Abstract
Airborne manganese (Mn) exposure can result in neurotoxicity and postural instability in occupationally exposed workers, yet few studies have explored the association ambient exposure to Mn in children and postural stability. The goal of this study was to determine the association between Mn and lead (Pb) exposure, as measured by blood Pb, blood and hair Mn and time weighted distance (TWD) from a ferromanganese refinery, and postural stability in children. A subset of children ages 7–9 years enrolled in the Marietta Community Actively Researching Exposure Study (CARES) were invited to participate. Postural balance was conducted on 55 children residing in Marietta, Ohio and the surrounding area. Samples of blood were collected and analyzed for Mn and Pb, and samples of hair were analyzed for Mn. Neuromotor performance was assessed using postural balance testing with a computer force platform system. Pearson correlations were calculated to identify key covariates. Associations between postural balance testing conditions and Mn and Pb exposure were estimated with linear regression analyses adjusting for gender, age, parent IQ, parent age. Mean blood Mn was 10 μg/L (SEM=0.36), mean blood Pb was 0.85 μg/dL (SEM=0.05), and mean hair Mn was 0.76 μg/g (SEM=0.16). Mean residential distance from the refinery was 11.5 km (SEM=0.46). All three measures of Mn exposure were significantly associated with poor postural balance. In addition, low-level blood Pb was also negatively associated with balance outcomes. We conclude that Mn exposure and low-level blood Pb are significantly associated with poor postural balance.
Keywords: postural balance, manganese, lead, children, academic-community partnership, air quality, Appalachian American
1. Introduction
Manganese (Mn) is an essential trace element, and plays a key role in bone mineralization, protein and energy metabolism, metabolic regulation and cellular protection from damaging free radical species (Aschner, 2000). Ingested Mn is under tight homeostatic control (Papavasiliou et al., 1966); however, inhaled Mn bypasses the biliary excretion mechanism and is capable of crossing the blood brain barrier via several pathways including facilitated diffusion and active transport (Rabin et al., 1993; Davis, 1999; Aschner and Gannon, 1994; Aschner, 2006; Crossgrove et al., 2003). Mn can also enter the brain following nasal inhalation through axonal transport from nerve endings in the nose, to the olfactory bulb and directly to the cerebral cortex (Brenneman et al., 2000; Dorman et al., 2002; Elder et al., 2006). Mn accumulates in iron rich brain regions of the basal ganglia: caudate-putamen, globus pallidus, substantia nigra, and subthalamic nuclei (Eriksson et al., 1992; Elder et al., 2006; Uchino et al., 2007; Bock et al., 2008; Aschner, 2006). The basal ganglia integrate sensory feedback from the visual proprioceptive and vestibular systems that control movement and postural balance (Visser et al., 2005). Occupational studies have associated Mn exposure with impaired motor function, such as difficulty performing alternating movements, hand tremor, postural instability, and prolonged reaction time (Bowler et al., 2007; Ellingsen et al., 2008; Zoni et al., 2007), yet very few have evaluated this measure of gross motor function in children exposed to environmental ambient Mn (Lucchini et al., 2012). Children and infants may be uniquely susceptible to the neurotoxic effects of environmental ambient Mn exposure as their brains are undergoing a dynamic process involving complex pathways of growth, differentiation, pathway direction, synaptic organization, and apoptosis, all of which can be influenced by environmental chemical factors (Dietrich, 2010; Landrigan et al., 2011).
Washington County Ohio is home to the longest operating ferromanganese refinery in North America, Eramet Marietta, Inc. (EMI). EMI is located in Marietta, Ohio, the county seat of Washington County. Based on self-reported annual emissions data to the U. S. Environmental Protection Agency (EPA), the refinery emitted nearly 600,000 lbs of Mn in 1999 and by 2010 had decreased to slightly over 100,000 lbs of Mn per year (EPA, 2012). We previously found a significant association between postural balance in non-occupationally exposed adults residing in Marietta, Ohio (Standridge et al., 2008). In response to community concern about the neurological consequences of ambient Mn exposure on children, a community-based participatory research study was initiated, Marietta Community Actively Researching Exposure Study (CARES) (Haynes et al., 2011). The purpose of this manuscript is to report the findings from the postural balance assessment conducted on a subset of the CARES cohort residing in Washington County.
2. Materials and Methods
2.1 Study Population
Children were recruited to participate in the postural balance assessment through their enrollment in CARES. Eligibility for CARES included children ages 7, 8 and 9 years who have resided in Washington County, Ohio throughout their life with no plans to move for at least one year. In addition, their biological mother must have resided in Washington County during her pregnancy with the child. Families were recruited for participation in CARES using a volunteer sampling strategy. Recruitment letters were sent home through schools and advertisements were aired on local radio and printed in local newspapers. Children were screened for eligibility in the balance assessment sub-study, if they had participated in CARES or were scheduled for participation in CARES within 8 weeks of the balance assessment testing dates. Children were excluded from the balance sub-study if they had parent-reported vision problems, scoliosis, seizures, epilepsy, any muscle or neurological disease, arthritis or joint disease, autism, recent broken bones in the legs, feet or back, difficulty standing without help or pain or discomfort when standing (i.e., health issues which may influence the postural test results). Postural balance assessment was conducted over five weekends (F.R., C.C., Dawn Wittberg noted in acknowledgements) between June 2009 and May 2010. The University of Cincinnati Institutional Review Board approved this study. All parents and children signed an informed consent and the children also signed an assent.
2.2 Internal Dose Measures of Manganese and Lead
2.2.1 Specimen collection procedures
A blood specimen was collected by venipuncture from the antecubital vein by a trained phlebotomist. Whole blood was collected into a 3-mL purple top (K2EDTA) tube certified by the analyzing laboratory for trace element analysis. To avoid potential metal contamination from the surgical steel needle, the second tube of blood collected was used for metal analyses. The tube was inverted gently 5–10 times and placed on an orbital mixer for 15 minutes. Blood specimens were refrigerated at 5° C until shipped to the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health's (NYS DOH) Wadsworth Center for analysis in Albany, New York.
Approximately 20 strands of hair were collected from the occipital region, cut with ceramic scissors as close to the scalp as possible. It was ensured that the hair was at least 1 cm in length for analysis. Long hair was trimmed to 6 cm and taped towards the non-scalp-side end of the hair shaft onto an index card with an arrow pointing in the direction of the scalp end on the index card. The card with the taped hair sample was placed into a pre-labeled envelope and stored at room temperature until shipped to the Channing Trace Metals Laboratory, Brigham and Women's Hospital, Harvard School of Public Health for analysis in Boston, Massachusetts.
2.2.2 Analytical Procedures
Whole blood specimens were analyzed for Mn content by graphite furnace atomic absorption spectrometry (GFAAS). Briefly, whole blood is diluted 1+9 in 0.015% (w/v) Mg(NO3)2, 0.1% (v/v) Triton X-100™, and 0.2% (v/v) double-distilled HNO3 and analyzed for Mn using a PerkinElmer® Model 4100 ZL GFAAS instrument equipped with a transversely-heated graphite atomizer and a longitudinal Zeeman background correction system (PerkinElmer® Life and Analytical Sciences, Shelton, CT). The method was well validated as described previously (Praamsma et al., 2011) and results for blood Mn compare well with other spectrometric methods based on analysis of National Institute of Standards and Technology (NIST) Standard Reference Material® (SRM) 955c Toxic Metals in Caprine Blood (Praamsma et al., 2012). While no SRM has yet been certified for Mn in blood, the GFAAS method used for this study was also used to assign a consensus value for Mn in SRM 955c (Praamsma et al., 2012).
Blood Pb was determined by inductively coupled plasma-mass spectrometry (ICP-MS) (Palmer et al., 2006) using a method optimized and validated for biomonitoring purposes as described elsewhere (McKelvey et al., 2007, Birdsall et al., 2010). A PerkinElmer® Sciex ELAN DRC Plus ICP-MS instrument equipped with a Burgener Teflon MiraMist® nebulizer (Burgener Research Inc., Mississauga, ON, Canada) and a Cinnabar spray-chamber (Glass Expansion, West Melbourne, VIC, Australia), and operated in standard mode was used for all blood Pb measurements. Whole blood was diluted 1+49 with a reagent containing 0.5% (v/v) double-distilled HNO3, 0.005% (v/v) Triton™ X-100 and 25 μg/L of rhodium and iridium as internal standards. Accuracy for blood Pb was verified against NIST SRM 955c Toxic Metals in Caprine Blood, which is certified for Pb (Murphy et al., 2009).
The Wadsworth Center is fully certified as a clinical trace elements laboratory under the Clinical Laboratory Improvement Amendments (CLIA '88) and successfully participates in numerous proficiency testing program and external quality assessment schemes including those operated by: (A) the College of American Pathologists (blood Pb), (B) Wisconsin State Laboratory of Hygiene (blood Pb), (C) Institut National de Santé Publique du Québec, Le Centre de Toxicologie du Québec (CTQ, blood Pb and blood Mn), (D) the Trace Elements External Quality Assessment Scheme, at the University of Surrey, United Kingdom (blood Pb and blood Mn), and (E) the German External Quality Assessment Scheme, operated by the Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine of the Friedrich-Alexander University, Erlangen-Nuremberg, Germany (blood Pb and blood Mn). Throughout each analytical run, four levels of internal quality control (IQC) materials were analyzed for blood Pb and three IQC materials were analyzed for blood Mn. The IQC materials for blood Pb were previously characterized NYS DOH proficiency testing materials, while for blood Mn a combination of in-house produced, spiked human blood and external quality assessment materials from CTQ were used. The between-run precision for blood Mn based on IQC data was 7.0% RSD at 8.5 μg/L, and 2.7 % RSD at 23.1 μg/L. The between-run precision for blood Pb was 2.8% at 3.5 μg/dL. The method detection limit for Mn in blood is 1.5 μg/L while for blood Pb the method detection limit is 0.04 μg/dL.
The hair samples were analyzed in the trace metals research laboratory at Brigham and Women's Hospital. The samples were first washed in a 1% Triton™ X-100 solution and then digested using concentrated HNO3. Acid digestates were then analyzed by ICP-MS. Hair was analyzed for Mn with a detection limit of <2 ng/g.
2.2.3 Time-Weighted Distance (TWD)
TWD was calculated based on time spent at home (TimeHome) and school (TimeSchool) and the distance of each (DistanceHome and DistanceSchool) from the ferromanganese refinery. The time spent at home and school were estimated as 70% and 30%, respectively, based on daily activity logs recorded by parents and children participating in the personal air Mn exposure study (n=38) within the CARES population (Haynes et al, 2012). These children did not participate in the balance portion of the study. The distance of each balance participant's home and school from the refinery was determined by measuring the distance between the GPS latitude and longitude coordinates from each location. TWD was calculated using the following equation:
2.2.4 Parent Intellectual Function and Education
Full scale parent IQ was used in this study. Psychology graduate students at Marietta College were trained by co-author, K.N.D., to administer the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). We assessed education of the child's parent/legal guardian using the Barratt Simplified Measure of Social Status (BSMSS) (Barratt, 2006).
2.2.5 Postural Balance Assessment
Postural balance was quantified using the Hall-Effect type portable force platform system, Accusway Plus (Model ACS Plus, Advanced Mechanical Technology Inc., Watertown, MA) connected to a laptop computer. The Accusway Plus forceplate provides forces and moments along three orthogonal axes associated with subjects' postural sway during the testing. This Accusway Plus system was used to collect the postural balance data that was processed using a custom designed software, “KineLysis”© (Copyright All Rights Reserved, University of Cincinnati). The KineLysis© software used the force and moment signals for the three axes for the calculation of the subjects' x-y coordinates of their center of pressure movement associated with postural balance testing (Bhattacharya et al., 1988). The postural balance outcomes were calculated as the sway area (SA), sway length (SL), anterior-posterior excursion (AP) and medio-lateral excursion (ML). SA is defined as the area encompassed by the x-y plot of the excursion or movement of the center of pressure on the horizontal plane of the force plate. SL is defined as the total distance traversed by the center of pressure. AP is defined as the maximum excursion of the center of pressure in the anterior-posterior direction, whereas ML is defined as the maximum excursion of the center of pressure in the medio-lateral direction. In order to maintain consistency with foot placement between tests, footprints were marked on a sheet of paper on the platform surface using a 30-degree wooden block separating the feet. Postural balance was measured for 30 seconds each under six different sensory conditions in which somatosensory or visual inputs were challenged (Table 1). The child's height and weight were assessed prior to testing using a portable stadiometer and electronic scale.
Table 1.
Postural Balance Testing Conditions
| Test Condition |
Sensory Afferents
|
|
|---|---|---|
| Challenged | Dependent | |
| Eyes Open (EO) | Visual | |
| Proprioceptive | ||
| Vestibular | ||
| Eyes Closed (EC) | Visual | Proprioceptive |
| Vestibular | ||
| Standing on Foam, Eyes Open (FO) | Proprioceptive | Visual |
| Vestibular | ||
| Standing on Foam, Eyes Closed (FC) | Visual | Vestibular |
| Proprioceptive | ||
| Bending, Eyes Open (BO) | Vestibular | Visual |
| Proprioceptive | ||
| Bending, Eyes Closed (BC) | Visual | Proprioceptive |
| Vestibular | ||
Participants were instructed to perform certain tasks while standing on the force platform including standing with eyes open (EO), standing with eyes closed (EC), standing on a 3-inch thick foam pad placed on the force platform with eyes open (FO), standing on a 3-inch thick foam pad placed on the force platform with eyes closed (FC), standing and then bending with eyes open (BO), and standing and then bending with eyes closed (BC). When standing on the force platform with eyes open, participants were instructed to focus on a large blue dot placed at eye level. The bending tasks (BO and BC) involved the participant standing upright for 12 seconds, bending over for 5 seconds with legs straight and hands just below the knee, and returning to and maintaining the upright position for 13 seconds. Repeat trials were done for each test condition, and the average of each was obtained for statistical analysis. The force plate was calibrated twice daily to ensure accuracy and the same testing conditions for all participants.
2.2.6 Statistical Analysis
All statistical analyses were conducted using SAS statistical software (Version 9.3 for Windows, SAS Institute Inc, Cary, NC) and R software (The R Project for Statistical Computing, Version 2.8.1 for Windows, Department of Statistics and Mathematics of the WU Wien). Internal dose markers and postural sway data were log-transformed for statistical analyses. Descriptive statistics were calculated by SAS PROC UNIVARIATE and PROC MEANS. Exposure variables included internal dose markers of Pb in blood, Mn in blood and hair, and TWD from the ferromanganese refinery. SAS PROC CORR was used to calculate Pearson correlations between the exposure variables and sway outcomes and to identify potential covariates. Covariates included in the analyses were child's age, height/weight ratio, gender, parental IQ, and parental education. Blood Pb was included also as a covariate in the Mn exposure models for Mn in hair, blood, and TWD. Mn in hair blood and TWD were included in the Pb exposure models. SAS PROC REG was used to fit the linear regression models. The final model was obtained by using backward elimination along with a single forward inclusion step for the final model. Variables that had a statistical significance level of p<0.10 were kept in the final model. In secondary analyses, we evaluated the interaction between blood Pb and blood and hair Mn in final regression models. We also examined the Mn-Pb interaction by Mn tertiles in the blood Pb final models and compared the differences between the regression coefficients between the tertiles. Raw data was plotted as a stabilogram to illustrate excursion of the center of pressure between two participants, one with higher hair Mn and another with lower hair Mn. Microsoft Excel was used to create scatterplots for the relationships between internal dose markers of Mn, Pb, TWD from the ferromanganese refinery, and postural balance.
3. Results
3.1 Demographics and Internal Dose Measures
A total of 55 children (35 females) residing in the Marietta area participated in the postural balance assessment sub-study (Table 2). Twelve children were found ineligible to participate based on parent reported poor vision/eye disorders (n=3), seizures (n=2), ADD/ADHD (n=2), speech/language disorders (n=3), recent surgery (n=1), and a recent blood transfusion (n=1). There were no significant differences in demographic characteristics or biological metal concentrations between those who were ineligible or the participants except parent education. Parent education was significantly higher in balance participants (p=0.03).
Table 2.
Descriptive Characteristics of Study Participants
| Characteristic | SEM | Range | Median | |
|---|---|---|---|---|
| Age (mean years), n=55 | 8.5 | 0.13 | 1–9 | 8.6 |
| Height (mean m), n=55 | 1.3 | 0.43 | 1.2–1.5 | 1.3 |
| Weight (mean kg), n=55 | 30.2 | 1.06 | 19.8–51.6 | 28.9 |
| Ht/Wt (mean m/kg), n=55 | 0.05 | 0.001 | 0.03–0.06 | 0.05 |
| Residential Distance from Refinery (mean km), n=55 | 11.5 | 0.46 | 4.9–18.3 | 11.9 |
| School Distance from Refinery (mean km), n=55 | 10 | 0.50 | 3.7–19.5 | 11.9 |
| Time Weighted Distance (mean km), n=55 | 10.0 | 0.39 | 4.2–15.1 | 11 |
| Participant IQ (mean), n=55 | 103.8 | 1.61 | 72–125 | 105 |
| Parent IQ (mean), n=55 | 107.3 | 2.12 | 65–132 | 109 |
| Parent Education (mean years), n=55 | 15.2 | 0.31 | 9–20 | 15.5 |
| Mean Percentage of Time Spent at Current | 92 | |||
| Address (%) | ||||
| Female (%) | 64 | |||
| Blood Mn (mean μg/L), n=48 | 10 | 0.36 | 6.1–18.8 | 9.75 |
| Ln Blood Mn (mean μg/L) | 2.3 | 0.03 | 1.8–2.9 | 2.28 |
| Blood Pb (mean μg/dL,) n=48 | 0.86 | 0.05 | <DL-2.0 | 0.79 |
| Ln Blood Pb (mean μg/dL) | −0.22 | 0.05 | −1.1–0.69 | −0.24 |
| Hair Mn (mean μg/g), n=48 | 0.77 | 0.16 | 0.1–7.4 | 0.44 |
| Ln Hair Mn (mean μg/g) | −0.72 | 0.13 | −2.3–2.0 | −0.082 |
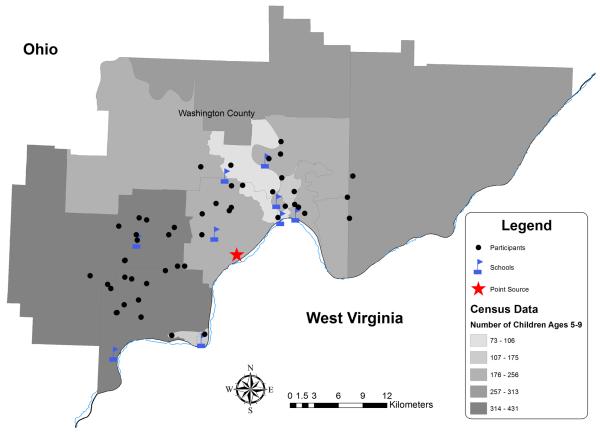
The mean age of balance sub-study participants was 8.5 years. The mothers of all postural balance participants reported residing in the community while pregnant with the child and remaining in the area until the day of testing. Of these children, 56% reported living at the same residential address their entire life. The participants' residential locations, schools, and location of the ferromanganese refinery are depicted in Figure 1. Mean residential distance from the ferromanganese refinery was 11.5 km (SEM=0.46). The WASI was administered to the parent or legal guardian who brought the child to the testing site. In our sample of 55, 47 (85%) were biological mothers, 6 (11%) biological fathers, 1 (2%) biological grandmother, and 1 (2%) female legal guardian. Parent education ranged from partial high school to graduate degree with a mean of partial college (at least one year).
Figure 1.
Map of CARES Postural Balance Participants in Washington County Ohio.
Approximately 87% of the participants provided a sample of blood, and although 100% of the participants provided a sample of hair, seven hair samples (13%) were of insufficient weight (≤5 mg) and were not included in the analyses. All of these insufficient weight samples were collected from males who had very short hair. Mean blood Mn concentration was 10 μg/L (SEM=0.36); mean blood Pb concentration was 0.86 μg/dL (SEM=0.05); and mean hair Mn was 0.77 μg/g (SEM=0.16) (Table 2). The Mn exposure measures were neither significantly correlated with each other nor blood Pb.
3.2 Postural Balance Assessment
In regression analyses measures of Mn exposure were statistically significant for nearly every postural balance testing condition. An increase in sway response outcomes (SL, SA, ML, and AP) implies poorer postural balance. Children with higher hair Mn concentrations had consistently larger SL, SA, and ML and AP excursion (Table 3). Hair Mn was also consistently associated with the FO and FC postural balance testing conditions (Table 3). Likewise, hair Mn was associated with EC under the SA, ML excursion, and AP excursion testing conditions. Higher blood Mn concentration was significantly associated with ML excursion under the EC and FO testing conditions.
Table 3.
Regression Coefficients from Linear Regression Models for Postural Balance Testin Conditions and Manganese and Lead Exposure Measures.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
|
| |||||
| Exposure | Postural Balance Testing Condition | β | SE | β | SE |
| ln Hair Mn a | |||||
| Sway Length | Standing on Foam, Eyes Open | 0.03 * | 0.03 | 0.07 ** | 0.03 |
| Standing on Foam, Eyes Closed | 0.04 | 0.03 | 0.08 ** | 0.03 | |
| Sway Area | Eyes Closed | 0.13 * | 0.08 | 0.11 | 0.08 |
| Standing on Foam, Eyes Open | 0.1 * | 0.06 | 0.14 ** | 0.06 | |
| Standing on Foam, Eyes Closed | 0.14 ** | 0.07 | 0.23 ** | 0.06 | |
| Medio-Lateral | Eyes Closed | 0.06 | 0.05 | 0.09 ** | 0.04 |
| Standing on Foam, Eyes Open | 0.07 * | 0.04 | 0.09 ** | 0.04 | |
| Standing on Foam, Eyes Closed | 0.1 ** | 0.04 | 0.09 ** | 0.04 | |
| Anterior-Posterior | Eyes Closed | 0.1 ** | 0.04 | 0.11 ** | 0.04 |
| Standing on Foam, Eyes Closed | 0.08 ** | 0.04 | 0.12 ** | 0.03 | |
| ln Blood Mn a | |||||
| Medio-Lateral | Eyes Closed | 0.32 ** | 0.16 | 0.36 ** | 0.14 |
| Standing on Foam, Eyes Open | 0.15 | 0.14 | 0.23 * | 0.13 | |
| TWD a | |||||
| Sway Length | Eyes Closed | −0.01 | 0.01 | − 0.02 * | 0.01 |
| Standing on Foam, Eyes Open | −0.01 | 0.01 | − 0.01 * | 0.01 | |
| Sway Area | Eyes Open | −0.02 | 0.02 | − 0.03 * | 0.02 |
| Eyes Closed | −0.04 | 0.02 | − 0.05 ** | 0.02 | |
| Bending, Eyes Open | −0.02 | 0.02 | − 0.03 * | 0.02 | |
| Medio-Lateral | Bending, Eyes Open | − 0.03 ** | 0.01 | −0.03** | 0.01 |
| Anterior-Posterior | Eyes Open | − 0.02 ** | 0.01 | − 0.03 ** | 0.01 |
| Eyes Closed | − 0.02 * | 0.01 | − 0.03 ** | 0.01 | |
| ln Blood Pb b | |||||
| Sway Length | Eyes Open | 0.25 ** | 0.07 | 0.12 * | 0.07 |
| Eyes Closed | 0.29 ** | 0.07 | 0.28 ** | 0.08 | |
| Standing on Foam, Eyes Open | 0.23 ** | 0.06 | 0.13 * | 0.06 | |
| Standing on Foam, Eyes Closed | 0.19 ** | 0.07 | 0.1 | 0.08 | |
| Bending, Eyes Open | 0.15 ** | 0.06 | 0.16 ** | 0.06 | |
| Sway Area | Eyes Open | 0.31 * | 0.16 | 0.22 | 0.16 |
| Eyes Closed | 0.5** | 0.17 | 0.43 ** | 0.18 | |
| Standing on Foam, Eyes Open | 0.40 ** | 0.15 | 0.19 | 0.16 | |
| Standing on Foam, Eyes Closed | 0.42 ** | 0.15 | 0.27 * | 0.15 | |
| Medio-Lateral | Eyes Open | 0.24 ** | 0.1 | 0.11 | 0.1 |
| Eyes Closed | 0.22 ** | 0.1 | 0.25 ** | 0.1 | |
| Standing on Foam, Eyes Open | 0.28 ** | 0.09 | 0.14 | 0.09 | |
| Anterior-Posterior | Eyes Closed | 0.31 ** | 0.1 | 0.28 ** | 0.1 |
| Standing on Foam, Eyes Closed | 0.27 ** | 0.08 | 0.19 ** | 0.09 | |
Adjusted for Gender, Height/Weight Ratio, Parent IQ, ln Blood Pb, Parent Education, Age
Adjusted for Gender, Height/Weight Ratio, Parent IQ, ln Hair Mn, ln Blood Mn, TWD, Parent Education, Age
p <0.10
p <0.05
A child's TWD from the ferromanganese refinery also had a significant inverse association with postural balance. TWD was statistically significant for the SL under the EC and EO testing conditions, and for SA under the EO, EC, and BO testing conditions. TWD was also statistically significant for ML under the BO testing condition, and AP under the EO and EC testing conditions. Ln blood Mn was significantly associated with ML excursion under the EC and FO testing conditions (p<0.05, p<0.10, respectively) (Table 3).
Children with higher blood Pb had poorer postural stability. After adjusting for confounders, ln blood Pb was significantly (p<0.05) associated with all four of the postural balance assessments: SL under the EC and BO testing conditions, SA under the EC testing condition, ML excursion under the EC testing condition, and AP excursion under the EC and FC testing conditions (Table 3). The interaction between hair and blood Mn and blood Pb was examined in the final regression models, but was not significantly associated with any of the postural balance outcomes. There was a significant interaction between blood Pb and hair Mn on both sway length and sway area in which the lowest hair Mn tertile modified the effect of blood Pb; however, the regression coefficients for each hair Mn tertile did not significantly differ from each other.
A child's age, gender, and height/weight ratio were also significant in many of the balance testing conditions. Older children had better postural balance demonstrating neuromuscular maturation consistent with findings by other researchers (Bhattacharya, 2006; Shumway-Cook and Woollacott, 1985). Female participants had significantly better postural balance than male participants, and those with a lower height/weight ratio also had significantly better postural balance. Other covariates that were significantly inversely associated with balance testing conditions were parent IQ and parent education.
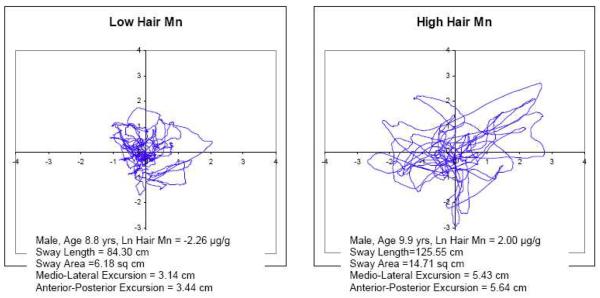
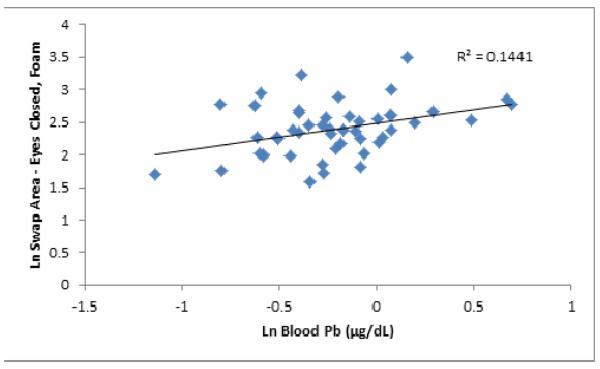
Examples of stabilograms from two participants with low and high hair Mn levels are presented (Figure 2) to illustrate the differences in center of pressure movement patterns associated with Mn exposure associated changes in postural balance. The stabilograms are from two male participants ages 8.8 years and 9.9 years with low ln hair Mn (−2.26 μg/g) and high ln hair Mn (2.2 μg/g), respectively. The participant with higher ln hair Mn has greater SL, SA, AP excursion and ML excursion. Scatterplots were produced to demonstrate the relationship between exposure and postural stability. Figure 3 shows sway area under the FC testing condition and hair Mn concentration, while Figure 4 shows SA under the EC testing condition and TWD. Figure 5 illustrates sway area under the FC testing condition and blood Pb.
Figure 2.
Stabilograms of Sway Length under the Eyes Closed on Foam Testing Condition Comparing two Participants with Low and High Ln Hair Mn Concentrations.
Figure 3.
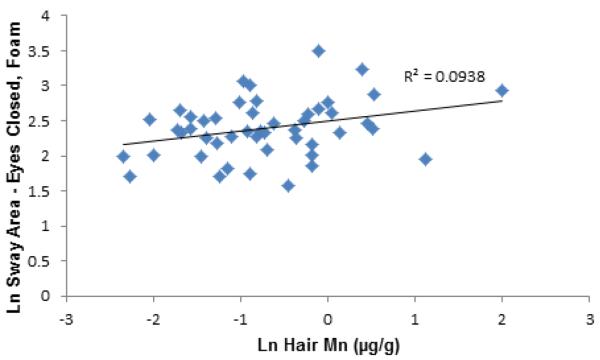
Scatter Plot of Ln Sway Area under the Standing on Foam with Eyes Closed Testing Condition and Ln Hair Mn.
Figure 4.
Scatter Plot of Ln Sway Area under the Standing with Eyes Closed Testing Condition and Time-Weighted Distance.
Figure 5.
Scatter Plot of Ln Sway Area under the Standing on Foam with Eyes Closed Testing Condition and Ln Blood Pb.
4. Discussion
Our study found significant associations between Mn exposure, as measured by hair Mn concentration and proximity to the refinery, and postural balance in children. Children with higher hair Mn concentrations had poorer postural stability than children with lower hair Mn concentrations. The mean hair Mn concentration in this cohort of children residing in Marietta was considerably higher than hair Mn concentrations reported previously in children residing near a hazardous waste site in Tar Creek, Oklahoma, where the average hair Mn value was 471.5 ppb (Wright et al., 2006). Although both the Tar Creek study and this present study used the same laboratory and methods of analysis for hair Mn, there remains controversy over trace element analysis of hair (ATSDR, 2001; Harkins and Susten, 2003) due to pre-analytical variables (e.g., hair color, exogenous contamination) and differences in collection protocols and laboratory procedures for washing hair. Recently, Eastman and colleagues (2013) demonstrated a rigorous hair cleaning methodology to remove exogenous Mn contamination; however, it is difficult to quantify the amount of Mn metabolically incorporated into the hair shaft. Validation of hair as a biomarker for Mn is promising and requires further collection and processing validation. As anticipated, blood Mn and hair Mn in this study were not correlated with TWD as internal dose markers reflect all environmental and internal sources of Mn, such as biologically stored Mn, diet, water, dust, soil, and potentially other sources of Mn not identified in this study. In addition, blood Mn levels are tightly regulated by normal homeostatic mechanisms (Finley et al., 2003), yet may reflect recent, active exposure (Zheng et al., 2011).
Our findings also indicate that children residing and attending schools closer to the ferromanganese refinery have significantly poorer postural balance than children living farther from the refinery. Residential distance from the refinery ranged from slightly less than 5 km to 18 km. This is consistent with previous work using the same postural balance assessment method by Standridge et al. (2008) which found an increase in neuromotor deficits in adults residing in Marietta, Ohio with shorter residential distance from the refinery.
Stationary air monitoring and personal air sampling was conducted in Marietta during two spring seasons during the same timeframe as the postural balance sub-study (Haynes et al., 2011). Mean stationary air Mn was 11 ng/m3 (Haynes et al., 2011). In 2006, ambient air Mn concentration in Marietta and the surrounding area was estimated as 20 ng/m3 to 2,610 ng/m3 using AERMOD, an EPA developed air pollution dispersion model (Haynes et al 2010). In comparison, Wallace and Slonecker (1997) conducted a survey of ambient air Mn (PM2.5) during the late 1980s and early 1990s and found mean ambient concentrations of 1 ng/m3 Mn in rural sites within national parks in the U.S. and 3 ng/m3 Mn within urban sites in California. The EPA reference concentration (RfC) for ambient air Mn is 50 ng/m3 Mn (EPA, 2012).
Although blood Mn may not be a reliable internal dose marker for environmental exposure to Mn, it is commonly use for assessing Mn exposure. The mean blood Mn concentration in this study population was 10 μg/L. This value is similar to concentrations reported previously in children exposed chronically to Mn in the air (Gulson et al., 2006; Menezes-Filho et al., 2009; Rodriguez-Agudelo et al., 2006; Röllin et al., 2005; Hernandez-Bonilla et al., 2011). The study on children in Mexico reported average blood Mn levels of 10.2 μg/L (Rodriguez-Agudelo et al., 2006). Children in Australia exposed to Mn had a mean blood Mn concentration of 11.6 μg/L (Gulson et al., 2006). These levels fall within the normal range in blood for adults, 4–15 μg/L (ATSDR, 2012).
The association between postural balance and exposure to Mn is biologically plausible as Mn accumulates in the basal ganglia (Dorman et al., 2006; Guilarte et al., 2006), a region of the brain that controls movement, along with the cerebellum (Nambu 2008; Kaneda et al., 2002). Postural imbalance associated with Mn exposure may be the result of its influence on the vestibular and proprioception systems that control postural muscle contractions (Bacsi and Colebatch, 2005; Bock, 2008; Uchino et al., 2007). Furthermore, like other heavy metals, such as Pb, Mn is a calcium channel blocker and could influence the postural muscle contraction pattern by inhibiting initiation of the calcium spike that leads to muscle contraction (Nasu et al., 1995; Hagiwara and Nakajima, 1966). In addition, cerebellar effects could be at play as demonstrated in a previous study in which workers exposed to 71 μg/m3 of Mn in total dust for about 15 years had deficits in vestibular function (Lucchini et al., 1999), possibly detrimentally influencing their postural balance.
The bending action incorporated into the dynamic bending postural balance tests BO and BC, evaluates the functionalities of all the relevant afferent motor neurons under more challenging conditions for the vestibular/ proprioceptive /visual systems. Bending is a dynamic task that challenges the proprioceptive function as an individual returns to the upright position (Table 1). In this study, SA and ML were significantly associated with TWD when the children were bending with their eyes open. This suggests that the remaining undisturbed afferents, the visual and vestibular systems, were not capable of maintaining appropriate postural balance.
Postural instability has been significantly associated with Mn exposure in adult and child cohorts (Bowler et al., 2007; Kim et al., 2007; Rodríguez-Agudelo et al., 2006; Standridge et al., 2008; Hernández-Bonilla et al., 2011; Lucchini et al. 2012; Takser et al., 2003). Using the Computer Assisted Tremor Analysis System (CATSYS) to determine postural instability, welders with higher exposure to Mn, as measured by air and blood Mn, had greater postural instability compared to age-matched production workers from the same facility (Kim et al., 2007). Using the same system, space bridge welders had significant impairment in the stability assessment among the workers (Bowler et al., 2007). Residents living near Mn mines in Mexico also demonstrated poor performance on motor tests; however the authors found no association between motor performance and blood Mn (Rodríguez-Agudelo Y et al., 2006). Postural instability in non-occupationally exposed adults residing in Marietta, Ohio was assessed using a Hall-Effect type Accusway force platform system (Standridge et al., 2008). This is the same type of system used in the current study with children. Standridge and colleagues (2008) found a positive association between hair Mn and the EO and EC test conditions for both sway area and sway length (p<0.05). The force platform system has also been used to evaluate neuromotor function on workers exposed to jet fuel (Smith et al., 1997) and children exposed to Pb (Bhattacharya et al., 1995, 2006). This quantitative posturagraphy method is highly sensitive as it can detect participants' movement pattern of center of pressure with an error of less than 2% (Bhattacharya et al., 1987) and thereby require a small sample size (Bhattacharya et al., 1988; Standridge et al., 2008).
Recently, Lucchini and colleagues (2012) reported that Italian adolescents chronically exposed to high levels of air Mn had significantly higher body sway with eyes closed than children living in an unexposed community, and a significant correlation between resting tremor intensity and blood and hair Mn concentrations (Lucchini et al., 2012). In a Mexican cohort, Hernández-Bonilla and colleagues (2011) found mixed results as children in the Mn exposed community performed faster on a test of manual dexterity and fine motor coordination, but had more errors than children in the comparison community. Takser and colleagues (2003) observed a negative relationship between umbilical cord blood Mn and hand skill at 3 years of age, but the association was only significant for boys.
The finding of a significant association between ln blood Pb and several balance outcomes is further evidence that Pb displays a no-threshold effect. Blood Pb concentration in children in this study did not exceed 1 μg/dL. The Centers for Disease Control and Prevention (CDC) recently concurred with a recommendation from their Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) that an elevated blood Pb level should be re-defined as a reference value based on the National Health and Nutrition Examination Survey (NHANES) 97.5th percentile for children age 1–5, currently 5 μg/dL (CDC, 2012). The results of this study are consistent with the CDC and ACCLPP statements that no level of Pb has been proven safe for children (CDC, 2012) as ln blood Pb was significantly associated with three postural balance assessment test conditions. Numerous studies have demonstrated the negative impacts of elevated blood Pb (15 μg/dL) in children on postural balance (Bhattacharya et al., 1988; Bhattacharya et al., 1990; Bhattacharya et al., 2006; Bhattacharya et al., 2007), yet, to our knowledge, this study is the first to demonstrate an association between these adverse health effects in children with blood Pb concentrations less than 1 μg/dL. Recently, blood Pb <2μg/dL was associated with postural balance dysfunction in adults participating in the NHANES 1999–2004 (Min et al., 2012). There is also evidence that exposure to Pb during early life is associated with fall related injury during adolescence (Kincl et al., 2006). The finding of an interaction between the lowest tertile of hair Mn and blood Pb in post-hoc analyses is exploratory, and may suggest that low Mn modifies the negative impact of Pb on balance. This finding is consistent with the known role of Mn as an essential nutrient.
This current study has several limitations. For example, residential and school distance from the refinery is based on the child's current home and school addresses. It does not represent a lifetime exposure measure; however, given that hair grows approximately 1 cm/month, we can assume that hair Mn would represent approximately 1–6 months of their exposure. Only three children in this sub-study reported a residential move within the catchment area within 6 months prior to the hair collection date. In addition, we did not determine individual variation in Mn metabolism. Variation in iron metabolism genes may contribute to variability in internal dose measures of Mn by influencing Mn absorption, distribution, and excretion (Claus Henn et al 2011; Haynes et al 2010). Another limitation is that our measure of exposure, TWD, does not include wind direction or wind speed. In our previous analyses, we found that wind direction and wind speed were significant variables when predicting personal air Mn (Haynes et al., 2011). We were also limited in this study by our exposure assessment as we did not include a measure of Mn in soil, water, food, or other unidentified source of Mn. In a recent analysis of adolescents chronically exposed to airborne Mn from ferroalloy production facilities in the province of Brescia, Italy, soil Mn levels were significantly associated with motor coordination (Lucchini et al., 2012). Cognitive deficits were observed in children drinking tap water with Mn concentrations within the highest quintile (median, 216 μg/L; range, 154 - 2,700 μg/L) in children from Québec (Bouchard et al., 2011). Full scale, performance and verbal IQ were significantly lower in children drinking well water with Mn concentrations >1,000 μg/L compared to children drinking well water with Mn concentrations <200 μg/L, in Bangladesh after adjusting for sociodemographic factors (Wasserman et al., 2006). The majority of our balance study participants (95%) reported receiving their drinking water from the public water system. Three of our participants reporting using well water. Of these, one well water source was analyzed for metals, and the Mn concentration was below the level of detection (1.25 μg/L). In the Québec study, estimated dietary Mn intake was not significantly associated with child IQ (Bouchard et al., 2011). Our volunteer selection method for CARES limits the study's ability to generalize the results to the general population; however, this sampling mechanism was selected to maximize our bidirectional research partnership with the community (Haynes et al., 2011) to ensure that all families interested in participating and met the inclusion criteria could participate. This sampling mechanism also provides strengths to the study as a volunteer sample is significantly more likely to be female, more educated and have higher cognitive scores than a random sample (Ganguli et al., 1998). Our data, which demonstrate a negative association between postural instability and exposure measures, may be biasing our results toward the null hypothesis and thus, under representing the impact of exposure on outcome.
In summary, our data demonstrate that all afferents relevant for maintenance of static postural balance (vestibular, visual, and proprioceptive) (Bhattacharya et al., 2006; Crowe and Horak, 1988) were detrimentally influenced by Mn and low-level Pb exposure. In particular, ln hair Mn was significantly, positively associated with postural instability in children exposed to airborne Mn. Children residing near the ferromanganese refinery had greater postural instability than children residing farther from the refinery. Additionally, airborne Mn measured during the testing period was four times lower than the EPA RfC for ambient air Mn (EPA, 2012) suggesting that the EPA RfC should be reevaluated to consider health effects of children exposed to continuous ambient air Mn. Given the cross-sectional study design, this study cannot support a conclusion that the observed gross motor function deficits observed in this study are attributable to recent or early life exposure to airborne Mn. A follow-up study is recommended to evaluate the impact of these early indicators of poor postural balance on later life falls and fall related injuries. Finally, ambient air Mn can affect regions of the brain known to control movement, and novel biomarkers of chronic exposure such as tooth Mn, or biomarkers of effect, such as or magnetic resonance imaging may be useful tools to characterize chronic exposure and neurological effects.
Highlights
Postural balance was assessed in children exposed to ambient air manganese.
Manganese exposure was significantly associated with poor postural balance.
Low-level lead exposure was significantly associated with poor postural balance.
Ambient air Mn can affect regions of the brain known to control movement.
Acknowledgements
This research study was supported by the National Institute of Environmental Health Sciences: 5T32ES10957, R01ES016531, R03 HD059615-01, and P30-ES06096. Support for this work was also received by an Institutional Clinical and Translational Science Award, NIH/NCRR 8UL1TR000077-04. The authors would like to thank Dawn Wittberg, Marietta College for use of their facilities to conduct the study, and the Marietta Community Actively Researching Exposure Community Advisory Board. This work was completed in partial fulfillment for the Doctor of Philosophy degree in Epidemiology in the Department of Environmental Health, Division of Epidemiology and Biostatistics, University of Cincinnati College of Medicine. All authors declare no actual or potential competing financial interests.
Abbreviations
- ACCLPP
Advisory Committee on Childhood Lead Poisoning Prevention
- AP
Anterior-Posterior
- ATSDR
Agency for Toxic Substances and Disease Registry
- BC
Standing on the force platform and bending over with eyes closed
- BO
Standing on the force platform and bending over with eyes open
- BSMSS
Barratt Simplified Measure of Social Status
- CARES
Community Actively Researching Exposure Study
- CATSYS
Computer Assisted Tremor Analysis System
- CDC
Centers for Disease Control and Prevention
- CLIA
Clinical Laboratory Improvement Amendments
- CTQ
Le Centre de Toxicologie du Québec
- EC
Standing on the force platform with eyes closed
- EO
Standing on the force platform with eyes open
- EPA
Environmental Protection Agency
- FC
Standing on a 3-inch thick foam pad placed on the force platform with eyes closed
- FO
Standing on a 3-inch thick foam pad placed on the force platform with eyes open
- GFAAS
Graphite Furnace Atomic Absorption Spectrometry
- ICP-MS
Inductively Coupled Plasma-Mass Spectrometry
- IQC
Internal Quality Control
- Ln
Natural Log
- ML
Medio-Lateral
- Mn
Manganese
- NHANES
National Health and Nutrition Examination Survey
- NIST
National Institute of Standards and Technology
- NYS
DOH New York State Department of Health
- Pb
Lead
- SA
Sway Area
- SAS
Statistical Analytical Software
- SL
Sway Length
- SRM
Standard Reference Material®
- RfC
Reference Concentration
- TWD
Time-Weighted Distance
- US
United States
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M. Manganese: Brain Transport and Emerging Research Needs. Environ Health Perspect. 2000;108:429–432. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345–9. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Aschner M. The transport of manganese across the blood-brain barrier. Neurotoxicology. 2006;27:311–44. doi: 10.1016/j.neuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Hair Analysis Panel Discussion: Exploring the State of the Science. Atlanta, Georgia: 2001. Division of Health Assessment and Consultation and Division of Health Education and Promotion. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012. [PubMed] [Google Scholar]
- Bacsi AM, Colebatch JG. Evidence for reflex and perceptual vestibular contributions to postural control. Exp Brain Res. 2005;160:22–8. doi: 10.1007/s00221-004-1982-2. [DOI] [PubMed] [Google Scholar]
- Barratt W. The Barratt Simplified Measure of Social Status. Indiana State University; Terre Haute, IN: 2006. [Google Scholar]
- Bhattacharya A, Shukla R, Dietrich K, Bornschein R, Berger O. Effect of early lead exposure on children's postural balance. Dev Med Child Neurol. 1995;37:861–78. doi: 10.1111/j.1469-8749.1995.tb11939.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Shukla R, Dietrich KN, Bornschein RL. Effect of early lead exposure on the maturation of children's postural balance: A longitudinal study. Neurotoxicol Teratol. 2006;28:376–85. doi: 10.1016/j.ntt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Shukla R, Auyang ED, Dietrich KN, Bornschein R. Effect of succimer chelation therapy on postural balance and gait outcomes in children with early exposure to environmental lead. Neurotoxicology. 2007;28:686–95. doi: 10.1016/j.neuro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Shukla R, Bornschein RL, Dietrich KN, Keith R. Lead Effects on Postural Balance of Children. Environ Health Perspect. 1990;89:35–42. doi: 10.1289/ehp.908935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Shukla R, Bornschein R, Dietrich K, Kopke JE. Postural disequilibrium quantification in children with chronic lead exposure: a pilot study. Neurotoxicology. 1988;9:327–40. [PubMed] [Google Scholar]
- Bhattacharya A, Morgan R, Shukla R, Ramakrishanan HK, Wang L. Noninvasive estimation of afferent inputs for postural stability under low levels of alcohol. Ann of Biomed Engineering. 1987;15:533–50. doi: 10.1007/BF02364247. [DOI] [PubMed] [Google Scholar]
- Birdsall RE, Kiley MP, Segu ZM, Palmer CD, Madera M, Gump BB, et al. Effects of lead and mercury on the blood proteome of children. J Proteome Res. 2010;9:4443–53. doi: 10.1021/pr100204g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock NA, Paiva FF, Nascimento GC, Newman JD, Silva AC. Cerebrospinal fluid to brain transport of manganese in a non-human primate revealed by MRI. Brain Res. 2008;1198:160–70. doi: 10.1016/j.brainres.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÉ, Bouffard T, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–43. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup and Environ Med. 2007;64:167–77. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Wong BA, Buccellato MA, Costa ER, et al. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169(3):238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) [Internet] CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention.”. 2012 Available from: http://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf.
- Claus Henn B, Kim J, Wessling-Resnick M, Téllez-Rojo MM, Jayawardene I, Ettinger AS, et al. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ Health. 2011;10:97. doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of manganese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- Crowe TK, Horak FB. Motor Proficiency Associated with Vestibular Deficits in Children with Hearing Impairments. Phys Ther. 1988;68:1493–9. [PubMed] [Google Scholar]
- Davis JM. Inhalation health risks of manganese: an EPA perspective. Neurotoxicology. 1999;20:511–8. [PubMed] [Google Scholar]
- Dietrich KN. Environmental toxicants. In: Yeates KO, et al., editors. Pediatric Neuropsychology. 2nd Edition Guilford; New York: 2010. pp. 211–264. [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65(20):1493–511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Wong BA, Dye JA, Robertson ID. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 2006;92:219–27. doi: 10.1093/toxsci/kfj209. [DOI] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol. 2013;47(3):1629–37. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, et al. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology. 2008;29:48–59. doi: 10.1016/j.neuro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–8. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) [Internet]. Reports. Integrated Risk Information System. [updated 2012 August 09; cited 2012 February 23]. Available from: www.eps.gov/iris/subst/0373.htm/
- Environmental Protection Agency (EPA) [Internet]. Reports. Toxic Release Inventory Explorer. Available; [updated 2012 October 16; cited 2012 February 23]. Available from: www.epa.gov/triexplorer/
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, et al. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66:403–7. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Finley JW, Penland JG, Pettit RE, Davis CD. Dietary manganese intake and type of lipid do not affect clinical or neuropsychological measures in healthy young women. J Nutr. 2003;133(9):2849–56. doi: 10.1093/jn/133.9.2849. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Lytle ME, Reynolds MD, Dodge HH. Random versus volunteer selection for a community-based study. J of Gerontology. 1998;53A(1):M39–46. doi: 10.1093/gerona/53a.1.m39. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan J, Degaonkar M, Chen MK, Barker PB, Syversen T, et al. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol. Sci. 2006;94:351–8. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Gulson B, Mizon K, Taylor A, Korsch M, Stauber J, Davis JM, et al. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline-preliminary results. Environ Res. 2006;100:100–14. doi: 10.1016/j.envres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966;49:793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins DK, Susten AS. Hair analysis: exploring the state of the science. Environ Health Perspect. 2003;111:576–8. doi: 10.1289/ehp.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, Heckel P, Ryan P, Roda S, Leung YK, Sebastian K, et al. Environmental manganese exposure in residents living near a ferromanganese refinery in Southeast Ohio: a pilot study. Neurotoxicology. 2010;31:468–74. doi: 10.1016/j.neuro.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, Beidler C, Wittberg R, Meloncon L, Parin M, Kopras EJ, et al. Developing a Bidirectional Academic-Community Partnership with an Appalachian American Community for Environmental Health Research and Risk Communication. Environ Health Perspect. 2011;119(10):1364–72. doi: 10.1289/ehp.1003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, Ryan P, Chen A, Brown D, Roda S, Kuhnell P, Wittberg D, Terrell M, Reponen T. Assessment of personal exposure to manganese in children living near a ferromanganese refinery. Sci Total Environ. 2012;427–428:19–25. doi: 10.1016/j.scitotenv.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Bonilla D, Schilmann A, Montes S, Rodríguez-Agudelo Y, Rodríguez-Dozal S, Solís-Vivanco R, et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32(5):615–21. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections. J Neurophysiol. 2002;88:1420–32. doi: 10.1152/jn.2002.88.3.1420. [DOI] [PubMed] [Google Scholar]
- Kincl LD, Dietrich KN, Bhattacharya A. Injury Trends for Adolescents with Early Childhood Lead Exposure. J of Adol Health. 2006;39(4):604–6. doi: 10.1016/j.jadohealth.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Kim EA, Cheong HK, Choi DS, Sakong J, Ryoo JW, Park I, et al. Effect of occupational manganese exposure on the central nervous system of welders: 1H magnetic resonance spectroscopy and MRI findings. Neurotoxicology. 2007;28:276–83. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Miodovnik A. Children's health and the environment: an overview. Mt Sinai J Med. 2011;78:1–10. doi: 10.1002/msj.20236. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, et al. Long-term exposure to `low levels' of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferromanganese emission. Neurotoxicology. 2012;33(4):687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey W, Gwynn RC, Jeffrey N, Kass D, Thorpe LE, Garg RK, et al. A biomonitoring study of lead, cadmium, and mercury, in the blood of New York City adults. Environ. Health Perspect. 2007;115:1435–41. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Bouchard M, Sarcinelli Pde N, Moreira JC, et al. Manganese exposure and the neuropsychological effect on children and adolescents: a review. Pan Am J Public Health. 2009;26:541–8. doi: 10.1590/s1020-49892009001200010. [DOI] [PubMed] [Google Scholar]
- Min K, Lee K, Park J, Min J. Lead and Cadmium Levels and Balance and Vestibular Dysfunction among adult participants in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Environ Health Perspect. 2012;120:413–7. doi: 10.1289/ehp.1103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Guthrie WF, Vetter TW, Turk GC, Palmer CD, Lewis ME, Jr, et al. Comparison of clinical methods with isotope dilution inductively coupled plasma mass spectrometry for the new Standard Reference Material 955c Lead in Caprine Blood. J Anal At Spectrom. 2009;24:1170–8. [Google Scholar]
- Nambu A. Seven problems of the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Nasu T, Oosako H. Dantrolene blocks the tonic contractions to carbachol and histamine with smaller effects on the phasic due to release of intracellular Ca2+ in ileal longitudinal muscle. Gen Pharmacol. 1995;26(7):1591–6. doi: 10.1016/0306-3623(95)00055-0. [DOI] [PubMed] [Google Scholar]
- Palmer CD, Lewis ME, Jr, Geraghty CM, Barbosa F, Jr, Parsons PJ. Determination of lead, cadmium and mercury in blood for assessment of environmental exposure: a comparison between inductively coupled plasma-mass spectrometry and atomic absorption spectrometry. Spect Acta B. 2006;61:980–90. [Google Scholar]
- Papavasiliou PS, Miller ST, Cotzias GC. Role of liver in regulating distribution and excretion of manganese. Am J Physiol. 1966;211(1):211–6. doi: 10.1152/ajplegacy.1966.211.1.211. [DOI] [PubMed] [Google Scholar]
- Praamsma ML, Arnason JG, Parsons PJ. Monitoring Mn in whole blood and urine: a comparison between electrothermal atomic absorption and inorganic mass spectroscopy. J Anal At Spectrom. 2011;26:1224–32. [Google Scholar]
- Praamsma ML, Jones DR, Jarrett JM, Dumas P, Cirtiu CM, Parsons PJ. A comparison of clinical laboratory data for assigning a consensus value for manganese in a caprine blood reference material. J Anal At Spectrom. 2012;27:1975–82. doi: 10.1039/C2JA30142C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. Retrieved from http://www.R-project.org. [Google Scholar]
- Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61:509–17. doi: 10.1111/j.1471-4159.1993.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Agudelo Y, Riojas-Rodríguez H, Ríos C, Rosas I, Sabido Pedraza E, Miranda J, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368:542–56. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Röllin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ Res. 2005;97:93–9. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. The growth of stability: postural control from a developmental perspective. Journal of Motor Behavior. 1985;17:131–47. doi: 10.1080/00222895.1985.10735341. [DOI] [PubMed] [Google Scholar]
- Smith LB, Bhattacharya A, Lemasters G, Succop P, Puhala E, 2nd, Medvedovic M, et al. Effect of chronic low-level exposure to jet fuel on postural balance of U.S. Air Force personnel. J Occup Environ Med. 1997;39:623–32. doi: 10.1097/00043764-199707000-00007. [DOI] [PubMed] [Google Scholar]
- Standridge JS, Bhattacharya A, Succop P, Cox C, Haynes E. Effect of Chronic Low Level Manganese Exposure on Postural Balance: A Pilot Study of Residents in Southern Ohio. J Occup Environ Med. 2008;50:1421–9. doi: 10.1097/JOM.0b013e3181896936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. NeuroToxicology. 2003;24(4–5):667–74. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Uchino A, Noguchi T, Nomiyama K, Takase Y, Nakazono T, Nojiri J, et al. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49:715–20. doi: 10.1007/s00234-007-0243-z. [DOI] [PubMed] [Google Scholar]
- Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12:161–74. doi: 10.1155/NP.2005.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L, Slonecker T. Ambient air concentrations of fine (PM2.5) manganese in U.S. national parks and in California and Canadian cities: the possible impact of adding MMT to unleaded gasoline. J Air Waste Manag Assoc. 1997;47(6):642–52. doi: 10.1080/10473289.1997.10463930. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment, Inc.; San Antonio, TX: 1999. [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–6. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32(1):1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Albini E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity : a review and a proposal. Am J Ind Med. 2007;50(11):812–830. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]