Abstract
Developing new strategies to treat cerebral ischemic-reperfusion injury will require a better understanding of the mechanisms that underlie vascular permeability. In this study we examined the temporal expression of Src and angiogenic factors in rat brain after focal cerebral ischemia and reperfusion and analyzed the relationships among those factors. We also investigated the effect of Src inhibitor PP1 in ischemic reperfusion. Rats were subjected to middle cerebral artery occlusion for 90 min followed by reperfusion with or without PP1 treatment. Src mRNA increased at 3 h after reperfusion and then gradually declined. Phosphorylation of Src at Y418 displayed a biphasic increase. Phosphorylation increased as early as 3 h and peaked at 6 h; after decreasing, it peaked again at 3 to 7 days. Increases in Src mRNA and phosphorylation correlated positively with levels of vascular endothelial growth factor (VEGF) and angiopoietin-2 (Ang-2), and negatively with levels of angiopoietin-1 (Ang-1) and zonula occludens-1 (ZO-1). Changes in the expression of these factors correlated with the progress of vascular permeability, especially early after reperfusion. Hence, dynamic temporal changes in Src Y418 phosphorylation may modulate vascular permeability after cerebral ischemia and reperfusion. PP1 effectively decreased Src Y418 phosphorylation and the expression of VEGF and Ang-2 and increased the expression of Ang-1 and ZO-1. It also reduced cerebral infarct size and neurologic dysfunction. Therefore, Src may represent a new therapeutic target for reducing tissue damage caused by increased vascular permeability.
Keywords: angiogenic factors, cerebral ischemia, PP1, Src, vascular permeability, zonula occludens-1
1. Introduction
The Src family comprises a subclass of membrane-associated non-receptor tyrosine kinases that are involved in a variety of cellular signal transduction pathways (Thomas and Brugge, 1997), including gene transcription, adhesion regulation, cell proliferation, and angiogenesis (Brown and Cooper, 1996; Theus et al., 2006; Tang et al., 2007; Schlessinger, 2000). One study has reported that Src kinase activity correlates with vascular endothelial growth factor (VEGF)-mediated vascular permeability and brain damage after permanent focal cerebral ischemia in mice (Paul et al., 2001). Using the same model in rats, we further identified that the expression of Src correlates with VEGF and angiopoietin expression, and with cerebral edema formation (Zan et al., 2011). However, the exact contribution of Src Y418 phosphorylation to vascular permeability after cerebral ischemia and reperfusion remains to be established.
VEGF is an endothelial mitogen as well as a potent mediator of vascular permeability (Schoch et al., 2002). Therefore, it may contribute to brain edema associated with ischemia and reperfusion. The angiopoietins represent another family of proteins that play an essential role in angiogenesis. Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) have been well characterized. Ang-1 is required for blood vessel stabilization and maturation and acts as an anti-permeability factor to prevent vascular leakage (Risau et al., 1998; Davis et al., 1996; Suri et al., 1996). In contrast, endogenous Ang-2 serves as a natural inhibitor of Ang-1 activity, disrupting vessel integrity and promoting disassembly of the cellular components (Pfaff et al., 2006). Although these angiogenic factors play critical roles in maintaining vascular integrity, their upstream regulators need to be identified.
To better understand the pathologic role of Src and its possible mechanism of action in cerebral ischemia and reperfusion, we characterized the temporal profile of Src mRNA expression and Y418 phosphorylation and the expression of angiogenic factors at mRNA and protein levels after focal cerebral ischemia and reperfusion in rats, and analyzed the possible correlations among them. Additionally, using the same model, we investigated the effect of Src inhibitor PP1 on Src mRNA expression and Y418 phosphorylation, vascular permeability, brain edema formation, lesion volume, and neurologic function.
2. Experimental procedures
2.1. Animals and ischemia-reperfusion model
Adult male Sprague-Dawley rats weighing 250–300 g were purchased from the Center of Experimental Animals. The rats were housed under standard conditions with free access to rat chow and tap water before and after surgery. All experimental procedures were approved by the Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to use alternatives to in vivo techniques.
The rats were randomized into two groups: the sham-operated control group and the ischemia-reperfusion model group. Animals were anesthetized with 10% chloral hydrate (400 mg/kg, i.p.) and then underwent transient middle cerebral artery occlusion (MCAO) with the filament model as previously described (Longa et al., 1989). Body temperature of the rats was continuously monitored and maintained at 37.0 ± 0.5°C with a heating pad during and after the surgery. Reperfusion was induced after 90 min of ischemia by removing the filament. Rats in the ischemia-reperfusion group were subdivided into seven subgroups based on duration of reperfusion: 3 h, 6 h, 12 h, 1 day, 3 days, 7 days, or 14 days. At each of these time points after reperfusion, one subgroup of rats was anesthetized; brains were quickly removed after decapitation. Sham-operated rats underwent anesthesia and surgery without the filament insertion.
2.2. Treatment with PP1
To address the role of Src in ischemia-reperfusion-induced brain damage and the potential underlying mechanisms, we investigated the effects of the Src inhibitor PP1. PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo [3,4-d] pyrimidine) is a cell-permeable pyrazolopyrimidine compound that inhibits Src family tyrosine kinases Lck, Fyn, Hck, and Src (IC50 = 5, 6, 20, and 170 nM, respectively). Its molecular weight (MW) is 281.36, and its chemical formula is C16H19N5. PP3 (4-amino-7-phenylpyrazolo[3,4-d]pyrimidine), an inactive analog of PP1 (MW = 211.2; C11H9N5), inhibits the activity of EGFR kinase (IC50 = 2.7 μM) and was used as a negative control for PP1. PP1 and PP3 were freshly dissolved in dimethyl sulfoxide (DMSO) and further diluted in phosphate-buffered saline (final dilution, 0.1% DMSO) just before surgery. Rats were randomly assigned to receive PP1 (1.5 mg/kg), PP3 (1.5 mg/kg), or an equal volume of vehicle (0.1% DMSO) by intraperitoneal injection 30 min before surgery (Kusaka et al., 2004). The rats were euthanized by decapitation 1 day after reperfusion.
2.3. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from ipsilateral cerebral cortex (n = 6/group) by TRIzol Reagent (Invitrogen, Carlsbad, CA). The Transcriptor First Stand cDNA Synthesis Kit (Roche, USA) was used for the reverse transcription reactions. Using 1 μg of total RNA for each sample, we synthesized the cDNA according to the manufacturer's instructions. Then 2 μL of the reverse transcription reaction was used for PCR amplification in a volume of 20 μL. The reactions contained gene-specific primers for Src forward: 5'-AGA GTG CCC TAT CCT GGG AT-3', SRC reverse: 5'-AAA GTA GTC TTC CAG GAA GGCC-3'; VEGF forward: 5'-CTG CTC TCT TGG GTG CACT-3', VEGF reverse: 5'-ATA CAC TAT CTC ATC GGG GTA CT-3'; Ang-1 forward: 5'-GAA AAT TAT ACT CAG TGG CTG GAA AAA- 3', Ang-1 reverse: 5'-TTC TAG GAT TTT ATG CTC TAA TAA ACT-3'; Ang-2 forward: 5'-AAA GAG TAC AAA GAG GGC TTC GGG AGC-3', Ang-2 reverse: 5'-GTA GTA CCA CTT GAT ACC GTT GAA CTT-3'; zonula occludens-1 (ZO-1) forward: 5'-CAG GAA AAT GAC CGA GTC GC-3', ZO-1 reverse: 5'-CCA ATG TGA CCT TGG TGG GT-3'; or β-actin forward: 5'-CCT CTG AAC CCT AAG GCC AAC-3', β-actin reverse: 5'-TGC CAC AGG ATT CCA TAC CC-3'. All reactions were carried out in a thermal cycler (Bio-Rad, USA) under the following conditions: 5 min at 94°C, 40 cycles of amplification (94°C for 30 s, 58°C for 30 s, and 72°C for 40 s), and 72°C for 10 min. All products were separated on 2% agarose gels containing 0.5 μg/mL ethidium bromide. Band intensities were evaluated by the Chemidoc XRS imaging densitometer system (Bio-Rad, Hercules, CA) and quantified with Quantity One software.
2.4. Western blot analysis
Protein was extracted from ipsilateral cerebral cortex (n = 6/group) with lysis buffer. Equal amounts of total protein extract were loaded onto sodium dodecyl sulfate (10%) polyacrylamide gels and separated by electrophoresis. Protein was then transferred to polyvinylidene difluoride membranes by electroblotting. After being blocked for 2 h with 5% nonfat dried milk in Tris-buffered saline containing 0.05% Tween 20, the membrane was incubated overnight at 4°C with rabbit polyclonal anti-Src (phospho-Y418, 1:600; Abcam, Cambridge, MA), rabbit polyclonal anti-Ang-2 (1:800; Abcam), rabbit polyclonal anti-ZO-1 (1:600; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-VEGF (1:1000; Abcam), or goat polyclonal anti-Ang-1 (1:800; Santa Cruz Biotechnology). Membranes were then washed and incubated with secondary antibodies for 1 h at room temperature. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was determined as an internal control. Protein bands were visualized by the Western blot detection system (Amersham Biosciences, Piscataway, NJ), and band intensity was quantified by NIH ImageJ analysis software. Three independent experiments were duplicated.
2.5 Immunohistochemistry
Rats were perfused transcardially with saline followed by 4% paraformaldehyde, and their brains were removed and post-fixed in 4% paraformaldehyde overnight before being embedded in paraffin. A series of 4-μm-thick sections was cut from the block and used for immunohistochemical staining. Selected sections at 2.0, 0, and −2.0 mm from the bregma were incubated overnight at 4°C with rabbit polyclonal anti-Src (phospho-Y418, 1:100; Abcam), rabbit polyclonal anti-Ang-2 (1:200; Abcam), rabbit polyclonal anti-ZO-1 (1:250; Santa Cruz Biotechnology), mouse monoclonal anti-VEGF (1:200; Abcam), or goat polyclonal anti-Ang-1 (1:200; Santa Cruz Biotechnology) and then in the appropriate secondary antibody for 1 h. Staining was visualized with diaminobenzidine (Maixin Bio, China). Sections used as negative controls were processed in parallel but were not exposed to primary antibody. Immunoreactive cells or microvessels were counted around the infarct region under a 40× objective for pSrc, Ang-1, Ang-2, VEGF, and ZO-1 from 18 locations per rat (6 fields per slice×3 slices per rat). Quantifications were averaged and expressed as positive cells or microvessels / 40x field (n = 7 rats/group). To target similar regions of interest, slices with similar lesion area were selected and analyzed by an investigator blinded to the experimental cohort.
2.6. Measurement of vascular permeability
Vascular permeability was quantitatively assessed by spectrophotometric measurement of Evans blue (EB) extravasation. The vascular permeability was assessed from 3 h to 14 days after reperfusion. EB (2%; 4 mL/kg) was injected over 2 min into the right femoral vein and allowed to circulate for 60 min. The animals were then killed after induction of deep anesthesia. The amount of extravasated EB in homogenate from ipsilateral cortex was determined spectrophotometrically. Measurements were conducted at an excitation wavelength of 620 nm, an emission wavelength of 680 nm, and a bandwidth of 10 nm.
2.7. Measurement of brain water content
To evaluate the effect of PP1 on brain edema after ischemia and reperfusion, we calculated the water content as the weight difference between wet and dry samples. Animals (n = 7 rats/group) were deeply anesthetized and decapitated 1 day after reperfusion. The brains were removed and immediately weighed to obtain the wet weight. Then they were dried at 100°C for 48 h and reweighed to obtain the dry weight. The percentage of water in the forebrain was calculated as follows: (wet weight - dry weight)/wet weight × 100% (Wang and Dore, 2007).
2.8. Measurement of infarct volume
To evaluate the effect of PP1 on infarct volume after ischemia and reperfusion, we administered intraperitoneal injections of PP1 30 min before the induction of cerebral ischemia. Rats were decapitated according to the scheduled time. Brains were removed and placed at −20°C for quick freezing. Brains were then sectioned from front to back into five 2-mm slices. The slices were stained in 2% TTC solution at 37°C and fixed in formaldehyde. Viable brain tissue was stained red, whereas the ischemic area remained unstained (white). Each slice was photographed under a microscope, and infarct volume was calculated with an HPIAS2100 image analysis system.
2.9. Measurement of neurologic function
To evaluate the effect of PP1 on neurologic function, we assessed the neurologic scores of the rats according to Longa's neurological score criteria (Longa et al., 1989). In this scoring system, 0 = no deficit, 1 = failure to extend left forepaw fully, 2 = circling to the left, 3 = falling to the left, 4 = no spontaneous walking and a depressed level of consciousness. Rats were eliminated from the study if they were bleeding, had difficulty breathing, had brain subarachnoid hemorrhage, or died prematurely.
2.10. Statistical analysis
Parametric data are presented as the mean ± standard deviation. Parametric data, including the expression of Src, VEGF, Ang-1, Ang-2, and ZO-1 at different time points after ischemia and reperfusion, EB extravasation, brain water content, and infarct volume, were analyzed by one-way ANOVA with the Bonferroni post hoc test. Neurologic deficit scores are presented as medians with interquartile ranges (25th and 75th percentiles) and were analyzed by using the non-parametric Kruskal-Wallis analysis of ranks. The Pearson correlation was used to determine the strength of association among the target proteins examined. SPSS 13.0 software was used for statistical analysis. Statistical significance was set at p < 0.05.
3. Results
3.1. Temporal changes of Src mRNA and Y418 phosphorylation in rat brain after ischemia and reperfusion
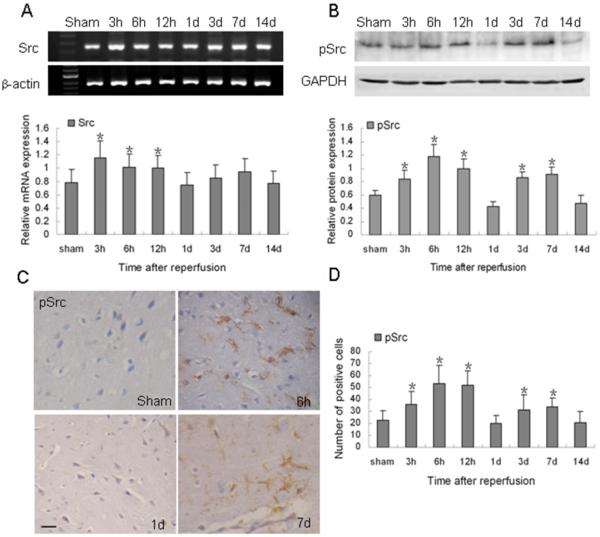
Compared with control levels, the expression of Src mRNA was markedly increased at 3 h after reperfusion (p < 0.05), persisted at a high level until 12 h, and then gradually declined to the basal level at 1 day (Fig. 1A). However, the increase in Src Y418 phosphorylation was biphasic. It increased significantly as early as 3 h, reached a maximum at 6 h and remained high for up to 12 h after reperfusion. After decreasing, it had a second peak at 3 to 7 days and then returned to the basal level at 14 days (p < 0.05; Fig. 1B). Immunohistochemistry showed that phosphorylated Src was nearly absent in sham-operated rat brain. However it was expressed in glial-like and endothelial-like cells at 6 h and 7 days after reperfusion, particularly in the peri-infarct region (Fig. 1C). Quantification analysis showed a similar trend of the protein expression of pSrc after reperfusion (Fig. 1.D).
Fig. 1.
Changes in Src mRNA and Y418 phosphorylation after ischemia and reperfusion. (A) Top: RT-PCR of Src mRNA at the indicated time points after reperfusion. Bottom: bar graph showing mRNA expression relative to that of β-actin. (B) Top: Western blot of Src Y418 phosphorylation at the indicated time points after reperfusion. Bottom: bar graph showing protein expression relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are representative of at least three independent experiments. (C) Immunohistochemical staining for Src (Y418) at the indicated time points after ischemic reperfusion. Scale bar = 20 μm. (D) Bar graph shows quantification analysis of pSrc-positive cells per 40x field at the indicated time points after ischemic reperfusion. Data in A (n = 6 rats/group), B (n = 6 rats/group), and D (n = 7 rats/group) are presented as means ± S.D. and were analyzed by one-way ANOVA followed by the Bonferroni post hoc test. *p < 0.05 compared with the sham group.
3.2. Expression of VEGF and angiopoietins after ischemia and reperfusion
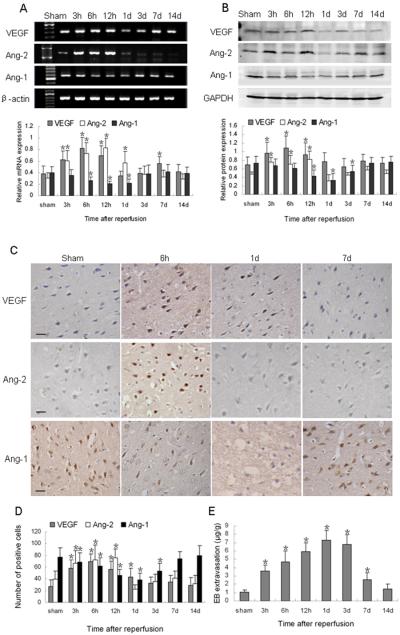
VEGF mRNA expression also exhibited upregulation after ischemia and reperfusion. It was markedly increased at 3 h, had a peak at 6 h, and then decreased gradually after 12 h; it had a second peak at 7 days and then returned to the basal level at 14 days (p < 0.05; Fig. 2A). Protein expression followed a similar trend. Western blot analysis indicated that VEGF protein underwent a marked increase at 6 h after reperfusion and then began to decrease gradually (p < 0.05; Fig. 2B). Immunoreactivity of VEGF was mainly observed in neuron-like and glial-like cells in the peri-infarct region (Fig. 2C) and followed a trend similar to that shown by Western blot analysis (Fig. 2D).
Fig. 2.
Changes in expression of VEGF, Ang-1, Ang-2, and Evans blue (EB) extravasation after ischemia and reperfusion. (A) Top: RT-PCR of VEGF, Ang-1, and Ang-2 mRNA at the indicated time points after reperfusion. Bottom: bar graph showing mRNA expression relative to that of β-actin. (B) Top: Western blot of VEGF, Ang-1, and Ang-2 at the indicated time points after reperfusion. Bottom: bar graph showing protein expression relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (C) Immunohistochemical staining shows VEGF and Ang-2 immunoreactivity in neuron-like and glial-like cells in the peri-infarct region. Ang-1 immunostaining was weak at 1 day after ischemic reperfusion. Scale bar = 20 μm. (D) Quantification analysis of VEGF-, Ang-2-, and Ang-1-positive cells per 40x field at the indicated time points after ischemic reperfusion. (E) EB extravasation increased at 3 h and peaked at 1 day after reperfusion before returning to baseline at 14 days. Data in A (n = 6 rats/group), B (n = 6 rats/group), D (n = 7 rats/group), and E (n = 7 rats/group) are presented as means ± S.D. and were analyzed by one-way ANOVA followed by the Bonferroni post hoc test. *p < 0.05 compared with the sham group.
Ang-2 mRNA increased significantly as early as 3 h after reperfusion, peaked at 12 h, and persisted at high levels until 1 day (p < 0.05). The level then decreased rapidly and remained at baseline from 3 days until the end of the experimental period at 14 days (Fig. 2A). Ang-2 protein also increased at 3 h and peaked at 12 h after reperfusion. It then decreased significantly to the basal level at 1 day (p < 0.05, Fig. 2B). Ang-2 was primarily expressed in neuron-like and glial-like cells in the peri-infarct region (Fig. 2C), and changes in the number of Ang-2-positive cells confirmed the results of Western blotting (Fig. 2D). Ang-1 mRNA was significantly decreased from baseline at 6 h after reperfusion (p < 0.05) and remained depressed for up to 1 day (p < 0.05). It returned to control levels by 3 days after reperfusion (Fig. 2A). Ang-1 protein also decreased after ischemia and reperfusion, reaching a minimum at 1 day (p < 0.05; Fig. 2B). It then gradually increased back to the baseline expression level. Ang-1 immunoreactivity was found mainly in neuron-like and glial-like cells in the ipsilateral hemisphere (Fig. 2C). Quantification analysis showed that changes in the number of Ang-1-positive cells were consistent with the results of Western blot analysis (Fig. 2D).
3.3. Vascular permeability after ischemia and reperfusion
Extravasation of EB was significantly greater in rats that underwent ischemic-reperfusion brain injury than in sham-operated rats. Extravasated EB was detected beginning at 3 h, peaked at 1 day, and remained significantly higher than baseline until 7 days after reperfusion (p < 0.05; Fig. 2E). Extravasation gradually decreased and returned to the control level at 14 days.
3.4 Expression of ZO-1 after ischemia and reperfusion
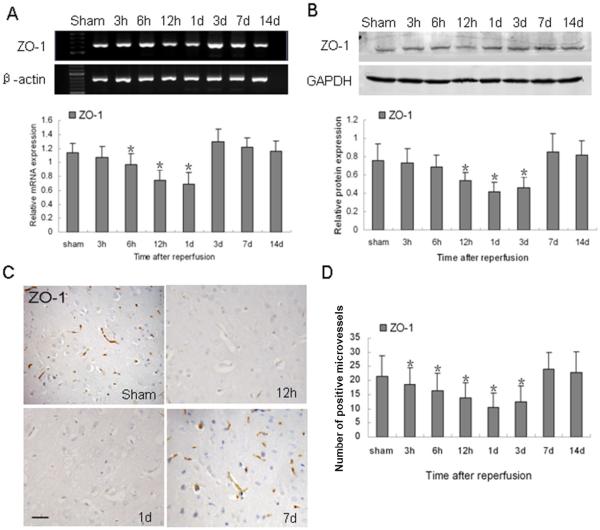
To examine the structural basis for the formation of edema, we examined the expression of the tight junction protein ZO-1. As shown in Fig. 3A, ZO-1 mRNA decreased beginning at 6 h and reached a minimum at 1 day (p < 0.05). ZO-1 protein also gradually decreased to a minimum 1 day after reperfusion (p < 0.05). It persisted at a low level until 3 days and then increased rapidly back to control levels (Fig. 3B). The results demonstrate that the decrease in ZO-1 expression was in accordance with the extent of vascular permeability. Immunostaining showed that ZO-1 was expressed in vascular-like structures. (Fig. 3C), indicating that ZO-1 is important in maintaining the vascular integrity. Quantification analysis confirmed that changes in the number of ZO-1-positive microvessels were in accordance with the results of Western blotting (Fig. 3D).
Fig. 3.
Changes in ZO-1 expression after ischemia and reperfusion. (A) RT-PCR of ZO-1 mRNA at the indicated time points after reperfusion. β-actin was used as a loading control. (B) Western blot of ZO-1 protein after reperfusion. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (C) ZO-1 immunoreactivity was observed in vascular-like structures, especially in the adjacent vessels. ZO-1 was strongly expressed in the brains of sham-operated rats, but it was weakly expressed at 12 h and 1 day after ischemic reperfusion. Scale bar = 50 μm. (D) Quantification analysis of ZO-1-positive microvessels per 40x field at the indicated time points after ischemic reperfusion. Data in A, B, and D are presented as means ± S.D. and were analyzed by one-way ANOVA followed by the Bonferroni post hoc test. n = 7 rats per group; *p < 0.05 compared with the sham group.
3.5. Src correlates with angiogenic factors and ZO-1
Our results indicate that after reperfusion, Src may modulate VEGF and angiopoietins, the levels of which correlated with vascular permeability. VEGF and Ang-2 were upregulated, especially early after reperfusion, whereas Ang-1 was downregulated. Src levels correlated positively with levels of VEGF and Ang-2, but negatively with those of Ang-1 and ZO-1; the decrease in Ang-1 paralleled that of ZO-1 (Tables 1 and 2). The results indicate that Src may play an important role in vascular permeability by modulating angiogenic factors and ZO-1.
Table 1.
Correlation of Src and angiogenic factors at the mRNA level after cerebral ischemia and reperfusion
| Factor | Ang-2 | Src | VEGF | ZO-1 |
|---|---|---|---|---|
| Ang-1 | −0.723** | −0.299** | −0.426** | 0.764** |
| Ang-2 | 0.394** | 0.747** | −0.767** | |
| Src | 0.605** | −0.171* | ||
| VEGF | −0.445** |
Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1.
Pearson correlation was used to analyze the correlations among the proteins at the mRNA level.
p < 0.05;
p < 0.01.
Table 2.
Correlation of Src Y418 phosphorylation and angiogenic factors at the protein level after cerebral ischemia and reperfusion
| Factor | Ang-2 | Src | VEGF | ZO-1 |
|---|---|---|---|---|
| Ang-1 | −0.262** | −0.408** | −0.427** | 0.787** |
| Ang-2 | 0.637** | 0.743** | −0.128 | |
| Src | 0.699** | −0.282** | ||
| VEGF | −0.234** |
Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1.
Pearson correlation was used to analyze the correlations among the proteins at the protein level.
p < 0.01.
3.6. PP1 reduces the expression of angiogenic factors and ZO-1
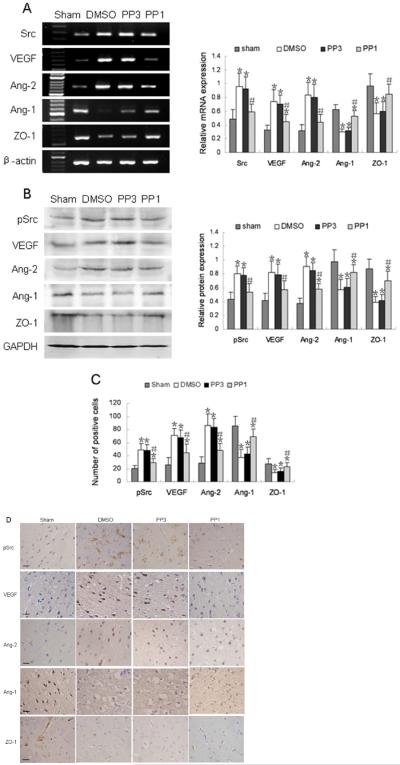
To address the effect of Src on angiogenic factors, we treated rats with PP1 before surgery; DMSO and PP3 were used as controls. The mRNA and protein expression levels of Src, VEGF, and Ang-2 were significantly increased, whereas Ang-1 was decreased in DMSO- and PP3-treated groups compared with levels in the sham group (p < 0.05, Fig. 4A, B) at 1 day after reperfusion. Pretreatment with PP1 significantly reversed the expression levels of these proteins. The results showed that Src mRNA and Src Y418 phosphorylation were significantly decreased in the PP1-treated group, compared with levels in the DMSO- and PP3-treated groups (p < 0.05, Fig. 4A, B) at 1 day after reperfusion. Like Src, VEGF and Ang-2 mRNA and protein levels also were decreased in the PP1-treated group compared with levels in the DMSO- and PP3-treated groups, whereas Ang-1 mRNA and protein were increased in the PP1-treated group (p < 0.05, Fig. 4A, B). ZO-1 mRNA and protein were also increased in the PP1-treated group compared with levels in the DMSO- and PP3-treated groups, although ZO-1 protein remained lower than that in the sham-operated group (p < 0.05, Fig. 4A, B). Consistent with the Western blotting results, immunostaining revealed that phosphorylated Src, VEGF, and Ang-2 were significantly decreased, while Ang-1 and ZO-1 were significantly increased in the PP1-treated group compared with the respective levels in the DMSO- and PP3-treated groups at 1 day after reperfusion (Fig. 4C, D).
Fig. 4.
Changes in expression of Src, angiogenic factors, and ZO-1 in PP1-treated rats after ischemia and reperfusion. (A) Left: RT-PCR of Src, VEGF, Ang-1, Ang-2, and ZO-1 mRNA in DMSO-, PP3-, and PP1-treated rats 1 day after reperfusion. Right: bar graph showing mRNA expression relative to that of β-actin. (B) Left: Western blot of Src (Y418), VEGF, Ang-1, Ang-2, and ZO-1 in DMSO-, PP3-, and PP1-treated rats 12 h after reperfusion. Right: bar graph showing protein expression relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (C) Bar graph shows quantification analysis of the immunopositive cells for pSrc, VEGF, Ang-2, Ang-1, and of the immunopositive microvessels for ZO-1.
Immunohistochemical staining revealed a decrease in immunoreactivity of Src (Y418), VEGF, and Ang-2, and an increase in immunoreactivity of Ang-1 and ZO-1 in PP1-treated rats, compared with that in the DMSO- and PP3-treated rats after ischemic reperfusion. Data in A, B, and C are presented as means ± S.D. and were analyzed by one-way ANOVA followed by the Bonferroni post hoc test. n = 7 rats per group; *p < 0.05 compared with the sham group, #p < 0.05 compared with DMSO-and PP3-treated groups. (D) Representative immunohistochemical staining for pSrc, VEGF, Ang-2, Ang-1, and ZO-1 in the sham group, DMSO-, PP3-, and PP1-treated groups at 1 day after ischemic reperfusion. Scale bar = 20 μm.
3.7. PP1 reduces vascular permeability, cerebral edema, and infarct volume
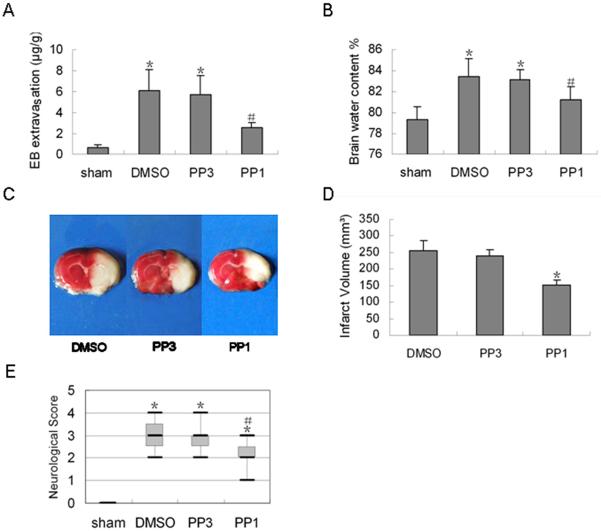
To further investigate the effects of Src on cerebral ischemic-reperfusion injury, we examined the effects of PP1 on vascular permeability, edema, and infarct volume. Treatment with PP1 markedly decreased the amount of EB extravasation in the ipsilateral cerebral cortex (p < 0.05, Fig. 5A) and decreased brain water content on day 1 after reperfusion (p < 0.05, Fig. 5B) compared with levels in the DMSO- and PP3-treated groups. Treatment with PP1 also decreased infarct volume by more than 30% on day 1 after reperfusion (p < 0.05, Fig. 5C, D).
Fig. 5.
Effect of Src inhibition with PP1 on Evans blue (EB) extravasation, brain water content, infarct volume, and neurologic score after ischemia and reperfusion. (A) Extravasation of EB was significantly reduced in the PP1-treated rats 1 day after reperfusion compared with that in DMSO- and PP3-treated rats. (B) PP1 treatment also significantly reduced post-ischemic water content. (C) Representative images of TTC-stained brain sections from control (left and middle panels) and PP1-treated rats. (D) The infarct volume was significantly decreased in the PP1-treated group compared to that in control groups. Data in A, B, and D are presented as means ± S.D. and were analyzed by one-way ANOVA followed by the Bonferroni post hoc test. (E) PP1 pretreatment decreased the neurologic score 1 day after ischemia and reperfusion. Data were analyzed with the non-parametric Kruskal-Wallis analysis of ranks and are represented as box-and-whisker plots. The upper and lower limits of the boxes indicate the 75th and 25th percentiles (interquartile range), respectively, and the horizontal line in each box represents the median. n = 7 rats per group; *p < 0.05 compared with the sham group, #p < 0.05 compared with DMSO- and PP3-treated groups.
3.8. Neurologic deficit scores
As shown in Figure 5E, PP1-treated rats exhibited significantly decreased neurologic deficit scores compared with scores of DMSO- and PP3-treated groups (p < 0.05) at 1 day after reperfusion. No difference in neurologic score was observed between DMSO- and PP3-treated rats. The results indicate that Src inhibition with PP1 improves functional outcome after focal cerebral ischemia and reperfusion.
4. Discussion
Ischemic-reperfusion injury causes blood-brain barrier (BBB) disruption, which accelerates the development of abnormal vascular permeability and exacerbates brain edema (Cole et al., 1991; Yang and Betz, 1994). Although the precise cellular mechanisms that underlie changes in vascular permeability after cerebral ischemia and reperfusion are unclear, several lines of evidence from our laboratory and those of others indicate the involvement of Src and angiogenic factors (Paul et al., 2001; Zan et al., 2011). An alteration in vascular permeability after cerebral ischemia and reperfusion may be important to the development of ischemic brain damage, especially in brain edema formation (Albayrak et al, 1997) Thus, elucidating the underlying mechanisms and molecules that regulate vascular permeability could lead to new therapies for the treatment of stroke and other brain injuries that are accompanied by brain edema. Here, we showed that Src mRNA and Y418 phosphorylation increase significantly after ischemia and reperfusion, and that Src signaling may regulate vascular permeability, possibly by modulating angiogenic factors and tight junction proteins. Furthermore, we provide novel evidence that PP1, an Src antagonist, ameliorates vascular permeability in rat brain after ischemia and reperfusion, an effect that may be mediated in part by direct protection against BBB disruption.
Src kinase has been implicated in brain injury resulting from permanent cerebral ischemia (Paul et al., 2001; Zan et al., 2011) and intracerebral hemorrhage (Ardizzone et al., 2007). Our results show for the first time that, compared with control levels, Src mRNA and Src Y418 phosphorylation are increased at 3 h after ischemic reperfusion and persist at a high level until 12 h. Moreover, Y418 phosphorylation demonstrates a biphasic response, peaking at 6 h and 7 days. The results indicate that the increase in Src Y418 phosphorylation is not solely due to the increase in Src mRNA. Vascular permeability increased after ischemic reperfusion, reaching a peak on day 1, before gradually returning to control levels. The results suggest that dynamic changes in Src kinase activity may contribute to cerebral ischemic-reperfusion injury.
Based on previous reports that Src inhibition reduces VEGF-induced vascular permeability and infarct volume (Paul et al., 2001) and protects the brain against focal ischemic injury (Lennmyr et al., 2004), we inferred that Src might modulate not only VEGF but also other angiogenic factors after ischemic-reperfusion injury. Here we showed that VEGF and Ang-2 increased in parallel with the increase in vascular permeability in the early period after ischemic reperfusion. By increasing endothelial permeability, VEGF enhances BBB disruption and produces vasogenic edema, which is particularly deleterious in ischemic-reperfusion injury. Ang-2 has been shown to destabilize the vessel, promote disassembly of cellular components (Asahara et al., 1998; Folkman and D'Amore, 1996; Lauren et al., 1998), and facilitate endothelial cell migration and vascular sprouting. Ang-2 may act synergistically with VEGF to promote vascular permeability after ischemic reperfusion. Ang-1 is a strong anti-permeability factor that can reduce vascular leakage. In this study, we observed that Ang-1 mRNA and protein were decreased significantly at 12 h and 1 day, when vascular permeability was at its greatest. This decrease in Ang-1 expression may explain the progression of vascular permeability after focal cerebral ischemia and reperfusion.
Reperfusion of the ischemic brain accelerates the development of abnormal vascular permeability, leading to brain edema formation and acceleration of brain injury (Cole et al., 1991; Yang and Betz, 1994). The increased vascular permeability is likely due, at least in part, to a decrease in tight junction proteins such as ZO-1, because absence of ZO-1 increases vascular permeability (Dvorak et al., 1999; Fischer et al., 2002; Wang et al., 2001). In this study, we found that, like Ang-1 expression, ZO-1 mRNA and protein were both decreased after ischemic reperfusion. The decrease in ZO-1 coincided with increases in EB extravasation, suggesting that ZO-1 might play a critical role in preserving BBB integrity.
Our analysis showed that Src correlated positively with VEGF and Ang-2 but negatively with Ang-1 and ZO-1. Ang-1 and ZO-1 did increase at later time points after reperfusion, but they may be regulated by other factors. We infer that Src may regulate vascular permeability, possibly by modulating angiogenic factors and tight junction proteins after ischemic reperfusion.
To address the role of Src in rat brain after ischemic reperfusion, we evaluated the effects of pharmacologic inhibition of Src activity with PP1 (Hanke et al., 1996; Liu et al., 1999). PP1 significantly reduced vascular permeability. This decrease was accompanied by an increase in ZO-1. Src inhibition with PP1 also decreased post-ischemic VEGF and Ang-2 expression and increased Ang-1 expression compared with that in control groups. Additionally, PP1 significantly reduced infarct volume, cerebral edema, and neurologic deficits. These results indicate that Src signaling may regulate the expression of angiogenic factors and tight junction proteins after ischemic reperfusion, thereby exacerbating cerebral injury and neurologic dysfunction. Therefore, inhibition of Src signaling could provide neurovascular protection against cerebral ischemic-reperfusion injury. However, it is possible that the efficacy of Src inhibition could have resulted from differences in baseline ischemic severity induced by PP1 pretreatment; therefore, the effects of post-treatment with PP1 still must be examined. If post-ischemic Src inhibition is not efficacious in the MCAO model, its utility might be limited to planned ischemic events, such as bypass surgery. PP1 would need to be tested in a global ischemia model to determine its efficacy in such cases.
Taken together, our novel findings indicate that Src signaling may promote vascular permeability after focal cerebral ischemia and reperfusion, possibly by modulating angiogenic factors, including VEGF, Ang-1, and Ang-2, and the tight junction protein ZO-1. Inhibition of Src signaling with PP1 reduced vascular permeability and infarct size and improved neurologic outcome in rats. Thus, Src signaling may provide a new therapeutic target for reducing tissue damage caused by increased vascular permeability.
Highlights
Src and angiogenic factor expression alters in focal cerebral ischemia/reperfusion.
Src inhibition with PP1 decreases Src, VEGF, and Ang-2; increases Ang-1 and ZO-1.
PP1 reduces cerebral edema, infarct size, and neurologic dysfunction.
Src regulates vascular permeability after ischemic reperfusion.
Src may provide a new therapeutic target for increased vascular permeability.
Acknowledgements
This work was supported by the Science Innovation Foundation of Shanxi Cancer Hospital (Dr201101), Basic research project of Shanxi Province (2013021034-2), NSFC (30973106), the Science Innovation Foundation of Harbin Medical University (HCXB2010013), AHA (13GRNT15730001), and NIH (K01AG031926, R01AT007317, R01NS078026). We thank Claire Levine for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albayrak S, Zhao Q, Siesjo BK, Smith ML. Effect of transient focal ischemia on blood-brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol. 1997;94:158–163. doi: 10.1007/s004010050688. [DOI] [PubMed] [Google Scholar]
- Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38:1621–1625. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Cole DJ, Matsumura JS, Drummond JC, Schultz RL, Wong MH. Time- and pressure-dependent changes in blood-brain barrier permeability after temporary middle cerebral artery occlusion in rats. Acta Neuropathol. 1991;82:266–273. doi: 10.1007/BF00308811. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gunji Y, Alitalo K. Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol. 1998;153:1333–1339. doi: 10.1016/S0002-9440(10)65717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennmyr F, Ericsson A, Gerwins P, Akterin S, Ahlstrom H, Terent A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol Scand. 2004;110:175–179. doi: 10.1111/j.1600-0404.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hou XY, Zhang GY, Xu TL. L-type voltage-gated calcium channel attends regulation of tyrosine phosphorylation of NMDA receptor subunit 2A induced by transient brain ischemia. Brain Res. 2003;972:142–148. doi: 10.1016/s0006-8993(03)02519-8. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Paul R, Zhang ZG, Eliceir BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol. 2006;80:719–726. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- Risau W, Esser S, Engelhardt B. Differentiation of blood-brain barrier endothelial cells. Pathol Biol (Paris) 1998;46:171–175. [PubMed] [Google Scholar]
- Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125:2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tang X, Feng Y, Ye K. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14:368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- Theus MH, Wei L, Francis K, Yu SP. Critical roles of Src family tyrosine kinases in excitatory neuronal differentiation of cultured embryonic stem cells. Exp Cell Res. 2006;312:3096–3107. doi: 10.1016/j.yexcr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434–440. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–1655. [DOI] [PubMed] [Google Scholar]
- Zan L, Wu H, Jiang J, Zhao S, Song Y, Teng G, Li H, Jia Y, Zhou M, Zhang X, Qi J, Wang J. Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema. Neurochem Int. 2011;58:872–879. doi: 10.1016/j.neuint.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]