Summary
Recent studies demonstrate that natural killer (NK) cells have adaptive immune features. Here, we investigated the role of the costimulatory molecule DNAM-1 in the differentiation of NK cells in a mouse model of cytomegalovirus (MCMV) infection. Antibody blockade of DNAM-1 suppressed the expansion of MCMV-specific Ly49H+ cells during viral infection and inhibited the generation of memory NK cells. Similarly, DNAM-1-deficient (Cd226−/−) Ly49H+ NK cells exhibited intrinsic defects in expansion and differentiation into memory cells. Src-family tyrosine kinase Fyn and serine -threonine protein kinase C isoform eta (PKCη) signaling through DNAM-1 played distinct roles in the generation of MCMV-specific effector and memory NK cells. Thus, cooperative signaling through DNAM-1 and Ly49H are required for NK cell-mediated host defense against MCMV infection.
Introduction
Natural killer (NK) cells are traditionally classified as innate immune cells because they rapidly respond to pathogens and are considered short-lived and unable to differentiate into memory cells (Lanier, 2005; Min-Oo et al., 2013; Sun et al., 2010). Accumulating evidence, however, indicates that NK cells have adaptive immune features (Cooper et al., 2009; Min-Oo et al., 2013; O'Leary et al., 2006; Paust et al., 2010; Sun et al., 2009, 2010, 2011). In some cases, NK cells are activated after exposure to pathogens, antigens, and cytokines, and subsequently differentiate into a long-lived population with enhanced effector function in response to a variety of secondary stimuli, as compared with naive NK cells (Cooper et al., 2009; O'Leary et al., 2006; Sun et al., 2009). The existence of memory NK cells in humans is suggested by the extensive expansion and persistence for months of NKG2C+ NK cells following infection with human cytomegalovirus (HCMV) (Foley et al., 2012a; Foley et al., 2012b; Lopez-Vergès et al., 2011; Min-Oo et al., 2013). We have previously demonstrated that mouse NK cells bearing the Ly49H receptor, which specifically recognizes the m157 mouse cytomegalovirus (MCMV) glycoprotein on the infected cells (Arase et al., 2002), undergo activation, a robust expansion, contraction, differentiation into a long-lived memory subset, and persistence in both lymphoid and non-lymphoid organs for several months after MCMV infection (Sun et al., 2009, 2010). These memory NK cells are capable of mounting a recall response to MCMV infection and protect the host better than naive NK cells (Sun et al., 2009). The kinetics of differentiation of Ly49H+ NK cells during MCMV infection are similar to virus-specific CD8+ T cells. NK cells and CD8+ T cells arise from a common lymphoid progenitor, express many shared cell surface antigens, secrete a similar array of cytokines, and most importantly, perform common functions including perforin and granzyme-mediated cytotoxicity (Sun et al., 2009, 2011; Sun and Lanier, 2011). Although DAP12 adaptor protein ITAM-mediated signaling through Ly49H and the pro-inflammatory cytokine interleukin-12 (IL-12) are known to be essential for optimal expansion of effector Ly49H+ NK cells and for the generation of memory Ly49H+ NK cells during MCMV infection (Sun et al., 2009, 2012), the contribution of signaling through other receptors required for the differentiation of MCMV-specific NK cells have not been defined.
DNAM-1 (DNAX accessory molecule-1, CD226) is a costimulatory molecule that is constitutively expressed by NK cells and CD8+ T cells (Shibuya et al., 1996). DNAM-1 recognizes CD155 and its related family member, CD112, as cognate ligands (Bottino et al., 2003; Tahara-Hanaoka et al., 2004), which are broadly expressed by hematopoietic, epithelial, and endothelial cells in many tissues (Aoki et al., 1997; Nabekura et al., 2010; Ravens et al., 2003). DNAM-1 has signal-transducing motifs in its cytoplasmic domain for the recruitment of the tyrosine kinases Fyn and serine-threonine kinase PKC (Shibuya et al., 1998; Shibuya et al., 1999; Shirakawa et al., 2005; Shirakawa et al., 2006). Interaction between DNAM-1 on NK cells and CD8+ T cells with its ligands on target cells triggers cell-mediated cytotoxicity and cytokine production (Bottino et al., 2003; Iguchi-Manaka et al., 2008; Shibuya et al., 1996). DNAM-1 is also involved in NK cell- and CD8+ T cell-mediated tumor rejection, as demonstrated using transplantable tumor models but also in immune surveillance of carcinogen-induced tumorigenesis (Iguchi-Manaka et al., 2008; Tahara-Hanaoka et al., 2006). Recently, DNAM-1 has been shown to co-stimulate the proliferation and interferon-γ (IFN-γ) production of alloantigen-specific CD8+ T cells (Nabekura et al., 2010). Thus, DNAM-1 plays a critical role in the functions of NK cells and CD8+ T cells for the elicitation of appropriate immune responses. Here, using the well-established model of MCMV infection (Sun et al., 2009), we investigated the role of DNAM-1 in the differentiation and function of MCMV-specific memory NK cells.
Results
DNAM-1 is expressed on immature and mature NK cells
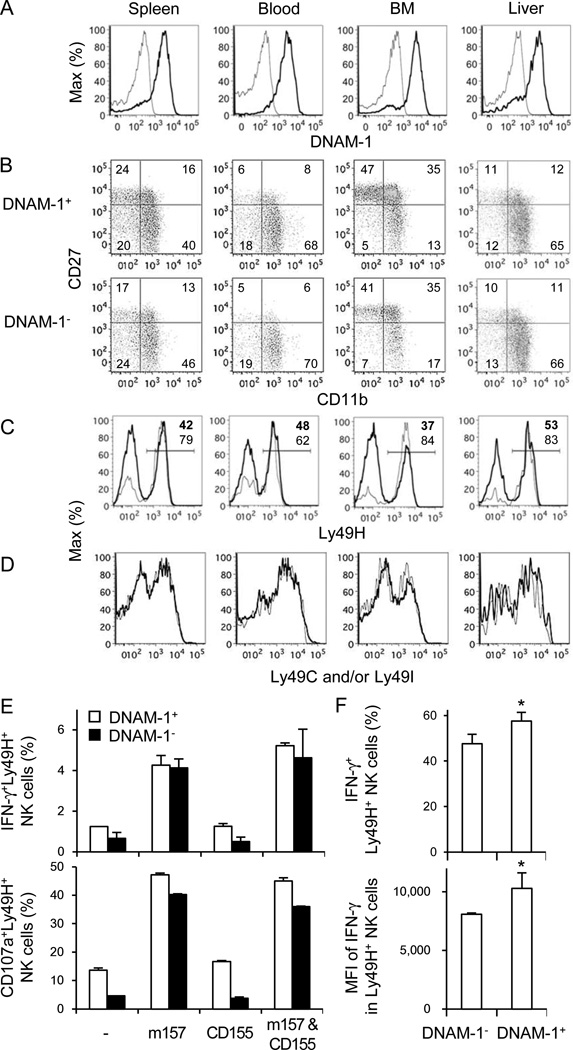
We examined whether DNAM-1 correlates with the maturation stage of NK cells or is required for NK cell-mediated effector functions in vitro. Approximately 80–90% of NK cells in the spleen, blood, bone marrow (BM), and liver of C57BL/6 mice expressed DNAM-1 (Figure 1A). Similar frequencies of immature (CD27+CD11b−), intermediate (CD27+CD11b+), and mature (CD27−CD11b+) NK cells were present within the DNAM-1+ and DNAM-1− subsets (Figure 1B). DNAM-1− and DNAM-1+ NK cells expressed equivalent amounts of IL-2 receptor β and γ subunits, IL-7Rα, and IFN-αR1 (not shown). The Ly49H receptor was expressed on a higher frequency of DNAM-1− NK cells (~60–85% Ly49H+) compared with DNAM-1+ NK cells (~40–50% Ly49H+) (Figure 1C). Within the DNAM-1− and DNAM-1+ Ly49H+ NK cell subsets the frequency of NK cells expressing Ly49C and/or Ly49I, inhibitory receptors that recognizes self H-2b ligands (marking licensed NK cells), was identical (Figure 1D).
Figure 1. Phenotype of DNAM-1+ and DNAM-1− NK cells.
(A) Expression of DNAM-1 on NK cells in the spleen, blood, BM, and liver, gating on TCRβ−NK1.1+ lymphocytes. Thin and bold lines represent staining with control Ig and anti-DNAM-1 mAb. (B) Developmental stages of DNAM-1+ and DNAM-1− NK cells as determined by co-staining with CD27 and CD11b, gating on TCRβ−NK1.1+ lymphocytes. (C) Ly49H expression on DNAM-1+ (thick lines) and DNAM-1− (thin lines) NK cells, gated on TCRβ−NK1.1+ lymphocytes. Percentages of Ly49H+ NK cells in DNAM-1+ (bold numbers) and DNAM-1− NK (thin numbers) cells are shown. (D) Expression of Ly49C and/or Ly49I on DNAM-1+ (thick lines) and DNAM-1− (thin lines) NK cells. Data are representative of 2–4 experiments. (E) IFN-γ production and degranulation of Ly49H+DNAM-1+ and Ly49H+DNAM-1− NK cells after co-culture with RMA cells expressing m157, CD155, or m157 and CD155. Data are representative of 2 independent experiments (n = 3–6 per experiment). (F) Twenty-five million WT splenocytes were transferred into DAP12-deficient mice and infected with 1 × 105 pfu MCMV. IFN-γ production by Ly49H+ NK cells was analyzed by intracellular staining on day 1.5 pi. Percentages of IFN-γ+Ly49H+ NK cells and mean fluorescent intensity (MFI) of IFN-γ of Ly49H+ NK cells within the DNAM-1+ and DNAM-1− Ly49H+ NK cell subsets are shown. Data are representative of 3 experiments (n = 3–4 mice per experiment). Error bars show s.e.m. *p <0.05.
Ly49H specifically recognizes the MCMV-encoded m157 glycoprotein (Arase et al., 2002; Smith et al., 2002). Similar frequencies of DNAM-1− and DNAM-1+ Ly49H+ degranulated and produced IFN-γ when co-cultured with RMA target cells transduced to express m157 (Figure 1E). Co-expression of CD155, a ligand of DNAM-1, on the m157+ RMA cells did not increase the frequency of Ly49H+ NK cells degranulating or producing IFN-γ. In a similar fashion we adoptively transferred wildtype (WT) Ly49H+ NK cells into syngeneic DAP12-deficient recipient mice, which lack functionally competent Ly49H+ NK cells and are unable to control early replication of MCMV (Sjölin et al., 2002; Sun et al., 2009). After infection with MCMV, both DNAM-1− and DNAM-1+ Ly49H+ NK cells produced IFN-γ on day 1.5 post-infection (pi) (Figure 1F). These findings demonstrate that DNAM-1 is not required for m157-induced degranulation or cytokine production by Ly49H+ NK cells.
DNAM-1 antibody blockade suppresses the NK cell response to MCMV
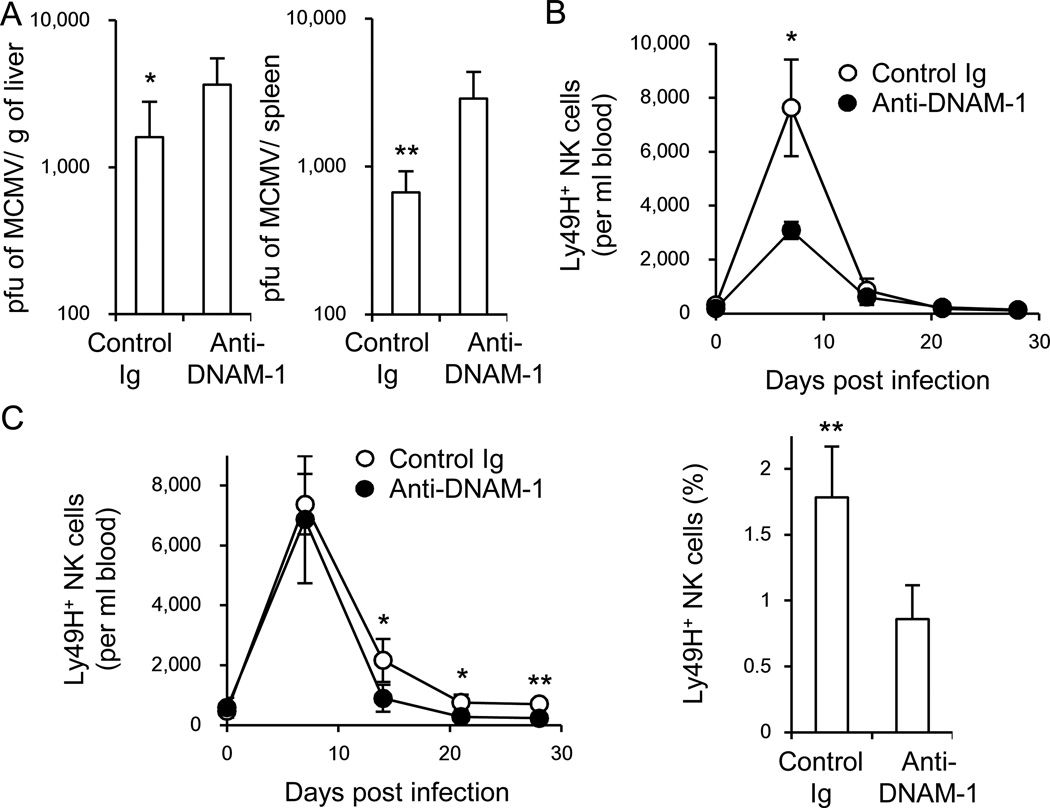
We addressed the role of DNAM-1 in the control of MCMV by treating C57BL/6 mice with a non-depleting, neutralizing anti-DNAM-1 mAb one day prior to infection and measured viral titers on day 3, when NK cells are predominantly responsible for antiviral immunity. Blocking DNAM-1 resulted in a significant increase in viral load in the spleen and liver (Figure 2A).
Figure 2. DNAM-1 antibody blockade suppresses the NK cell response to MCMV.
(A) WT mice were inoculated with control Ig or a neutralizing mAb against DNAM-1 on the day before infection with 5 × 105 pfu MCMV. Viral burden in the liver and spleen was measured on day 3 pi. (B) One hundred thousand WT Ly49H+ NK cells were transferred into DAP12-deficient mice and infected with 1 × 105 pfu MCMV. Mice were inoculated with 100 µg control Ig or anti-DNAM-1 mAb on the day before infection and day 3 pi. The absolute number of Ly49H+ NK cells per ml blood. (C) DAP12-deficient mice receiving 1 × 105 WT Ly49H+ NK cells were infected with MCMV, and treated with 100 µg control Ig or anti-DNAM-1 mAb on days 7, 14, and 21 pi. The absolute number of Ly49H+ NK cells per ml blood and percentages of Ly49H+ memory NK cells in the spleen on day 28 pi. Data were pooled from 2 experiments (n = 6 mice per mAb group). Error bars show s.e.m. *p <0.05, **p <0.01. See also Figure S1.
Ly49H+ NK cells preferentially expand after MCMV infection and are required for early control of viral replication (Brown et al., 2001; Dokun et al., 2001; Lee et al., 2001). When a limiting number of Ly49H+ NK cells are adoptively transferred into Ly49H-deficient mice and infected with MCMV, these Ly49H+ NK cells undergo extensive expansion and give rise to memory Ly49H+ NK cells (Sun et al., 2009). Monitoring of donor Ly49H+ NK cells in the blood reflects the responses in the spleen and liver, as previously demonstrated (Sun et al., 2010). Memory Ly49H+ NK cells can be operationally defined as KLRG1high, CD11b+, CD27−, Ly6Chigh Ly49H+ NK cells that persist for more than 25 days after infection with MCMV. To determine the effect of DNAM-1 blockade on the clonal expansion of Ly49H+ NK cells and generation of memory NK cells, we enriched NK cells and adoptively transferred wildtype (WT) Ly49H+ NK cells into syngeneic DAP12-deficient recipient mice (Figure S1). These mice were injected with a neutralizing anti-DNAM-1 mAb on the day before infection and on day 3 pi. Expansion of donor Ly49H+ NK cells at the peak of the NK cell response during MCMV infection was suppressed by anti-DNAM-1 antibody (Figure 2B); however, Ly49H+ NK cells were detected at day 28 in the mice treated with anti-DNAM-1 on days −1 and 3 pi. These NK cells were able to undergo a secondary response when adoptively transferred into naive Ly49H-deficient recipients and challenged with MCMV (not shown). Therefore, to address whether DNAM-1 antibody blockade affects the persistence of Ly49H+ NK cells after the peak of the response, Ly49H+ NK cells were adoptively transferred into DAP12-deficient recipients, infected with MCMV, and mice were treated with control Ig or neutralizing anti-DNAM-1 on days 7, 14, and 21 pi, and analyzed on day 28 pi. Blockade of DNAM-1 during the late phase of infection resulted in significantly fewer Ly49H+ NK cells at day 28, suggesting that sustained DNAM-1 signaling is required for establishment of an optimal pool of memory NK cells (Figure 2C).
Intrinsic lack of DNAM-1 impairs Ly49H+ NK cell response to MCMV
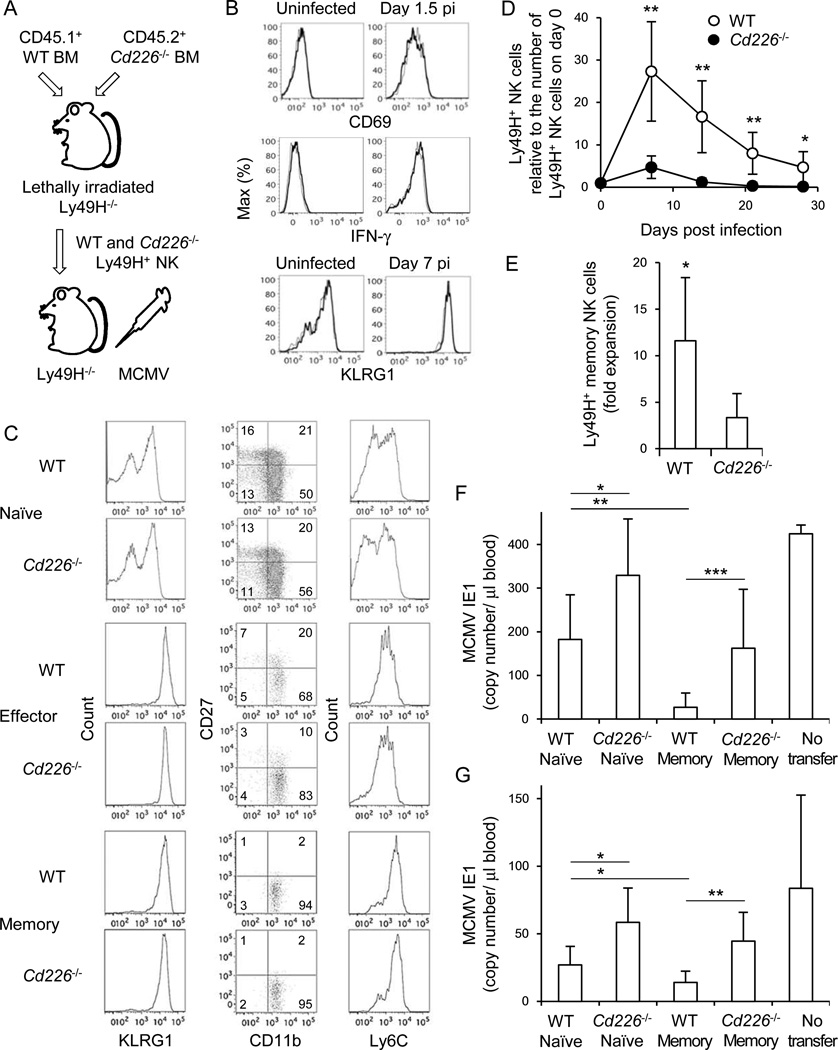
In addition to NK cells, DNAM-1 is expressed on CD8+ T cells and subsets of CD4+ T cells and dendritic cells (DCs) (Dardalhon et al., 2005; Iguchi-Manaka et al., 2008). Therefore, treatment with the blocking anti-DNAM-1 might affect the response of other immune cells during MCMV infection. To determine whether an intrinsic lack of DNAM-1 affects NK cells we reconstituted lethally irradiated recipient mice with CD45.1+ WT and CD45.2+ Cd226−/− BM cells and allowed NK cells to reconstitute in the recipient mice for 5 weeks. At this time, an equivalent frequency of mature WT and Cd226−/− NK cells was detected in the blood and spleen of the chimeric mice, consistent with prior studies documenting normal development and numbers of NK cells in Cd226−/− mice (Gilfillan et al., 2008). Ly49H+ NK cells were isolated from the chimeric mice, adoptively transferred into Ly49H-deficient mice, and then infected with MCMV (Figure 3A). During the early phase of the response to MCMV infection, Cd226−/− Ly49H+ NK cells were activated comparably to WT Ly49H+ NK cells, as reflected by up-regulation of Sca-1, CD69, and KLRG1, and WT and Cd226−/− Ly49H+ NK cells produced identical amounts of IFN-γ on day 1.5 pi (Figure 3B and data not shown). Ly49H+ effector and memory NK cells highly expressed KLRG1 and predominantly displayed a mature phenotype (CD11b+, CD27−, and Ly6Chigh) on day 7 and day 25 pi, respectively (Figure 3C). However, Cd226−/− Ly49H+ NK cells showed severe defects in expansion of effector NK cells, as well as in the generation of long-lived NK cells (Figure 3D and Figure S2). Cd226−/− Ly49H+ NK cells isolated from the MCMV-infected mice and adoptively transferred into naive Ly49H-deficient recipient mice expanded poorly when re-challenge with MCMV (Figure 3E). Moreover, when Cd226−/− Ly49H+ memory NK cells were adoptively transferred into a naive Ly49H-deficient or DAP12-deficient host they showed an impaired protective effect against MCMV challenge on days 3 pi and 7 pi (Figures 3F and 3G; Figure S3), although MCMV-specific T cell responses are mounted and decrease the viral burden on day 7 pi (Schlub et al., 2011). These results demonstrate that DNAM-1 is essential for the optimal differentiation of effector and memory Ly49H+ NK cells during MCMV infection, but is dispensable for the initial activation of NK cells.
Figure 3. DNAM-1 is required for expansion of effector NK cells and differentiation of memory NK cells during MCMV infection.
(A) NK cells were purified from WT and Cd226−/− mixed BM chimeric mice. One hundred fifty thousand WT and Cd226−/− Ly49H+ NK cells were transferred into Ly49H-deficient mice and infected with 1 × 105 pfu MCMV. (B) Activation markers CD69 and KLRG1 on Ly49H+ NK cells at day 1.5 and 7 pi, respectively, and IFN-γ production on day 1.5 pi. Thin and bold lines represent Cd226−/− Ly49H+ and WT Ly49H+ NK cells. (C) Phenotype of WT and Cd226−/− naïve, effector (day 7 pi), and memory (day 25 pi) NK cells. Expression of KLRG1, CD11b, CD27, and Ly6C on Ly49H+ NK cells is shown. (D) The kinetics of the absolute number of Ly49H+ NK cells in blood were represented as the ratio relative to the number of Ly49H+ NK cells in the blood on day 0 (before infection). Data are representative of 3 experiments (n = 6–7 mice). (E) Memory NK cells were isolated from recipient mice 30 days after primary infection, transferred into naïve Ly49H-deficient mice, and infected with MCMV. Secondary expansion of Ly49H+memory NK cells in the spleen on day 7 after infection with MCMV was represented as the fold expansion relative to the number of memory NK cells detected in the spleen of mice adoptively transferred with an aliquot of Ly49H+ memory NK cells but not infected with MCMV. Data are pooled from 2 experiments (n = 6 mice). (F and G) WT and Cd226−/− memory NK cells were purified from recipient mice 25–28 days after primary infection, and WT and Cd226−/− naïve NK cells were purified from BM chimeric mice. Ten thousand WT and Cd226−/− naïve and memory Ly49H+ NK cells were transferred separately into Ly49H-deficient or DAP12-deficient mice and infected with MCMV. The copy number of MCMV IE1 gene in blood on day 3 pi (F) and day 7 pi (G) was analyzed by quantitative PCR. Data were pooled from 2 experiments (n = 6–8 mice per group). *p <0.05, **p <0.01, and ***p <0.005. Error bars show s.e.m. See also Figure S2 and S3.
DNAM-1 signaling is mediated by Fyn and PKCη
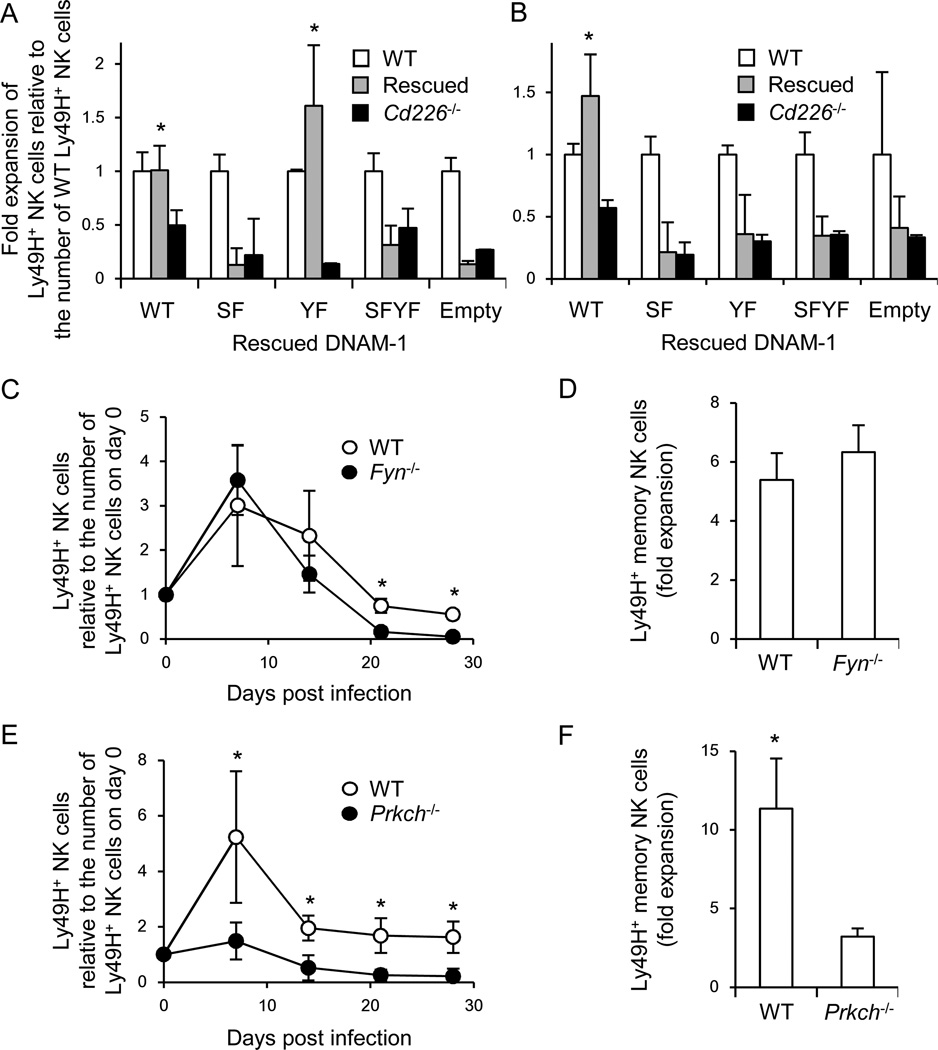
Previous studies have demonstrated that signaling through DNAM-1 is mediated by Fyn and PKC (Shibuya et al., 1999; Shirakawa et al., 2005), although which PKC isozymes are responsible had not been identified. We speculated that PKCη might be responsible for DNAM-1-dependent PKC signaling. This possibility is based on prior studies where phosphorylation of the cytoplasmic motifs of DNAM-1 was assessed in the presence and absence of PKC (Shibuya et al., 1998; Shirakawa et al., 2005), kinome analysis after cross-linking of DNAM-1 (Konig et al., 2012), transcriptional profiling of PKC isozymes in mouse and human NK cells (http://www.immgen.org/), and analysis of PKCθ-deficient (Prkcq−/−) mice after MCMV infection (Tassi et al., 2008). To address whether Fyn and PKC are directly involved in downstream signaling of DNAM-1, Cd226−/− hematopoietic progenitor cells (HPCs) were transduced with retroviral vectors encoding WT DNAM-1, a S326F mutant in a PKC-binding motif, a Y319F mutant in a Fyn-binding site, or a SFYF mutant with both mutations. Lethally irradiated recipient mice were reconstituted with the Cd226−/− BM cells transduced with WT or mutant DNAM-1 (hereafter referred to as “DNAM-1 rescued”) mixed with WT BM cells. WT DNAM-1-rescued and Y319F (Fyn-binding) mutant DNAM-1-rescued Cd226−/− Ly49H+ NK cells expanded similarly to WT Ly49H+ NK cells in Ly49H-deficient recipient mice after MCMV infection (Figure 4A). By contrast, the S326F (PKCη-binding) and SFYF double mutant DNAM-1-rescued Cd226−/− Ly49H+ NK cells showed a lower magnitude of initial response compared to WT DNAM-1-rescued Ly49H+ NK cells (Figure 4A). On the other hand, Cd226−/− Ly49H+ NK cells expressing any of the three mutant forms of DNAM-1 failed to efficiently differentiate into long-lived NK cells (Figure 4B).
Figure 4. DNAM-1 signaling is required for optimal differentiation of Ly49H+ NK cells during MCMV infection.
(A and B) NK cells were purified from BM chimeric mice reconstituted with WT, Cd226−/−, and DNAM-1-rescued Cd226−/− HPCs. Ly49H+ NK cells were isolated from chimeric mice and transferred into Ly49H-deficient mice, and then infected with 1 × 105 pfu MCMV. The number of Cd226−/− and DNAM-1-rescued Ly49H+ effector NK cells on day 7 pi (A) and memory NK cells on day 25 pi (B) in the spleen was represented as the fold expansion relative to the number of WT Ly49H+ NK cells on days 7 and 25 pi in the spleen. Data are pooled from 2 experiments (n = 4 mice per rescued construct). *p <0.05. (C-F) NK cells were purified from BM chimeric mice of WT and Fyn−/− mice, or WT and Prkch−/− mice. WT and Fyn−/− Ly49H+ NK cells (4 × 105 cells), or WT and Prkch−/−Ly49H+ NK cells (1 × 105 cells) were transferred into Ly49H-deficient mice and infected with MCMV. (C and E) The kinetics of the absolute number of Ly49H+ NK cells in blood were represented as the ratio relative to the number of Ly49H+ NK cells in blood on day 0 (before infection). (D and F) Memory NK cells were transferred into naïve Ly49H-deficient mice and infected with MCMV. Secondary expansion of Ly49H+ memory NK cells in the spleen on day 7 after infection with MCMV was represented as the fold expansion relative to the number of memory NK cells detected in the spleen of mice adoptively transferred with an aliquot of Ly49H+ memory NK cells but not infected with MCMV. Data are representative of 2–3 experiments (n = 3–5). Error bars show s.e.m. *p <0.05. See also Figure S4.
To confirm the roles of Fyn and PKCη in the differentiation of Ly49H+ NK cells during MCMV infection, we produced mixed BM chimeras of WT and Fyn-deficient (Fyn−/−) cells, or WT and PKCη-deficient (Prkch−/−) cells, so that WT and Fyn−/− or Prkch−/− NK cells would develop in the same environment. After reconstitution, Ly49H+ NK cells sufficient or deficient in Fyn or PKCη were purified from mixed BM chimeric mice, transferred into Ly49H-deficient mice, and infected with MCMV. Fyn-deficient Ly49H+ NK cells proliferated equivalently to WT Ly49H+ NK cells during the first week post-infection, but differentiated poorly into a memory subset (Figure 4C and Figure S4A); however, Fyn-deficient memory NK cells did expand when adoptively transferred into naïve Ly49H-deficient recipient mice and re-challenged with MCMV (Figure 4D). In contrast, PKCη-deficient Ly49H+ NK cells showed a dampened initial response, and impaired differentiation into long-lived NK cells with a defective secondary MCMV-induced expansion of these memory NK cells when transferred into naïve Ly49H-deficient recipients (Figures 4E and 4F and Figures S4B). The results with the DNAM-1-rescued donor Cd226−/− Ly49H+ NK cells are consistent with the phenotypes of Ly49H+ NK cells deficient in Fyn and PKCη, and strongly suggest that the DNAM-1-Fyn signaling axis is required for the efficient generation of memory NK cells but is dispensable for expansion of both effector NK cells and memory NK cells, whereas DNAM-1-dependent PKCη signaling is required for both the expansion of effector NK cells and memory NK cell generation.
DNAM-1 on Ly49H+ NK cells is dynamically regulated during MCMV infection
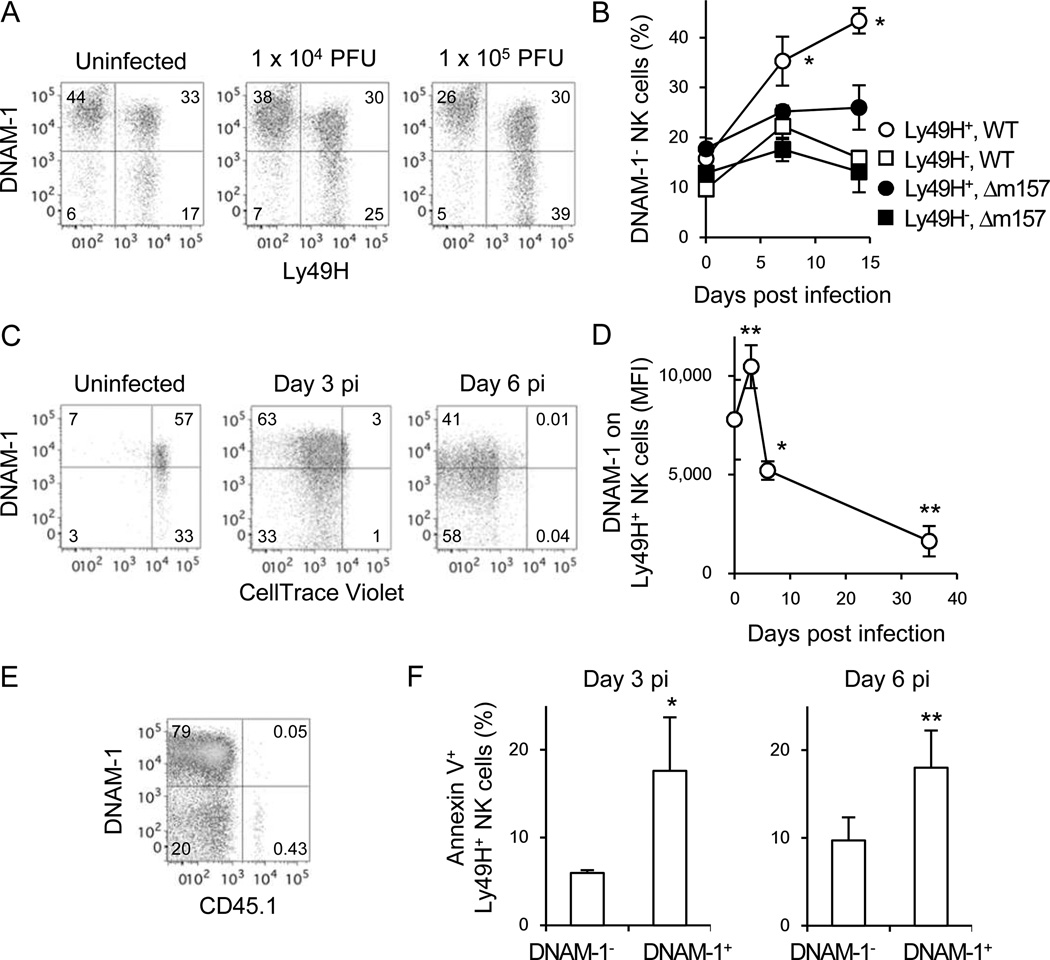
In WT mice, the frequency of Ly49H+DNAM-1− NK cells increased after MCMV infection in a virus dose-dependent manner (Figure 5A). The down-modulation of DNAM-1 on DNAM-1+ NK cells or expansion of DNAM-1− NK cells was observed only on Ly49H+ NK cells after infection with MCMV, but was not down-modulated on Ly49H− NK cells (Figure 5A). Moreover, the loss of DNAM-1 was not observed when mice were infected with a MCMV mutant strain lacking m157 (Figure 5B).
Figure 5. DNAM-1 on Ly49H+ NK cells is modulated during MCMV infection.
(A) DNAM-1 on Ly49H+ NK cells in the spleen of mice infected with MCMV on day 7 pi. Data are representative of 2 experiments (n =2–3 in each group). (B) Percentages of DNAM-1-negative NK cells within the Ly49H+ and Ly49H− NK cell subsets in the blood of mice infected with 5 × 105 pfu WT or Δm157 MCMV. *p <0.05 vs. the other 3 groups. (C) Twenty-five million CellTrace Violet-labeled splenocytes were transferred into DAP12-deficient mice and infected with 1 × 105 pfu MCMV. Expression of DNAM-1 on Ly49H+ NK cells in the spleen was analyzed on days 3 and 6 pi. Data are representative of 3 experiments (n = 3–4 mice per time point) (B and C). (D and E) One or two hundred thousand CD45.1+Ly49H+ NK cells were transferred into Ly49H-deficient mice and infected with MCMV. (D) MFI of DNAM-1 on Ly49H+ NK cells in the spleen. Data were pooled from 6 experiments (n = 3–5 mice per time point). *p <0.05, **p <0.01 vs. day 0. (E) DNAM-1 on CD45.1+Ly49H+ memory NK cells in the spleen on day 35 pi. Data are representative of more than 5 experiments. (F) Twenty-five million WT splenocytes were transferred into DAP12-deficient mice and infected with MCMV. Cell death of Ly49H+ NK cells was analyzed by Annexin V staining on days 3 and 6 pi. Data are representative of 3 experiments (n = 3–4 mice per experiment). *p <0.05, **p <0.01 vs. DNAM-1-. Error bars show s.e.m. See also Figure S5.
To determine whether the increase of Ly49H+DNAM-1− NK cells is caused by preferential expansion of Ly49H+DNAM-1− NK cells, WT NK cells labeled with the CellTrace Violet dye were adoptively transferred into Ly49H-deficient mice. The transferred NK cells did not undergo homoeostatic proliferation in the recipient in the absence of infection because the Ly49H-deficient mice express normal numbers of endogenous NK cells (data not shown). The adoptively transferred DNAM-1− and DNAM-1+ Ly49H+ NK cells divided equivalently early during MCMV infection (Figure 5C). We observed that the expression of DNAM-1 on Ly49H+ NK cells was transiently up-regulated after infection, but thereafter DNAM-1-negative or low Ly49H+ NK cells accumulated during the course of infection (Figure 5D), consequently most memory NK cells were DNAM-1-negative (Figure 5E). Interestingly, a higher frequency of DNAM-1+ Ly49H+ NK cells stained for Annexin V at days 3 and 6 pi compared with DNAM-1−Ly49H+ NK cells, suggesting that the predominance of DNAM-1− Ly49H+ NK cells might be due to preferential survival of DNAM-1−NK cells (Figure 5F; Figure S5).
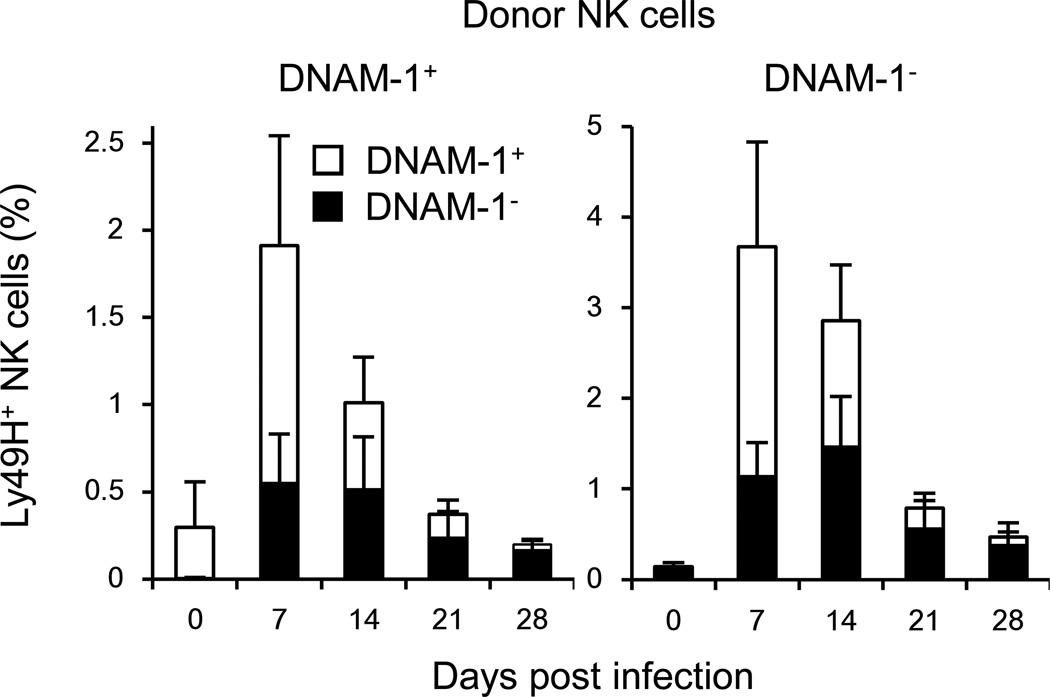
When naive DNAM-1− and DNAM-1+ NK cells were purified and transferred into Ly49H-deficient hosts and infected with MCMV, the sorted DNAM-1+ NK cells progressively lost expression of DNAM-1 (Figure 6). The sorted DNAM-1− NK cell population transiently up-regulated DNAM-1, but over the course of infection DNAM-1− Ly49H+ NK cells accumulated and predominated at day 28 pi (Figure 6). Similarly, when memory DNAM-1− Ly49H+ NK cells were isolated and adoptively transferred into a Ly49H-deficient naive host and re-challenged with MCMV, there was transient up-regulation of DNAM-1 (data not shown). These results indicate that DNAM-1 on Ly49H+ NK cells is dynamically regulated during MCMV infection.
Figure 6. DNAM-1− and DNAM-1+ NK cells expand and differentiate into memory NK cells during MCMV infection.
One hundred thousand purified Ly49H+DNAM-1+ or Ly49H+DNAM-1− NK cells were transferred separately into DAP12-deficient mice and infected with 1 × 105 MCMV. Percentages of Ly49H+DNAM-1+ and Ly49H+DNAM-1− NK cells in blood are shown. Data are representative of 3 experiments (n = 4–5 mice). Error bars show s.e.m.
DNAM-1 ligands are up-regulated after MCMV infection in vivo
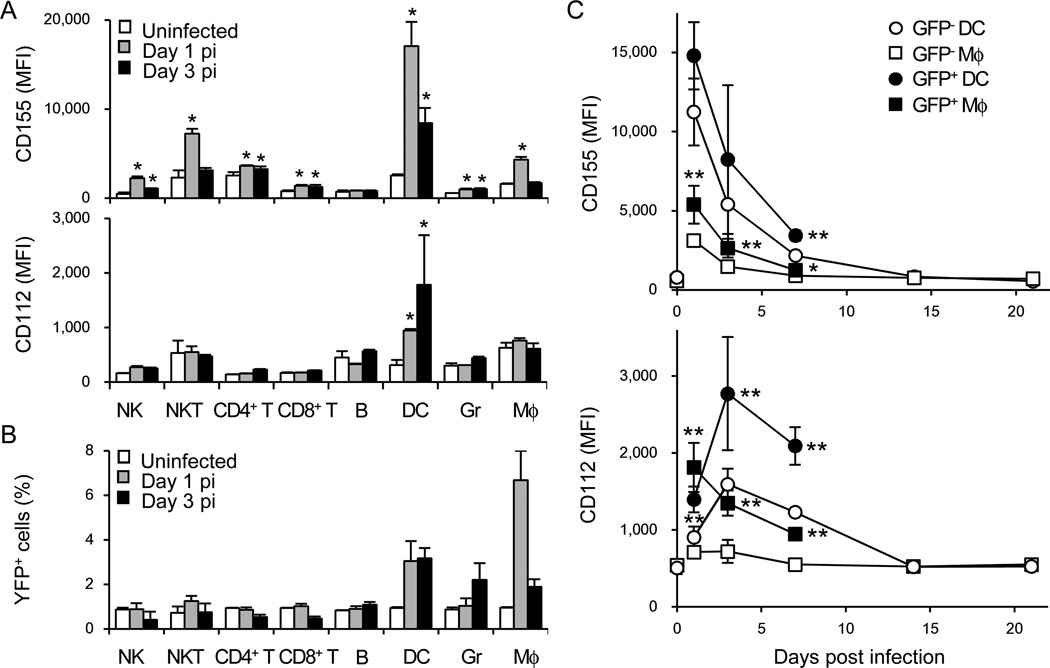
We investigated whether the DNAM-1 ligands are modulated in the host during MCMV infection. Although HCMV down-regulates DNAM-1 ligands on human cells infected in vitro (Prod'homme et al., 2010; Tomasec et al., 2005), changes in DNAM-1 ligand expression in vivo after MCMV infection had not been determined. CD155 and CD112 were rapidly up-regulated on splenic DCs and macrophages after MCMV infection (Figure 7A). Infection of floxed YFP reporter mice with MCMV encoding Cre demonstrated that a small percentage of DCs and macrophages was positive for YFP on days 1 and 3 pi (Figure 7B), consistent with a previous study using a MCMV encoding GFP (Hsu et al., 2009). Furthermore, infection with GFP-MCMV revealed that infected DC and macrophages highly expressed DNAM-1 ligands as compared with non-infected cells (Figure 7C). These results suggest that DNAM-1 ligands are up-regulated on DCs and macrophages during MCMV infection, allowing DNAM-1+ Ly49H+ NK cells to engage DNAM-1 ligand-expressing cells in lymphoid tissues. Collectively, our findings reveal that DNAM-1 signaling through Fyn and PKCη works in conjunction with the m157-specific Ly49H receptor to enhance the immune response against MCMV and generate long-lived memory NK cells.
Figure 7. DNAM-1 ligands are upregulated after MCMV infection in vivo.
(A) WT mice were infected with 1 × 106 pfu MCMV. Expression of CD155 and CD112 on hematopoietic cells in the spleen of infected mice on days 1 and 3 pi was analyzed by flow cytometry. NK cells (TCRβ−NK1.1+), NKT cells (TCRβ+NK1.1+), CD4+ T cells (TCRβ+CD4+), CD8+ T cells (TCRβ+CD8α+), B cells (CD11b−B220+), DCs (CD11chighI-AE+), granulocytes (Gr; CD11b+Gr-1high), macrophages (Mφ; CD11b+Gr-1low) were assessed. (B) Cre-dependent YFP reporter mice were infected with 2 × 104 pfu Cre-MCMV. YFP+ hematopoietic cells in the spleen of infected mice on days 1 and 3 pi were analyzed. Data are representative of 2 experiments (n = 3 mice per time point) (A and B). *p <0.05 vs. uninfected. (C) WT mice were infected with 1 × 106 pfu GFP-MCMV. Expression of CD155 and CD112 on GFP− and GFP+ DCs and macrophages in the spleen was analyzed. Data are pooled from 3 experiments (n = 5–6 mice per time point). *p <0.05, **p <0.01 vs. GFP-. Error bars show s.e.m
Discussion
Here, we have demonstrated that DNAM-1 is essential for the optimal function and differentiation of Ly49H+ NK cells during MCMV infection. Although the precise molecular mechanisms underlying adaptive immune features of MCMV-specific NK cells remain mostly unknown, we have determined that signaling through DNAM-1 is one of the critical factors for the initial expansion of effector Ly49H+ NK cells, as well as the generation and secondary expansion of memory Ly49H+ NK cells.
The expansion and generation of memory Ly49H+ NK cells induced by DAP12-mediated, immunoreceptor tyrosine-based activation motif (ITAM)-dependent signaling and the kinetics of the Ly49H+ NK cell response are quite similar to the adaptive immune features of virus-specific CD8+ T cells (Sun et al., 2009). We have identified Fyn and PKCη as downstream components of the DNAM-1 transmitted signals necessary for the differentiation of Ly49H+ NK cells during MCMV infection. Remarkably, these signaling molecules are differentially required at distinct stages of NK cell differentiation. DNAM-1-induced Fyn signaling contributed to the efficient generation of memory NK cells, but was dispensable for the initial proliferation of NK cells as well as the secondary expansion of memory NK cells, whereas the DNAM-1-PKCη signaling axis was essential for both expansion of effector NK cells and the efficient generation of memory NK cells. In T cells, Fyn and Lck are required for optimal T cell receptor (TCR)-mediated signaling (Salmond et al., 2009; Stein et al., 1992). Fyn-deficient T cells demonstrate a reduced calcium influx and proliferative responses, but normal Akt kinase phosphorylation, after engagement of their TCRs because of the functional redundancy of Lck (Salmond et al., 2009). In contrast to T cells, the role of Src family kinases in NK cells is still not understood well. In mouse NK cells, Fyn and Lck are physically and functionally associated with the DAP12 and Ly49D signaling complex (Mason et al., 2006), another activating receptor similar to Ly49H (Smith et al., 1998). Although Fyn-deficient mice display alternation in their Ly49 repertoire (Lowin-Kropf et al., 2002), Fyn-deficient Ly49D+ NK cells efficiently lyse YAC-1 and CHO target cells and secrete IFN-γ comparably to WT Ly49D+ NK cells (Mason et al., 2006). Fyn is capable of phosphorylating DAP12, and Ly49D stimulation reciprocally promotes phosphorylation of Fyn (Mason et al., 2006). Because an activation signal through DNAM-1 is mediated by Fyn but not Lck (Shibuya et al., 1999), activation of Fyn through DNAM-1 might contribute to enhanced Ly49H-induced phosphorylation of DAP12, leading to optimal signaling for the differentiation of memory Ly49H+ NK cells. Unlike Ly49H+ NK cells deficient in PKCη and DNAM-1, Fyn-deficient Ly49H+ NK cells mount a normal proliferation responses to MCMV, similar to the intact, albeit reduced, proliferation of Fyn-deficient T cells responding to antigenic challenge (Stein et al., 1992).
DNAM-1 co-stimulates the proliferation of CD8+ T cells (Nabekura et al., 2010) and DNAM-1-induced activation is partly mediated by PKC (Shibuya et al., 1998; Shirakawa et al., 2005). Fu et al. demonstrated a pivotal role of PKCη in the proliferation, calcium influx, and NF-κB signaling in T cells, and PKCη and PKCθ share functional redundancies in T cells (Fu et al., 2011). By contrast, PKCθ in NK cells is dispensable for cytotoxicity and control of early MCMV infection (Tassi et al., 2008). Although our findings do not exclude the possibility that other PKC isozymes participate in NK cell activation, our results clearly demonstrate a critical role of PKCη in the adaptive immune features of NK cells responding to MCMV infection. With regard to the virus-specific expansion of effector NK cells, the phenotypes of Ly49H+ NK cells deficient in DNAM-1, Fyn, and PKCη during MCMV infection are shared with the antigen-specific proliferation of T cells deficient in these molecules (Fu et al., 2011; Nabekura et al., 2010; Stein et al., 1992). However, the role of DNAM-1 in the differentiation of memory NK cells and T cells has not been appreciated previously. Previous studies have reported that a deficiency of Fyn has no significant impact on survival of naïve and memory T cells (Seddon and Zamoyska, 2002), and PKCη-deficient T cells exhibit identical sensitivity to cell death as WT T cells (Fu et al., 2011). Thus, NK cells and T cells may possess unique cell-specific molecular requirements for the generation and persistence of memory cells.
An optimal differentiation of MCMV-specific NK cells is governed not only by the distinct downstream signaling components of DNAM-1 but also by the temporal regulation of DNAM-1 signaling during the infection. Because DNAM-1 ligands are constitutively expressed by hematopoietic and epithelial cells (Aoki et al., 1997; Nabekura et al., 2010; Ravens et al., 2003), up-regulation of either DNAM-1 or its ligands might enable Ly49H+ NK cells bearing DNAM-1 to receive activating signal through DNAM-1 in addition to Ly49H during MCMV infection. Infection of human cells with HCMV in vitro results in the down-regulation of DNAM-1 ligands (Prod'homme et al., 2010; Tomasec et al., 2005). In contrast, DNAM-1 ligands on DCs and macrophages are strongly up-regulated by signaling through TLR3 and TLR9 (Kamran et al., 2013; Pende et al., 2006), which are essential for innate immune defense against MCMV infection (Tabeta et al., 2004). Our results demonstrate that DNAM-1 ligands are rapidly up-regulated on DCs in MCMV-infected mice, while DNAM-1 on Ly49H+ NK cells is temporally up-regulated at the same timing. One possible scenario is that the kinetics of expression of DNAM-1 enable Ly49H+ NK cells to receive adequate DNAM-1 signaling in the context of up-regulated DNAM-1 ligands on infected DCs during MCMV infection, consequently allowing Ly49H+ NK cells to control viral burden in the early phase, as well as to receive sustained DNAM-1 signaling for optimal expansion and differentiation of memory NK cells. Our findings indicate that cooperative signaling through multiple activating receptors regulates the adaptive immune features of NK cells. Further studies of the signaling adaptor molecules, cytokines, transcriptional factors, and activating and inhibitory receptors to more deeply understand the molecular mechanisms underlying the differentiation of NK cells will pave the way for the development of new NK cell-based vaccines and therapies against infectious diseases and malignancies.
Experimental procedures
Mice and MCMV
C57BL/6 and congenic CD45.1+ mice were purchased from the National Cancer Institute. DNAM-1-deficient (Cd226−/−) mice (Iguchi-Manaka et al., 2008) on a C57BL/6 background were bred and maintained at University of Tsukuba in accordance with the guidelines of the Institutional Review Committee of University of Tsukuba. Congenic CD45.1+, Fyn (Fyn−/−)-deficient (Stein et al., 1992), Ly49H-deficient (Fodil-Cornu et al., 2008), DAP12 (Tyrobp−/−)-deficient (Bakker et al., 2000), flox-stop-flox ROSA26-YFP, Cre-dependent YFP reporter mice (Srinivas et al., 2001), and PKCh (Prkch−/−)-deficient mice (Fu et al., 2011) on a C57BL/6 background were maintained at the University of California, San Francisco and The Scripps Research Institute in accordance with the guidelines of the Institutional Animal Care and Use Committee. Mixed BM chimeric mice were generated as described previously (Sun et al., 2009). Smith strain WT MCMV, Δm157 MCMV, and GFP-MCMV were prepared in C57BL/6 3T3 cells, and Cre-MCMV was prepared in BALB/c mice as described previously (Bubić et al., 2004; Cicin-Sain et al., 2005; Henry et al., 2000). Mice were infected by intraperitoneal injection of 1 × 105, 5 × 105, and 1 × 106 plaque-forming units (pfu) WT MCMV, Δm157 MCMV, GFP-MCMV, respectively, or 2 × 104 pfu Cre-MCMV. In some experiments, mice were inoculated intraperitoneally with 100 mg of TX42.1, a non-depleting, neutralizing rat mAb against mouse DNAM-1 (Nabekura et al., 2010), or an isotype-matched rat IgG2a on the day before infection and day 3 pi, or days 7, 14, and 21 pi.
NK cell enrichment and adoptive transfer
NK cells were enriched by incubating splenocytes with purified rat mAbs against mouse CD4, CD5, CD8, CD19, Gr-1, and Ter119, followed by anti-rat IgG antibodies conjugated to magnetic beads (Qiagen). NK cells were enriched from BM chimeric mice 5 weeks or later post-BM transplantation. DNAM-1+ and DNAM-1− NK cells, or WT and Cd226−/− NK cells were purified by using a FACSAria III (BD Biosciences). One to four hundred thousand Ly49H+ NK cells were injected intravenously into syngeneic Ly49H-deficient or DAP12-deficient recipient mice on the day before MCMV infection. 2.5 × 107 splenocytes were labeled with 10 mM CellTrace Violet according to the manufacturer’s instructions (Invitrogen) before intravenous injection of adoptively transferred NK cells.
Transplantation of transduced HPCs
Retroviral vectors MCSV2.2-IRES-GFP encoding WT DNAM-1, DNAM-1 mutated at a PKC-binding site (S326F mutant), DNAM-1 mutated at a Fyn-binding site (Y319F mutant), and SFYF mutant DNAM-1 with both mutations (Shirakawa et al., 2006) were prepared by calcium phosphate co-transfection of these retroviral constructs and expression vectors encoding MuLV gag-pol and vesicular stomatitis virus G protein into 293T cells according to the manufacturer’s instructions (Promega). Retroviruses were concentrated by overnight centrifugation and viral titers were determined by infection of 293T cells. BM cells were isolated from Cd226−/− mice, and HPCs were enriched by Ficoll-Paque gradient centrifugation of BM cells, and then incubated with purified rat mAbs against mouse CD4, CD5, CD8, CD11b, CD19, Gr-1, Ter119, and NKp46, followed by anti-rat IgG antibodies conjugated to magnetic beads (Qiagen). HPCs were cultured in StemPro34 (Invitrogen) in the presence of 10 ng/ml mouse IL-3, 30 ng/ml human IL-6, and 50 ng/ml mouse stem cell factor (PeproTech) on Retronectin-coated culture plates (Clontech). HPCs were transduced with retroviruses on days 1 and 2 (Nabekura et al., 2006). One to ten thousand transduced Cd226−/− HPCs were mixed with CD45.1+CD45.2+ WT HPCs, and transplanted into lethally irradiated recipient mice (550 rad × 2, 4 h interval) on day 2.5.
Flow cytometry
Fc receptors were blocked with 2.4G2 mAb before surface or intracellular staining with the indicated mAbs or isotype-matched control antibodies (BD Biosciences, eBioscience, or BioLegend). For measuring apoptosis, freshly isolated splenocytes were stained with antibodies against cell surface molecules and APC-conjugated Annexin V (BD Biosciences) according to the manufacturer’s protocol. Samples were acquired on an LSRII or a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Measurement of MCMV load
C57BL/6 mice were inoculated intraperitoneally with 100 µg of TX42.1, a neutralizing mAb against DNAM-1, or an isotype-matched rat IgG2a on the day before infection, followed by infection with 5 × 105 pfu MCMV. On day 3 pi, one lobe of each liver and spleen were homogenized in DMEM. Cell homogenates were plated on C57BL/6 3T3 cells in ten-fold serial dilutions and incubated for 2 h at 37° C. DMEM with 10% FCS and 0.75% carboxymethyl cellulose was added and samples were incubated for 9 days. Plaques were counted under a light microscopy. Viral titers in the spleen were calculated for the whole organ and titers in the liver were adjusted for weight of the tissue. The copy number of MCMV IE1 gene in DNA prepared from peripheral blood on days 3 and 7 pi was determined by quantitative PCR analysis using a SYBR green master mix reagent (Invitrogen) and the following primer pair; MCMV IE1 Forward: 5'-AGCCACCAACATTGACCACGCAC-3', MCMV IE1 Reverse: 5'-GCCCCAACCAGGACACACAACTC-3'. A plasmid encoding MCMV IE1 was used for the standard curve.
Ex vivo stimulation of NK cells
One million splenocytes were co-cultured with 1 × 105 RMA transfectants expressing m157, CD155, or m157 and CD155 for 6 h at 37°C in the presence of PE-conjugated anti-CD107a mAb, GolgiStop, and GolgiPlug (BD Biosciences), followed by staining for surface molecules and intracellular IFN-γ.
Statistical methods
Student’s t-test was used to compare results. The Mann–Whitney U test was used to compare groups in a PCR-based viral titer assay. p <0.05 was considered statistically significant.
Supplementary Material
Highlights.
DNAM-1 is required for the expansion and generation of memory NK cells
Fyn and PKCη play distinct role in DNAM-1 signaling in Ly49H+ NK
DNAM-1 is dynamically regulated on NK cells during MCMV infection
MCMV infection up-regulates DNAM-1 ligands on dendritic cells and macrophages
Acknowledgments
We thank the Lanier laboratory for comments and discussions. We are grateful to Dr. Arthur Weiss for Fyn-deficient mice. The work was supported by National Institutes of Health grants AI068129 to L.L.L. and GM065230 to N.R.J.G. L.L.L. is an American Cancer Society Professor and the Uehara Memorial Foundation, the Natio Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and Japan Society for the Promotion of Science support T.N..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- Bubić I, Wagner M, Krmpotić A, Saulig T, Kim S, Yokoyama WM, Jonjić S, Koszinowski UH. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol. 2004;78:7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L, Podlech J, Messerle M, Reddehase MJ, Koszinowski UH. Frequent coinfection of cells explains functional in vivo complementation between cytomegalovirus variants in the multiply infected host. J Virol. 2005;79:9492–9502. doi: 10.1128/JVI.79.15.9492-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, Sabatos CA, Ahuja R, Nguyen K, Freeman GJ, Greenfield EA, et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol. 2005;175:1558–1565. doi: 10.4049/jimmunol.175.3.1558. [DOI] [PubMed] [Google Scholar]
- Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- Fodil-Cornu N, Lee SH, Belanger S, Makrigiannis AP, Biron CA, Buller RM, Vidal SM. Ly49H-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012a;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012b;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Hu J, Niederberger-Magnenat N, Rybakin V, Casas J, Yachi PP, Feldstein S, Ma B, Hoerter JA, Ampudia J, et al. Protein kinase C η is required for T cell activation and homeostatic proliferation. Sci Signal. 2011;4:ra84. doi: 10.1126/scisignal.2002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SC, Schmader K, Brown TT, Miller SE, Howell DN, Daley GG, Hamilton JD. Enhanced green fluorescent protein as a marker for localizing murine cytomegalovirus in acute and latent infection. J Virol Methods. 2000;89:61–73. doi: 10.1016/s0166-0934(00)00202-0. [DOI] [PubMed] [Google Scholar]
- Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama WM. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol. 2009;90:33–43. doi: 10.1099/vir.0.006668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205:2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran N, Takai Y, Miyoshi J, Biswas SK, Wong JS, Gasser S. Toll-like receptor ligands induce expression of the costimulatory molecule CD155 on antigen-presenting cells. PLoS One. 2013;8:e54406. doi: 10.1371/journal.pone.0054406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig S, Nimtz M, Scheiter M, Ljunggren HG, Bryceson YT, Jansch L. Kinome analysis of receptor-induced phosphorylation in human natural killer cells. PLoS One. 2012;7:e29672. doi: 10.1371/journal.pone.0029672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lee SH, Girard S, Macina D, Busà M, Zafer A, Belouchi A, Gros P, Vidal SM. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin-Kropf B, Kunz B, Schneider P, Held W. A role for the src family kinase Fyn in NK cell activation and the formation of the repertoire of Ly49 receptors. Eur J Immunol. 2002;32:773–782. doi: 10.1002/1521-4141(200203)32:3<773::AID-IMMU773>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mason LH, Willette-Brown J, Taylor LS, McVicar DW. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006;176:6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, Otsu M, Nagasawa T, Nakauchi H, Onodera M. Potent vaccine therapy with dendritic cells genetically modified by the gene-silencing-resistant retroviral vector GCDNsap. Mol Ther. 2006;13:301–309. doi: 10.1016/j.ymthe.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Nabekura T, Shibuya K, Takenaka E, Kai H, Shibata K, Yamashita Y, Harada K, Tahara-Hanaoka S, Honda S, Shibuya A. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc Natl Acad Sci U S A. 2010;107:18593–18598. doi: 10.1073/pnas.1005582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- Prod'homme V, Sugrue DM, Stanton RJ, Nomoto A, Davies J, Rickards CR, Cochrane D, Moore M, Wilkinson GW, Tomasec P. Human cytomegalovirus UL141 promotes efficient downregulation of the natural killer cell activating ligand CD112. J Gen Virol. 2010;91:2034–2039. doi: 10.1099/vir.0.021931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens I, Seth S, Förster R, Bernhardt G. Characterization and identification of Tage4 as the murine orthologue of human poliovirus receptor/CD155. Biochem Biophys Res Commun. 2003;312:1364–1371. doi: 10.1016/j.bbrc.2003.11.067. [DOI] [PubMed] [Google Scholar]
- Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Schlub TE, Sun JC, Walton SM, Robbins SH, Pinto AK, Munks MW, Hill AB, Brossay L, Oxenius A, Davenport MP. Comparing the kinetics of NK cells, CD4, and CD8 T cells in murine cytomegalovirus infection. J Immunol. 2011;187:1385–1392. doi: 10.4049/jimmunol.1100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon B, Zamoyska R. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol. 2002;169:2997–3005. doi: 10.4049/jimmunol.169.6.2997. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J Immunol. 1998;161:1671–1676. [PubMed] [Google Scholar]
- Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, Nakauchi H, Shibuya A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11:615–623. doi: 10.1016/s1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- Shirakawa J, Shibuya K, Shibuya A. Requirement of the serine at residue 329 for lipid raft recruitment of DNAM-1 (CD226) Int Immunol. 2005;17:217–223. doi: 10.1093/intimm/dxh199. [DOI] [PubMed] [Google Scholar]
- Shirakawa J, Wang Y, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. LFA-1-dependent lipid raft recruitment of DNAM-1 (CD226) in CD4+ T cell. Int Immunol. 2006;18:951–957. doi: 10.1093/intimm/dxl031. [DOI] [PubMed] [Google Scholar]
- Sjölin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Kärre K, Vivier E, Cerboni C. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med. 2002;195:825–834. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, Honda S, Shibuya A. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- Tassi I, Cella M, Presti R, Colucci A, Gilfillan S, Littman DR, Colonna M. NK cell-activating receptors require PKC-theta for sustained signaling, transcriptional activation, and IFN-gamma secretion. Blood. 2008;112:4109–4116. doi: 10.1182/blood-2008-02-139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C, McSharry BP, Morris RJ, Llewellyn-Lacey S, Rickards C, et al. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol. 2005;6:181–188. doi: 10.1038/ni1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.