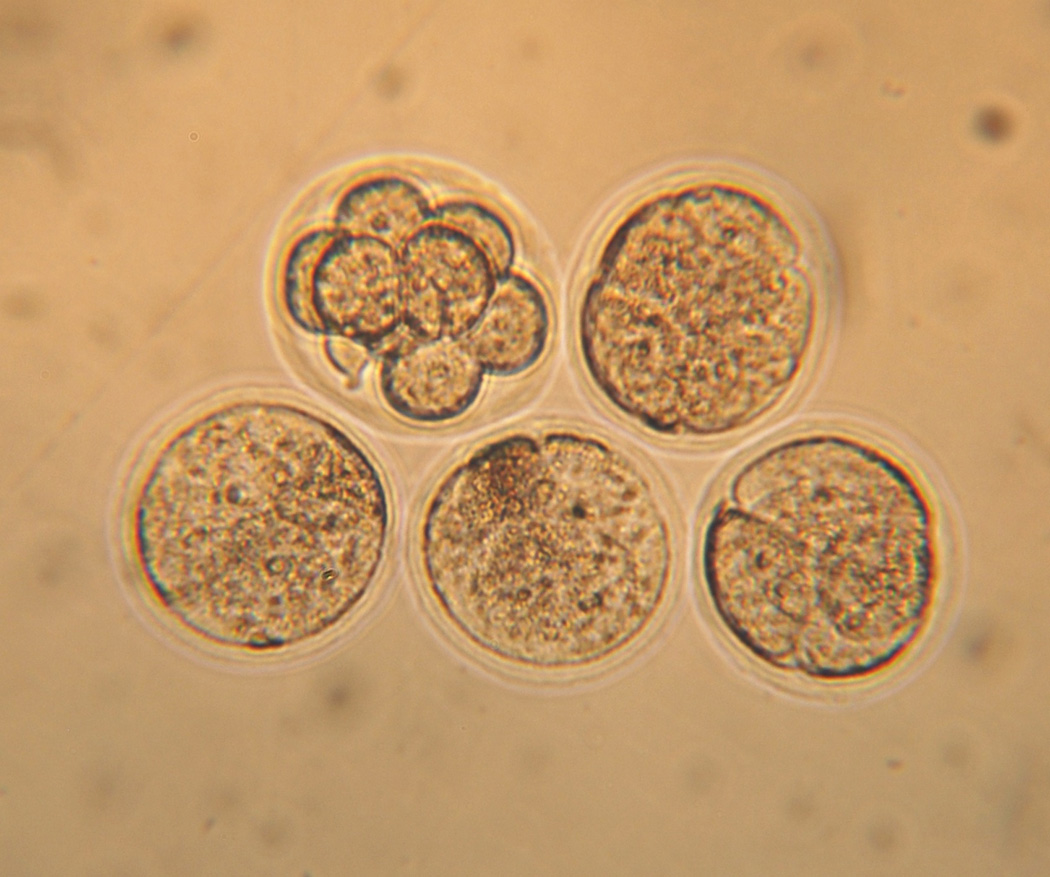
After the completion of warming, the embryos were transferred to fresh 0.5 M sucrose/PB1 solution at 23°C, and then approximately 10 min later, they were transferred to PB1 lacking sucrose and cultured in modified M16 medium [13] for 2 h at 37°C. Viability was assessed at three time points based on osmotic responsiveness and morphological normality. First, the 8-cell embryos were examined during the 10 min in PB1/sucrose. Membrane-intact embryos were expected to shrink with time because the sucrose is hypertonic. Second and third, they were examined after being placed in M16 and after 2 h incubation. The photomicrograph in Fig. 1 shows that they fall into two clearly separable groups: One embryo is indistinguishable morphologically and cytologically from an untreated 8-cell embryo. But four of them have lost all boundaries between the individual blastomeres and are clearly non-viable.
Fig. 1.
Photomicrograph of five 8-cell embryos after being suspended in 0.5 × EAFS, cooled at 69.000°C/min to −196°C, warmed at 4,050°C/min, and incubated in M16 at 37°C for 2 hrs. We score one embryo as viable, and four as dead, for a survival of 20%. The mean survival in Table 4 for the 7 replicates and 35 embryos subjected to that treatment (line 19) was 34%. The diameter of 8-cell embryos is 72 µm.
Determination of the Developmental Ability of 8-cell Mouse Embryos After “Thawing”
After determining the morphological survival, the 8-cell embryos were incubated an additional 48 hours in modified M16 medium under 5% CO2/95% air at 37 °C and the percentage developing into blastocysts was determined.
Statistical analysis
Error figures in tables and error bars in graphs are standard errors (standard deviations of the mean). Tests of significance were carried out by one-way ANOVA using Graphpad Software’s Instat, V. 3.02 followed by the Tukey-Kramer Multiple Comparison Test.
Results
Survival of embryos in full-strength EAFS10/10 vs. the cooling rate and warming rate
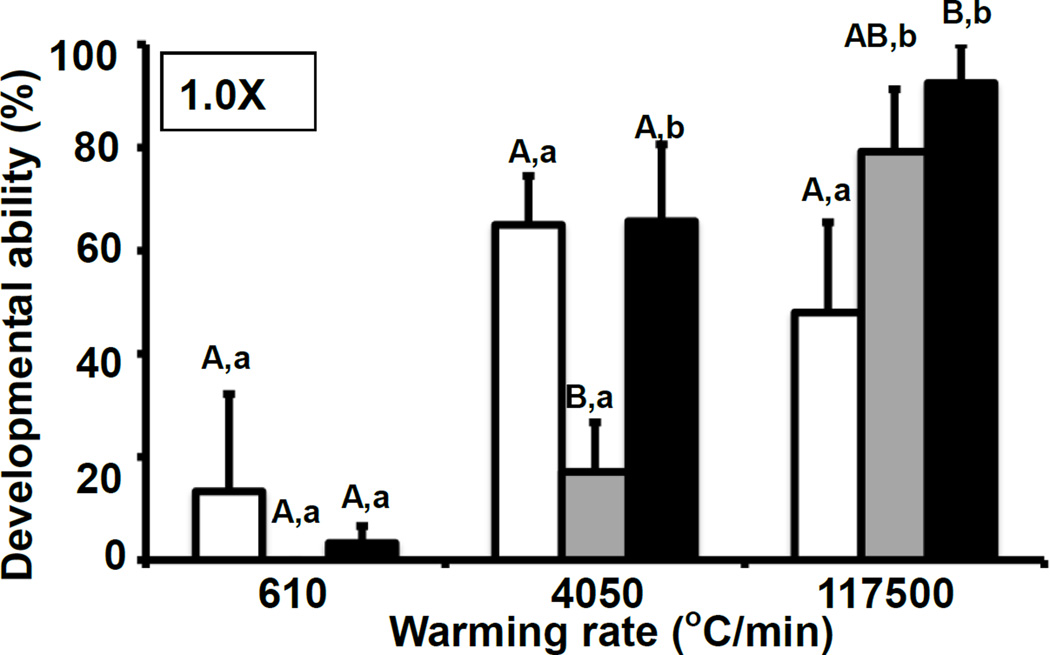
Fig.2 shows the developmental survival of 8-cell mouse embryos vitrified in full-strength EAFS10/10 for each of the three cooling rates and each of the three warming rates. The top nine rows of Table 4 compare these results with those for morphological survival and give the statistics. The first three rows of the table and the three right hand bars of Fig.2 show the survivals when the samples were warmed at the highest rate of 117,500°C/min. Both morphological and functional survivals were equal or greater than 90% when the cooling rate was 69,000°C/min (black bar). Survival only dropped slightly when the cooling rate was lowered to 880°C/min (gray bar), but it dropped to about 50% when the cooling was slowed to 95°C/min. (white bar).
Fig.2.
Developmental ability of mouse 8-cell embryos as a function of the cooling and warming rate after vitrification with 1.0× EAFS10/10. The cooling rates (°C/min) were 95 (□), 880 (■), or 69,000 (■), and the warming rates (°C/min) were 610, 4050, or 117,500. The protocols are given in Tables 2 and 3. Developmental ability is the percentage of “thawed” embryos that developed to blastocysts after 2 days incubation in M16 medium. Values with different superscripts were significantly different (P < 0.05) by one-way ANOVA or two-tailed t-tests. Different capital letters show statistically significant differences in developmental ability with various cooling rates and the same warming rate, and different lower case letters show the differences with same cooling rate and various warming rates.
Table 4.
Survival of mouse 8-cell embryos after freezing in EAFS10/10 with cryotop at each cooling rate and warming rate.
| Concn. EAFS |
Cooling rate (°C/min) |
Warming rate (°C/min) |
Survivals ± S.E. | % of Morphol. survivors develop to blasto. |
|
|---|---|---|---|---|---|
| Morphological observation | 8-cell to blastocysto | ||||
| 1× | 69,000 | 117,500 | 92.5 ± 7.5 (41/44, N = 8) | 92.5 ± 7.5 (41/44, N = 8) | 100 |
| 880 | 117,500 | 88.9 ± 12.2 (27/29, N = 6) | 90.0 ± 6.7 (25/30, N = 6) | 92.3 | |
| 95 | 117,500 | 64.0 ± 17.2 (16/25, N = 5) | 48.0 ± 17.5 (12/25, N = 5) | 75 | |
| 1× | 69,000 | 4,050 | 74.3 ± 16.7 (26/35, N = 7) | 65.7 ± 14.9 (23/35, N = 7) | 88.5 |
| 880 | 4,050 | 44.2 ± 17.4 (18/41, N = 8) | 17.1 ± 9.6 (7/41, N = 8) | 38.9 | |
| 95 | 4,050 | 71.1 ± 9.5 (24/34, N = 6) | 65.0 ± 9.5 (22/34, N = 6) | 91.7 | |
| 1× | 69,000 | 610 | 23.3 ± 16.4 (7/30, N = 6) | 3.3 ± 3.3 (1/30, N = 6) | 14.3 |
| 880 | 610 | 0.0 ± 0.0 (0/25, N = 5) | 0.0 ± 0.0 (0/25, N = 5) | 0 | |
| 95 | 610 | 20.0 ± 30.6 (6/30, N = 6) | 13.3 ± 18.9 (4/30, N = 6) | 66.7 | |
| 0.75× | 69,000 | 117,500 | 100.0 ± 0.0 (38/38, N = 7) | 92.4 ± 3.6 (35/38, N = 7) | 92.1 |
| 880 | 117,500 | 100.0 ± 0.0 (32/32, N = 6) | 96.7 ± 3.3 (31/32, N = 6) | 96.9 | |
| 95 | 117,500 | 88.0 ± 12.0 (22/25, N = 5) | 64.0 ± 22.3 (16/25, N = 5) | 72.8 | |
| 0.75× | 69,000 | 4,050 | 55.5 ± 8.8 (25/45, N = 9) | 33.3 ± 10.0 (15/45, N = 9) | 60 |
| 880 | 4,050 | 31.4 ± 14.8 (12/36, N = 7) | 25.7 ± 13.6 (10/36, N = 7) | 83.3 | |
| 95 | 4,050 | 59.0 ± 14.6 (23/38, N = 7) | 46.2 ± 13.0 (18/38, N = 7) | 78.3 | |
| 0.5× | 69,000 | 117,500 | 85.7 ± 14.3 (34/39, N = 7) | 83.3 ± 14.1 (33/39, N = 7) | 97.1 |
| 880 | 117,500 | 91.7 ± 8.3 (31/34, N = 6) | 86.1 ± 8.0 (29/34, N = 6) | 93.5 | |
| 95 | 117,500 | 100 ± 0.0 (25/25, N = 5) | 92.0 ± 5.5 (23/25, N = 5) | 92 | |
| 0.5× | 69,000 | 4,050 | 34.3 ± 14.3 (12/35, N = 7) | 14.3 ± 14.3 (5/35, N = 7) | 41.7 |
| 880 | 4,050 | 30.0 ± 12.0 (10/32, N = 6) | 12.8 ± 9.8 (4/32, N = 6) | 40 | |
| 95 | 4,050 | 31.1 ± 11.8 (10/32, N = 6) | 21.1 ± 8.4 (7/32, N = 6) | 70 | |
| 0.33× | 69,000 | 117,500 | 74.8 ± 11.5 (29/39, N = 7) | 39.5 ± 14.2 (15/39, N = 7) | 51.7 |
| 880 | 117,500 | 48.0 ± 21.2 (12/28, N = 6) | 40.0 ± 24.5 (10/28, N = 6) | 83.3 | |
| 95 | 117,500 | 28.6 ± 17.8 (10/35, N = 7) | 10.0 ± 10.0 (3/30, N = 6) | 30 | |
| 0.33× | 69,000 | 4,050 | 0.0 ± 0.0 (0/20, N = 4) | 0.0 ± 0.0 (0/20, N = 4) | 0 |
| 880 | 4,050 | 5.0 ± 10.0 (1/20, N = 4) | 0.0 ± 0.0 (0/20, N = 4) | 0 | |
| 95 | 4,050 | 40.0 ± 23.1 (10/25, N = 5) | 8.0 ± 4.9 (2/25, N = 5) | 20 | |
The data are % survival ± SEM. The first sets of parentheses are the ratios of the number of surviving oocytes to the number frozen or vitrified. N is the number of replicates.
At the other extreme, when the embryos were warmed at the lowest rate (610°C/min)[Rows 7–9 in Table 4 and the left-hand set of bars in Fig.2], the survivals were 0 to 23% for morphological/osmotic survival and 0 to 13 % for developmental survival regardless of the cooling rate. The middle group of bars in the figure and lines 4–6 in the table give the survivals with an intermediate warming rate of 4,050°C/min. The survivals after slow and rapid warming (white and blacks bars) are also intermediate; i.e., about 50 to 60%. However, the survivals depicted by the gray bar representing an intermediate cooling rate of 880°C/min (row 5 of Table 4) are anomalously low (17%). We have no explanation to suggest. This anomaly is not present when using 0.75× or 0.5× EAFS (Fig. 3).
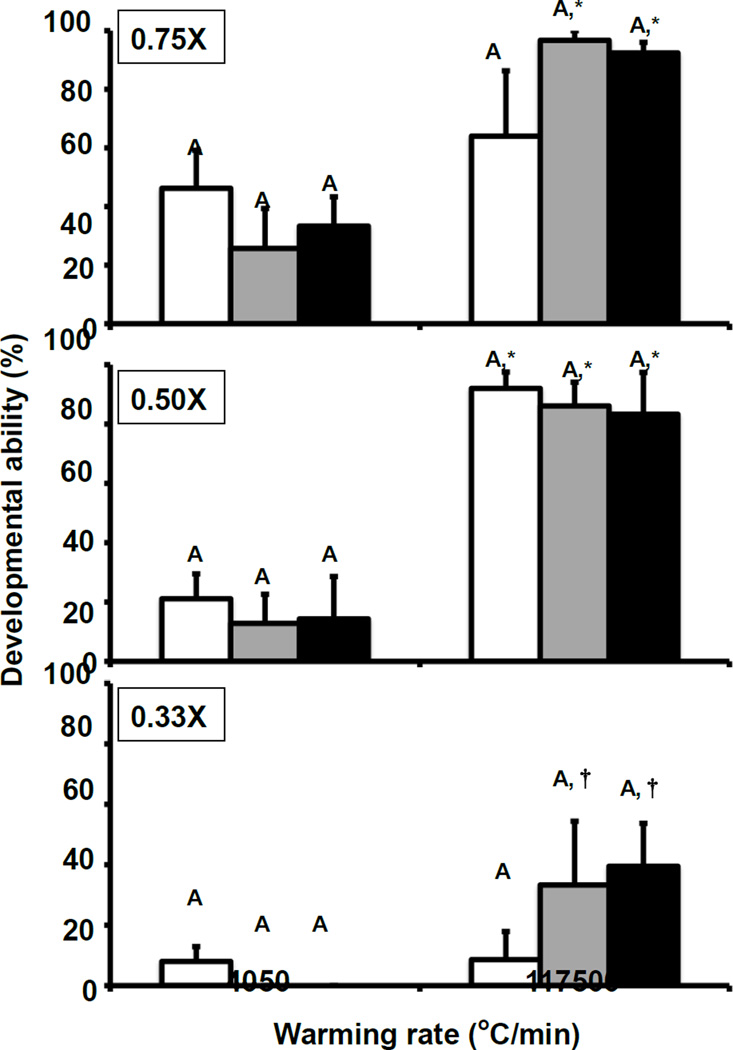
Fig. 3.
Developmental ability of 8-cell mouse embryos as a function of the extent of dilution of EAFS and the cooling and warming rate. The dilutions were 0.75×, 0.5×, or 0.33×. The cooling rates (°C/min) were 95 (□), 880 (■), or 69,000 (■), and the warming rates (°C/min) were 4050 or 117,500. The protocols are given in Tables 2 and 3. Developmental ability is the percentage of “thawed” 8-cell embryos that developed to blastocysts after 2 days incubation in M16 medium. There are no significant differences in developmental ability with various cooling rates and the same warming rate and the same dilution of EAFS. There were, however, significant differences in survival between the two warming rates for the same cooling rate and dilution of EAFS. These cases of differences are shown by the asterisks. For example, in the top graph, the survivals in the two white bars do not differ significantly, but as indicated by the asterisks, the survivals in the two gray bars and in the two black bars do.
Survival of embryos in various dilutions of EAFS10/10 vs. the cooling rate and warming rate
The upper two panels of Fig. 3 show the developmental survival of 8-cell mouse embryos after suspension in 0.75× and 0.5× EAFS, cooling at three rates (69,000, 880, and 95°C/min), and warming at two rates (117,500 and 4050°C/min). Rows 10–21 of Table 4 compare these functional survivals with morphological survivals and list the standard errors. The survivals after warming the 0.75× and 0.5× vitrified solutions at the highest rate of 117,500°C/min (Black and gray right-hand bars in the upper two panels of Fig. 3) are very similar to the results for 1× EAFS in Fig.2. Morphological survivals range from 86 to 100% and developmental survivals from 83 to 97% with little difference between the results for the three cooling rates.
When the warming rate is lowered to 4,050°C/min, functional survivals are far lower than those found after warming at 117,500°C/min; namely, 26– 46% and 13–21% for embryos in 0.75× and 0.5× EAFS, respectively. This is similar to but slightly lower than with 1× EAFS (Fig.2).
Freezing in 0.33× EAFS is decidedly more damaging than in the more concentrated EAFS (Fig. 3, bottom panel; Table 4, last six rows). Even with the fastest warming, functional survivals dropped to 40%, which is about half that obtained with 0.5×, 0.75×, and 1× EAFS. And again, cooling rates of 69,000°C and 880°C/min gave about the same results. When the warming rate was lowered to 4,050°C/min., only 8% or none survived, regardless of the cooling rate.
Morphological/osmotic survival vs. functional/developmental survival
The right-hand column of Table 4 gives the percentage of morphological survivors that developed to blastocysts. In 10/24 cases, that percentage exceeded 80%; in 14/24 cases, it exceeded 60%. In these fourteen cases, the absolute survivals also tended to be high. In the remaining 10 cases where the ratio of functional/morphological was less than 60%, the absolute survivals were also low.
Discussion
Effects of cooling rate, warming rate, and their interactions on the functional survival of 8-cell embryos
The three groups of bars in Fig. 2 show from left to right the functional survivals of 8-cell embryos cooled in 1× EAFS vitrification solution and warmed at 610, 4050, and 117,500°C/min. The mean developmental survivals for each group were 5.5%, 49.3%, and 73.2%, respectively. Clearly, the rate of warming exerts a major effect. The white, gray, and black bars within each group represent survivals with cooling rates of 95, 880, and 69,000°C/min. In seven of the nine cases, the cooling rate had little effect on survival.
Fig. 3 shows the survivals in three dilutions of the EAFS 10/10. The results with 0.75× and 0.5× EAFS are similar to each other and to those for the 1× EAFS in Fig.2. With 0.75×, the mean survivals after warming at 4,050 and 117,500°C/min were 35% and 84%; with the 0.5×, they were 16% and 87%. The three bars within each group show even less effect of cooling rate than in the case of the 1× EAFS in Fig.2.
The fact that an average of 73% of the 8-cell embryos are functionally viable after warming from −196°C/min at 117,500°C/min has to mean that that percentage was viable after being cooled to −196°C. However, nearly all of these are killed if the warming is slowed to 610°C/min. What is the process occurring during warming that is accelerated by slower warming? We have hypothesized previously [19], and continue to do so now, that the process is the recrystallization of small ice crystals that formed during cooling. Small ice crystals have higher surface free energies than larger crystals. As a consequence if one has a mixture of different size crystals, there will be a thermodynamic driving force for water molecules to move from the smaller crystals to the larger ones, thus further increasing the size of the larger ones at the expense of the smaller ones. This process is referred to as recrystallization and the rate at which it occurs depends on the temperature, the time at a given temperature, and the activation energy of the process. In oocytes, the activation energy for recrystallization is 27.5 kcal/mole [19] and the rate increases about four-fold for each five degree rise in temperature. We hypothesize that a warming rate of 117,500°C/min is high enough to prevent the recrystallizing ice crystals from reaching a lethal size. A warming rate of 4,050°C/min is not high enough to prevent lethal recrystallization in embryos cooled in 0.75× or 0.5× EAFS (Fig. 3) and a warming rate of 610°C/min does not prevent it in embryos cooled in 1× EAFS (Fig.2).
Is cooling rate critical to survival or is it of tertiary importance? Is warming rate critical to survival or is it of tertiary importance?
As we stated in the Introduction, if one cools cells slowly enough, their internal water remains in near thermodynamic equilibrium with the outside ice by osmotic outflow, and under these conditions the water in the interior of the cells can not freeze in situ and high survivals are possible. If one does not cool slowly enough, the unfrozen intracellular water departs more and more from equilibrium and eventually freezes in the cell–usually with lethal results. In 1963, Mazur applied physical chemical relationships to quantify what is meant by “slowly enough” or “not slowly enough”[5]. Subsequent papers tested these predictions experimentally and found them accurate. The most relevant to the present paper was the 1972 publication by Whittingham, Leibo, and Mazur [24] on the successful cryopreservation of 1-cell and 8-cell embryos, and papers by Leibo, McGrath, and Cravallo, [3] and by Rall, Mazur, and McGrath [16] on microscope observations on IIF in mouse oocytes and 8-cell embryos vs. cooling rate and temperature. These studies, and publications from other laboratories, indicated that a cooling rate of 0.5°C/min was “slow enough” to avoid IIF in mouse oocytes and embryos, and a cooling rate of ≥ 4°C/min essentially guaranteed IIF and death. The more general conclusion was that the cooling rate is critical.
The other experimental observation made in that same period was that if the cells are cooled “slowly enough” to cryogenic temperatures, the rate at which they are subsequently warmed and thawed usually makes relatively little difference. From the results of our present study on the vitrification of 8-cell embryos and from our previous studies on oocytes [9,19], we conclude the exact opposite; namely, that the cooling rate is relatively unimportant and the warming rate is critical. The answer to this apparent paradox is that the range of cooling rates that we are talking about here is about 100 to 70,000 times higher than the range that was analyzed in the ‘70’s, and the physical phenomena that are occurring are entirely different. In slow equilibrium freezing, the phenomena are the degree of exosmosis of intracellular water and whether it is sufficient to prevent IIF.
In this and our other recent vitrification studies, the cooling rates are 20 to several thousand times above those required to maintain equilibrium, and the oocytes and embryos effectively undergo zero osmotic shrinkage during cooling. If they can not equilibrate by dehydration, their water must either freeze or vitrify. Theory predicts and experiments show that the faster cells are cooled, the smaller are the resulting ice crystals. The corollary hypothesis is that if the crystals are small enough, they are harmless. But as we stated above, these small crystals are thermodynamically unstable, and unless they are warmed extremely rapidly, they will grow to damaging size by recrystallization.
Vitrification represents the extreme of the above. With it, the intracellular viscosity is raised to such a high level by a combination of partial dehydration and the loading of high concentrations of exogenous solutes, and the cooling rate is so high that the stable ice nuclei formed below the homogeneous nucleation temperature do not have time to grow before the temperature drops below the glass transformation temperature, Tg. Below Tg, the viscosity rises to extremely high values and the resulting solid is a noncrystalline glass. During warming the process is reversed. Above Tg, the ice nuclei begin to grow into crystals, and the crystals become subject to further growth by recrystallization.
There are thermodynamic differences between the scenario of supercooled viscous liquid water converting to glass below Tg, converting to small ice crystals when warmed above Tg, and undergoing size-differential growth by recrystallization and the scenario where the cell water is converted to small ice crystals during cooling which in turn are subject to growth from recrystallization during warming. But from the viewpoint of survival, they are probably indistinguishable.
Survival as a function of the concentration of solutes in the EAFS vitrification solution
As mentioned in the Introduction, one of the tenets in vitrification has been that the solute concentrations in the vitrification medium have to be exceedingly high to avoid ice formation. Since most vitrification procedures only expose oocytes or embryos to the vitrification media for 1 to 2 min before being plunged into LN2, very little of the permeating solutes (here, EG and acetamide) has time to permeate; hence, the cells will be exposed to an osmotic force equal to the sum of the osmolalities of both non-permeating and permeating solutes in the medium (approcimately, the sums of columns D through G in Table 1) minus the isotonic osmolality initially within the cell. The resulting osmosis will pull out an extensive proportion of their water. The proportion is the isotonic osmolality (0.28) divided by the total external osmolality of columns D–G. The external osmolality is approximately equal to the total external molality plus 0.13 to account for the fact that the osmolality of isotonic NaCl is nearly double its molality because of ionization. Perhaps, it is this dehydration that is responsible for some of the changes observed in vitrified oocytes and embryos, such as hardening of the zona pellucida
A comparison of the right hand set of three bars in Fig.2 and in the upper two panels of Fig. 3 shows that when samples are warmed at 117,500°C/min, developmental survivals are almost identical in 1 ×, 0.75×, and 0.5 × EAFS over cooling rates ranging from 95 to 60,900°C/min. Furthermore, when the cooling rate is 880 or 69,000°C/min (black and gray bars) and the warming is 117,500°C/min, the developmental survivals exceed 90% in the three dilutions of EAFS. But if the warming rate is reduced to 4,050°C/min (middle set of bars in Fig.2 and left-hand set of bars in the upper two panels in Fig. 3), survivals decrease progressively as the EAFS concentration is reduced from 1× to 0.75× and 0.5×. However, vitrification in 0.33×EAFS (bottom panel of Fig. 3) is clearly damaging even when using the highest warming rate.
The dilution of the EAFS solutes has two physical effects. One is an increase in the relative amount of water in the embryos prior to the onset of cooling; the other is a decrease in the viscosity of the cytoplasm. EAFS contains two solutes, ethylene glycol and acetamide, that can rapidly permeate the embryos, and it contains three solutes that can not; namely, salts (mostly NaCl), sucrose, and Ficoll. If the embryos were allowed to fully equilibrate, the relative equilibrium volume of intracellular water would be determined by the osmolality of the non-permeating solutes in the medium relative to the isotonic osmolality of PBS and the embryos. That ratio is given in the right-hand column of Table 1. One sees that the ratio increases as the extent of EAFS dilution increases. Thus, in 1× EAFS, the equilibrium ratio is 0.28; in 0.75×, 0.5×, and 0.33×, the ratio increases to 0.38, 0.52, and 0.63, respectively.
But more important is what we brought up in the beginning of this section; namely, because cells are much more permeable to water than to most solutes, when placed in the EAFS solutions (or other hyperosmotic solutions), they initially shrink rapidly to a minimum volume, the value of which depends on the summed osmolalities of both the permeating and non-permeating solutes. Unpublished studies in Dr. Keisuke Edashige’s laboratory are finding that this minimum occurs in 1–2 minutes. This is consistent with the experimental finding that survivals of vitrified oocytes and embryos are highest when the pre-vtrification exposure time is 2 min, not 5 min [11].
We know from our previous studies on MII mouse oocytes frozen in 1 or 1.5 M EG, that if their water content is above 40%, they undergo a flashing type of IIF during cooling and are dead upon warming [6]. If the water content is reduced to 23% beforehand, they show no flashing during cooling, but undergo blackening during warming, presumably from recrystallization. Our hypothesis is that the 8-cell embryos frozen in 0.33× EAFS in the present study are like the former. That is to say, because of their high water content, they undergo lethal IIF during cooling, and therefore are not capable of being “rescued” by warming at 117,500°C/min.
We have identified two previous reports over the past 25 years of 8-cell mouse embryos surviving vitrification procedures in diluted vitrification media. In the earliest, Rall [15] achieved good survival of 8-cell mouse embryos in a slightly diluted (0.85×) VS1 vitrification solution. More recently, Yavin et al. [25] obtained rather good survivals of mouse embryos in closed pulled straws subjected to very rapid cooling in a liquid/solid nitrogen slush rather than LN2.. They attributed their success to these very high cooling rates. The data in our present report, however, clearly disagree with that view. Our data show that high percentages of embryos in extensively diluted vitrification media will survive being cooled at moderate rates provided that the subsequent warming rate is exceedingly high.
Rall [15] was also the first to report that the survival of 8-cell embryos vitrified in straws in a full strength vitrification solution medium was highly dependent on the warming rate over a range of 10 to 200°C/min, but was totally independent of the cooling rate over a range of 20 to 2500°C/min.
Comparison between morphological and functional survivals
When our studies on IIF and vitrification of oocytes were initiated seven years ago, we based survival on the morphological/osmotic criteria detailed in Mazur et al. [8]. There were several reasons for doing so. One was that although high morphological survivals did not guarantee high functional survival, poor morphological survival guaranteed poor or no functional survival. Thus, morphological survival served as a triage to determine which treatments should be subsequently tested for functional survival. Second, oocytes and early embryos are all-or-none with respect to morphological and osmotic normality. Either they are indistinguishable from untreated controls or they show gross changes in appearance. Third, unpublished experiment indicated that MII oocytes from the ICR strain could not be fertilized by IVF with an intact zona present, and would not develop in vitro from the 2-cell stage to blastocyst. Recently, however, Seki & Mazur [21] have succeeded in obtaining high percentages of 2-cell embryos after the IVF of MII oocytes in which the zona has been partially dissected, thus providing a measure of functional survival. Fortunately, this complication was not present in the present study; here, our measure of functional survival has been in vitro development from the 8-cell stage to blastocyst.
Table 6 of Seki and Mazur [21] gives the functional survival of MII oocytes as a percentage of their morphological survival. Table 5 of the present paper compares these data with overlapping data for 8-cell embryos. The cases depicted are ones where the morphological survival with one exception is greater than 80%.(Note that the warming rate was 117,500°C/min in all of these.) And in all 18 cases except one, the percent of morphological survivors that were functional exceeded 50%. Thus, when the morphological survival is high, it is a rather reliable indicator of high functional survival. Conversely, as predicted, the data for the 0.33× dilution in Table 4 show that when the morphological survival of 8-cell embryos is low or erratic, the functional survival is low or zero.
Table 5.
Comparison of morphological survival and functional survival of oocytes and 8-cell embryos as a function of the dilution of EAFS and the cooling rate. The warming rate was 117,500°C/min.
| Concn. EAFS |
Cooling rate (°C/min) |
% Morphol. Surv. Oocytea |
% Morphol. Surv. 8-cellb |
% Morphol. Surv. Developing to 2-cella |
% Morphol. Surv. Developing to blasto.b |
|---|---|---|---|---|---|
| 1× | 69,000 | 92 | 92 | 88 | 100 |
| 880 | 82 | 89 | 63 | 92 | |
| 95 | 77 | 64 | 39 | 75 | |
| 0.75× | 69,000 | 97 | 100 | 78 | 92 |
| 880 | 97 | 100 | 67 | 97 | |
| 95 | 83 | 88 | 52 | 73 | |
| 0.5× | 69,000 | 90 | 86 | 71 | 97 |
| 880 | 88 | 92 | 97 | 86 | |
| 95 | 87 | 100 | 59 | 92 | |
Comparison between 8-cell embryos and MII oocytes
The data in Table 5 also allow us to compare the survivals of Mouse 8-cell embryos when they are exposed to the same set of conditions as previously used for MII oocytes. The agreement for morphological survivals is exceedingly close (Columns 3 vs. 4). The agreement between the functional survivals of oocytes and 8-cell embryos (Columns 5 and 6) although somewhat less striking, is still quite good. The discrepancy is greatest with a cooling rate of 95°C/min. If one calculates the mean functional survivals omitting those three data points, the mean ratios of functional survival to morphological survival are 77% and 94% for oocytes and 8-cell embryos, respectively. The greater sensitivity of the oocytes to vitrification is not surprising in view of their greater sensitivity overall, which is partly a result of their being locked into meiotic metaphase II at the time of vitrification.
Conclusions
Vitrification is trending towards becoming the preferred method for cryopreserving mammalian oocytes, especially human. Two tenets are believed to underlie success. One is that the oocytes be suspended in vitrification solutions containing high concentrations of solutes. The second is that the solutions be cooled very rapidly. The purpose of both is to prevent the occurrence of ice crystals. However, we have recently published data for mouse oocytes and, here, for mouse 8-cell embryos that are counter to those views. First, we have found that a very high warming rate is considerably more important to the attainment of high survivals than is a very high cooling rate. Second, we report that when oocytes or 8-cell embryos are warmed at the highest available rate (117,500°C/min), we obtain high survivals even when the concentration of solutes in EAFS 10/10 vitrification solution is cut in half. Our explanation for the importance of a high warming rate is that it prevents or reduces the recrystallization of intracellular ice during warming. These findings, we believe, are important to our understanding of fundamental cryobiology. Furthermore, they are likely to be germane to the cryopreservation of other cell types of fundamental and applied interest.
Acknowledgments
This research was supported by NIH grant R01-OD011201 (P. Mazur, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of funding: Supported by National Institutes of Health Grant R01-OD011201
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- 1.Kleinhans FW, Seki S, Mazur P. Simple inexpensive attainment and measurement of very high cooling and warming rates. Cryobiology. 2010;61:231–233. doi: 10.1016/j.cryobiol.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduced cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction. 2006;131:53–61. doi: 10.1530/rep.1.00878. [DOI] [PubMed] [Google Scholar]
- 3.Leibo SP, McGrath JJ, Cravalho EG. Microscopic observation of intracellular ice formation in unfertilized mouse ova as a function of cooling rate. Cryobiology. 1978;15:257–271. doi: 10.1016/0011-2240(78)90036-6. [DOI] [PubMed] [Google Scholar]
- 4.Liu XH, Zhang T, Rawson DM. Effect of cooling rate and partial removal of yolk on the chilling injury in zebrafish (Danio rerio) embryos. Theriogenology. 2001;55:1719–1731. doi: 10.1016/s0093-691x(01)00515-5. [DOI] [PubMed] [Google Scholar]
- 5.Mazur P. Kinetics of water loss from cells at subzero temperature and the likelihood of intracellular freezing. Journal of General Physiology. 1963;47:347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazur P, Pinn IL, Kleinhans FW. Intracellular ice formation in mouse oocytes subjected to interrupted rapid cooling. Cryobiology. 2007;55:158–166. doi: 10.1016/j.cryobiol.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazur P, Schneider U, Mahowald AP. Characteristics and kinetics of subzero chilling injury in Drosophila embryos. Cryobiology. 1992;29:39–68. doi: 10.1016/0011-2240(92)90005-m. [DOI] [PubMed] [Google Scholar]
- 8.Mazur P, Seki S, Pinn IL, Kleinhans FW, Edashige K. Extra- and intracellular ice formation in mouse oocytes. Cryobiology. 2005;51:29–53. doi: 10.1016/j.cryobiol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to −196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: A new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62:1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paredes E, Mazur P. The survival of mouse oocytes shows little or no correlation with the vitrification or freezing of the external medium, but the ability of the medium to vitrify is affected by its solute concentration and by the cooling rate. Cryobiology. doi: 10.1016/j.cryobiol.2013.09.003. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedro PB. Ph.D. thesis. Chap. 3. Japan: Ehime University; 2003. Studies on the cryopreservation of mammalian oocytes and embryos with reference to some cryobiological characteristics. [Google Scholar]
- 12.Pedro PB, Yokoyama E, Zhu SE, Yoshida N, Valdez DM, Jr, Tanaka M, Edashige K, Kasai M. Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. Journal of Reproduction and Development. 2005;51:235–246. doi: 10.1262/jrd.16079. [DOI] [PubMed] [Google Scholar]
- 13.Pedro PB, Zhu SE, Makino N, Sakurai T, Edashige K, Kasai M. Effects of hypotonic stress on the survival of mouse oocytes and embryos of various stages. Cryobiology. 1997;35:150–158. doi: 10.1006/cryo.1997.2034. [DOI] [PubMed] [Google Scholar]
- 14.Pollard JW, Leibo SP. Chilling sensitivity of mammalian embryos. Theriogenology. 1994;41:101–106. [Google Scholar]
- 15.Rall WF. Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology. 1987;24:387–402. doi: 10.1016/0011-2240(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 16.Rall WF, Mazur P, McGrath JJ. Depression of the ice-nucleation temperature of rapidly cooled mouse embryos by glycerol and dimethyl sulfoxide. Biophysical Journal. 1983;41:1–12. doi: 10.1016/S0006-3495(83)84399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki S, Kouya T, Tsuchiya R, Valdez DM, Jr, Jin B, Koshimoto C, Kasai M, Edashige K. K, Cryobiological properties of immature zebrafish oocytes assessed by their ability to be fertilized and develop to term. Cryobiology. 2011;62:8–14. doi: 10.1016/j.cryobiol.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Seki S, Mazur P. Effect of warming rate on the survival of vitrified mouse oocytes and on the recrystallization of intracellular ice. Biology of Reproduction. 2008;79:727–737. doi: 10.1095/biolreprod.108.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki S, Mazur P. Kinetics and activation energy of recrystallization of intracellular ice in mouse oocytes subjected to a vitrification procedure. Cryobiology. 2008;56:171–180. doi: 10.1016/j.cryobiol.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59:75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to −196°C in dilutions of a standard vitrification solution. PLoS One. 2012;7:e36058. doi: 10.1371/journal.pone.0036058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Songsasen N, Yu I, Ratterree M, VandeVoort C, Leibo SP. Effect of chilling on the organization of tubulin and chromosomes in rhesus monkey oocytes. Fertility and Sterility. 2002;77:818–825. doi: 10.1016/s0015-0282(01)03240-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai S, Rawson DM, Zhang T T. Studies on chilling sensitivity of early stage zebrafish (Danio rerio) ovarian follicles. Cryobiology. 2009;58:279–286. doi: 10.1016/j.cryobiol.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Whittingham DG, Leibo SP, Mazur P. Surivival of mouse embryos frozen to −196 and −269 C. Science. 1972;178:411–414. [PubMed] [Google Scholar]
- 25.Yavin S, Aroyo A, Roth Z, Arav A. Embryo cryopreservation in the presence of low concentration of vitrification solution with sealed pulled straws in liquid nitrogen slush. Human Reproduction. 2009;24:797–804. doi: 10.1093/humrep/den397. [DOI] [PubMed] [Google Scholar]
- 26.Zenzes MT, Bieleck R, Caspe R, Leibo SP. Effects of chilling to 0 degrees C on the morphology of meiotic spindles in human metaphase II oocytes. Fertility and Sterility. 2001;75:769–777. doi: 10.1016/s0015-0282(00)01800-8. [DOI] [PubMed] [Google Scholar]