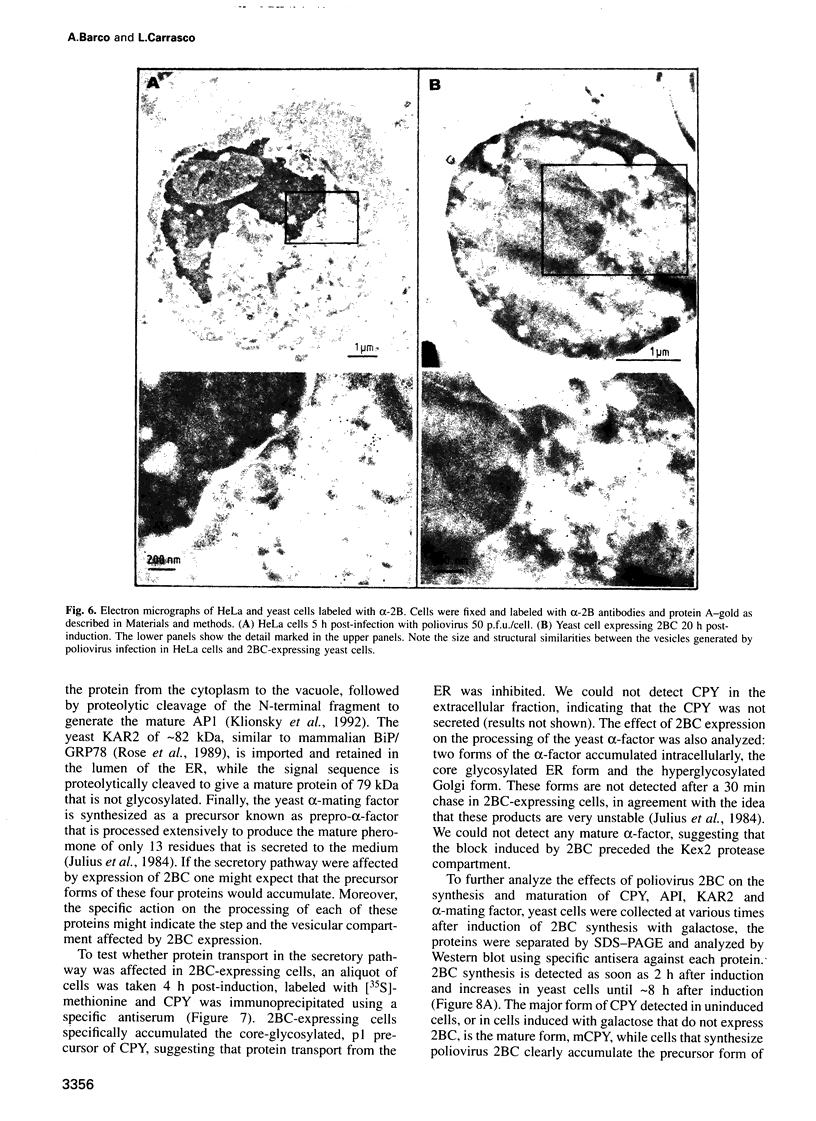
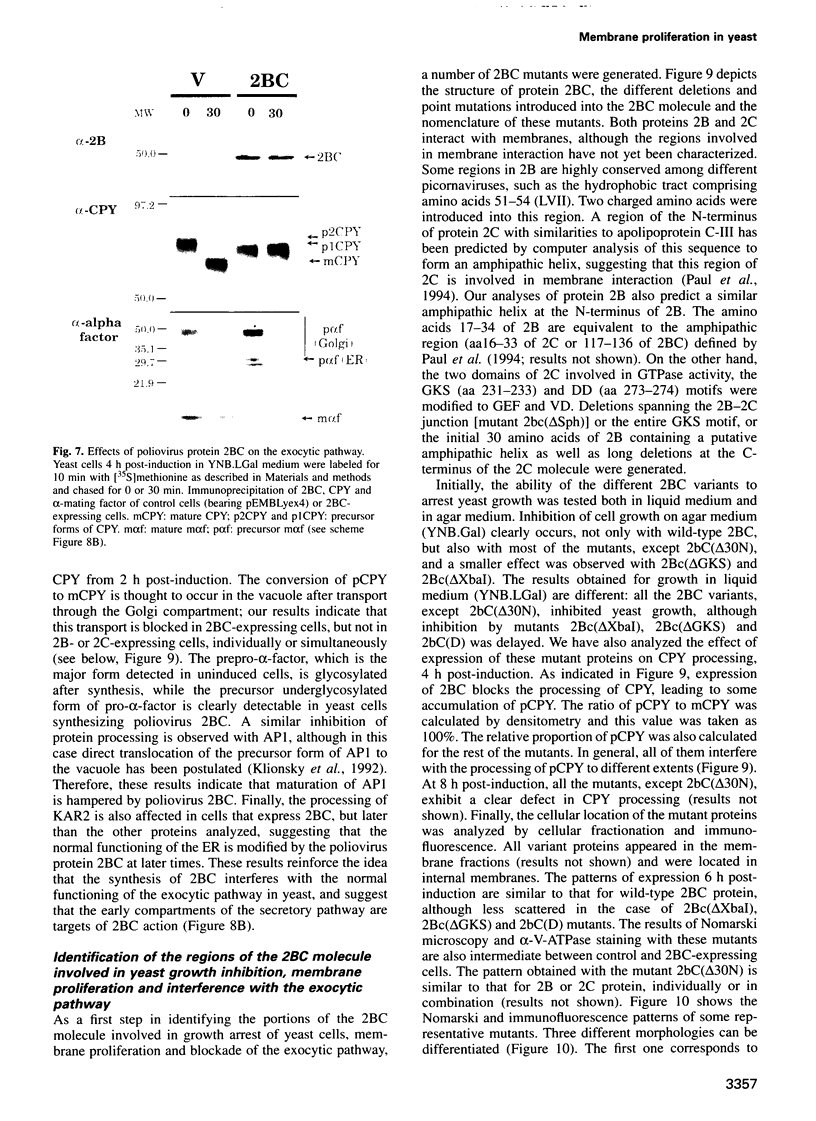
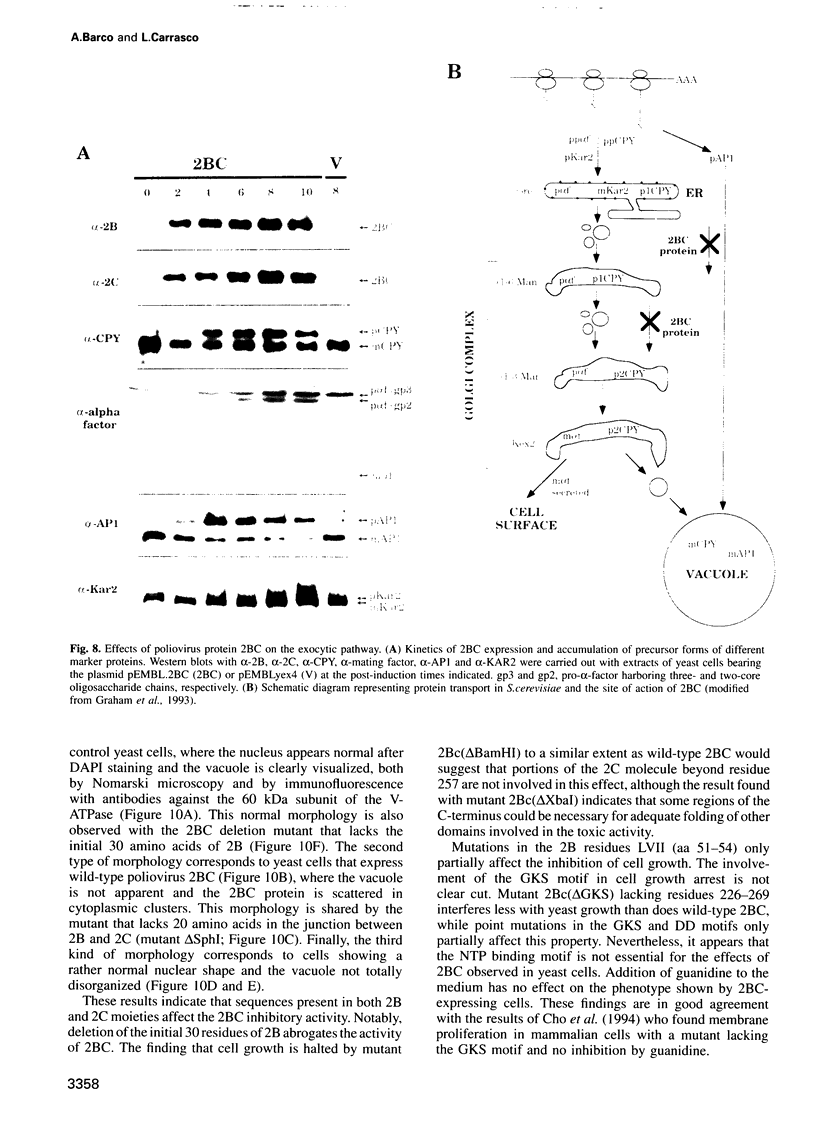
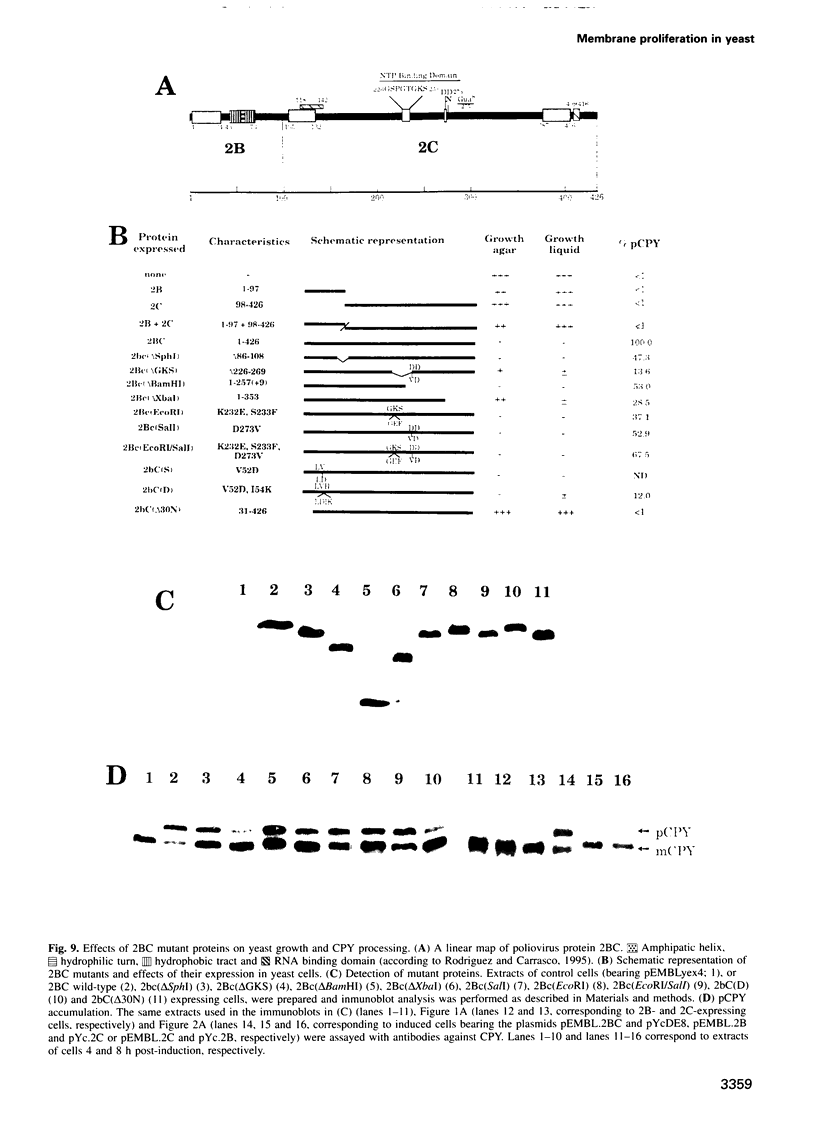
Abstract
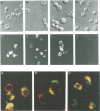
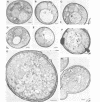
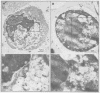
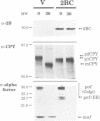
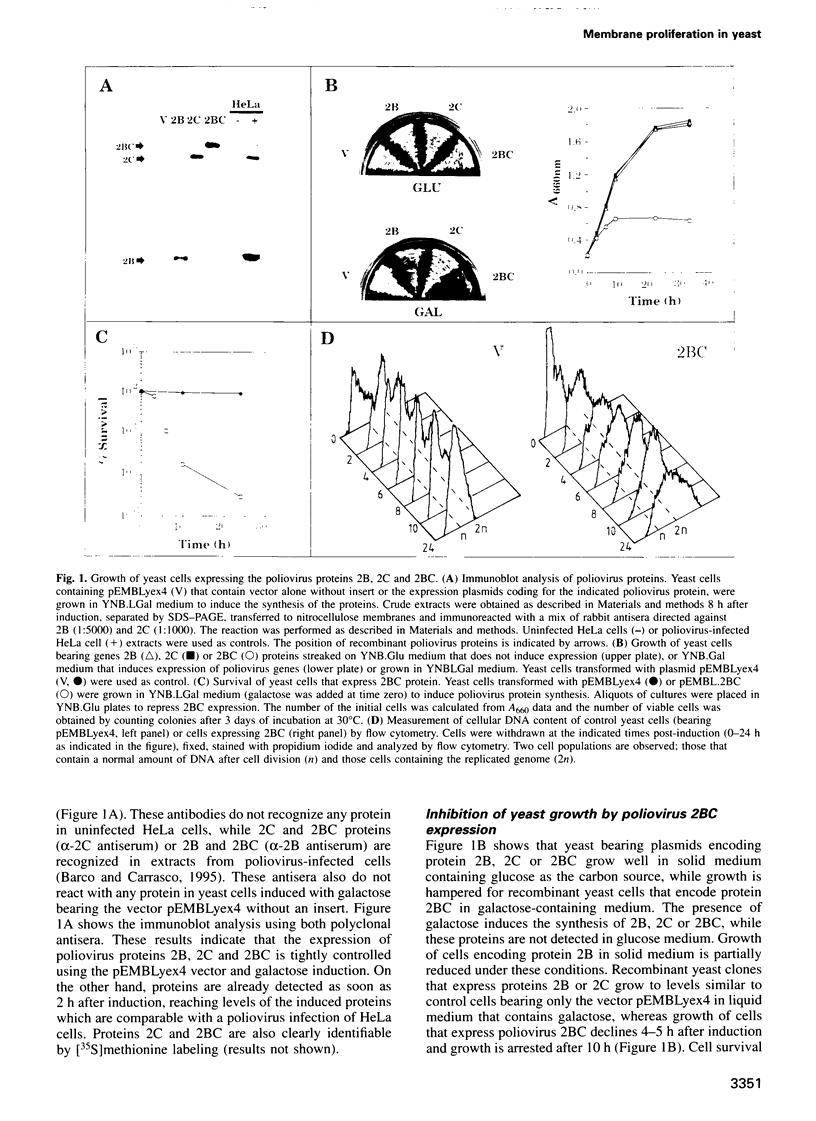
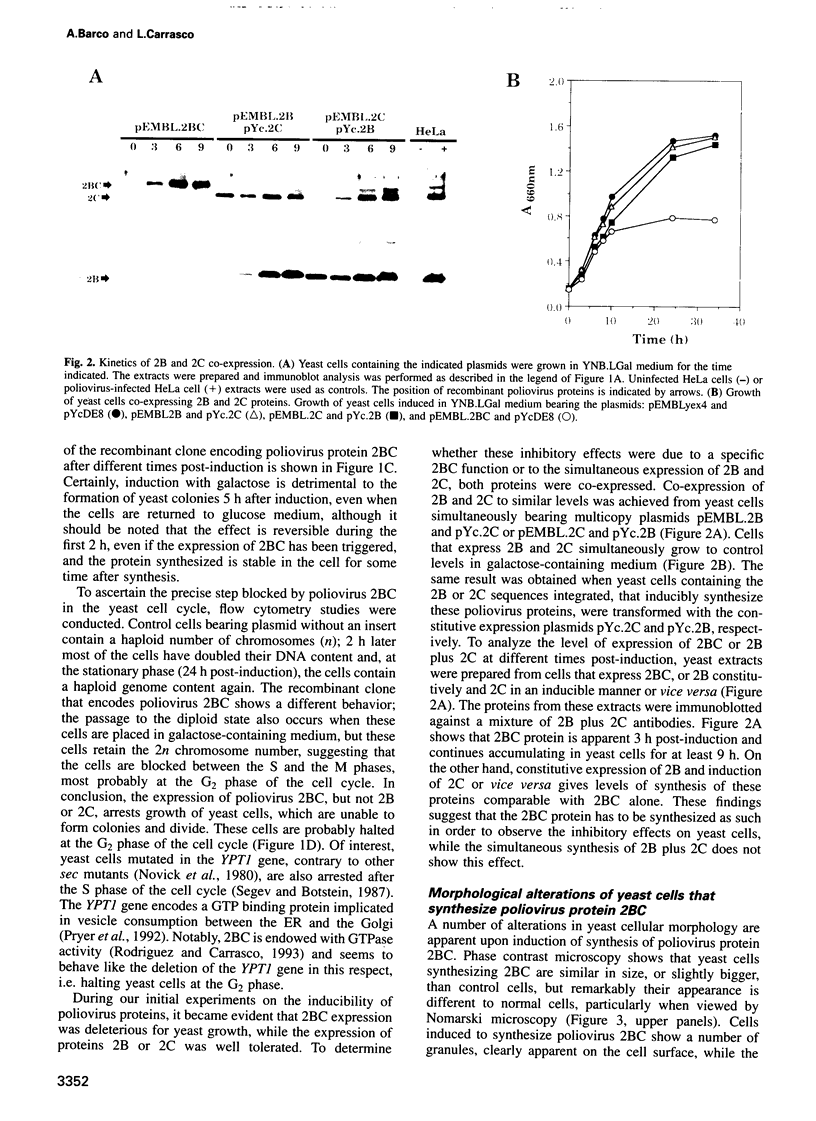
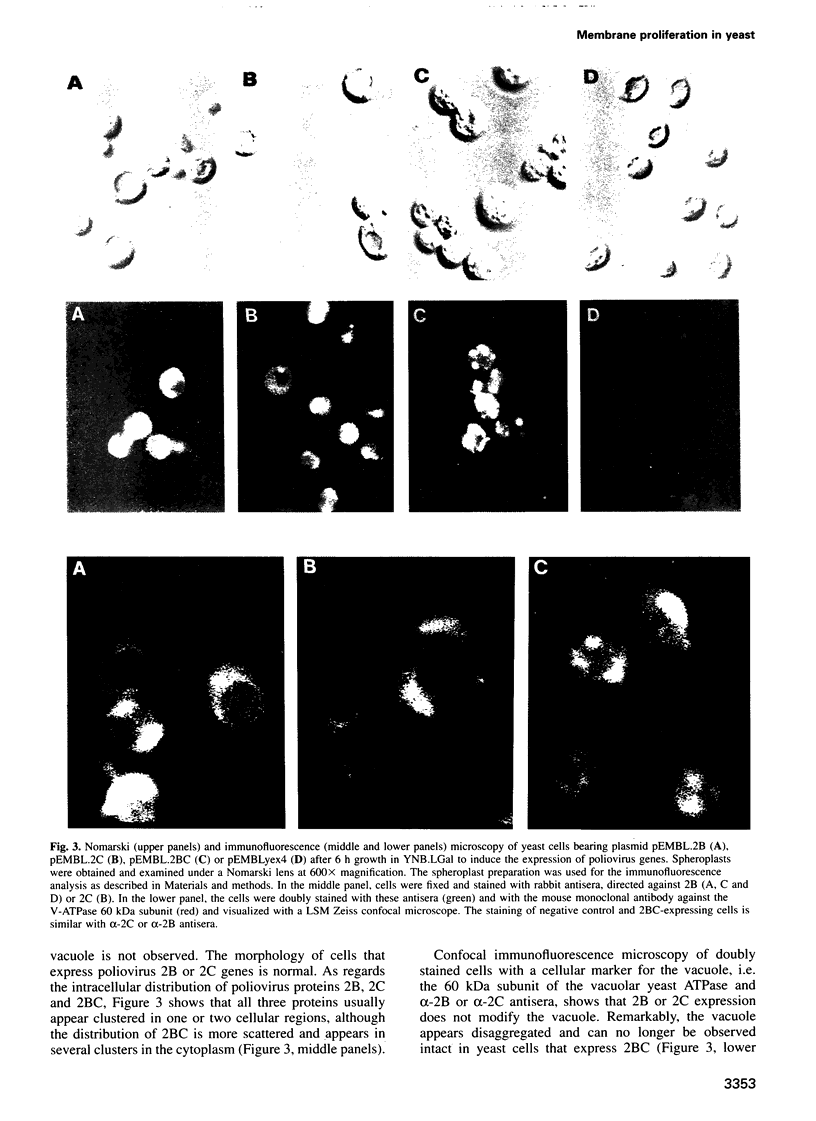
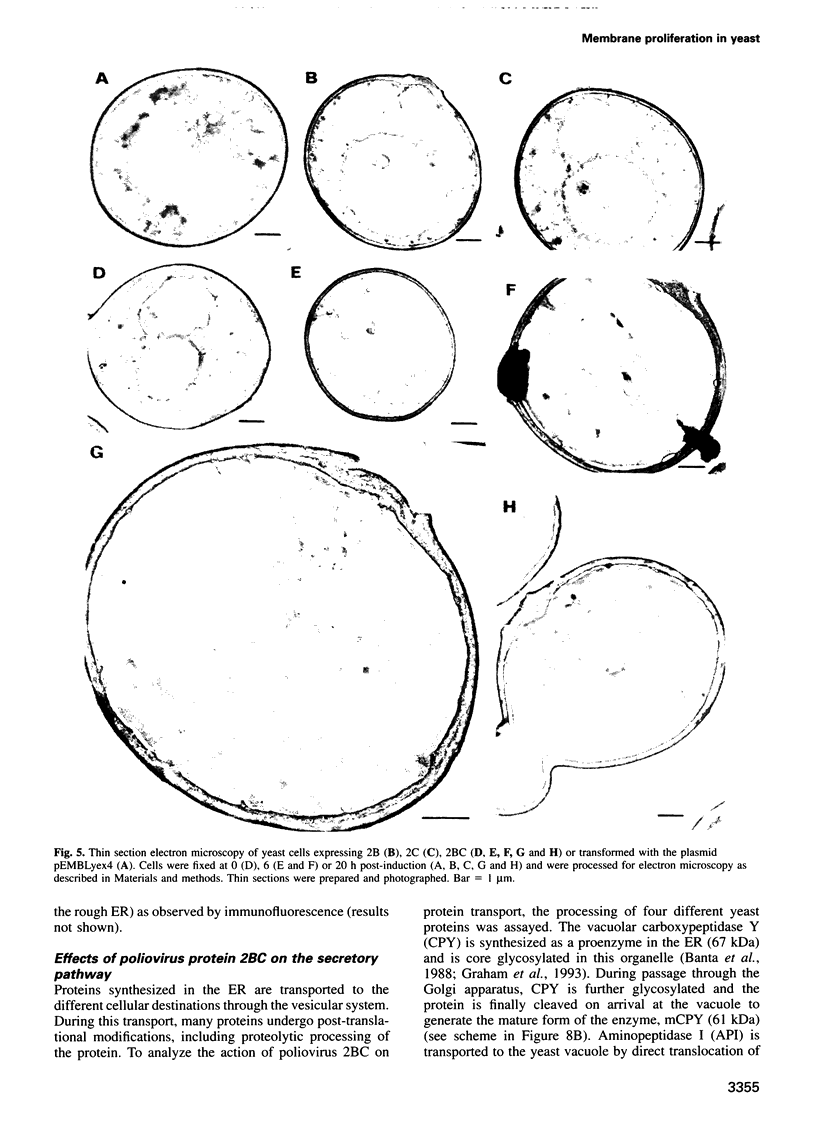
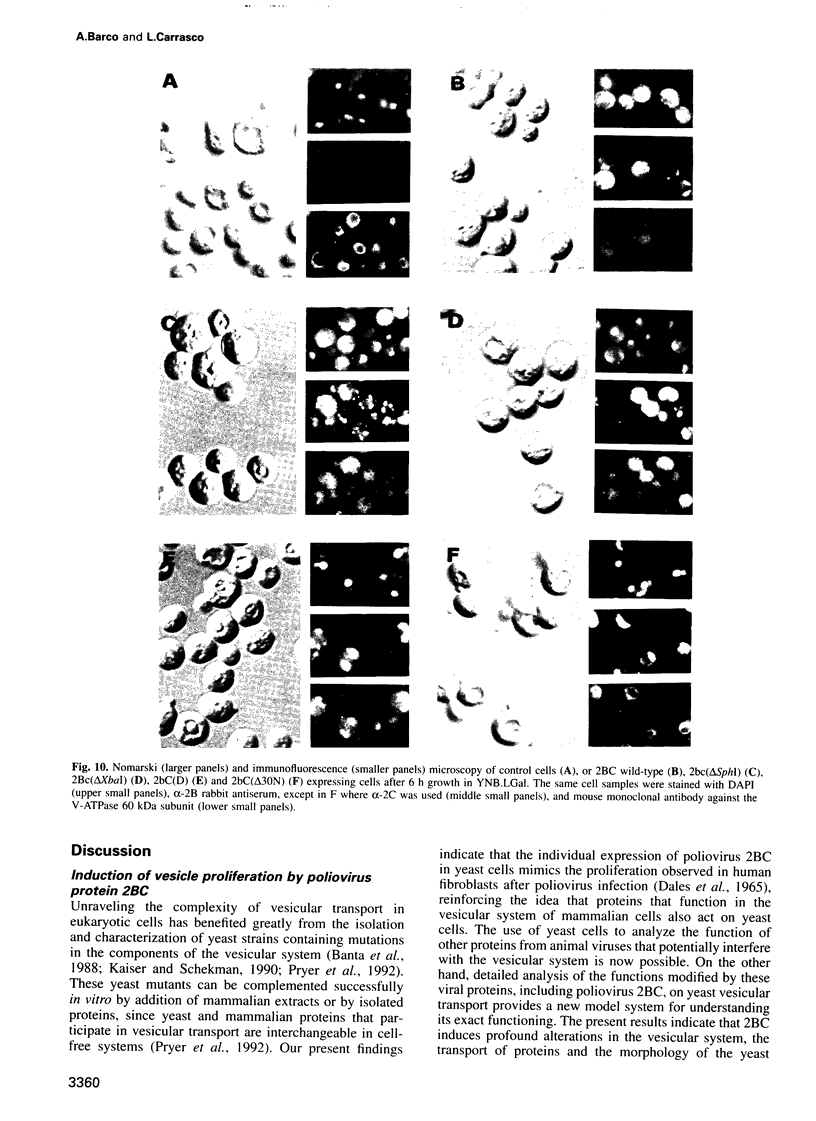
Inducible synthesis of poliovirus protein 2BC in Saccharomyces cerevisiae arrests cell growth in the G2 phase of the cell cycle, while no effects are observed upon expression of poliovirus genes 2B or 2C, either individually or in combination. Expression of 2BC induces a number of morphological modifications in yeast cells, one of the most striking being the proliferation of small membranous vesicles that fill most of the cytoplasm. These vesicles are morphologically similar to the cytopathic vacuoles that proliferate during the infection of human cells by poliovirus. The transport and processing of several yeast proteins, including vacuolar carboxypeptidase Y, aminopeptidase I or yeast alpha-mating factor, is hampered upon expression of poliovirus 2BC, suggesting that transport of proteins through the Golgi apparatus is impaired by this viral protein. Finally, a number of 2BC variants were generated and the effects of their expression on yeast growth, cellular morphology and protein processing were analyzed. 2BC variants defective in the NTPase activity were still able to interfere with yeast growth and the exocytic system, while deletion of 30 amino acids at the N-terminus of 2BC impairs its function. These findings lend support to the idea that 2BC, but not 2B or 2C, is the protein responsible for vesicle proliferation in poliovirus-infected cells. In addition, the activity of a human virus protein in yeast cells opens new avenues to investigate the exact location at which poliovirus 2BC interferes with the vesicular system and to test the action of other animal virus proteins potentially involved in modifying the vesicular system in mammalian cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldabe R., Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun. 1995 Jan 5;206(1):64–76. doi: 10.1006/bbrc.1995.1010. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Malehorn D. E., Emr S. D., Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989 Apr;108(4):1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta L. M., Robinson J. S., Klionsky D. J., Emr S. D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988 Oct;107(4):1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A., Carrasco L. Cloning and inducible synthesis of poliovirus non-structural proteins in Saccharomyces cerevisiae. Gene. 1995 Apr 14;156(1):19–25. doi: 10.1016/0378-1119(94)00872-p. [DOI] [PubMed] [Google Scholar]
- Benichou S., Bomsel M., Bodéus M., Durand H., Douté M., Letourneur F., Camonis J., Benarous R. Physical interaction of the HIV-1 Nef protein with beta-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J Biol Chem. 1994 Dec 2;269(48):30073–30076. [PubMed] [Google Scholar]
- Bernstein H. D., Sarnow P., Baltimore D. Genetic complementation among poliovirus mutants derived from an infectious cDNA clone. J Virol. 1986 Dec;60(3):1040–1049. doi: 10.1128/jvi.60.3.1040-1049.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Pfister T., Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992 May;66(5):2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983 Nov;131(1):39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Boschelli F. Expression of p60v-src in Saccharomyces cerevisiae results in elevation of p34CDC28 kinase activity and release of the dependence of DNA replication on mitosis. Mol Cell Biol. 1993 Aug;13(8):5112–5121. doi: 10.1128/mcb.13.8.5112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Butterworth B. E., Shimshick E. J., Yin F. H. Association of the polioviral RNA polymerase complex with phospholipid membranes. J Virol. 1976 Aug;19(2):457–466. doi: 10.1128/jvi.19.2.457-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Carrasco L. Modification of membrane permeability by animal viruses. Adv Virus Res. 1995;45:61–112. doi: 10.1016/S0065-3527(08)60058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. W., Teterina N., Egger D., Bienz K., Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994 Jul;202(1):129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. R., Scott P. A., Emr S. D. Brefeldin A reversibly blocks early but not late protein transport steps in the yeast secretory pathway. EMBO J. 1993 Mar;12(3):869–877. doi: 10.1002/j.1460-2075.1993.tb05727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Fuller S. D., Back R., Hollinshead M., Pfeiffer S., Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989 Feb;108(2):277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea R., Carrasco L. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 1990 Jun;9(6):2011–2016. doi: 10.1002/j.1460-2075.1990.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky B. F., Emr S. D. The VPS16 gene product associates with a sedimentable protein complex and is essential for vacuolar protein sorting in yeast. J Biol Chem. 1993 Mar 5;268(7):4953–4962. [PubMed] [Google Scholar]
- Irurzun A., Pérez L., Carrasco L. Brefeldin A blocks protein glycosylation and RNA replication of vesicular stomatitis virus. FEBS Lett. 1993 Dec 28;336(3):496–500. doi: 10.1016/0014-5793(93)80863-p. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Roof L. L. Cloning of the orotidine 5'-phosphate decarboxylase (ODC) gene of Schwanniomyces occidentalis by complementation of the ura3 mutation in S. cerevisiae. Curr Genet. 1988;13(1):29–35. doi: 10.1007/BF00365753. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Cueva R., Yaver D. S. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992 Oct;119(2):287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Baltimore D. An intragenic revertant of a poliovirus 2C mutant has an uncoating defect. J Virol. 1990 Mar;64(3):1102–1107. doi: 10.1128/jvi.64.3.1102-1107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Murray J. A., Scarpa M., Rossi N., Cesareni G. Antagonistic controls regulate copy number of the yeast 2 mu plasmid. EMBO J. 1987 Dec 20;6(13):4205–4212. doi: 10.1002/j.1460-2075.1987.tb02768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Sikorski R., Reed S. I., Vogelstein B. Human p53 and CDC2Hs genes combine to inhibit the proliferation of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Mar;12(3):1357–1365. doi: 10.1128/mcb.12.3.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novoa I., Feduchi E., Carrasco L. Hybrid proteins between Pseudomonas exotoxin A and poliovirus protease 2Apro. FEBS Lett. 1994 Nov 21;355(1):45–48. doi: 10.1016/0014-5793(94)01157-5. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh R., Morrow C. D. A poliovirus minireplicon containing an inactive 2A proteinase is expressed in vaccinia virus-infected cells. J Virol. 1993 Aug;67(8):4621–4629. doi: 10.1128/jvi.67.8.4621-4629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. V., Molla A., Wimmer E. Studies of a putative amphipathic helix in the N-terminus of poliovirus protein 2C. Virology. 1994 Feb 15;199(1):188–199. doi: 10.1006/viro.1994.1111. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Raymond C. K., Yamashiro C. T., Stevens T. H. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- Rodríguez P. L., Carrasco L. Poliovirus protein 2C contains two regions involved in RNA binding activity. J Biol Chem. 1995 Apr 28;270(17):10105–10112. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- Rodríguez P. L., Carrasco L. Poliovirus protein 2C has ATPase and GTPase activities. J Biol Chem. 1993 Apr 15;268(11):8105–8110. [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Jacobson S. J., Najita L. Poliovirus genetics. Curr Top Microbiol Immunol. 1990;161:155–188. doi: 10.1007/978-3-642-75602-3_6. [DOI] [PubMed] [Google Scholar]
- Segev N., Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol. 1987 Jul;7(7):2367–2377. doi: 10.1128/mcb.7.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B., Doms R. W., Ericsson M., Hiller G., Machamer C. E., van 't Hof W., van Meer G., Moss B., Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993 May;121(3):521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershak D. R. Association of poliovirus proteins with the endoplasmic reticulum. J Virol. 1984 Dec;52(3):777–783. doi: 10.1128/jvi.52.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler M., Egger D., Pfister T., Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructural level using single-stranded riboprobes. Virology. 1992 Dec;191(2):687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Griff I. C., Rothman J. E. Proteins involved in vesicular transport and membrane fusion. Curr Opin Cell Biol. 1991 Aug;3(4):615–620. doi: 10.1016/0955-0674(91)90031-s. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Hellen C. U., Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- Wright R., Basson M., D'Ari L., Rine J. Increased amounts of HMG-CoA reductase induce "karmellae": a proliferation of stacked membrane pairs surrounding the yeast nucleus. J Cell Biol. 1988 Jul;107(1):101–114. doi: 10.1083/jcb.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihisa T., Anraku Y. A novel pathway of import of alpha-mannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae. J Biol Chem. 1990 Dec 25;265(36):22418–22425. [PubMed] [Google Scholar]