Abstract
Our understanding of chronic pain involves complex brain circuits that include sensory, emotional, cognitive and interoceptive processing. The feed-forward interactions between physical (e.g., trauma) and emotional pain and the consequences of altered psychological status on the expression of pain have made the evaluation and treatment of chronic pain a challenge in the clinic. By understanding the neural circuits involved in psychological processes, a mechanistic approach to the implementation of psychology-based treatments may be better understood. In this review we evaluate some of the principle processes that may be altered as a consequence of chronic pain in the context of localized and integrated neural networks. These changes are ongoing, vary in their magnitude, and their hierarchical manifestations, and may be temporally and sequentially altered by treatments, and all contribute to an overall pain phenotype. Furthermore, we link altered psychological processes to specific evidence-based treatments to put forth a model of pain neuroscience psychology.
Keywords: chronic pain, psychology, anxiety, depression, anhedonia, cognition, attention, perception, interoception, motivation, fear, catastrophizing, imaging, reward, brain, allostatic load, nociception, neurocircuits, behavior, insula, amygdala, prefrontal cortex, hippocampus, parietal cortex, anterior cingulate cortex
Introduction
As organisms, we respond to external and internal milieu. In humans, such behavioral and physiological responses may be adaptive or maladaptive. In chronic pain, alterations in physical systems can result in changes in psychological processes observed as neural-circuit defined changes in behavior. Control of these processes may be viewed as a balance of excitatory and inhibitory events. Some processes may contribute to an escalating cascade or deescalating cascade of neural systems to produce altered behaviors in a sensitized or desensitized state. Chronic pain provides an ideal model to attempt to understand these types of processes in the context of localized and integrated neural networks. Brain regions consistently implicated include the primary and secondary somatosensory cortex (S1 and S2), spinal cord, thalamus, insula, anterior cingulate cortex, prefrontal cortex (Apkarian et al., 2005; Peyron et al., 2000; Tracey, 2008); midbrain areas including the periaqueductal gray (Linnman et al., 2011) and cerebellum (Moulton et al., 2010), and subcortical structures including the hippocampus, basal ganglia, and amygdala (Borsook et al., 2010; Maleki et al., 2011; Schweinhardt and Bushnell, 2010; Simons et al., 2012a); and see (Bushnell et al., 2013) for a recent review on brain regions involved in cognitive and emotional aspects of chronic pain) (see Figure 1). The brain's interconnected networks contribute to resting and active functions that are orchestrated with varying degrees of contributions from these and other brain regions.
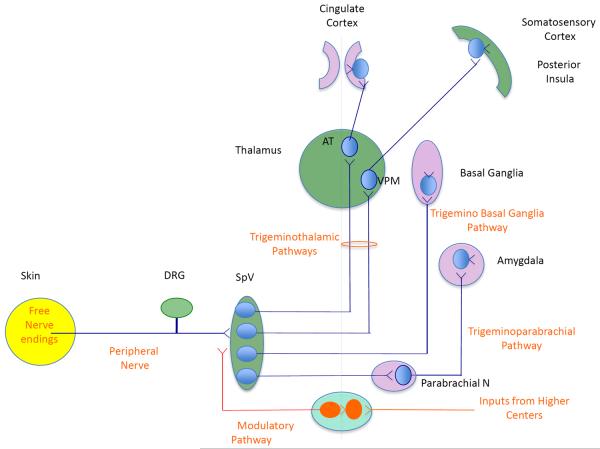
Figure 1. Neural Pathways involved in Pain.
The figure conceptualizes brain regions involved in sensory and emotional processing of chronic pain. DRG = dorsal root ganglion, SpV = spinal nucleus of trigeminal ganglion (dorsal horn), AT = anterior thalamus; VPM = ventroposteromedial thalamus
Pain is a response to nociceptive stimuli, often the driving force leading individuals to seek treatment, when they ache, hurt, and/or suffer. As pain becomes chronic, there is a tendency to be different - ones psychological state of being (and mind) is altered. Physical and emotional pain exist on the same continuum (Borsook et al., 2007; Elman et al., 2011; Perl, 2007) with common brain networks involved (Bendelow and Williams, 2008), with duplication and redundancy abounding. For example, using both noxious heat and unpleasant pictures, overlapping activation was observed in the posterior cerebellum with significant inverse correlations with limbic structures (viz., anterior hypothalamus, subgenual anterior cingulate, and parahippocampal gyrus), suggesting that the cerebellum contains specific regions involved in encoding generalized aversive processing, not necessarily unique to affective or sensorimotor functions (Moulton et al., 2011). Figure 2 conceptualizes physiological and psychological processing of nociception and pain related processes that may lead to altered behaviors. There are two major points that need to be considered when trying to understand a brain-systems approach to psychological processes in pain: (1) experiencing pain can trigger a cascade of neurological (initially sensory) events that lead to an altered psychological state; and (2) prior psychological states can confer a heightened risk for pain chronicity due to processes such as cross sensitization, where exposure to stress in the past results in greater sensitivity to other seemingly unrelated stimuli (e.g., childhood trauma, loss of a parent, and addiction) (Elman et al., 2013; Goldberg et al., 1999; Nicolson et al., 2010).
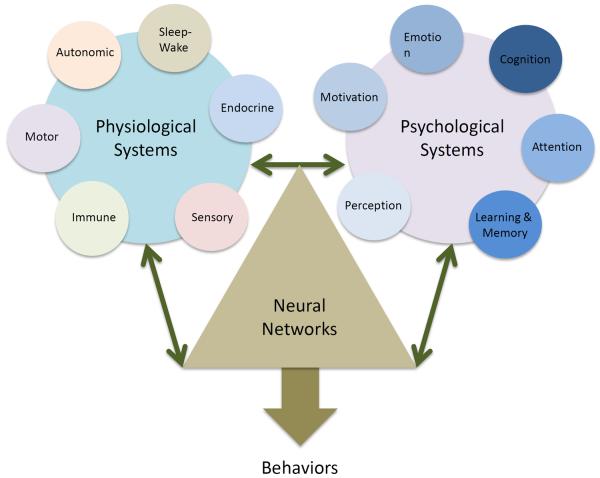
Figure 2. Physiological and Psychological Systems that integrate Pain Behaviors.
Physiological systems including stress-related hormones and brain systems that control these, sleep-wake processes as well as those sensory systems that respond to tissue damage clearly contribute to alterations in neural networks. Some physiological changes can be either objectively measured or reasonably easily interpreted (e.g., pain intensity). Psychological systems to so in a less obvious manner until a forme fruste behavioral aberration is present. These changes alter our normal network profile that is dependent on genetic, epigenetic and live experience to modify behaviors. Clearly the two processes in pain are integrated and result in aberrant behaviors.
In this review we evaluate some of the principle behavioral alterations in the context of neural circuits and how these may be altered as a consequence of chronic pain. Data extracted from original research and from seminal review articles were critically assessed and summarized within the following main sections: (1) Pain, Neural Circuits, and Maladaptive Changes that contribute to central sensitization and perturbations in allostasis (allostatic load); (2) Alteration of Psychological Processes in Chronic Pain with evidence-based treatments that map onto key areas such as cognition, reward state and fear learning; (3) Premorbid Risk Factors and Pain where vulnerabilities such as psychological trauma can confer heightened risk; and lastly, we bring together these concepts with (4) Integrating Dysfunction: Complex Behaviors with Reciprocal, or Multiple and Multiplying Effects and provide suggestions for (5) Treatment and Training Approaches aimed at psychologists' training in the basic tenets of pain neuroscience.
1: Pain, Neural Circuits, and Maladaptive Changes
1.1. Chronic Pain, Neurocircuits and Behavior
The evolution or transition to chronic pain is not obvious. For many chronic pain conditions, the transition follows a specific insult (e.g., complex regional pain syndrome, post herpetic neuralgia, diabetic neuropathy, spinal cord injury, etc.), relates to a missing enzyme (e.g., Fabry's Disease) or is the result of a genetic condition (e.g., hemiplegic migraine), while in some cases pain emerges spontaneously. In cases of a specific insult the evolution of altered behaviors that are comorbid with pain unfold. Take for example, the individual who has a traumatic amputation from war or civilian injuries; prior to the injury, they were considered `normal'. Following their injury a sequence of altered behaviors that relate to or are comorbid with their pain unfold with varying temporal frequency. Thus, the subject who was not previously depressed is now. The subject who had no sleep-wake issues does now. The subject who had no problems related to anxiety may have components of post-traumatic stress disorder (PTSD). Similarly alteration in memory, cognition and even subtle changes in relating spatially to the environment may change. How do we understand these psychological changes in the context of neural plasticity, maladaptation and allostatic load?
Allostasis refers to the ability of complex physiological systems to maintain stability through change when confronted with a stressor (McEwen, 1998). Regulatory changes often engage multiple physiological systems, including the cardiovascular, neuronal, immunologic, and endocrine systems. When these processes are compromised, allostatic load occurs; manifested as chronic dysregulation of physiologic systems. Allostatic load has a direct impact on brain function. The brain reacts to stressors by modulating physiology and behavior, which in turn results in chemical and structural brain changes (McEwen, 2000a, b). Although pain is an inherently adaptive signal that warns an individual of real or potential damage, persistent pain leads to cumulative strain on the brain, allostatic load, and alterations in normative psychological processes such as perception, emotion, cognition, and motivation that are closely intertwined with physiological responses of interoception, brain system rest (sleep), and autonomic function (see Figure 3). Pre-clinical models are beginning to take into consideration the broad scope of behavioral responses associated with persistent pain and pain relief. A number of behaviors suppressed by pain in rodents (rearing, burrowing) mimic more closely the alterations in behavior observed in patients with chronic pain and are becoming targets to measure analgesic response. Specifically, burrowing has been conceptualized as an indicator of global `wellbeing' for the rat (Deacon, 2006). Andrews and colleagues successfully examined spontaneous burrowing behavior to detect the effects of traumatic peripheral nerve injury and tissue inflammation and were able to measure the analgesic efficacy of gabapentin (30mg/kg) and ibuprofen (30mg/kg), both of which reversed the burrowing deficits (Andrews et al., 2011). In other words, some behaviors may be difficult to evaluate, but clearly contribute to the process of either seeking pain relief or sequestering ourselves as a protective process from stress, the elements, social situations, etc.
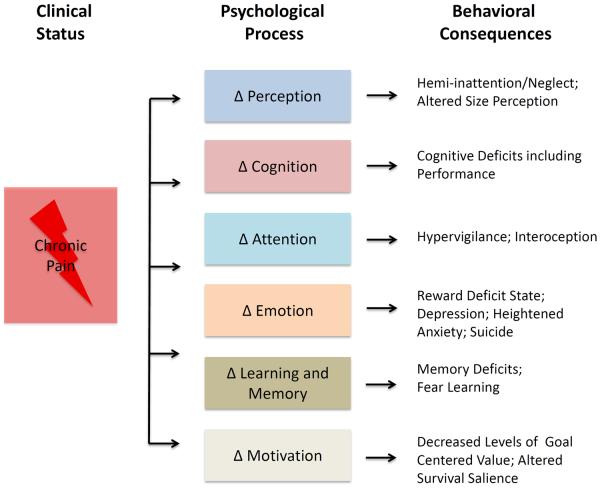
Figure 3. Pain, Psychological Processes and Behavioral Consequences.
Following a pain (a sensory or emotional experience to an actual trauma or perceived bodily threat), a number of psychological processes including those listed here are involved in response. These change processes may be resilient or resistant to the inciting events or become altered as noted in examples of behavioral consequences. Additionally, alterations in one system may have consequences in another. The understanding of how these systems interact and can be targeted will have significant implications on treatment approaches.
1.2. Pain Behavior – Adaptive or Maladaptive
Escape or avoidance from painful or potentially painful stimuli is a normal process (i.e., even rapid withdrawal from a noxious stimuli) for acute threats. In chronic pain this drive is no longer adaptive. Clinical examples include resting the painful limb in complex regional pain syndrome that may be considered protective to limit pain sensory exacerbation with movement. However, the nature of this behavior is maladaptive, as the condition may be made worse with immobility. Similar, but perhaps more complex processing relates to how individuals have emotional equivalents of `protecting the limb' in trying to protect (or avoid) affective experiences. The high comorbidity of chronic pain and PSTD exemplifies this process where similar mechanisms (e.g., attentional biases, avoidance coping) “mutually maintain” (Liedl et al., 2010; Sharp and Harvey, 2001) both conditions/disorders.
A clear example of adaption and maladaptation related to pain is conditioned fear. It may be intimately linked to the onset of pain such as in the case of trauma or triggered by the experience of increased pain with activity. Such processes contribute to an ongoing actual and perceived `threat' to the individual. In an adaptive process, individuals would allow for neuronal repair of neurons and functional circuits or implement physical and behavioral functions to restore their ability to interact with the environment. In a maladaptive process, feed forward mechanisms result in system failure (McEwen, 2012). Some of these perturbations are relatively easily measured such as alterations in sensation (sensitization, allodynia, hyperalgesia, hyperpathia, etc.) and may encompass peripheral and/or central sensitization. This dynamic and relatively quantitative approach is more difficult in non-sensory aspects of pain. As such, the term centralization of pain may be used to capture the process where the initial stimulus (e.g., surgical trauma) progresses to a chronic pain state (neuropathic pain) but also alters cognition (Berryman et al., 2013), affective and motivational states (Wiech and Tracey, 2013), potential for suicide (Elman et al., 2013) and may diminish normal inhibitory and enhanced facilitatory pain modulatory processes (Chalaye et al., 2012; Porreca et al., 2002). Taken together these may set up an unstable emotional state, frequently manifested as emerging comorbid conditions, most commonly, anxiety and depression. While certain vulnerability factors are clearly at play (see 3.0 Premorbid Risk Factors and Pain), most non-sensory processes (i.e., motivation, cognition, memory, etc.) are altered in pain as a consequence of centralization of nociceptive signaling. Nociception eventually invades neural processes that are altered to produce the centralized brain state of chronic pain. Figure 3 and Figure 4 encompasses two main themes: (1) that some psychological systems when altered have deleterious effects on others (e.g., cognition on memory and vice versa); and (2) that progressive cumulative failures of psychological function, as exemplified by the diminishing energy of activation in a bouncing ball, applies to chronification of pain where patients' neural systems are no longer adaptive and systems fail.
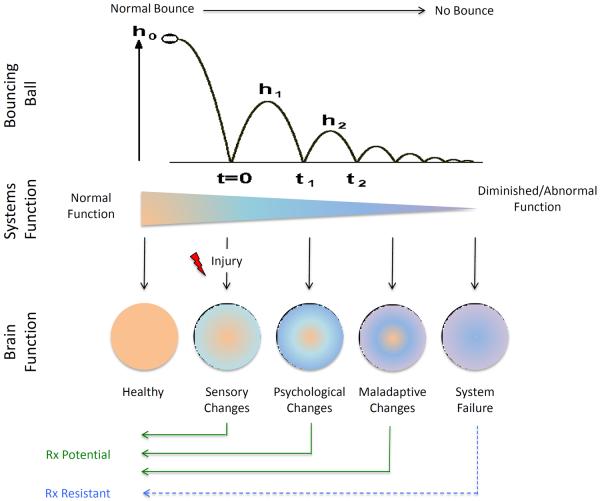
Figure 4. Pain Progression – Complex interactions leading to maladaptive changes and system failure (allostatic load).
The upper part of the figure shows the loss of energy in a bouncing ball – after a while all potential/kinetic energy (height, h) diminishes over time. The comparison is similar for chronic pain (middle figure) where normal function evolves to diminished normal or increased aberrant or abnormal function. Multiple changes in brain function (altered networks, see Figure 6) occur over time following injury as shown in the lower part of the figure where there is a sequential alteration in function (physiological and psychological) that eventually leads to maladaptive changes and system failure.
2. Alteration of Psychological Processes in Chronic Pain
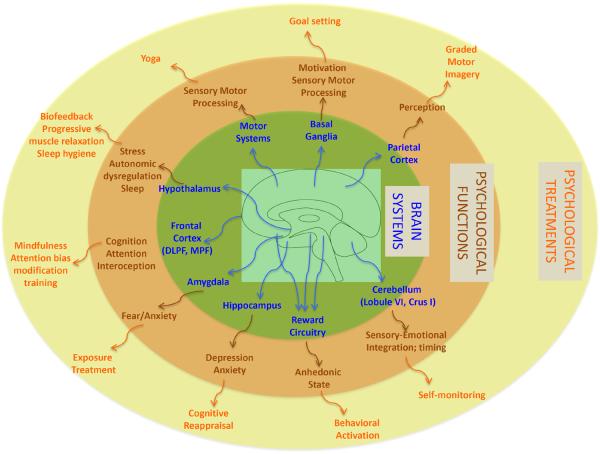
In the following sections we describe how pain itself drives changes in psychological processes. While segregation of some of these processes is somewhat artificial since they are integrative or may overlap, the understanding of brain network involvement in these processes affords a model of how individual processes may contribute to altered behaviors in chronic pain. We also use this approach to explore how integrated system-focused treatments for chronic pain should be considered as depicted in Figure 5.
Figure 5. From Brain Systems to Psychotherapeutic Targets.
A network of brain systems underlies alterations in psychological function in the chronic pain state. This figure shows specific psychological treatments that target alterations in psychological function across brain systems.
2.1. Cognition and Attention
Cognition is defined as the act of knowing (http://www.merriam-webster.com) and includes awareness, perception, reasoning, decision-making (van der Meer et al., 2012), and judgment. These processes are altered in chronic pain (for reviews in this area see (Hart et al., 2003; Moriarty et al., 2011; Wiech et al., 2008). Overall, the evidence supports the notion that pain has a negative effect on cognitive performance and attentional processes (Eccleston and Crombez, 1999) and that it may affect brain regions involved in cognition. For example, activation of the anterior cingulate cortex (ACC) and its role in attention can be altered by hypnotic suggestion in healthy subjects (Rainville et al., 1997). Cognitive changes have been reported in chronic pancreatitis (Jongsma et al., 2011), complex regional pain syndrome (CRPS) (Kolb et al., 2012), and fibromyalgia (Glass, 2008) amongst others (Hart et al., 2003). In another study comparing healthy participants to patients with osteoarthritis using a computerized sustained attention task (Continuous Performance Task [CPT]) and painful stimulus, investigators observed increased ACC activity when the painful stimuli was applied during the CPT task across all subjects, but activation patterns in the ACC were different between patients and healthy participants (Buffington et al., 2005). In acute pain models, cognitive processing is not significantly disturbed (Seminowicz and Davis, 2007; Van Essen et al., 2012)). In ongoing pain, alterations in cognition may relate to alteration in the dorsolateral prefrontal cortex (DLPFC) (Apkarian et al., 2004b), the ventrolateral prefrontal cortex (VLPC) (Jensen et al., 2012), and reduced connectivity from the medial prefrontal cortex (mPFC) to the default mode network (Loggia et al., 2013). It is as if ongoing pain drives alterations in these regions to alter synaptic connectivity or function to diminish cognitive abilities resulting in altered perceptive and interoceptive states and inhibition of top-down cognitive control of emotional states (as described further in section 2.4.1.).
2.2. Altered Perception
Perhaps the best examples of the importance of bodily interpretation and chronic pain relate to the perception of missing limbs (Flor et al., 2013) where treatments such as mirror training and sensory stimulation provide visual or proprioceptive inputs to the brain. These seem highly salient as correction of these perceptual abnormalities can provide pain relief (Mercier and Sirigu, 2009; Moseley and Flor, 2012). More subtle forms of altered perception are seen in other chronic pain syndromes and include specific processes such as hemineglect.
Hemineglect has been defined as “a deficit in processing or responding to sensory stimuli in the contralateral hemispace, a part of the own body, the part of an imagined scene, or may include the failure to act with the contralesional limbs despite intact motor functions” (Kerkhoff, 2001). In chronic pain altered perception including hemiinattention-hemineglect are present. This was first reported in CRPS patients by Galer and colleagues wherein a series of 11 selected patients uniformly described an inability to move their affected limb without significant focus and effort and a sense of disconnection from their affected body part (Galer et al., 1995; Galer and Jensen, 1999). Now observed in others including those with chronic low back pain (Moseley et al., 2012), these patients have altered perception of the external environment and also, more difficult to define, alterations in their perception of their internal physiological environment. The latter is captured in the writings of Craig on `interoception' (Craig, 2002). Thus, these patients have diminished awareness of their body schema and surroundings (Kolb et al., 2012) that have been termed neglect-like symptoms. Other studies have also shown impaired evaluation of hand size in CRPS (Forderreuther et al., 2004; Peltz et al., 2011). More recent studies have not only confirmed neglect symptoms in CRPS patients, but observed this same phenomena in other chronic pain patients, although to a lesser degree (Frettloh et al., 2006; Kolb et al., 2012). Such disturbances, it has been argued, contribute to chronic pain (Lewis et al., 2007). Such alterations may have significant implications for treatment, endorsing the benefits of treatment plans that include physical therapy or the direct targeting of affected cortical areas. The main focus, based on our understanding from the neurological literature on hemi-inattention and neglect (Rizzo, 1993; Vallar, 1998), would be the parietal cortex that is clearly involved in functional changes in CRPS as defined in neuroimaging studies (Cohen et al., 2012; Lebel et al., 2008). The parietal lobe is involved in perception of both real and illusionary experimental pain (Seckel et al., 2012; Zaretskaya et al., 2013). Neglect like symptoms are more frequently observed in right parietal changes (i.e., in the case of pain patients, affecting the left painful limb). Given our understanding of cerebral dominance, such processes feed into the overall notion of right sided processing in affective changes vs. left. Alternatively, hyperfunctional changes in the intact left hemisphere may be a driver of neglect like symptoms in CRPS/chronic pain (Koch et al., 2008). Neglect symptomology, seen predominantly after lesions of the right hemisphere, may be the result of an imbalance of lateral orienting processes that have a bias towards the ipsilesional side and a deficit in orienting attention (Gainotti et al., 1989; Karnath, 1988; Kucyi et al., 2012).
Altered perception has been relatively under-evaluated in the clinic, but when one considers the existence of these changes in other significant disease states, including schizophrenia (Cavezian et al., 2011) and Parkinson's disease (Ebersbach et al., 1996), the process seems fundamental to altered neurological function in diseases that affect the brain. Furthermore, the deficits in visospatial processing can be modulated by other intersecting processes. For example, emotionally negative stimuli can overcome visiospatial neglect through activation of frontal and limbic networks (Dominguez-Borras et al., 2012; Grabowska et al., 2011) suggesting that emotionally laden information may overcome the deficit. Clearly, the neural pathways are complex and in a recent meta-analysis, multiple regions of the right hemisphere (viz., middle and superior temporal gyrus, inferior parietal lobule, intra-parietal sulcus, precuneus, middle occipital gyrus, caudate nucleus, and posterior insula) were found to be involved in spatial neglect (Molenberghs et al., 2012). It is unknown if sensory perceptual alterations in chronic pain can also manifest as psychological neglect (e.g., individuals are unaware of their depression). In hemi-neglect patients, with parietal lobe damage, emotional interpretation of sensory stimuli are reported to be intact (Dominguez-Borras et al., 2012). While these alterations of the perceptual circuitry may not be explicitly defined, evidence furthers the notion that psychological alterations such as attention and emotion are intimately connected.
Treatment approaches to address perceptual alterations can include sensory stimulation (Marshall, 2009), specific visio-spatial training (Piccardi et al., 2006; Thimm et al., 2009), graded motor imagery (Moseley, 2006), and enhancing hypokinesia of the affected limb (Sapir et al., 2007). A fMRI study examined differential brain activation patterns after optokinetic stimulation training compared to alertness training; greater bilateral activation of the precuneus was observed after optokinetic training, whereas there was more activation bilaterally in the frontal cortex after alertness training. The data suggest that these two trainings may have differential effects on attentional intensity and spatial attention (Thimm et al., 2009). These treatment modalities align closely with physical therapy and psychological treatment.
2.3. Interoception
An individual's sense of the physiological condition of the physical body is directly associated with emotional well-being and stress levels (Craig, 2002). The anterior insula appears to play a significant role in this evaluative process (Craig, 2009). In the context of pain, this encompasses heightened awareness of or attentional bias toward general (e.g., increased heart rate) or threat-related (e.g., muscle tension) physiological cues. Interoceptive sensitivity can not only heighten acute pain sensitivity and decrease pain tolerance (Pollatos et al., 2012), it is theorized to be one of several mechanisms of fear learning for chronic pain patients (De Peuter et al., 2011). A related interoceptive bias is anxiety sensitivity (AS) where an individual interprets interoceptive information as potentially aversive or dangerous. AS has been found to have large effects on fear of pain and small to moderate effects on pain and disability (Ocanez et al., 2010). In the context of treatment, interoceptive sensitivity and AS can be manipulated to help patients improve their responses to the pain experience. In a randomized clinical trial for patients with irritable bowel syndrome, cognitive behavioral therapy with interoceptive exposure to visceral sensations was found to be superior to control conditions (Craske et al., 2011). This attentional bias can also be manipulated through mindfulness-based techniques where patients are taught to “pay attention in a particular way; on purpose, in the present moment, nonjudgmentally” (Kabat-Zinn, 2009). The benefits of this approach with chronic pain patients date back almost 30 years (Kabat-Zinn et al., 1985) and are now grounded in functional imaging. Meditation induced changes in pain have been associated with activity in the anterior insula and anterior cingulate cortex (Lutz et al., 2013; Zeidan et al., 2011), alterations which seem to be modulated top-down by cortical alpha rhythms (Kerr et al., 2013).
2.4. Reward, Aversion and Motivation
Rewarding and aversive stimuli have profound and dynamic abilities to modify behavior. In animals, a rewarding stimulus is defined by its ability to reinforce behaviors that produce the stimulus. In humans, we define the conscious “reward state” as an affect bias towards positive emotions and motivation and “aversive state” or “anti-reward” as the opposite. The ability to derive pleasure from ordinary activities is important, as is aversion to potentially damaging stimuli and environments (Cabanac, 1971). The dopaminergic and opioidergic reward pathways of the brain are critical for survival, as they provide the pleasure drives, for example, the drives for food or sex (Gardner, 2011; Wise, 1989). These 'natural rewards' involve the release of dopamine in the nucleus accumbens and frontal lobes. In a general theory of reward and aversion processing, neural systems obtain information from the external (e.g., auditory, visual, or touch or pain) and internal (e.g., thirst, hunger, thermoregulation) environments to derive characteristics of the stimulus (Schultz, 2004; Wise, 2004). Integration of this information, including the specific weighting of properties (e.g. rate, intensity, delay, amount, proximity), allows the assignment of emotional salience (degree of motivational drive (Borsook et al., 2013)) and valence (rewarding or aversive) to the stimulus. Both conscious and unconscious processes are involved in the evaluation of the internal and external environments (Wise, 2002), including prediction of deficit states (e.g. lack of food or water), planning to obtain goal objects, and decision-making. Prior experience is a major determinant of the motivational impact of any given stimulus or trigger. In a normal state, the organism utilizes this information in a manner that optimizes biological fitness over time.
Emerging research suggests that even among healthy individuals, there is variability in reward circuitry activity that can directly predict opioid analgesia. Using fMRI, trait reward responsiveness and the response of brain reward circuitry (viz., orbitofrontal cortex, nucleus accumbens, and the ventral tegmental area) to predict analgesic effects of the μ-opioid remifentanil was evaluated (Wanigasekera et al., 2012). Significantly, the magnitude of opioid-induced behavioral analgesia (as measured by change in pain intensity) was positively correlated with trait reward responsiveness and was predicted by reward circuitry neuronal responses as measured by fMRI prior to the remifentanil infusion (Wanigasekera et al., 2012). A potential baseline reward circuitry deficit would only compound the impact of an ongoing aversive pain state producing alterations in mood, social interactions, cognition and potentially leading to a persistent hedonic deficit state (Elman et al., 2011). Individuals in chronic pain may seek pain relief (Leknes et al., 2013; Navratilova et al., 2012) and if this is not obtained, deterioration of adaptive systems (allostatic failure) may occur.
2.4.1. Depression and Anhedonia
In chronic pain the process of a `reward deficit syndrome' or anti-reward processes, may relate to ongoing circuit dysfunction. Increasing evidence suggests that plasticity of neural circuits are responsible for subtle changes over time that contribute to the behavioral manifestation of altered affective processes including blunting of reward or enhancing depression (Becker et al., 2012; Elman et al., 2011). Unquestionably, affective changes may evolve from normal reactive depression that relates to stress (Hammen, 2005) to more clinically related comorbid depression associated with chronic pain (Bair et al., 2003; DeVeaugh-Geiss et al., 2010). From an evolutionary point of view the down-regulation of affect is adaptive (Gilbert, 2006). While this may promote functioning in daily life, under circumstances of chronic pain altered affect is a common problem, and may be viewed as a loss of control over the aversive nature of the underlying pain problem. As such the loss of control unfolds into a failure to adapt to social and other environmental processes. Individuals may be preordained to be more sensitive to developing depression based on genetic (Lohoff, 2010), epigenetic (Sun et al., 2013), metabolic (Kim et al., 2012), or other less defined mechanistic processes. It is unclear what factors contribute to kindling (increased vulnerability after repeated exposure to stress) or confer direct diathesis (Willner et al., 2012) as depression evolves as a comorbid process in chronic pain. The vulnerability to depression in chronic pain is not well defined, but processes such as chronic stress contribute to the allostatic load of the disease (Borsook et al., 2012; McEwen, 2004). Whatever the etiology, the classification of the depressive state clearly has implications for treatment (Krishnan et al., 1985). The psychological tenant of reward deficiency syndrome present in chronic pain has been evaluated as anhedonia (the inability to feel pleasure) and is a core feature of depression. The evolution of sadness or mild mood alterations in chronic pain to meeting clinical criteria for major depressive disorder can be indistinct (Table 1). Some assert that the cognitive focus of negative thoughts can potentially distinguish `normal' depressed individuals marked with self-hatred and loathing from patients with pain and depressed mood whose negative thoughts center on their pain (Pincus and Morley, 2001). Regardless, preventing the progression from symptoms to full disorder is clearly an issue. Brain regions considered to be involved in depressive symptomatology involve an extensive network including the medial prefrontal cortex, limbic, striatal, thalamic, and basal forebrain structures (Price and Drevets, 2012). In a study where negative mood was induced in healthy volunteers, increased activity in prefrontal areas, ACC, and hippocampus, as well as significantly less deactivation in response to noxious thermal stimuli when compared to pain responses in a neutral mood (Berna et al., 2010). Subjects who reported greater increases in pain unpleasantness in this study showed greater inferior frontal gyrus and amygdala activation. Such findings underscore the compounding effect of the co-occurrence of depressed mood and a persistent pain state, suggesting that these systems may rapidly adapt, with persistent co-activation leading to altered circuits and behavior (emotional regulation).
Table 1.
Diagnostic criteria for Major Depression and Generalized Anxiety and the altered psychological state in chronic pain
| Process | Psychiatric Condition | Altered Psychological State in Pain |
|---|---|---|
| Depression | Major Depressive Disorder* | |
| 1. Depressed mood or irritable. | Depressed mood (Ohayon and Schatzberg, 2010; Tang et al., 2008) | |
| Anger (Bruehl et al., 2009) | ||
| Catastrophizing (Wood et al., 2012) | ||
| 2. Decreased interest or pleasure in activities | Anhedonia (Nicholas et al., 2009) | |
| 3. Weight or appetite change | Weight gain/obesity (Ursini et al., 2011) | |
| 4. Insomnia or hypersomnia | Sleep disturbance (Palermo et al., 2012; Palermo et al., 2011; Tang et al., 2012a) | |
| 5. Psychomotor agitation or retardation | Motor control (Gerdle et al., 2010; Tang et al., 2012a) | |
| 6. Fatigue or loss of energy | Fatigue (Dansie et al., 2012) | |
| 7. Feelings of worthlessness or excessive guilt | Self-perceived burden (Kanzler et al., 2012; Kowal et al., 2012) | |
| 8. Difficulty concentrating | Cognitive impairment (Reyes Del Paso et al., 2012) | |
| Decision-making deficits (Apkarian et al., 2004a) | ||
| 9. Thoughts of death or suicide | Suicidal ideation (Fishbain et al., 2012; Ilgen et al., 2008; van Tilburg et al., 2011) | |
|
| ||
| Anxiety/Fear | Generalized Anxiety Disorder* | |
| “Excessive anxiety and worry” that is difficult to control | Anxiety (Lucchetti et al., 2013; Simons et al., 2012b) | |
| Catastrophizing (Sullivan et al., 2001) | ||
| Pain-related anxiety/fear (Vlaeyen and Linton, 2012) | ||
| 1. Feeling wound-up, tense, or restless | Anxiety sensitivity (Ocanez et al., 2010; Payne et al., 2013) | |
| 2. Easily becoming fatigued or worn-out | #6 above | |
| 3. Concentration problems | #8 above | |
| 4. Irritability | #1 above | |
| 5. Significant tension in muscles | Muscle tension (Klinger et al., 2010) | |
| 6. Difficulty with sleep | #4 above | |
To meet DSM-IV-TR diagnostic criteria must have 5 of 9 symptoms for MDD and 3 of 6 symptoms (1 of 6 for children) for GAD.
Psychological treatment of depressive-like symptoms are likely most effective when mapped appropriately to the underlying mechanisms driving the dysfunction, such as interpersonal psychotherapy for individuals for whom relational strain or social isolation are prominent features (Klerman, 1984). As humans regularly use thoughts to generate more adaptive emotional and social reactions (Gross, 1998), cognitive regulation strategies, such as reappraisal and selective attention are typically taught within cognitive-behavioral therapy for depressive symptoms (Beck and Beck, 2011) and have a critical function in emotional responses (Fontaine et al., 2007). Reappraisal of negative scenes has been associated with increased activation of both dlPFC and vlPFC regions along with dorsal anterior cingulate, and decreased activation of a region of the orbitofrontal cortex and the amygdala (Ochsner et al., 2002) with other studies echoing these results (Ochsner and Gross, 2008). Additional findings implicate increased activity in the vmPFC (Delgado et al., 2008), underscoring the importance of reinitiating top-down cognitive control of emotion centers in the brain. For some, attempts to challenge their dysfunctional thought patterns can prove difficult, thus behavioral activation (BA), which aims to increase engagement in activities often associated with the experience of pleasure or mastery can be a more effective. BA predicates on the assertion that depressive symptoms persist through low rates of response-contingent positive reinforcement/pleasant activities (Dimidjian et al., 2011). Meta-analyses examining BA support this treatment approach among depressed patients (Cuijpers et al., 2008) and its application among individuals suffering with chronic pain fits well with the overall goals of pain rehabilitation to return to important activities of daily living.
2.5. Learning and Memory
Pain is an emotionally salient stressor that can trigger memory consolidation and learning. Both explicit and implicit learning processes have been implicated in the maintenance of chronic pain, with implicit processes more pronounced, and due to their unconscious nature, more difficult to extinguish (Flor, 2012). Prominent implicit processes include operant conditioning, social learning, and classical conditioning. Under operant contingencies, pain behavior (such as limping) can be directly maintained by escape from actual/anticipated noxious stimulation (negative reinforcement (Iwata, 1987)) or increased attention in the `sick role' (positive reinforcement). For example, social learning or modeling has been observed in pediatric chronic pain with an increased incidence of pain problems among children of parents who suffer with pain problems (Hoftun et al., 2013). Lastly, classical conditioning underlies the acquisition of fear learning where a previously neutral stimulus (a medical exam) when initially paired with pain later elicits a conditioned response (increased heart rate when the physician approaches). Fear learning in the context of pain typically develops after few repetitions, generalizes quickly, and can be maintained simply through anticipation of increased pain transitioning to an operant process of reinforcement (Vlaeyen and Linton, 2012). In chronic pain, prediction error may be a key way to monitor stress and aversive situations. Heightened in pain sensitization (see (Ploghaus et al., 2000)), prediction error leads to impaired learning as well as the inability to attend to more constructive stimuli (Schultz and Dickinson, 2000). Under healthy conditions, coding of prediction errors may represent a basic mode of brain function in adaptive behaviors including normal learning.
2.5.1. Fear learning and pain-related fear
Broadly, fear is a normative response to a real or imagined threat with the primary function of promoting survival (e.g., (Gullone, 2000)). From separation anxiety manifested during infancy to fear of bodily injury emerging in school-age children and continuing into adulthood, we humans are hardwired to experience fear. These fears are protective, and in most cases, adaptive. Pain (an unconditioned, universally noxious stimulus; US) triggers our fear response and alerts our flight-or-fight system to act (an unconditioned response; UR). This is where pain and fear can collide. After exposure to painful stimuli or an injury, a previously neutral experience, such as movement or even anticipation of movement (a conditioned stimulus; CS) can elicit fear (a conditioned response; CR) even in the absence of pain. Although this can be adaptive in the short-term to promote healing, individuals who continue to perceive movement as threatening after the painful stimuli is no longer present or the initial injury has healed (conditioned fear) can experience a number of psychological and physical sequelae, including hypervigilance, muscular reactivity, escape/avoidance, and guarding behaviors that maintain or exacerbate pain and promote pain-related disability (Verbunt et al., 2003). The brain circuitry of fear learning has been extensively studied in animals (Johansen et al., 2010) and humans (Schiller and Delgado, 2010), although not explicitly among patients with chronic pain. Regions most prominently implicated are the amygdala, hippocampus, and prefrontal cortex (dlPFC and mPFC), with the amygdala serving as the primary hub of action. The amygdala circuitry provides an ideal example of pathways that may inform new approaches and metrics related to treatment. The CS and US learning pathways converge at the lateral nucleus of the amygdala (LA), where the LA receives afferent sensory input. The LA then connects with the central nucleus (CE) of the amygdala that controls the expression of the conditioned fear responses (CR), involving behavioral (e.g., freezing), autonomic nervous system (e.g., heart rate) and endocrine (pituitary-adrenal hormones) responses. Projections from the hippocampus to the basal nucleus (B) of the amygdala process contextual information during conditioning, and may gate fear expression through the CE (Hartley and Phelps, 2010). Results from fMRI studies of fear conditioning in healthy individuals show increased activity in the amygdala (Buchel et al., 1998; LaBar et al., 1998) with the magnitude of this response predictive of the strength of the conditioned response (LaBar et al., 1998; Phelps et al., 2004). Additionally, cognitive interpretation of a previously neutral stimulus through verbal instruction (LaBar et al., 1998; Phelps et al., 2004) or reappraising the meaning of an ambiguous scene so that it is more fearful (Ochsner et al., 2004) enhances amygdala activation. As many fears associated with pain are imagined and anticipated, but never actually experienced, these results support the neural pathways of these processes.
Reversing the impact of fear learning is complex and difficult. Extinction, or learning of an inhibitory response, of acquired fear is resistant to automatic generalization, requiring massed rehearsal in a variety of contexts during stressful and non-stressful circumstances to prevent renewal (Orsini and Maren, 2012; Quirk and Mueller, 2008). Graded in-vivo exposure, a CBT developed by Vlaeyen and colleagues (Vlaeyen et al., 2002a; Vlaeyen et al., 2002b) effectively targets fear of pain and disability through exposing patients to activities previously avoided due to fear of pain or re-injury (de Jong et al., 2005). When patients experience how disengagement from safety behaviors does not lead to catastrophic consequences, their misinterpretations are challenged and disconfirmed and they correct their fear expectancies leading to fear extinction and cognitive regulation (Goubert et al., 2005; Leeuw et al., 2007; Smeets et al., 2006). Acceptance-based approaches also encourage exposure to a broader repertoire of avoided internal and external stimuli and are grounded in solid empirical evidence for their effectiveness among children (Wicksell et al., 2009) and adults (Vowles et al., 2011; Wicksell et al., 2013). FMRI results demonstrate increased top-down cognitive control (i.e., enhanced pain-evoked prefrontal cortex activation) after acceptance-based treatment (Jensen et al., 2012). From pre-clinical studies, we know that during extinction learning, inhibitory connections between the ventromedial prefrontal cortex (vmPFC) and the intercalcated (ITC) cell masses are established. During extinction recall, these connections inhibit fear expression through projections to the CE. Inhibitory connections between the vmPFC and the LA may also regulate fear expression through the CE. Contextual modulation of extinction expression is mediated by projections from the hippocampus to the vmPFC and/or LA. Imaging studies of extinction learning in healthy humans report a decrease in amygdala activation (Gottfried and Dolan, 2004; Knight et al., 2004; LaBar et al., 1998; Phelps et al., 2001) while BOLD signals in the vmPFC increase during extinction learning and extinction retrieval (Phelps et al., 2004). Preclinical data has provided further insights into the role fo the mPFC in these changes. For example, using a chronic neuropathic pain model, repeated administered D-cycloserine, a partial agonist of the NMDA receptor, can ehance learning and potentiate the extinction of acquired fear. Not only did D-cycloserine reduce mechanical sensitivity of the injured limb, the effect persisted after discontinuation of the drug (Millecamps et al., 2007). Specific infusions into the mPFC and amygdala acutely induced antinociception and infusions into the mPFC reversed place avoidance behavior induced by mechanical stimulation of the injured paw, together suggesting the potential efficacy of D-cycloserine in enhancing fear extinction and reducing neuropathic pain behavior (Millecamps et al., 2007). Persistent changes have also been observed in patients who have received a similar drug, ketamine that may reorder or restore neural circuits including the anterior cingulate (Becerra et al., 2009). Similarly, preliminary results suggest that the partial NMDAR glycine-site agonist, GLYX-13, may be a successful treatment agent for both tonic and neuropathic pain, but with an added antidepressant quality (Wood et al., 2008). Unlike ketamine, a non-competitive NMDAR antagonist, GLYX-13 does not seem to have the same sedative effect or abusive potential (Burgdorf et al., 2013). The increased positive emotional learning that occurs as result of mPFC infusions, suggests potential efficacy of GLYX-13 in deminishing neuropathic pain and it's associated pyschological consequences (Burgdorf et al., 2011). Overall, extinction learning in humans seems to depend on the integrated functioning of a neural circuit that is predominated by the amygdala and vmPFC. Such studies are insights to potential reordering of brain connectivity in a manner that may be therapeutic.
3. Premorbid Risk Factors and Pain
Some individuals seem more resistant to alterations in psychological function in the presence of persistent pain whereas others seems to swiftly transition into a downward spiral of dysfunction. Premorbid vulnerability factors likely contribute to some of the disparities in these outcomes. Here we briefly review some of the key risk factors that have emerged from the literature including prior physical and psychological trauma, social dysfunction, catastrophizing, social status, and gender.
3.1. Prior Physical and Psychological Trauma
While initially put forward in the late 1950's, the connection between psychological (e.g., emotional abuse, neglect) and physical (e.g., sexual) abuse in childhood has a strong connection with the subsequent development of chronic pain (Paras et al., 2009; Saariaho et al., 2011; Symes et al., 2013; Tietjen et al., 2010). Among types of abuse, sexual abuse amongst both men and women appears to confer the greatest diathesis or predisposition to chronic pain (Hart-Johnson and Green, 2012; Walling et al., 1994). It is unclear whether the heightened risk is derived in a similar fashion to that involving depression and pain where kindling of the underlying process of neural sensitization leads to the comorbidity (e.g., generalized pain in MDD patients who have not had pain previously).
3.2. Social Dysfunction
Recent studies support the notion that social pain (feelings associated with social disconnection) may have the same neurobiological underpinnings of physical pain (Eisenberger, 2012). Specifically, brain areas that are activated by social distress parallel those activated by acute experimental pain (Eisenberger et al., 2003; Onoda et al., 2009). The critical issue here in the clinical context is that social processes such as social exclusion (or bullying), isolation and lack of support may contribute to cross sensitization and affect physical pain i.e., one process may affect the other and vice versa. This is particularly applicable in the unfolding of a common process frequently encountered by chronic pain patients – rejection or social separation (Eisenberger and Lieberman, 2004; Macdonald and Leary, 2005). Although many of these studies have focused on regions such as the anterior cingulate cortex, the integration of social hurt may relate to how this region is involved in salience and how our salience network is disturbed in chronic pain. When socially rejected, psychological process of emotion and cognition may be adversely affected and pain exacerbated (Kashikar-Zuck et al., 2007) or behaviors such as intimacy and family relationships severely affected (Smith, 2003). Such changes may reinforce pain behaviors. This issue is also particularly important in children where early socializing, schooling, and interactions with family are so important.
3.3. Catastrophizing
Pain catastrophizing is a cognitive attributional style characterized by a negative mindset, magnification, and rumination about pain (Sullivan et al., 2001). For the patient, the worst is feared and the individual is left feeling helpless (Edwards et al., 2006). Catastrophizing is consistently and robustly associated with poor outcomes in adults and children post-surgically (Page et al., 2012; Sullivan et al., 2011) and across a broad spectrum of chronic pain conditions (Hirsh et al., 2010; Osborne et al., 2007; Turner et al., 2002). Catastrophizing is a cognitive vulnerability factor often found to precipitate depressive symptoms (Lee et al., 2008; Simons and Kaczynski, 2012). Furthermore, persistent pain catastrophizing may contribute to the ongoing pain process (intensity, disability, and other psychological changes) (Severeijns et al., 2001). While the importance of catastrophizing and the prediction of chronic pain are now established across many studies, a better understanding of its neurobiological correlates will provide insights into those components that may be more amenable to preventive treatments/psychological interventions (Campbell and Edwards, 2009). For example, a very recent study found increased dorsolateral prefrontal gray matter volume associated with reduced pain catastrophizing among patients who completed an 11-week CBT pain coping intervention (Seminowicz et al., 2013)
3.4. Social Status and Education Attainment
Across studies, low social standing usually has a negative effect on epidemiology of chronic pain (Gale et al., 2012; Gore et al., 2012; Rodarte et al., 2012; Zarei et al., 2012). Some issues that correlate with social status include obesity, lower level of education, and inequalities in the distribution of health care. Pre-clinical work has demonstrated decreased dopamine transporter binding and increased D2 reception binding in the caudate putamen and nucleus accumbens in subordinate rats (Lucas et al., 2004) and increased dopamine D2 receptor availability/amount among socially dominant macaque monkeys (Morgan et al., 2002). Perhaps dopaminergic deficits in individuals of low social standing are predisposing them to chronic pain (Jarcho et al., 2012).
3.5. Gender
Post-pubertal women generally have a higher prevalence of chronic pain compared with men (Bjornsdottir et al., 2013; Fillingim, 2000; Keogh and Eccleston, 2006). A number of physiological factors including hormones (Kuba and Quinones-Jenab, 2005) and menopause (Martinez-Jauand et al., 2013) contribute to this discrepancy. Specifically, reduced mu opioid receptor activation (Zubieta et al., 2002) and the anti-dopaminergic effect of estrogen (Euvrard et al., 1980) (McEwen, 2001) may play such a role. It may also relate to issues such as a woman being more inclined to report their pain, especially considered the self-report nature of many chronic pain measures. While numerous reports of gender differences have been published, sex differences in psychological factors in chronic pain are inconclusive. Heightened pain-related anxiety has been identified in men with chronic back pain (Robinson et al., 2005), while recent work has identified pain-related anxiety as influential on pain intensity ratings in men and pain tolerance in women (Thibodeau et al., 2013). Thus the relationship between gender and pain is complex (Fillingim, 2013; Racine et al., 2012). This is further underscored by observations of heightened pain sensitivity and thalamocortical activity in women compared to men that is eliminated after controlling for trait anxiety (Goffaux et al., 2011). Comorbidities may contribute to a higher female prevalence including disorders of mood as estrogen has been implicated in depression (Fernandez-Guasti et al., 2012; Ryan and Ancelin, 2012). Recent data from imaging has shown differences in brain systems in response to chronic pain (Maleki et al., 2012) and this type of measure together with further epidemiological data may provide further insight into sex differences. What is clear however, is that more women present to pain clinics, particularly with certain pain disorders (migraine, fibromyalgia) and so at a practical level, we need to evaluate and understand psychological perturbations across men and women.
4. Integrating Dysfunction: Complex Behaviors with Reciprocal, or Multiple and Multiplying Effects
Together, the chronic pain state can trigger changes in psychological or mental functions that manifest as alterations in perception, attention, mood, motivation, learning and memory. The changes are ongoing, vary in their magnitude, and their hierarchical manifestations, and may be temporally and sequentially altered by treatments, and all contribute to an overall pain phenotype with some components of behavior having a stronger phenotypic influence. One may consider intact, normal behavior as that similar to an orchestra playing in harmony. In chronic pain, elements of the orchestra begin to fail, are missing or are playing out of tune. Obviously the nature of the quality of individual players is important, akin to the modifying effects of an individual's prior history – emotional, social, biological, education/intellectual, and other components that form the composite of who we are and how we behave in response to chronic pain. These processes are integrated in what has now been termed the “The Connectome” – representing who we are intellectually, behaviorally and emotionally (D'Angelo, 2012; Van Essen et al., 2012).
As previously mentioned in Figure 4, the response to chronic pain may be akin to a bouncing ball, in that if left alone (no treatments) the system fails from energetic and resilient (healthy) to a non-energetic and dysfunctional status (clinical failure). In some respects this is akin to the cardiac failure model that continues to spiral out of control towards an untreatable or difficult to treat syndrome (i.e., allostatic load). Taking this view, early definition and understanding of those neural systems that are most salient in each chronic pain patient needs to be identified and targeted. One of the missing keys to this is a lack of understanding of interactive processes that may be positive or negative. An example of this is complexities surrounding the use of opioids in chronic pain. In many cases they have short-term beneficial effects, but can have poor long-term effects on brain systems (Upadhyay et al., 2010).
4.1. When the Butterfly Flaps its Wings
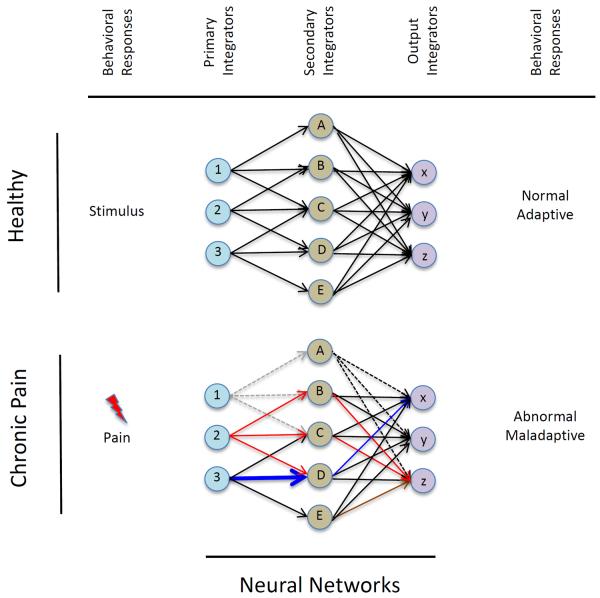
While speculative, local and distant changes in brain systems may be considered in the concept of ”When a butterfly flaps its wings”. These changes may be dependent on the current conditions, in this case brain networks that define these conditions. Thus a small change may produce significant changes in a non-linear process, particularly in pain where cross-sensitization may occur and a psychological effector (e.g., fear) enhances the pain experience. The effect is also determined by prior psychological and other factors (genetic, pain condition, age, gender etc.) that define baseline conditions that may contribute to this effect. These processes may result in relative resistance or resilience to a potential multiplying effect. Given the multiple psychological processes that may be altered in chronic pain, these interactions further contribute to the `butterfly effect' in complex yet ill-defined ways. Another way of looking at this is how these processes may contribute to a self-healing effect – the so-called Gaia effect that relates to how organisms interact to have self-regulating system that parallel allostasis in physiological systems – adapting to a stressor. Even In practical terms, the interaction of a patient's perceived limb pain and gaining motor function may alter other brain networks through these effects (see Figure 6).
Figure 6. Altered Networks.
The figure shows a model of brain network changes as a consequence of a stimulus in a healthy subject (top) and the same networks in response to pain (bottom). Using tissue damage as an example of a pain stimulus (viz., surgery), there are alterations in sensory inputs (Primary Integrators) that produce changes in Secondary Integrators (these may initially be adaptive e.g., enhanced modulation of pain by higher cortical centers such as the anterior cingulate cortex (See Figure 1), but eventually become maladaptive through individual networks or across networks resulting in altered behavioral responses.
4.2. Cross-Sensitization
Cross sensitization refers to how one process may exacerbate or enhance another. Common examples include drugs of abuse and repeated stress increasing glutamate receptors in the ventral tegmental area that alter dopamine function (Fitzgerald et al., 1996). Thus there are common, long term effects on function that occur in chronic pain. Pain-, stress-, and analgesic drug-induced opponent and proponent states of the mesolimbic dopaminergic pathways may render, in a similar manner altered reward/motivation dysfunction that is now vulnerable to sensitization, cross-sensitization and aberrant learning. The affects are further amplified through the long-term neuroplasticity process of sensitization at the cortical and subcortical limbic structures (Rome and Rome, 2000). This is an autonomous, self-sustaining feed-forward loop generated by a variety of intermittent sensory (e.g., pain), emotional (e.g., altered mood, fear) and behavioral (e.g., social isolation) stimuli and characterized by conditionability, interchangeability as well as progressive intensification of the duration and magnitude of the responses (Pierce and Kalivas, 1997). Cross sensitization has significant implications in illness progression and treatment resistance that are independent of genetic or environmental (including epigenetic changes (e.g., (Hoffmann and Spengler, 2012)) vulnerability and increase the affliction level (Post, 2010; Post et al., 2012).
4.3. Allostatic Failure: Unsuccessful Perseverative Behavior
The concept of allostatic load in disease, initially promulgated by authors such as Bruce McEwen (recently reviewed in: (Karatsoreos and McEwen, 2011; McEwen and Gianaros, 2010) is increasingly being applied to disease states including chronic pain (Borsook et al., 2012). The notion of psychobiological allostasis has received attention (Karatsoreos and McEwen, 2011). Taken together, the homeostatic balance is altered in chronic pain and becomes unstable at a physiological (stress) and psychological level. When there is no adaptation or resilience to stressors, there are systemic failures that manifest as alteration in behaviors. Thus the comorbidity of a primary inciting process (i.e., pain) and the multitude of psychobiological processes that may subsequently ensue, contribute to alterations in brain plasticity that while part of resistance and allostatic load, may eventually lead to allostatic failure. Such failure can be measured in a number of ways including progression from altered mood states to defined major depressive disorder (MDD).
4.4. Early Forme Fruste Markers of PsychoNeurological Changes
It is clear that alterations in neural networks occur with chronic pain. Many of these changes confer alterations in our psychobiological or psychoneurological behaviors. However, we need to have a better understanding of how we can define brain changes early, before these plastic changes, assumed to be adaptive become maladaptive. One example is when pain drives individuals to suicidal ideation and the act of suicide itself (Ritchie, 2012). The use of brain imaging has enormous potential in defining markers that may predate the evolution of behavioral changes or define relative states (e.g., state of resiliency to treatments; at risk states (Wood et al., 2013). Such information would have enormous implications for how we treat patients since early interventions have by and large proved to be better (since brain plasticity has not fully evolved into a resistant state) and innovative approaches for new therapies may become possible to enhance resiliency (Russo et al., 2012).
5. Treatment and Training Approaches
5.1. Integrating Psychological Science into Pain Neurobiology
It would seem that targeted research would contribute to a better understanding of psychological processes in chronic pain. Clearly, Neuroscience Psychology is advancing into clinical translation. However as in many fields some data sets such as functional imaging, while hugely appealing in terms of objective measures need reproducing to gain further acceptance. Clinical and research programs, and for that matter, drug trials, should further integrate psychological issues in pain to improve research strategies. Of course chronic diseases with multiple factors of primary and secondary (comorbid) etiologies are difficult studies to perform. As previously mentioned Figure 5 shows a correlation of psychological changes with brain function (regions, circuits and interactive circuits or networks) and treatments and Figure 7 summarizes the potential contributions of specific psychological processes to pain and behavioral responses to pain.
Figure 7. Treatment Paradigm – Neural Network-Directed Decreases in Allostatic Psychological Load with Readout Measures (from (Borsook and Kalso, 2013) with permission).
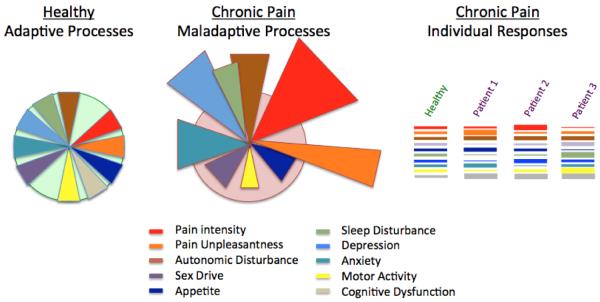
The figure shows containment and normal adaptive processing to various stressors (noted below in the key); these normal responses are balanced and adaptive (adapt to `homeostatic set-point') over time. In chronic pain responses may be exaggerated (out of `homeostatic set-point') or inhibited. In a multidimensional biological process such as chronic pain each of these stressors may affect an individual differently as represented in the `bar-code' noted on the right.
One approach to evaluating and planning for readapting the chronic brain using psychological and other approaches is `Stripping Allostatic Load, One piece at a time'. Table 2 links altered psychological processes to specific evidence-based psychological treatments. Current behavioral therapy approaches (e.g., Cognitive Behavioral Therapy, Acceptance and Commitment Therapy) have some, but relatively weak effects in controlled clinical trials (Wetherell et al., 2011; Williams et al., 2012) although somewhat better among pediatric patients (Eccleston et al., 2012). Therapeutic response can be enhanced by targeting the most susceptible processes initially that could then have the reversal of the `Butterfly Effect'. Just as most people adapt to a stressful situation, unadapting from a chronic condition needs a focused mechanism based, data driven approach.
Table 2.
Psychological Processes and Treatment Approaches
| Process | Psychological Treatment | References |
|---|---|---|
| Physiological Systems | ||
| Autonomic dysregulation | Progressive muscle relaxation | (Emery et al., 2008) |
| Stress | Biofeedback | (Nestoriuc and Martin, 2007) |
| Sleep | Sleep Hygiene | (Tang et al., 2012b) |
| Sensory-Emotional Integration Timing | Self-monitoring; self-regulation | (Sauer et al., 2010) |
| Sensory motor processing | Yoga | (Ward et al., 2013) (Cramer et al., 2012) |
| Psychological Systems | ||
| Cognition | Mindfulness and acceptance | (Veehof et al., 2011) |
| Interoception | Attention modification | (Carleton et al., 2011; Sharpe et al., 2012) |
| Anxiety Sensitivity | ||
| Perception | Graded motor imagery | (Bowering et al., 2013; Walz et al., 2013) |
| Pain-related fear | Exposure | (Bailey et al., 2010) |
| Anxiety | Cognitive reappraisal | (Ochsner et al., 2006) |
| Motivation | Goal setting | (Sauer et al., 2010) |
| Reward and Aversion | Values based action | (Vowles et al., 2011) |
| Motivational enhancement therapy | (Vong et al., 2011) | |
| Anhedonic state Depression | Behavioral activation | (Dimidjian et al., 2011) |
| Catastrophizing | Cognitive reappraisal | (Lawrence et al., 2011; Thorn et al., 2007) |
5.2. Training – Sub-Specialty in `Pain Neuroscience Psychology'
While one could argue that most of clinical psychology may relate to `pain' or hurt, few clinical training programs have a focus on the neurobiology of pain. Given the tremendous advances in understanding the brain and the impact that this has and will have on our clinical practice, psychology is well positioned to take advantage of these in applying new developments in treating brain-related changes in chronic pain. It is here, with the huge advantages of a basic understanding of the brain and a sound basis in neurobehavioral processes that can be specifically targeted in patients with chronic pain. Thus, psychology could be a major contributor to advancing our understanding and treatment of chronic pain.
Conclusions
Although pain epitomizes the indivisible nature of physical and emotional suffering, it is rarely addressed from the psychological standpoint. This, however, is a timely endeavor because modern pain neuroscience research consistently implicates the key brain limbic and cortical structures respectively engaged in the generation and control of drives, impulses and affects experienced among chronic pain patients. Cyclical processes whereby pain-induced deterioration in cognitive, emotional, behavioral, perceptive and interoceptive functions worsens pain problems that in turn cause a transition from negative affective states and maladaptive behaviors to bona fide mental health disorders. While acute pain activates mesolimbic reward-related pathways and engenders sudden alterations in psychological states (e.g., fear, stress and avoidance), chronic pain is associated with progressive changes and relentless decline in psychological wellbeing paralleled by reward deficiency (i.e., diminished dopaminergic effects), pain sensitization and cross-sensitization (e.g., depression, anxiety, addiction, etc.) along with anti-reward (i.e., stress) allostatic neuroadaptations. Then again, negative affective states substantially worsen pain conditions further deteriorating psychological outcomes.
The notion of integrative psychological processes is not new (see Ryan, 1995). While the cognitive neurosciences have contributed enormous insights into brain function (e.g., consciousness (Feinberg, 2011; Goldfine and Schiff, 2011), chronic stress (Gourley et al., 2013; Hill et al., 2012), and resilience (Wu et al., 2013); while psychiatric research has furthered our understanding of disease states such as pathological anxiety and depression (Willner et al., 2012), the implementation of an integrative neural systems approach of psychological tenets into treatments for chronic pain are relatively limited. In some ways this is surprising. More data from combination treatments that involve drugs, behaviors, physical therapy and the like are making a bold statement: we need to view the brain as an orchestra and bring to it components that harmonize and enhance.
Highlights
-
! !
The chronic pain state can trigger a cascade of changes in psychological processes.
-
! !
Changes occur in perception, attention, mood, motivation, learning and memory.
-
! !
We describe processes in the context of localized and integrated neural networks.
-
! !
We link altered behavioral processes to evidence-based psychological treatments.
-
! !
Pain neuroscience psychology can enhance our treatment of chronic pain.
Acknowledgements
Supported by a K23 career development award (HD067202) to LS, a K24 Mentoring Grant (NINDS NS064050) to DB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. European journal of pain. 2011 doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004b;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KM, Carleton RN, Vlaeyen JW, Asmundson GJ. Treatments addressing pain-related fear and anxiety in patients with chronic musculoskeletal pain: a preliminary review. Cogn Behav Ther. 2010;39:46–63. doi: 10.1080/16506070902980711. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain medicine. 2009 doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- Beck J, Beck A. Cognitive Behavior Therapy. 2nd ed Guilford Press; New York, NY, New York, NY: 2011. [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci Lett. 2012;520:182–187. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Bendelow GA, Williams SJ. Transcending the dualisms: towards a sociology of pain. Society of Health & Illness. 2008;17:139–165. [Google Scholar]
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain. 2013;154:1181–1196. doi: 10.1016/j.pain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir S, Jonsson S, Valdimarsdottir U. Functional limitations and physical symptoms of individuals with chronic pain. Scand J Rheumatol. 2013;42:59–70. doi: 10.3109/03009742.2012.697916. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr., Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. European journal of pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and Analgesia: The Value of Salience Circuits. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Kalso E. Transforming pain medicine: Adapting to science and society. European journal of pain. 2013 doi: 10.1002/j.1532-2149.2013.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowering KJ, O'Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, Stanton TR. The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. The journal of pain : official journal of the American Pain Society. 2013;14:3–13. doi: 10.1016/j.jpain.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neuroscience and biobehavioral reviews. 2009;33:475–491. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113:172–184. doi: 10.1016/j.pain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, Panksepp J, Moskal JR. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews. Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res. 2009;153:97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN, Richter AA, Asmundson GJ. Attention modification in persons with fibromyalgia: a double blind, randomized clinical trial. Cogn Behav Ther. 2011;40:279–290. doi: 10.1080/16506073.2011.616218. [DOI] [PubMed] [Google Scholar]
- Cavezian C, Michel C, Rossetti Y, Danckert J, d'Amato T, Saoud M. Visuospatial processing in schizophrenia: does it share common mechanisms with pseudoneglect? Laterality. 2011;16:433–461. doi: 10.1080/13576501003762758. [DOI] [PubMed] [Google Scholar]
- Chalaye P, Goffaux P, Bourgault P, Lafrenaye S, Devroede G, Watier A, Marchand S. Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin J Pain. 2012;28:519–526. doi: 10.1097/AJP.0b013e31823ae69e. [DOI] [PubMed] [Google Scholar]
- Cohen H, McCabe C, Harris N, Hall J, Lewis J, Blake DR. Clinical evidence of parietal cortex dysfunction and correlation with extent of allodynia in CRPS type 1. European journal of pain. 2012 doi: 10.1002/j.1532-2149.2012.00213.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Haller H, Dobos G. A Systematic Review and Meta-analysis of Yoga for Low Back Pain. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e31825e1492. [DOI] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Labus J, Wu S, Frese M, Mayer EA, Naliboff BD. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behaviour research and therapy. 2011;49:413–421. doi: 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, van Oppen P, Andersson G. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. J Clin Psychiatry. 2008;69:1675–1685. doi: 10.4088/jcp.v69n1102. quiz 1839–1641. [DOI] [PubMed] [Google Scholar]
- D'Angelo E. Toward the connectomic era. Funct Neurol. 2012;27:77. [PMC free article] [PubMed] [Google Scholar]
- Dansie EJ, Furberg H, Afari N, Buchwald D, Edwards K, Goldberg J, Schur E, Sullivan PF. Conditions comorbid with chronic fatigue in a population-based sample. Psychosomatics. 2012;53:44–50. doi: 10.1016/j.psym.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116:264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Van Diest I, Vansteenwegen D, Van den Bergh O, Vlaeyen JW. Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. European journal of pain. 2011;15:889–894. doi: 10.1016/j.ejpain.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nature protocols. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVeaugh-Geiss AM, West SL, Miller WC, Sleath B, Gaynes BN, Kroenke K. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain medicine. 2010;11:732–741. doi: 10.1111/j.1526-4637.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Jr., Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- Dominguez-Borras J, Saj A, Armony JL, Vuilleumier P. Emotional processing and its impact on unilateral neglect and extinction. Neuropsychologia. 2012;50:1054–1071. doi: 10.1016/j.neuropsychologia.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Trottenberg T, Hattig H, Schelosky L, Schrag A, Poewe W. Directional bias of initial visual exploration. A symptom of neglect in Parkinson's disease. Brain. 1996;119(Pt 1):79–87. doi: 10.1093/brain/119.1.79. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Palermo TM, de CWAC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2012;12:CD003968. doi: 10.1002/14651858.CD003968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Bingham CO, 3rd, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55:325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Volkow ND. Pain and suicidality: Insights from reward and addiction neuroscience. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery CF, France CR, Harris J, Norman G, Vanarsdalen C. Effects of progressive muscle relaxation training on nociceptive flexion reflex threshold in healthy young adults: a randomized trial. Pain. 2008;138:375–379. doi: 10.1016/j.pain.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Euvrard C, Oberlander C, Boissier JR. Antidopaminergic effect of estrogens at the striatal level. J Pharmacol Exp Ther. 1980;214:179–185. [PubMed] [Google Scholar]
- Feinberg TE. The nested neural hierarchy and the self. Conscious Cogn. 2011;20:4–15. doi: 10.1016/j.concog.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44:607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- Fillingim RB. Complex associations among sex, anxiety and pain. Pain. 2013;154:332–333. doi: 10.1016/j.pain.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Bruns D, Meyer LJ, Lewis JE, Gao J, Disorbio JM. Exploration of the relationship between disability perception, preference for death over disability, and suicidality in patients with acute and chronic pain. Pain medicine. 2012;13:552–561. doi: 10.1111/j.1526-4637.2012.01358.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. New developments in the understanding and management of persistent pain. Curr Opin Psychiatry. 2012;25:109–113. doi: 10.1097/YCO.0b013e3283503510. [DOI] [PubMed] [Google Scholar]
- Flor H, Diers M, Andoh J. The neural basis of phantom limb pain. Trends Cogn Sci. 2013;17:307–308. doi: 10.1016/j.tics.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Fontaine JR, Scherer KR, Roesch EB, Ellsworth PC. The world of emotions is not two-dimensional. Psychol Sci. 2007;18:1050–1057. doi: 10.1111/j.1467-9280.2007.02024.x. [DOI] [PubMed] [Google Scholar]
- Forderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS) Pain. 2004;110:756–761. doi: 10.1016/j.pain.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184–189. doi: 10.1016/j.pain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Gainotti G, De Bonis C, Daniele A, Caltagirone C. Contralateral and ipsilateral tactile extinction in patients with right and left focal brain damage. Int J Neurosci. 1989;45:81–89. doi: 10.3109/00207458908986219. [DOI] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Cooper C, Batty GD. Intelligence in childhood and chronic widespread pain in middle age: the National Child Development Survey. Pain. 2012;153:2339–2344. doi: 10.1016/j.pain.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (Complex Regional Pain Syndrome-1) J Pain Symptom Manage. 1995;10:385–391. doi: 10.1016/0885-3924(95)00061-3. [DOI] [PubMed] [Google Scholar]
- Galer BS, Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. J Pain Symptom Manage. 1999;18:213–217. doi: 10.1016/s0885-3924(99)00076-7. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdle B, Gronlund C, Karlsson SJ, Holtermann A, Roeleveld K. Altered neuromuscular control mechanisms of the trapezius muscle in fibromyalgia. BMC Musculoskelet Disord. 2010;11:42. doi: 10.1186/1471-2474-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. Evolution and depression: issues and implications. Psychol Med. 2006;36:287–297. doi: 10.1017/S0033291705006112. [DOI] [PubMed] [Google Scholar]
- Glass JM. Fibromyalgia and cognition. J Clin Psychiatry. 2008;69(Suppl 2):20–24. [PubMed] [Google Scholar]
- Goffaux P, Michaud K, Gaudreau J, Chalaye P, Rainville P, Marchand S. Sex differences in perceived pain are affected by an anxious brain. Pain. 2011;152:2065–2073. doi: 10.1016/j.pain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disabil Rehabil. 1999;21:23–30. doi: 10.1080/096382899298061. [DOI] [PubMed] [Google Scholar]
- Goldfine AM, Schiff ND. Consciousness: its neurobiology and the major classes of impairment. Neurol Clin. 2011;29:723–737. doi: 10.1016/j.ncl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012;37:E668–677. doi: 10.1097/BRS.0b013e318241e5de. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Goubert L, Crombez G, Danneels L. The reluctance to generalize corrective experiences in chronic low back pain patients: a questionnaire study of dysfunctional cognitions. Behaviour Research and Therapy. 2005;43:1055–1067. doi: 10.1016/j.brat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska A, Marchewka A, Seniow J, Polanowska K, Jednorog K, Krolicki L, Kossut M, Czlonkowska A. Emotionally negative stimuli can overcome attentional deficits in patients with visuo-spatial hemineglect. Neuropsychologia. 2011;49:3327–3337. doi: 10.1016/j.neuropsychologia.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Gullone E. The development of normal fear: a century of research. Clin Psychol Rev. 2000;20:429–451. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hart RP, Wade JB, Martelli MF. Cognitive impairment in patients with chronic pain: the significance of stress. Curr Pain Headache Rep. 2003;7:116–126. doi: 10.1007/s11916-003-0021-5. [DOI] [PubMed] [Google Scholar]
- Hart-Johnson, Green CR. The impact of sexual or physical abuse history on pain-related outcomes among blacks and whites with chronic pain: gender influence. Pain medicine. 2012;13:229–242. doi: 10.1111/j.1526-4637.2011.01312.x. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neuroscience and biobehavioral reviews. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh AT, Kupper AE, Carter GT, Jensen MP. Psychosocial factors and adjustment to pain in individuals with postpolio syndrome. Am J Phys Med Rehabil. 2010;89:213–224. doi: 10.1097/PHM.0b013e3181c9f9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Spengler D. The lasting legacy of social stress on the epigenome of the hypothalamic-pituitary-adrenal axis. Epigenomics. 2012;4:431–444. doi: 10.2217/epi.12.34. [DOI] [PubMed] [Google Scholar]
- Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT Study. JAMA Pediatr. 2013;167:61–69. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Zivin K, McCammon RJ, Valenstein M. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30:521–527. doi: 10.1016/j.genhosppsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata BA. Negative reinforcement in applied behavior analysis: an emerging technology. J Appl Behav Anal. 1987;20:361–378. doi: 10.1901/jaba.1987.20-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153:744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma ML, Postma SA, Souren P, Arns M, Gordon E, Vissers K, Wilder-Smith O, van Rijn CM, van Goor H. Neurodegenerative properties of chronic pain: cognitive decline in patients with chronic pancreatitis. PLoS One. 2011;6:e23363. doi: 10.1371/journal.pone.0023363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Foreward. In: Didonna F, editor. Clinical handbook of mindfulness. Springer; New York, NY: 2009. pp. xxv–xxxii. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kanzler KE, Bryan CJ, McGeary DD, Morrow CE. Suicidal ideation and perceived burdensomeness in patients with chronic pain. Pain Pract. 2012;12:602–609. doi: 10.1111/j.1533-2500.2012.00542.x. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Karnath HO. Deficits of attention in acute and recovered visual hemi-neglect. Neuropsychologia. 1988;26:27–43. doi: 10.1016/0028-3932(88)90028-0. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum. 2007;57:474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- Keogh E, Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain. 2006;123:275–284. doi: 10.1016/j.pain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front Hum Neurosci. 2013;7:12. doi: 10.3389/fnhum.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–2954. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman GL. Basic Books; New York: 1984. Interpersonal psychotherapy of depression. [Google Scholar]
- Klinger R, Matter N, Kothe R, Dahme B, Hofmann UG, Krug F. Unconditioned and conditioned muscular responses in patients with chronic back pain and chronic tension-type headaches and in healthy controls. Pain. 2010;150:66–74. doi: 10.1016/j.pain.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb L, Lang C, Seifert F, Maihofner C. Cognitive correlates of “neglect-like syndrome” in patients with complex regional pain syndrome. Pain. 2012;153:1063–1073. doi: 10.1016/j.pain.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kowal J, Wilson KG, McWilliams LA, Peloquin K, Duong D. Self-perceived burden in chronic pain: relevance, prevalence, and predictors. Pain. 2012;153:1735–1741. doi: 10.1016/j.pain.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, France RD, Pelton S, McCann UD, Davidson J, Urban BJ. Chronic pain and depression. I. Classification of depression in chronic low back pain patients. Pain. 1985;22:279–287. doi: 10.1016/0304-3959(85)90028-4. [DOI] [PubMed] [Google Scholar]
- Kuba T, Quinones-Jenab V. The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: clinical versus preclinical studies. Brain Res Bull. 2005;66:179–188. doi: 10.1016/j.brainresbull.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Hodaie M, Davis KD. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS One. 2012;7:e35589. doi: 10.1371/journal.pone.0035589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Hoeft F, Sheau KE, Mackey SC. Strategy-dependent dissociation of the neural correlates involved in pain modulation. Anesthesiology. 2011;115:844–851. doi: 10.1097/ALN.0b013e31822b79ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Wu MY, Lee GK, Cheing G, Chan F. Catastrophizing as a cognitive vulnerability factor related to depression in workers' compensation patients with chronic musculoskeletal pain. J Clin Psychol Med Settings. 2008;15:182–192. doi: 10.1007/s10880-008-9118-7. [DOI] [PubMed] [Google Scholar]
- Leeuw M, Houben RM, Severeijns R, Picavet HS, Schouten EG, Vlaeyen JW. Pain-related fear in low back pain: a prospective study in the general population. Eur J Pain. 2007;11:256–266. doi: 10.1016/j.ejpain.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: When relative relief renders pain pleasant. Pain. 2013;154:402–410. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR. Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS) Pain. 2007;133:111–119. doi: 10.1016/j.pain.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Liedl A, O'Donnell M, Creamer M, Silove D, McFarlane A, Knaevelsrud C, Bryant RA. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med. 2010;40:1215–1223. doi: 10.1017/S0033291709991310. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2011 doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lucchetti G, Peres MF, Lucchetti AL, Mercante JP, Guendler VZ, Zukerman E. Generalized anxiety disorder, subthreshold anxiety and anxiety symptoms in primary headache. Psychiatry Clin Neurosci. 2013;67:41–49. doi: 10.1111/j.1440-1819.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS. Rehabilitation approaches to hemineglect. Neurologist. 2009;15:185–192. doi: 10.1097/NRL.0b013e3181942894. [DOI] [PubMed] [Google Scholar]
- Martinez-Jauand M, Sitges C, Femenia J, Cifre I, Gonzalez S, Chialvo D, Montoya P. Age-of-onset of menopause is associated with enhanced painful and non-painful sensitivity in fibromyalgia. Clin Rheumatol. 2013 doi: 10.1007/s10067-013-2212-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochem Res. 2000a;25:1219–1231. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000b;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabilitation and neural repair. 2009;23:587–594. doi: 10.1177/1545968308328717. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2007;132:108–123. doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Sale MV, Mattingley JB. Is there a critical lesion site for unilateral spatial neglect? A meta-analysis using activation likelihood estimation. Front Hum Neurosci. 2012;6:78. doi: 10.3389/fnhum.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology. 2006;67:2129–2134. doi: 10.1212/01.wnl.0000249112.56935.32. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabilitation and neural repair. 2012;26:646–652. doi: 10.1177/1545968311433209. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Gallagher L, Gallace A. Neglect-like tactile dysfunction in chronic back pain. Neurology. 2012;79:327–332. doi: 10.1212/WNL.0b013e318260cba2. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65:14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111–127. doi: 10.1016/j.pain.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Nicholas MK, Coulston CM, Asghari A, Malhi GS. Depressive symptoms in patients with chronic pain. Med J Aust. 2009;190:S66–70. doi: 10.5694/j.1326-5377.2009.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Nicolson NA, Davis MC, Kruszewski D, Zautra AJ. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosom Med. 2010;72:471–480. doi: 10.1097/PSY.0b013e3181d9a104. [DOI] [PubMed] [Google Scholar]
- Ocanez KL, McHugh RK, Otto MW. A meta-analytic review of the association between anxiety sensitivity and pain. Depress Anxiety. 2010;27:760–767. doi: 10.1002/da.20681. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120:69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Chronic pain and major depressive disorder in the general population. J Psychiatr Res. 2010;44:454–461. doi: 10.1016/j.jpsychires.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007;127:52–62. doi: 10.1016/j.pain.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Page MG, Stinson J, Campbell F, Isaac L, Katz J. Pain-related psychological correlates of pediatric acute post-surgical pain. J Pain Res. 2012;5:547–558. doi: 10.2147/JPR.S36614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Law E, Churchill SS, Walker A. Longitudinal course and impact of insomnia symptoms in adolescents with and without chronic pain. The journal of pain : official journal of the American Pain Society. 2012;13:1099–1106. doi: 10.1016/j.jpain.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Wilson AC, Lewandowski AS, Toliver-Sokol M, Murray CB. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 2011;152:89–94. doi: 10.1016/j.pain.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- Payne LA, Seidman LC, Lung KC, Zeltzer LK, Tsao JC. Relationship of neuroticism and laboratory pain in healthy children: Does anxiety sensitivity play a role? Pain. 2013;154:103–109. doi: 10.1016/j.pain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz E, Seifert F, Lanz S, Muller R, Maihofner C. Impaired hand size estimation in CRPS. The journal of pain : official journal of the American Pain Society. 2011;12:1095–1101. doi: 10.1016/j.jpain.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Piccardi L, Nico D, Bureca I, Matano A, Guariglia C. Efficacy of visuo-spatial training in right-brain damaged patients with spatial hemineglect and attention disorders. Cortex. 2006;42:973–982. doi: 10.1016/s0010-9452(08)70203-x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychol Bull. 2001;127:599–617. doi: 10.1037/0033-2909.127.5.599. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci U S A. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Fustos J, Critchley HD. On the generalised embodiment of pain: how interoceptive sensitivity modulates cutaneous pain perception. Pain. 2012;153:1680–1686. doi: 10.1016/j.pain.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18:256–271. doi: 10.1007/s12640-010-9182-2. [DOI] [PubMed] [Google Scholar]
- Post RM, Fleming J, Kapczinski F. Neurobiological correlates of illness progression in the recurrent affective disorders. J Psychiatr Res. 2012;46:561–573. doi: 10.1016/j.jpsychires.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153:619–635. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Reyes Del Paso GA, Pulgar A, Duschek S, Garrido S. Cognitive impairment in fibromyalgia syndrome: the impact of cardiovascular regulation, pain, emotional disorders and medication. European journal of pain. 2012;16:421–429. doi: 10.1002/j.1532-2149.2011.00032.x. [DOI] [PubMed] [Google Scholar]
- Ritchie EC. Suicide and the United States Army: Perspectives from the Former Psychiatry Consultant to the Army Surgeon General. Cerebrum. 2012;2012:1. [PMC free article] [PubMed] [Google Scholar]
- Rizzo M. `Balint's syndrome' and associated visuospatial disorders. Baillieres Clin Neurol. 1993;2:415–437. [PubMed] [Google Scholar]
- Robinson ME, Dannecker EA, George SZ, Otis J, Atchison JW, Fillingim RB. Sex differences in the associations among psychological factors and pain report: a novel psychophysical study of patients with chronic low back pain. The journal of pain : official journal of the American Pain Society. 2005;6:463–470. doi: 10.1016/j.jpain.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Rodarte RR, Asmus CI, Camara VD, Meyer A. Epidemiological assessment of the socioeconomic status as a factor associated with the occurrence of musculoskeletal disorders. Acta Reumatol Port. 2012;37:233. [PubMed] [Google Scholar]
- Rome HP, Jr., Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain medicine. 2000;1:7–23. doi: 10.1046/j.1526-4637.2000.99105.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Ancelin ML. Polymorphisms of estrogen receptors and risk of depression: therapeutic implications. Drugs. 2012;72:1725–1738. doi: 10.2165/11635960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Saariaho TH, Saariaho AS, Karila IA, Joukamaa MI. Early maladaptive schemas in Finnish adult chronic pain patients and a control sample. Scand J Psychol. 2011;52:146–153. doi: 10.1111/j.1467-9450.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- Sapir A, Kaplan JB, He BJ, Corbetta M. Anatomical correlates of directional hypokinesia in patients with hemispatial neglect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4045–4051. doi: 10.1523/JNEUROSCI.0041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer SE, Burris JL, Carlson CR. New directions in the management of chronic pain: self-regulation theory as a model for integrative clinical psychology practice. Clin Psychol Rev. 2010;30:805–814. doi: 10.1016/j.cpr.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? The Journal of clinical investigation. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckel E, Krause B, Ramachandran VS. Interpolation of illusory pain in the human somatosensory system. Perception. 2012;41:878–880. doi: 10.1068/p7019. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. The journal of pain : official journal of the American Pain Society. 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Ianiello M, Dear BF, Nicholson Perry K, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain. 2012;153:722–731. doi: 10.1016/j.pain.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: examination for pediatric application. The journal of pain : official journal of the American Pain Society. 2012;13:827–835. doi: 10.1016/j.jpain.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: Evidence from neuroimaging. Human brain mapping. 2012a doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Sieberg CB, Claar RL. Anxiety and impairment in a large sample of children and adolescents with chronic pain. Pain Res Manag. 2012b;17:93–97. doi: 10.1155/2012/420676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets RJ, Wittink H, Hidding A, Knottnerus JA. Do patients with chronic low back pain have a lower level of aerobic fitness than healthy controls?: are pain, disability, fear of injury, working status, or level of leisure time activity associated with the difference in aerobic fitness level? Spine (Phila Pa 1976) 2006;31:90–97. doi: 10.1097/01.brs.0000192641.22003.83. discussion 98. [DOI] [PubMed] [Google Scholar]
- Smith AA. Intimacy and family relationships of women with chronic pain. Pain Manag Nurs. 2003;4:134–142. doi: 10.1016/s1524-9042(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain. 2011;152:2287–2293. doi: 10.1016/j.pain.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes L, McFarlane J, Nava A, Gilroy H, Maddoux J. The association of pain severity and pain interference levels with abuse experiences and mental health symptoms among 300 mothers: baseline data analysis for a 7-year prospective study. Issues Ment Health Nurs. 2013;34:2–16. doi: 10.3109/01612840.2012.709916. [DOI] [PubMed] [Google Scholar]
- Tang NK, Goodchild CE, Hester J, Salkovskis PM. Pain-related insomnia versus primary insomnia: a comparison study of sleep pattern, psychological characteristics, and cognitive-behavioral processes. Clin J Pain. 2012a;28:428–436. doi: 10.1097/AJP.0b013e31823711bc. [DOI] [PubMed] [Google Scholar]
- Tang NK, Goodchild CE, Salkovskis PM. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behaviour research and therapy. 2012b;50:814–821. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138:392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Thibodeau MA, Welch PG, Katz J, Asmundson GJ. Pain-related anxiety influences pain perception differently in men and women: A quantitative sensory test across thermal pain modalities. Pain. 2013;154:419–426. doi: 10.1016/j.pain.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Thimm M, Fink GR, Kust J, Karbe H, Willmes K, Sturm W. Recovery from hemineglect: differential neurobiological effects of optokinetic stimulation and alertness training. Cortex. 2009;45:850–862. doi: 10.1016/j.cortex.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Thorn BE, Pence LB, Ward LC, Kilgo G, Clements KL, Cross TH, Davis AM, Tsui PW. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. The journal of pain : official journal of the American Pain Society. 2007;8:938–949. doi: 10.1016/j.jpain.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA. Childhood maltreatment and migraine (part I). Prevalence and adult revictimization: a multicenter headache clinic survey. Headache. 2010;50:20–31. doi: 10.1111/j.1526-4610.2009.01556.x. [DOI] [PubMed] [Google Scholar]
- Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–39. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- Turner JA, Jensen MP, Warms CA, Cardenas DD. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98:127–134. doi: 10.1016/s0304-3959(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F, Naty S, Grembiale RD. Fibromyalgia and obesity: the hidden link. Rheumatol Int. 2011;31:1403–1408. doi: 10.1007/s00296-011-1885-z. [DOI] [PubMed] [Google Scholar]
- Vallar G. Spatial hemineglect in humans. Trends Cogn Sci. 1998;2:87–97. doi: 10.1016/s1364-6613(98)01145-0. [DOI] [PubMed] [Google Scholar]
- van der Meer M, Kurth-Nelson Z, Redish AD. Information processing in decision-making systems. Neuroscientist. 2012;18:342–359. doi: 10.1177/1073858411435128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E, Consortium WU-MH. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilburg MA, Spence NJ, Whitehead WE, Bangdiwala S, Goldston DB. Chronic pain in adolescents is associated with suicidal thoughts and behaviors. The journal of pain : official journal of the American Pain Society. 2011;12:1032–1039. doi: 10.1016/j.jpain.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152:533–542. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA. Fear of injury and physical deconditioning in patients with chronic low back pain. Archives of physical medicine and rehabilitation. 2003;84:1227–1232. doi: 10.1016/s0003-9993(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin. J. Pain. 2002a;18:251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, De Jong JR, Onghena P, Kerckhoffs-Hanssen M, Kole-Snijders AM. Can pain-related fear be reduced? The application of cognitive-behavioural exposure in vivo. Pain Res Manag. 2002b;7:144–153. doi: 10.1155/2002/493463. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Vong SK, Cheing GL, Chan F, So EM, Chan CC. Motivational enhancement therapy in addition to physical therapy improves motivational factors and treatment outcomes in people with low back pain: a randomized controlled trial. Arch Phys Med Rehabil. 2011;92:176–183. doi: 10.1016/j.apmr.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McCracken LM, O'Brien JZ. Acceptance and values-based action in chronic pain: a three-year follow-up analysis of treatment effectiveness and process. Behaviour research and therapy. 2011;49:748–755. doi: 10.1016/j.brat.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Walling MK, Reiter RC, O'Hara MW, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: I. Prevalences of sexual abuse and physical abuse. Obstet Gynecol. 1994;84:193–199. [PubMed] [Google Scholar]
- Walz AD, Usichenko T, Moseley GL, Lotze M. Graded Motor Imagery and the Impact on Pain Processing in a Case of CRPS. Clin J Pain. 2013;29:276–279. doi: 10.1097/AJP.0b013e318250f4e8. [DOI] [PubMed] [Google Scholar]
- Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, Tracey I. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17705–17710. doi: 10.1073/pnas.1120201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L, Stebbings S, Cherkin D, Baxter GD. Yoga for Functional Ability, Pain and Psychosocial Outcomes in Musculoskeletal Conditions: A Systematic Review and Meta-Analysis. Musculoskeletal Care. 2013 doi: 10.1002/msc.1042. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Solomon BC, Lehman DH, Liu L, Lang AJ, Atkinson JH. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152:2098–2107. doi: 10.1016/j.pain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, Ingvar M, Olsson GL. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. European journal of pain. 2013;17:599–611. doi: 10.1002/j.1532-2149.2012.00224.x. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci. 2013;7:46. doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Scheel-Kruger J, Belzung C. The neurobiology of depression and antidepressant action. Neuroscience and biobehavioral reviews. 2012 doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neuroscience and biobehavioral reviews. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wood BM, Nicholas MK, Blyth F, Asghari A, Gibson S. Catastrophizing Mediates the Relationship Between Pain Intensity and Depressed Mood in Older Adults With Persistent Pain. The journal of pain : official journal of the American Pain Society. 2012 doi: 10.1016/j.jpain.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Wood PL, Mahmood SA, Moskal JR. Antinociceptive action of GLYX-13: an N-methyl-D-aspartate receptor glycine site partial agonist. Neuroreport. 2008;19:1059–1061. doi: 10.1097/WNR.0b013e32830435c9. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58:13–18. doi: 10.1177/070674371305800104. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathe AA. Understanding resilience. Front Behav Neurosci. 2013;7:10. doi: 10.3389/fnbeh.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei S, Bigizadeh S, Pourahmadi M, Ghobadifar MA. Chronic Pain and Its Determinants: A Population-based Study in Southern Iran. Korean J Pain. 2012;25:245–253. doi: 10.3344/kjp.2012.25.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaya N, Anstis S, Bartels A. Parietal cortex mediates conscious perception of illusory gestalt. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:523–531. doi: 10.1523/JNEUROSCI.2905-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]