Abstract
Parkinson's disease (PD) is marked by the loss of dopamine neurons in the substantia nigra (SN). Although the exact etiology is unknown, sporadic PD is hypothesized to be a result of genetic susceptibility interacting with environmental insult. Epidemiological studies suggest that pesticide exposure is linked to higher PD risk, but there are no studies demonstrating SN changes with chronic pesticide exposure in human subjects. Thus, high resolution T2-weighted magnetic resonance imaging (MRI) and diffusion tensor (DTI) images were obtained from 12 agricultural workers with chronic pesticide exposure, 12 controls, and 12 PD subjects. Neither controls, nor pesticide-exposed subjects, had any parkinsonian symptoms. Exposure history to pesticides was assessed by a structured questionnaire. DTI measures in the SN, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), were obtained for all subjects and compared among groups. Compared to controls, PD patients showed the expected significant changes in all DTI measurements in the SN. The pesticides-exposed subjects, compared to controls, had significantly lower FA values (p=0.022, after multiple comparisons correction), but no significant differences in RD, MD, or AD measures. The study is the first to demonstrate microstructural changes in the SN of human subjects with chronic pesticide exposure. The changes detected by MRI may mark “one of the hits” leading to PD, and underlie the increased risk of PD in pesticide users found in epidemiological studies. Further human studies assisted by these imaging markers may be useful in understanding the etiology of PD.
Keywords: Parkinson's disease (PD), substantia nigra (SN), diffusion tensor imaging (DTI), pesticides, paraquat, magnetic resonance imaging (MRI)
1. Introduction
Parkinson's disease (PD) is marked primarily by the loss of dopaminergic neurons in the substantia nigra (SN) (Fearnley and Lees, 1991; Dickson et al., 2009). The fact that identical twins frequently are discordant for PD suggested that environmental factors may be associated with the etiology of sporadic PD through mechanisms such as oxidative stress, neuroinflammation, and/or cytotoxicity (Tanner et al., 1999; Wirdefeldt et al., 2004). Recent epidemiologic data support that pesticide exposure is associated with increased risk of PD (McCormack et al., 2002; Tanner et al., 2011; Pezzoli and Cereda, 2013). In addition, a number of pesticides (e.g., paraquat, rotenone, maneb) have been shown to cause direct dopaminergic toxicity in animal models (Uversky, 2004; Desplats et al., 2012). There has been no direct evidence, however, suggesting that chronic pesticide exposure leads to SN changes in human subjects due to lack of in vivo markers for the pathological process occurring in PD.
Recently, magnetic resonance imaging (MRI) has been explored extensively to study PD-related pathological changes in human subjects (Chan et al., 2007; Martin et al., 2008; Vaillancourt et al., 2009). In particular, diffusion tensor imaging (DTI), by measuring microstructural disorganization due to dopamine cell loss, has shown promise as a tool of PD-related changes (Chan et al., 2007; Peran et al., 2010; Du et al., 2011). In the MPTP mouse model, DTI changes have been associated with dopamine neuron loss in the SN (Boska et al., 2007). Furthermore, several human studies have demonstrated reduced FA values in the SN of early PD patients, consistent with the notion that DTI changes may be able to detect nigral changes in vivo (Vaillancourt et al., 2009; Du et al., 2012).
The current study is the first to investigate potential DTI changes in the SN of asymptomatic pesticide-exposed subjects, testing the hypothesis that chronic pesticide exposure may lead to microstructural changes in the SN, possibly explaining the increased risk of PD in pesticide users.
2. Methods
2.1. Subjects
Twelve male subjects with chronic pesticide exposure were recruited using IRB-approved flyers. Pesticide exposure was evaluated by using a structured questionnaire from the Agriculture Health Study (http://www.niehs.nih.gov) for both pesticide-exposed and control subjects. The 12 idiopathic PD subjects and 12 healthy controls came from the male subgroups of ongoing cohorts at our center that had been recruited either from our tertiary movement disorders clinic and/or using IRB approved flyers (see Table 1 and Table 2 for detailed demographic information). The control and PD subjects were selected impartially from the existing cohorts by matching for age with the pesticide-exposed group using a greedy matching algorithm (http://mayoresearch.mayo.edu/mayo/research/biostat).
Table 1. Demographics of control, pesticide-exposed, and PD subjects.
| Controls | Pesticide-exposed | PD | P-valuesa | |
|---|---|---|---|---|
| Number | 12 | 12 | 12 | - |
| Age (y) | 53.9±11.9 | 55.8±11.7 | 57.1±9.1 | 0.772 |
| Pesticide exposure (y) | 0 | 19.4±6.1 | n/a | - |
| Parkinson's evaluation: | ||||
| Hoehn & Yahr stage (number at I/II) | - | - | 5/7 | - |
| Disease duration (years) | - | - | 4.4(4.7) | - |
| UPDRS scores: | ||||
| Part I | 2.3±3.4 | 4.3±4.3 | 9.3±4.9b | 0.003 |
| Part II | 0.1±0.3 | 0.7±0.9 | 7.9±6.1b | 0.001 |
| Part III | 1.0±1.7 | 3.5±1.8 | 25.9±13.7b | <0.001 |
| Grooved pegboard test: | ||||
| Dominant | 79.5±18.3 | 90.8±20.5 | 125.3±40.8b | 0.001 |
| Non-dominant | 88.3±26.4 | 89.2±18.3 | 208.3±188.6b | 0.016 |
All values are means ±SD.
p-value is calculated from one-way ANOVA.
Significantly different from Control and PD-exposed subjects.
Table 2. Pesticide exposure information.
| ID | Occupation | Exposure (years) | # of Pesticides |
|---|---|---|---|
| Subject 1 | Farmer | 25 | 7 |
| Subject 2 | Commercial Pesticide Applicator | 15 | 6 |
| Subject 3 | Commercial Pesticide Applicator | 15 | 5 |
| Subject 4 | Commercial Pesticide Applicator | 25 | 10 |
| Subject 5 | Farmer | 25 | 12 |
| Subject 6 | Farmer | 15 | 11 |
| Subject 7 | Farmer | 15 | 9 |
| Subject 8 | Farmer | 25 | 21 |
| Subject 9 | Commercial Pesticide Applicator | 25 | 16 |
| Subject 10 | Farmer | 8 | 7 |
| Subject 11 | Farmer | 25 | 16 |
| Subject 12 | Farmer | 15 | 10 |
| Mean (SD) | n/a | 19.4 (6.1) | 10.8 (4.8) |
The control subjects had essentially no history of pesticide exposure, and all of the control and pesticide-exposed subjects were free of any subjective or objective PD symptoms or signs. PD diagnosis was made by a movement disorder specialist (XH) according to published criteria (Hughes et al., 1992; Hughes et al., 2001). All subjects were assessed using the Hoehn & Yahr staging, Unified Parkinson's Disease Rating Scale (UPDRS) sections I-III, and Grooved Pegboard Test (GPT). For PD subjects, disease duration from time of diagnosis was obtained from subject history. All subjects were free of major and acute medical issues such as liver, kidney, or thyroid abnormalities and deficiencies of B12 and folate, and had Mini Mental Status Exam (MMSE) scores >24. All subjects gave written informed consent that was reviewed and approved by the Penn State Hershey Institutional Review Board and was consistent with the Declaration of Helsinki.
2.2. Magnetic resonance imaging (MRI) data acquisition
All subjects were scanned with a 3.0 Tesla MRI system (Trio, Siemens Magnetom, Erlangen, Germany, 8-channel phased array head coil) with high-resolution T2-weighted and diffusion tensor imaging (DTI) sequences. A fast-spin-echo sequence was used to obtain T2-weighted images with TR/TE=2500/316, FOV=256 mm × 256 mm, matrix=256 × 256, slice thickness=1 mm (with no gap), and slice number=176. For DTI, acquisition parameters were as follows: TR/TE=8300/82 ms, b value=1000 s/mm2, diffusion gradient directions=42 and 7 b=0 scans, FOV=256 mm × 256 mm, matrix=128 × 128, slice thickness=2 mm (with no gap), and slice number=65.
2.3. Image processing and analysis
2.3.1. Segmentation of the SN
The SN was defined manually on T2-weighted images using ITK-SNAP (Yushkevich et al., 2006) by an investigator blinded to subject diagnosis. The region of interest was defined as a hypointense band between the red nucleus and cerebral peduncle in axial sections (Vaillancourt et al., 2010; Massey and Yousry, 2010), as illustrated in Figure 1. The most superior extent of the SN segmentation was defined at one slice inferior to the axial section of the red nucleus showing the largest radius (Figure 1). A total of four to six slices (from the rostral to caudal part of the SNpc) were used as the SN region of interest (ROI) (Yushkevich et al., 2006). The right and left ROIs of the SN were summed in order to obtain an average measurement from both sides. Another set of ROIs also was obtained by a second rater (GD) using the same segmentation protocol, thus providing an estimate of the inter-rater reliability using intra-class correlation coefficients.
Figure 1.
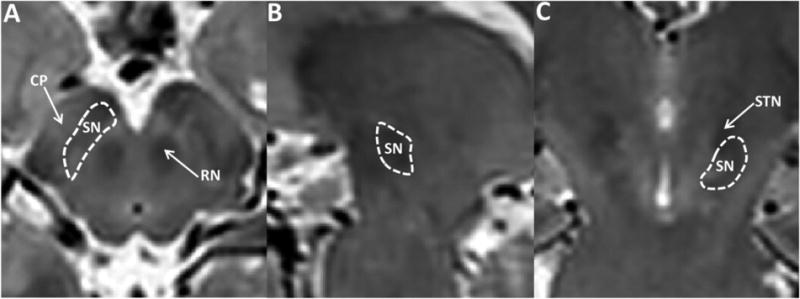
Illustration of region of interest (ROI) definition of the substantia nigra. A, B, and C represent axial (A), sagittal (B) and coronal (C) views of the midbrain in T2-weighted images. The yellow arrows in A and C indicate the substantia nigra (SN), red nucleus (RN), cerebral peduncle (CP), and subthalamic nucleus (STN).
2.3.2. DTI estimation
DTI image quality control and tensor reconstruction was performed using DTIPrep (Neuro Image Research and Analysis Laboratory, University of North Carolina, Chapel Hill, NC USA) that first checks DWI images for appropriate quality by calculating the inter-slice and inter-image intra-class correlation, and then corrects for the distortions induced by eddy currents and head motion (Liu et al., 2010). Diffusion tensor images then were estimated via weighted least squares (Salvador et al., 2005). DTI is thought to reflect the diffusion of water molecules in tissues and thus is an indicator of the structural organization in a region. Several measures typically are used, including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). For the current study, FA, MD, AD, and RD maps all were generated for subsequent analysis. FA is a non-linear combination of three eigenvalues of the tensor matrix calculated by the following equation to represent the local degree of diffusion anisotropy:
where λ1, λ2, λ3 and represent the degree of diffusion in the three main axes of the tensor (Basser and Pierpaoli, 1996). It can range in value from 0-1, with 0 meaning water is free to move in any direction (no structural integrity/boundaries) and 1 reflecting all water molecules aligned in the same direction (e.g., along an axon). MD is a simple average of the three eigenvalues (MD = (λ1 + λ2 + λ3)/3) representing local mean diffusivity. AD (AD = λ1) and RD (RD = (λ2 + λ3)/2) separate spatial diffusivity into a main component along the axis of maximum diffusion and a remaining diffusivity component for the other two directions. These two measures help yield a better understanding of local diffusivity changes that contribute to FA and/or MD changes.
After manual segmentation, the SN ROIs were mapped to DTI maps (FA, MD, AD, and RD) by co-registering the T2-weighted images to the B0 images of DTI data using an affine registration followed by a b-spline non-rigid registration implemented in 3D Slicer (www.slicer.org) (Rueckert et al., 1999). Mean DTI (FA, MD, AD, and RD) values in the SN of each subject were calculated and used for subsequent statistical analyses.
2.4. Inter-rater reliability validation of DTI measurements
There was strong agreement between the two raters regarding the segmentation and resulting extraction of DTI measures in the SN. The intra-class correlation coefficients (ICC) were 0.97 for FA, 0.93 for MD, 0.91 for AD, and 0.93 for RD values. The independent analysis of the ROIs from the second rater yielded similar results (data available upon request) to those from the first rater that are reported below.
2.5. Statistical analyses
The mean values of demographic factors (age, UPDRS, and GPT) were compared among the three groups by one-way analysis of variance (ANOVA) followed by post-hoc comparisons with Tukey-Kramer multiple comparison correction to probe for specific differences between the groups. SN DTI measures (FA, MD, AD, and RD) were compared among the three groups by one-way analysis-of-covariance (ANCOVA) with adjustment for age and significant results also probed for specific group differences using the Tukey-Kramer multiple comparison correction. We explored the association between pesticide exposure (in the pesticide group), motor function (UPDRS and GPT in both the pesticide and PD groups), and SN DTI measures via Spearman correlation coefficients. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographics
The demographic and clinical data are summarized in Table 1. All subjects were male, and there were no significant differences in age between pesticide exposed, PD, and control groups (p=0.772). Detailed exposure information for pesticide-exposed subjects, including exposure time, number of chemicals and occupation, are summarized in Table 2. All pesticide-exposed subjects had a minimum of eight years history of chronic application of multiple pesticides, all control and pesticide-exposed subjects were free of any subjective PD symptom, and had UPDRS scores less than six. All PD subjects were at Hoehn & Yahr stage I or II in the “off” drug state, indicating early and mild PD. There were significant differences in UPDRS and GPT scores among the three groups. Post-hoc tests revealed, however, that only PD subjects performed significantly worse on these measures compared to controls and pesticide-exposed subjects, and that there were no significant differences between control and pesticide-exposed subjects (see Table 1).
3.2. Comparison of DTI measures among groups
As shown in Figure 2, there were significant differences in the SN FA [F2,32 = 8.30, p < 0.001], MD [F2,32 = 5.77, p = 0.003], and RD [F2,32 = 6.02, p = 0.002] values, with a trending difference in the AD [F2,32 = 2.81, p = 0.055] value among the three groups. Post hoc comparisons showed that, compared to controls, PD subjects had significantly lower FA, higher MD and RD values, and a trend for a higher AD value in the SN [p<0.001 for FA, p=0.004 for MD, p=0.003 for RD, and p=0.059 for AD].
Figure 2.
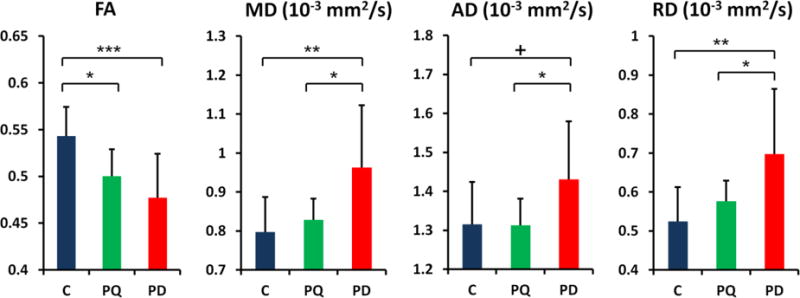
Comparison of DTI values in the substantia nigra for Control (C), pesticide-exposed, and Parkinson's disease (PD) subjects. Error bars represent the standard deviation. FA: Fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity Significant differences between groups are represented as: +: p<0.1; *: p<0.05; **: p<0.01;***: p<0.001.
Compared to controls, pesticide-exposed subjects showed a significantly lower FA value (p=0.022, after correction for multiple comparison) in the SN, but no significant differences in MD, RD, or AD measures (Figure 2). The FA change in pesticide-exposed subjects most likely was driven primarily by an increased RD value and not by a change in the AD value. Compared to PD subjects, pesticide exposed subjects showed no significant difference in FA (p=0.307), yet were significantly lower on the other three DTI measures (p=0.017 for MD, p=0.046 for AD, and p=0.037 for RD).
3.3. Clinical and behavioral correlations of DTI measurements
Among either pesticide-exposed subjects or PD patients, there were no correlations between DTI measures and motor functional measurements (data available upon request). Similarly among the pesticide-exposed group, we did not find any significant correlations between DTI measurements, duration of exposure to pesticides, or whether the exposure was as a farmer or applicator (see Table 2).
4. Discussion
Sporadic PD is probably a result of genetic susceptibility interacting with environmental insult. One such environmental factor has been pesticide exposure (Kasten et al., 2007; Cannon and Greenamyre, 2011), and consistent with these epidemiologic data, animal studies have demonstrated that a number of pesticides may be toxic to dopamine neurons (Richardson et al., 2005). The current study is the first to demonstrate directly the microstructural changes in the SN of human subjects having had chronic, low-dose exposure of a diverse group of pesticides. The changes detected by MRI may mark “one of the hits” leading to PD. Moreover, our study demonstrated that DTI could be a potential in vivo marker for microstructural changes caused by potential environmental risk factors of PD.
In the current study, we recruited a group of agricultural workers who had extensive histories of chronic, multiple pesticide exposure, several of whom were professional pesticide applicators (Table 2). During our recruitment, we purposefully insured that this entire group had a history of paraquat exposure. This was done because there is strong epidemiological data linking paraquat exposure to PD risk (Hatcher et al., 2008; Ritz et al., 2009; Elbaz et al., 2009; Weisskopf et al., 2010; Tanner et al., 2011), and because of the fact that paraquat is both structurally similar to the dopamine cytotoxicant 1-methyl-4-phenylpyridinium (MPP+) and can cause dopamine neuron toxicity in animal models (Richardson et al., 2005; Cui et al., 2009; Rappold et al., 2011), despite that the toxicity of paraquat in animal models still is a controversial topic (Breckenridge et al., 2013). Although this was a part of the experimental design, the current study was neither designed to, nor was powered adequately, to assess if these PD-like SN changes were primarily due to any specific pesticide.
Recently, dopamine neuron loss in a mouse model has been correlated with DTI changes (Boska et al., 2007), and such DTI changes also have been reported in early stage PD subjects (Chan et al., 2007; Vaillancourt et al., 2009; Peran et al., 2010; Du et al., 2011). The present study is the first to apply DTI in asymptomatic human subjects and demonstrate SN microstructural changes associated with chronic pesticide exposure. The exact mechanism and implications of the DTI changes are unknown. Given the evidence from animal and epidemiological studies, it is plausible that the microstructural changes detected by DTI reflect dopaminergic cell loss similar to that in PD except with a milder magnitude. In recent years, there is a growing realization that PD may be caused by dual or multiple hits (Hawkes et al., 2007; Sulzer, 2007). Thus, the microstructural changes detected by DTI in the current study may mark one of the “hits” leading to PD in pesticide users as predicted by the cited epidemiological studies. Namely, the SN changes do not have to represent PD pathology per se, but rather a proximal event that may make exposed individuals more susceptible to a “next” hit that will cumulatively lead to PD.
Diffusion tensor imaging, as a measure of local water diffusivity and microstructure integrity, has shown high sensitivity to detect mild structure alternations in multiple neurological disorders including PD (Vaillancourt et al., 2009; Du et al., 2012). The nonlinear nature of the FA measurement and small sample size, however, limit our ability to detect correlations between brain microstructural changes and exposures. Furthermore, previous research has indicated that FA values are affected by age and factors such as diet and socio-economic status (Salat et al., 2005; Vaillancourt et al., 2010; Wu et al., 2011). The small sample size in our study did not allow us to control for confounders other than age. Nevertheless, the current study does strongly suggest the value of in vivo neuroimaging to investigate the roles of environmental factors in the etiology of PD.
In summary, we demonstrate that subtoxic, chronic pesticide exposure may lead to microstructural changes in the SN, and may represent the consequence of pesticide-induced toxicity to nigral circuitry. These findings are consistent with the previously proposed role of pesticide exposure in initiating or accelerating pathological processes that are similar to those occurring in PD. More importantly, our study demonstrated that DTI could detect microstructural changes in the SN possibly caused by pesticides. This approach may be useful in determining if other environmental chemicals alter brain microstructure, and/or can assist in focusing on specific chemicals that affect PD susceptibility. Because of the potential impact, replication of our results using larger cohorts and longitudinal designs is warranted.
Highlights.
Chronic pesticide exposure is associated with microstructural changes in midbrain.
Asymptomatic agricultural workers had similar, but less severe, changes than in PD.
MRI may be useful as a structural marker for potential changes caused by pesticides.
Acknowledgments
This work was funded by National Institutes of Health (NS060722, ES019672, and NS082151), the Hershey Medical Center GCRC (NIH M01RR10732) and GCRC Construction Grant (C06RR016499), and the National Center for Advancing Translational Sciences (UL1 TR000127). Dr. Chen is funded by the Intramural Research Program of the NIH, the National Institute of Environmental Health Sciences.
We also would like to thank all the participants in the study, as well as support from the study coordinator Ms. Melissa Santos and MRI technical support from Mr. Jeffery Vesek. We also would like to thank Drs. James Connor, Byron Jones, and Freya Kamel for their input during the planning phase of the study.
Footnotes
Contributors: GD: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, statistical analysis. MML: drafting/revising the manuscript, study concept and design, study supervision. NS: drafting/revising the manuscript, analysis and interpretation of data. LK: drafting/revising the manuscript, statistical analysis, study concept and design, analysis and interpretation of data. HC: drafting/revising the manuscript, study concept and design, analysis and interpretation of data. RBM: drafting/revising the manuscript, study concept and design. XH: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, study supervision, and obtaining funding.
Conflict interests: The authors report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, et al. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson's disease. Neurobiol Dis. 2007;26:590–596. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124:225–250. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LL, Rumpel H, Yap K, Lee E, Loo HV, Ho GL, et al. Case control study of diffusion tensor imaging in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, et al. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8048. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Patel P, Kosberg K, Mante M, Patrick C, Rockenstein E, et al. Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson's disease. Mol Neurodegener. 2012;7:49. doi: 10.1186/1750-1326-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Du G, Lewis MM, Sen S, Wang J, Shaffer ML, Styner M, et al. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov Disord. 2012;27:1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Lewis MM, Styner M, Shaffer ML, Sen S, Yang QX, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson's disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del TK, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- Kasten M, Chade A, Tanner CM. Epidemiology of Parkinson's disease. Handb Clin Neurol. 2007;83:129–151. doi: 10.1016/S0072-9752(07)83006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, et al. Quality control of diffusion weighted images. 2010 doi: 10.1117/12.844748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology. 2008;70:1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- Massey LA, Yousry TA. Anatomy of the substantia nigra and subthalamic nucleus on MR imaging. Neuroimaging Clin N Am. 2010;20:7–27. doi: 10.1016/j.nic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, et al. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Peran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, et al. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain. 2010;133:3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80:2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- Rappold PM, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, et al. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci U S A. 2011;108:20766–20771. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, et al. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salvador R, Pena A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Hum Brain Mapp. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Knekt P, O'Reilly EJ, Lyytinen J, Reunanen A, Laden F, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74:1055–1061. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- Wu YC, Field AS, Whalen PJ, Alexander AL. Age- and gender-related changes in the normal human brain using hybrid diffusion imaging (HYDI) Neuroimage. 2011;54:1840–1853. doi: 10.1016/j.neuroimage.2010.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


