Abstract
Although sterilizing immunity to malaria can be elicited by irradiated sporozoite vaccination, no clinically practical subunit vaccine has been shown to be capable of preventing the approximately 600,000 annual deaths attributed to this infection. DNA vaccines offer several potential advantages for a disease that primarily affects the developing world, but new approaches are needed to improve the immunogenicity of these vaccines. By using a novel, lipid-based adjuvant, Vaxfectin, to attract immune cells to the immunization site, in combination with an antigen-chemokine DNA construct designed to target antigen to immature dendritic cells, we elicited a humoral immune response that provided sterilizing immunity to malaria challenge in a mouse model system. The chemokine, MIP3αCCL20, did not significantly enhance the cellular infiltrate or levels of cytokine or chemokine expression at the immunization site but acted with Vaxfectin to reduce liver stage malaria infection by orders of magnitude compared to vaccine constructs lacking the chemokine component. The levels of protection achieved were equivalent to those observed with irradiated sporozoites, a candidate vaccine undergoing development for further large scale clinical trial. Only vaccination with the combined regimen of adjuvant and chemokine provided 80–100% protection against the development of bloodstream infection. Treating the immunization process as requiring the independent steps of 1) attracting antigen-presenting cells to the site of immunization and 2) specifically directing vaccine antigen to the immature dendritic cells that initiate the adaptive immune response may provide a rational strategy for the development of a clinically applicable malaria DNA vaccine.
Introduction
The 1967 report by Nussenzweig et al [1] that immunization with irradiated sporozoites could induce sterilizing immunity to malaria in mice has provided the foundation for current efforts at malaria vaccine development. A recent clinical trial of this approach demonstrated the need for five rounds of intravenous immunizations in order to uniformly attain sterilizing immunity [2] Intensive study of the immune mechanisms mediating efficacy of irradiated sporozoite vaccines has led to a focus on the important role of liver-resident CD8 T cells in the development of the observed protection [3]–[10].
Because of the impracticality of generating and administering the large quantities of irradiated sporozoites that would be needed for protection within malaria endemic areas, efforts to develop subunit vaccines have persisted. The recently completed, and most extensive to date, clinical trial of a subunit vaccine capable of eliciting both humoral and T-cell mediated immune responses demonstrated only 35% protection against the development of severe malaria [11].
Given the paucity of examples of clinically successful vaccines acting through cell-mediated immune mechanisms, it becomes important to acknowledge the obstacles that confront the development of any vaccine targeting that mechanism of protection. Elicitation of effective T-cell mediated protective immunity targeting liver stage parasites may be problematic because of evidence that 1) persistent antigen exposure is most effective at eliciting effector T cell memory [3], [12] and 2) exposure to malaria sporozoites gives rise to a short burst of CD8 T cell responses that are refractory to re-stimulation [13], [14]. Further, critical to T-cell mediated protection is the necessity for sustaining local memory T cells at the site of parasite invasion, in this case, the liver. A recent study with a candidate herpes virus type 2 vaccine has demonstrated that localized application of chemokine preparations to the potential site of virus exposure can ensure local maintenance of effector memory T cells and enhanced resistance to infection within the female genital tract of mice [15]. It is unclear how such localization and maintenance of memory T cells could be obtained within the liver. Indeed, the prodigious, but unsuccessful, effort to develop a T cell vaccine to prevent human immunodeficiency virus infection, culminating in the large STEP study [16] provides ample testimony to the difficulty of achieving this goal.
In view of these experiences, our laboratory has undertaken an effort to develop a malaria vaccine that can provide protective immunity simply on the basis of its ability to elicit a profound humoral immune response. That humoral immunity can protect against infection initiated with as many as 100 sporozoites inoculated intravenously has been demonstrated with passive administration of both monoclonal and polyclonal antibodies, as well as with immunization with adjuvanted peptide vaccines [17]–[20]. However, elicitation of equivalent protective humoral immunity with DNA vaccines has been difficult to achieve (reviewed in [21].
For these studies we employ a mouse strain, C57Bl/6 (H-2b) that poses a high bar to the development of protective immunity. C57Bl/6 mice are known to lack T cells within their repertoire capable of generating a Class I-restricted T cell response to the Plasmodium yoelii malaria circumsporozoite protein [22], [23]. Further, in studies with Plasmodium berghei this mouse strain is more susceptible to infection than the Balb/c strain often used in malaria vaccine studies [24], [25], rendering the demonstration of protective immunity more difficult. C57Bl/6 mice have been identified, therefore, as the preferred mouse strain for use in studies of immune protection against P. berghei liver stage infection [25] and in the current studies we will be analyzing the ability of these mice to be protected against parasite challenge that is an order of magnitude greater than that used in the previously cited humoral immunity studies.
The immunodominance of the circumsporozoite protein in sporozoite-elicited immunity to malaria has been well-described [22]. The use of adjuvants to enhance the immune response represents an effort to attract to the site of immunization those cells that initiate the development of acquired immunity, without eliciting the adverse inflammatory effects associated with true infection. However, many of the cells attracted by adjuvants play no direct role in the development of an immune response [26]. As part of the effort to attract cells involved in the initiation of the immune response without producing excessive inflammation, considerable interest has developed in using specific chemokines to replace the more non-specifically acting group of adjuvants based primarily on bacterial lipid derived products [27]–[29]. To enhance the efficiency of bringing the antigen into contact with appropriate antigen presenting cells, several studies have examined generating vaccines by fusing candidate vaccine antigens to chemokines that attract the appropriate antigen presenting cells to the site of immunization [28], [30], [31]. While many of these studies have focused on the ability of specific cytokines or chemokines to enhance the inflammatory infiltrate or promote differentiation of specific subsets of immune cells [27], [28], [32]–[34], others have used chemokines to target pathogen proteins to specific immune cell populations. In many of these studies, the chemokine was expected to perform the dual function of attracting cells to the site of immunization and then targeting the pathogen-specific antigen to an immune cell of interest, frequently antigen-presenting dendritic cells [35]–[40].
In the current study we have examined the merit of treating these two functions of adjuvants, chemoattractant and cell-targeting, as independent variables in the development of a DNA vaccine. This approach allowed us to develop vaccine strategies that optimize both variables independently. To stimulate the innate immune system, we employed the adjuvant Vaxfectin, which has been demonstrated to function well as an adjuvant for DNA vaccines and has specifically been shown to enhance the response to candidate malaria DNA vaccines [41]–[44]. Because the initiation of the immune response requires efficient engagement of antigen with immature dendritic cells, we fused DNA encoding portions of the circumsporozoite protein (CSP) from either the mouse malaria strain Plasmodium yoelii or the human malaria strain Plasmodium falciparum to DNA encoding the chemokine macrophage inflammatory protein-3α(MIP3α), also known as CCL20.
Our studies indicate that the addition of DNA encoding MIP3α to the CSP DNA construct provided no significant alteration of the cellular or cytokine or chemokine profile obtained at the immunization site over that achieved with Vaxfectin and CSP DNA alone. As used in this vaccine construct, the MIP3α component lacked any of the traditional adjuvant functions. However, the presence of the MIP3α component in the vaccine DNA construct, when combined with the use of Vaxfectin, resulted in profound enhancement in mice of the antibody response and levels of protection against malaria sporozoite challenge equivalent to those observed after immunization with irradiated sporozoites, the gold standard in the field. Vaccine design which incorporates the independent roles of the chemoattractant and cell-targeting components in eliciting immune responses can lead to dramatic improvements in the humoral immune response and the resulting vaccine efficacy.
Materials and Methods
Animals
Six- to eight-week-old female C57BL/6 (H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a pathogen-free micro-isolation facility in accordance with the National Institutes of Health guidelines for the humane use of laboratory animals. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University (Protocol Number MO6H319).
Plasmids
The plasmid DNA encoding regions of the P. yoelii or P. falciparum circumsporozoite protein (pCSP) fused with murine or human MIP-3α (pMCSP) and control plasmids are described in Figure 1. Plasmid (pM) containing DNA encoding MIP-3α fused to DNA encoding an irrelevant lymphoma immunoglobulin variable region protein [39] was used as a negative control. The leader sequence for the P. yoelii construct is the interferon-γ-induced protein 10 (IP-10) leader sequence. The P. yoelii and P. falciparum circumsporozoite nucleotide and amino acid sequences are provided in the supplementary materials. For construction of the P. falciparum DNA vaccine, plasmid (phMfCSP) encoding human MIP-3α fused with a codon-optimized P. falciparum (3D7) CSP DNA sequence containing deletions of the N-terminal 20 aa signaling sequence and the C-terminal 23 aa anchor region was synthesized and cloned into the DNA vaccine plasmid VR1012 [45]. Human MIP3α was employed in this construct to validate its efficacy since it would be the component employed in any human vaccine targeting P. falciparum. Both mouse and human MIP3α can serve as ligands for mouse CCR6. The human tissue plasminogen activator (TPA) signal sequence [46] was included in the synthetic construct of hMfCSP and fCSP to obtain more efficient protein secretion than that observed with the P. falciparum construct using the IP-10 leader sequence (data not shown). Control plasmids (pfCSP and phM) were constructed using the same strategies as the constructs pCSP and pM. Plasmids were purified using Endofree purification columns (Qiagen, Hilden, Germany) and stored at -20° C in PBS. Endotoxin contaminates were determined using the ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit (Genscript, NJ). All plasmids used for immunization had final endotoxin levels below, the lower limit of detection of the kit, 0.1 EU/DNA. The DNA sequences for the leader, MIP3α spacer and CSP components of the vaccine plasmids are provided in Figures S1 and S2.
Figure 1. Diagrammatic representation of the DNA constructs, including control plasmids, used for immunization.
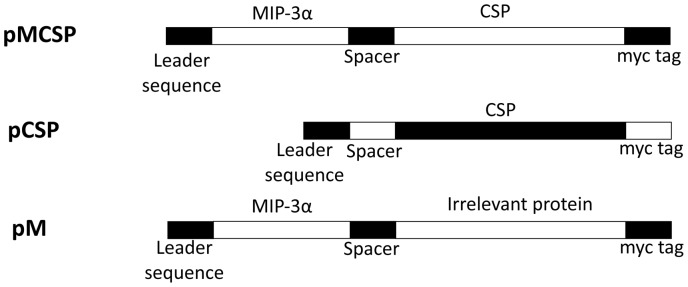
The construct for P. yoelii used mouse MIP3α, while the P. falciparum construct employed the sequence for human MIP3α. M refers to MIP3α. CSP refers to a segment of the Plasmodium yoelii circumsporozoite protein; and MCSP refers to the fusion protein of MIP-3α and a segment of the P. yoelii circumsporozoite protein. Also shown are the positions of the leader sequence, spacer, and the myc tag. The leader sequence for the P. yoelii construct is the interferon–γ induced protein 10 secretion leader sequence; the leader sequence for the P. falciparum construct is the human tissue plasminogen activator leader sequence [46]. The MCSP and hMfCSP nucleotide sequences are provided in the supplementary materials.
Transfection and Western Blot
DNA transfection in 293T cells was carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described by the manufacturer. Cells and supernatant were collected 48 h after transfection. Lysed cells (105) and supernatant (10µl) were placed in loading buffer (0.08 M Tris (pH 6.8), 2.0% sodium dodecyl sulfate (SDS), 10% glycerol, 0.1 M dithiothreitol, 0.2% bromophenol blue). The samples were boiled for 10 min, and proteins were separated by SDS-polyacrylamide gel electrophoresis. Proteins were transferred onto nitrocellulose membranes, and the membranes were probed with anti-cMyc antibodies, kindly provided by Dr. Michael Matunis, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. Secondary antibodies were alkaline phosphatase-conjugated anti-mouse antibodies (Jackson Immunoresearch, Inc., West Grove, PA), and staining was carried out with 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitro blue tetrazolium (NBT) solutions prepared from chemicals obtained from Sigma (St. Louis, MO).
Vaxfectin formulation
The adjuvant Vaxfectin has been previously described [47] and was provided by Vical Inc. (San Diego, CA). Vaccine formulations were prepared by adding 1 ml of 0.9% NaCl solution to 2.18 mg of Vaxfectin, which was then mixed in an equivalent volume with 2 mg/ml DNA. The mixture was then diluted to the desired DNA concentration with PBS. The final DNA/Vaxfectin molar ratio was 4∶1.
Immunization of mice
C57BL/6 mice were immunized with 2 µg (P. yoelii) or 5 µg (P. falciparum) of the constructs described above, and were delivered as a single intramuscular injection in the anterior tibialis muscle in 100µl of PBS formulated with Vaxfectin. Mice received three immunizations at bi-weekly intervals. For the positive control group for the P. yoelii studies, 105 (initial immunization) and 5×104 (booster immunizations) with irradiated P. yoelii sporozoites (17XN) obtained from Anopheles stephensi mosquitoes maintained in the Johns Hopkins Malaria Research Institute insectary were inoculated by tail-vein injection at the same time-points.
Parasites for challenge
P. yoelii and transgenic P. berghei sporozoites [48] were used for challenge. Sporozoites were obtained by hand dissection of salivary glands of Anopheles stephensi mosquitoes maintained in the Johns Hopkins Malaria Research Institute insectary. The isolated sporozoites were suspended in HBSS medium containing 1% normal mouse serum. Challenges to evaluate vaccine effect on hepatic parasite load were accomplished by injecting 5×103 sporozoites in the tail vein. Challenges to evaluate protection from blood stage malaria were accomplished by injecting 1×103 sporozoites in the tail vein.
ELISA assay
Humoral immune responses to the immunodominant B cell epitope of the relevant CSP protein were measured using a previously described CSP-specific ELISA, with minor modifications [49]. ELISA plates were coated with 2 µg of recombinant PyCSP or recombinant PfCSP. Recombinant PyCSP was a GST-tagged segment of PyCSP protein that includes 15 copies of the central GPGAPQ repeat region [50]. Recombinant PfCSP was a 6-His-tagged segment of the PfCSP protein that includes 21 copies of the central NANP repeat region and was kindly provided by Dr. Gary Ketner, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. The endpoint ELISA titer is reported as the highest dilution of serum at which the average absorbance was twice the value obtained using pre-immunization serum. For quantitation of the antibody concentration, monoclonal 2A10 [51], [52] (IgG2b, κ), which recognizes the (NANP)3 sequence of P. falciparum was used as the standard. Known concentrations of this standard were diluted to obtain O.D.405 readings in the linear range and compared to equivalent O.D.405 readings with dilutions of sera from immunized mice. For statistical analysis of differences in antibody concentrations among different groups, direct O.D.405 readings of individual mice were used.
To obtain ELISA readings, plates were placed inside a humidity chamber and incubated overnight at 4°C. Plates were blocked with 2% BSA for 30 min at room temperature (RT) and then serially diluted serum samples were added and incubated at RT for 2 h. After washing six times, peroxidase labeled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, INC) was added at a dilution of 1∶1,000 and incubated at RT for 1 h. After washing six times, ABTS Peroxidase substrate (KPL, Gaithersburg, MD) was added for development and incubated for 1 h. The data were collected using the Synergy HT (BioTek Instruments, Inc, Winooski, VT).
Real-time PCR for liver stage parasites
Real-time PCR was used for the detection and quantification of the liver stage of Plasmodium parasites [53]. Two pairs of specific primers were designed to amplify the parasite 18s rRNA sequence. For P. yoelii parasites (17XNL), the forward primer was 5′-GGGGATTGGTTTTGACGTTTTTGCG-3′ and the reverse primer was 5′-AAGCATTAAATAAAGCGAATACATCCTTAT-3′. For transgenic P.berghei parasites, the forward primer was 5′-TGGGAGATTGGTTTTGACGTTTATGT-3′ and the reverse primer was 5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′. Values were normalized against measurements of mouse actin mRNA in the same samples. The primers used for mouse actin were as follows: 5′-GTCCCTCACCCTCCCAAAAG-3′ (forward) and 5′-GCTGCCTCAACACCTCAACCC-3′ (reverse). The reactions were performed in a final volume of 20 µl using SYBR green PCR Master Mix (2X) from Applied Biosystems and processed with ABI StepOne Real-time PCR system (Applied Biosystems). Amplification was performed using a 2-min step at 50°C and a 10-min denaturation step at 95°C, followed by 40 cycles of 15 s denaturation at 95°C, 1 min of primer annealing, and polymerization at 60°C.
In vivo depletion of T cell subsets
To deplete the CD4+, CD8+, or both T cell subsets, immunized mice were injected i.p. with anti-CD4 (GK1.5) and/or anti-CD8 (2.4.3) or both mAbs, purchased from American Type Culture Collection (ATCC, Rockville, MD]. Each mouse received daily doses of 200 µg of anti-CD4 or anti-CD8 or both antibodies for two days. Twenty-four hours after the last mAb treatment, the efficacy of the depletion was estimated by two-color flow cytometry analysis of peripheral blood lymphocytes, using FITC-conjugated anti-mouse CD4 or APC-conjugated anti-CD8 mAbs (eBioscience,San Diego, CA).
Real-time PCR analysis of cytokine and chemokine levels of muscle samples
Total RNA was isolated from tissue samples (n = 3 per group). Reverse transcription was done using total RNA (2 µg) as template and the High-Capacity cDNA reverse transcription kit (Applied Biosystems; Foster City, CA) reagents. Relative transcript levels for CXCL-2, IFN-γ, CXCL-9, TGF-β, CCL-2, TNF-α, IL-2, IL-6, IL-10, IL-12b, and MIP-3α were determined in tissue samples collected 24 h and 48 h after vaccination. For this purpose commercially available TaqMan Gene Expression Assays (Applied Biosystems) were used. These cytokines and chemokines were selected for analysis based on preliminary exploratory work on biomarkers associated with Vaxfectin formulations [54]. Analysis was performed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) and cycling conditions suggested by the manufacturer. A comparative method was used to determine relative quantitation. Briefly, Ct values for target gene amplification were normalized by subtracting the Ct values for the house keeping gene GAPDH.
Extraction and analysis of infiltrating DCs
Anterior tibialis muscles from immunized mice were harvested after euthanization at the indicated time points, minced, and incubated with digestion buffer (Hanks' Balanced Salt Solution (Invitrogen, Carlsbad, CA), supplemented with 0.5% collagenase, 0.2% BSA, and 0.025% trypsin) for 30 minutes at 37°C with vortexing every 5 minutes to facilitate tissue dissociation. Cell suspensions were filtered, washed twice with PBS containing 2% FBS. The cells numbers were counted and 1×106 were stained with APC-labeled CD11c antibodies (clone N418, eBioscience, San Diego, CA), and analyzed by flow cytometry on a Becton-Dickinson FACScan (Becton-Dickinson, Franklin Lakes, New Jersey).
Statistical analysis
Differences in sporozoite rRNA and antibody concentrations among groups were analyzed by an ANOVA test (Stata Corp, College Station, TX). A value of p<0.05 was considered to be significant. Correlations between antibody concentration and P. yoelii rRNA levels in the liver were determined using Pearson's r correlation (pwcorr) function (Stata Corp, College Station, TX). The significance of differences in development of bloodstream infection among groups of challenged mice was determined using a Fisher exact test (Stata Corp, College Station, TX).
Results
Expression of vaccine constructs
The efficacy of any DNA vaccine targeting the elicitation of a humoral immune response and dependent on the activity of incorporated targeting ligands will depend on achieving extracellular expression of the encoded proteins. Figure 2 demonstrates that both the P. yoelii and P. falciparum constructs could be expressed into the extracellular environment from transformed 293T cells. In order to achieve efficient expression of the P. falciparum construct, the human tissue plasminogen activator leader sequence was used. Both constructs with and without the MIP3α component were expressed as soluble proteins in the extracellular environment. The fCSP lane shows the presence of proteolytic fragments, as has been observed by others expressing this construct [55].
Figure 2. Western blot analysis of expression of constructs of malaria DNA vaccines and controls.
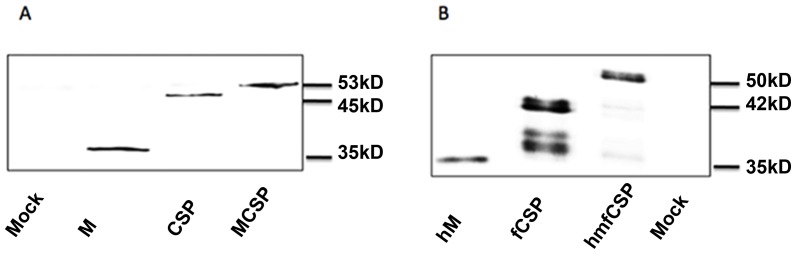
P. yoelii (A) and P. falciparum (B) DNA vaccine candidates and control constructs were transfected into and expressed from 293T cells. Protein expression into culture supernatant was detected with anti-myc antibody 48 hours after transfection. In each lane the vaccine constructs are the uppermost bands with lower bands of fCSP representing proteolytic fragments of the expressed proteins. CSP = a fragment of the P. yoelii circumsporozoite protein, fCSP = a fragment of the P. falciparum circumsporozoite protein, M = murine MIP3α, hM = human MIP3α.
Comparison of immunization regimens for elicitation of antibody responses
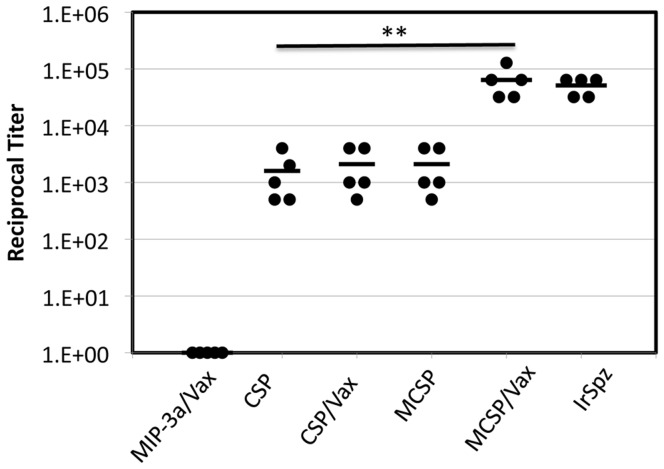
To test the relative immunogenicity of the different P. yoelii vaccine constructs with and without Vaxfectin, C57BL/6 mice were immunized three times with a low dose (2 µg) of the different vaccine or control plasmids according to the schedule outlined in the Methods. For the positive control group (IrSpz), 105 (initial immunization) and 5×104 (booster immunizations) irradiated P. yoelii sporozoites (17XN) were inoculated at the same time-points. Mice were bled two weeks after the final immunization to determine specific antibody concentrations. Figure 3 demonstrates the differences in endpoint dilution titers from individual mice and the mean endpoint dilution titer for each group. The results indicate that fusion of DNA encoding MIP3α to the CSP DNA vaccine construct or use of the CSP DNA vaccine construct in combination with Vaxfectin both resulted in similar enhancement of the antibody response which, in this experiment, was marginally, but not significantly improved over the response to CSP alone (p = 1.000). However the response to the combination of Vaxfectin plus the MIP3α-CSP DNA construct was significantly greater than responses to the other CSP-containing vaccine regimens (p<0.001) and did not differ significantly from the response to irradiated sporozoites (p>0.9).
Figure 3. Antibody titers in C57Bl/6 mice immunized with P. yoelii CSP DNA constructs Specific antibody concentrations in sera obtained two weeks after the final immunization.
Values shown represent the reciprocal of the endpoint ELISA titer for each mouse (closed circles) and the mean of the reciprocal titers from the 5 mice in each group (horizontal broken lines). The endpoint titer is reported as the highest dilution of serum at which the absorbance was twice the value obtained using pre-immunization serum. The responses to irradiated sporozoite and MIP3α-CSP/Vaxfectin immunization regimens did not differ (p>0.9) and were significantly greater than those to the other immunization regimens (p<0.001), as indicated by ** in the figure.
Protective efficacy of different vaccine regimens to sporozoite challenge
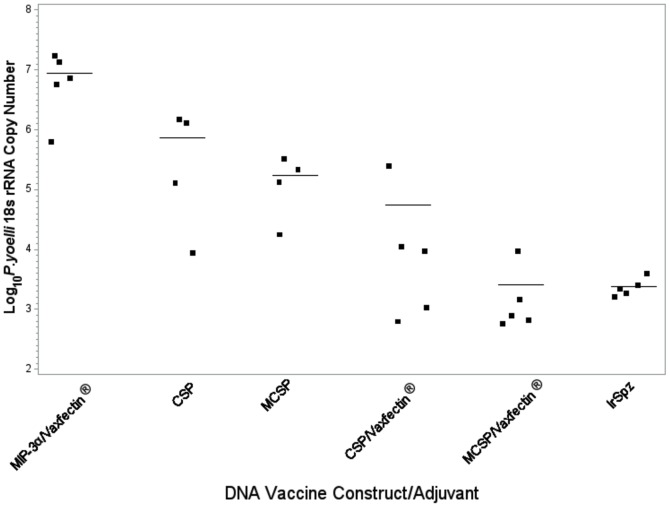
To determine if the differences in antibody responses among the different regimens correlated with protection, the immunized mice were challenged two weeks after the final immunization with 5×103 P. yoelii sporozoites obtained by dissection of the salivary glands of infected A. stephensi mosquitoes. Liver sections recovered 48 hours post-challenge from five mice from each of the immunization groups were assayed by qRT-PCR for the level of P. yoelii 18s rRNA present (Figure 4). Compared to mice immunized with a construct lacking CSP, mice immunized with CSP alone had an approximately one log reduction in the P. yoelii rRNA load in the liver, while recipients of either MCSP alone or CSP plus Vaxfectin had their parasite load reduced 100 fold. Recipients of the MCSP plus Vaxfectin or irradiated sporozoites vaccines had an essentially equivalent 3.5 order of magnitude reduction in the liver parasite load. The protection achieved by MCSP + Vaxfectin or irradiated sporozoites differed significantly from any of the other CSP immunization groups (p<0.002) but did not differ from each other (p = 1.000).
Figure 4. Effect of immunization with the combination of Vaxfectin and MCSP DNA on protection achieved against in vivo challenge with P. yoelii sporozoites.
C57BL/6 mice were immunized with the indicated DNA vaccine or irradiated sporozoites as described in the Methods section. Two weeks after the final immunization, mice were challenged with 5×103 P. yoelii sporozoites, and parasite-specific rRNA levels in the liver were determined by quantitative RT-PCR on samples obtained 48 hours post challenge. All results were normalized against the expression of actin. Results indicate the copy numbers from individual mice (closed squares) and the mean from the 5 mice in each group (horizontal lines). The MCSP/Vaxfectin or irradiated sporozoite groups differed significantly from any other group (p<0.002), but not from each other (p>1.000).
We next examined the correlation between antibody concentrations in the sera of individual mice and the copies of rRNA that were detected in the liver sections. Figure 5 shows a scatter plot and regression line of the antibody concentrations and associated P. yoelii rRNA levels in the livers of immunized mice and demonstrates a strong correlation between the two measurements (r = .64, p = 0.0002).
Figure 5. Association between anti-CSP antibody concentration and sporozoite infection of liver.
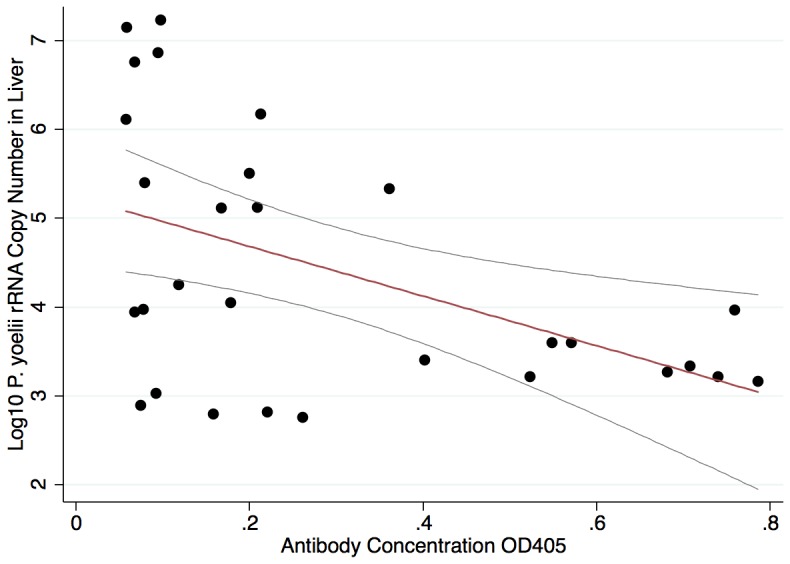
Pearson correlation plot with regression line and 95% CI demonstrating the relationship between anti-CSP antibody concentration within individual mice and the log10 standardized copy number of P. yoelii rRNA recovered from the liver of the corresponding mouse, (r = 0.64, p<0.001).
Role of T lymphocytes as effectors of MCSP-Vaxfectin elicited protective immunity
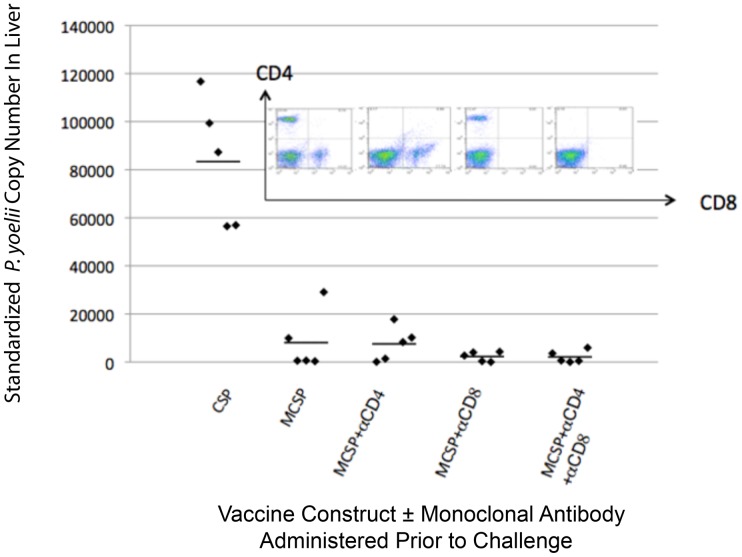
Because there is considerable evidence from irradiated sporozoite vaccine studies that T lymphocytes serve as important mediators of the elicited protective immunity at the pre-erythrocytic stage of infection [3]-[5], [8]-[10], [56]–[62], we sought to confirm further that in C57Bl/6 mice T cells were playing no role at the effector stage of the protective response elicited by our vaccine construct. This becomes important due to studies showing that C57Bl/6 mice are capable of generating a Class II-restricted effector T cell response [63]. Mice were immunized as previously described and two weeks after the final immunization were depleted of CD4+, CD8+ or both classes of T cells, as described in the Methods. A control group was immunized with Vaxfectin plus the CSP plasmid without the chemokine fusion component. The flow cytometric images in the inset of Figure 6 demonstrate that the respective monoclonal antibody treatments effectively eliminated the targeted T cell populations. Figure 6 also shows that the MCSP plus Vaxfectin immunized groups did not differ significantly from each other independent of T cell depletion (p = 1.000) and all MCSP + Vaxfectin immunized mice had significantly fewer rRNA copies in the liver than the CSP plus Vaxfectin immunized mice (p<0.001). These results support the conclusion that T lymphocytes play no role at the effector level in the observed protection.
Figure 6. Effect of T cell depletion just prior to challenge on protection provided by Vaxfectin plus MCSP immunization.
Two weeks after the final immunization, recipients of MCSP DNA were depleted of CD4, CD8 or both T cell subsets. The efficacy of the T-cell depletions is indicated by the flow cytometry analysis shown in the inset. The mice were then challenged with 2×103 P. yoelii sporozoites. The standardized P. yoelii rRNA copy number in the livers of individual mice (5/group) and the mean for each group are shown. Not shown in the figure is that all immunized mice received Vaxfectin in addition to the indicated DNA constructs. The MCSP plus Vaxfectin immunized groups did not differ significantly from each other independent of T cell depletion (p = 1.000) and all MCSP + Vaxfectin immunized mice had significantly fewer rRNA copies than the CSP plus Vaxfectin immunized mice (p<0.001).
Characterization of the role of the adjuvant and the chemokine in eliciting enhanced responses
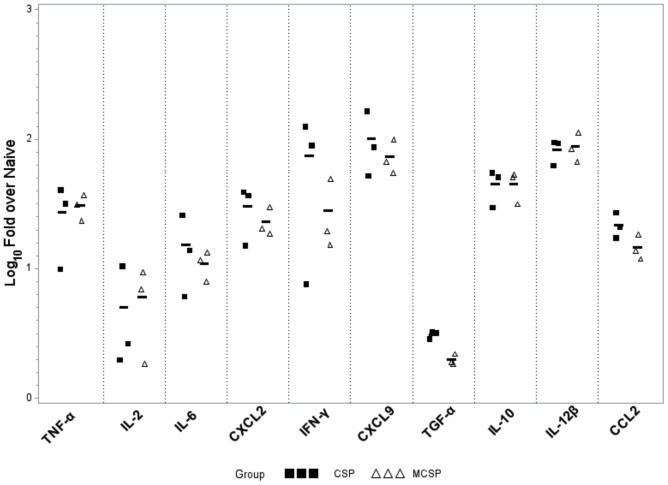
The previous experiments demonstrate the marked enhancement of the immune response by use of the combination of Vaxfectin and the fusion of DNA encoding the chemokine MIP3α to that encoding CSP. To determine the role of these two vaccine components in eliciting the enhanced immune response, the relative ability of the Vaxfectin-CSP and Vaxfectin–MCSP regimens to elicit accumulation of reactive cells and enhanced cytokine and chemokine expression at the site of immunization 24 and 48 hours post-inoculation was evaluated. For this purpose, the entire tibialis major muscle at the immunization site was harvested from euthanized mice 24 and 48 hours post immunization with the adjuvant and the two constructs. Figure 7 demonstrates at 48 hours the expression at the site of immunization of a representative group of cytokines and chemokines associated with the initiation of an immune response. At both time points (24 hours not shown) the addition of MIP3α to the vaccine construct did not enhance the studied cytokine or chemokine levels over those observed with Vaxfectin and DNA encoding CSP alone (p>0.5).
Figure 7. Expression of vaccine-induced cytokines and chemokines at immunization site 48 hours after immunization with and without MIP3α.
Scatterplot showing results of an experiment in which 2 µg of Vaxfectin-formulated MCSP or Vaxfectin-formulated CSP were inoculated into the tibialis anterior muscle of C57BL/6 mice. The entire muscle was resected and processed as described in the Methods. Cytokine and chemokine levels of injected muscle samples were analyzed by real-time PCR 24 h (not shown) and 48 h after injection. There were no significant differences between the two time points. In all cases, the cytokine or chemokine levels did not differ between recipients of the CSP vs. the MCSP construct (p>0.5). All cytokine or chemokine levels for either DNA construct were significantly above control levels (p<0.002) except for levels of IL-2, and TGF-β, which did not differ significantly from control levels at either 24 or 48 hours (p>0.2).
We also examined the impact of the presence or absence of MIP3α in the vaccine construct on the cellular infiltrate that accumulated at the site of immunization. Table 1 shows that the combination of Vaxfectin and CSP DNA is the most potent attractant of cells to the site of inoculation. The recruitment of cells, and specifically dendritic cells, was not enhanced by inoculating with Vaxfectin and the fusion MIP3α-CSP DNA construct compared to Vaxfectin and CSP DNA without the MIP3α component (p = 1.000). The immunological enhancement observed with the combination of Vaxfectin and the MCSP construct therefore cannot be attributed to the chemoattractant functions of the chemokine. Further, the proportion of infiltrate contributed by CD11c+ cells was equivalent among all groups, other than that receiving only PBS, which differed significantly from the CSP/Vaxfectin group in the percentage of CD11c+ cells attracted.
Table 1. Impact of Different Immunization Regimens on Cellular Infiltrate at Site of Immunization.
| Total cell number (104) (mean±SEM) | %CD11c+ cell(mean±SEM) | Total CD11c+ cell number (104) (mean±SEM) | |
| PBS | 17.67±1.20 | 2.77±0.63 | 0.49±0.11 |
| CSP | 27.66±4.33 | 5.64±0.22 | 1.56±0.24 |
| CSP/Vaxfectin | 79.33±4.26* | 6.46±0.97 | 5.05±0.52 |
| MCSP | 22.66±1.45 | 4.91±0.52 | 1.10±0.57 |
| MCSP/Vaxfectin | 83.66±8.57* | 5.19±0.84 | 4.21±0.43 |
Immune response to and protection provided by a vaccine construct targeting P. falciparum
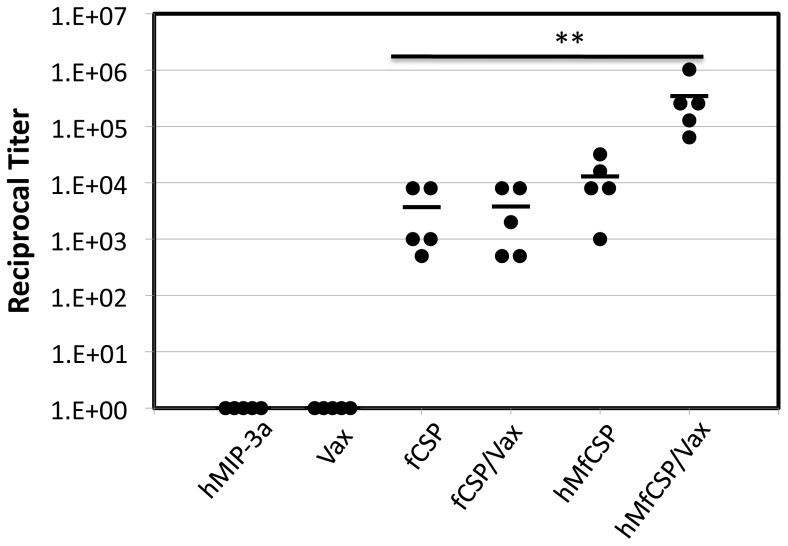
A vaccine construct incorporating a portion of P. falciparum CSP DNA instead of P. yoelii CSP DNA was then developed and used to confirm the efficacy of this vaccine platform for reducing hepatic infection. This construct contained DNA encoding human MIP3α instead of murine MIP3α, both of which bind to murine CCR6. This vaccine construct would be one which would have potential clinical application by using the human MIP3α construct in a vaccine targeting the human malaria pathogen. In order to ensure adequate secretion of the MIP3α-fCSP protein complex, DNA encoding the tissue plasminogen activator signal sequence was incorporated into this vaccine construct. Mice immunized with the hMIP3α-CSP construct plus Vaxfectin had significantly higher antibody concentrations in their serum than did mice receiving any of the other Vaxfectin-containing combinations, including fCSP DNA plus Vaxfectin (p<0.01, Figure 8).
Figure 8. Antibody titers in mice immunized with the P. falciparum construct.
C57BL/6 mice were immunized with 5 µg of the P. falciparum constructs of Figure 1 with or without Vaxfectin. Specific antibody concentrations. (Closed circles), which are reported as the highest dilution of serum at which the absorbance was twice the value obtained using pre-immunization serum, were determined two weeks after the final immunization. Also shown is the mean of the titers from the 5 mice in each group (lines). The response to the MIP3α-CSP/Vaxfectin immunization regimen was significantly greater than those to the other immunization regimens (p<0.01), as indicated by **.
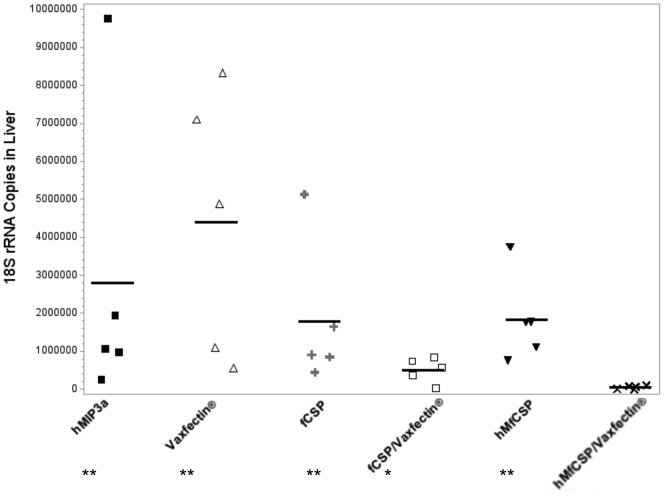
Two weeks following the third immunization mice were challenged with 5×103 transgenic P. berghei expressing CSP from P. falciparum. The hMIP3α-fCSP plus Vaxfectin protocol reduced the standardized quantity of P. berghei rRNA recovered from the liver by almost two orders of magnitude compared to the Vaxfectin alone control and one order of magnitude compared to the fCSP plus Vaxfectin immunization regimen (Figure 9, p<0.001 and p<0.05, respectively). In a separate experiment the ability of this vaccination protocol to protect against the development of blood-stage malaria was evaluated and compared to that in recipients of PBS, Vaxfectin plus a DNA construct encoding only hMIP3α without the fCSP component, and Vaxfectin and a DNA construct encoding CSP without hMIP3α. Only the hMfCSP plus Vaxfectin protocol provided significant protection in two independent challenge experiments (Table 2, Experiment 1 or 2, p = 0.05 vs. PBS). Although the same number of transgenic P. berghei sporozoites were used in both challenge experiments, sporozoites may vary in virulence among different harvests from the salivary gland of infected mosquitoes [43], as evidenced by the marked differences in outcomes among the groups receiving suboptimal vaccine regimens.
Figure 9. Protective efficacy of different vaccine constructs in C57BL/6 mice challenged with transgenic P. berghei expressing P. falciparum CSP.
C57BL/6 mice were immunized with 5 µg of the indicated DNA vaccine as described in the Methods section. Two weeks after the final immunization, mice were challenged with 5×103 transgenic P.berghei sporozoites, and parasite-specific rRNA levels in the liver were determined by quantitative RT-PCR on samples obtained 48 hours post challenge. All results were normalized against the expression of actin. Results indicate the copy numbers from individual mice (closed squares) and the mean from the 5 mice in each group (lines). ** Compared to hMfCSP/Vaxfectin p<0.001. *Compared to hMfCSP/Vaxfectin p<0.05. No other differences among groups were statistically significant.
Table 2. Ability of different vaccine constructs to protect against the development of blood stage malaria infection.
| Experiment 1 | |
| Immunization | No. Infected/No. Challenged |
| PBS | 4/5 |
| hMIP3α/Vaxfectin | 4/5 |
| fCSP/Vaxfectin | 2/5 |
| hMfCSP/Vaxfectin | 0/5 |
To determine the actual concentration of specific antibody elicited by the different immunization protocols, pooled sera from mice used in the second experiment from Table 3 were assayed for antibody concentration by ELISA, using the fCSP-specific monoclonal antibody 2A10 as a reference standard. The results indicate a greater than 15-fold higher concentration of specific antibody in the group receiving the Vaxfectin-hM-fCSP regimen compared to the other immunization groups.
Table 3. CSP-specific Antibody Concentration in Pooled Sera from Different Groups of Immunized Mice.
| Immunization Regimen | Antibody concentration (μg/ml) |
| fCSP/Vax | 11.1 |
| hMfCSP | 105.3 |
| hMfCSP/Vax | 1638.9 |
| PBS | 0 |
Discussion
The current studies demonstrate that a novel plasmid DNA-based immunization regimen can profoundly enhance the protective antibody response against malaria sporozoite challenge. The regimen used adjuvant to attract antigen-presenting cells to the site of immunization and, in addition, fused the vaccine antigen to a ligand that would specifically target it to immature dendritic cells. Use in a murine challenge model of the adjuvant Vaxfectin with a DNA construct encoding either the murine or human chemokine MIP3α used to CSP from P. yoelii or P. falciparum resulted in a greater than one log reduction in liver parasite burden compared to use of the construct without the chemokine and a reduction of greater than three orders of magnitude compared to that observed in CSP naïve mice. Where protection was compared to that achieved by immunization with irradiated sporozoites, the level of protection was equivalent. Further, the chemokine-CSP-adjuvant combination provided significantly enhanced protection against the development of blood stage malaria, a result not obtained with the regimen lacking the chemokine component.
MIP3α was included in the vaccine construct for the express purpose of targeting the vaccine antigen to the CCR6 receptor present on the immature dendritic cells that initiate an immune response. To determine the basis for the enhanced responses observed by use of the combination of adjuvant and chemokine, we examined the relative ability of the different compositions to attract cells involved in the immune response, particularly dendritic cells, to the site of immunization. These studies revealed that the combination of Vaxfectin and DNA encoding CSP was the most potent for attracting dendritic cells to the immunization site and that fusion of the chemokine encoding DNA to the CSP encoding DNA did not enhance the migration of those cells to the site. Although the chemotactic capability of MIP3α has been well documented [64]–[70], it is, in our studies, clearly less potent than Vaxfectin in attracting cells to the site of immunization. Further, the addition of the chemokine component to the vaccine construct did not alter the cytokine or chemokine profile within the inoculated tissue, compared to that observed with the use of Vaxfectin and vaccine encoding the CSP antigen alone. These observations would suggest that the role of the chemokine in this vaccine platform is simply to target the vaccine antigen to immature dendritic cells. In this vaccine platform, the traditional role of the adjuvant, attracting immune cells to the immunization site and creating an immunological milieu that favors the generation of adaptive immunity, is dependent on Vaxfectin. That MIP3α is acting solely as a targeting molecule is supported by previous studies indicating that actual fusion of the chemokine to the vaccine antigen was required for enhancement of immune responses observed with chemokine-antigen constructs without Vaxfectin [39].
Because of its demonstrated efficacy in the clinical setting, protection elicited by irradiated sporozoites has been the focus of much of the study of the immune mechanisms involved in protective immunity at the pre-erythrocytic stage of malaria infection [2], [3], [5], [13], [60], [71]–[77]. While previous studies have clearly demonstrated the protective potential of antibodies elicited by subunit vaccines [20], studies of vaccination with irradiated sporozoites have shown a critical role for CD8+ T cells in the elicited protection [3], [5], [8], [60], [78]–[81]. It has previously been demonstrated that C57Bl/6 mice fail to develop a Class I-restricted T cell response to P. yoelii CSP. The current studies further confirmed that neither CD4+ nor CD8+ T cells had any role at the effector stage in the observed protection.
The malaria vaccine we have described demonstrates a platform that can employ DNA to elicit highly protective humoral immune responses in mice. While some earlier studies have suggested poor immunogenicity of DNA vaccines in humans [82], more recent studies have indicated that use of adjuvants and alternative delivery systems have resulted in greatly enhanced immunogenicity in humans and/or non-human primates [83]–[90]. While further studies will be required to confirm the efficacy of this approach for sustained protection in non-murine vaccination settings, the protection observed with this vaccine platform demonstrates the potential for a DNA-based subunit vaccine to provide potent humoral immunity that prevented bloodstream infection after an intravenous challenge of C57Bl/6 mice with 1000 transgenic P. berghei sporozoites. Sedegah et al [43] studied protection using only Vaxfectin with a CSP DNA construct that lacked the MIP3α component and obtained maximum bloodstream protection of 54%, similar to what we observed with that construct, but clearly less than the 80–100% protection observed with the addition of the MIP3α component. In a more recent trial, Limbach et al. [91] could protect only 57% of mice challenged intravenously with 300 P. yoelii sporozoites after immunization with plasmid DNA followed by a vaccinia virus vector boost that expressed one, two or three different P. yoelii vaccine antigens. In one of the few studies to show a high level of protection, Scheiblhofer et al. demonstrated that DNA immunization with a CSP construct from which the glycosylphosphatidylinositol signal sequence was deleted elicited 100% protection of Balb/c mice from intradermal challenge with P. berghei [92]. In all of these studies, challenge occurred 14 days after the final immunization.
C57Bl/6 mice, in addition to being unable to generate a Class I-restricted T cell response to P. yoelii CSP, are also more susceptible to P. berghei infection than the Balb/c mice used by Scheiblhofer and others [24], [25]. Although intradermal or mosquito-based challenge may appear more representative of the clinical setting, it is more difficult to protect against direct intravenous challenge [93], [94]. Thus the potent antibody-based protection against bloodstream infection with transgenic P. berghei elicited by this subunit DNA vaccine was obtained under the most stringent challenge conditions.
Our study, like many others, demonstrates that DNA vaccines, properly formulated and delivered are capable of eliciting profound humoral immune responses. DNA vaccines also offer the advantages of rapid formulation and production, a long shelf life, an inability to revert into virulent forms, and ease of storage and transport, likely not requiring a cold chain, all issues highly relevant to the development of a malaria vaccine. However, the profound immune responses elicited by the platform described here supports its potential for application to other pathogens, as well.
Supporting Information
DNA sequence of pMCSP plasmid. The IP-10 leader sequence: red; MIP3α: blue; CSP: black; Myc-Hisx6 tag: orange; Multiple cloning site/spacer: green. Translation start codon and stop codon are marked with boxes.
(TIF)
DNA sequence of phMfCSP plasmid. TPA leader sequence: red; hMIP3α: blue; fCSP: black; Myc tag: orange; Multiple cloning site/spacer: green. Translation start codon and stop codon are marked with boxes.
(TIF)
Acknowledgments
The authors wish to thank Dr. Gerald Medoff, Washington University in St. Louis Medical School, St. Louis, MO, and Sean Sullivan, Vical, Inc., San Diego, CA, for helpful discussions. We also wish to acknowledge Ms. Susan Langan for assistance with graphics, and Dr. Sze-Wah Tse for technical support.
Funding Statement
This work was supported by grants from the National Institutes of Health (Grant #AI073619) and the Johns Hopkins University Malaria Research Institute (http://malaria.jhsph.edu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nussenzweig RS, Vanderberg J, Most H, Orton C (1967) Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature 216: 160–162. [DOI] [PubMed] [Google Scholar]
- 2. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, et al. (2013) Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 3. Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, et al. (2010) Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog 6: e1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cockburn IA, Chakravarty S, Overstreet MG, Garcia-Sastre A, Zavala F (2008) Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J Immunol 180: 64–71. [DOI] [PubMed] [Google Scholar]
- 5. Tsuji M, Zavala F (2003) T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol 19: 88–93. [DOI] [PubMed] [Google Scholar]
- 6. Tsuji M, Zavala F (2001) Peptide-based subunit vaccines against pre-erythrocytic stages of malaria parasites. Mol Immunol 38: 433–442. [DOI] [PubMed] [Google Scholar]
- 7. Carvalho LH, Hafalla JC, Zavala F (2001) ELISPOT assay to measure antigen-specific murine CD8(+) T cell responses. J Immunol Methods 252: 207–218. [DOI] [PubMed] [Google Scholar]
- 8. Nardin E, Zavala F, Nussenzweig V, Nussenzweig RS (1999) Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia 41: 397–402. [PubMed] [Google Scholar]
- 9. Rodrigues EG, Zavala F, Nussenzweig RS, Wilson JM, Tsuji M (1998) Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 16: 1812–1817. [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, et al. (1991) CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol 3: 579–585. [DOI] [PubMed] [Google Scholar]
- 11. Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, et al. (2011) First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365: 1863–1875. [DOI] [PubMed] [Google Scholar]
- 12. Finn JD, Bassett J, Millar JB, Grinshtein N, Yang TC, et al. (2009) Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J Virol 83: 12027–12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hafalla JC, Morrot A, Sano G, Milon G, Lafaille JJ, et al. (2003) Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J Immunol 171: 964–970. [DOI] [PubMed] [Google Scholar]
- 14. Hafalla JC, Sano G, Carvalho LH, Morrot A, Zavala F (2002) Short-term antigen presentation and single clonal burst limit the magnitude of the CD8(+) T cell responses to malaria liver stages. Proc Natl Acad Sci U S A 99: 11819–11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin H, Iwasaki A (2012) A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, et al. (2008) Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charoenvit Y, Leef MF, Yuan LF, Sedegah M, Beaudoin RL (1987) Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun 55: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charoenvit Y, Mellouk S, Cole C, Bechara R, Leef MF, et al. (1991) Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol 146: 1020–1025. [PubMed] [Google Scholar]
- 19. Charoenvit Y, Sedegah M, Yuan LF, Gross M, Cole C, et al. (1990) Active and passive immunization against Plasmodium yoelii sporozoites. Bull World Health Organ 68 Suppl: 26–32 [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R, Charoenvit Y, Corradin G, Porrozzi R, Hunter RL, et al. (1995) Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J Immunol 154: 2784–2793. [PubMed] [Google Scholar]
- 21. Doolan DL, Hoffman SL (2001) DNA-based vaccines against malaria: status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int J Parasitol 31: 753–762. [DOI] [PubMed] [Google Scholar]
- 22. Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, et al. (2006) The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444: 937–940. [DOI] [PubMed] [Google Scholar]
- 23. Weiss WR, Good MF, Hollingdale MR, Miller LH, Berzofsky JA (1989) Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol 143: 4263–4266. [PubMed] [Google Scholar]
- 24. Jaffe RI, Lowell GH, Gordon DM (1990) Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am J Trop Med Hyg 42: 309–313. [DOI] [PubMed] [Google Scholar]
- 25. Scheller LF, Wirtz RA, Azad AF (1994) Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect Immun 62: 4844–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neimert-Andersson T, Hallgren AC, Andersson M, Langeback J, Zettergren L, et al. (2011) Improved immune responses in mice using the novel chitosan adjuvant ViscoGel, with a Haemophilus influenzae type b glycoconjugate vaccine. Vaccine 29: 8965–8973. [DOI] [PubMed] [Google Scholar]
- 27. Lapteva N, Huang XF (2010) CCL5 as an adjuvant for cancer immunotherapy. Expert Opin Biol Ther 10: 725–733. [DOI] [PubMed] [Google Scholar]
- 28. Loudon PT, Yager EJ, Lynch DT, Narendran A, Stagnar C, et al. (2010) GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS One 5: e11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen-Hoai T, Hohn O, Vu MD, Baldenhofer G, Sayed Ahmed MS, et al. (2012) CCL19 as an adjuvant for intradermal gene gun immunization in a Her2/neu mouse tumor model: improved vaccine efficacy and a role for B cells as APC. Cancer Gene Ther 19: 880–887. [DOI] [PubMed] [Google Scholar]
- 30. Zheng Q, Fan D, Gao N, Chen H, Wang J, et al. (2011) Evaluation of a DNA vaccine candidate expressing prM-E-NS1 antigens of dengue virus serotype 1 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in immunogenicity and protection. Vaccine 29: 763–771. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez AM, Pascutti MF, Maeto C, Falivene J, Holgado MP, et al. (2012) IL-12 and GM-CSF in DNA/MVA Immunizations against HIV-1 CRF12_BF Nef Induced T-Cell Responses With an Enhanced Magnitude, Breadth and Quality. PLoS One 7: e37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kodama S, Abe N, Hirano T, Suzuki M (2011) A single nasal dose of CCL20 chemokine induces dendritic cell recruitment and enhances nontypable Haemophilus influenzae-specific immune responses in the nasal mucosa. Acta Otolaryngol 131: 989–996. [DOI] [PubMed] [Google Scholar]
- 33. Ma X, Li J, Zhang F (2012) Intranasal co-delivery with the mouse zona pellucida 3 and GM-CSF expressing constructs enhances humoral immune responses and contraception in mice. Scand J Immunol 76: 521–527. [DOI] [PubMed] [Google Scholar]
- 34. Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill LB 2nd, et al. (2012) The vaccine-site microenvironment induced by injection of incomplete Freund's adjuvant, with or without melanoma peptides. J Immunother 35: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, et al. (2002) DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 100: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 36. Biragyn A, Ruffini PA, Coscia M, Harvey LK, Neelapu SS, et al. (2004) Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood 104: 1961–1969. [DOI] [PubMed] [Google Scholar]
- 37. Biragyn A, Schiavo R, Olkhanud P, Sumitomo K, King A, et al. (2007) Tumor-associated embryonic antigen-expressing vaccines that target CCR6 elicit potent CD8+ T cell-mediated protective and therapeutic antitumor immunity. J Immunol 179: 1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, et al. (2001) Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol 167: 6644–6653. [DOI] [PubMed] [Google Scholar]
- 39. Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW (1999) Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol 17: 253–258. [DOI] [PubMed] [Google Scholar]
- 40. Perales MA, Fantuzzi G, Goldberg SM, Turk MJ, Mortazavi F, et al. (2002) GM-CSF DNA induces specific patterns of cytokines and chemokines in the skin: implications for DNA vaccines. Cytokines Cell Mol Ther 7: 125–133. [DOI] [PubMed] [Google Scholar]
- 41. Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, et al. (2012) A Vaxfectin-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 30: 7046–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shlapobersky M, Marshak JO, Dong L, Huang ML, Wei Q, et al. (2012) Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J Gen Virol 93: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sedegah M, Rogers WO, Belmonte A, Belmonte M, Banania G, et al. (2006) Vaxfectin enhances immunogenicity and protective efficacy of P. yoelii circumsporozoite DNA vaccines. Vaccine 24: 1921–1927. [DOI] [PubMed] [Google Scholar]
- 44. Sedegah M, Rogers WO, Belmonte M, Belmonte A, Banania G, et al. (2010) Vaxfectin enhances both antibody and in vitro T cell responses to each component of a 5-gene Plasmodium falciparum plasmid DNA vaccine mixture administered at low doses. Vaccine 28: 3055–3065. [DOI] [PubMed] [Google Scholar]
- 45. Luke CJ, Carner K, Liang X, Barbour AG (1997) An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis 175: 91–97. [DOI] [PubMed] [Google Scholar]
- 46. Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, et al. (1983) Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature 301: 214–221. [DOI] [PubMed] [Google Scholar]
- 47. Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, et al. (2001) Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine 19: 1911–1923. [DOI] [PubMed] [Google Scholar]
- 48. Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, et al. (2002) Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol 169: 6681–6685. [DOI] [PubMed] [Google Scholar]
- 49. Del Giudice G, Lambert PH, Mendis K, Pessi A, Tanner M (1990) Antibody responses to Plasmodium falciparum and P. vivax sporozoites in areas with stable and unstable malaria. Bull World Health Organ 68 Suppl: 191–196 [PMC free article] [PubMed] [Google Scholar]
- 50. Noland GS, Chowdhury DR, Urban JF Jr, Zavala F, Kumar N (2010) Helminth infection impairs the immunogenicity of a Plasmodium falciparum DNA vaccine, but not irradiated sporozoites, in mice. Vaccine 28: 2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, et al. (1982) Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med 156: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, et al. (1985) Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 228: 1436–1440. [DOI] [PubMed] [Google Scholar]
- 53. Witney AA, Doolan DL, Anthony RM, Weiss WR, Hoffman SL, et al. (2001) Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol Biochem Parasitol 118: 233–245. [DOI] [PubMed] [Google Scholar]
- 54. Shlapobersky M, Wei Q, Sullivan S, Vilalta A (2009) Vaxfectin-adjuvanted seasonal influenza protein vaccine: correlation of systemic and local immunological markers with formulation parameters. Vaccine 27: 6404–6410. [DOI] [PubMed] [Google Scholar]
- 55. Hedstrom RC, Doolan DL, Wang R, Kumar A, Sacci JB Jr, et al. (1998) In vitro expression and in vivo immunogenicity of Plasmodium falciparum pre-erythrocytic stage DNA vaccines. Int J Mol Med 2: 29–38. [DOI] [PubMed] [Google Scholar]
- 56. Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, et al. (2001) Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med 194: 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M (1997) Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol 158: 1268–1274. [PubMed] [Google Scholar]
- 58. Zavala F (1991) The multifactorial character of the protective immunity induced by immunization with sporozoites. Res Immunol 142: 654–658. [DOI] [PubMed] [Google Scholar]
- 59. Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, et al. (1989) Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341: 323–326. [DOI] [PubMed] [Google Scholar]
- 60. Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, et al. (2008) Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A 105: 14017–14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amante FH, Good MF (1997) Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol 19: 111–126. [DOI] [PubMed] [Google Scholar]
- 62. Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke ED, et al. (1996) Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol 157: 4061–4067. [PubMed] [Google Scholar]
- 63. Renia L, Grillot D, Marussig M, Corradin G, Miltgen F, et al. (1993) Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol 150: 1471–1478. [PubMed] [Google Scholar]
- 64. Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, et al. (2005) Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 64: 706–715. [DOI] [PubMed] [Google Scholar]
- 65. Cremel M, Berlier W, Hamzeh H, Cognasse F, Lawrence P, et al. (2005) Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction. J Leukoc Biol 78: 158–166. [DOI] [PubMed] [Google Scholar]
- 66. Zhao X, Jain S, Benjamin Larman H, Gonzalez S, Irvine DJ (2005) Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials 26: 5048–5063. [DOI] [PubMed] [Google Scholar]
- 67. Wu YY, Tsai HF, Lin WC, Hsu PI, Shun CT, et al. (2007) Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect Immun 75: 4357–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. He S, Cao Q, Yoneyama H, Ge H, Zhang Y, et al. (2008) MIP-3alpha and MIP-1alpha rapidly mobilize dendritic cell precursors into the peripheral blood. J Leukoc Biol 84: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, et al. (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181: 8391–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, et al. (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10: 514–523. [DOI] [PubMed] [Google Scholar]
- 71. Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, et al. (2010) Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 6: 97–106. [DOI] [PubMed] [Google Scholar]
- 72. Hafalla JC, Rai U, Morrot A, Bernal-Rubio D, Zavala F, et al. (2006) Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur J Immunol 36: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 73. Mueller AK, Labaied M, Kappe SH, Matuschewski K (2005) Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433: 164–167. [DOI] [PubMed] [Google Scholar]
- 74. Sun P, Schwenk R, White K, Stoute JA, Cohen J, et al. (2003) Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol 171: 6961–6967. [DOI] [PubMed] [Google Scholar]
- 75. Sedegah M, Belmonte M, Epstein JE, Siegrist CA, Weiss WR, et al. (2003) Successful induction of CD8 T cell-dependent protection against malaria by sequential immunization with DNA and recombinant poxvirus of neonatal mice born to immune mothers. J Immunol 171: 3148–3153. [DOI] [PubMed] [Google Scholar]
- 76. Luke TC, Hoffman SL (2003) Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol 206: 3803–3808. [DOI] [PubMed] [Google Scholar]
- 77. Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, et al. (2002) Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 185: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 78. Vichier-Guerre S, Lo-Man R, BenMohamed L, Deriaud E, Kovats S, et al. (2003) Induction of carbohydrate-specific antibodies in HLA-DR transgenic mice by a synthetic glycopeptide: a potential anti cancer vaccine for human use. J Pept Res 62: 117–124. [DOI] [PubMed] [Google Scholar]
- 79. Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, et al. (2004) Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol 172: 2487–2495. [DOI] [PubMed] [Google Scholar]
- 80. Doolan DL, Hoffman SL (2000) The complexity of protective immunity against liver-stage malaria. J Immunol 165: 1453–1462. [DOI] [PubMed] [Google Scholar]
- 81. Bonelo A, Valmori D, Triponez F, Tiercy JM, Mentha G, et al. (2000) Generation and characterization of malaria-specific human CD8(+) lymphocyte clones: effect of natural polymorphism on T cell recognition and endogenous cognate antigen presentationby liver cells. Eur J Immunol 30: 3079–3088. [DOI] [PubMed] [Google Scholar]
- 82. Rosenberg SA, Yang JC, Sherry RM, Hwu P, Topalian SL, et al. (2003) Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum Gene Ther 14: 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Otten G, Schaefer M, Doe B, Liu H, Srivastava I, et al. (2004) Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 22: 2489–2493. [DOI] [PubMed] [Google Scholar]
- 84. Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, et al. (2007) Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol 81: 5257–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, et al. (2008) Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 26: 440–448. [DOI] [PubMed] [Google Scholar]
- 86. Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, et al. (2009) DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A 106: 15831–15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, et al. (2010) Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine 28: 4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, et al. (2011) Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis 203: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smith LR, Wloch MK, Ye M, Reyes LR, Boutsaboualoy S, et al. (2010) Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 28: 2565–2572. [DOI] [PubMed] [Google Scholar]
- 90. Epstein JE, Gorak EJ, Charoenvit Y, Wang R, Freydberg N, et al. (2002) Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum Gene Ther 13: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 91. Limbach K, Aguiar J, Gowda K, Patterson N, Abot E, et al. (2011) Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar J 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Scheiblhofer S, Chen D, Weiss R, Khan F, Mostbock S, et al. (2001) Removal of the circumsporozoite protein (CSP) glycosylphosphatidylinositol signal sequence from a CSP DNA vaccine enhances induction of CSP-specific Th2 type immune responses and improvesprotection against malaria infection. Eur J Immunol 31: 692–698. [DOI] [PubMed] [Google Scholar]
- 93.Vanderberg J, Mueller AK, Heiss K, Goetz K, Matuschewski K, et al. (2007) Assessment of antibody protection against malaria sporozoites must be done by mosquito injection of sporozoites. Am J Pathol 171: : 1405-1406; author reply 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vanderberg JP, Frevert U (2004) Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol 34: 991–996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA sequence of pMCSP plasmid. The IP-10 leader sequence: red; MIP3α: blue; CSP: black; Myc-Hisx6 tag: orange; Multiple cloning site/spacer: green. Translation start codon and stop codon are marked with boxes.
(TIF)
DNA sequence of phMfCSP plasmid. TPA leader sequence: red; hMIP3α: blue; fCSP: black; Myc tag: orange; Multiple cloning site/spacer: green. Translation start codon and stop codon are marked with boxes.
(TIF)