Abstract
Background
Rheumatoid arthritis (RA) is associated with a variety of kidney disorders. However, it is unclear whether the development of reduced kidney function (RKF) is higher in patients with RA compared to the general population.
Study Design
Retrospective review.
Setting & Participants
Incident adult-onset RA cases (813) and a comparison cohort of non-RA subjects (813) in Olmsted County, Minnesota, from 1980-2007.
Predictor
Baseline demographic and clinical variables.
Outcomes
RKF: 1) Estimated GFR (eGFR) <60 ml/min/1.73m2 and 2) eGFR <45 on two consecutive occasions at least 90 days apart; cardiovascular disease (CVD);, and death.
Measurements
The cumulative incidence of RKF was estimated adjusting for the competing risk of death.
Results
Of 813 RA patients and 813 non-RA subjects, mean age was 56±16 (SD) years, 68% were female, and 9% had RKF at baseline. The 20-year cumulative incidence of RKF was higher in RA patients compared to non-RA participants for eGFR <60 ml/min/1.73m2 (25% vs. 20%; p=0.03) but not eGFR <45 ml/min/1.73m2 (9% vs. 10%; p=0.8). The presence of CVD at baseline (HR, 1.77; 95% CI, 1.14-2.73; p=0.01) and elevated erythrocyte sedimentation rate in RA patients (HR per 10-mm/h greater, 1.08; 95% CI, 1.00-1.16; p=0.04) was associated with increased risk of eGFR <60 ml/min/1.73m2. An eGFR <60 ml/min/1.73m2 was not associated with an increased risk of CVD development in RA patients (HR, 0.99; 95% CI, 0.63-1.57; p=0.9), however, a greater reduction in GFR (eGFR <45 ml/min/1.73m2) was associated with an increased risk of CVD (HR, 1.93; CI, 1.04-3.58; p=0.04).
Limitations
RKF was defined by estimating equations for kidney function. We are limited to deriving associations from our findings.
Conclusions
RA patients were more likely to develop RKF over time. CVD and associated factors appear to play a role. The presence of RA in individuals with RKF may lead to an increase in morbidity from CVD development, for which awareness may provide a means for optimizing care.
Keywords: cardiovascular disease, glomerulonephritis, mortality, renal progression, kidney dysfunction, RA, eGFR
Chronic kidney disease (CKD) affects more than 26 million adults in the United States1. While diabetes mellitus and hypertension remain the two primary causes of kidney failure, other conditions including autoimmune processes contribute to the burden of kidney disease. The reported kidney disease prevalence in patients with rheumatoid arthritis (RA) ranges from 5% to 50% based on studies of different designs2,3, and the true prevalence of kidney disease remains unclear.
RA has been associated with a variety of kidney disorders due principally to chronic inflammation and drug exposure or toxicity. The most commonly observed kidney diseases in RA patients who have undergone kidney biopsy include mesangial proliferative glomerulonephritis, membranous nephropathy, IgA nephropathy, minimal change disease, pauci-immune glomerulonephritis, analgesic nephropathy, interstitial nephritis, and AA amyloidosis4,5.
As treatment patterns for RA have changed over the years, the incidence of kidney disease may be altered. Agents such as gold salts and d-penicillamine more commonly used in the past were directly linked to proteinuria and kidney disease6,7. Cyclosporine therapy is associated with a dose-related nephrotoxicity in patients with RA8,9. More recently, biologic agents including tumor necrosis factor α inhibitors have emerged as effective treatment. A variety of case reports suggesting a link to etanercept and glomerulonephritides indicate that kidney disease continues to be a disease- and treatment-related feature of RA10. Finally, long-term maintenance anti-inflammatory therapy with non-steroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase 2 inhibitors is a well-recognized cause of kidney injury11.
Patients with kidney disease face increased morbidity and mortality, most of which is attributed to an increased burden of cardiovascular disease (CVD) 12-15. Historically, patients with RA-associated renal amyloidosis had higher mortality rates relative to the general population10. The occurrence of hematuria, proteinuria or CKD was associated with a 3- to 4-fold increased risk of death16. Patients with RA have an excess burden of CVD for which CV risk has been previously underestimated by standard risk scores17. Subclinical decreased kidney function has been identified as an independent risk factor for CV events in RA patients18.
The objective of this study was to determine the rate of and associated factors related to the development of reduced kidney function (RKF) in patients with RA.
Methods
Study Population
An inception cohort of all cases of RA first diagnosed between January 1, 1980 and December 31, 2007 (n=813) among Olmsted County, Minnesota, residents aged18 years or older was assembled as previously described19. The incidence date was defined as the earliest date at which the patient fulfilled at least 4 of the 7 American College of Rheumatology 1987 classification criteria for RA20. A comparison cohort of subjects without RA with similar age, sex and calendar year was also assembled. All patients in both cohorts were followed up longitudinally through their entire medical records until death, migration from Olmsted County, or December 31, 2008.
All clinically obtained creatinine values were collected. The serum creatinine assay was standardized 21 in October 2006, and we adjusted previous serum creatinine levels by subtracting 0.14 mg/dL, the mean change in serum creatinine levels after standardization22. RKF was classified by glomerular filtration rate (GFR). RKF was defined as two consecutive estimated GFRs (eGFRs) <60 mL/min/1.73 m2 at least 90 days apart using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation23.
The date the patient was considered to have RKF is the second date of eGFR <60 mL/min/1.73 m2. Similarly, more advanced RKF was defined as two consecutive eGFRs <45 mL/min/1.73 m2 at least 90 days apart, and the date the patient had the second eGFR <45 mL/min/1.73 m2 was the date of this classification. If more than 2 creatinine tests were performed in a 90-day period, all tests in a 90-day period had to be below the cutoff to satisfy the definition. RKF at index date was defined using the closest creatinine measurement to the index date within ±90 days. CVD includes myocardial infarction (MI; hospitalized or silent), revascularization procedures, angina, and heart failure as previously described17.
Statistical Methods
The cumulative incidence of RKF adjusted for the competing risk of death was estimated24. These methods are similar to Kaplan-Meier method with censoring of patients who are still alive at last follow-up. However, patients who die before having RKF are appropriately accounted for to avoid the overestimation of the rate of occurrence of RKF, which can occur if such subjects are simply censored. Patients diagnosed with RKF prior to the diagnosis of RA, or prior to the index date for the non-RA comparison cohort, were excluded from the analysis of cumulative incidence. Cumulative incidence comparisons between the cohorts were performed using methods by Gray25.
Cox proportional hazards models were used to compare the rate of development of RKF between patients with RA and the non-RA comparison cohort. In addition, Cox proportional hazards models were used to assess the association of risk factors with the development of RKF among patients with RA.
Cox models were also used to assess the impact of RKF on the development of CVD or mortality among the RA and non-RA cohorts. Age was used as the time scale for these models to provide optimal adjustment for age, under the assumption that age is likely the most important time determinate of CVD. Subjects entered the model at the age they met criteria for RA and remained in the model until the age of each CVD event. Subjects without events were censored at death or last follow-up. The models were stratified by sex.
Traditional CVD risk factors were included in these models as adjustors. Time-dependent covariates were used to model risk factors that developed over time. These time-dependent covariates allowed patients to be modeled as unexposed to the risk factor during the follow-up time prior to development of the risk factor, then change to exposed following development of the risk factor. Interactions between cohort and RKF were examined.
Results
Study Participants
The study population included 813 RA patients and 813 non-RA subjects. The 2 cohorts had similar characteristics (Table 1). The average age at RA incidence (index date for the non-RA cohort) was 56±16 (standard deviation) years and 556 (68%) were female. There was a statistically significant difference between smoking status in RA patients (45% never smokers) compared to in individuals in the non-RA cohort (54%). There was no difference in the presence of RKF at RA incidence/ index date (p=0.9), but the mean eGFR was higher among the RA cohort compared to the non-RA cohort (86±20 vs 83±20 ml/min/1.73m2; p=0.008). There were no end-stage renal disease events.
Table 1.
Characteristics of RA and Non-RA Cohorts.
| Characteristic | RA (n=813) | Non-RA (n=813) | p |
|---|---|---|---|
| At incidence/index | |||
| Ageˆ (y) | 55.9 ± 15.7 | 55.9 ± 15.7 | 0.9 |
| Female sex | 556 (68%) | 556 (68%) | 0.9 |
| White race | 761 (94%) | 771(96%) | 0.1 |
| BMI (kg/m2) | 27.8 ± 6.0 | 27.7 ± 6.0 | 0.8 |
| Smoking status | 0.002 | ||
| Never | 364 (45%) | 435 (54%) | |
| Current | 178 (22%) | 144 (18%) | |
| Former | 271 (33%) | 234 (29%) | |
| Diabetes Mellitus | 79 (10%) | 67 (8%) | 0.3 |
| Hypertension | 307 (38%) | 275 (34%) | 0.1 |
| CVD | 87 (11%) | 89 (11%) | 0.9 |
| Heart failure | 23 (3%) | 23 (3%) | 0.9 |
| eGFR | 0.008 | ||
| Mean (mL/min/1.73 m2) | 85.7 ± 19.7 | 83.1 ± 19.8 | |
| Median (mL/min/1.73 m2) | 87.1 [73.4-98.9] | 83.5 [70.8-96.8] | |
| Presence of eGFR <60* | 74 (9%) | 74 (9%) | 0.9 |
| Presence of eGFR <45** | 19 (2%) | 22 (3%) | 0.8 |
| Length of follow-up (y) | 9.6 ± 6.9 | 10.9 ± 7.2 | -- |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range].
CVD: Cardiovascular disease; eGFR: estimated glomerular filtration rate; CKD: chronic kidney disease; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; RA, rheumatoid arthritis
eGFR <60 was defined as two consecutive eGFRs <60 mL/min/1.73 m2 at least 90 days apart using the CKD-EPI creatinine equation.
eGFR <45 was defined as two consecutive eGFRs <45 mL/min/1.73 m2 at least 90 days apart using the CKD-EPI creatinine equation.
eGFR <60 mL/min/1.73 m2
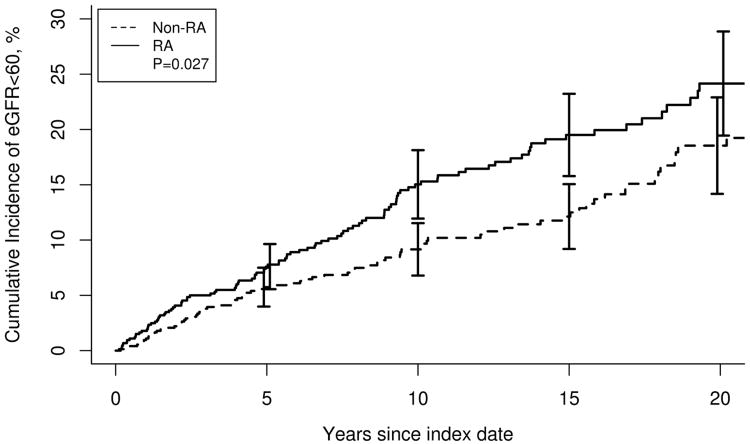
During follow-up (in the non-prevalent with eGFR <60 mL/min/1.73 m2), 111 and 88 people in the RA and non-RA cohorts, respectively, developed eGFR<60 mL/min/1.73 m2. The cumulative incidence of eGFR <60 mL/min/1.73 m2 was higher in RA patients compared to the non-RA cohort (25% ± 2.5% vs. 20% ± 2.3% at 20 years in RA and non-RA cohorts, respectively; p=0.03; Table 2). As illustrated in Figure 1, patients with RA were more likely to develop eGFR <60 mL/min/1.73 m2 during follow-up (hazard ratio [HR], 1.63; 95% confidence interval [CI], 1.23-2.16; analysis adjusted for age, sex and calendar year). This difference between RA and non-RA cohorts persisted following adjustment for smoking and baseline eGFR (HR, 1.95; 95% CI, 1.45-2.62).
Table 2. Cumulative incidence of reduced kidney function by eGFR in RA versus non-RA cohorts.
| eGFR Category | No. of Events After Incidence/Index | Cumulative incidence (95% CI) | p-value | ||
|---|---|---|---|---|---|
| RA | Non-RA | RA | Non-RA | ||
| <60 mL/min/1.73 m2 | 111 | 88 | 10 y: 15.0 (11.9-18.1) 20 y: 25.1 (20.2-30.0) |
10 y: 9.9 (7.5-12.3) 20 y: 20.2 (15.7-24.7) |
0.03 |
| <45 mL/min/1.73 m2 | 46 | 51 | 10 y: 4.2 (2.4-6.0) 20 y: 9.3 (6.2-12.4) |
10 y: 5.6 (4.0-7.2) 20 y: 10.4 (7.3-13.5) |
0.8 |
eGFR, estimated glomerular filtration rate; RA, rheumatoid arthritis; CI, confidence interval
Figure 1.
Cumulative incidence of eGFR<60 mL/min/1.73 m2 in rheumatoid arthritis (RA) vs. non-RA. The solid line represents patients with RA and the dashed line those without RA (p=0.03).
The rate of creatinine testing was higher during follow-up in the RA cohort (20363 tests/ 7827.7 person-years = 2.6 tests per patient per year) than in the non-RA cohort (13887 tests /8886.3 person-years = 1.6 per patient per year). To examine the possibility that the differences in testing rate influenced the results, a sensitivity analysis was performed. More frequent testing among the RA patients may have led to earlier dates of meeting RKF definitions. The time between consecutive abnormal tests at least 90 days apart was compared and was found to be shorter in the RA cohort compared to the non-RA cohort (mean, 255 ± 183 vs 322 ± 223 days; p=0.01). In sensitivity analyses the date of RKF was defined to be exactly 90 days after the first abnormal creatinine test instead of the actual date of the second abnormal test, and the results comparing cumulative incidence of RKF in the RA vs non-RA cohort did not change. Patients with RA remained more likely to develop eGFR <60 mL/min/1.73 m2 during follow-up after adjustment for number of serum creatinine measures (HR, 1.53; 95% CI, 1.14-2.05).
eGFR <45 mL/min/1.73 m2
There was no difference in the development of eGFR <45 mL/min/1.73 m2 between the RA cohort and non-RA cohort (HR, 1.13; 95% CI, 0.76-1.70; p=0.6; analysis adjusted for age, sex and calendar year). The cumulative incidence of eGFR <45 mL/min/1.73 m2 at 20 years of follow up was not different in the RA compared to the non-RA group (9% ± 1.6% vs. 10% ± 1.6%; p=0.8).
Risk Factors for RKF in RA
The potential association of both RA disease characteristics and CV risk factors with the development of RKF in patients with RA was also examined (Table 3). An elevation in the inflammatory marker erythrocyte sedimentation rate (highest value in the first year after RA diagnosis) was associated with eGFR <60 mL/min/1.73 m2 (HR per 10-mm/h greater, 1.08; 95% CI, 1.00-1.16; p=0.05). The presence of CVD was associated with a nearly 2-fold increased risk of eGFR <60 mL/min/1.73 m2 (HR, 1.77; 95% CI, 1.14-2.73; p=0.01). Patients with obesity (body mass index ≥30 kg/m2) and dyslipidemia were also more likely to develop eGFR <60 mL/min/1.73 m2. Erythrocyte sedimentation rate (highest value in the first year), CVD, severe extra-articular manifestations, and use of corticosteroids were each associated with the development of eGFR <45 mL/min/1.73 m2 in RA patients.
Table 3. Association between RA disease characteristics and reduced kidney function in patients with RA.
| eGFR <60* | eGFR <45** | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| RA characteristic | ||||
| ESR at index (per 10-mm/h greater) | 1.05 (0.96, 1.15) | 0.3 | 1.07 (0.93, 1.25) | 0.4 |
| Highest ESR in 1st y (per 10-mm/h greater) | 1.08 (1.00, 1.16) | 0.05 | 1.14 (1.02, 1.26) | 0.02 |
| RF positiveˆ | 0.80 (0.55, 1.16) | 0.2 | 0.95 (0.52, 1.74) | 0.9 |
| Current smokerˆˆ | 1.13 (0.71, 1.79) | 0.6 | 1.70 (0.86, 3.39) | 0.1 |
| Former smokerˆˆ | 1.05 (0.70, 1.57) | 0.8 | 0.74 (0.37, 1.50) | 0.4 |
| Time-dependent characteristic# | ||||
| Hypertension | 2.05 (0.81, 5.17) | 0.1 | 3.12 (0.41, 23.78) | 0.3 |
| Diabetes Mellitus | 1.51 (0.97, 2.36) | 0.07 | 1.59 (0.82, 3.09) | 0.2 |
| Dyslipidemia | 1.99 (1.17, 3.37) | 0.01 | 1.74 (0.76, 3.99) | 0.2 |
| CVD | 1.77 (1.14, 2.73) | 0.01 | 3.62 (1.92, 6.82) | <0.001 |
| BMI Category | ||||
| ≥30 kg/m2 | 1.87 (1.28, 2.73) | 0.001 | 1.75 (0.96, 3.17) | 0.07 |
| <20 kg/m2 | 0.51 (0.29, 0.90) | 0.02 | 0.77 (0.35, 1.70) | 0.5 |
| Nodules | 1.06 (0.69, 1.64) | 0.8 | 1.16 (0.60, 2.24) | 0.7 |
| Erosions/destructive changes on radiographs | 0.94 (0.64, 1.36) | 0.7 | 1.53 (0.84, 2.80) | 0.2 |
| Severe extra-articular manifestations | 1.06 (0.55, 2.04) | 0.9 | 2.25 (1.06, 4.77) | 0.04 |
| Large joint swelling | 1.06 (0.69, 1.65) | 0.8 | 1.67 (0.74, 3.79) | 0.2 |
| Joint surgery | 0.93 (0.61, 1.43) | 0.7 | 1.46 (0.78, 2.71) | 0.2 |
| MTX | 1.22 (0.82, 1.79) | 0.3 | 1.13 (0.60, 2.14) | 0.7 |
| HCQ | 0.82 (0.56, 1.21) | 0.3 | 1.03 (0.56, 1.89) | 0.9 |
| Other DMARD | 1.01 (0.64, 1.57) | 0.9 | 0.91 (0.45, 1.86) | 0.8 |
| Biologic | 0.46 (0.14, 1.45) | 0.2 | 1.82 (0.53, 6.25) | 0.3 |
| Steroids | 1.49 (0.96, 2.31) | 0.08 | 2.49 (1.18, 5.23) | 0.02 |
| NSAID | 0.59 (0.35, 0.98) | 0.04 | 1.84 (0.56, 6.10) | 0.3 |
| COX-2 | 0.92 (0.60, 1.41) | 0.7 | 0.78 (0.38, 1.59) | 0.5 |
Note: Systemic glucocorticoids included either oral or intravenous forms (e.g., prednisone, methylprednisolone, hydrocortisone, dexamethasone). Other DMARDs included sulfasalazine, leflunomide, and azathioprine. Biologic included tumor necrosis factor inhibitors, anakinra, abatacept, and rituximab.
ESR: erythrocyte sedimentation rate; RF: rheumatoid factor; CVD: cardiovascular disease; BMI: body mass index; MTX: methotrexate; HCQ: hydroxychloroquine; DMARD: disease-modifying antirheumatic drugs; NSAID: nonsteroidal anti-inflammatory drugs; COX-2: cyclooxygenase 2 inhibitor. eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; RA, rheumatoid arthritis
eGFR <60 was defined as two consecutive eGFR <60 mL/min/1.73 m2 at least 90 days apart using the CKD-EPI creatinine equation.
eGFR <45 was defined as two consecutive eGFR <45 mL/min/1.73 m2 at least 90 days apart using the CKD-EPI creatinine equation.
Reference: RF negative
Reference: nonsmokers
Reference: patients without each characteristic
Some characteristics were “protective” for the development of RKF, including body mass index <20 kg/m2 and use of NSAIDs. NSAID use was common in RA patients over the study period. Nearly all RA patients took NSAIDs with 588 of 813 (72%) taking them at the time of RA diagnosis and another 149 (18%) using NSAIDs during follow-up. Additionally, we found no association between NSAID exposure and the development of CVD, hypertension or diabetes mellitus during follow-up (p=0.9 for all). Given the extensive NSAID use, there was no difference in eGFR at baseline between RA patients who did and did not use NSAIDs at baseline (mean, 85.5 ± 19.6 vs 86.4 ± 20.1; p=0.5).
RKF Outcomes
During follow-up, a total of 392 patients (RA, 229; non-RA, 163) died. eGFR <60 mL/min/1.73 m2 was associated with an increased risk of mortality among the non-RA (HR, 1.59; 95% CI, 1.13-2.24) and RA (HR, 1.25; 95% CI, 0.92-1.68) cohorts, but the effect of eGFR <60 mL/min/1.73 m2 on mortality was significantly less among patients with RA compared to non-RA individuals (HR for interaction, 0.66; 95% CI, 0.43-0.97; p=0.04). Results were similar for eGFR <45 mL/min/1.73 m2 (HRs of 2.06 [95% CI, 1.41-2.99] and 1.62 [95% CI, 1.15-2.28] for RA and non-RA cohorts, respectively [HR for interaction, 0.66; 95% CI, 0.41-1.06; p=0.09]).
During followup, a total of 245 patients (RA, 137; non-RA, 108) developed CVD. eGFR <60 mL/min/1.73 m2 was not significantly associated with an increased risk of CVD among the non-RA (HR, 1.37; 95% CI, 0.83-2.24; p=0.2) or RA (HR, 0.99; 95% CI, 0.63-1.57; p=0.9) cohorts, and the effect of eGFR <60 mL/min/1.73 m2 on mortality was not different among the RA compared to non-RA cohorts (HR for interaction, 0.71; 95% CI, 0.39-1.29; p=0.3). Among patients with RA, eGFR <45 mL/min/1.73 m2 was associated with an increased risk of CVD development (HR, 1.93; 95% CI, 1.04-3.58; p=0.04). Results were similar, but not statistically significant, for the non-RA cohort (HR, 1.41; 95% CI, 0.67-2.98; p=0.4 [HR for interaction, 1.25; 95% CI, 0.50-3.16; p=0.6]).
Discussion
Patients with RA are more likely to develop RKF than persons without RA. In our study, at baseline, the prevalence of eGFR <60 ml/min/1.73 m2 among cohort groups was approximately 9%. Over time, the cumulative incidence of eGFR <60 mL/min/1.73 m2 in RA patients reached 15% at 10 years and 25% at 20 years of follow up, which was close to 5% more than in the non-RA cohort at these time points. The rate of development of more advanced kidney disease, eGFR <45 ml/min/1.73m2, was similar between groups. Several factors in patients with RA were associated with RKF development. Many of these were related to CVD and associated risk factors.
Historically, there are only a few studies which describe the occurrence of RKF in patients with RA. A cross-sectional population-based cohort study of 604 Finnish patients with RA yielded evidence of nephropathy (defined as hematuria, proteinuria or kidney failure) in 17%26. Of 604 patients, 54 had isolated hematuria, 27 had isolated proteinuria, and 7 had both hematuria and proteinuria. Chronic kidney failure defined by sex-based serum creatinine cutoffs was found in a total of 29 of which 15 had neither hematuria nor proteinuria.
In a long term 15-year follow up study of Finnish patients with RA and nephropathy (n=103), the endpoints of death and kidney failure requiring dialysis were reached in 56% and 10%, respectively16. In a subset of patients (n=102) with RA from the original cohort who did not have nephropathy, 28% developed CKD, 49% died, and only two patients required dialysis therapy by study end. Kidney disease appeared in patients with RA most often during the first 10-15 years of RA disease; however, a significant percentage of patients with RA without baseline nephropathy progressed to CKD over time16.
In our study, eGFR <60 mL/min/1.73 m2 was prevalent in 9% of patients at baseline, and we observed a 10-year cumulative incidence rate of eGFR <60 mL/min/1.73 m2 among patients without prevalent eGFR <60 of 15%, which further increased to 25% by 20 years. Given the comparison cohort observed, we can now appreciate that these rates are higher than those found in the general population of similar individuals. This insight should alert providers to the increased burden of disease experienced by patients with RA.
In our analysis a variety of characteristics and CV risk factors were associated with the development of RKF in patients with RA including dyslipidemia, CVD, body mass index, and use of NSAIDs. As expected, patients with CVD had a nearly 2-fold increased risk of developing eGFR <60 mL/min/1.73 m2. Daoussis et al found that female sex, advanced age, increased serum uric acid levels, the presence of extra-articular disease, and increased cholesterol levels were independently associated with decreased kidney function in a cross-sectional, single-center study of 400 consecutive patients with RA27. An increased erythrocyte sedimentation rate in their study was not strongly associated with CKD, and in our study the relationship reached marginal significance (HR, 1.08 per 10-mm/h increase in erythrocyte sedimentation rate during first year; 95% CI, 1.00-1.16; p=0.05). This relationship is relatively surprising given that in the past, chronic inflammation in patients with RA led to prevalent kidney disease; however, this was primarily due to AA amyloidosis. Immonen et al recently reported the findings of a marked decline in incidence of renal replacement therapy for amyloidosis associated with inflammatory rheumatic disease between 1995 and 200828. Taken together, we may speculate that in the modern treatment era, the rate of amyloidosis associated with RA has decreased, which is consistent with the dampened association of sedimentation rate with CKD in recent studies.
Similar to the Daoussis group27, we were able to detect an association between anti-inflammatory drugs and RKF development in patients with RA. Interestingly, they found a higher GFR in NSAID users compared to non-users (eGFR, 88.6±23.9 vs 80.2±20.2 mL/min/1.73 m2; p=0.002). We observed that the use of NSAIDs was “protective” (HR, 0.59; 95% CI, 0.35-0.98; p=0.04) for the development of CKD. One might infer that NSAIDs were prescribed or taken by individuals with fewer comorbidities or better kidney function at baseline. We cannot exclude the possibility of residual confounding, which might explain this finding. However, in our analysis, there was a high prevalence (>90%) of NSAID use at baseline and over the study period which was not associated with GFR or other comorbidities. In our study, only two factors, body mass index <20 kg/m2 and NSAID use, were associated with a reduced risk of developing eGFR <60 mL/min/1.73 m2; however this association did not extend to eGFR <45 mL/min/1.73 m2. Only the highest value of erythrocyte sedimentation rate in the first year, CVD, severe extra-articular manifestations, and corticosteroids were associated with an increased risk of eGFR <45 mL/min/1.73 m2. These factors likely manifest an additive effect on small vessel disease contributing to decreased kidney function in individuals with RA.
The progression of CKD and its impact on mortality was described in a recent Department of Veterans Affairs national database cohort study of over 4000 patients with RA with early stage CKD (eGFR, 45-60 ml/min)29. Over a median of 2.6 years, 38% maintained stable kidney function (eGFR decline of 0-1 ml/min) while 24% had severe progression of CKD (eGFR decline > 4 ml/min per year). After a median of 5.7 years, patients with RA and severe progression had an increased risk for mortality compared to patients with RA who had mild CKD progression (HR, 1.54; 95% CI, 1.30-1.82)29. Similar to other studies, the presence of concurrent kidney disease was associated with mortality in patients with RA in the population based cohort study from Finland (HR, 1.78 [95% CI, 1.34-2.31] in those with nephropathy compared to controls)16.
We examined the effect of RKF on mortality in patients with and without RA. eGFR <60 mL/min/1.73 m2 was associated with an increased risk of death among those in the non-RA cohort (HR, 1.59; 95% CI, 1.13-2.24) and perhaps among RA patients (HR, 1.25; 95% CI, 0.92-1.68). However, the effect of eGFR <60 mL/min/1.73 m2 on mortality was significantly less among the RA cohort compared to the non-RA cohort (HR for interaction, 0.66; 95% CI, 0.43-0.97; p=0.04). The presence of other factors that contribute to mortality in RA but not in the absence of RA might result in this dilution effect making the relative contribution of RKF seem smaller among RA patients.
It is well appreciated that individuals with RA have an increased burden of CVD30-32. Inflammation is believed to play a pivotal role in the increased risk of CVD, in addition to traditional risk factors, in patients with RA and has recently been proposed to be a primary component for the underestimation of CVD risk prediction by standard CVD risk assessment tools17. In our study, eGFR <60 mL/min/1.73 m2 was not significantly associated with an increased risk of CVD development in either the RA or non-RA cohort. However, a greater reduction in kidney function (eGFR <45 mL/min/1.73 m2) was associated with an increased risk of CVD in RA patients (HR, 1.93; 95% CI, 1.04-3.58). This relationship was similar in the non-RA cohort, but did not reach statistical significance.
The primary strength of this study was the use of a population-based design with a large RA cohort and equally large population-based non-RA comparison cohort. Furthermore, we were able to perform a comprehensive review of all inpatient and outpatient medical records from the community, which allowed for accurate assessment of RKF and CVD. Our study has some potential limitations. We used serum creatinine and the CKD-EPI creatinine equation to estimate kidney function. Some studies have suggested that use of estimating equations such as the MDRD (Modification of Diet in Renal Disease) Study equation may underestimate kidney function in patients with RA primarily because this and other equations have not yet been validated in patients with RA or lupus nephritis 33-35. Acknowledging this limitation, we utilized the CKD-EPI equation, which has been found in a recent meta-analysis to more accurately estimate GFR than the MDRD Study equation 36 to improve our estimation of RKF prevalence. In addition, we did not utilize urine protein excretion rates or report the prevalence of hematuria to aid in classification of RKF. Therefore an understanding of the true prevalence and risk of CKD or RKF in RA patients may not have been fully explored. Nonetheless, any underestimation of kidney disease and/or its association with mortality was uniformly made in the comparison cohort. Given the observational nature of this study, we are limited to deriving associations only from our findings. More prospective studies are needed to gain more conclusive insight into the relationship between RA and RKF. Finally, the majority of participants in the study were white, thus limiting the generalization of the results to other ethnic and racial groups. However, they are representative of patients with this disease and the general population37.
In conclusion, RKF is common in patients with RA and increases in prevalence over time. A combination of RA disease characteristics and CVD-associated factors appear to play a role in RKF development. The presence of RA disease did not appear to affect survival among those who developed RKF. The presence of RA disease in individuals with RKF may lead to an increase in morbidity from CVD development, primarily in those with advanced kidney disease. Based upon these findings, therapeutic strategies to modify non-traditional risk factors for CVD and consistent kidney function monitoring where appropriate may be employed to minimize the risk of RKF, and subsequent complications including CVD and death in our RA population.
Acknowledgments
Support: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) (grant R01AR46849) and by the National Institute on Aging of the NIH (grant R01AG034676). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):242–247. doi: 10.1002/art.1780380213. [DOI] [PubMed] [Google Scholar]
- 3.Karie S, Gandjbakhch F, Janus N, et al. Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford) 2008 Mar;47(3):350–354. doi: 10.1093/rheumatology/kem370. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Higashi S, Tomoda K, Tsukano M, Shono M. Etanercept can induce resolution of renal deterioration in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Rheumatol. 2010 Dec;29(12):1395–1401. doi: 10.1007/s10067-010-1469-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakano M, Ueno M, Nishi S, et al. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol. 1998 Sep;50(3):154–160. [PubMed] [Google Scholar]
- 6.Hall CL, Fothergill NJ, Blackwell MM, Harrison PR, MacKenzie JC, MacIver AG. The natural course of gold nephropathy: long term study of 21 patients. Br Med J (Clin Res Ed) 1987 Sep 26;295(6601):745–748. doi: 10.1136/bmj.295.6601.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CL, Jawad S, Harrison PR, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed) 1988 Apr 16;296(6629):1083–1086. doi: 10.1136/bmj.296.6629.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkmans BA, van Rijthoven AW, Goei The HS, Boers M, Cats A. Cyclosporine in rheumatoid arthritis. Semin Arthritis Rheum. 1992 Aug;22(1):30–36. doi: 10.1016/0049-0172(92)90046-g. [DOI] [PubMed] [Google Scholar]
- 9.Yocum DE, Klippel JH, Wilder RL, et al. Cyclosporin A in severe, treatment-refractory rheumatoid arthritis. A randomized study. Ann Intern Med. 1988 Dec 1;109(11):863–869. doi: 10.7326/0003-4819-109-11-863. [DOI] [PubMed] [Google Scholar]
- 10.Laakso M, Mutru O, Isomaki H, Koota K. Mortality from amyloidosis and renal diseases in patients with rheumatoid arthritis. Ann Rheum Dis. 1986 Aug;45(8):663–667. doi: 10.1136/ard.45.8.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999 May 31;106(5B):13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 12.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004 Sep 23;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005 Feb;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sihvonen S, Korpela M, Mustonen J, Laippala P, Pasternack A. Renal disease as a predictor of increased mortality among patients with rheumatoid arthritis. Nephron Clin Pract. 2004;96(4):c107–114. doi: 10.1159/000077372. [DOI] [PubMed] [Google Scholar]
- 17.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012 Aug 1;110(3):420–424. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Sijl AM, van den Oever IA, Peters MJ, et al. Subclinical renal dysfunction is independently associated with cardiovascular events in rheumatoid arthritis: the CARRE Study. Ann Rheum Dis. 2012 Mar;71(3):341–344. doi: 10.1136/annrheumdis-2011-200051. [DOI] [PubMed] [Google Scholar]
- 19.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010 Jun;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007 Apr;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 22.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010 May 4;152(9):561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999 Mar 30;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of Competing Risk. The Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 26.Karstila K, Korpela M, Sihvonen S, Mustonen J. Prognosis of clinical renal disease and incidence of new renal findings in patients with rheumatoid arthritis: follow-up of a population-based study. Clin Rheumatol. 2007 Dec;26(12):2089–2095. doi: 10.1007/s10067-007-0625-y. [DOI] [PubMed] [Google Scholar]
- 27.Daoussis D, Panoulas VF, Antonopoulos I, et al. Cardiovascular risk factors and not disease activity, severity or therapy associate with renal dysfunction in patients with rheumatoid arthritis. Ann Rheum Dis. 2010 Mar;69(3):517–521. doi: 10.1136/ard.2008.105049. [DOI] [PubMed] [Google Scholar]
- 28.Immonen K, Finne P, Gronhagen-Riska C, et al. A marked decline in the incidence of renal replacement therapy for amyloidosis associated with inflammatory rheumatic diseases - data from nationwide registries in Finland. Amyloid. 2011 Mar;18(1):25–28. doi: 10.3109/13506129.2010.549252. [DOI] [PubMed] [Google Scholar]
- 29.Al-Aly Z, Zeringue A, Fu J, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010 Nov;21(11):1961–1969. doi: 10.1681/ASN.2009121210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008 Dec 15;59(12):1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum. 2008 Sep;58(9):2612–2621. doi: 10.1002/art.23811. [DOI] [PubMed] [Google Scholar]
- 32.Davis JM, 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008 Sep;58(9):2603–2611. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders HJ, Rihl M, Loch O, Schattenkirchner M. Prediction of creatinine clearance from serum creatinine in patients with rheumatoid arthritis: comparison of six formulae and one nomogram. Clin Rheumatol. 2000;19(1):26–29. doi: 10.1007/s100670050006. [DOI] [PubMed] [Google Scholar]
- 34.Anders HJ, Rihl M, Vielhauer V, Schattenkirchner M. Assessment of renal function in rheumatoid arthritis: validity of a new prediction method. J Clin Rheumatol. 2002 Jun;8(3):130–133. doi: 10.1097/00124743-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Estimating renal function in lupus nephritis: comparison of the Modification of Diet in Renal Disease and Cockcroft Gault equations. Lupus. 2007;16(11):887–895. doi: 10.1177/0961203307084167. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Jama. 2012 May 9;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012 Feb;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]