Abstract
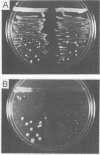
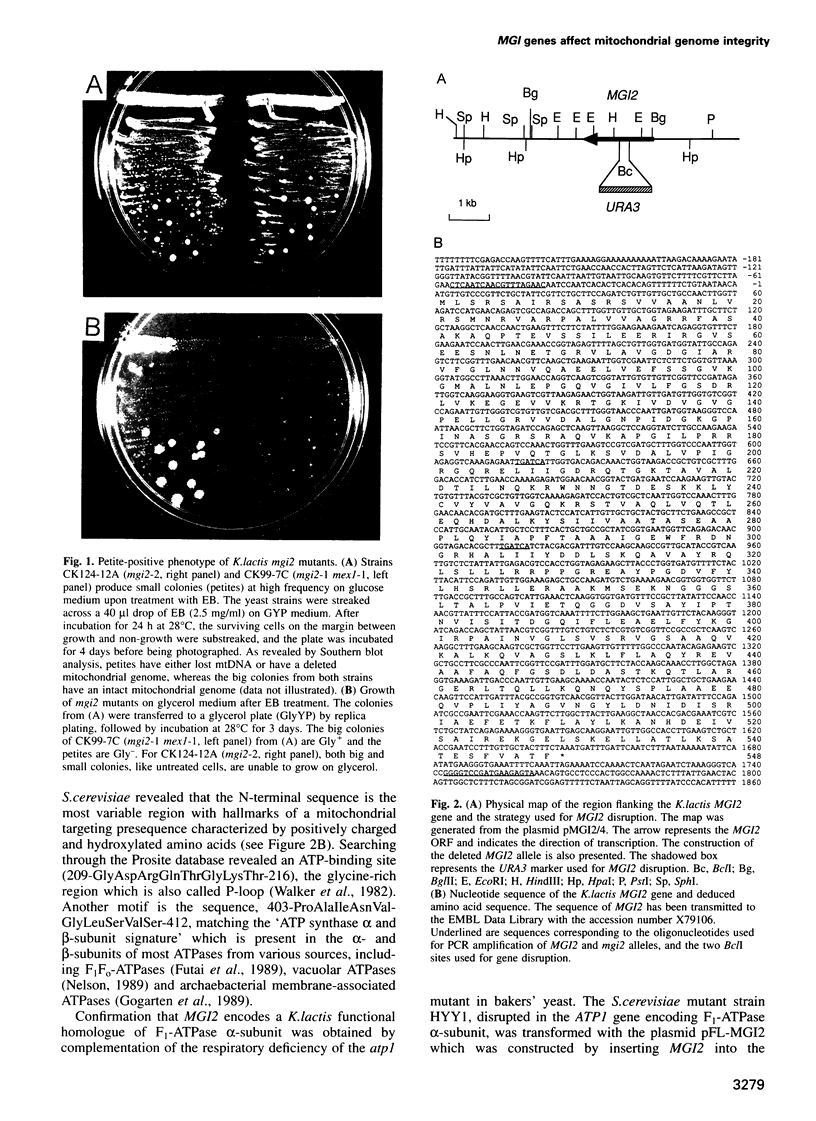
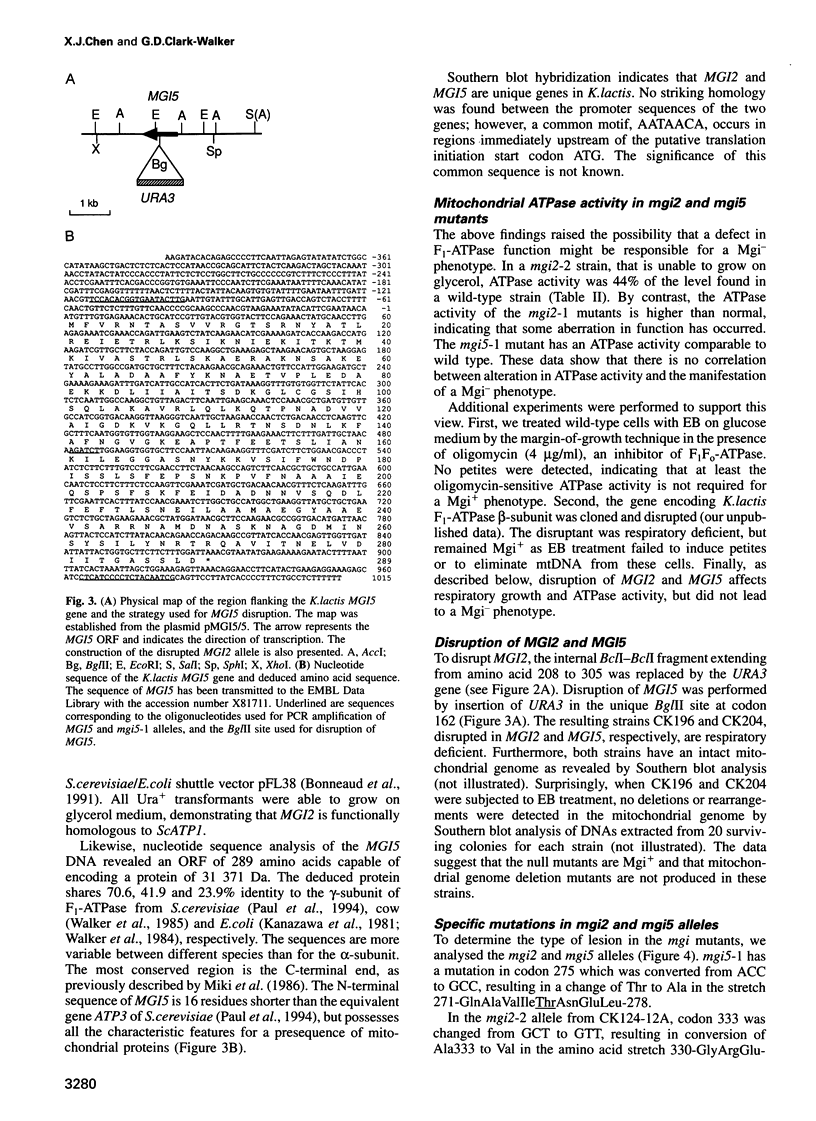
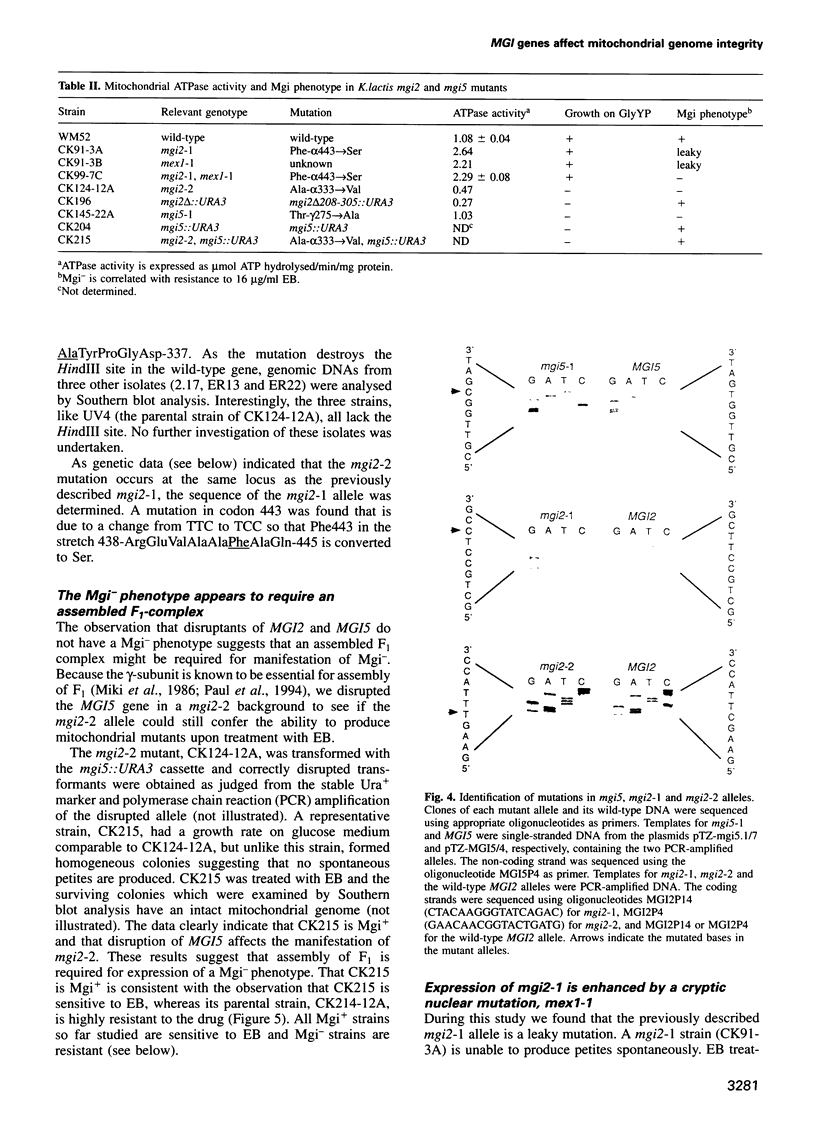
We have shown previously that mutations in nuclear genes, termed MGI, for mitochondrial genome integrity, can convert the petite-negative yeast Kluyveromyces lactis into a petite-positive form with the ability to produce mitochondrial genome deletion mutants (Chen and Clark-Walker, Genetics, 133, 517-525, 1993). Here we describe that two genes, MGI2 and MGI5, encode the alpha- and gamma-subunits of mitochondrial F1-ATPase. Specific mutations, Phe443-->Ser and Ala333-->Val in MGI2, and Thr275-->Ala in MGI5, confer on cells the ability to produce petite mutants spontaneously with deletions in mitochondrial (mt) DNA and the capacity to lose their mitochondrial genomes upon treatment with ethidium bromide. Structural integrity of the F1 complex seems to be needed for expression of the Mgi- phenotype as null mutations in MGI2 and MGI5 remove the ability to form mtDNA deletions. It is suggested that mgi mutations allow petites to survive because an aberrant F1 complex prevents collapse of the mitochondrial inner membrane potential that normally occurs on loss of mtDNA-encoded F0 subunits.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avni A., Avital S., Gromet-Elhanan Z. Reactivation of the chloroplast CF1-ATPase beta subunit by trace amounts of the CF1 alpha subunit suggests a chaperonin-like activity for CF1 alpha. J Biol Chem. 1991 Apr 25;266(12):7317–7320. [PubMed] [Google Scholar]
- BULDER C. J. INDUCTION OF PETITE MUTATION AND INHIBITION OF SYNTHESIS OF RESPIRATORY ENZYMES IN VARIOUS YEASTS. Antonie Van Leeuwenhoek. 1964;30:1–9. doi: 10.1007/BF02046695. [DOI] [PubMed] [Google Scholar]
- BULDER C. J. LETHALITY OF THE PETITE MUTATION IN PETITE NEGATIVE YEASTS. Antonie Van Leeuwenhoek. 1964;30:442–454. doi: 10.1007/BF02046758. [DOI] [PubMed] [Google Scholar]
- Bonneaud N., Ozier-Kalogeropoulos O., Li G. Y., Labouesse M., Minvielle-Sebastia L., Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991 Aug-Sep;7(6):609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- Brusilow W. S. Assembly of the Escherichia coli F1F0 ATPase, a large multimeric membrane-bound enzyme. Mol Microbiol. 1993 Aug;9(3):419–424. doi: 10.1111/j.1365-2958.1993.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Douglas M. G. Phosphodiester bond cleavage outside mitochondria is required for the completion of protein import into the mitochondrial matrix. Cell. 1987 Jun 5;49(5):651–658. doi: 10.1016/0092-8674(87)90541-1. [DOI] [PubMed] [Google Scholar]
- Chen X. J., Clark-Walker G. D. Mutations in MGI genes convert Kluyveromyces lactis into a petite-positive yeast. Genetics. 1993 Mar;133(3):517–525. doi: 10.1093/genetics/133.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. J., Clark-Walker G. D. sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol Cell Biol. 1994 Jul;14(7):4501–4508. doi: 10.1128/mcb.14.7.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. J., Fukuhara H. A gene fusion system using the aminoglycoside 3'-phosphotransferase gene of the kanamycin-resistance transposon Tn903: use in the yeast Kluyveromyces lactis and Saccharomyces cerevisiae. Gene. 1988 Sep 30;69(2):181–192. doi: 10.1016/0378-1119(88)90429-5. [DOI] [PubMed] [Google Scholar]
- Chen X. J., Wésolowski-Louvel M., Fukuhara H. Glucose transport in the yeast Kluyveromyces lactis. II. Transcriptional regulation of the glucose transporter gene RAG1. Mol Gen Genet. 1992 May;233(1-2):97–105. doi: 10.1007/BF00587566. [DOI] [PubMed] [Google Scholar]
- Chen X. J., Wésolowski-Louvel M., Tanguy-Rougeau C., Bianchi M. M., Fabiani L., Saliola M., Falcone C., Frontali L., Fukuhara H. A gene-cloning system for Kluyveromyces lactis and isolation of a chromosomal gene required for killer toxin production. J Basic Microbiol. 1988;28(4):211–220. doi: 10.1002/jobm.3620280402. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Mitochondrial genetics, circular DNA and the mechanism of the petite mutation in yeast. Genet Res. 1974 Aug;24(1):43–57. doi: 10.1017/s0016672300015068. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Langman L., Senior A. E., Ash G., Fayle D. R., Gibson F. Assembly of the adenosine triphosphatase complex in Escherichia coli: assembly of F0 is dependent on the formation of specific F1 subunits. J Bacteriol. 1981 Oct;148(1):30–42. doi: 10.1128/jb.148.1.30-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. L. The mechanism and regulation of ATP synthesis by F1-ATPases. Annu Rev Biochem. 1981;50:681–714. doi: 10.1146/annurev.bi.50.070181.003341. [DOI] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effects and its relation to the petite mutation. J Gen Microbiol. 1966 Aug;44(2):157–165. doi: 10.1099/00221287-44-2-157. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Protein unfolding and the energetics of protein translocation across biological membranes. Cell. 1988 Feb 26;52(4):481–483. doi: 10.1016/0092-8674(88)90458-8. [DOI] [PubMed] [Google Scholar]
- Faye G., Fukuhara H., Grandchamp C., Lazowska J., Michel F., Casey J., Getz G. S., Locker J., Rabinowitz M., Bolotin-Fukuhara M. Mitochondrial nucleic acids in the petite colonie mutants: deletions and repetition of genes. Biochimie. 1973;55(6):779–792. doi: 10.1016/s0300-9084(73)80030-6. [DOI] [PubMed] [Google Scholar]
- Futai M., Noumi T., Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu Rev Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P., Fox T. D. Nuclear mutations in the petite-negative yeast Schizosaccharomyces pombe allow growth of cells lacking mitochondrial DNA. Genetics. 1992 Jun;131(2):255–260. doi: 10.1093/genetics/131.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy C. M., Galeotti C. L., Clark-Walker G. D. Deletions and rearrangements in Kluyveromyces lactis mitochondrial DNA. Curr Genet. 1989 Dec;16(5-6):419–427. doi: 10.1007/BF00340721. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. ATP synthesis in mitochondria. Eur J Biochem. 1993 Dec 15;218(3):759–767. doi: 10.1111/j.1432-1033.1993.tb18431.x. [DOI] [PubMed] [Google Scholar]
- Humbert R., Altendorf K. Defective gamma subunit of ATP synthase (F1F0) from Escherichia coli leads to resistance to aminoglycoside antibiotics. J Bacteriol. 1989 Mar;171(3):1435–1444. doi: 10.1128/jb.171.3.1435-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto A., Miki J., Maeda M., Futai M. H(+)-ATPase gamma subunit of Escherichia coli. Role of the conserved carboxyl-terminal region. J Biol Chem. 1990 Mar 25;265(9):5043–5048. [PubMed] [Google Scholar]
- Kanazawa H., Kayano T., Mabuchi K., Futai M. Nucleotide sequence of the genes coding for alpha, beta and gamma subunits of the proton-translocating ATPase of Escherichia coli. Biochem Biophys Res Commun. 1981 Nov 30;103(2):604–612. doi: 10.1016/0006-291x(81)90494-0. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luis A. M., Alconada A., Cuezva J. M. The alpha regulatory subunit of the mitochondrial F1-ATPase complex is a heat-shock protein. Identification of two highly conserved amino acid sequences among the alpha-subunits and molecular chaperones. J Biol Chem. 1990 May 15;265(14):7713–7716. [PubMed] [Google Scholar]
- Miki J., Takeyama M., Noumi T., Kanazawa H., Maeda M., Futai M. Escherichia coli H+-ATPase: loss of the carboxyl terminal region of the gamma subunit causes defective assembly of the F1 portion. Arch Biochem Biophys. 1986 Dec;251(2):458–464. doi: 10.1016/0003-9861(86)90352-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nakamoto R. K., Maeda M., Futai M. The gamma subunit of the Escherichia coli ATP synthase. Mutations in the carboxyl-terminal region restore energy coupling to the amino-terminal mutant gamma Met-23-->Lys. J Biol Chem. 1993 Jan 15;268(2):867–872. [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Paul M. F., Ackerman S., Yue J., Arselin G., Velours J., Tzagolof A., Ackermann S [corrected to Ackerman S. ]. Cloning of the yeast ATP3 gene coding for the gamma-subunit of F1 and characterization of atp3 mutants. J Biol Chem. 1994 Oct 21;269(42):26158–26164. [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J. 1985 Nov;4(11):2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Perrodin G., Croft J. H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal "petites". Biochem Biophys Res Commun. 1968 Feb 15;30(3):232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Soga S., Noumi T., Takeyama M., Maeda M., Futai M. Mutational replacements of conserved amino acid residues in the alpha subunit change the catalytic properties of Escherichia coli F1-ATPase. Arch Biochem Biophys. 1989 Feb 1;268(2):643–648. doi: 10.1016/0003-9861(89)90332-9. [DOI] [PubMed] [Google Scholar]
- Takeda M., Chen W. J., Saltzgaber J., Douglas M. G. Nuclear genes encoding the yeast mitochondrial ATPase complex. Analysis of ATP1 coding the F1-ATPase alpha-subunit and its assembly. J Biol Chem. 1986 Nov 15;261(32):15126–15133. [PubMed] [Google Scholar]
- Tingle M., Herman A., Halvorson H. O. Characterization and mapping of histidine genes in Saccharomyces lactis. Genetics. 1968 Mar;58(3):361–371. doi: 10.1093/genetics/58.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Fearnley I. M., Gay N. J., Gibson B. W., Northrop F. D., Powell S. J., Runswick M. J., Saraste M., Tybulewicz V. L. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985 Aug 20;184(4):677–701. doi: 10.1016/0022-2836(85)90313-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski-Louvel M., Tanguy-Rougeau C., Fukuhara H. A nuclear gene required for the expression of the linear DNA-associated killer system in the yeast Kluyveromyces lactis. Yeast. 1988 Mar;4(1):71–81. doi: 10.1002/yea.320040108. [DOI] [PubMed] [Google Scholar]
- Yuan H., Douglas M. G. The mitochondrial F1ATPase alpha-subunit is necessary for efficient import of mitochondrial precursors. J Biol Chem. 1992 Jul 25;267(21):14697–14702. [PubMed] [Google Scholar]