Abstract
Objective
Endothelial colony-forming cells (ECFCs) are a subset of circulating endothelial progenitor cells that are particularly abundant in umbilical cord blood. We sought to determine whether ECFC abundance in cord blood is associated with maternal body-mass index (BMI) in non-pathological pregnancies.
Study design
We measured the level of ECFCs in the cord blood of neonates (n=27) born from non-obese healthy mothers with non-pathological pregnancies and examined whether ECFC abundance correlated with maternal BMI. We also examined the effect of maternal BMI on ECFC phenotype and function using angiogenic and vasculogenic assays.
Results
We observed variation in ECFC abundance among subjects and found a positive correlation between pre-pregnancy maternal BMI and ECFC content (r=0.51, P=0.007), which was independent of other obstetric factors. Despite this variation, ECFC phenotype and functionality were deemed normal and highly similar between subjects with maternal BMI <25 kg/m2 and BMI between 25–30 kg/m2, including the ability to form vascular networks in vivo.
Conclusions
This study underlines the need to consider maternal BMI as a potential confounding factor for cord blood levels of ECFCs in future comparative studies between healthy and pathological pregnancies.
Endothelial colony-forming cells (ECFCs) are a subset of progenitor cells that circulate in peripheral blood and can give rise to endothelial cells (1,2), contributing to the formation of new vasculature and the maintenance of vascular integrity (3–5). The mechanisms that regulate the abundance of these cells in vivo remain poorly understood.
ECFCs are rare in adult peripheral blood (1,2,10). In contrast, there is an elevated number of these cells in fetal blood during the third trimester of pregnancy (11–13). Emerging evidence indicates that deleterious conditions during fetal life can impair ECFC content and function. For instance, offspring of diabetic mothers have been shown to have reduced number of circulating ECFCs and impaired cell functionality (14), which may contribute to the long-term cardiovascular complications. Similar observations have been reported in neonates with bronchopulmonary dysplasia (15,16).
The adverse association between maternal weight and the outcome of pregnancy is well known (17,18). Epidemiologic studies have shown that cardiovascular disease may have origins during fetal development (19). Excessive maternal pre-pregnancy weight and gestational weight gain are associated with adverse cardiovascular risk factors in the offspring (20). The fetal adaptations that occur in response to changes in maternal weight during pregnancy and whether these adaptations affect the level of ECFCs is completely unknown. In this study we quantified the baseline variation in ECFC abundance and function among neonates born from non-obese healthy mothers with non-pathological pregnancies and examined whether this normal variation was associated with differences in maternal weight.
Keywords: endothelial progenitor cells, cord blood, body mass-index, pregnancy
METHODS
Twenty-seven Caucasian mother-offspring pairs were included in this study. Exclusion criteria included pre-pregnancy obesity (ie, maternal body-mass index (BMI) >30 kg/m2), severe pre-pregnancy underweight (ie, maternal BMI <16 kg/m2), maternal infections, pre-existing and gestational diabetes, hypertensive disorders of pregnancy, multiple gestation, asthma and/or respiratory diseases, thyroid disease, intrauterine growth retardation, and women who carried fetuses with chromosomal abnormalities or congenital malformations. The study included five preterm deliveries (<37 gestational weeks) that were not due to either maternal or fetal pathologies. This research was approved by the local ethics committee at the Hospital Universitario Virgen del Rocío. All the parents gave written informed consent for abstraction of data from their obstetric records and for the use of umbilical cord blood in accordance with the Declaration of Helsinki.
The following maternal and neonate data were obtained from the obstetric records: maternal age; mode of delivery (cesarean/vaginal delivery); mode of conception (natural/in vitro fertilization); parity (primipara/multipara); evidence of intrauterine meconium exposure; offspring sex; offspring birth weight; offspring birth height; maternal height; pre-pregnancy (6–8 weeks gestation) maternal weight; end-of-pregnancy (right before delivery) maternal weight; and postpartum (6 month postpartum) maternal weight. Gestational age was recorded according to the obstetricians' best estimate of gestation. Maternal BMI was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2). Gestational weight gain was calculated as the difference between the weight at the end of pregnancy and the weight at first consultation. Postpartum weight retention was calculated as the difference between the weight at 6 months postpartum and the weight at first consultation. Maternal pre-pregnancy tobacco use was dichotomized into never- and ever-users.
Umbilical cord blood samples were collected ex utero using heparinized tubes. Cells were removed from plasma by centrifugation for 5 min at 3,000 rpm using a refrigerated centrifuge. The resulting supernatant (plasma) was apportioned into 0.5 mL aliquots and stored at −80°C until analysis. Total cholesterol (CHOD-PAP), high-density lipoprotein (HDL) cholesterol (HDL-C-plus), low-density lipoprotein (LDL) cholesterol (LDL-C-plus), and triglycerides (GPO-PAP) were measured by enzymatic spectrophotometry using a Roche Cobas 8000 Modular Analyzer (Roche Diagnostics).
Cord blood samples (30–50 mL) were processed within 2 h of blood collection. Mononuclear cells were obtained as previously described (6,21). Mononuclear cells were cryopreserved in aliquots equivalent to 10 mL cord blood as previously described (21) and stored in liquid nitrogen for comparative analysis. Cryopreserved mononuclear cells were thawed in a 37°C bath, thoroughly washed, and seeded on fibronectin-coated 6-well tissue culture plates (BD Bioscience) using endothelial cell-medium (EGM-2 without hydrocortisone, Lonza; 20% FBS; 1X glutamine-penicillin-streptomycin) (6). Unbound cells were removed at 48 h and the bound fraction maintained in endothelial cell-medium, with media being replenished every 2–3 days. Endothelial colonies were identified as well-circumscribed monolayers of ≥50 cells with cobblestone morphology. Colonies were enumerated on days 7, 14, 21, and 28 by visual inspection using an inverted microscope.
Endothelial cell phenotype was characterized by testing (1) cloning-forming ability, (2) proliferation and migration towards VEGF and FGF-2, and (3) regulation of leukocyte adhesion upon inflammatory stimuli using methods previously described by our laboratory (22). The vasculogenic ability of ECFCs was evaluated in vivo using a previously developed xenograft model of human endothelial cell transplantation into immunodeficient mice (6,7). Animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital.
Statistical analyses
Data were analyzed with IBM SPSS version 19.0 software. Categorical variables were expressed by absolute frequencies and percentages (n,%). Non-categorical variables were expressed by mean±SD. Data from subjects in the maternal BMI <25 kg/m2 group were compared with those in the 25–30 kg/m2 group. Categorical variables were compared with Pearson chi-squared test except for tobacco use that was analyzed with Fisher exact test. Non-categorical variables were compared with Mann-Whitney U tests except for maternal age that was normally distributed and was analyzed with two-tailed unpaired student's t-test. Shapiro-Wilk tests were used to determine Normality. Univariate correlations were performed with use of Spearman correlation co-efficient. Data from experiments performed in vitro and in mice were analyzed using GraphPad Prism version 5 software. These data were expressed as mean±SE and means were compared using unpaired Student t tests. For all analyses, P<0.05 was considered significant.
RESULTS
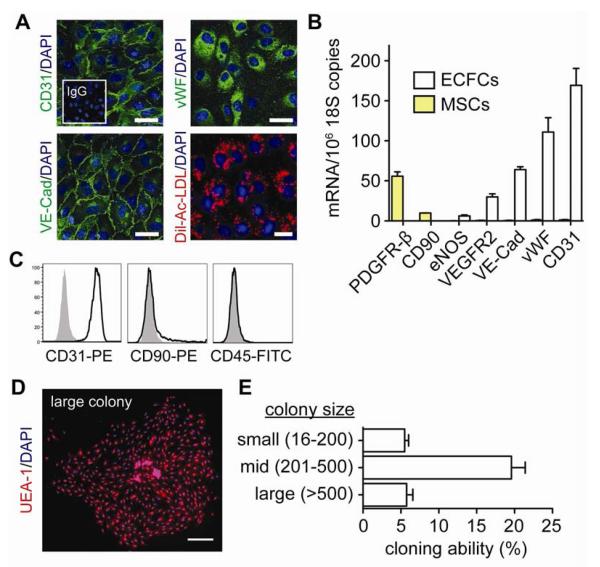
Cord blood-derived ECFCs were identified in culture as outgrown colonies containing ≥50 endothelial cells (Figure 1, A). Colonies emerged in culture as early as one week, although the majority of the colonies appeared between 2 and 3 weeks (Figure 1, B), which is consistent with previous reports (1). ECFCs were allowed to grow and were then purified by expression of CD31. The endothelial phenotype of CD31-selected cells was verified via their expression of CD31 and VE-cadherin at the cell-cell borders, the expression of vWF in a punctuate pattern in the cytoplasm, and specific affinity for uptake of acetylated low-density lipoproteins (Figure 2, A). Flow cytometry confirmed uniform expression of CD31 and negative expression of mesenchymal marker CD90 and pan-hematopoietic marker CD45 (Figure 2, C). Additionally, qRT-PCR analyses showed the expression of endothelial markers CD31, vWF, VE-Cadherin, VEGF-R2, and eNOS at the mRNA level as well as the absence of mRNA transcripts of mesenchymal markers CD90 and PDGF-Rβ (Figure 2, B). Finally, colony-forming ability was confirmed at clonal density, which reflected the expected presence of highly-proliferative clonogenic cells in each ECFC population (Figure 2, D and 2E).
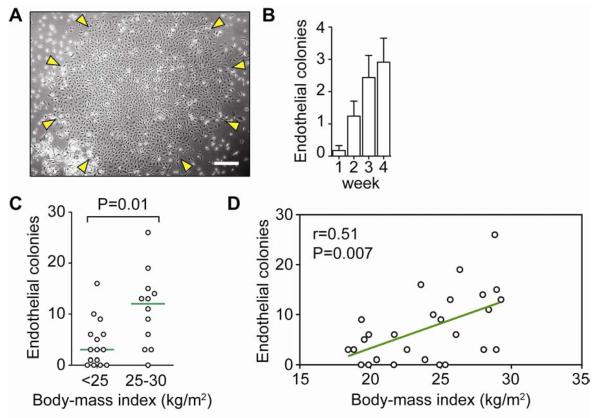
Figure 1. Association between maternal BMI and ECFC count in cord blood.
A, Phase contrast micrograph of an ECFC colony (scale bar: 200 μm). B, Cumulative number of ECFC colonies at 4 weeks from 10 mL of cord blood (mean±SE). C, ECFC abundance in cord blood from subjects with pre-pregnancy maternal BMI <25 kg/m2 and 25–30 kg/m2 (green line represents median abundance). D, Univariate correlation between pre-pregnancy maternal BMI and ECFC count performed with Spearman's Rho test.
Figure 2. Assessment of ECFC phenotype.
A, ECFCs express CD31, vWF, VE-Cadherin, and uptake Dil-Ac-LDL. Cell nuclei were counterstained with DAPI (scale bar: 50 μm). B, Quantitative RT-PCR analyses of ECFCs for endothelial (CD31, vWF, VE-Cadherin, VEGFR2, eNOS) and mesenchymal (CD90, PDGFRβ) markers. C, Flow cytometric analysis of ECFCs for CD31, CD90, and CD45 (black line histograms). Isotype-matched controls are overlaid in solid gray histograms. D, Cloning-forming ability of ECFCs. The endothelial nature of the colonies was confirmed by binding of UEA-1 lectin (scale bar: 500 μm). E, Outgrown colonies were categorized by size. Bars represent mean±SE (n=6).
The abundance of ECFCs in each cord blood sample was determined after 4 weeks in culture. We found a median abundance of 6 colonies per 10 mL of cord blood with a broad 25th–75th interquartile range of 1.0–13 colonies. To address whether ECFC abundance was associated with maternal weight, we categorized the study into pre-pregnancy maternal BMI <25 kg/m2 (normal weight; n=15) and 25–30 kg/m2 (overweight; n=12). We found that the level of these cells in cord blood was significantly higher in samples categorized as pre-pregnancy maternal overweight than in samples categorized as normal maternal weight, with median abundance of 12 and 3 colonies, respectively (P=0.01; Figure 1, C). Moreover, multivariate regression analysis revealed a statistically significant positive correlation between maternal pre-pregnancy BMI and the level of ECFCs in the umbilical cord blood (r=0.51, P=0.007; Figure 1, D). This correlation remained statistically significant when maternal BMI was measured at both the end-of-pregnancy (r=0.45, P=0.02) and at 6 month postpartum (r=0.55, P=0.004). In contrast, we did not observe statistical association between ECFC abundance and gestational weight gain (r=0.02, P=0.93), the number of mononuclear cells in cord blood (r=−0.08, P=0.73), or postpartum weight retention at 6 months (r=0.25, P=0.23). The correlation between ECFC count and maternal BMI was independent of other obstetric factors, including maternal age, mode of delivery, parity, pre-pregnancy use of tobacco, gestational age, offspring birth weight, and the lipid profile of the cord blood (Table). There were differences in the percentage of male/female offspring in each group. However, no statistical difference by sex was found(P=0.60).
Table.
Obstetric characteristics according to pre-pregnancy maternal BMI*
| Maternal BMI | ||||
|---|---|---|---|---|
| All Subjects (N=27) | <25 kg/m2 (N=15) | 25–30 kg/m2 (N=12) | P Value ‡ | |
| Maternal | ||||
| Age - years | 30.4±5.7 | 30.7±5.6 | 30.1±6.0 | 0.79 |
| Primipara - no. (%) | 18 (66.6) | 10 (66.7) | 8 (66.6) | 0.99 |
| In vitro fertilization - no. (%) | 2 (7.4) | 2 (13.3) | 0 (0.0) | 0.48 |
| Cesarean delivery - no. (%) | 3 (11.1) | 2 (13.3) | 1 (8.3) | 0.99 |
| Tobacco use - no. (%) | 11 (40.7) | 5 (33.3) | 6 (50.0) | 0.45 |
| Gestational weight gain - kg | 12.6±4.8 | 12.2±2.7 | 13.0±6.7 | 0.84 |
| Postpartum weight retention - kg † | 0.0±4.4 | −0.6±4.1 | 0.7±4.9 | 0.35 |
| Neonatal | ||||
| Male - no. (%) | 19 (70.4) | 8 (53.3) | 11 (91.6) | 0.04 |
| Gestation age - weeks | 38.8±2.3 | 38.8±1.6 | 38.7±3.0 | 0.46 |
| Preterm birth - no. (%) | 5 (18.5) | 2 (13.3) | 3 (25.0) | 0.63 |
| Birth weight - kg | 3.2±0.7 | 3.3±0.6 | 3.2±0.8 | 0.88 |
| Lipid profile in cord blood † | ||||
| Total cholesterol - mg/dL | 45.8±14.4 | 44.3±14.6 | 47.6±14.7 | 0.58 |
| HDL cholesterol - mg/dL | 20.2±7.2 | 20.2±7.3 | 20.2±7.4 | 0.99 |
| LDL cholesterol - mg/dL | 15.5±7.1 | 15.2±5.7 | 15.8±8.8 | 0.84 |
| Triglycerides - mg/dL | 26.4±12.9 | 25.2±12.2 | 27.7±14.0 | 0.61 |
Categorical variables are represented by absolute frequencies and percentages (n,%). Non-categorical variables are represented by mean±SD.
Values for postpartum weight retention and lipid profile are from N=25 subjects.
P values are from comparison of the maternal BMI <25 kg/m2 and the 25–30 kg/m2 groups. Categorical variables were analyzed with Pearson chi-squared tests except for tobacco use that was analyzed with Fisher exact test. Non-categorical variables were analyzed with Mann-Whitney U tests except for maternal age, Total cholesterol, HDL, LDL that were normally distributed and were analyzed with student's t-test.
Our study included mostly deliveries (Table) and thus we found no significant correlation between gestational age and ECFC content. Only when subjects were categorized as either preterm (<37 gestational weeks; n=5) or term (≥37 weeks; n=22), we found differences in ECFC abundance (increased in preterm) (11).
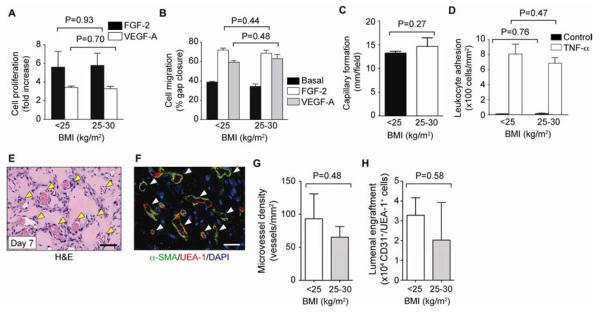
We then examined whether there were functional differences among ECFCs from subjects with maternal BMI <25 kg/m2 and 25–30 kg/m2. We found no statistical difference (P>0.05) in (1) the mitogenic and migratory response to angiogenic growth factors VEGF-A and FGF-2 (Figure 3, A and B); (2) the ability to assemble into capillary-like structures onto Matrigel; and (3) the ability to up-regulate leukocyte adhesion molecules E-selectin and ICAM-1 upon exposure to the inflammatory cytokine TNF-α and the subsequent increase in leukocyte binding (Figure 3, C and D). We also found no statistical difference (P>0.05) in the vasculogenic ability of ECFCs (Figure 3). Quantitative evaluation of microvessel density by histological and flow cytometric methods revealed no statistical difference between the groups (Figure 3, G and H).
Figure 3. Assessment of ECFC function.
ECFCs were categorized into pre-pregnancy maternal BMI <25 kg/m2 and 25–30 kg/m2. A, Cell proliferation in response to VEFG-A (10 ng/mL) and FGF-2 (1 ng/mL). B, Cell migration in response to VEGF-A and FGF-2 expressed as percentage of gap closure. C, Capillary-like network formation on Matrigel expressed as total tube length per field. D, Adhesion of leukocytes onto ECFCs after stimulation with TNF-α. E, Vasculogenic properties of ECFCs compared in nude mice after subcutaneous implantation. H&E staining reveals numerous blood vessels (yellow arrowheads; scale bar: 100 μm). F, Human-specific lumens confirmed by staining with UEA-1 (red) and perivascular coverage indicated by α-SMA (green) (white arrowheads; scale bars: 50 μm). G, Microvessel density. H, ECFCs expressing UEA-1 and human CD31. Bars represent mean±SE (n=3 each group).
DISCUSSION
ECFCs have enormous therapeutic potential due to their inherent proliferative and vasculogenic properties (6,9). However, current knowledge regarding what governs the abundance of these cells in vivo is still very limited.
The main implication of this study is the need to consider maternal BMI as a potential confounding factor for cord blood levels of ECFCs. Emerging evidence indicates that deleterious conditions during fetal life can impair ECFC content and function (14–16,24), and the level of these cells may vary extensively. Understanding what constitutes a normal baseline level of ECFC abundance will facilitate the interpretation of future comparative studies between pathological and non-pathological pregnancies.
Epidemiological studies have shown that there is a relationship between BMI and several chronic conditions, including type 2 diabetes, hypertension, and coronary heart disease (25). Although these risks are notably increased in obese subjects, there is also an approximately linear relationship between BMI and cardiovascular risks in non-obese subjects (26). Additionally, excessive weight during gestation is generally associated with higher risk for negative neonatal outcomes (17,18). Considering that our study was focused on non-obese healthy mothers, and with the limitation of not being able to establish a definitive cause-and-effect, we believe the correlation between maternal BMI and level of ECFC in cord blood could be interpreted as a normal physiological adaptation that occurs in the rapidly growing fetus in order to maintain adequate formation of new vasculature and/or to preserve vascular integrity. Further studies will be needed to determine the mechanisms by which ECFCs are mobilized. Further studies also should examine the influence of ethnical, environmental, and genetic factors on the association between maternal BMI and ECFCs in umbilical cord blood.
ACKNOWLEDGMENTS
We thank Dan Lin and Dr Shou-Ching Jaminet (Center for Vascular Biology, Department of Pathology, Beth Israel Deaconess Medical Center) for quantitative RT-PCR analyses; María Jesús Domínguez-Simeón (Laboratorio de Hipertensión Arterial y Lípidos), Rafael Torrejon, Lucas Cerrillo, and the study midwives (Servicio de Paritorio de la Unidad Gestión Clínica de Ginecología Obstetricia y Patologías Mamarias del Hospital de la Mujer, Hospital Universitario Virgen del Rocio) for assistance in the collection of umbilical cord blood samples.
Funded by the National Institutes of Health (R00EB009096 to J.M.-M.), Consejería de Salud de la Junta de Andalucía, Sistema Andaluz de Salud (SAS111241 to R.M.-L.), and the Instituto de Salud Carlos III FIS (PI10/02473 to P.S.).
ABBREVIATIONS AND ACRONYMS
- AcLDL
Acetylated low density lipoprotein
- BMI
Body-mass index
- DAPI
4',6-diamidino-2-phenylindole
- ECFC
Endothelial colony-forming cell
- FGF
Fibroblast growth factor
- H&E
Hematoxilin and Eosin
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- MSC
Mesenchymal stem cell
- TNF-α
Tumor necrosis factor-α
- α-SMA
α smooth muscle actin
- UEA-1
Ulex Europaeus Agglutinin I
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 2.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 4.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melero-Martin J, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 7.Melero-Martin J, De Obaldia ME, Kang S-Y, Khan ZA, Yuan L, Oettgen P, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Aledia AS, Popson SA, Him L, Hughes CCW, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16:585–94. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–20. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 10.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–5. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 11.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–61. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, et al. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64:68–73. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 13.Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood. 2011;118:1699–709. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, et al. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes. 2008;57:724–31. doi: 10.2337/db07-1507. [DOI] [PubMed] [Google Scholar]
- 15.Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–6. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 16.Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan BSL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–22. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 18.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–70. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 20.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion M-J, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–64. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin R-Z, Dreyzin A, Aamodt K, Dudley AC, Melero-Martin J. Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell Transplant. 2011;20:515–22. doi: 10.3727/096368910X532729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin R-Z, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin J. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–55. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–37. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acosta JC, Haas DM, Saha CK, Dimeglio LA, Ingram DA, Haneline LS. Gestational diabetes mellitus alters maternal and neonatal circulating endothelial progenitor cell subsets. Am J Obstet Gynecol. 2011;204:254.e8–254.e15. doi: 10.1016/j.ajog.2010.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopelman PG. Obesity as a medical problem. Nature. Nature Publishing Group. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the “normal” weight range. JAMA. 1995;273:461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]