Abstract
Endotoxemia correlates with the degree of liver failure and may participate in worsening of liver diseases. Lipopolysaccharide (LPS, synonymous as endotoxin) treatment in mice lowered hepatic glutathione (GSH) level, which in turn is a variable that determines susceptibility to LPS-induced injury. We previously showed that LPS treatment in mice lowered hepatic expression of the rate-limiting enzyme in GSH synthesis, glutamate-cysteine ligase (GCL). The aims of our current work were to determine the molecular mechanism(s) responsible for these changes. Studies were done using RAW cells (murine macrophage), in vivo LPS treated mice, and mouse hepatocytes. We found that LPS treatment lowered GCL catalytic and modifier (Gclc and Gclm) subunit expression at the transcriptional level, which was unrelated to alteration in nitric oxide production or induction in NFkB/p65 subunit. The key mechanism was due to decreased sumoylation of nuclear factor-erythroid 2 related factor 2 (Nrf2) and MafG, which is required for their heterodimerization and subsequent binding and trans-activation of the anti-oxidant response element (ARE) present in the promoter region of these genes that is essential for their expression. LPS treatment lowered markedly the expression of ubiquitin-conjugating enzyme 9 (Ubc9), which is required for sumoylation. Similar findings also occurred in liver after in vivo LPS treatment and LPS-treated mouse hepatocytes. Overexpression of Ubc9 protected against LPS-mediated inhibition of Gclc and Gclm expression in RAW cells and hepatocytes.
Conclusions
LPS-mediated lowering of GCL expression in hepatocyte and macrophage is due to lowering of sumoylation of Nrf2 and MafG, leading to reduced heterodimerization, binding and trans-activation of ARE.
Keywords: Glutamate-cysteine ligase, anti-oxidant response element, sumoylation, Nrf2, MafG
INTRODUCTION
Lipopolysaccharide (LPS, synonymous as endotoxin) is a complex glycolipid that is a major constituent of the outer cell wall of all gram-negative bacteria [1]. LPS signals through Toll-like receptor 4 (TLR4)-myeloid differentiation protein 2 (MD2) complex to trigger the synthesis and release of pro-inflammatory cytokines, chemokines, reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) [1–4]. Endotoxemia is frequently observed in cirrhotic patients and the degree of endotoxemia correlates with the degree of liver failure [2,3]. This is because the liver is where gut-derived endotoxin is cleared [2]. Endotoxemia in turn participates in worsening of many liver diseases [2–5]. Endotoxemia has been shown to lower glutathione (GSH) levels in liver [6,7], peritoneal macrophages and lymphocytes [8]. GSH is an important cellular antioxidant that also determines susceptibility to LPS-induced injury in multiple tissues [7,9,10]. This may be related to GSH’s ability to influence TLR4 signaling, which is supported by the finding that LPS-induced mortality and tumor necrosis factor a (TNF ) secretion were higher when GSH level was reduced [11]. In addition, exogenous GSH treatment suppressed LPS-induced systemic inflammatory response and reduced mortality in rats [9]. The fall in hepatic GSH level is multifactorial. Increased oxidative stress [4] and GSH efflux [12] have both been implicated. We reported that mice treated with LPS exhibited a marked reduction in hepatic GSH level which coincided with a marked reduction in the expression of GSH synthetic enzymes, glutamate cysteine ligase (GCL) and GSH synthase (GS) at the mRNA and protein levels [6]. The current study was undertaken to elucidate the molecular mechanisms responsible for LPS-mediated inhibition of GSH synthetic enzymes expression.
MATERIALS AND METHODS
Materials
LPS (E. coli 0111:B4), S-nitroso-N-acetylpenicillamine (SNAP, nitric oxide (NO) donor), NG-monomethyI-L-arginine methyl ester (L-NAME, NO synthase (NOS) inhibitor) were obtained from Sigma-Aldrich (St. Louis, MO). All other reagents were of analytical grade and obtained from commercial sources.
Animal experiments
Four-month-old male C57/B6 mice (Harlan, Indianapolis, IN) were fed ad libitum a standard diet (Harland Teklad irradiated mouse diet 7912, Madison, WI) and housed in a temperature-controlled animal facility with 12-hour light-dark cycles. Mice were treated with LPS (15mg/kg body weight) or vehicle control intraperitoneally (ip) and sacrificed 4 hours afterwards. Livers were snap frozen immediately in liquid nitrogen for subsequent analyses described below. Animals were treated humanely and all procedures were in compliance with our institutions guidelines for the use of laboratory animals.
Cell culture
RAW 264.7 murine macrophage cells and isolated mouse hepatocytes were obtained from the Cell Culture Core of the USC Research Center for Liver Diseases. RAW cells were grown according to instructions provided by American Type Cell Collection (ATCC, Manassas, VA). Primary mouse hepatocytes were plated in DMEM medium supplemented with 10% fetal calf serum, 2mM L-glutamine, 50mM penicillin, and 50mg/ml streptomycin sulfate. RAW cells and mouse hepatocytes were stimulated with LPS (500ng/ml, in the presence of serum) or an equal volume of solvent (water) for 4 to 18 hours. In other experiments, RAW cells were treated with LPS alone or with NO donor SNAP (200μM) or NOS inhibitor L-NAME (1mM) for 4 to 24 hours.
RNA isolation and gene expression analysis
Total RNA was isolated by the TRIzol reagent (Invitrogen) from liver tissues and cells. Gene expression was assessed using real-time PCR. Total RNA was subjected to reverse transcription (RT) by using M-MLV Reverse transcriptase (Invitrogen, CarlsBad, CA). Two μl of RT product was subjected to real-time PCR analysis. The primers and TaqMan probes for murine Gclc, Gclm, GS, ubiquitin-conjugating enzyme 9 (Ubc9), SUMO-1,2,3, p65, MafG, MafF, MafK and the Universal PCR Master Mix were purchased from Applied Biosystems (Foster City, CA). 18S rRNA was used as housekeeping gene. The thermal profile consisted of initial denaturation at 95°C for 3 minutes followed by 40 cycles at 95°C for 3 seconds and at 60°C for 30 seconds. The cycle threshold (Ct value) of the target genes was normalized to that of 18S rRNA to obtain the delta Ct (ΔCt). The ΔCt was used to find the relative expression of target genes according to the formula: relative expression= 2−ΔΔCt, where ΔΔCt= ΔCt of target genes in experimental condition – ΔCt of target gene under control condition.
RNA interference
The predesigned small interfering RNA (siRNA) targeting mouse p65 and Ubc9 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and negative control siRNA were purchased from Ambion (Austin, TX). RAW cells were cultured in 6-well plate (0.5× 106 cells/well) and transfected using RNAiMax (6μl/well) from Invitrogen (Carlsbad, CA) with p65 siRNA (30nM) or negative control siRNA for 48 hours for mRNA or promoter activity assay, following the manufacturer's manual.
Overexpression of Ubc9
Ubc9 overexpression vector (Ubc9-pCMV4-HA) and negative control empty vector (pCMV4-HA) were purchased from Addgene (Cambridge, MA). RAW cells and primary mouse hepatocytes were cultured in 6-well plate (0.5×106 cells/well), transfected using 6μl of Superfect from Qiagen (Valencia, CA) and 5μg of target plasmid per well. After 4 hours, the transfection medium was changed to normal medium. Protein expression was examined 24 hours later.
Promoter constructs and transient transfection assays
Murine Gclc (6.5kb) and Gclm (1.29kb) promoter-luciferase (LUC) constructs containing functional ARE were kindly provided by Rosenfeld and colleagues [13]. NF B promoter-LUC construct was obtained from Stratagene (cat. #219078-51, Santa Clara, CA). RAW cells were transfected with these constructs for 24 hours and treated with LPS (500ng/ml) or vehicle for the last 4 hours. In some experiments, RAW cells were co-transfected with siRNA against p65 or scramble control, Ubc9 overexpression vector or negative empty vector control prior to LPS treatment. Luciferase activity was determined as we described [14] and reported as fold of control vector pGL3-enhancer (cat. # E1771, Promega, Madison, WI) for Gclc and Gclm, and pLuc-MCS (cat.# 219087, Agilent, Santa Clara, CA) for NFκB promoter-LUC constructs, respectively. The luciferase activity was normalized to Renilla luciferase activity (pRL-CMV, cat. # E2261, Promega, Madison, WI). Each experiment was done in triplicates.
Western blot and Co-immunoprecipitation (Co-IP) analysis
Protein extracts (total and nuclear) from RAW cells, primary mouse hepatocytes and liver samples were prepared as described [15] and immunoprecipitated by specific SUMO-1 agarose-conjugated and Nrf2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and processes as reported [15]. Immunoprecipitated proteins were subjected to Western blotting following standard protocols (Amersham BioSciences, Piscataway, NJ), and the membrane were probed with the anti-Gclc, anti-Gclm (Aviva System Biology, San Diego, CA), anti-Ubc9 (Genetex, Irvine, CA), anti-Nrf2, anti-MafG (Abcam, Cambridge, MA), anti-sumoylated RanGAP1 (Cell Signaling, Danvers, MA) antibodies. Blots were developed using enhanced chemoluminescence. Membranes were developed by chemiluminescence using the ECL detection system (Amersham BioSciences, Piscataway, NJ). Densitometric analysis was done using the Quantity One™ densitometry program (Bio-Rad laboratories, Hercules, CA) after normalizing to housekeeping control (b-actin for total, Histone H3 for nuclear).
ChIP and sequential ChIP (SeqChIP) assay
ChIP and SeqChIP were done to examine changes in protein binding to the ARE of the mouse Gclc and Gclm promoters in an endogenous chromatin configuration, and co-localization of two proteins on the same region of the Gclc and Gclm promoters, respectively following the ChampionChIP™ kit protocol (SABioscences, Frederick, MD). Briefly, DNA immunoprecipitated by Nrf2 antibody was processed for a second round of immunoprecipitation using anti-MafG antibody. The purified DNA was detected by PCR analysis. Antibodies used for ChIP and SeqChIP were anti-Nrf2 and anti-MafG (Abcam), respectively. PCRs of the mouse Gclc promoter region across ARE (GCGCTGAGTCAC, −3708/-3697bp relative to ATG start site) (GenBank® accession no. AY382195) used forward primer 5'-ACGGCTGCTACGACAACGGCCCTC-3’ (bp −3830 to −3806) and reverse primer 5'-ACCCAGCGGTGCAAACTCCGCGC-3' (bp −3729 to −3706). PCRs of the mouse Gclm promoter region across ARE (− 344 to/−305 bp relative to ATG start site) (GenBank® accession no. NC-000069) used forward primer 5'-TCCTCTCGAAGAGGGCGTGTCCAG-3’ (bp −631 to −608) and reverse primer 5'-AGGGAGGGAAGGAAGGGAGGGAG-3' (bp −243 to −220). All PCR products were run on 2% agarose gels. Since mouse Gclc and Gclm promoters are GC-rich, advantage GC 2 polymerase mix was used to amplify the ARE region. The thermal profile consisted of initial denaturation at 94°C for 3 minutes followed by 28 cycles at 94°C for 30 seconds and at 67°C for 1.5 min.
Protein stability assay
Cycloheximide (15μg/ml) was added to primary mouse hepatocytes for 15 minutes prior LPS treatment (500ng/ml). Protein levels were determined at indicated time points by Western blotting as described above using the anti-Ubc9 antibody. The relative amount of Ubc9 protein was evaluated by densitometry and normalized to actin.
GSH and nitric oxide (NO) levels
GSH levels were measured as we described [13]. NO levels in RAW cells were measured using a NO assay kit, with detection limit of 0.1 nmol/well (cat. # ab65328, Abcam, Cambridge, MA).
Apoptosis measurement
Apoptosis was measured using Hoechst staining as we described [6].
Statistical Analysis
Data are given as mean ± standard error of the mean (SEM). Statistical analysis was performed using analysis of variance followed by Fisher's test for multiple comparisons. For changes in mRNA and protein levels, ratios of genes or proteins to housekeeping genes or proteins densitometric values were compared. Significance was defined by p<0.05.
RESULTS
Changes in the expression of GSH synthetic enzymes following LPS in RAW cells
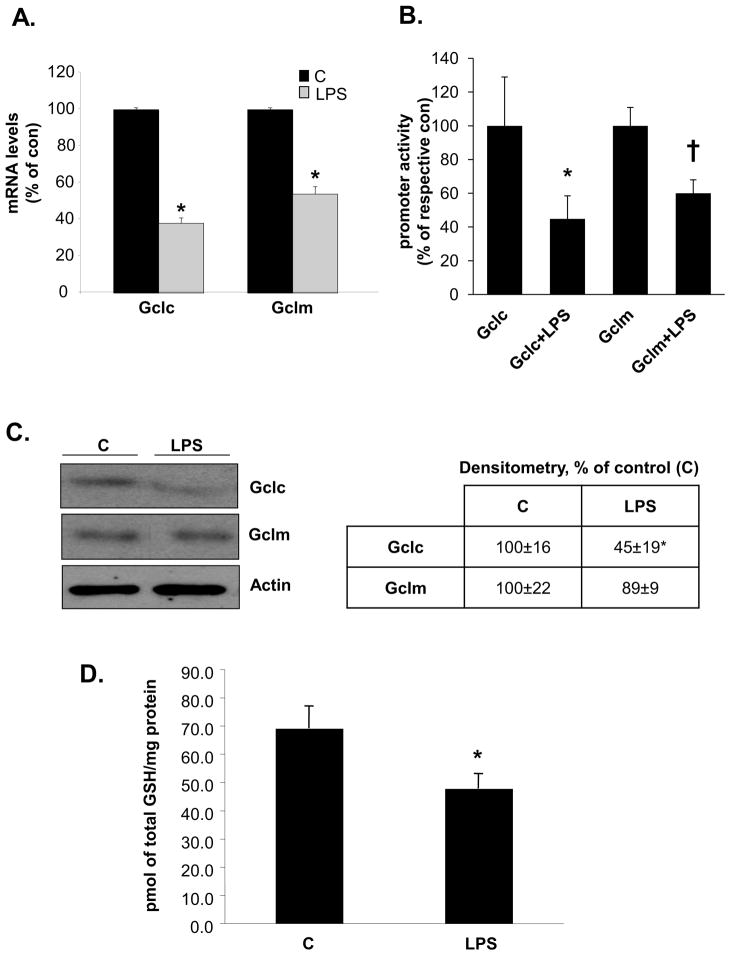
We examined whether LPS can affect the expression of these genes in RAW cells, a murine macrophage cell line for which LPS signaling is well characterized [16]. LPS treatment for 4 hours lowered the mRNA levels of Gclc and Gclm by about 50–60% (Fig. 1A). This was due to inhibition of their transcription since LPS reduced the promoter activity of both Gclc and Gclm by a comparable magnitude (Fig. 1B). This translated also to a 55% fall in the protein levels of Gclc but Gclm protein level was not significantly changed (Fig. 1C). Cellular GSH levels fell by about 30% (Fig. 1D). Under these experimental conditions, LPS had no influence on the mRNA level of GS (data not shown).
Figure 1. Effect of LPS on GCL subunits and GSH levels in RAW cells.
RAW cells were treated with LPS and processed for real-time PCR, promoter activity analysis, Western blotting and GSH level measurements as described in Methods. Part A) shows mRNA levels expressed as % of control (C) from six experiments after 4 hours of LPS treatment, *p<0.05 vs. C. Part B) shows Gclc and Gclm promoter activities expressed as % of respective control from nine experiments after 4 hours of LPS treatment, *p<0.05 vs. Gclc, †p<0.02 vs. Gclm. Part C) shows Western blot of Gclc and Gclm at 18 hours after LPS treatment. Representative blots from four separate experiments are shown and densitometric values expressed as % of control are shown in box, *p<0.05 vs. control. Part D) shows GSH levels at 18 hours after LPS treatment from three experiments, *p<0.05 vs. control.
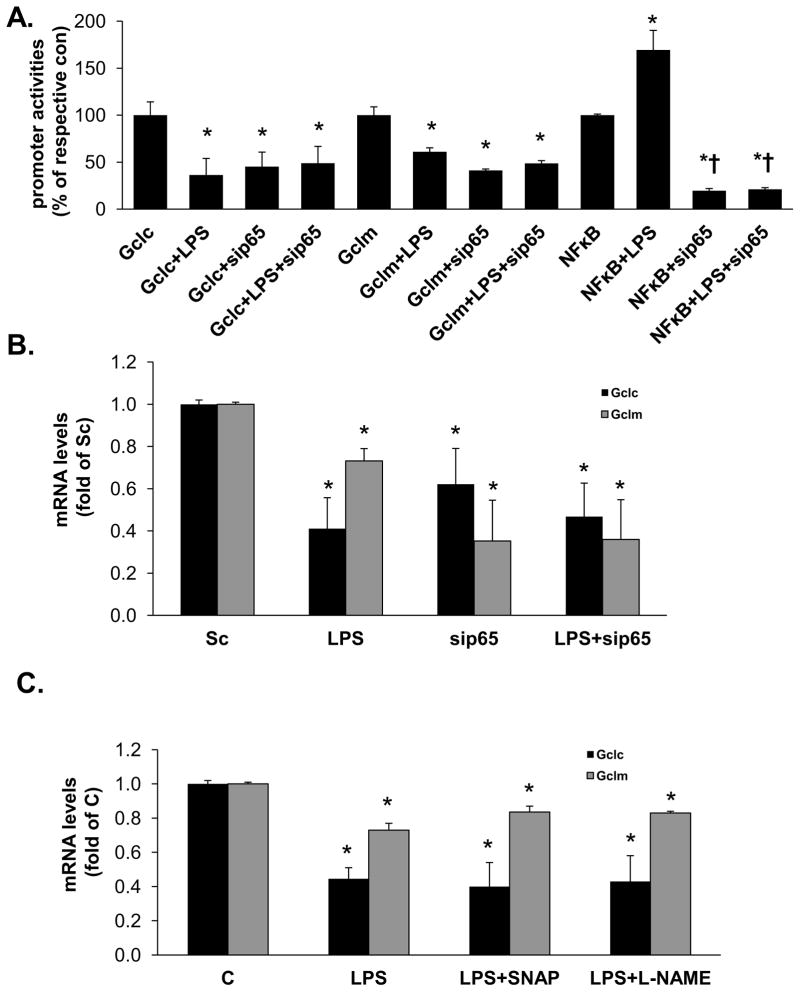
LPS treatment-mediated suppression of Gclc and Gclm promoter activity is independent of p65 or NO
LPS treatment activates NFkB [17] and p65 subunit of NFkB has been shown to inhibit Nrf2 signaling [18]. To examine whether this might be the mechanism, Gclc and Gclm promoter activities were measured in RAW cells treated with either scramble or p65 siRNA to block the increase in p65. Treatment with p65 siRNA for 48 hours lowered p65 mRNA levels to 15% of scramble controls, lowered NFkB promoter activity by 80% and completely blocked the ability of LPS to induce the NFkB promoter (Fig. 2A). Lowering p65 expression in RAW cells reduced basal Gclc and Gclm promoter activities (Fig. 2A) and mRNA levels (Fig. 2B) but had no influence on the LPS-mediated suppression. A previous study suggested that down-regulation of hepatic Gclc expression during endotoxemia is due to increased NO [7]. To examine this possibility, RAW cells were treated with LPS plus either NO donor SNAP or NOS inhibitor L-NAME for 4 hours. Neither SNAP nor L-NAME exerted any influence on LPS-mediated lowering of Gclc or Gclm mRNA levels (Fig. 2C). Treatment with LPS, with or without L-NAME for 4 hours did not result in measurable NO levels; while LPS+SNAP treatment raised NO level to 20.7±6.1μmol/L. LPS treatment for 24 hours raised NO level to 23.3±2.1μmol/L (all values are mean±SEM from three determinations).
Figure 2. Effect of p65 knockdown and NO on LPS-mediated inhibition of Gclc and Gclm expression in RAW cells.
RAW cells were treated with p65 siRNA or scramble siRNA control prior to LPS, or NO donor SNAP or NOS inhibitor L-NAME in conjunction with LPS as described in Methods. Part A) shows effect of knocking down p65 on basal promoter activity and after LPS treatment. Results are from four experiments, expressed as % of Gclc, Gclm, or NFkB basal promoter activity. *p<0.05 vs. respective promoter basal activity, †p<0.05 vs. LPS treatment. Part B) shows the effect of p65 knockdown on basal and LPS-treated Gclc and Gclm mRNA levels. Results are expressed as fold of scramble (Sc) from five experiments, *p<0.05 vs. Sc. Part C) shows the effect of varying NO on LPS-mediated suppression of Gclc and Gclm mRNA levels by co-treatment with SNAP or L-NAME. Results are from three determinations, *p<0.05 vs. control (C).
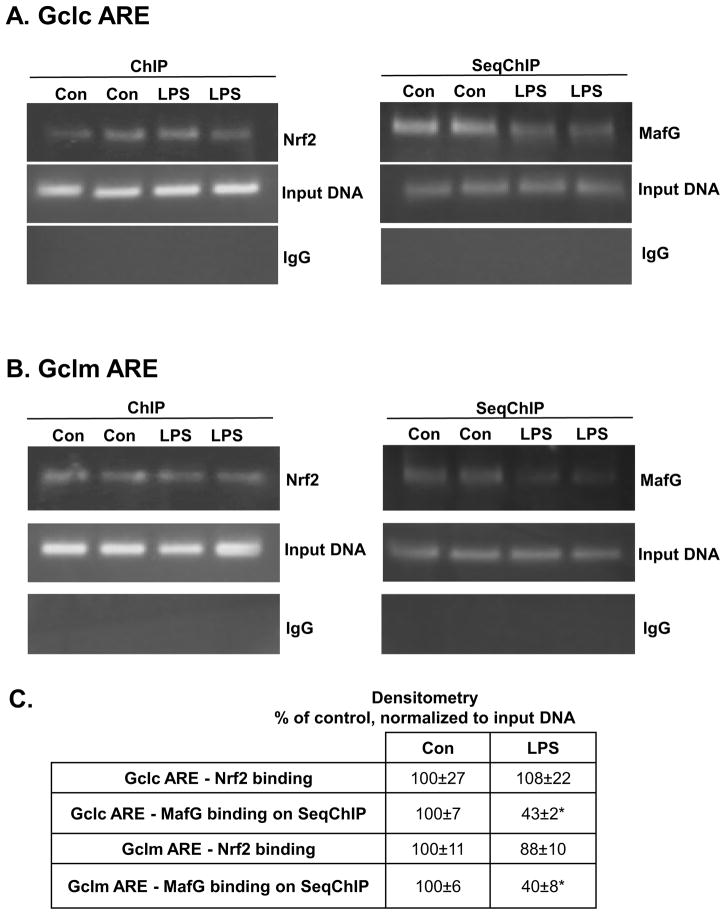
Effect of LPS on Nrf2 and MafG heterodimer formation and binding to ARE
Both Gclc and Gclm gene expression is positively regulated by ARE, which is trans-activated by Nrf2 as a heterodimer with small Maf (MafG, MafK and MafF) or Jun (c-Jun, Jun-D, and Jun-B) proteins [19,20]. In RAW cells MafF and MafK are minimally expressed (CT value in the mid 30s), whereas MafG’s CT value is in the 20s (data not shown). We therefore focused on MafG. We next examined Nrf2 and MafG binding to the ARE region of murine Gclc and Gclm. While Nrf2 binding to the ARE region remained essentially unchanged in both Gclc and Gclm (Fig. 3A and B, left panels), Nrf2-MafG heterodimer binding as assessed by SeqChIP was markedly reduced to the ARE region in both promoters (Fig. 3A and B, right panels, and densitometry shown in Fig. 3C).
Figure 3. Effect of LPS treatment on Gclc and Gclm ARE nuclear binding activity by Nrf2 and MafG in RAW cells.
ChIP and SeqChIP were done in RAW cells treated with LPS for 4 hours as described in Methods. Part A) ChIP (left) and SeqChIP (right) analysis of Gclc ARE after LPS treatment shows Nrf2 binding is unchanged but MafG co-occupancy is reduced. Input genomic DNA (Input DNA) was used as a positive control and a non-specific antibody IgG was used as a negative control. Part B) shows the same for Gclm ARE. Part C) summarizes the densitometric changes as % of control after normalizing to input DNA. *p<0.05 vs. control.
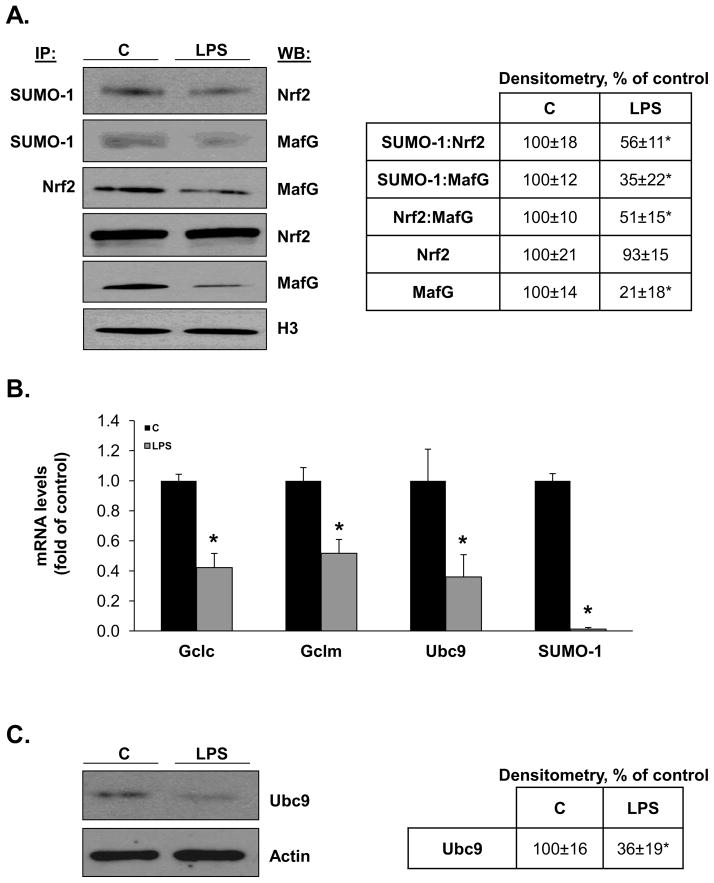
Role of sumoylation on LPS-mediated inhibition of Gclc and Gclm expression
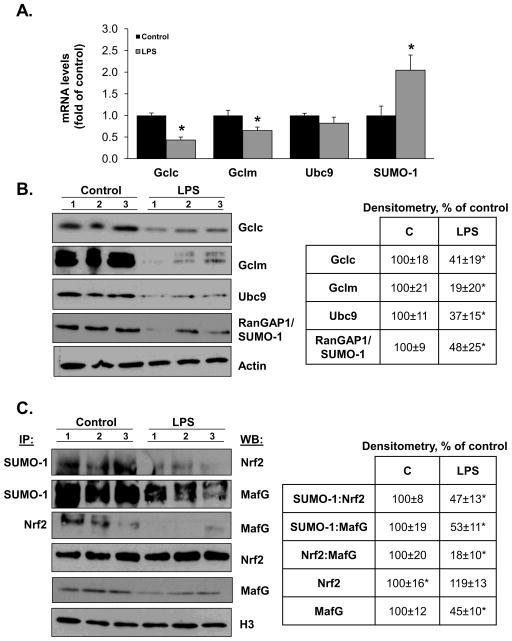
We recently showed that Nrf2 and MafG are sumoylated and this is required for their heterodimerization and trans-activation of the ARE [21]. We next examined whether LPS treatment caused a change in Nrf2 and MafG sumoylation. Figure 4A shows that following LPS treatment, nuclear total Nrf2 level is unchanged but sumoylated Nrf2 level fell by 44%. Interestingly, both nuclear total and sumoylated MafG levels fell dramatically after LPS and nuclear Nrf2-MafG heterodimer fell by 50%. Sumoylation can occur with SUMO-1,2,3 and Ubc9 is the sole E2 enzyme responsible for sumoylation [15]. Figure 4B shows that LPS treatment reduced Ubc9 mRNA level by 70% and SUMO-1 mRNA level by more than 90% (SUMO-2 and SUMO-3 mRNA levels were unchanged, data not shown). Reduced Ubc9 expression was confirmed at the protein level (Fig. 4C). Although LPS treatment did not cause a significant increase in apoptosis up to 24 hours, treatment of RAW cells with siUbc9 for 48 hours (lowered Ubc9 mRNA level by 70%) resulted in a 60% increase in apoptosis (160±9.9%, p<0.05 vs. SC control).
Figure 4. Effects of LPS treatment on sumoylation machinery, nuclear levels of total and sumoylated Nrf2 and MafG and Nrf2:MafG heterodimerization in RAW cells.
Part A) shows the effect of LPS on nuclear levels of total and sumoylated Nrf2 and MafG and their heterodimer formation. Sumoylated Nrf2 and MafG were measured by co-IP using anti-SUMO-1 antibody followed by blotting for Nrf2 or MafG. Nrf2:MafG heterodimer formation was measured by co-IP using anti-Nrf2 antibody followed by blotting for MafG. Total nuclear Nrf2 and MafG were measured by Western blotting. All were normalized to H3 housekeeping control. See Methods for details. Densitometric changes are summarized in the box on right, expressed as % of control. Results are from three experiments, *p<0.05 vs. control. Part B) shows the effect of LPS on mRNA levels of Gclc, Gclm, Ubc9 and SUMO-1. *p<0.05 vs. control from three experiments. Part C) shows the effect of LPS on total cellular Ubc9 protein levels. Representative Western blot is shown on left and densitometric changes are shown in box on right from five experiments after normalizing to the housekeeping control actin. *p<0.02 vs. control.
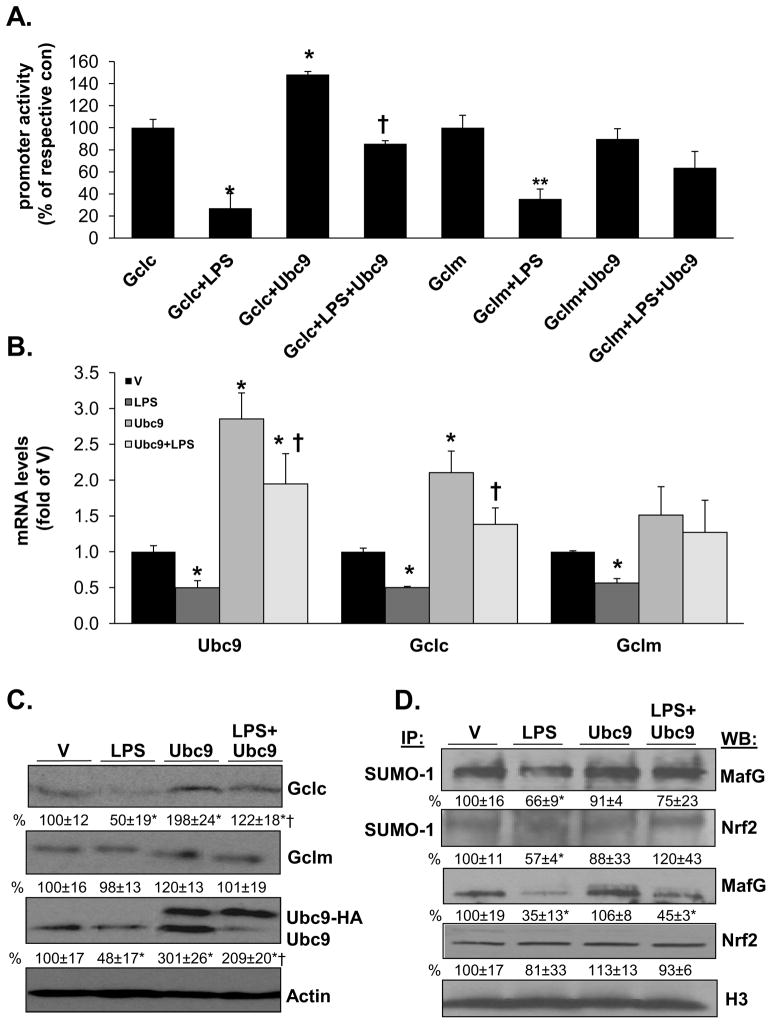
To critically examine the role of reduced Ubc9 expression and sumoylation on LPS-mediated suppression of Gclc and Gclm expression, RAW cells were first treated with an Ubc9 overexpression vector and subsequently with LPS. Figure 5A shows that Ubc9 overexpression raised the basal Gclc promoter (but not Gclm) activity and importantly, prevented LPS’s inhibitory effect on both promoters. Ubc9 overexpression prevented LPS-mediated lowering of Gclc and Gclm mRNA (Fig. 5B) and protein (Fig. 5C) levels. Similar to the effect of Ubc9 overexpression on the promoter activity, Gclc mRNA and protein levels (but not Gclm) were higher. Finally, Ubc9 overexpression protected against the fall in Nrf2 and MafG sumoylation (Fig. 5D).
Figure 5. Effects of Ubc9 overexpression on LPS-mediated suppression of Gclc and Gclm expression, and sumoylation of MafG and Nrf2 in RAW cells.
Part A) shows the effect of Ubc9 overexpression on basal promoter activity and after LPS treatment. Ubc9 overexpression raised basal Gclc (but not Gclm) promoter activity and prevented LPS-mediated inhibition of both promoter activity. Results are from three experiments, expressed as % of respective basal promoter activity, *p<0.05 vs. Gclc basal promoter activity, †p<0.05 vs. Gclc+LPS, **p<0.05 vs. Gclm basal promoter activity. Part B) shows the effect of overexpressing Ubc9 or empty vector (V) on Ubc9, Gclc and Gclm mRNA levels at baseline and after LPS treatment. Results are from three experiments, *p<0.05 vs. V, †p<0.05 vs. LPS. Part C) shows the same experimental design as part B on protein levels of the same genes. Representative Western blots are shown with densitometric values shown below the blots expressed as % of V. Note the higher molecular weight band of Ubc9-HA. Both Ubc9 bands were included in their densitometric measurement and all were normalized to actin. Part D) shows nuclear levels of total and SUMO-1 sumolyated MafG and Nrf2. Results are mean±SE from three experiments expressed as % of V, *p<0.05 vs. V, †p<0.02 vs. LPS.
Changes in Ubc9, Gclc, Gclm expression and sumoylation in mouse liver after LPS
We next examined whether a similar mechanism may be operative in LPS-treated mice liver, which we previously showed had reduced expression of Gclc and Gclm [6]. Consistent with our previous results, LPS treatment lowered Gclc and Gclm mRNA levels (Fig. 6A). However, unlike RAW cells, LPS treatment had no influence on Ubc9 mRNA levels and actually raised SUMO-1 mRNA levels (Fig. 6A). Although Ubc9 mRNA levels were unchanged, its protein levels fell in the liver by more than 60% following LPS treatment (Fig. 6B). Gclc, Gclm protein levels and SUMO-1 protein sumoylation as indicated by RanGAP1/SUMO-1 all fell significantly after LPS (Fig. 6B). Similar to RAW cells, nuclear levels of sumoylated Nrf2, MafG and their heterodimerization fell markedly following LPS treatment. Also similar to RAW cells, nuclear levels of total Nrf2 was unchanged but MafG levels were reduced (Fig. 6C).
Figure 6. Effect of LPS treatment on Gclc, Gclm, Nrf2, MafG expression, sumoylation and Nrf2:MafG heterodimerization in mice livers.
Part A) shows Gclc, Gclm, Ubc9 and SUMO-1 mRNA levels from mice livers after LPS treatment for 4 hours. Results are expressed as % of control from three mice per group, *p<0.03 vs. control. Part B) shows the effect of LPS on hepatic protein levels of the same genes and protein sumoylation as indicated by RanGAP1/SUMO-1. Western blots are shown on left and densitometry is summarized in the box on right. Results are from three mice per group, *p<0.03 vs. control group. Part C) shows effect of LPS on hepatic nuclear levels of total and sumoylated Nrf2 and MafG and their heterodimer formation. See Methods for details. Co-IP and Westerns are shown on left from three mice per group and densitometric changes are summarized in the box on right. *p<0.05 vs. control group.
Effect of LPS on Ubc9, Gclc, Gclm expression and sumoylation in mouse hepatocytes
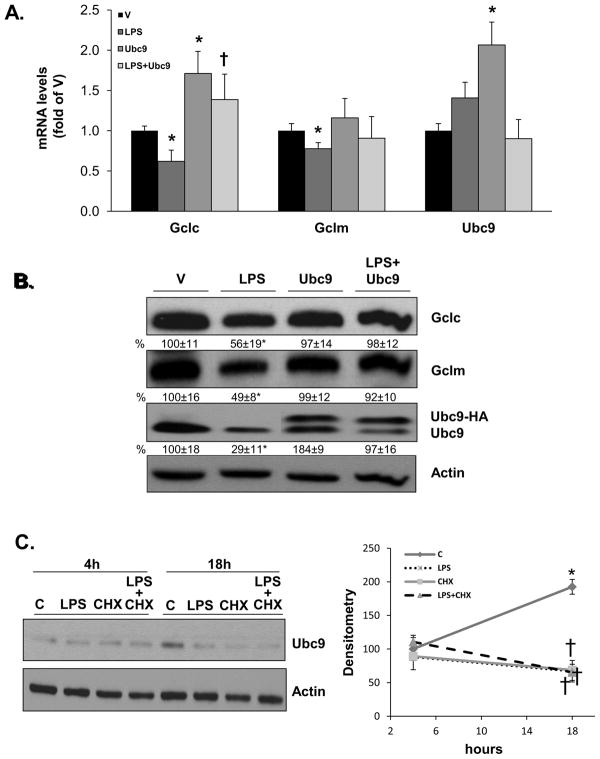
Since hepatocytes make up most of the liver and they also respond to LPS [22], we examined whether similar effects on Ubc9, Gclc and Gclm expression occur in mouse hepatocytes. LPS treatment also lowered the mRNA and protein levels of Gclc and Gclm (Fig. 7A and B). Like whole liver, LPS had no influence on Ubc9 mRNA level but it reduced Ubc9 protein level by 71% (Fig. 7A and B). Similar to RAW cells, overexpression of Ubc9 completely protected against LPS-mediated lowering of Gclc and Gclm mRNA and protein levels (Fig. 7A and B).
Figure 7. Effect of LPS and Ubc9 overexpression on Ubc9, Gclc and Gclm expression in mouse hepatocytes.
Primary cultures of mouse hepatocytes were treated with Ubc9 overexpression vector or empty vector (V) and then subjected to LPS treatment as described in Methods. Part A) shows the effect of Ubc9 overexpression on basal Gclc and Gclm mRNA levels and after LPS treatment for 4 hours. Ubc9 overexpression raised Gclc (but not Gclm) mRNA level and prevented LPS-mediated lowering of both. Results are expressed as % of V from three experiments, *p<0.05 vs. V, †p<0.05 vs. LPS. Part B) shows the same treatments as A) on protein levels of the same genes after 18 hours. Representative Western blots are shown and densitometric changes are expressed as % of V in the box below. Results are from three experiments, *p<0.02 vs. V, †p<0.05 vs. LPS. Part C) shows the effect of LPS on Ubc9 protein level is not due to increased protein degradation. Hepatocytes were treated with cycloheximide prior to LPS for 4 to 18 hours. Graph on the right summarizes changes in densitometry of Ubc9 as a function of time, expressed as % of 4-hour control. Results are from 3 experiments, *p<0.05 vs. 4 hour control, †p<0.05 vs. 18-hour control.
To see whether LPS treatment affected Ubc9 protein stability, hepatocytes were first treated with cycloheximide to block de novo protein synthesis. Ubc9 protein level increased in mouse hepatocytes cultured for 18 hours as compared to 4 hours (200% higher), but was reduced to 35% of control at 18 hours in cycloheximide and LPS treated, alone or combined (Fig. 7C).
DISCUSSION
Liver plays a central role in both clearing gut-derived LPS and GSH homeostasis [2,19]. GSH is highly concentrated in the liver and is synthesized in the cytosol via two enzymatic steps, the formation of g-glutamylcysteine from glutamate and cysteine catalyzed by GCL; and the formation of GSH from g-glutamylcysteine and glycine catalyzed by GS [19]. GSH protects against oxidative stress, and regulates cell death, inflammatory and fibrotic responses [19]. Others and we have shown that hepatic GSH level falls during endotoxemia [4,6,7,12]. Septic patients have lower blood GSH:GSSG ratios [23]. Lower GSH has been shown to sensitize to TLR4 signaling [11] and up-regulate cell surface molecules and allo-stimulatory capacity [24]. Exogenous GSH corrected these abnormalities [11,24], suppressed LPS-induced systemic inflammatory response and reduced mortality [9]. Since GSH level plays a key role in the inflammatory response to endotoxin, it is important to better understand how LPS lowers GSH level.
Although oxidative stress and enhanced release have both been implicated in LPS-induced fall in hepatic GSH level [4,12], our study using in vivo treated LPS mice showed a marked reduction of hepatic Gclc and Gclm expression at the mRNA level [6]. This agrees with the finding of Payabvash et al [7] who reported reduced hepatic Gclc mRNA level and GCL activity 24 hours after LPS treatment. However, these investigators found that blocking NO synthesis protected against the fall in GSH level and Gclc mRNA level, suggesting that increased NO production was responsible [7]. This possibility was critically examined in our study.
Our study examined the effect of LPS in RAW cells, hepatocytes and in vivo treated livers. This is because RAW cells have been used extensively as a convenient model of LPS signaling in macrophages [16,17] and hepatocytes make up the bulk of the liver. LPS initiates intracellular signaling cascade through TLR4 and TLR2 [1,25]. At least three LPS-binding proteins, including LPS-binding protein (LBP), CD14, and MD2 are required for optimal TLR-4 mediated LPS signaling [26]. LBP, a plasma acute phase protein that is primarily synthesized in the liver, potentiates LPS signaling by transferring LPS released from the outer membrane of gram-negative bacteria to membrane-bound and soluble forms of CD14 [26]. Hepatocytes express CD14, TLR2, TLR4 (as well as all other TLRs) and respond to LPS [22]. They can further enhance the effect of LPS on Kupffer cells [26]. We found that in all three models (RAW cells, hepatocytes and whole liver), LPS lowered the mRNA and protein levels of Gclc. Interestingly, although LPS lowered the mRNA level of Gclm in all three models, it lowered Gclm protein levels in liver and hepatocytes but not in RAW cells. This suggests the half-life of Gclm protein may be cell type specific. In RAW cells, the mechanism for LPS-mediated inhibition of Gclc and Gclm expression lies at the transcriptional level. The mechanism was not due to a change in NO level since at the 4-hour time point when their expression fell, no measurable increase in NO level had occurred. In addition, iNOS knockout mice were not protected from LPS-induced lethality in mice [27], which suggests an increase in NO is probably not a key mechanism for LPS-mediated injury. Another potential mechanism we explored is repression of Nrf2-ARE signaling by LPS-mediated activation of NFkB/p65 [18]. Specifically Liu et al reported that in HepG2 and HEK293 cells p65 can repress Nrf2 by competing for transcription co-activator CREB binding protein (CBP) and promote recruitment of histone deacetylase 3 (HDAC3), a corepressor, to ARE by facilitating the interaction of HDAC3 with either CBP or MafK [18]. If increase in p65 was responsible, blocking its induction should prevent LPS-mediated suppression of Gclc and Gclm expression. However, we found that lowering p65 expression lowered basal Gclc and Gclm expression and had no influence on LPS-mediated inhibition. These results confirmed our previous finding that basal Gclc and Gclm expression requires NFkB [14] and showed increased p65 was not the mechanism responsible for LPS-mediated suppression.
Having ruled out two potential mechanisms, we focused on ARE, the key cis-acting element that positively regulates the expression of GCL subunits [19]. Nrf2 is the transcription factor most well characterized to activate ARE [20]. Under normal non-stressful conditions, Nrf2 is kept in the cytosol by Keap1 and undergoes proteosomal degradation [20]. Under many stressful conditions, Nrf2 is released from Keap1, escapes proteosomal degradation and translocates to the nucleus to induce genes involved in defense and survival [28]. Nrf2 forms heterodimers with small Maf (MafG, MafK and MafF) and Jun (c-Jun, Jun-D, and Jun-B) proteins to bind to ARE [20]. We focused on MafG because in both liver and RAW cells, we found that MafG is the dominant small Maf expressed. This is consistent with what others have reported [29]. A recent report showed triple MafF/G/K knockout mice exhibit severe growth retardation and die by E13.5 [30]. These mice have severe liver hypoplasia and markedly reduced expression of ARE-dependent cytoprotection genes in the liver. Importantly, this phenotype could be completely rescued by transgenic expression of exogenous MafG under MafG gene regulatory control, suggesting MafG is the most critical of the three small Mafs in adult stages [30]. In addition, we recently reported that Nrf2 and MafG are sumoylated and this facilitates their heterodimerization and trans-activation of the ARE [21]. This prompted us to examine Nrf2 and MafG expression and their heterodimerization and binding to the ARE.
Although Nrf2 expression and binding to the ARE of Gclc and Gclm remained unchanged, nuclear sumoylated Nrf2 level and importantly, heterodimerization with MafG and their co-occupancy on the same promoter region were markedly reduced following LPS treatment in RAW cells and in mouse liver. Total nuclear MafG level was also lower after LPS treatment. MafG is one of the small Maf proteins (MafF/G/K) that are under complex control, both transcriptional and post-translational and are responsive in particular to stressful stimuli [31]. Effect of LPS on small Maf expression has been reported in a murine microglial cell line N9 [32]. The expression of the small Maf proteins increased in the nuclear fraction after 15 minutes to one hour of LPS treatment [32]. However, the antibody used in that study did not distinguish among the small Maf proteins. Different time points and cell types in our studies may also have contributed to the difference in the results. The mechanism for the fall in MafG expression will require further study.
While reduced MafG sumoylation likely reflects reduced total MafG level, the mechanism for reduced Nrf2 sumoylation was due to LPS-mediated inhibition of Ubc9 and SUMO-1 expression in RAW cells. Ubc9 is the sole E2 enzyme in the sumoylation pathway [15] so its reduced expression would impact on overall protein sumoylation, which occurred in both RAW cells and livers of LPS-treated mice. However, there is an interesting difference in that in RAW cells, reduced Ubc9 expression occurred at the mRNA level but in whole liver and hepatocytes, it occurred at the protein level since the mRNA level was unchanged. We also did not find a change in the Ubc9 protein stability after LPS treatment in hepatocytes. These results suggest the possibility that LPS may have induced translational repression of Ubc9 in hepatocytes, which will require further investigation. SUMO-1 expression fell more than 90% at the mRNA level in RAW cells but it actually increased following LPS treatment in mouse liver. How LPS affects Ubc9 and SUMO-1 expression in these different model systems will require further investigation but the observation that overexpression of Ubc9 prevented LPS-mediated inhibition of Gclc and Gclm expression in both RAW cells and mouse hepatocytes supports the notion that Ubc9 down-regulation (whether at the mRNA or protein level) plays a key role in reduced expression of GCL subunits and GSH biosynthesis.
It should be noted that there are conflicting reports in the literature regarding the effect of LPS on GSH levels in RAW cells that included increased [33], unchanged [34], to lower levels [35]. Different doses and duration of treatment may have been responsible for some of these differences. Our study examined an acute effect, which occurred not only in RAW cells but also hepatocytes and the whole liver. In terms of LPS’s effect on sumoylation, one prior study reported that LPS lowered the expression of Ubc9 and SUMO-1 expression at the mRNA level in astrocytes [36]. To our knowledge, our work is the first that examined the effect of LPS on sumoylation in macrophages, hepatocytes and whole liver and its impact on the anti-oxidant defense system.
In conclusion, our current work delineated the molecular mechanism of how LPS treatment suppresses the expression of Gclc and Gclm in macrophages, hepatocytes and whole liver. The mechanism lies mainly in its inhibitory effect on the sumoylation machinery via suppressing the expression of the sole E2 enzyme Ubc9. This results in reduced Nrf2 and MafG sumoylation, their heterodimerization and trans-activation of the ARE present in Gclc and Gclm promoters. Since ARE is an element that is present in the promoter region of many genes involved in anti-oxidant defense and survival [20], our findings have important implications regarding the pathogenesis of endotoxemia. It also raises the possibility of targeting Ubc9 dysregulation as a therapeutic approach.
Highlights.
Lipopolysaccharide (LPS) reduced glutamate-cysteine ligase (GCL) subunits expression.
LPS reduced ubiquitin-conjugation enzyme 9 (Ubc9) expression and protein sumoylation.
LPS reduced Nrf2 and MafG sumoylation, heterodimerization and binding to the ARE.
Overexpression of Ubc9 protected from LPS-mediated inhibition of GCL expression.
Similar findings occurred in RAW cells, hepatocytes and livers of LPS treated mice.
Acknowledgments
This work was supported by NIH grants R01DK092407 (to SC Lu and H Yang), and F32AA020150 (ML Tomasi). RAW cells and mouse hepatocytes were provided by the Cell Culture Core of the USC Research Center for Liver Diseases (P30DK48522).
List of abbreviations (in alphabetical order)
- ARE
antioxidant response element
- CBP
CREB binding protein
- ChIP
chromatin immunoprecipitation
- CNC
cap ‘n’ collar
- Co-IP
co-immunoprecipitation
- GCL
glutamate-cysteine ligase
- Gclc
GCL-catalytic subunit
- Gclm
GCL-modifier subunit
- GSH
reduced glutathione
- GS
GSH synthase
- HDAC3
histone deacetylase 3
- iNOS
inducible nitric oxide synthase
- LBP
LPS-binding protein
- L-NAME
NG-monomethyI-L-arginine methyl ester
- MD2
myeloid differentiation protein 2
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear factor-erythroid 2 related factor 2
- ROS
reactive oxygen species
- RT
reverse transcription
- SC
scramble
- SEM
standard error of the mean
- SeqChIP
sequential ChIP
- siRNA
small interfering RNA
- SNAP
S-nitroso-N-acetylpenicillamine
- TNFa
tumor necrosis factor a
- TLR4
Toll-like receptor 4
- Ubc9
ubiquitin-conjugating enzyme 9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryant CE, Spring DR, Gangloff M, Gay NJ. The molecular basis of the host response to lipopolysaccharide. Nature Rev Microbiology. 2010;8:8–14. doi: 10.1038/nrmicro2266. [DOI] [PubMed] [Google Scholar]
- 2.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffercell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 3.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Walker LM, Hinson JA, Mayeux PR. Oxidant stress in rat liver after lipopolysaccharide administration: effect of inducible nitric-oxide synthase inhibition. J Pharmacol Exp Therap. 2000;293:968–972. [PubMed] [Google Scholar]
- 5.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko KS, Yang HP, Noureddin M, Iglesia-Ara A, Xia M, Wagner C, Luka Z, Mato JM, Lu SC. Changes in S-adenosylmethionine and glutathione homeostasis during endotoxemia in mice. Lab Invest. 2008;88:1121–1129. doi: 10.1038/labinvest.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payabvash S, Ghahremani MH, Goliaei A, Mandegary A, Shafaroodi H, Amanlou M, Dehpour AR. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Rad Biol Med. 2006;41:1817–1828. doi: 10.1016/j.freeradbiomed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Victor VM, De La Fuente M. Immune cells redox state from mice with endotoxin-induced oxidative stress. Involvement of NF-κB. Free Rad Res. 2003;37:19–27. doi: 10.1080/1071576021000038522. [DOI] [PubMed] [Google Scholar]
- 9.Sun S, Zhang H, Xue B, Wu Y, Wang J, Yin Z, Luo L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 10.Gould NS, Min E, Day BJ. Macropinocytosis of extracellular glutathione ameliorates tumor necrosis factor release in activated macrophages. PLoS One. 2011;6:e25704. doi: 10.1371/journal.pone.0025704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaeschke H. Enhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivo. Am J Physiol Gastrointest Liver Physiol. 1992;263:G60–68. doi: 10.1152/ajpgi.1992.263.1.G60. [DOI] [PubMed] [Google Scholar]
- 13.Bea F, Hudson FN, Chait A, Kavanagh TJ, Rosenfeld ME. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circulation Res. 2003;92:386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- 14.Yang HP, Magilnick N, Ou XP, Lu SC. Tumor necrosis alpha induces coordinated activation of rat GSH synthetic enzymes via NF B and AP-1. Biochem J. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomasi ML, Tomasi I, Ramani K, Pascale RM, Xu J, Giordano P, Mato JM, Lu SC. S-adenosylmethionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in human cancers. Hepatology. 2012;56:982–993. doi: 10.1002/hep.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veal N, Hsieh CL, Xiong S, Lu SC, Tsukamoto H. Inhibition of lipopolysaccharide-stimulated TNFa promoter activity by S-adenosylmethionine and 5'-methylthioadenosine. Am J Physiol. 2004;287:G352–362. doi: 10.1152/ajpgi.00316.2003. [DOI] [PubMed] [Google Scholar]
- 17.Iglesias Ara A, Xia M, Ramani K, Mato JM, Lu SC. S-Adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu GH, Qu J, Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim et Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Lu SC. Glutathione synthesis. BBA-Gen. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radical Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 21.Ramani K, Tomasi ML, Yang HP, Ko K, Lu SC. Mechanism and significance of changes in glutamate-cysteine ligase expression during hepatic fibrogenesis. J Biol Chem. 2012;287:36341–36355. doi: 10.1074/jbc.M112.370775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Németh I, Boda D. Xanthine oxidase activity and blood glutathione redox ration in infants and children with septic shock syndrome. Int Care Med. 2001;27:216–221. doi: 10.1007/s001340000791. [DOI] [PubMed] [Google Scholar]
- 24.Yamada H, Arai T, Endo N, Yamashita K, Fukuda K, Sasada M, Uchiyama T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sciences. 2006;78:926– 933. doi: 10.1016/j.lfs.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of Toll-Like Receptors in changes in gene expression and NF-kB activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immunity. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott MJ, Liu S, Su GL, Vodovotz Y, Billiar TR. Hepatocytes enhance effects of lipopolysaccharide on liver nonparenchymal cells through close cell interactions. Shock. 2005;23:453–458. doi: 10.1097/01.shk.0000160939.08385.f1. [DOI] [PubMed] [Google Scholar]
- 27.Laubach V, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol, Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 29.Onodera K, Shavit JA, Motohashi H, Katsuoka F, Akasaka J, Engel JD, Yamamoto M. Characterication of the murine MafF gene. J Biol Chem. 1999;274:21162–21169. doi: 10.1074/jbc.274.30.21162. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki H, Katsuoka F, Motohashi H, Engel JD, Yamamoto M. Emryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins. Mol Cell Biol. 2012;32:808–816. doi: 10.1128/MCB.06543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? J Mol Biol. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 32.Dodd CA, Filipov NM. Manganese potentiates LPS-induced heme oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output. Neurotoxicology. 2011;32: 683–692. doi: 10.1016/j.neuro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferret PJ, Soum E, Negre O, Fradelizi D. Auto-protective redox buffering systems in stimulated macrophages. BMC Immunology. 2002;3:3. doi: 10.1186/1471-2172-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndengele MM, Bellone CJ, Lechner AJ, Matuschak GM. Brief hypoxia differentially regulates LPS-induced IL-1β and TNF-α gene transcription in RAW 264.7 cells. Am J Physiol: Lung Cell Mol Physiol. 2000;278:L1289–L1296. doi: 10.1152/ajplung.2000.278.6.L1289. [DOI] [PubMed] [Google Scholar]
- 35.Butzer U, Weidenbach H, Gansauge S, Gansauge F, Beger HG, Nussler AK. Increased oxidative stress in the RAW 264.7 macrophage cell line is partially mediated via the S-nitrosothiol-induced inhibition of glutathione reductase. FEBS Letts. 1999;445:274–278. doi: 10.1016/s0014-5793(99)00139-8. [DOI] [PubMed] [Google Scholar]
- 36.Akar CA, Feinstein DL. Modulation of inducible nitric oxide synthase expression by sumoylation. J Neuroinflammation. 2009;6:12. doi: 10.1186/1742-2094-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]