Abstract
In the human upper extremity (UE), unintended effects of proximal muscle activation on muscles controlling the hand could be an important aspect of motor control due to the necessary coordination of distal and proximal segments during functional activities. This study aimed to elucidate the effects of concurrent activation of elbow muscles on the coordination between hand muscles performing a grip task. Eleven healthy subjects performed precision grip tasks while a constant extension or flexion moment was applied to their elbow joints, inducing a sustained submaximal contraction of elbow muscles to counter the applied torque. Activation of four hand muscles was measured during each task condition using surface electromyography (EMG). When concurrent activation of elbow muscles was induced, significant changes in the activation levels of the hand muscles were observed, with greater effects on the extrinsic finger extensor (23.2 % increase under 30 % elbow extensor activation; p = 0.003) than extrinsic finger flexor (14.2 % increase under 30 % elbow flexor activation; p = 0.130). Elbow muscle activation also induced involuntary changes in the intrinsic thumb flexor activation (44.6 % increase under 30 % elbow extensor activation; p = 0.005). EMG–EMG coherence analyses revealed that elbow muscle activation significantly reduced intermuscular coherence between distal muscle pairs, with its greatest effects on coherence in the β-band (13–25 Hz) (average of 17 % decrease under 30 % elbow flexor activation). The results of this study provide evidence for involuntary, muscle-specific interactions between distal and proximal UE muscles, which may contribute to UE motor performance in health and disease.
Keywords: Hand, Upper extremity, Muscle, Proximal–distal interaction, EMG–EMG coherence
Introduction
In human motor tasks, unintended muscle activity, often triggered by activation of adjacent muscles, is frequently found to emerge under certain task conditions. During tasks that require a high level of effort, for example, the high activation of the muscles involved in the task often induces activation of nearby muscles within the limb (Dimitrijevic et al. 1992; Gandevia et al. 1993; Zijdewind and Kernell 2001). The degree of such ‘motor overflow’ increases under conditions such as fatigue (Bodwell et al. 2003) and intersegmental instability (Cholewicki et al. 2000) among healthy subjects.
Though the effects of muscle contraction on nearby muscles have been studied, unintended effects in more distant muscles have not been fully examined. The effects of proximal arm muscle contraction on distal arm muscles, for example, would be of particular interest given the functional importance of coordination between proximal and distal UE musculature. Previous studies of the neural connections and interactions between the hand muscles and proximal arm muscles have mostly focused on spinal reflex mechanisms (e.g., Rothwell et al. 1980; Marsden et al. 1981; Manning and Bawa 2011), which may not fully explain interactions during goal-oriented movements that do not occur in response to sudden unexpected perturbations or other sensory inputs that elicit reflex responses. Investigations of ‘involuntary’ interactions between the arm and hand that do not involve reflex mechanism are relatively sparse. Previous studies of involuntary muscle interactions during UE motor tasks have focused on muscle groups controlling a single joint (i.e., agonist–antagonist muscles). For example, co-contraction of agonist/antagonist pairs at the elbow or shoulder joints has been examined in relation to task-related parameters such as endpoint accuracy (Gribble et al. 2003), dynamic joint perturbation (Buchanan et al. 1989), and stability maintenance (Milner 2002). Other studies expanded the scope of inquiry to include interactions between muscles controlling different proximal arm joints, such as shoulder and elbow joints, examining the effects of task-related parameters (e.g., endpoint stiffness: Franklin et al. 2007) on the muscle activation patterns. As of yet, however, potential ‘involuntary’ neural interactions between proximal and distal UE muscles during multi-joint motor tasks have not been examined in detail.
Most human functional activities require the synergistic use of task-relevant muscles with proper spatiotemporal coordination (e.g., Zajac 1993). Underlying interactions between UE muscles could have significant implications for UE functionality as they could facilitate multi-joint movements (e.g., by effectively reducing degrees-of-freedom to be controlled) or potentially compromise task performance. Involuntary interactions between the muscles controlling distal and proximal UE joints, in particular, could influence UE motor performance, since precise coordination of hand and arm movements is central to most UE functional tasks (Paulignan et al. 1990; Flanagan and Wing 1993; Lemon et al. 1995). Furthermore, as many UE muscles cross-multiple joints (i.e., bi-articular or multi-articular), some mono-articular muscles need to be coordinated accordingly in order to counteract or balance the kinetic impact of such multi-articular muscles (Kurtzer et al. 2006; Herter et al. 2007). Therefore, proper coordination of multiple UE muscles, either at the supraspinal (Donoghue et al. 1992; Holdefer and Miller 2002) or spinal (Tresch et al. 1999; Hart and Giszter 2004) level, would be crucial in functional UE task performance.
Indeed, the functional impact of involuntary interactions between distal and proximal UE muscles is often observed in patients with neurological disorders. Increased co-activation of muscles crossing multiple joints has been reported in subjects with neurological disorders such as stroke (Dewald et al. 1995; Zackowski et al. 2004; McCrea et al. 2005; Sukai et al. 2007) and cerebral palsy (Nashner et al. 1983; Thelen et al. 2003). Involuntary patterns of co-activation between hand and arm muscles are often manifested as kinematic abnormalities, or so-called flexion and/or extension synergies of the affected arm and hand (Brunnstrom 1966; Gowland et al. 1993). A recent study also showed that, in individuals with stroke, a shoulder abduction load can induce involuntary wrist and finger flex-ion forces (Miller and Dewald 2012). It appears that involuntary multi-joint coupling patterns can occur throughout the UE musculature following neurological injury.
Abnormal muscle activation that emerges after neurological injuries sometimes represents an abnormal augmentation of preexisting neural mechanisms. Stretch reflex responses in both upper and lower limbs, for example, are often exaggerated post-stroke (Ada et al. 1998; Sangani et al. 2007), resulting in an increased level of ‘velocity-dependent’ muscle activation within the affected limb (Levin et al. 2000; Hoffmann et al. 2009; Trumbower et al. 2010). However, the obligatory coupling often observed between the hand and arm post-stroke may not be fully explained by exaggerated reflex responses (Burne et al. 2005; Sheean and McGuire 2009). Instead, the flexion or extension synergy patterns often observed during voluntary movement generation could also be indirect evidence for the existence of a ‘velocity-independent’ proximal–distal interaction between UE musculature that was present before the disease.
As an initial inquiry into this possibility, we aimed to elucidate potential interactions between muscles controlling proximal and distal UE joints in healthy subjects under isometric conditions. Specifically, we examined the effects of isometric activation of the elbow extensor or flexor muscles on the activation level of the distal hand muscles controlling the index finger and thumb during a precision grip task. We hypothesized that concurrent activation of elbow muscles would increase the activation levels of the hand muscles, similar to synergy patterns observed post-stroke (Brunnstrom 1966; Gowland et al. 1993).
Further, we examined the oscillatory activities of the electromyography (EMG) signals from the distal muscles and the intermuscular (EMG–EMG) coherence in different frequency ranges (frequency-domain), as well as their cumulant density functions (time-domain). These time–frequency analyses can provide information regarding the origin of common oscillatory drive to different motor units and assess the degree of synchronous oscillations among different muscles (Farmer et al. 1998; Fisher et al. 2002; Lowery et al. 2007; Norton and Gorassini 2006; Nishimura et al. 2009; Poston et al. 2010). This allowed us to assess the degree of interruption of the common drive to distal muscles caused by the elbow muscle activation. Inter-muscular coherence of stroke survivors during functional movements, for example, is found to be lower than that of control subjects (Kisiel-Sajewicz et al. 2011), indicating a loss of common drive to the task-relevant muscles. We hypothesized that the EMG–EMG coherence between hand muscles will be reduced during concurrent elbow muscle activation.
Methods
Subjects
Eleven subjects with no history of neurological disorders (six males and five females; mean ± SD age = 27.1 ± 5.0 years; nine right-handed) participated in the study. The experimental protocol was approved by the MedStar Health Institutional Review Board, and written informed consent was obtained from each subject prior to participation.
Instrumentation
Six pairs of disposable, self-adhesive silver/silver chloride surface electrodes (diameter 15 mm, center spacing 20 mm; Noraxon, AZ, USA) were used for the surface EMG recordings. Two pairs were placed on the hand to record the activities of intrinsic hand muscles [flexor pollicis brevis (FPB) and first dorsal interosseous (FDI)], two pairs on the forearm to record extrinsic hand muscle activities [1st compartment of the flexor digitorum superficialis (FDS) and extensor digitorum communis (EDC)], and two pairs on the upper arm to record the activity of elbow flexor and extensor muscles [biceps brachii (BB) and triceps brachii (TB)]. Here, short head (medial) of the biceps brachii muscle and lateral head of the triceps brachii muscle were targeted. To ensure the accurate placement of each electrode, EMG signals from the electrodes were inspected while subjects performed several thumb and finger movements associated with the target muscle and adjacent muscles after the placement. The electrode location was adjusted if the EMG signal recorded from a muscle changed during isolated contraction of any neighboring muscle. For the electrode that targeted FPB, the EMG signal was monitored during thumb abduction to see whether abductor pollicis brevis muscle activity was captured. Similarly, for the extrinsic hand muscles, EMG signals were monitored during wrist movements (i.e., extensor carpi radialis/ulnaris and flexor carpi radialis/ulnaris).
The four target hand muscles (FPB, FDI, FDS, and EDC) were selected because they are the major agonists and antagonists of the thumb and index finger for the pinch grip task performed during the experiment. The EMG signals were sampled at 1,000 Hz and band-pass filtered between 10 Hz and 500 Hz.
Target tasks
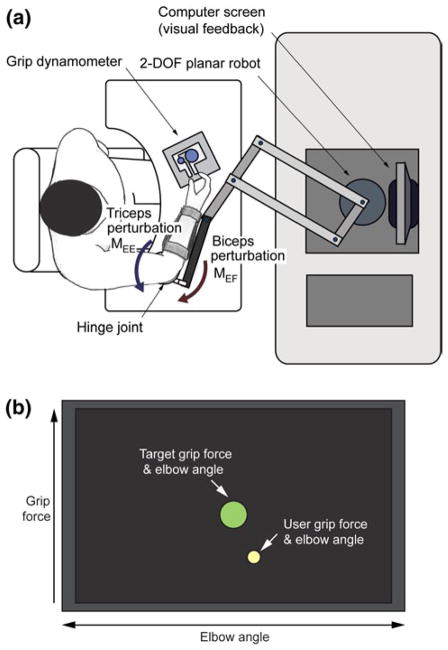
Subjects performed a pinch grip task with their dominant hands while a planar 2-degrees-of-freedom robot (InMo-tion2; Interactive Motion Technologies, Cambridge, MA, USA) applied a constant extension or flexion moment about their elbow joints. Subjects were seated in front of the system, with their dominant forearm placed on a custom-made apparatus, which was connected to the end effector (handle) of the robot (Fig. 1a). The base of the apparatus was mounted to the table, and the elbow joint was aligned parallel to the pivot joint of the apparatus. This setup required the subject to produce a sustained elbow flexion or extension moment to resist the force delivered by the robot, while the shoulder joint remained relatively unaffected during the experiment. As the hinge joint was attached to the table, the location of the elbow joint remains unchanged throughout the experiment and subjects did not move their upper arms. The location and height of the chair was adjusted so that the elbow joint of each subject was at his/her shoulder level and the entire upper arm was positioned within a plane parallel to the table. Here, shoulder flexion angle was maintained at 90°, and the abduction angle was approximately set to 45°.
Fig. 1.
Experimental setup. a Schematic of the setup; b graphical user interface (GUI) providing information regarding elbow angle and grip force. The GUI is based on the simplest design that can provide the necessary real-time information (grip force and elbow angle). To users, the target task is simply to ‘keep the yellow circle inside the green target circle’(color figure online)
Once the forearm was secured to the apparatus, a pinch dynamometer with a custom-made interface was placed on the table, and its position was adjusted for each subject’s arm length so that the corresponding upper arm posture resulted in an elbow joint angle of approximately 70°–80°. Once determined, the location of the dynamometer was marked on the table and constantly monitored during the experiment to ensure consistent arm and hand posture. This arm posture was maintained throughout all of the subsequent testing conditions. During the experiments, subjects were instructed to produce a target grip force while maintaining elbow position, which required them to resist the force delivered by the robot by producing a constant elbow flexion or extension moment. A custom-made graphical user interface provided subjects with real-time visual feedback regarding current values of elbow joint angle and grip force relative to the target levels (Fig. 1b) (see “Experimental protocol” section below for the details of the protocol).
Subjects used their thumb and index finger to grip the dynamometer for the pinch task. They were instructed to maintain full extension of the distal–interphalangeal (DIP) joint of the index finger while flexing the proximal–inter-phalangeal (PIP) and metacarpophalangeal (MCP) joints to produce the target grip force. Note that FDS was one of the major agonist muscles of the index finger for this task, as the FDS produces a PIP joint flexion moment (An et al. 1983; Valero-Cuevas et al. 1998) and its involvement in grip increases as the DIP joint extends (Vigouroux et al. 2006).
Experimental protocol
Before the experimental sessions started, maximum EMG activity was recorded for each muscle. Subjects performed maximal isometric contractions of the six muscles by performing each of the following tasks for approximately 2 s; maximum finger extension (for EDC), maximum index finger flexion with DIP joint extended (i.e., mainly PIP joint flexion; for FDS), maximum thumb interphalangeal flex-ion (for FPB), maximum index finger abduction (for FDI), maximum elbow flexion (for BB), and maximum elbow extension (for TB). During maximal contraction, EMG data were recorded for 4 s; only data recorded during the middle 2 s of the contraction were used to calculate the maximum EMG level. During the first second, subjects increased their muscle activities to the maximum level (ramp-up phase), then they sustained the maximum contraction for the next 3 s. As our observation of the data showed that some subjects did not maintain the maximum activation level for the rest of the recording (from 1- to 4-s), we also excluded the last 1-s (i.e., 3- to 4-s). For maximum index finger flexion force, subjects were asked to resist the experimenter’s palm with their index finger. Movements of the other fingers were not constrained.
Maximum elbow moments generated by BB and TB, and maximum pinch grip force were also measured. Subjects were seated in front of the device and placed their forearm on the apparatus to perform maximum isometric elbow flexion (for BB) and extension (for TB) tasks, during which the device was locked in position. A six degrees-of-freedom (DOFs) load cell mounted on the handle of the robot recorded the forces generated during maximum elbow flexion and extension. These values were used to estimate maximum elbow flexion and extension moments, MEFmax and MEEmax. Then, the maximum pinch grip force (FGmax) of the subject was recorded by the pinch dynamometer. The estimated maximum elbow flexion/extension moments and maximum pinch force values were used to determine the force level delivered by the robot to induce sustained BB/TB contractions and the target grip force presented to subjects, respectively.
The entire experiment for each subject consisted of three identical sessions. In each session, subjects were asked to perform the grip task, i.e., to produce the target grip force displayed on the screen (see Fig. 1b), while simultaneously producing either elbow flexion or extension moments to prevent elbow movement. Within each grip task block, target grip force (FG) was increased from 8 to 32 % of the maximal grip force, in increments of 8 %, resulting in four target force levels. Target grip force was increased to the next level when the subject generated and maintained each target force for three consecutive seconds; thus, the duration of a trial for each grip force level was 3 s.
Throughout each grip task block, one of the following elbow moments was applied; low and high levels of elbow flexion moment (to induce TB contraction), low and high levels of elbow extension moment (to induce BB contraction), and no moment. Low and high elbow extension or flexion moments corresponded to 15 and 30 % of the subject’s maximum elbow extension (EEmax) or flexion (EFmax) moments, respectively. Thus, there were five elbow joint moments (ME) applied: 15 % MEEmax, 30 % MEEmax, 15 % MEFmax, 30 % MEFmax, and no moment. Note that target grip force was displayed on the monitor only after each subject produced the required elbow joint moment against the robot and stabilized his/her arm posture, maintaining the target elbow joint angle for 3 s. The time to complete each grip task block (four grip force levels) under different elbow moment conditions was also estimated.
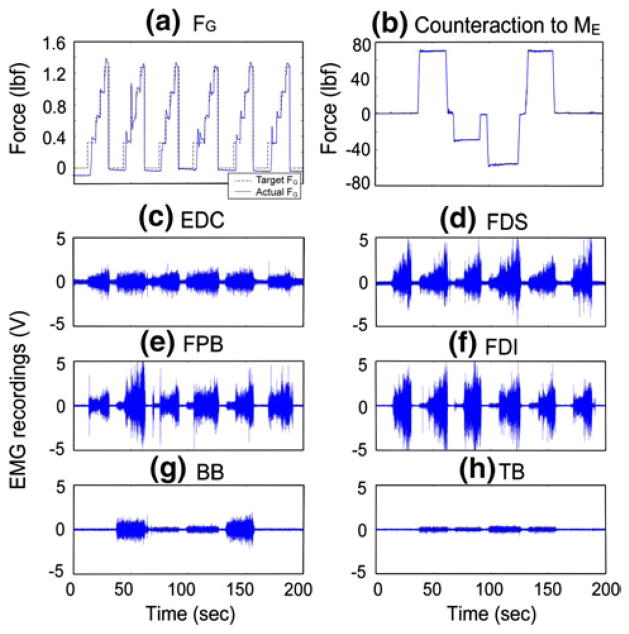
In each of the three sessions, each elbow joint moment condition was tested three times in random order. Thus, each elbow joint moment condition was tested nine times in total. To prevent fatigue, subjects were asked to take a mandatory break of 1–2 min after every six grip force blocks. In addition, 5-min rest periods were administered between the experimental sessions. Typical force and EMG profiles during one experimental block are depicted in Fig. 2.
Fig. 2.
Typical force and EMG measurements during one experimental block (subject 1, session 2). Temporal trajectories of a grip force FG (dotted line target grip force; solid line measured grip force), b force counteracting the imposed elbow moment ME (positive: resisting elbow extension; MEF, negative: resisting elbow flexion; MEE), and c–h EMG recordings. In the experimental block shown, the following six elbow joint moments were applied: no moment, 30 % MEFmax, 15 % MEEmax, 30 % MEEmax, 30 % MEFmax, and no moment, which is also indicated by b. Note that only a subset of the EMG data (final 3-s at each FG level) were used for the data analysis
Data analysis
Activation level
For each condition, the activation levels of the four distal hand muscles were estimated from the recorded EMG signals by calculating the root-mean-square (RMS) values of the EMG signals over the 3 s of task performance at each grip force level. For each hand muscle (EDC, FDS, FPB, and FDI), a repeated-measures analysis of variance (ANOVA) was implemented to determine the effect of elbow moment condition (ME condition: no perturbation, MEE, and MEF), elbow moment magnitude (ME magnitude: 15, 30 %), and grip force (FG) on the activation level of the muscle (IBM SPSS Statistics version 20; IBM Corp., New York, NY, USA). Gender was defined as a between-subject variable as gender differences have been reported in hand muscle coordination patterns (e.g., Endo and Kawahara 2011). A significance level was set to 0.05. Pairwise comparisons between different ME conditions (no perturbation vs. MEE vs. MEF) were also made.
In addition, to quantify the change in activation level of the distal muscles under different proximal muscle activation conditions, the percent change (PC) in the muscle activation under each of the elbow joint moment conditions, normalized to the activation level with no elbow moment, was estimated for each hand muscle:
| (1) |
where denotes the RMS values of the EMG signal at the grip force level of k (k = 1: 8 % FGmax, 2: 16 % FGmax, 3: 24 % FGmax, 4: 32 % FGmax) during four elbow moment conditions (i = 1: 15 % MEFmax, 2: 30 % of MEFmax, 3: 15 % MEEmax, 4: 30 % MEEmax), and the RMS value in the no elbow moment condition. For each subject, the percent change (PC1–PC4) values were estimated for each of the four distal muscles (EDC, FDS, FPB, and FDI).
Time–frequency analysis
Coherence and cumulant density functions between two EMG signals were computed to quantify correlations between muscle activations in the time and frequency domains. EMG–EMG coherence between six pairs of hand muscles (EDC–FDS, EDC–FPB, EDC–FDI, FDS–FPB, FDS–FDI, FPB–FDI), was estimated for each of the five elbow moment conditions. For each condition, EMG data for all five grip force levels (3-s each) from all nine trials were concatenated (a total of 135 s) since the EMG–EMG coherence level of hand muscle pairs is generally not affected by grip force level (Poston et al. 2010).
EMG–EMG coherence values between muscle pairs were estimated using non-overlapping segments (rectangular window) that resulted in a frequency resolution of 2 Hz within the MATLAB environment (MathWorks, Inc.; Natick, MA, USA), employing a script developed by Neurospec (www.neurospec.org; Halliday et al. 1995). Briefly, given two EMG signals x and y, let the power spectra of the two signals be denoted as fxx(λ) and fyy(λ), and their cross-spectrum as fxy(λ). The coherence between two signals at frequency λ, Rxy(λ), is defined as (Halliday et al. 1995):
| (2) |
The cumulant density function in the time-domain qxy(t) is defined as the inverse Fourier transform of the cross-spectrum fxy(λ):
| (3) |
The EMG–EMG coherence was estimated in the following four frequency bands; δ/θ (0–5 Hz), α (6–15 Hz), β (16–35 Hz), and γ (36–55 Hz). We focused our analyses on the α-, β-, and γ-bands. The coherence values at the lowest frequency band, i.e., δ and θ bands (0–5 Hz), were not examined in detail, since the coherence in this frequency band is not thought to originate from the corticospinal system (Farmer et al. 1993). Then, the coherence estimates were z-transformed as follows,
| (4) |
as the ‘z-transformed’ coherence values will be normally distributed with a standard deviation of approximately one (Rosenberg et al. 1989). Then, the integral of z-transformed coherence was estimated within three frequency bands. For each frequency band, paired t tests were performed to compare the integral of z-transformed coherence estimates between three elbow moment condition pairs (i.e., no elbow moment vs. 30 % MEFmax, no elbow moment vs. 30 % MEEmax, and 30 % MEFmax vs. 30 % MEEmax).
Additionally, for all muscle pairs, we estimated coherence values pooled across all subjects for each elbow moment conditions (i.e., no elbow moment, 30 % MEEmax, and 30 % MEFmax) to examine the overall trend in the intermuscular coherence under different proximal muscle activation conditions.
Post hoc analysis
Two post hoc analyses were performed on the experimental data in order to assess whether assumptions made in the data analyses were violated.
First, to test for an effect of the grip force level on intermuscular coherence, intermuscular coherence values between the lowest (8 % of the FGmax) and the highest (32 % of the FGmax) grip force levels were compared. Intermuscular coherence values integrated within the three frequency bands (α-, β-, and γ-bands) at the lowest grip force and the highest grip force were computed for each subject. A paired t test was then performed to verify the significance of the grip force level on the intermuscular coherence values.
In addition, an EMG cross talk analysis was performed to test for any potential cross talk between the EMG channels. For this analysis, the EMG signals recorded when subjects produced 24 % and 32 % of their maximum grip force (0.24FGmax and 0.32FGmax) were used. To minimize any potential effect of proximal muscle activation on the correlation coefficient estimation, we used EMG signals from the first two trials obtained under no proximal muscle activation conditions. The EMG signal used in the cross-correlation analysis was thus 12 s in duration (3 s/grip force level × 2 grip force levels × 2 trials) for each subject. The raw EMG signal from one channel was cross-correlated with the signal from the other channel. We computed cross-correlation of all muscle pairs (i.e., EDC–FDS, EDC–FDI, EDC–FPB, FDS–FDI, FDS–FPB, and FDI–FPB), and the peak cross-correlation value for each muscle pair, Rxy, was estimated. Then, its squared value ( ) was computed, which represents the percentage of the common signal existing in the two EMG signals (Winter et al. 1994).
Results
All subjects successfully performed the targeted grip force tasks under the different elbow moment conditions. When the elbow muscles were concurrently activated, subjects took slightly more time to reach the target grip force, specifically during production of an elbow extension moment (mean 22.9 s during 30 % MEEmax vs. mean 21.6 s during no ME; p = 0.006). Once the target grip force level was reached, however, they experienced no difficulty in maintaining the force level for 3 s.
Activation level of distal hand muscles
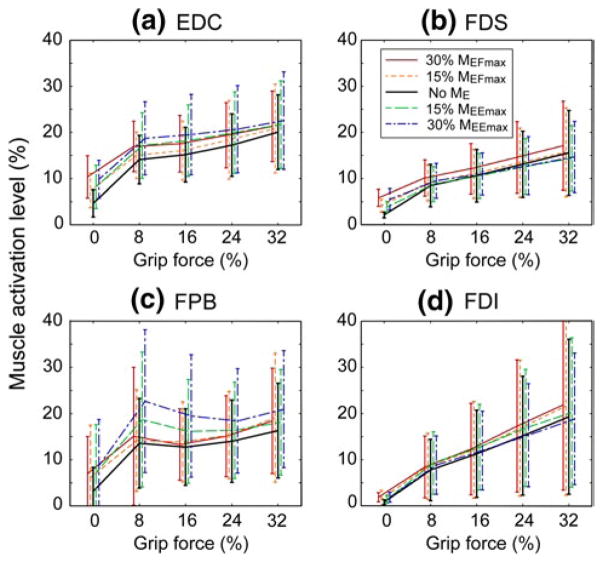
For the extrinsic hand muscles, there was a main effect of ME direction on the activation level of the finger extensor (EDC; p = 0.003; Table 1; Fig. 3) during the pinch grip tasks. For the intrinsic hand muscles, ME direction had a significant effect on FPB (p = 0.005; Table 1; Fig. 3).
Table 1.
Results of three-way repeated-measures ANOVA: p values for the effects of factors and their interaction on muscle activation
| EDC | FDS | FPB | FDI | |
|---|---|---|---|---|
| Within-subject factor | ||||
| ME condition | 0.003 | 0.132 | 0.005 | 0.260 |
| ME level | 0.013 | 0.011 | 0.067 | 0.363 |
| FG | 0.001 | 0.040 | 0.029 | 0.008 |
| Between-subject factor | ||||
| Gender | 0.048 | 0.352 | 0.329 | 0.008 |
| Interaction | ||||
| ME condition × ME level | 0.447 | 0.303 | 0.052 | 0.001 |
| ME condition × FG | 0.017 | 0.292 | 0.624 | 0.584 |
| ME condition × gender | 0.240 | 0.748 | 0.783 | 0.010 |
| ME level × FG | 0.353 | 0.050 | 0.632 | 0.883 |
| ME level × gender | 0.347 | 0.425 | 0.918 | 0.701 |
| FG × gender | 0.337 | 0.081 | 0.251 | 0.025 |
Bold values indicate p ≤ 0.05
EDC extensor digitorum communis, FDS flexor digitorum superficialies, FDI first dorsal interosseous, FPB flexor pollicis brevis, ME elbow moment, FG grip force
Fig. 3.
EMG activation level of four muscles (a EDC, b FDS, c FPB, d FDI) during grip force generation (FG) and varying elbow moment conditions (ME) expressed as a percentage of each muscle’s maximum activation. Error bars indicate one standard deviation from the mean values
There was also a main effect of the target grip force on the activation level of all four distal muscles (FG; Table 1), confirming their involvement in the pinch grip task. Post hoc pairwise comparisons showed that the activation level of all muscles were significantly different between all grip force levels (all p’s < 0.05).
The mean percent change in the activation level of the extrinsic finger muscles showed two different patterns of distal–proximal coupling (Table 2). The increase in activation of the EDC was greater with elbow extensor activation (MEE) (23.2 % increase during 30 % MEFmax vs. 14.7 % increase during 30 % MEFmax), while the increase in the FDS activation level was greater during elbow flexor activation (14.0 % increase during 30 % MEFmax vs. 2.0 % increase during 30 % MEEmax), although the effects of ME direction did not reach statistical significance for FDS (p =0.132).
Table 2.
Mean (SD) % change in the activation level of the four distal muscles (PC1–PC4) during elbow muscle activation
| Muscle | Gender | Elbow joint moment conditions
|
|||
|---|---|---|---|---|---|
| PC1: 15 % MEFmax | PC2: 30 % MEFmax | PC3: 15 % MEEmax | PC4: 30 % MEEmax | ||
| EDC | Male | 13.8 % (3.0 %) | 22.0 % (6.7 %) | 11.8 % (3.9 %) | 13.5 % (7.5 %) |
| Female | −2.0 % (0.4 %) | 6.1 % (4.9 %) | 21.8 % (8.1 %) | 34.9 % (11.0 %) | |
| Mean | 6.6 % (1.8 %) | 14.7 % (5.9 %) | 16.3 % (5.8 %) | 23.2 % (9.1 %) | |
| FDS | Male | 5.0 % (4.5 %) | 21.2 % (6.6 %) | 1.7 % (4.4 %) | 4.0 % (9.3 %) |
| Female | 1.7 % (2.2 %) | 5.7 % (2.6 %) | −5.1 % (5.2 %) | −0.3 % (7.0 %) | |
| Mean | 3.5 % (3.5 %) | 14.2 % (4.8 %) | −1.4 % (4.8 %) | 2.0 % (8.3 %) | |
| FPB | Male | 12.3 % (5.6 %) | 10.3 % (5.5 %) | 24.5 % (8.4 %) | 42.5 % (10.4 %) |
| Female | 9.2 % (10.8 %) | 9.2 % (6.9 %) | 22.8 % (18.8 %) | 47.1 % (30.7 %) | |
| Mean | 10.9 % (7.9 %) | 9.8 % (6.1 %) | 24.6 % (13.1 %) | 44.6 % (19.6 %) | |
| FDI | Male | 11.1 % (9.1 %) | 19.1 % (2.3 %) | 47.5 % (9.7 %) | 43.9 % (11.5 %) |
| Female | 8.8 % (1.2 %) | 8.5 % (2.2 %) | −1.1 % (1.1 %) | 9.4 % (1.6 %) | |
| Mean | 10.1 % (5.5 %) | 14.3 % (2.3 %) | 25.4 % (5.8 %) | 28.2 % (7.0 %) | |
For the intrinsic hand muscle, similar to EDC, elbow extensor activation was associated with larger changes in the FPB activation (44.6 % increase during 30 % MEEmax vs. 9.8 % increase during 30 % MEFmax).
The activation level of the FDI muscle was not significantly affected by ME level (p = 0.260). However, a number of interactions between other experimental factors were significant. For this muscle, there were significant effects for ME direction × ME level (p < 0.001), ME direction × FG (p = 0.025), and ME direction × gender (p = 0.010).
Gender was also found to be a significant between- subject factor for the activation level of FDI (p =0.008) and EDC (p = 0.048) muscles. Activation levels of the FDI in female subjects were significantly higher than those in male subjects during grip task performance, regardless of the elbow moment condition. Note that male subjects showed a significant increase in FDI activation level with elbow muscle activation, specifically during elbow extension (MEE), but the change in the FDI activation level with MEE among female subjects was much smaller (see Table 2). EDC activation level differed between male and female subjects, but the difference was not as large as that of FDI.
EMG–EMG coherence between hand muscles
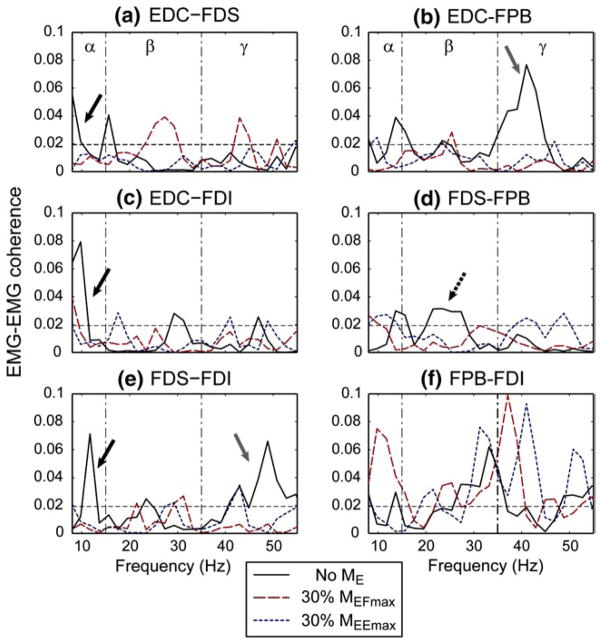
Proximal muscle activation affected the coherence between a subset of distal muscle pairs. The degree of change and the specific frequency bands affected by the elbow moment generation varied slightly across subjects, but in general, the EMG–EMG coherence values between the hand muscles decreased under elbow moment generation conditions. Figure 4 shows an exemplary case of the change in coherence values of the six muscle pairs under three elbow moment conditions in a single subject (Subject 1). For this subject, a reduction in the coherence values under BB activation (30 % MEFmax) was observed mostly in the α-band; specifically, α-band coherence between EDC–FDS, EDC–FDI, and FDS–FDI pairs was greatly reduced during BB 30 % activation (30 % MEFmax).
Fig. 4.
EMG–EMG coherence of the six muscle pairs for a single subject under three elbow moment conditions, i.e., no elbow moment, 30 % of maximum elbow flexion (30 % MEFmax), and 30 % of maximum elbow extension (30 % MEEmax): a EDC–FDS, b EDC–FPB, c EDC–FDI, d FDS–FPB, e FDS–FDI, and f FPB–FDI. The dotted horizontal lines denote the upper 95 % confidence limit. For this subject, a significant reduction in coherence values in the α-band of a subset of muscle pairs was apparent under elbow joint moment conditions (a, c, e solid black arrows). Coherence between the FDS–FPB pair was decreased mainly in the β-band (d dotted black arrow), and coherence between EDC–FPB and FDS–FDI pairs were affected in the γ band (b, e solid gray arrows). In contrast, for the intrinsic muscle pair (f FPB–FDI), the coherence value slightly increased under elbow joint moment conditions for this subject
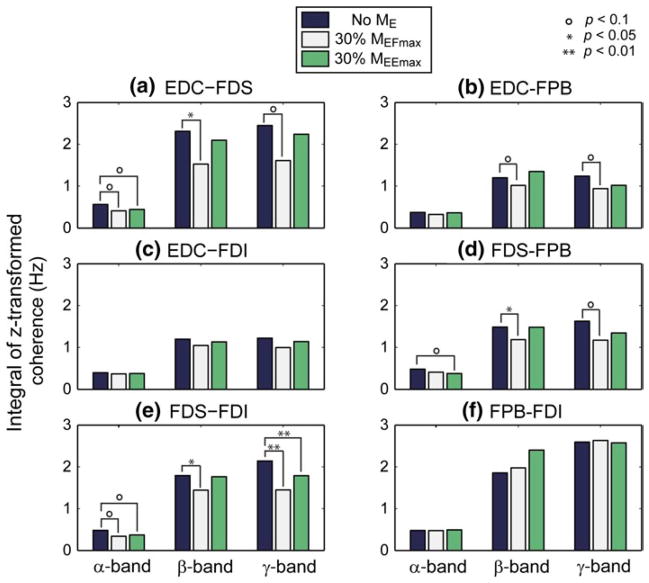
Figure 5 shows the integral of z-transformed coherence for the six muscle pairs, averaged across all subjects, at three different frequency ranges (α: 6–15 Hz, β: 16–35 Hz, and γ: 36–55 Hz) under the three elbow joint moment conditions, no ME, 30 % MEFmax, and 30 % MEEmax. Here, the muscle pairs with statistically significant differences between conditions are indicated. As stated above, we specifically examined the change in coherence in the α-, β-, and γ-bands due to their significance in human motor control.
Fig. 5.
Integral of z-transformed coherence for six muscle pairs at the three frequency ranges under three elbow moment conditions: a EDC–FDS, b EDC–FPB, c EDC–FDI, d FDS–FPB, e FDS–FDI, f FPB–FDI
In the β-band (16–35 Hz), proximal muscle activation, especially MEE, significantly affected distal muscle coherence. The coherence values of most muscle pairs in the α-band decreased except FPB–FDI pair (Fig. 5f). The decrease in the β-band induced by MEF was significant for all of the muscle pairs that included FDS, i.e., index finger flexor-thumb flexor pair (FDS-FPB; p = 0.01), an agonist–antagonist muscle pair of the index finger (EDC–FDS; p = 0.04), and a synergist pair of the index finger (FDS–FDI; p = 0.05).
In other frequency bands, elbow joint moment affected the intermuscular coherence of a relatively smaller number of muscle pairs. In the α-band (8–15 Hz), the coherence values of two muscle pairs, EDC–FDS and FDS–FDI, showed non-significant trends under MEF and MEE (p < 0.1). In the γ-band (36–55 Hz), both MEF and MEE had significant effects on the FDS–FDI pair (p < 0.01). In addition, three other muscle pairs (EDC–FDS, EDC–FPB, FDS–FPB) showed a non-significant trend (p < 0.1) toward a reduction in γ-band coherence under MEF.
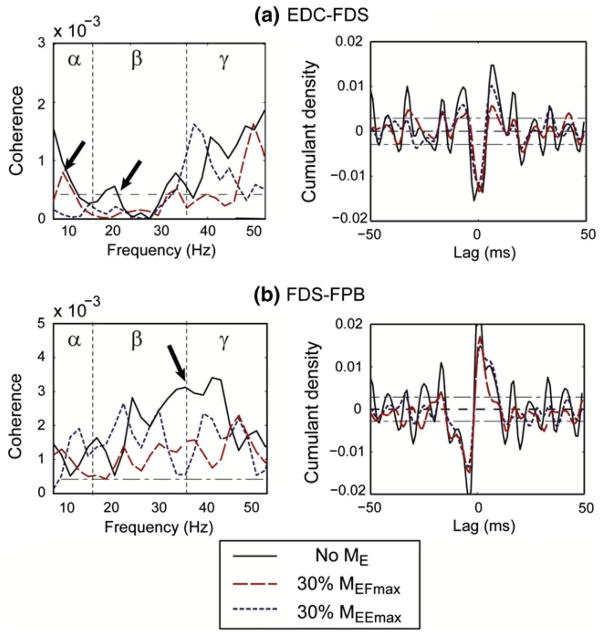
Pooled coherence analyses, which compare the group difference between the three elbow moment conditions, also highlighted a significant effect of proximal muscle activation on distal muscle pairs. Figure 6a shows the results of the pooled coherence analysis for the EDC–FDS pair, the agonist–antagonist muscle pair of the index finger. The intermuscular coherence values were decreased by both elbow joint moments (MEE and MEF) in the α- and β-bands. The magnitude of the cumulant density estimate also decreased under MEF and MEE.
Fig. 6.
Pooled analysis: effects of elbow joint moment on coherence between a EDC–FDS and b FDS–FPB. The dashed horizontal line denotes the upper 95 % confidence limit based on the assumption of independence. a EDC–F8DS: biceps brachii activation (i.e., elbow flexion moment) visibly reduced the coherence values across β- and γ-bands (see arrow). b FDS–FPB: a significant reduction in coherence value in the α- and β-bands was observed (see arrow). A noticeable decrease in the magnitude of the cumulant density estimate is observed under biceps brachii activation, while the reduction was not as significant under triceps brachii activation
Figure 6b delineates the coherence analysis results for the FDS–FPB pair, major flexor muscles for the index finger (FDS) and the thumb (FPB), and major agonist muscles of the target task (i.e., pinch grip). For this pair, MEF reduced the coherence values over the entire frequency range, particularly in the β- and γ-bands. The coherence values were also reduced under MEE, but the degree of reduction was smaller compared to MEF. Note that, unlike in the EDC–FDS pair, an asymmetric cumulant density was obtained for the FDS–FPB pair (Fig. 6a vs. b), indicating an absence of coherence at the lowest frequency (<5 Hz). The magnitude of the cumulant density of the FDS–FPB pair was also decreased under MEF and MEE. Here, as indicated in the coherence reduction, both low- and high-frequency oscillatory components of the cumulant density of the FDS–FPB pair were decreased.
Our post hoc analysis confirmed the assumptions of our primary data analysis approach. First, the effects of grip force level on intermuscular coherence of most muscle pairs were indeed found to be small, similar to the findings of Poston et al. (2010). Out of 18 comparisons (six muscle pairs × three frequency bands), only one case was found to be statistically significant (EDC–FDS pair, β-band; p = 0.01) due to a slightly larger coherence value at the highest force level (average of 12.6 % increase). The difference in the other coherence values (i.e., all three bands of the other five muscle pairs, and α- and γ-bands of the EDC–FDS pair) was not statistically significant. Second, cross talk between closely placed EMG channels (i.e., hand intrinsic muscles) was not detected. Peak cross-correlation values between EMG signals during grip force production, which represent the amount of cross talk present in the muscle pairs, were found to be low for all pairs. The peak cross-correlation values across all subjects was the lowest for the EDC–FDI pair (mean ± SD: 0.076 ±0.025; 0.6 % cross talk) and the highest for the FDI–FPB pair (mean ± SD: 0.133 ±0.089; 1.8 % cross talk).
Discussion
Our results demonstrate that proximal muscle activation can induce a significant change in the activation of distal hand muscles performing a precision grip task. Interestingly, activation of the elbow extensor had a greater effect on the extrinsic finger extensor (EDC) and the intrinsic thumb flexor (FPB), while activation of the elbow flexor affected the extrinsic finger flexor (FDS) to a greater degree. The activation of elbow muscles also reduced intermuscular coherence between a subset of distal hand muscle pairs.
Muscle activation levels
The activation level of the extrinsic finger extensor, EDC, was significantly changed by concurrent activation of the elbow muscles (p = 0.003), and the increase in EDC activation level was greater under elbow extension than elbow flexion (23.2 % increase during 30 % MEEmax vs. 14.7 % increase during 30 % MEFmax). The activation of the extrinsic finger flexor, FDS, showed a contrasting pattern to the EDC, as the increase in its activation level was greater under elbow flexion than elbow extension (14.2 % increase during 30 % MEFmax vs. 2.0 % increase during 30 % MEEmax), although this difference did not reach statistical significance (p = 0.132); the extrinsic finger extensor showed higher degree of covariation with the elbow extensor, while the extrinsic finger flexor mainly covaried with the elbow flexor.
Similar to EDC, the intrinsic thumb flexor (FPB) activation level was more affected by elbow extension than flex-ion (44.6 % increase during 30 % MEEmax vs. 9.8 % increase during 30 % MEFmax). Thus, it appears that, overall, elbow extensor activation led to a greater change in the distal hand muscle activation level than elbow flexion did.
The observed change in the hand muscle activation level cannot be explained simply by multi-joint reflex responses mediated through polysynaptic pathways that involve multiple muscles of the hand and arm. The grip task was initiated only after subjects stabilized the handle location by producing a sustained contraction of the elbow muscles against the constant force provided by the robot (see “Methods” section). Therefore, a sustained submaximal activation (as low as 30 % of the maximal contraction) of proximal elbow muscles can induce a change in the multi-muscle coordination patterns of the hand, supporting our hypothesis that proximal muscle activity can alter the activation patterns of distal hand muscles.
EMG–EMG coherence
Examination of the coherence values in this study provides insight into the potential neural mechanism underlying the observed patterns of coupling between the distal and proximal UE muscles. An EMG–EMG coherence estimate can quantify an oscillatory synchrony between pairs of muscles, providing an objective measure of common synaptic drive shared between motoneurons controlling the muscles of interest (Gibbs et al. 1997; Farmer et al. 1998; Grosse et al. 2003; Perez et al. 2006).
EMG–EMG coherence between hand muscles decreased during elbow muscle activation, with the greatest effects occurring in the β-band (16–35 Hz; Fig. 5). A significant decrease in the coherence values in this band was observed in three out of the six muscle pairs (EDC–FDS, FDS–FPB, FDS–FDI; p < 0.05), as well as a non-significant trend in EDC–FPB (p < 0.1), indicating that their common β-band neural input was substantially reduced with elbow muscle activation. Coherence values in the α- and γ-bands between distal muscle pairs were also affected by proximal muscle activation, although these effects often did not reach statistical significance and a relatively small number of muscle pairs were involved (α-band: EDC–FDS, FDS–FPB, and FDS–FDI; p < 0.1, γ-band: FDS–FDI; p < 0.01, EDC–FDS, EDC–FPB, and FDS–FPB; p < 0.1).
β-Band coherence
The origin of β-band (16–35 Hz) coherence is typically regarded to be cortical (Salenius et al. 1997; Halliday et al. 1998; Grosse et al. 2003), and a number of studies have shown that the primary motor cortex is involved in the generation of EMG–EMG coherence between hand muscles in the β-band during grip tasks. Previous studies have shown that the EMG–EMG coherence in the β-band during grip tasks is modulated by different factors, such as age (Gibbs et al. 1997; Farmer et al. 2007), task phase (i.e., ramp vs. hold; Kilner et al. 1999), object property (Kilner et al. 2000), and sensory feedback (Fisher et al. 2002). Reduced intermuscular coherence in the β-band can lead to less efficient motor unit recruitment, e.g., increased co-contraction of agonist–antagonist muscles as observed in this study, and often subsequent motor performance degradation (Baker et al. 1999; Farmer et al. 2007).
Here, we extend these findings by showing that the proximal UE muscle activation, specifically elbow flexor activation, can decrease the degree of synchrony in the hand muscles. Importantly, the muscle pairs that showed β-band coherence reduction account for the major motor control mechanisms of the grip task: i.e., agonist–antagonist co-contraction (EDC–FDS), between-digit coordination of the major flexors (FDS–FPB), and index finger synergist muscles (FDS–FDI).
Coherence in other bands
In the α-band (6–15 Hz), elbow muscle activation also affected intermuscular coherence of the same muscle pairs that showed a reduction in β-band coherence (EDC–FDS, FDS–FPB, FDS–FDI). Although the significance of coherence reduction in the β-band was marginal (0.05 < p < 0.1), such reduction in the common inputs to these muscles may also affect the motor control of the hand due to the importance of proper coordination of the muscle pairs affected.
Elbow muscle activation appears to have had a more significant effect on the EMG–EMG coherence of the distal hand muscles in the lower γ-band (36–55 Hz) than on the coherence in the α-band, as a total of four muscle pairs were either significantly (one pair with p < 0.01) or marginally (three pairs with p < 0.1) affected. A recent study on cortico- and intermuscular coherence of spinal injury patients (Nishimura et al. 2009) suggested that γ-band coherence may represent inputs from subcortical pathways such as reticulospinal and/or rubrospinal tracts, which were found to play an important role in motor control of various functional hand movements (Baker 2011). γ-band coherence was also found to increase specifically during strong isometric grip exertions and/or grasping movements (Brown 2000), which indicates the importance of intermuscular coherence in this band for grip tasks.
Methodological considerations
It must be acknowledged that not all of the hand muscles involved in the pinch grip task were examined in this study, since the objective of this study was not to fully describe the muscle coordination patterns employed to perform the task, but to identify the effects of proximal muscle activation on selected key hand muscles involved in the task. Our findings provide evidence of ‘involuntary’ proximal–distal interactions in the selected UE muscles, and further analyses that examine the entire muscle set could provide a more comprehensive description of this phenomenon.
It should be acknowledged that the extrinsic finger muscles examined in this study, EDC and FDS, also cross the elbow joint and may be involved in moment production about the elbow joint. The multi-articular nature of these muscles may have affected the coordination patterns observed during concurrent elbow moment conditions. However, these muscles have relatively small moment arms around the elbow joint (An et al. 1981) and usually act at the elbow only when the finger and wrist joints are fully extended or flexed (Standring 2009). Thus, we do not expect that the change in extrinsic finger muscle occurred because they were being recruited for elbow moment production.
In addition, we observed some degree of between-subject variability in the grip posture. Although subjects were instructed to keep a specific finger posture (i.e., PIP and MCP joint flexion with DIP joint extended) during the grip task, some subjects unconsciously altered their finger posture during the experimental session. As finger posture could have a significant impact on muscle coordination patterns of the hand (Kamper et al. 2006; Qui et al. 2009), such deviation from the instructed posture could affect the activation level of certain muscles, contributing to the within- and between-subject variability in the muscle coordination patterns. However, we attempted to minimize such postural effects by monitoring and correcting hand posture throughout the experiment and do not believe this affected the results in any systematic way.
Given the size of the electrode, the electrodes placed on some muscles may have picked up activity from adjacent muscles; for instance, the EMG signals of the FPB muscle may have contained activity of the neighboring abductor pollicis brevis muscle. However, we attempted to minimize such cross talk during electrode placement by testing several movements associated with the muscle (for instance, monitoring the channel acquiring FPB activity during thumb abduction).
Implications
The results of this study provide evidence for the presence of involuntary interactions between proximal and distal UE muscles. Due to the nature of functional UE activities, which often require simultaneous and coordinated movements of the distal (hand) and proximal (elbow/shoulder) segments, this underlying proximal–distal coupling could potentially affect task performance, as evidenced by obligatory proximal–distal interactions between patients with neurological disorders (e.g., Miller and Dewald 2012).
The activation patterns of the thumb intrinsic flexor (FPB) and the index finger extrinsic extensor (EDC) were found to be most affected by concurrent elbow muscle activation. We postulate that a rather complex neuromechanical adjustment could underlie the observed change in the coordination pattern of the hand muscles. Note that the increase in EDC activation level was considerably higher than that of the FDS during concurrent elbow extension (i.e., 30 % MEEmax; Table 2). Such an imbalance could be the result of interrupted multi-muscle coordination of the index finger with proximal muscle activation, as evidenced by the changes in the intermuscular coherence values (e.g., reduction in EDC–FDS coherence in the α-band and/or reduction in FDS-FDI coherence in the α- and γ-bands; Fig. 5a). Theoretically, a larger increase in EDC activation (compared to that of FDS and FDI) would result in a reduction in the palmar-directional fingertip force magnitude produced by the index finger. At the same time, joint impedance of the index finger may also have increased due to the increased activation of both the agonist and antagonist muscles of the index finger (both EDC and FDS increased activation). In such a case, to meet the target grip force level, the thumb would have to produce a larger amount of flexion force, which may explain the increased activation level of the thumb flexor (FPB) that we observed. The increased joint impedance of the index finger, due to increased co-contraction of the EDC and FDS muscles, could have assisted in resisting the increased thumb tip force. We acknowledge that such modification could have led to slight changes in the thumb/index finger postures (compared to those obtained during no elbow moment conditions), which could have induced small movement of the force transducer, but we observed neither significant change in the thumb/finger postures nor visible movement of the transducer across the different elbow moment conditions.
Note that, for the target task (i.e., pinch grip), there are two groups of muscles for each digit, agonist muscles (e.g., FDS, FDI for the index finger; FPL, FPB for the thumb) and antagonist muscles (e.g., EDC for the index finger; EPB, EPL for the thumb), thus a total of four muscle groups (agonist/antagonist for the index finger, agonist/antagonist for the thumb) are involved in the task. In order to produce the same level of grip force while maintaining the finger/thumb posture, the change in the activation level of these four muscle groups needs to be mechanically balanced as described above.
As mentioned above, previous studies have shown that intermuscular coherence in the β-band during grip tasks is modulated by various factors. Here, we showed that one such factor is the activation of proximal muscles. The observed degradation in motor synchrony in the β-band may be an underlying mechanism of the observed changes in distal hand muscle activation under proximal muscle activation, especially those caused by elbow flexor activation. Conversely, the activation levels of some muscles (i.e., EDC and FPB) were more affected by elbow extensor activation (Table 2). Since the intermuscular coherence in the α- and γ-bands between the finger and thumb muscles (i.e., EDC–FDS, FDS–FPB, and FDS–FDI) was mainly affected by elbow extension (see Fig. 5a, d, e), our results may suggest that the interaction between the elbow extensor and the distal hand muscles involves either a spinal pathway (e.g., Christou et al. 2007; Takei and Seki 2010) or other subcortical pathways such as brainstem pathways (Baker 2011).
In summary, concurrent activation of proximal UE muscles during grip task performance induced significant changes in the coordination patterns of the distal hand muscles. There was a clear difference between the effects of elbow flexor versus extensor activation on the extrinsic finger muscles. Elbow extensor activation led to a greater increase in activation of the extrinsic finger extensor (EDC) and intrinsic thumb flexor (FPB) compared to the other distal muscles, while elbow flexor activation affected all four distal muscles to a similar degree. The coherence analysis suggests that the effects of elbow extensor and flexor activation on distal hand muscles may involve different pathways (i.e., β-band vs. α- and γ-bands). The outcome of this study confirms the existence of involuntary coupling between distal and proximal UE muscles. Such involuntary proximal–distal coupling in UE musculature could have a significant impact on motor performance and consequent UE functionality and may emerge more strongly in neurological diseases such as stroke.
Acknowledgments
The authors thank Dr. B. Bregman for her invaluable input to the study. This work was partially supported by a USAMRMC Grants W81XWH-11-1-0632 (SWL) and NICHD K01HD-60886 (MHL).
Contributor Information
Sang Wook Lee, Department of Biomedical Engineering, Catholic University of America, Washington, DC, USA. Center for Applied Biomechanics and Rehabilitation Research, Medstar National Rehabilitation Hospital, Washington, DC, USA.
Katlin Landers, Department of Biomedical Engineering, Catholic University of America, Washington, DC, USA. Center for Applied Biomechanics and Rehabilitation Research, Medstar National Rehabilitation Hospital, Washington, DC, USA.
Michelle L. Harris-Love, Email: michelle.l.harris-love@medstar.net, Interdisciplinary Program in Neuroscience, Department of Rehabilitation Medicine, Georgetown University, Washington, DC, USA. Neuroscience Research Center, Medstar National Rehabilitation Hospital, 102 Irving Street NW, Washington, DC 20010, USA
References
- Ada L, Vattansilp W, O’Dwyer N, Crosbie J. Does spasticity contribute to walking dysfunction after stroke? J Neurol Neurosurg Psychiatry. 1998;64:628–635. doi: 10.1136/jnnp.64.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An KN, Hui FC, Morrey RL, Linschied RL, Chao EY. Muscles cross the elbow joint: a biomechanical analysis. J Biomech. 1981;14:659–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- An KN, Ueba Y, Chao EY, Cooney WP, Linschied RL. Tendon excursion and moment arm of index finger muscles. J Biomech. 1983;16:419–425. doi: 10.1016/0021-9290(83)90074-x. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Bodwell JA, Mahurin RK, Waddle S, Price R, Cramer SC. Age and features of movement influence motor overflow. J Am Geriatr Soc. 2003;51:1735–1739. doi: 10.1046/j.1532-5415.2003.51557.x. [DOI] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol. 1989;62:1201–1212. doi: 10.1152/jn.1989.62.6.1201. [DOI] [PubMed] [Google Scholar]
- Burne JA, Carleton VL, O’Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry. 2005;76:47–54. doi: 10.1136/jnnp.2003.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewicki J, Simons APD, Radebold A. Effects of external trunk loads on lumbar spine stability. J Biomech. 2000;33:1377–1385. doi: 10.1016/s0021-9290(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Christou EA, Rudroff T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res. 2007;178:285–295. doi: 10.1007/s00221-006-0739-5. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Mckay WB, Sarjanovic I, Sherwood AM, Svirtlih L, Vrbova G. Co-activation of ipsi- and contralateral muscle groups during contraction of ankle dorsiflexors. J Neurol Sci. 1992;109:49–55. doi: 10.1016/0022-510x(92)90092-y. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Leibovic S, Sanes JN. Organization of the fore-limb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89(1):1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- Endo H, Kawahara K. Gender differences in hand stability of normal young people assessed at low force levels. Ergonomics. 2011;54:273–281. doi: 10.1080/00140139.2010.547607. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Brenner FD, Halliday DM, Rosenberg JR, Stephans JA. The frequency content of common synaptic inputs to motoneurons studies during isometric voluntary contraction in man. J Physiol. 1993;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Sheean GL, Mayston MJ, Rothwell JC, Marsden CD, Conway BA, Halliday DM, Rosenberg JR, Stephens JA. Abnormal motor unit synchronization of antagonist muscles underlies pathological co-contraction in upper limb dystonia. Brain. 1998;121:801–814. doi: 10.1093/brain/121.5.801. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Gibbs J, Halliday DM, Harrison LM, James LM, Mayston MJ, Stephens JA. Changes in EMG coherence between long and short thumb abductor muscles during human development. J Physiol. 2007;579:389–402. doi: 10.1113/jphysiol.2006.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Galea MP, Brown P, Lemon RN. Digital nerve anaesthesia decreases EMG–EMG coherence in a human precision grip task. Exp Brain Res. 2002;145:207–214. doi: 10.1007/s00221-002-1113-x. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. Modulation of grip force with load force during point-to-point arm movements. Exp Brain Res. 1993;95:131–143. doi: 10.1007/BF00229662. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Liaw G, Milner TE, Osu R, Burdet E, Kawato M. Endpoint stiffness of the arm is directionally tuned to instability in the environment. J Neurosci. 2007;27:7705–7716. doi: 10.1523/JNEUROSCI.0968-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Macefield VG, Bigland-Ritchie B, Gorman RB, Burke D. Motoneuronal output and gradation of effort in attempts to contract acutely paralysed leg muscles in man. J Physiol (Lond) 1993;471:411–427. doi: 10.1113/jphysiol.1993.sp019907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cross-correlation analysis of motor unit activity recorded from separate thumb muscles in man. J Physiol. 1997;499:255–266. doi: 10.1113/jphysiol.1997.sp021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster stroke assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. 2003;89:2396–2405. doi: 10.1152/jn.01020.2002. [DOI] [PubMed] [Google Scholar]
- Grosse P, Guerrini R, Parmeggiani L, Bonanni P, Pogosyan A, Brown P. Abnormal corticomuscular and intermuscular coupling in high-frequency rhythmic myoclonus. Brain. 2003;126:326–342. doi: 10.1093/brain/awg043. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SFA. Framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci. 2004;24(22):5269–5282. doi: 10.1523/JNEUROSCI.5626-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter TM, Kurtzer I, Cabel DW, Haunts KA, Scott SH. Characterization of torque-related activity in primary motor cortex during a multijoint postural task. J Neurophysiol. 2007;97(4):2887–2799. doi: 10.1152/jn.00757.2006. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Kamper DG, Kahn JH, Rymer WZ, Schmit BD. Modulation of stretch reflexes of the finger flexors by sensory feedback from the proximal upper limb poststroke. J Neurophysiol. 2009;102:1420–1429. doi: 10.1152/jn.90950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE. Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res. 2002;146(2):233–243. doi: 10.1007/s00221-002-1166-x. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Fischer HC, Cruz EG. Impact of finger posture on mapping from muscle activation to joint torque. Clin Biomech. 2006;21:361–369. doi: 10.1016/j.clinbiomech.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmäki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516:559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel-Sajewicz K, Fang Y, Hrovat K, Yue GH, Siemionow V, Sun CK, et al. Weakening of synergist muscle coupling during reaching movement in stroke patients. Neurorehabil Neural Repair. 2011;25:259–268. doi: 10.1177/1545968310388665. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH. Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol. 2006;95(1):493–504. doi: 10.1152/jn.00706.2005. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. Corticospinal control during reach, grasp, and precision lift in man. J Neurosci. 1995;15:6145–6156. doi: 10.1523/JNEUROSCI.15-09-06145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MF, Selles RW, Verheul MH, Meijer OG. Deficits in the coordination of agonist and antagonist muscles in stroke patients: implications for normal motor control. Brain Res. 2000;853:352–369. doi: 10.1016/s0006-8993(99)02298-2. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Lyers LJ, Erim Z. Coherence between motor unit discharges in response to shared neural inputs. J Neurosci Methods. 2007;163:384–391. doi: 10.1016/j.jneumeth.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Manning CD, Bawa P. Heteronymous reflex connections in human upper limb muscles in response to stretch of forearm muscles. J Neurophysiol. 2011;106:1489–1499. doi: 10.1152/jn.00084.2011. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain. 1981;104:513–534. doi: 10.1093/brain/104.3.513. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Saturated muscle activation contributes to compensatory reaching strategies after stroke. J Neurophysiol. 2005;94:2999–3008. doi: 10.1152/jn.00732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Dewald JP. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123:1216–1225. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TE. Adaptation to destabilizing dynamics by means of muscle cocontraction. Exp Brain Res. 2002;143:406–416. doi: 10.1007/s00221-002-1001-4. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Shumway-Cook A, Marin O. Stance posture control in select groups of children with cerebral palsy: deficits in sensory organization and muscular coordination. Exp Brain Res. 1983;49:395–409. doi: 10.1007/BF00238781. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Morichika Y, Isa T. A subcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Brain. 2009;132:709–721. doi: 10.1093/brain/awn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–2589. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- Paulignan Y, MacKenzie C, Marteniuk R, Jeannerod M. The coupling of arm and finger movements during prehension. Exp Brain Res. 1990;79:431–435. doi: 10.1007/BF00608255. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurons following visuo-motor skill learning in humans. J Physiol. 2006;573:843–855. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston B, Danna-Dos Santos A, Jesunathadas M, Hamm TM, Santello M. Force-independent distribution of correlated neural inputs to hand muscles during three-digit grasping. J Neurophysiol. 2010;104:1141–1154. doi: 10.1152/jn.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui D, Fischer HC, Kamper DG. Muscle activation patterns during force generation of the index finger. Engineering in medicine and biology society; Annual international conference of the IEEE; 2009. pp. 3987–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature. 1980;286:496–498. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Sangani SG, Starsky AJ, McGuire JR, Schmit BD. Multijoint reflexes of the stroke arm: neural coupling of the elbow and shoulder. Muscle Nerve. 2007;36:694–703. doi: 10.1002/mus.20852. [DOI] [PubMed] [Google Scholar]
- Sheean G, McGuire JR. Spastic hypertonia and movement disorders: pathophysiology, clinical presentation, and quantification. PM R. 2009;1:827–833. doi: 10.1016/j.pmrj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray’s Anatomy. 40. Elsevier/Churchill Livingston; Edinburgh: 2009. [Google Scholar]
- Sukai TM, Ellis MD, Dewald JPA. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183:215–223. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei T, Seki K. Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J Neurosci. 2010;30:17041–17050. doi: 10.1523/JNEUROSCI.4297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen DD, Riewald SA, Asakawa DS, Sanger TD, Delp SL. Abnormal coupling of knee and hip moments during maximal exertions in persons with cerebral palsy. Muscle Nerve. 2003;27:486–493. doi: 10.1002/mus.10357. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci. 1999;2(2):162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Contributions of altered stretch reflex coordination to arm impairments following stroke. J Neurophysiol. 2010;104:3612–3624. doi: 10.1152/jn.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation. J Biomech. 1998;31:693–703. doi: 10.1016/s0021-9290(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Vigouroux L, Quaine F, Labarre-Vila A, Moutet F. Estimation of finger muscle tendon tensions and pulley forces during specific sport-climbing grip techniques. J Biomech. 2006;39:2583–2592. doi: 10.1016/j.jbiomech.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: theoretical and practical estimates. J Electromyogr Kinesiol. 1994;4:15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle coordination of movement: a perspective. J Biomech. 1993;26(S1):109–124. doi: 10.1016/0021-9290(93)90083-q. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol. 2001;85:1907–1913. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]