Abstract
There are multiple roles for purinergic signalling in both male and female reproductive organs. ATP, released as a cotransmitter with noradrenaline from sympathetic nerves, contracts smooth muscle via P2X1 receptors in vas deferens, seminal vesicles, prostate and uterus, as well as in blood vessels. Male infertility occurs in P2X1 receptor knockout mice. Both short- and long-term trophic purinergic signalling occurs in reproductive organs. Purinergic signalling is involved in hormone secretion, penile erection, sperm motility and capacitation, and mucous production. Changes in purinoceptor expression occur in pathophysiological conditions, including pre-eclampsia, cancer and pain.
Keywords: Vas deferens, Testes, Penile erection, Ovary, Uterus, Placenta
Synopsis
Male reproductive organs
Penis and penile erection
Testis
Vas deferens
Seminal vesicles
Epididymis
Prostate
Sperm
Female reproductive organs
Ovary
Oocytes
Fallopian (uterine) tubes/oviduct
Uterus
Myometrium
Endometrium
Cervix
Amnion
Placenta
Vagina
Mammary glands
Conclusions
There is increasing recognition for purinergic signalling in both male and female reproductive organs, which have not been reviewed recently. For full accounts of cancer of various reproductive organs see [62].
Male reproductive organs
Smooth muscle function and secretion in the organs of the male reproductive system are under autonomic nervous control. The seminal vesicles, prostate and related glands as well as the vas deferens and penile vessels are innervated by short sympathetic neurons in the pelvic plexus. There are earlier reviews that include discussion of the involvement of nucleotides and nucleosides in the complex innervation of the male reproductive system ([14,157]; see also [66]). There are also studies of P2X receptor immunoreactivity in male reproductive organs [227] and of the localisation of plasma membrane-bound NTPDases [260]. A high expression and activity of ecto-5′ nucleotidase (CD73) in the male murine reproductive tract has also been reported that may impact male fertility [261].
Penis and penile erection
The actions of adenosine 5′-triphosphate (ATP) on the corpus cavernosum of a wide spectrum of different mammalian species were first described in 1997 [209]. ATP can induce significant relaxations of rabbit cavernosal tissue which was unaffected by removal of the endothelium [402]. ATP has a powerful relaxant effect on corporal smooth muscle at high tone or pre-stimulated tissue, but causes contractions at low tone [443]. Both human and rabbit corpus cavernosal pre-contracted strips relaxed to ATP [232]. The powerful relaxant effect of ATP on the smooth muscle of the dog, rabbit and human corpus cavernosum is about the same as that produced by nitric oxide (NO), and it was suggested that a combination of ATP with an NO donor might be an effective therapeutic approach for erectile disorders [172]. The relaxant effect of ATP and adenosine was greater in mature (24 months old) rabbits than at 3 and 7 months [332].
Intracavernosal injection of adenosine induced a full erection in cats [394,395]. Adenosine also had prejunctional inhibitory actions on excitatory sympathetic cotransmission to the rabbit corpus cavernosum, probably acting via A2B receptors [79]. The effect of long-term testosterone propionate therapy on endothelium-dependent and -independent relaxations of corpus cavernosum strips to purines was examined [192]. It was shown that the NO synthase (NOS) inhibitor, nitro-l-arginine methylester (l-NAME), inhibited the relaxations to adenosine 5′-diphosphate (ADP), but not to ATP or adenosine (suggesting that P2Y1 receptors on endothelial cells might be involved). Relaxations in response to ADP (but not ATP or adenosine) were significantly enhanced by testosterone therapy. In fact, P2Y1 receptor transcripts were later identified on the endothelial cells which line the lacunar space and blood vessels in the penis, but not on corpus cavernosum smooth muscle cells [308]. Functional studies showed that the P2Y1 selective agonist, adenosine-5′-(β-thio)-diphosphate, relaxed the human corpus cavernosum via endothelial release of NO [364]. Nerve-mediated relaxation of human corpus cavernosal smooth muscle strips pre-contracted with phenylephrine is amplified by stimulating P2Y1 and P2Y2 receptors, suggesting a purinergic relaxing mechanism separate from the endothelium NO-mediated relaxing pathway [158]. ATP (in contrast to ADP) acts as a potent and NO-independent relaxant agent of human and rabbit corpus cavernosum, although part of its relaxant effect is attributable to adenosine, after breakdown of ATP, acting via A2A receptors [122].
Two distinct layers of smooth muscle were identified in the penile bulb of rats, an inner layer (parenchyma) and an outer sheet (sac). Transmural stimulation initiated non-adrenergic, non-cholinergic (NANC) inhibitory junction potentials in parenchymal muscle, but excitatory junction potentials (EJPs) in the sac smooth muscle [161]. It was claimed that ATP released from nerves produces relaxation of cavernosum smooth muscle via P2Y4 receptors on smooth muscle, while ADP acting via P2Y1 receptors on endothelial cells produces relaxation via NO [72]. Modulation of cavernosum smooth muscle relaxation occurs by activation of P2Y6 receptors via non-neuronal and non-NO-dependent mechanisms [224]. It was suggested that ADP-sensitive P2Y12 and/or P2Y13 receptors might mediate relaxation of corpus cavernosum through the release of prostanoids [112].
Both ATP and adenosine produced penile tumescence in anaesthetised dogs, probably via adenosine A2A receptors; pelvic nerve stimulation also produced tumescence, but this did not appear to be via A2A receptors [307]. Experiments on isolated rabbit corpus cavernosum suggested that A2B receptors were also involved in relaxation [189]. In another paper, it was claimed that adenosine directly relaxes cavernosal smooth muscle via both A2A and A2B receptor subtypes, while it modulates sympathetic neurotransmission via A1 receptors [404]. Activation of A1 receptors during penile erection results in reduced noradrenaline (NA) release and reduced cavernosal smooth muscle contraction, thereby facilitating penile erection [303]. Impaired adenosine signalling via A1 receptors has been claimed to contribute to erectile dysfunction [304]. The possibility has been raised that elevated adenosine signalling contributes to priapism, a condition of persistent penile erection lasting at least 4 h in the absence of sexual excitation [86,305]. Excess adenosine in penile erectile tissue in mice lacking adenosine deaminase contributes to priapism via A2B receptor signalling [272]. A recent review highlights adenosine signalling pathways operating in penile tissue as significant therapeutic targets for the treatment of erectile disorders [430].
ATP was shown to dilate isolated bovine and canine penile arteries [50]. However, EJPs were recorded in smooth muscle cells of both penile artery and vein in response to nerve stimulation [206], resembling the ATP-mediated EJPs recorded in the vas deferens.
The incidence of erectile dysfunction among patients with renal failure is significantly higher than in the general population. Clinical complaints include an inability to initiate and maintain an erection. The purinergic relaxation responses in an experimental rabbit model of chronic renal failure were examined and the adenosine- and ATP-induced relaxations were not impaired [203]. Corpus cavernosum from men with vasculogenic impotence is partially resistant to adenosine relaxation due to endothelial A2B receptor dysfunction [110]. Activation of A2B receptors on endothelial cells contributes to penile erection via PI3K/AKT signalling cascade-mediated endothelial NOS activation [432]. It was shown in a study of alloxan-induced diabetic rabbits that there was impairment of both direct P2Y4 receptor-induced muscle relaxation and endothelium-dependent ADP P2Y1 receptor-mediated relaxations [72]. The possibility that the responses of the vas deferens to ATP via P2X1 receptors are heightened in overactive ejaculatory reflex associated with premature ejaculation has been considered [1].
Hypothyroidism, where testosterone levels are low, has been shown to lead to impotence in some men. In an experimental rabbit model of hypothyroidism, it was shown that relaxation responses to ATP, α,β-methylene ATP (α,β-meATP) and electrical field stimulation of corpus cavernosum strips pre-treated with phenylephrine were reduced significantly, while relaxations to adenosine were unchanged [448]. Impaired erectile function has been reported in CD73-deficient mice that resulted in reduced endogenous penile adenosine production [431]. Human corpus cavernosum from men with vasculogenic erectile dysfunction exhibit decreased ectonucleotidase NTPDase/CD39 activity leading to persistent extracellular ATP accumulation [111]. As a consequence, alteration of vascular responses of strips of corpus cavernosum from impotent patients to stable ATP analogues may be due to P2 purinoceptor desensitisation. In an earlier paper, this group showed that endothelial dysfunction in men with vasculogenic erectile dysfunction is associated with the loss of adenosine A2B receptor activity in penile vessels [110]. In a later study by Faria and colleagues, it was shown that relaxation of human corpus cavernosum by P2 receptor agonists was severely attenuated in erectile dysfunction patients [113]. They suggest further that relaxation in control subjects may be mediated by ADP-sensitive P2Y12 receptors, with a shift towards activation of P2Y1 and perhaps P2Y13 receptors in erectile dysfunction patients. ATP release from cavernosal tissue is greater in patients following prostatectomy as compared to patients with organic erectile dysfunction [202]. Severe bladder dysfunction secondary to chronic partial bladder outlet obstruction induced morphological, physiological and molecular dysfunction of corpus cavernosum smooth muscle, including significantly decreased relaxation responses to nerve stimulation and to ATP [240].
ATP can contract corpus cavernosum smooth muscle by activating P2X receptors, while it evokes relaxation via P2Y1 and P2Y2 receptors in the diabetic rat [158]. It was suggested that activating ATP based pathways can restore erectile function when NO bioavailability is impaired by diabetes.
Testis
Sperm cells are generated in the testis. Adenosine A1 receptors were identified in rat testis using labelling with 3H-cyclohexyladenosine (CHA) [296]. Steroid production in isolated Leydig cells was induced by adenosine [347]. The rat testes contain a dense population of A1 receptors mediating inhibition of adenylate cyclase activity [387]. Binding studies with CHA in rat testes showed that A1 receptors were localised in Sertoli cells of the seminiferous tubules [284]. A later study by this group showed that treatment of cultured Sertoli cells with pertussis toxin reversed the adenosine-mediated inhibition of cyclic AMP (cAMP) accumulation and potentiated the cAMP response to follicle stimulating hormone (FSH) [185,285]. A low affinity binding site for N6-substituted adenosine derivatives in a rat testis membrane preparation showed the typical pharmacological profile of the cloned rat A3 receptor [265]. The TM4 cell line is derived from a primary culture of Sertoli cells isolated from mouse testis. Selective agonists to both A1 and A2 receptors inhibited proliferation of the TM4 cells [362].
The distribution of P2X receptor subtypes in the rat testis was examined immunohistochemically [145]. P2X1, P2X2, P2X3 and P2X5 and P2X7, but not P2X4 and P2X6, receptors were localised in the testes. Blood vessels displayed P2X1 and P2X2 receptor immunoreactivity. P2X2, P2X3 and P2X5 receptors were expressed in the various germ cell types throughout the different stages of the cycle of the seminiferous epithelium. P2X4 receptor mRNA was expressed in the rat testis [44]. Sertoli cells also showed differential staining for P2X2 and P2X3 receptors during the cycle of the seminiferous epithelium, while P2X7 receptor expression was present throughout all stages. It was suggested that purinergic signalling may play a role in controlling the maturation of germ cell subsets of different developmental ages that exist alongside each other in the adult testis.
The Sertoli cells from the mammalian testis release several proteins and fluid into the lumen of the seminiferous tubules to play a key role in germ cell development. The main messenger of the response of immature Sertoli cells is FSH. A fast and biphasic increase in [Ca2+]i was evoked when Sertoli cells were exposed to ATP [221]. P2 receptors on Sertoli cells are associated with phosphoinositide turnover and are activated by ATP and uridine 5′-triphosphate (UTP) suggesting that P2Y2 or P2Y4 receptors are involved. ATP and UTP had profound effects on FSH responsiveness [123]. Rapid and large accumulation of inositol 1,4,5-trisphosphate (InsP3) in primary cultures of rat and mouse Sertoli calls were evoked by ATP, in keeping with P2Y2 or P2Y4 receptor activation [355]. ATP depolarises and produces an increase in [Ca2+]i from intracellular stores in rat Sertoli cells, consistent with P2Y receptor activation, but it also induces a selective Na+ influx from the extracellular medium consistent with activation of P2X receptors [126]. Prolonged treatment of cultured Sertoli cells with purinergic agonists for A1 and P2 receptors led to an increase in aromatase, γ-glutamyl transpeptidase activities and transferrin secretion [271]. Oestradiol secretion in rat Sertoli cells was produced by extracellular ATP via both P2X and P2Y receptors, which led to increases in both [Ca2+]i and [Na+]i and membrane depolarisation [351]. mRNA for P2Y1, P2Y2 and P2X4 and P2X7 receptors was expressed in cultured rat Sertoli cells [214]. It has been claimed that mitochondria are essential components of Sertoli cell signalling that control purinergic Ca2+ responses, mediated by P2X2 and P2Y2 receptors [414].
During spermatogenesis, germinative cells are supported by Sertoli cells which have their secretory activity precisely regulated by germinative and myoid peritubular cells of the seminiferous tubules that make up the bulk of the testis. Extracellular ATP and its breakdown product adenosine are both involved in this process after ATP and adenosine are released from Sertoli cells during paracrine regulation of the maturation process [141]. Extracellular inosine increases ERK1/2 and p38 phosphorylation in Sertoli cells, possibly through A1 receptor activation [383]. Sertoli cells are key cells in the development and maintenance of stem cell spermatogenesis as well as in the secretion of ATP activated Cl−- and K+-rich fluid into the lumen of seminiferous tubules [20]. Extracellular inosine participates in tumour necrosis factor-α (TNF-α)-induced NO production in cultured Sertoli cells [89]. EctoATPases and adenosine deaminase have been identified and characterised in rat Sertoli cells [26,73].
Leydig cells, lying between the seminiferous tubules in the testis, secrete androgens in response to luteinising hormone (LH) from the anterior pituitary gland. Activation by ATP of rat Leydig cells stimulates testosterone secretion via a mechanism dependent on the influx of Ca2+ from the external medium [128], implying mediation via a P2X receptor subtype. The pharmacological features of the P2X receptor involved were most similar to the P2X2 subtype [324]. Leydig cells produce androgen, which is dependent on androstenedione, the precursor of testosterone synthesis and on activation of the microsomal enzyme 17β-hydroxysteroid dehydrogenase (17βHSD). ATP generation is via the glucose transport system, which is required for the activation of 17βHSD in the last step of androgen biosynthesis [200]. Extracellular pyridine dinucleotides and adenosine modulate the activity of 17βHSD [120]. Sympathetic innervation of human Leydig cells and its influence on the secretion of testosterone probably involves ATP release as a cotransmitter with NA [126].
In patients with cystic fibrosis transmembrane regulator (CFTR)-mediated infertility with azoospermia or severe oligozoospermia, change in the gene expression of the ATP-binding cassette superfamily transporter is involved [223].
Important regulators of the male reproductive system are thyroid hormones, which modulate the extracellular ATP levels in hypothyroid cultured Sertoli cells. The effect of congenital hypothyroidism and thyroid hormone supplementation on NTPDase activities in Sertoli cells influences the actions of adenosine and ATP on reproductive functions throughout development [455].
Germ cell death is part of the regulation of normal testicular function and disruption of this orderly process is associated with several male reproductive disorders. It was considered that the mitochondrial ATP production machinery plays an important role in regulating primary pathways of human male germ apoptosis, although there seems to be a secondary pathway of testicular apoptosis that does not require mitochondrial ATP production [106] and perhaps involves P2X7 receptors. The antiviral drug, acyclovir, a synthetic purine nucleoside analogue, induces testicular toxicity by adverse effects on the sperm parameters and serum level of testosterone in male rats [293].
Sympathetic and sensory nerves supply the rodent testicular artery and the pampiniform plexus, a venous network that surrounds it. Innervation is largely confined to the capsule of the testes and superficial blood vessels, suggesting a role in the control of temperature. Smooth muscle is present in the testicular capsule of the rat, mouse, rabbit and man. ATP, released as a cotransmitter from sympathetic nerves, stimulates contraction of testicular smooth muscle, probably via P2X1 and/or P2X2 receptors [23]. Using Western blots it has been shown that mouse Leydig cells express P2X2, P2X4, P2X6 and P2X7 receptor subunits and heteromeric P2X2/4/6 receptors may also be present [17].
Vas deferens
The vas deferens is an important organ of the male reproductive system mainly concerned with transporting sperm from the testes to the penis during ejaculation. The vas deferens store mature spermatozoa and is responsible for dispersing sperm into the urethra to be combined with other components of sperm prior to ejaculation. The sympathetic nerve-vas deferens preparation, introduced by Huković in [171] has been used as a model to study autonomic neuromuscular transmission ([64,65]; see [60,67,331,435]).
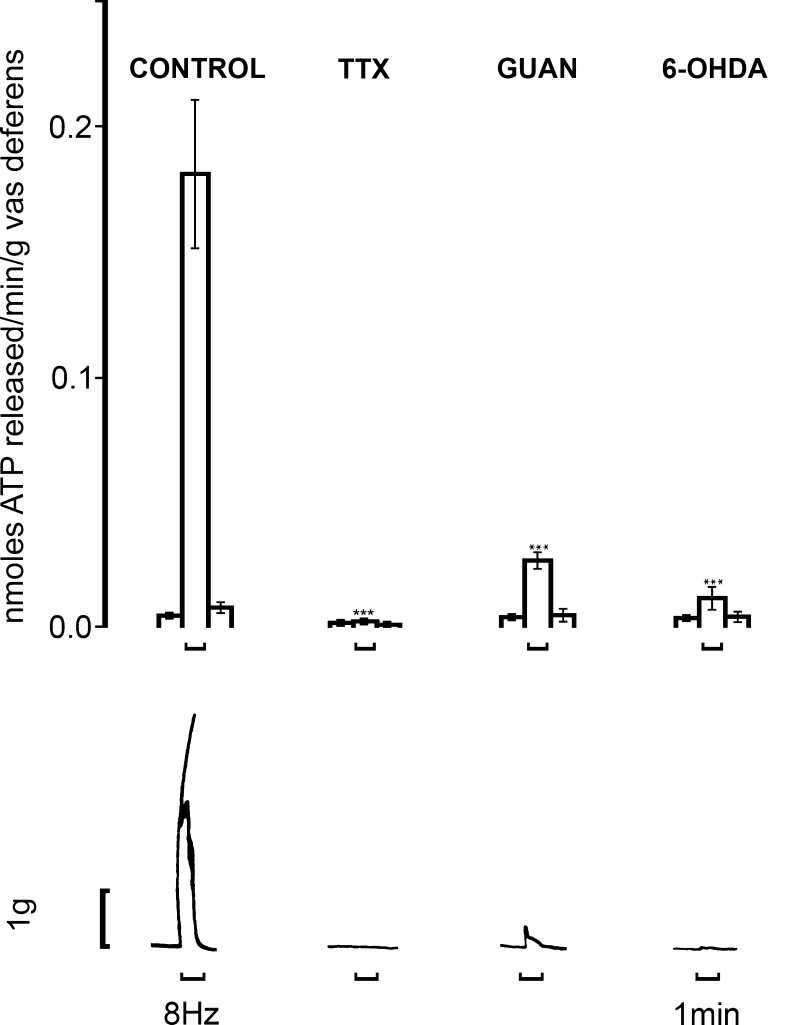
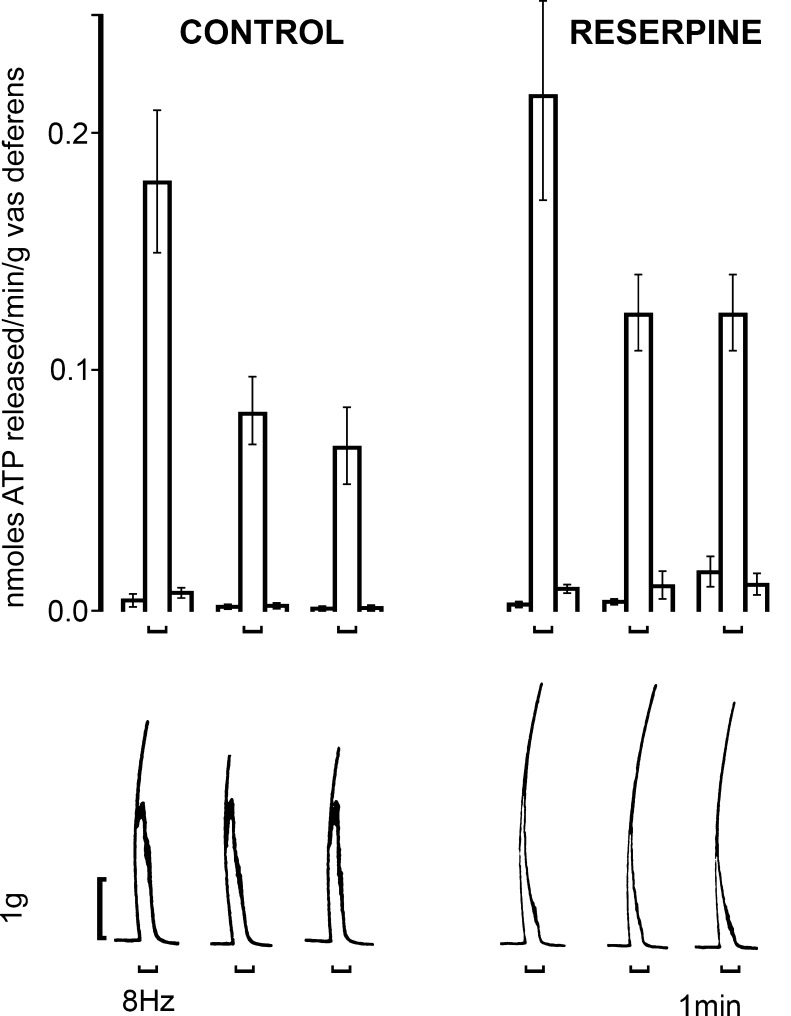
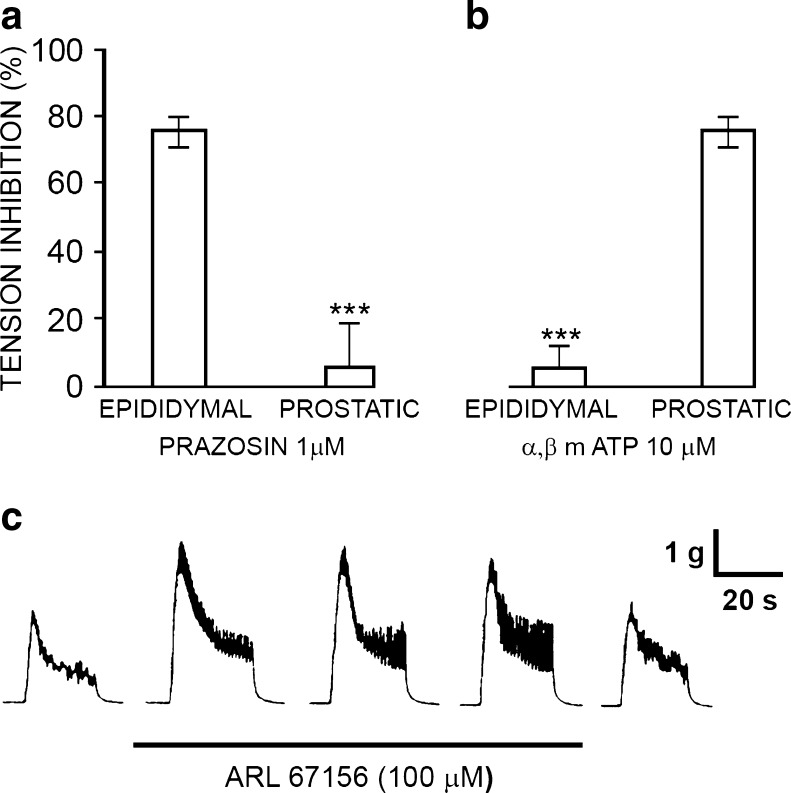
Burnstock and Holman [63–65] studied the electrophysiology of sympathetic neurotransmission in the guinea-pig vas deferens. They showed that EJPs in smooth muscle in response to single nerve pulses summed and facilitated, until at a critical depolarisation threshold spikes were initiated associated with contraction. However, they were puzzled that adrenoceptor antagonists did not abolish the EJPs, NA being established as the sympathetic neurotransmitter at that time. Over 20 years later, it was recognised that ATP, acting as a cotransmitter with NA, was responsible for the EJPs, which were blocked by the ATP receptor desensitiser α,β-meATP [375]. David Westfall and colleagues were the first to demonstrate that ATP produced contraction of the vas deferens when released as a cotransmitter with NA from sympathetic nerves [114,377,434]. The non-adrenergic contractile component of the responses to sympathetic stimulation was antagonised by arylazido aminopropionyl ATP [115], suramin [102,258,374,422], pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; [266]), NF023 [378] and desensitised by α,β-meATP both in vitro and in vivo [12,57,268,375]. 6-Hydroxydopamine (6-OHDA) abolished both adrenergic and purinergic components, supporting the view that they were cotransmitters in sympathetic nerves (Fig. 1; [9]). ATP and its breakdown products ADP, adenosine monophosphate (AMP) and adenosine were detected in the surfusate during transmural stimulation of nerves in the vas deferens [234,235]. As an alternative explanation Neild and Hirst proposed in the mid 1980s that EJPs were due to NA acting on hypothetical γ-adrenoceptors [301]. This was much debated at the time [35,166,376]. However, it was shown that NA, unlike ATP, did not mimic the EJP [377] and that reserpine, which depleted NA but not ATP, failed to affect the rapid component of sympathetic nerve-modulated responses (Fig. 2; [208]), and the γ-hypothesis was abandoned. Direct evidence was presented for concomitant release of NA, ATP and neuropeptide Y (NPY) from sympathetic nerves supplying the guinea-pig vas deferens [187]. A more recent paper described a purinergic component of sympathetic nervous control of the human vas deferens [22]. Rapid emission of sperm into the urethra prior to ejaculation is mediated by the fast purinergic component of the contraction, while the sustained noradrenergic contraction prevents any reflux into the vas deferens during ejaculation. Electrophysiological studies of the vas deferens to study packaged release of ATP from sympathetic nerve varicosities have been carried out (see [34,41,51,53,388,452]). Secretion of transmitters from a single varicosity was shown to be intermittent, with only a small percentage of varicosities releasing transmitters during sympathetic nerve stimulation. All varicosities secrete ATP [186], release is quantal [226] and Ca2+-dependent [251]. There is regional variation, with dominance of purinergic signalling at the prostatic segment of the vas deferens, while noradrenergic signalling was more prominent in the epididymal segment (Fig. 3a and b) [97,211,345].
Fig. 1.
Release of endogenous ATP from control (n = 32), 6-OHDA-pretreated (n = 7), TTX- (16 μM, n = 7) and guanethidine (GUAN)-exposed (5 μg/ml, n = 7) guinea-pig vasa deferentia during field stimulation at 8 Hz (0.5 ms, 20 V). Upper panel: mean ± SEM nmol of ATP released/min per g of vas deferens. Lower panel: contractile responses to field stimulation. The vasa deferentia were stimulated for 1 min as denoted by the upward bracket. ***P < 0.001. (Reproduced from [208], with permission from Elsevier)
Fig. 2.
Release of endogenous ATP from control (n = 32) and reserpine-pretreated (n = 12) guinea-pig vasa deferentia during field stimulation at 8 Hz (pulse width 0.5 ms, 20 V). Upper panel: mean ± SEM nmol of ATP released/min per g of vas deferens. Lower panel: contractile responses to field stimulation. The vasa deferentia were stimulated for 1 min as denoted by the upward bracket. Note that the second slow phase of the mechanical response has gone in the reserpine-pretreated tissue. (Reproduced from [208], with permission from Elsevier)
Fig. 3.
a and b Antagonism of adrenergic and purinergic activity. b Inhibition of the electrically evoked twitches after incubation of the tissues for 10 min with 10 μM α,β-mATP. c Inhibition of the electrically evoked twitches after incubation of the prostatic (n = 10) and epididymal (n = 8) segments with 1 μM prazosin for 20 min. Bars represent SEM. *P < 0.005. Seven separate prostatic and epididymal segments of rat ductus were utilized. Symbols as in b. (Modified from [345], with permission from Elsevier.) c The time course of the effect of ARL 67156 on neurogenic contractions evoked by trains of pulses for 20 s at 2 Hz. The trace shows contractions recorded at 10-min intervals. ARL 67156 (100 μM) was added immediately after the end of the control response (first panel), left in contact with the tissue for 30 min (second, third and fourth panels), then washed out and stimulation repeated (fifth panel). (Reproduced from [436], with permission from John Wiley & Sons)
Neuromodulation of transmitter release is via prejunctional A1 adenosine receptors [255,398] as well as receptors to NA (see [400]), nicotine, γ-aminobutyric acid, NPY, histamine, prostaglandins, angiotensin, opioids, calcitonin gene-related peptide and cannabinoids (see [67,99]). There is postjunctional synergism by the sympathetic cotransmitters NA and ATP [165,201]. NA potentiates the contractile responses of the vas deferens to ATP via a protein kinase C (PKC) mechanism that involves the inhibition of myosin light chain phosphatase and subsequent calcium sensitisation [373]. Prejunctional P2Y receptors have also been shown to inhibit, while P2X receptors facilitate transmitter release [330]. ATP and NA appear to be released from different populations of vesicles (see [104]). Postjunctional neuromodulation also occurs [96,290].
Ectonucleotidase activity has been described on smooth muscle membranes of the vas deferens, including 5′-nucleotidase [219,274]. Sympathetic purinergic neurotransmission was enhanced by the ecto-ATPase inhibitor, ARL67156, in the guinea-pig vas deferens (Fig. 3c; [143,436]). Soluble nucleotidases were shown to be released together with transmitters from sympathetic nerves supplying the vas deferens as a novel mechanism for neurotransmitter inactivation [399,437].
The main smooth muscle receptor to ATP is the P2X1 ion channel receptor, leading to increase in intracellular calcium and the fast component of contraction. P2Y2 receptors mediating contraction of the rat vas deferens have also been claimed [58], while P2Y1 receptors mediate relaxation to ATP [47]. Nifedipine blocked P2X-mediated responses to ATP, but not to NA [250,389]. Clusters of P2X1 receptors on smooth muscle opposite close sympathetic terminal varicosities were described in the mouse vas deferens [27]. P2X1 receptor internalisation has been reported after exposure to the agonist α,β-meATP [105], perhaps underlying the mechanism of desensitisation. Perinuclear immunoreactivity for P2X7-like receptors has been reported in smooth cells of the guinea-pig vas deferens [270]. It has been claimed that P2X2 receptors expressed by interstitial cells of Cajal in vas deferens are involved in semen emission [68].
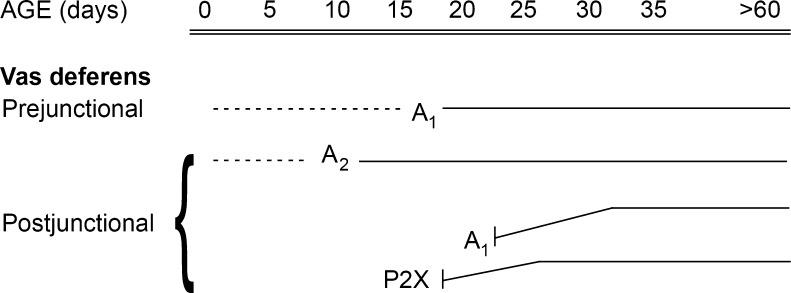
During developmental, changes in purinergic signalling in the vas deferens occur later than in the gut, because rats are not sexually active until about 10 weeks, although the muscle morphology of the vas deferens appeared to be mature by day 35 [131]. EJPs in response to nerve stimulation and ATP were not observed in the vas deferens of mice of less than 18 days postnatal [139]. Another early study showed that at 3 weeks postnatal in the rat (the earliest time studied) the responses of the vas deferens to field stimulation with single or trains of pulses lacked the adrenergic component, although the non-adrenergic (purinergic) component was present [248]. Responses to ATP first appeared at day 15 and increased with age [169]. Adenosine, acting via prejunctional A1 receptors, inhibited nerve-mediated contractions when they were first seen at day 15, but its actions decreased with age [169]. Inhibitory postjunctional A2-like receptors and prejunctional A1 receptors were present from days 10 and 15, respectively ([318]; Fig. 4). They also identified postjunctional excitatory A1 receptors that only appeared after day 20. Expression of P2X receptors also occurred at day 20 [168]. Changes in sympathetic nerve-evoked contractions of the circular muscle layer of the guinea-pig vas deferens showed a significant decrease with increasing age, apparently due to postjunctional rather than prejunctional mechanisms; responses to α,β-meATP decreased in parallel [338]. An increase in P2X1 receptor mRNA expression was shown between postnatal days 10 and 42 [238]. Both pre- and post-junctional mechanisms caused the maturation of fast purinergic junctional transmission of the longitudinal muscle of the mouse vas deferens between 21 and 42 days postnatal [239].
Fig. 4.
Diagram summarizing the development of functional responses mediated by purine receptors in the rat vas deferens. The dashed lines represent ages at which it was not possible to study functional responses, and the solid lines show when responses were observed, with the slope of the line indicating whether a response, in general, increased or decreased with age. (Modified and reproduced from [168], with permission from Elsevier)
By analogy with the release of transmitters from endothelial cells lining blood vessels (see [61]) and urothelial cells in bladder and ureter [212,421], it was shown that prostaglandin E2 was released from epithelial cells of the rat vas deferens in response to neurally released ATP acting via P2Y receptors to mediate neurogenic contractions [353].
Data about the role of ATP as a cotransmitter with NA in the vas deferens from humans is conflicting. Early studies suggested that sympathetic neuromuscular transmission was largely by NA [16,38,163]. However, staining with quinacrine, which indicates high ATP levels, was described in nerve terminals in human vas deferens [11] and the involvement of both P2X1 receptors and α1-adrenoceptors in neurotransmission was reported [22]. A recent paper has shown that there is purinergic transmission to the longitudinal, but not circular muscle, which might explain some of the earlier ambiguities [13]. P2X1 receptor antagonists acting as non-hormonal male contraceptives has been proposed (see [101,294]), but the effectiveness of such drugs in man is not yet established. However, a purinergic cotransmitter pathway in the vas deferens of man appears to be present [101]. In P2X1 receptor-deficient mice, contractions of the vas deferens to sympathetic nerve stimulation were reduced by up to 60 % and there was a 90 % decrease in male fertility [294].
An enhanced initial fast component of cotransmission of the vas deferens in response to sympathetic nerve stimulation from spontaneously hypertensive rats (SHR) was described in 1985 [415]. This appears to be in keeping with reports of a significant increase in the purinergic component of cotransmission from sympathetic nerves supplying blood vessels of SHR (see [333]). A reduction in purinergic prejunctional neuromodulation via A1 receptors in SHR has also been described [176] and other possible sites of adenosine malfunction in hypertension discussed [21]. In streptozotocin diabetic rats after 8 and 12 weeks, there was an increase in the purinergic component of the responses to sympathetic nerve stimulation in the vas deferens [427], although an earlier paper concluded that sympathetic neuropathy occurred in the vas deferens of the streptozotocin-diabetic mouse [188]. Chronic alcohol treatment differentially affects noradrenergic and purinergic responses in the rat vas deferens, perhaps modifying male reproductive tract function [49,197].
Seminal vesicles
Seminal vesicles are a pair of male accessory sex glands that open into the vas deferens before it joins the urethra. They secrete most of the liquid component of semen.
ATP was considered in an early paper as an excitatory transmitter in response to hypogastric nerve stimulation to the guinea-pig seminal vesicle [297]. EJPs in response to nerve stimulation were recorded in the circular muscle of the guinea-pig seminal vesicle, resembling those mediated by ATP in the vas deferens [309]. Evidence was presented that ATP was involved as a cotransmitter in the hypogastric nerves supplying the guinea-pig seminal vesicles [269,320,424]. Endothelin (ET)-1 causes modest tonic contractions of the rat seminal vesicle via ETA receptors and selectively potentiates the motor responses to ATP [246].
Epididymis
The epididymis is a highly convoluted tube about seven metres long in man that connects the testes to the vas deferens. Spermatozoa, entering the epididymis from the efferent ducts of the testes, undergo maturation as they progress through the epididymis in response to contractions.
ATP stimulates via P2 receptors short circuit currents in primary monolayer cultures of rat epididymal cells when applied to the apical, but not to the basolateral side of the monolayer and ATP included in the luminal perfusion solution caused an increase in water and Cl− secretion [231,441]. The authors noted that spermatozoa contain high levels of ATP and it has been proposed that ATP released from spermatozoa may affect anion and fluid secretion by the epididymis. ATP-activated cation conductance in human epididymal cells may be responsible for secreting K+ across the epididymal epithelia, resulting in a much higher K+ concentration in the lumen of the epididymis than in the blood [74]. ATP activates both Ca2+ and cAMP-dependent Cl− conductances in rat epididymal cells [75]. ATP is released from principal cells in the cauda epididymis of mice and evidence presented that the CFTR is involved in the release mechanism [354]. Since mutations of CFTR are a leading cause of infertility, the authors propose that defective ATP signalling in the epididymis might contribute to the dysfunction. It has been claimed that pannexins may play a role in ATP secretion into the epididymal lumen and basal extracellular spaces for functions involving sperm transport and maturation [405].
Evidence was presented that both ATP and NA act as cotransmitters in sympathetic nerves supplying the smooth muscle of the rat cauda epididymis involving P2X and α1-adrenoceptors, respectively [416]. Prejunctional inhibitory neuromodulation of nerve-mediated contractions by P1 and possibly P2Y receptors was later proposed [417]. Both A1 and A2A receptor subtypes appear to be involved [162]. The sympathetic nerves supplying the cauda epididymis mediate the ejaculatory contraction at orgasm, with both cotransmitters NA and ATP involved. When α,β-meATP, which is a selective desensitiser of purinergic transmission, was injected directly into the cauda epididymis, fertility of male rats was impaired [335].
Using immunohistochemistry and RT-PCR, P2X1, P2X2, P2X4, P2X5 and P2Y1 and P2Y2 receptor subtype protein and mRNA were identified in the mouse epididymis and shown to mediate Cl− fluid secretion from epididymal epithelium and sperm maturation [365]. In a more recent paper, P2X3 and P2X6 receptor genes were also identified in rat epididymal epithelial clear cells as well as A1, A2B and A3 adenosine receptor genes [33]. They suggest that activation of these receptors may play a significant role in luminal acidification in the epididymis, a process that is crucial for the establishment of male fertility.
Prostate
The fluid secreted by the prostate during ejaculation consists of about a third of the volume of semen. Nerve stimulation responses of the smooth muscle of the prostate gland were inhibited by the prejunctional action of adenosine [327]. ATP and UTP caused an increase in outward current and hyperpolarisation of isolated rat prostate secretary epithelial cells, perhaps controlling exocrine secretions [204].
ATP is an excitatory cotransmitter with NA to the smooth muscle and fibromuscular stroma of the prostate gland of rat and guinea-pig [56,418]. The P2 receptor antagonist, suramin, attenuates nerve-mediated contractions of the guinea-pig prostate [225]. P2 receptors have been shown to be present in the human prostate, including P2Y1 receptors [181] and P2X1 receptors [205,244]. P2X1 receptors were also expressed in the rat prostate [227]. Human prostate epithelial cells express P2X1, P2X2 and P2X7 receptors [371]. It was shown further that expression of these receptors is low in healthy men, but with the development of prostatic cancer their expression increases. Prostate expression of P2X1, P2X2 P2X5 and P2X7 receptors in rats decreases with age, while expression of P2X3, P2X4 and P2X6 receptors increases [369]. Ectonucleotidases have been identified in the human prostate [215]. Histamine, acting via H1 receptors, potentiates nerve-mediated contractions of the guinea-pig prostate by postjunctional enhancement of the response to ATP [196]. ATP modulates the release of NA from sympathetic nerves supplying the prostate via two different prejunctional receptors, namely A1 and P2Y receptor subtypes [289]. Activation of adrenergic receptors in the rat prostate triggers the release of ATP both in vitro and in vivo [295].
A2B receptors were claimed to be present on human prostatic stromal cells [83]. Adenosine A1 and A2A receptors modulate α1-adrenoceptor-mediated contractions of human cultured prostate stromal cells [328]. Transgenic mice with disrupted A2A receptors have reducted nerve-mediated contractions of the prostate [155], suggesting that prejunctional A2A receptors facilitate sympathetic neurotransmitter release.
Purinergic drugs have been considered for the treatment of benign prostatic hyperplasia [15]. Injection of botulinum toxin, which is known to reduce release of ATP as well as acetylcholine [249], into the prostate have been used to treat bladder obstruction hyperactivity, since it decreases prostate size and thereby improves urine flow rate [82].
Multiple P1 and P2 receptors are expressed by prostate cancer cells [78,180,363,428]. For a detailed coverage of the involvement of purinergic signalling in malignant prostatic hyperplasia see [62].
Sperm
ATP is obligatory for sperm movement. Sperm develop their motile capacity during maturation in the epididymis, during which sperm exonemes change their sensitivity to ATP [447]. Measurement of the ATP concentration in semen has been used to estimate the motility and energy status of human spermatozoa and has been recommended as a method to define optimal conditions for sperm function [325,380], although other groups concluded that ATP measurement had only limited value in the evaluation of semen quality [267,273]. Deficient generation of ATP may cause low sperm motility in some, but not all, conditions of sperm infertility [242]. In P2X1 receptor knockout mice, there was diminished fertility with decreased numbers of spermatozoa in the ejaculate [294].
Changes in ATP content of sperm depends on temperature [71,379]. Spermatozoal ATP concentrations decrease during passage through the epididymis [129]. High flagellar beat efficiency that occurs during hyperactivation is related to a fall in intracellular ATP levels [182]. ATP-supplemented medium has been used for the conservation of human semen for use in artificial insemination [91]. ATP concentration and ATP/ADP ratios in human sperm appear to be unrelated to fertility [419]. Reactive oxygen species, which inhibit sperm motility, is associated with loss of intracellular ATP and motility of spermatozoa ceased when the concentration of ATP was reduced by 85 ± 5 % [88]. Prostasomes transmit signalling complexes between acinar epithelial cells of the prostate and sperm cells and have the capacity for ATP formation [349].
Evidence was presented that an ATP receptor in the egg membrane may be the recipient target for ATP released from sperm, where ATP-induced increase in sodium permeability mediates the initial sperm and egg signal in the fertilisation process [218]. Guanethidine-induced sympathectomy delayed epididymal transit, but did not produce qualitative changes in sperm [195].
In the trout, extracellular ATP and adenosine were shown to influence the in vitro proliferation of spermatogonia and it was suggested that they may be released from nerves to influence the induction, speeding up, then slowing down of spermatogenesis [243]. In humans, extracellular ATP induces significant increase of sperm fertilising potential and the use of ATP for in vitro treatment of spermatozoa during in vitro fertilisation (IVF) for male factor infertility was proposed [350]. Preincubation of fresh spermatozoa with adenosine before the transient co-incubation IVF can also improve the monospermy rate in pigs [137]. Freeze-thawing spontaneously activated sperm motility did not negatively affect fertility in the carp [48]. The post-thaw motility of avian sperm was improved with a combination of ATP and dimethylacetamide [42]. Cryopreserved sperm penetrated rat oocytes when the gametes were cultured in an ATP- and dibutyryl cAMP-containing medium and the resultant embryos formed blastocysts [445].
While early papers considered the role of ATP in terms of its role largely as an energy source for mitochondrial respiration in sperm, from the early 1990s the possible roles of ATP and its breakdown product, adenosine, were also considered as extracellular signalling molecules. For example, the stimulatory affect of adenosine by regulating adenylate cyclase activity in the fertilising ability of mouse sperm in the early stages of capacitation, i.e., the physiological changes needed for sperm to penetrate and fertilise an egg [164,386]. Ecto-5′ nucleotidase was abundantly detected in the corpora lutea of the ovaries; it showed changes during the oestrous cycle, being maximum coinciding with female sexual receptivity. It was speculated that the high levels of adenosine generated at that time might contribute to sperm capacitation, thus significantly influencing fertility [7]. Adenosine was shown to act via A2 receptors and adenosine stimulates sperm motility via A2 receptors [119,132] and to stimulate human sperm motility via A2 receptors [134,366]. In a later study A1 receptors were also identified on epididymal bovine spermatozoa [277–279]. A1 receptor agonists induce calcium release and transient InsP3 increase, capacitance and increase in acrosome reaction rate in human sperm cells [10]. A significant reduction in the number of pups produced by A1 receptor knockout mice suggests that A1 receptors must be fully operative to accomplish the optimal degree of capacitation and thereby fertilisation [280] involving modulation of classical Ca2+-dependent PKC isoforms and upregulation of the ERK1/2 phosphorylation [281]. In the hamster, an influx of calcium accompanies capacitation [439] and this may be induced by extracellular ATP. Extracellular ATP was shown to be a trigger for the acrosome reaction in human spermatozoa, via P2 receptors but the subtype involved was not clear at that time [125]. In a later paper from this group it was suggested that a P2X receptor was involved that mediated transient Na+ influx [127]. Later it was claimed that only a limited population of human spermatozoa has the potential to undergo the acrosome reaction stimulated by ATP and progesterone [401]. Another study suggested that ATP induces acrosomal exocytosis via a P2Y receptor. This leads to an elevation of [Ca2+]i and to activation of PKCα, which phosphorylates proteins, participating in the cascade leading to the sperm acrosome reaction [247].
Guanine, guanosine, inosine and adenosine were found in large amounts in human seminal plasma and more significantly higher in the seminal plasma of oligozoo- and azoospermic men [109]. Guanosine triphosphate had earlier been shown to regulate sperm adenylate cyclase activity [164]. Both fertilising promoting peptide and adenosine stimulate capacitation and inhibit spontaneous acrosome loss in epididymal mouse spermatozoa [138].
A detailed study of the expression of P2X receptor subtypes on the various germ cell types throughout the different stages of the cycle in the seminiferous epithelium within which spermatogenesis takes place from sperm spermatogonia through spermatocytes to spermatids [145]. P2X2 and P2X3 receptors were always observed together on the same cells at the same stages; P2X7 receptor expression was present throughout all stages, P2X5 receptors were expressed in the later stages. It was suggested that purinergic signalling may play a role in the maturation of germ cells at the different developmental ages that exist alongside each other in the adult testis (see Table 1). P2X1, P2X2, P2X3 and P2X4 receptors were localised in the head, probably on the acrosome, of immature sperm in human, mouse and hamster caput epididymis, but this staining was reduced progressively through the epididymis and was absent on mature sperm in the cauda epididymis, except in humans where P2X4 receptors were retained in the cauda epididymis [24] These changes in localisation of P2X receptors were coincident with the maturational changes seen in sperm as they travel through the epididymis, suggesting a role for purinergic signalling in sperm maturation and possibly fertility. P2X2 receptors have been identified on mouse epididymal sperm, localised to the sperm midpiece [241]. ATP-induced current on mouse spermatozoa was mediated by P2X2 receptors; however, despite the loss of ATP-gated current, spermatozoa from P2X2 knockout mice had normal progressive motility, hyperactivated motility and acrosome reactions [300]. Nevertheless, fertility of male P2X2 knockout mice declined with frequent mating over days, suggesting that P2X2 receptors add a selective advantage under these conditions.
Table 1.
Summary of P2X-immunopositive cells in the seminiferous tubules throughout the l4 stages of the seminiferous epithelium
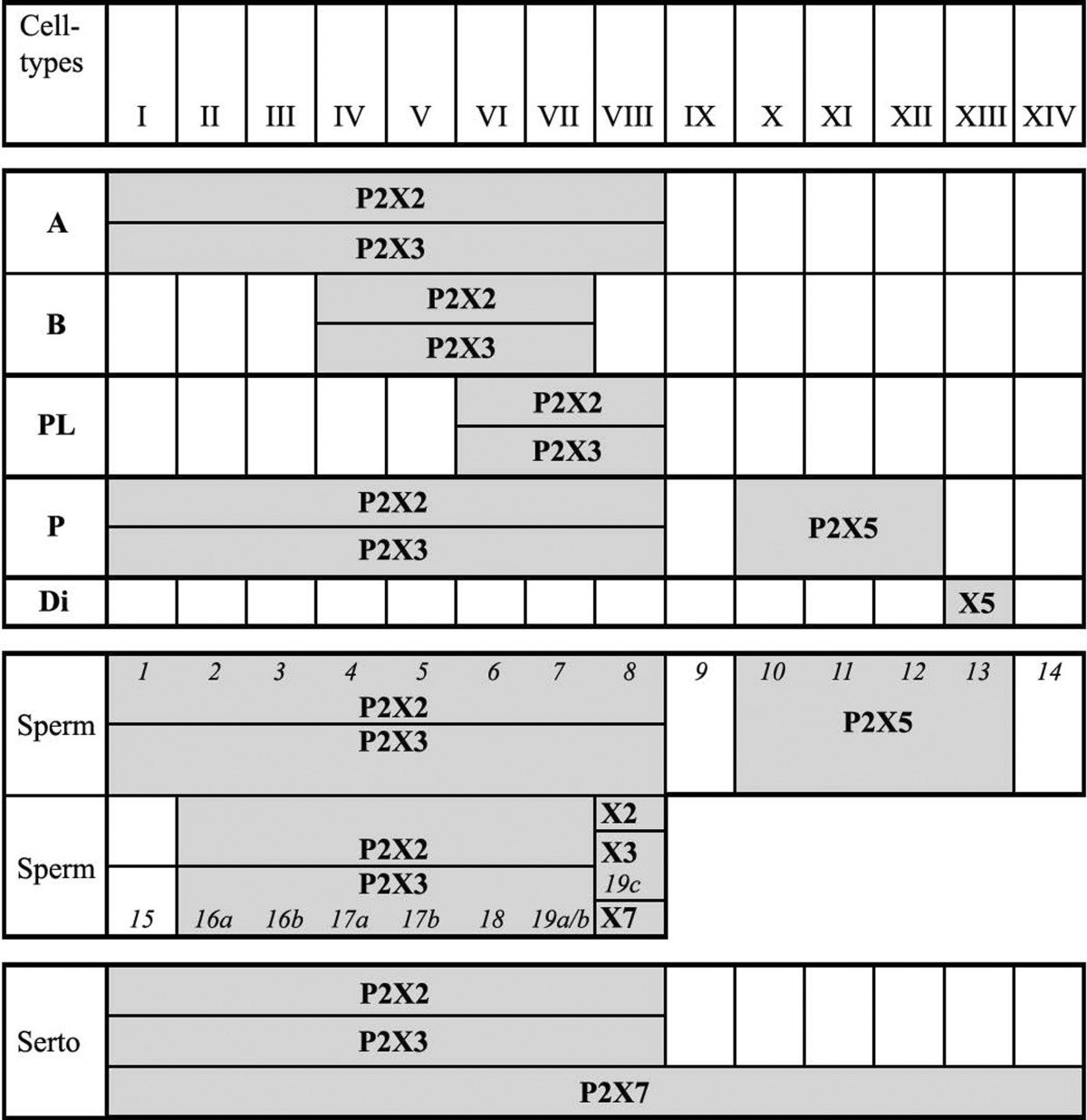
Reproduced from [145], with permission from Karger
The stages of the cycle of the seminiferous epithelium are given in roman numerals. Shaded boxes indicate the presence of immunopositive cells for a single P2X receptor subtype throughout the respective stages of the cycle. Italic numerals indicate the developmental steps of spermatid maturation. Throughout stages I to VIII younger (1–8) and older (16a–19c) generations of spermatids coexist, whereas through stages IX to XIV only one generation of developing spermatids is present
X2 P2X2 receptor, X3 P2X3 receptor, X5 P2X5 receptor, X7 P2X7 receptor, A type A spermatogonia, B type B spermatogonia, P pachytene spermatocytes, Di diplotene spermatocytes, PL preleptotene spermatocytes, Sperm spermatids, Serto Sertoli cells
Sperm preincubation with oestradiol 17βE2 inhibited the effects of extracellular ATP on sperm plasma membrane potential variations and the acrosome reaction [352]. Extracellular ATP improves human sperm motility parameters and provides a rational explanation for increased IVF percentages when sperm is treated with ATP [103]. The effects of ATP on acrosomal exocytosis, protein tyrosine phosphorylation and sperm maturity parameters were quantified. While ATP did not affect acrosomal exocytosis or protein tyrosine phosphorylation in sperm from healthy donors, it significantly altered several motility parameters, with the largest effect manifested in increased curvilinear velocity and percentage hyperactivation. ATP similarly affected sperm selected for poor motility and thawed cryopreserved sperm, although to a lesser extent on sperm with normal motility. These important findings constitute a novel therapeutic modality in the treatment of male infertility. Extracellular ATP was shown to similarly alter motility and improve the fertilising capacity of mouse sperm [344].
Spermatozoa possess a high level of ecto-ATPase activity [256]. The effect of ATP and adenosine on sperm function could be regulated by hydrolysis of ATP, ADP and AMP by ecto-NTPDases and ecto-5′-nucleotidases identified in human spermatozoa [346].
In asthenozoospermic or oligoasthenozoospermic patients with less than 1,000,000 round spermatid cells/ml, the concentration of ATP is significantly lower than normal [70]. Semen ATP content was not helpful in predicting the occurrence of pregnancy in which the female partner was normal and the male partner had sperm concentrations of 720 × 106/ml [442]. The enzymatic treatment of spermatozoa with a trypsin solution enhanced ATP concentration and improved motility [121]. Control of anion and fluid secretion by ATP released from sperm during transit was acting via P2 receptors [441].
In summary it appears that ATP is involved in sperm motility and fertilising ability by two independent mechanisms: on the presence of high intracellular levels of ATP to supply the energy for mitochondrial respiration; and release of ATP from sperm during transit to regulate their microenvironment by acting on receptors for both ATP (probably via P2Y1 receptors) and its breakdown product adenosine (via A1 receptors) to activate the acrosome reaction to facilitate motility and fertilisation. Treatment of sperm with ATP used for IVF is now being explored. For example, a recent paper showed, with IVF experiments in mice, that treatment of sperm with ATP improved the in vitro fertility rate of genetically modified (transgenic and knockout) mice with low fertility [411].
Female reproductive organs
Purinergic signalling has been identified in the ovary, oviduct, amnion, oocytes, uterus and cervix, placenta and umbilical vein, vagina and mammary glands.
Ovary
The principal source of estrogens and androgens in the female are ovarian granulosa and theca cells.
A surge of pituitary LH 12 h prior to ovulation stimulates oocyte maturation. In rabbit isolated ovarian follicles ATP inhibited LH-stimulated testosterone accumulation [245]. A 7-fold amplification of LH-stimulated cAMP accumulation and progesterone secretion in rat luteal cells was produced by adenosine, but it did not have a similar effect on LH-stimulated cAMP accumulation and androgen secretion in Leydig cells [159]. Adenosine had predominantly inhibitory actions on hormone-induced granulosa cell differentiation [210]. Adenosine acting via A2 receptors stimulated adenylate cyclase in rat ovarian membrane preparations and preovulatory granulosa cells [37]. Progesterone secretion by rat granulosa cells was regulated by AMP-activated protein-kinase [403]. Adenosine and prostaglandin F2α are possible regulators of luteal cell function, acting by local control of the action of LH [31]. This group later showed that there was no effect of adenosine on androgen secretion in Leydig cells. Rather, adenosine produced marked amplification of FSH-stimulated cAMP accumulation and steroid secretion from granulosa cells from both rat and human ovaries [32,323]. Luteal cell ATP is rapidly depleted by LH. This appears to be a physiological event as a result of cells undergoing apoptosis (i.e., luteolysis) at the end of the pseudopregnant cycle [382].
Gonadotrophins as well as adenosine can affect ATP levels in granulosa cells [36]. The effect of purine nucleotides on human and porcine granulosa cells was examined using luteinised cells [184]. The authors suggested that the results indicated the presence of P2U (i.e., P2Y2 and/or P2Y4) receptors on human granulosa cells and P2U and P2T (i.e., P2Y12) receptors on porcine granulosa cells, but the functional roles of these receptors was unclear. P2U receptor mRNA was found in human granulosa cells and ATP/UTP was shown to cause rapid transient increase in [Ca2+]i [391]. ATP has an antigonadotrophic action in human granulosa cells [392]. This group later showed that ATP induced nuclear translocation of phosphorylated ERKs and the induction of egr-1 and c-raf-i expression in the human ovary [393]. This suggested that the mitogen-activated protein kinase signalling pathway plays a role in mediating the effects of ATP on gonadotrophin-induced progesterone secretion in the human ovary. P2, but not P1, receptors were shown to be expressed on chicken granulosa cells [292]. Calcium oscillations were mediated by P2Y2 and/or P2Y4 receptors in human granulosa-luteal cells [228,385].
ATP acts on granulosa cells via a mechanism that involves P2Y2 receptor stimulation and the participation of ryanodine receptors [288]. UTP-sensitive P2Y receptors are expressed in cultured murine theca/interstitial cells and occupation of these receptors leads to the activation of mitogenic signalling pathways to promote cell proliferation [413]. The authors concluded that regulation of proliferation of these cells and steroidogenesis plays an important role in ovarian pathophysiology, since theca hyperplasia is found in rats with polycystic ovarian syndrome [357]. Signalling to ovarian perifollicular smooth muscle changes from mediation via P2X2 receptors to P2X1 receptors during pregnancy, and there is an increase in P2X2 receptor expression on ovarian vascular smooth muscle [191].
Menopause is associated with a decline in ovarian function. In a murine menopause model, ovarian levels of P2X2 receptor protein increased with ageing, suggesting that the P2X2 receptor is involved with menopause/ageing-related decline in ovarian function [464].
Thecal cells, which form the external layer surrounding the ovarian follicle, are involved in the synthesis of androgens. Apoptotic cell death of porcine ovarian theca cells is caused by ATP acting via P2X7 receptors [412].
The cat ovary is densely innervated by sympathetic nerves, which release NA and ATP, as well as by intrinsic neurons, most of which are parasympathetic. The storage and release of NA and ATP decreased after ovulation, but sympathetic nerve activity appears to increase during ovulation [222]. Sympathetic neurotransmitters are present in the rat ovary during early postnatal development and affect steroidogenesis before the ovary becomes responsive to gonadotrophins and before the first primordial follicles are formed.
Chinese hamster ovary (CHO) cells contain very few endogenous receptors, which makes them ideal transfection recipients. However, a constitutive ATP receptor linked to arachidonic acid release has been reported [116]. A two-phase response of acid extrusion triggered by P2Y2 purinoceptors on CHO cells was later reported [310]. This ATP receptor is also linked to the mobilisation of [Ca2+]i and through pharmacological characterisation it appears to be a P2Y2 and/or P2Y4 receptor [178]. Exposure to ATP or UTP on the apical side of confluent CHO cell monolayers produced an increase in [Ca2+]i [407]. It was also shown that functional expression of human CFTR in the plasma membrane of CHO cells infected with adenovirus regulated the increase in intracellular Ca2+ produced by ATP released from the cells. A2B receptors have also been identified on CHO cells [6].
The hydrolysis of ATP and ADP is decreased by ovariectomy and oestradiol replacement therapy [322]. Ovarian tumours arise mainly from the surface of simple squamous-to-cuboid mesothelium that covers the ovary. Mitogen-activated kinase in pre-neoplastic and neoplastic surface epithelial cells is stimulated by ATP, suggesting that co-released ATP from sympathetic nerves may play a role in regulating cell proliferation in both normal and neoplastic ovarian surface epithelial cells [80]. FSH has a stimulatory effect on ATP release and platelet aggregation [25]. Phosphodiesterase 8 has been found in the mammalian ovarian follicle and may be involved in hormonal regulation of folliculogenesis [359].
Oocytes
K+ current responses to FSH or adenosine in monolayers of small follicular cells surrounding a single large oocyte of Xenopus are suppressed by ATP [136]. P2 receptors are expressed by the follicular cells of Xenopus [207,286]. Since UTP and ATP are equipotent, this effect could be mediated by a P2Y2 or P2Y4 receptor subtype [136]. Moreover, it has been suggested that there are close interactions between A2 and P2Y receptors in Xenopus follicles [19]. Cultured CHO cells express a P2 receptor equi-reactive to ATP and UTP [178], probably the P2Y2 and/or P2Y4 subtype. Extracellular ATP has been shown to facilitate the development of parthenogenetically activated bovine oocytes [444]. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation [453]. The Xenopus oocyte can release ATP, both in basal conditions and in response to mechanical stimulation or osmotic changes to have paracrine actions [5,46,160,259].
P1 receptors on follicular cells mediate K+ current responses, while P2Y2 receptors are involved in the activation of Cl− currents [356]. These authors and others [329] suggested that these effects are consistent with intrafollicular ATP signalling that modulates oocyte maturation.
In Xenopus oocytes ectonucleotidases rapidly and locally convert ATP to adenosine, which leads to activation of A2B receptors [264]. A study in human ovaries has shown a relationship between the higher follicular fluid adenosine levels and follicular/oocyte maturity [433]. The ATP content and fertilisation outcome of bovine oocytes were shown to increase with maternal age [179].
Cumulus cells consist of a cluster of follicle cells that surround a freshly ovulated ovum and they are dispersed at fertilisation by the contents of the acrosome. Data has shown that cumulus cells express P2Y2 and possibly P2Y12 receptor subtypes, but not P2X receptors (Bains, unpublished data).
Fallopian (uterine) tubes/oviduct
Released oocytes are captured by the fallopian tubes/oviduct and transported in an appropriate condition to the site of fertilisation in the distal ampullary region of the tube. They also provide tubal secretions for sperm to migrate proximally from the uterus. The sperm acrosomal reaction occurs mainly at the cervix and uterus. Purinergic receptor-mediated increases in [Ca2+]i were described in single isolated epithelial cells of the human uterine tube [92,384]. P2X1 and P2X2 receptors are expressed on blood vessels in rat fallopian tubes, but not peritubular smooth muscle [28]. ATP-mediated contraction of the human fallopian tube is enhanced with acute purulent inflammation, probably by upregulation of P2X1 and P2X2 receptors [463]. A functional purinergic receptor was identified on primary cultures of bovine oviduct epithelia [84,420]. ATP evoked a rapid increase in [Ca2+]i in oviductal endosalpingeal cells isolated from heifers at different reproductive stages [397]. The receptor involved in increases of [Ca2+]i and regulation of ion transport on the basal surface of bovine oviduct cells is likely to be the P2Y2 or P2Y4 subtype since UTP and ATP were equipotent [85]. Both ATP and adenosine receptors are present on single ciliated oviductal cells acting via P2Y2 an A2A receptor subtypes, respectively [287]. The ciliated cells from oviduct play an important role in the control of mucociliary transport velocity of gametes and embryos. ATP increases ciliary beat frequency [29] and this effect is modulated by adenosine [30]. Purinergic signalling via P2Y2 receptors also constitutes a key mechanism for regulating chloride secretion and thus fluid formation in the bovine oviduct [193]. In a later paper, they claimed that purinergic activation of a calcium-dependent, apamin-sensitive potassium conductance was essential to promote chloride secretion [194].
Uterus
The uterus is the site of sperm migration, implantation of the fertilised ovum, placental development, embryo and foetus development and the generation of contractions at term for delivery. The myometrium and endometrium are the main uterine functional tissue types.
Myometrium
ATP and ADP contract the uterus [100,311] partly through the action of prostaglandins induced by P2Y receptor occupation [4,291,390]. In the longitudinal muscle of the mouse myometrium, ATP produced a biphasic response, an initial hyperpolarisation followed by a depolarisation, which remained the same in both non-pregnant uterus and throughout pregnancy [306]. In the cat uterus reversal of the ATP response during gestation was reported, namely inhibition of spontaneously generated electrical and mechanical activity in the virgin uterus, but excitation in the early stages of gestation [55]. Whole cell voltage clamp studies of cultured smooth muscle cells from the pregnant rat myometrium showed that ATP, but not adenosine, AMP or ADP, activated a monovalent cation-selective and oestrogen-sensitive conductance [167].
Data from experiments using isolated uteri of non-pregnant guinea-pigs suggests that myometrial cells contain a mixture of P2 receptors, a UTP-sensitive receptor (probably P2Y2 or P2Y4), another P2Y receptor and an α,β-meATP-sensitive receptor (probably P2X1, P2X2/3 or P2X3) [321]. Using immunohistochemistry, P2X2 receptors were expressed by the smooth muscle of the rat uterus, with only weak immunostaining of P2X1 receptors [28]. P2X1 and/or P2X2/3 receptor-mediated contractions of isolated human pregnant uterus were evoked by α,β-meATP and shown to be term-dependent, increasing during pregnancy [462]. P2X1 and P2X2 receptors were identified in uterine smooth muscle, suggesting that P2X1 and P2X2/3 receptors mediate contractions of the pregnant human uterus, although it is likely that P2Y2 and/or P2Y4 receptors are also involved, to account for the high potency of UTP [461].
P2X1 receptors have been shown to be closely associated with connexin 43 (Cx43) in the human myometrium, and may be involved in gap junction formation [183]. Expression of Cx43 is upregulated in the later stages of pregnancy and peaks near parturition, perhaps playing a role in the synchronised and coordinated uterine contractions at parturition. It was suggested that the high levels of ATP released from uterine cells during childbirth may act on P2X1 receptors in gap junctions to inhibit expression of Cx43, thereby reducing gap junctions and coordinated contractions in the post-partum uterus, when P2X1 receptor expression peaks [199]. Enhanced expression of P2X4 and P2X7 receptors has been observed in the myometrium of pregnant rats in preterm delivery models, which could be related to uterine contraction leading to term and preterm delivery [406].
It has been claimed that extracellular ATP is essential for the initiation of contractions and control of their frequency (but not contractile force) and may be involved in the pacemaking mechanism for the generation of uterine contractions [173], perhaps via P2Y2 receptors [174]. Neocuproine, a copper chelator, can affect uterine contractile activity by modulation of purinergic excitatory responses [217]. Two different ectonucleotidase phosphohydrolases have been described in the rat myometrium [252]. Changes in apyrase activity were shown to occur during pregnancy [409].
Adenosine acting via A1 receptors contracts the smooth muscle of the virgin guinea-pig uterus [361,372]. It has been suggested that there are two types of receptors for adenosine, the breakdown product of ATP, in the myometrium, one mediating excitatory, the other inhibitory responses [124]. It was later claimed that A1 receptors mediate excitatory responses mainly during the proliferation phase of the menstrual cycle, while A2 receptors, mediating excitation, are largely involved during the secretory phase [358]. Adenosine is also a prejunctional modulator of adrenergic neurotransmission to the human uterus [440]. Evidence for the presence of the A2B subtype of the adenosine (P1) receptor in the rat myometrium has also been presented [144]. A purinergic receptor was identified in human myometrium membranes using 5′-N-[3H]ethylcarboxamide-adenosine as a radioligand [348]. The binding site had the characteristics of the A2 adenosine receptor and some of those of P2 receptors too. Later pharmacological studies suggested that both P1 and P2 receptors are present in the guinea-pig myometrium, both mediating contraction [372].
It has been shown that ATP induced ion currents and contractions via P2X7 receptors in freshly isolated myometrial cells from pregnant rats and that P2X7 receptor mRNA was localised in these cells [282,283], supporting the earlier findings of Urabe et al. [406]. These authors showed further that Mg2+ blocked the P2X7 receptor-mediated contraction in tocolysis and suggested that targeting P2X7 receptors could lead to novel treatments for the prevention of uterine contractions in preterm deliveries. The ectonucleotidase, NTPDase 1, has been localised on the surface of myometrial smooth muscle cells and blood vessel endothelium [275].
Endometrium
ATP, released from nerves innervating the uterus and uterine blood vessels and by autocrine or paracrine release from epithelial cells, plays an important role in regulating endometrial functions such as local (paracrine) coordination of sperm migration and capacitation, implantation of the fertilised egg, endometrial fluid formation and composition; cell proliferation and differentiation in post-partum reorganisation; and endometrial epithelial cell differentiation and apoptosis.
Human endometrial epithelial cells express P2X7 receptors [236]. P2X5 and P2X7 receptors were shown to be immunoreactive in the rat uterine epithelium [28]. Changes in the expression of P2X receptor subtypes in uterine epithelial cells were shown during the endometrial cycle and early pregnancy in the rat [368] and in humans [150]. These changes are related to, and mediate differentiation and apoptosis of the endometrium.
One of the functions of ATP is regulation of the steroid-binding activities of oestradiol receptors [220]. Extracellular nucleotides may play an important role in the fine-tuning of the uterine fluid microenvironment by regulating both Cl− secretion and Na+ absorption across the endometrium [92,425], since over-expression of Na+ channels in mouse endometrial epithelium suppresses ATP-induced Cl- secretion [426]. Oestrogen and progesterone differentially regulate UTP-stimulated anion secretion in endometrial epithelial cells by altering the expression of P2Y2 receptors and basolateral K+ channels [314].
Extracellular ATP activates nuclear translocation of ERK1/2 leading to the induction of matrix metalloproteinase expression in human endometrial stromal cells [76]. In a later paper, these authors showed that the P2Y2 receptor is expressed by stromal cells, suggesting that it mediates the expression of the early growth response 1 and inhibits stromal cell viability [77]. The distribution of ecto-nucleotidases in human cyclic and post-menopausic endometrium has been described [8]. Ecto-NPP3 was identified as a new biological marker of tubal metaplasia. It has been proposed that uridine diphosphate-glucose and its P2Y14 receptor are key players able to trigger innate uterine mucosal immunity in human endometrial epithelial cells by inducing interleukin-8 [18].
In the rat, P2X7 receptors are localised on eosinophils, macrophages and fibroblasts of the endometrium during oestrus [216]. The authors speculated that ATP-mediated responses may be important in uterine preparation and remodelling before implantation, and that the presence of P2X7 receptors in stromal cells may indicate their involvement in immune and inflammatory responses. There is a strong transitory expression of A2B adenosine receptors on the mouse uterus after blastocyst implantation during early post-implantation development [40].
In the rat uterine epithelium on day 1 of pregnancy, there was no expression of P2X7, P2Y2 or P2Y4 receptors, although there was some diffuse immunolabelling of P2Y1 receptors. On day 3, P2X7 and P2Y2 receptors were present and confined to the lateral plasma membrane of the epithelium. At the time of implantation on day 6, there was strong labelling for P2X7 and P2Y2 receptors, found along the entire surface of the apical epithelium, suggesting a role for calcium-modified events proceeding and facilitating attachment and implantation of the blastocyst [370].
In the pig uterus, epithelial cells of the endometrial gland expressed P2Y2 and P2Y4 receptors [313]. P2X7 receptors were shown to be expressed on the luminal surface of endometrial cells prior to implantation in rats [396] and in the periovulatory period in humans [150]. In the rat, at the time of implantation on day 6, apoptosis was reduced in the non-implantation uterine epithelium, but was markedly increased adjacent to the implanting blastocyst [396]. It was proposed that P2X7 receptor-mediated apoptotic cell death is an important regulatory factor involved in uterine remodelling prior to and during implantation. It was later proposed that P2X7 receptor-mediated apoptotic cell death and endometrial remodelling facilitate the attachment and implantation of the blastocyst to the endometrium [233].
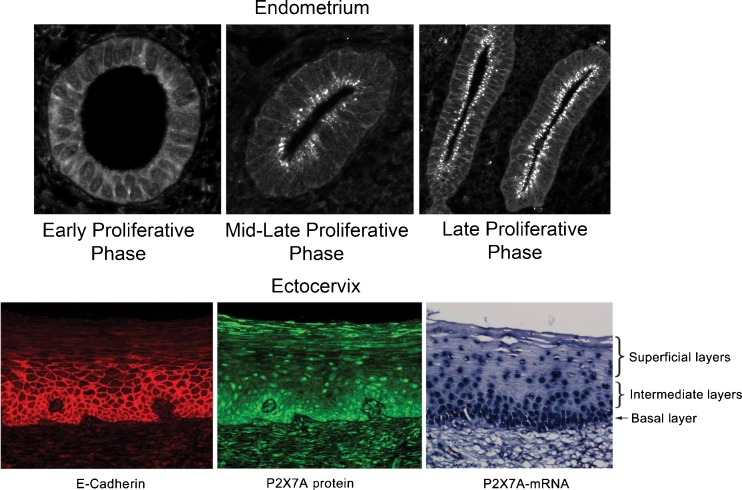
In the human endometrium, as in the cervix and vagina, P2X7 receptors regulate terminal differentiation and are the main physiological pro-apoptotic mechanism in vivo [150]. Activation of the P2X7 receptor induces pore formation in the plasma membrane and facilitates terminal differentiation and apoptosis [117]. In the human endometrium in vivo, expression of the P2X7 receptor depends on the state of tissue differentiation. In the early proliferative phase, the receptor is diffusely expressed in the plasma membrane, while in more advanced stages of maturation, i.e., mid-late proliferative phases, expression of the P2X7 receptor increases in regions of the apical (luminal) plasma membrane (Fig. 5). Treatment of cultured epithelial cells with ATP or 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP) induces pore formation preferentially in the apical membrane, suggesting that the terminal differentiation in vivo is mediated by formation of P2X7 receptor pores, and that the apical localisation of P2X7 receptors in the endometrium in vivo reflects the accumulation of P2X7 receptor aggregates within apical P2X7 receptor pores. The apical localisation of P2X7 receptor pores in vivo is not specific to the endometrium, and is found also in other normal human monolayered epithelia.
Fig. 5.
Upper panel (endometrium): P2X7 receptor expression in cross sections of human endometrial glands at different phases of the menstrual cycle. The increases in P2X7 receptor immunoreactivity in apical regions of the plasma membranes (facing the lumen) represent P2X7 aggregates within pores. (Reproduced from [237], with permission from Springer.) Lower panel: full-length P2X7 receptor type-A (P2X7A) expression in cross sections of human ectocervix. P2X7A receptor immunoreactivity in reserve cells (basal layer) represents de novo-synthesized P2X7A molecules. P2X7A receptor immunoreactivity in intermediate layers represents mainly P2X7A molecules aggregating into pores. P2X7A immunoreactivity in superficial layers represents P2X7A aggregates within pores that are retained in clumps of squames. (Reproduced from [150], with permission from Wiley)
Dysregulation of the P2X7 proapoptotic receptor plays a role in the development of endometrial cancers, as well as in other types of cancers of epithelia derived from the ectoderm, the urogenital sinus and the distal paramesonephric duct [236]. Decreased levels of P2X7 receptors are associated with cancer development, and in women decreased expression of the P2X7 receptor can be found in pre-cancerous endometrial lesions [237]. Mechanisms that could lead to decreased expression of the P2X7 receptor include epigenetic dysregulation through hypermethylation of the P2X7 receptor gene [457]; enhanced degradation of the P2X7 transcript [456]; and decreased glycosylation [117]. Of the naturally occurring P2X7 splice variants, the P2X7j receptor, a dominant negative form that blocks P2X7 receptor-mediated actions [118], is co-expressed with the P2X7 receptor in epithelia, primarily those of the female reproductive tract. The P2X7j isoform can hetero-oligomerise in the plasma membrane with the P2X7 receptor, and form non-functional complexes. Co-expression of P2X7 receptors plus P2X7j receptors blocks ATP-induced pore formation, and abolishes baseline and agonist-induced apoptosis.
Cervix
The cervix is the distal part of the uterus, which opens into the vagina. Columnar (endocervical) epithelium lines the proximal region of the cervix bordering the endometrial epithelium, while stratified squamous epithelium lines the distal part of the cervix, the ectocervix, which is continuous with the vaginal epithelium. A major function of the cervical epithelium is the synthesis and secretion of cervical mucous. The cervical mucous controls access to the uterine cavity for microorganisms and particulate matter. The content and composition of the cervical mucous change throughout the menstrual or oestrous cycles, and is influenced by changes in levels of ovarian oestrogen, androgens and progesterone [153]. During the pre-ovulatory phase, changes in mucous facilitate sperm movement towards the fallopian tube, and provide an optimal milieu for sperm capacitation. During other phases of the cervical cycle, changes in mucous characteristics impede sperm penetration and capacitation.
Cervical cells express different subtypes of purinoceptors. HeLa cervical epithelial cells express P2Y1, P2Y2, P2Y6 and P2X7 receptors [429]. In the rat cervix, expression of P2X3 receptors on afferent nerves increases during pregnancy, and this increase is probably related to coordination of cervical tone and contraction/dilation during labour [317]. Primary cultures of human endocervical and ectocervical epithelial cells express P2Y2 and P2X4 receptors, which are involved in epithelial transport [148] as well as P2X7 receptors, which are involved in the regulation of terminal differentiation and apoptosis of endocervical and ectocervical epithelial cells. The P2X7 receptor may also be involved in the control of cervical infections, since P2X7 receptor-mediated activation of cervical epithelial cells inhibits infection by Chlamydia and mycobacteria [87].
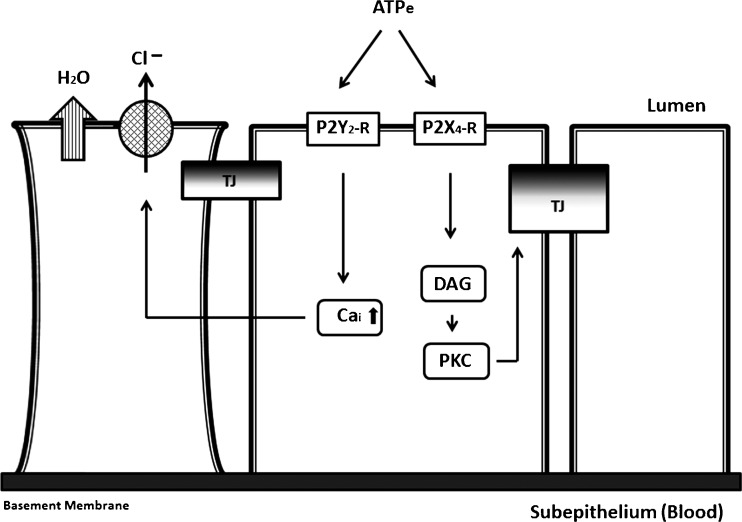
Data from experiments using three-dimensional culture models of human normal epithelial cervical cells suggest that the purinergic system controls cervical mucous secretion in vivo. Activation of the P2Y2 receptor stimulates [Ca2+]i-dependent chloride secretion, osmotic water efflux and increased transepithelial fluid secretion [146]. Activation of the P2X4 receptor, induces a slower and longer increase in tight junctional resistance and decreased transepithelial fluid secretion [147]. The P2X4 receptor effect is mediated by [Ca2+]i-dependent activation of phospholipase (PL)-D, upregulation of diacylglycerol and activation of PKC-mediated threonine dephosphorylation of the tight-junctional protein, occludin [153,458]. Both P2Y2 and P2X4 receptors are located on the apical (luminal) surface of the epithelial cells [146,147,151] (Fig. 6).
Fig. 6.
P2Y2 and P2X4 receptor regulation of epithelial permeability and transepithelial transport across human cervical cultures (compiled from data in [146,147,152,153,458]). Extracellular ATP (ATP e) modulates paracellular permeability by activating two distinct apically localized receptor–effector mechanisms. The P2Y2 receptor stimulates acute release of calcium from internal stores, active Cl− secretion and osmotic water efflux, resulting in cell shrinkage and reciprocal increase in the pericellular intercellular space volume; this leads to decreased paracellular resistance and an increase in the paracellular permeability. P2X4 receptors stimulate sustained release of diacylglycerol (DAG) and activation of protein kinase C (PKC). PKC modulates the tight junctional resistance by dephosphorylation of the tight junctional protein occluding, and stimulates a slow and long increase in the tight junctional resistance and a reciprocal decrease in paracellular permeability. The net effect on epithelial permeability is dependent on the spatial and temporal effects of the P2Y2 and P2X4 mechanisms. (Schematic courtesy of G.I. Gorodeski)
The dual regulation of transepithelial fluid secretion suggests that net fluid secretion (and thus cervical mucous secretion) in vivo is determined by the combined actions of the P2Y2 and P2X4 receptors. It was also suggested that P2X4 receptor-induced decrease in permeability and a decrease in mucous secretion in the micro-environment of the endocervix in vivo could result in retention of sperm cells in endocervical crypts [152]. Longer retention and longer exposure to endocervical fluid can improve capacitation and provide for graded transport of sperm cells to the uterus and tubes [177].
In the mammalian male reproductive tract, the epididymal and vas deferens epithelial cells acidify the lumen via an apical V-H+-ATPase mechanism; the epididymal and vas deferens V-H+-ATPase transporter is regulated by the P2X4 receptor [52]. A similar mechanism is present in the cervix and vagina, where baseline active proton secretion occurs constitutively throughout life, and the acidification is upregulated by oestrogen ([154]; see [133]).
Endocervical and ectocervical epithelial cells express the P2X7 receptors and the main role of P2X7 receptors in these epithelia is regulation of terminal differentiation and control of apoptosis [236,237]. Expression and function of the P2X7 receptor in the monolayered endocervical epithelium are similar to those in the endometrium, and decreased expression of the P2X7 receptor is associated with the development of endocervical cancer.
In the ectocervix, i.e., the distal part of the cervix which projects into the vagina, the P2X7 receptor is expressed by the stratifying ectocervical epithelial cells. In vivo, there is an abundance of P2X7 receptor expression by reserve cells in the basal layer of the epithelium and an abundance of expression of P2X7 receptor aggregates in more superficial cells undergoing terminal differentiation in vivo [150]. Data from cultured ectocervical cells showed preferential apical localisation of P2X7 receptors and preferential P2X7 receptor pore formation in the apical membrane. Similar data were found also in the epidermis [135], suggesting that P2X7 receptor pore formation initiates terminal differentiation in stratifying epithelia in vivo. Decreased levels of the P2X7 receptor are associated with the development of ectocervical cancers, and in women decreased expression of the P2X7 receptor can be found already in pre-cancerous lesions [236,237]. In cultured human ectocervical cells treatment with ATP or BzATP augments apoptosis [118], and in mice local application of BzATP on the skin augments apoptosis mainly of epidermal reserve cells, and blocks carcinogen-induced skin cancer [135]. The authors suggested that activation of P2X7 receptor-dependent apoptosis could be a novel chemotherapeutic growth-preventive modality for pre-cancerous and early cancerous epithelial lesions [135,149].
Amnion
The amniotic membrane forms the sac which contains the embryo, and it connects to the embryo at the umbilical cord. ATP activates the PLC cascade system in human amnion cells without increasing prostaglandin production [410].
Placenta
The placenta and umbilical vessels are involved in regulation of blood flow and control of transport of materno–foetal fluid and solutes, as well as in steroidogenesis. High plasma circulating levels of adenosine have been measured at birth in sheep and it was suggested that the adenosine, probably derived from the placenta, inhibits thermogenesis before birth. After cord occlusion, adenosine decreases, allowing thermogenesis to begin. These data suggest that adenosine plays a role in regulating foetal metabolism [360]. Increases in plasma levels of adenosine were also found in pregnant women, and the authors suggested that the adenosine is derived, in part, from platelet activation and an increase in 5′-nucleotidase activity during pregnancy [450]. Hyperemesis gravidorum is characterised by an overaction of sympathetic nerves and enhanced production of TNF-α. Plasma adenosine is significantly increased in hyperemesis gravidarum, and could serve as a prejunctional modulator of sympathetic neurotransmission and to counteract further progression of the disease [451].
Chronic caffeine or theophylline intake during pregnancy inhibits A1 receptor function in both maternal and foetal brains [230]. Adenosine is important as a metabolite of ATP in the human term placenta in ischaemic or hypoxic conditions [254]. Binding sites were identified in human placenta with characteristics of an adenosine receptor, probably the A2 receptor subtype [130]. Adenosine uptake by human placenta has been described, including its metabolism both intra- and extracellularly [2]. Adenosine uptake at both the maternal and foetal sides of the syncytiotrophoblast was demonstrated, and shown to be inhibited by dipyridamole; this suggested that the placenta is involved in the regulation of adenosine-related function [438]. Adenosine induced relaxations in segments of human chorionic arteries and veins pre-contracted with K+ and pharmacological analysis led to the conclusion that both A1 and A2 receptor subtypes were involved [339]. Adenosine causes a biphasic response (vasoconstriction followed by vasodilation) in the ovine foetal placental vasculature [337]. Chromosomal abnormality exhibited in TR21 (Down syndrome) pregnancies often end in abortion and represents a good model for examining the mechanism regulating miscarriage. Downregulation of A1 and A2B receptors in human trisomy 21 mesenchymal cells from first-trimester chorionic villi has been reported [142].
ATP (and adenosine) caused vasoconstriction of the human foetoplacental vascular bed, but there was reduced sensitivity to adenosine in cotyledons from some placentae that may represent downregulation of adenosine receptors as a result of exposure to adenosine during delivery [253]. ATP, α,β-meATP and β,γ-methylene ATP constricted the perfused human placental cotyledon via P2X receptors, but vasodilation via P2Y receptor-mediated NO release from endothelial cells was also demonstrated [94,336]. ATP increased Ca2+ uptake by microvillous boarder vesicles formed from term human placental syncytiotrophoblasts [312]. Continuous measurement of ATP was examined by 31P-nuclear magnetic resonance spectroscopy in human perfused placenta in vitro during ischaemia [257]. It was shown that after 4 h of ischaemia, ATP decreased by 85 % of controls, but after reperfusion ATP levels increased. Stimulated increase in [Ca2+]i by ATP and UTP in human term placental calls [319] suggests that a P2Y2 and/or P2Y4 receptor is involved.
In a study of P2 receptor subtypes in the arterial vascular bed of human perfused placental cotyledons [334], it was shown that:
The contractile responses mediated by α,β-meATP and ATP of the placental smooth muscle was resistant to desensitisation and insensitive to antagonism by PPADS, thus showing dissimilarity to the classic P2X1 receptor.
A smooth muscle P2Y2 or P2Y4 receptor also mediates vasoconstriction to UTP and ATP.
Vasodilation responses are mediated by P2Y1 receptors located on endothelial cells that, when occupied by ADP, lead to release of NO.
Evidence has been presented that a similar, novel P2X receptor is present in human placental chorionic surface arteries [95]. mRNAs for P2X1, P2X4, P2X5, P2X6 and P2X7 receptors were identified in the vascular smooth muscle from chorionic (and umbilical) arteries and veins, while mRNA for P2Y1, P2Y2 and P2Y6 receptors was found on the endothelium of these vessels [170] highlighting the role of extracellular nucleotides as regulators of foetal blood flow. Adenosine, acting via A2B receptors, has also been claimed to produce human chorionic vasoconstriction in perfused cotyledons and that this involves synthesis of a thromboxane receptor activator or a related prostanoid [98]. In a study of P2 receptor mRNA and protein expression in human placental villus fragments at two stages of gestation, the first trimester and term [342], P2X4, P2X7 and P2Y2 (but not P2Y4) receptor subtypes were found and fluorescent calcium imaging experiments showed that ATP, UTP and 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate caused significant elevation of [Ca2+]i in term compared to the first trimester. Systematic pharmacological studies of purinergic signalling in human placenta showed functionally active P2X4, P2X7, P2Y2 and P2Y6 receptors on cytotrophoblast cells and it was proposed that activation of these receptors and subsequent elevation of [Ca2+]i modulates syncytiotrophoblast homeostasis and maternofoetal ion exchange [341]. P2Y1 and P2Y2 receptors are unevenly distributed along the human placental vascular tree; there is a 6- to 8-fold increase in these receptors from the cord to the chorionic or cotyledon vessels [69]. In the cord and chorionic vessels the receptors are mainly on smooth muscle, while in the cotyledon vessels P2Y1 and P2Y2 receptors were equally distributed between endothelial and smooth muscle cells. mRNA for P2Y4, P2Y6 and P2Y11 receptors was also found. P2X1, P2X4 and P2X7 receptors were also present on the smooth muscle of human chorionic blood vessels [408].
Cytotrophoblast cells isolated from human term placenta were maintained in culture for 18 h (relatively undifferentiated mononuclear cells) or 66 h (differentiated multinucleate syncytiotrophoblast-like cells) [340]. P2X1 and P2X4, but not P2X3 or P2X5, receptors were expressed by all the cell types studied, while P2X2 receptors were found to be present in term placenta, and P2X7 receptors were found in both the first trimester and term. P2X7 receptors mediate production of PLD in human placental trophoblasts, implying involvement in the regulation of trophoblast function [93]. The varying P2X2 and P2X7 receptor expression may be correlated with gestational development and cytotrophoblast cell differentiation. ATP stimulates placental 11β-hydroxysteroid dehydrogenase type 2 activity, which is believed to play a key role in foetal development [446].
In late human pregnancy there is an increase in placental 5′-nucleotidase, and it was suggested that this may be associated with enhanced oestrogen synthesis and facilitation of uterine contractions during labour [59]. 5′-Nucleotidase was localised on the external surface of the microvillous plasma membrane of the syncytiotrophoblast, perhaps playing a role in regulation of the foeto–placental–maternal microcirculation in the human term placenta [262].
ADP-degrading properties of the placenta were described [175]. Later, ATP diphosphohydrolase (or apyrase) was identified in human term placenta [81,198,316]. Two isoforms (enzymes I and II) of placental ecto-ATP diphosphohydrolases were later identified by cDNA cloning [263]. Four ATPases, which bind to ATP at the external surface of the membrane of syncytiotrophoblast brush boarder membranes, were described [54]. Adenosine deaminase activity is differentially regulated in the maternal and foetal placental compartments. There are marked spatial and temporal changes in the profile of adenosine deaminase distribution in the antimesometrial decidua and basal zone of the chorioallentoic placenta and a rapid transition from maternal to foetal expression in mid-gestation [213]. Placental adenosine deaminase has been shown to be important for foetal development in mice [39]. Multiple regulatory protein binding motifs are necessary for adenosine deaminase gene expression in the placenta [367].
A study was undertaken to evaluate the relationship between utero–placental circulatory insufficiency and the foeto–placental release of adenosine in pregnancies complicated by pre-eclampsia [449]. It was concluded that foetal plasma adenosine increases before utero–placental insufficiency becomes severe enough to cause generalised foetal hypoxia. It was postulated that enhanced adenosine formation in the foetus, umbilical cord vessels and particularly the placenta may, at least in part, contribute to the control and maintenance of placental blood flow. P2X receptors have been identified on the smooth muscle of umbilical vessels [45].
Pre-eclampsia is characterised by elevated maternal blood pressure, proteinuria and altered foetal growth. It has been suggested that the pathophysiology of pre-eclampsia begins with shallow trophoblast invasion leading to placenta hypoxia. Hypoxia is a potent stimulus for the release of ATP, which is rapidly broken down by ectonucleotidases to adenosine. Women with pre-eclampsia and foetuses show increased circulating concentrations of adenosine. Adenosine A2A receptors expression is elevated in placental biopsies, villous explants and placental microvillous membranes [423]. A2B receptors on microvascular endothelial cells have also been implicated in pre-eclampsia [108]. Adenosine A2A receptor stimulation decreases NO synthesis in placental microvascular endothelial cells from women with pre-eclampsia and intrauterine growth retardation [107]. Post-translational modifications of the P2X4 receptor in the human placenta occur in pre-eclampsia and the authors propose that there is increased release of ATP in pre-eclampsia in response to hypoxia and oxidative/nitrative stress to act on P2X4 receptors to influence placental cell homeostasis [343].
Evidence has been presented that suggests that placental adenosine mediates the placental disturbances elicited by alcohol, which may contribute to the pathogenesis of foetal alcohol syndrome [3].
NO released from endothelial cells following occupation of P2 receptors in response to ATP and ADP may play a major role in control of foetal vessel tone in human placenta and NOS activity is markedly reduced in pre-eclampsia. NO may also regulate the release of corticotrophin-releasing hormone from human placental syncytiotrophoblast cells. However, while it was considered that NO released from syncytiotrophoblasts prevented platelet aggregation in both maternal and foetal circulations, there is no evidence that platelet aggregating agents other than ADP can affect the production of NO [90].
The P2Y6 receptor has been shown to be expressed in normal placenta and in gestational trophoblastic disease [381]. The authors showed that P2Y6 mRNA production is highly characteristic of the epithelial-like cytotrophoblast and syncytiotrophoblast, whereas expression is absent in the mesenchymal-like intermediate trophoblast, suggesting that P2Y6 receptors may play an important role in trophoblast development, differentiation and neoplasia.
Vagina
P2X5 and P2X7 receptors have been localised immunohistochemically in the stratified squamous epithelium of rat vagina, and it was suggested that they play a role in the physiological turnover of continuously regenerating cells [28,156]. Adenosine A2A and P2 receptors are present in the wall of the rabbit vagina that mediate relaxation, but it was suggested that they are not involved in the NANC relaxant responses that remained after removal of NO transmission by the NOS inhibitor, l-NAME [459,460].
P2Y2 receptor-selective agonists stimulate vaginal moisture in ovariectomised rabbits and P2Y2 receptor mRNA is localised in stratified squamous epithelium of the vagina [276]. It was suggested that P2Y2 receptor agonists produce a potential non-hormonal alternative for treating vaginal dryness in postmenopausal women. In the human vagina, similar to the ectocervix and epidermis, the P2X7 receptor plays an important role in the regulation of terminal differentiation and apoptosis [150].
Mammary glands
The epithelial cells lining the ducts of the human mammary gland are responsible for modifying sodium and potassium concentrations in milk by actively absorbing Na+ from and secreting K+ into the ductal fluid. ATP increased [Ca2+]i leading to contraction of cultured mouse mammary myoepithelial cells [298]. These effects were unaffected by Ca2+ removal, suggesting that P2Y receptors were involved and the responses were blocked by suramin. In a later paper from this group, synergistic effects of ATP on oxytocin-induced increases in [Ca2+]i were demonstrated in these cells and it was suggested that purinoceptors may facilitate myoepithelial cell contraction in milk-ejection responses [299]. In another study, ATP and growth hormone were shown to cause prompt responses in H+ efflux rate and increase in [Ca2+]i in an opposite direction by treatment with lactogenic hormones [190]. Mechanically-induced calcium waves mediating purinergic coupling between myo- and secretary epithelial cells in mammary glands have been reported [140]. P2Y2 receptors on the apical and basolateral membranes of 31EG4 mammary epithelia mediate apical Ca2+-dependent Cl− channels and cause fluid secretion that appears to be involved in the regulation of milk composition in vivo [43]. P2Y receptor regulation of sodium transport in human mammary epithelial cells has been described [229]. Adenosine, in concert with epidermal growth factor, stimulates mammary epithelial cell growth and DNA synthesis [454]. ATP and UTP stimulate Na+ absorption and K+ secretion via P2Y2 receptors that increase [Ca2+]i and activate Ca2+-dependent K+ channels [315]. They showed further that addition of the nucleotides to the luminal surface stimulated K+ secretion, while their action on the basolateral surface led to increase in Na+ absorption. Mastitis in cows is a problem to the dairy industry. A high level of ATP was found to be associated with mastitis in the udders of cows [302]. Ca2+-ATPase was elevated in mammary tissue and milk fat globule membranes in cows with milk fever [326]. There is upregulation of P2 receptors in breast cancer (see [62]).
Conclusions
There is widespread functional expression of purinoceptors in reproductive organs: P2X1 and P2X2 receptors mediate contraction of smooth muscle in organs and blood vessels; P2X2 receptors mediate capacitation of sperm; P2X3 receptors mediate activation of sensory nerve fibres involved in reflex activities and pain; P2X4 receptors mediate luminal acidification in the epididymis and vas deferens, and probably also in the vagina and ectocervix; P2X5 receptors mediate differentiation and consequently have antiproliferative effects; P2Y1 and P2Y2 receptors mediate cell proliferation; P2Y2 and P2X4 mediate regulation of epithelial transport in female reproductive tract epithelia; and P2X7 receptors mediate terminal differentiation and apoptotic cell death in epithelia.
Acknowledgements
The comments by George Gorodeski about the female reproductive system were much appreciated. The author is very grateful to Dr. Gillian E. Knight for her invaluable assistance in the preparation of this review article.
References
- 1.Abdel-Hamid IA, Jannini EA, Andersson KE. Premature ejaculation: focus on therapeutic targets. Expert Opin Ther Targets. 2009;13:175–193. doi: 10.1517/14728220802663549. [DOI] [PubMed] [Google Scholar]
- 2.Acevedo CG, Rojas S, Ramirez M, Bravo I. Transport and metabolism of adenosine in the perfused human placenta. Placenta. 1995;16:611–622. doi: 10.1016/0143-4004(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 3.Acevedo CG, Huambachano A, Perez E, Rojas S, Bravo I, Contreras E. Effect of ethanol on human placental transport and metabolism of adenosine. Placenta. 1997;18:387–392. doi: 10.1016/s0143-4004(97)80038-0. [DOI] [PubMed] [Google Scholar]
- 4.Aitken H, Poyser NL, Hollingsworth M. The effects of P2Y receptor agonists and adenosine on prostaglandin production by the guinea-pig uterus. Br J Pharmacol. 2001;132:709–721. doi: 10.1038/sj.bjp.0703848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleu J, Martin-Satue M, Navarro P, Perez dL I, Bahima L, Marsal J, Solsona C. Release of ATP induced by hypertonic solutions in Xenopus oocytes. J Physiol. 2003;547:209–219. doi: 10.1113/jphysiol.2002.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander SP, Cooper J, Shine J, Hill SJ. Characterization of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B4) cells. Br J Pharmacol. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliagas E, Torrejón-Escribano B, Lavoie EG, Gómez de Aranda I, Sévigny J, Solsona C, Martín-Satué M. Changes in expression and activity levels of ecto-5′-nucleotidase/CD73 along the mouse female estrous cycle. Acta Physiol (Oxf) 2010;199:191–197. doi: 10.1111/j.1748-1716.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- 8.Aliagas E, Vidal A, Torrejón-Escribano B, Taco MR, Ponce J, de Aranda I, Sévigny J, Condom E, Martín-Satué M. Ecto-nucleotidases distribution in human cyclic and postmenopausic endometrium. Purinergic Signal. 2013;9:227–237. doi: 10.1007/s11302-012-9345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allcorn RJ, Cunnane TC, Kirkpatrick K. Actions of α, β-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986;89:647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allegrucci C, Liguori L, Mezzasoma I, Minelli A. A1 adenosine receptor in human spermatozoa: its role in the fertilization process. Mol Genet Metab. 2000;71:381–386. doi: 10.1006/mgme.2000.3054. [DOI] [PubMed] [Google Scholar]
- 11.Alm P. On the autonomic innervation of the human vas deferens. Brain Res Bull. 1982;9:673–677. doi: 10.1016/0361-9230(82)90172-1. [DOI] [PubMed] [Google Scholar]
- 12.Amobi N, Smith IC. Effects of α, β-methylene ATP on biphasic responses of rat vas deferens. Eur J Pharmacol. 1987;133:75–82. doi: 10.1016/0014-2999(87)90207-x. [DOI] [PubMed] [Google Scholar]
- 13.Amobi NI, Guillebaud J, Smith IC. Perspective on the role of P2X-purinoceptor activation in human vas deferens contractility. Exp Physiol. 2012;97:583–602. doi: 10.1113/expphysiol.2011.063206. [DOI] [PubMed] [Google Scholar]
- 14.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Andersson KE, Chapple CR, Höfner K. Future drugs for the treatment of benign prostatic hyperplasia. World J Urol. 2002;19:436–442. doi: 10.1007/s00345-002-0253-8. [DOI] [PubMed] [Google Scholar]
- 16.Anton PG, McGrath JC. Further evidence for adrenergic transmission in the human vas deferens. J Physiol. 1977;273:45–55. doi: 10.1113/jphysiol.1977.sp012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonio LS, Costa RR, Gomes MD, Varanda WA. Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal. 2009;5:277–287. doi: 10.1007/s11302-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, Asada H, Yoshimura Y, Maruyama T. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol. 2009;182:7074–7084. doi: 10.4049/jimmunol.0900001. [DOI] [PubMed] [Google Scholar]
- 19.Arellano RO, Garay E, Vázquez-Cuevas F. Functional interaction between native G protein-coupled purinergic receptors in Xenopus follicles. Proc Natl Acad Sci U S A. 2009;106:16680–16685. doi: 10.1073/pnas.0905811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auzanneau C, Norez C, Noel S, Jougla C, Becq F, Vandebrouck C. Pharmacological profile of inhibition of the chloride channels activated by extracellular acid in cultured rat Sertoli cells. Reprod Nutr Dev. 2006;46:241–255. doi: 10.1051/rnd:2006013. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo I, Osswald W. Does adenosine malfunction play a role in hypertension? Pharmacol Res. 1992;25:227–236. doi: 10.1016/s1043-6618(05)80071-4. [DOI] [PubMed] [Google Scholar]
- 22.Banks F, Knight G, Calvert RC, Thompson CS, Mikhailidis DP, Morgan R, Burnstock G. The purinergic component of human vas deferens contraction. Fertil Steril. 2006;85:932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Banks FCL, Knight GE, Calvert RC, Turmaine M, Thompson CS, Mikhailidis DP, Morgan RJ, Burnstock G. Smooth muscle and purinergic contraction of the human, rabbit, rat, and mouse testicular capsule. Biol Reprod. 2006;74:473–480. doi: 10.1095/biolreprod.105.044602. [DOI] [PubMed] [Google Scholar]
- 24.Banks FCL, Calvert RC, Burnstock G. Changing P2X receptor expression on maturing sperm in the epididymides of mice, hamsters, rats and man. Fertil Steril. 2010;93:1415–1420. doi: 10.1016/j.fertnstert.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 25.Bar J, Orvieto R, Lahav J, Hod M, Kaplan B, Fisch B. Effect of urinary versus recombinant follicle-stimulating hormone on platelet function and other hemostatic variables in controlled ovarian hyperstimulation. Fertil Steril. 2004;82:1564–1569. doi: 10.1016/j.fertnstert.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 26.Barbacci E, Filippini A, De Cesaris P, Ziparo E. Identification and characterization of an ecto-ATPase activity in rat Sertoli cells. Biochem Biophys Res Commun. 1996;222:273–279. doi: 10.1006/bbrc.1996.0734. [DOI] [PubMed] [Google Scholar]
- 27.Barden JA, Cottee LJ, Bennett MR. Vesicle-associated proteins and P2X receptor clusters at single sympathetic varicosities in mouse vas deferens. J Neurocytol. 1999;28:469–480. doi: 10.1023/a:1007053004771. [DOI] [PubMed] [Google Scholar]
- 28.Bardini M, Lee HY, Burnstock G. Distribution of P2X receptor subtypes in the rat female reproductive tract at late pro-oestrus/early oestrus. Cell Tissue Res. 2000;299:105–113. doi: 10.1007/s004419900138. [DOI] [PubMed] [Google Scholar]
- 29.Barrera NP, Morales B, Villalón M. Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca2+ increase associated with the ATP-induced increase in ciliary beat frequency. Am J Physiol Cell Physiol. 2004;287:C1114–C1124. doi: 10.1152/ajpcell.00343.2003. [DOI] [PubMed] [Google Scholar]
- 30.Barrera NP, Morales B, Villalón M. ATP and adenosine trigger the interaction of plasma membrane IP3 receptors with protein kinase A in oviductal ciliated cells. Biochem Biophys Res Commun. 2007;364:815–821. doi: 10.1016/j.bbrc.2007.10.104. [DOI] [PubMed] [Google Scholar]
- 31.Behrman HR, Hall AK, Preston SL, Gore SD. Antagonistic interactions of adenosine and prostaglandin F2α modulate acute responses of luteal cells to luteinizing hormone. Endocrinology. 1982;110:38–46. doi: 10.1210/endo-110-1-38. [DOI] [PubMed] [Google Scholar]
- 32.Behrman HR, Polan ML, Ohkawa R, Laufer N, Luborsky JL, Williams AT, Gore SD. Purine modulation of LH action in gonadal cells. J Steroid Biochem. 1983;19:789–793. doi: 10.1016/0022-4731(83)90013-4. [DOI] [PubMed] [Google Scholar]
- 33.Belleannée C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 2010;298:C817–C830. doi: 10.1152/ajpcell.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett MR, Gibson WG. On the contribution of quantal secretion from close-contact and loose-contact varicosities to the synaptic potentials in the vas deferens. Philos Trans R Soc Lond B Biol Sci. 1995;347:187–204. doi: 10.1098/rstb.1995.0021. [DOI] [PubMed] [Google Scholar]
- 35.Bevan JA. The γ-connection’: are we ready to throw out the α-adrenoceptor in sympathetic vasoconstriction? Trends Pharmacol Sci. 1984;5:53–55. [Google Scholar]
- 36.Billig H, Rosberg S. Gonadotropin depression of adenosine triphosphate levels and interaction with adenosine in rat granulosa cells. Endocrinology. 1986;118:645–652. doi: 10.1210/endo-118-2-645. [DOI] [PubMed] [Google Scholar]
- 37.Billig H, Thelander H, Rosberg S. Adenosine receptor-mediated effects by nonmetabolizable adenosine analogs in preovulatory rat granulosa cells: a putative local regulatory role of adenosine in the ovary. Endocrinology. 1988;122:52–61. doi: 10.1210/endo-122-1-52. [DOI] [PubMed] [Google Scholar]
- 38.Birmingham AT. The human isolated vas deferens: its response to electrical stimulation and to drugs. Br J Pharmacol. 1968;34:692P–693P. [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn MR, Wakamiya M, Caskey CT, Kellems RE. Tissue-specific rescue suggests that placental adenosine deaminase is important for fetal development in mice. J Biol Chem. 1995;270:23891–23894. doi: 10.1074/jbc.270.41.23891. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn MR, Wubah JA, Chunn JL, Thompson LF, Knudsen TB. Transitory expression of the A2b adenosine receptor during implantation chamber development. Dev Dyn. 1999;216:127–136. doi: 10.1002/(SICI)1097-0177(199910)216:2<127::AID-DVDY4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 41.Blakeley AG, Cunnane TC. The packeted release of transmitter from the sympathetic nerves of the guinea-pig vas deferens: an electrophysiological study. J Physiol. 1979;296:85–96. doi: 10.1113/jphysiol.1979.sp012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanco JM, Long JA, Gee G, Wildt DE, Donoghue AM. Comparative cryopreservation of avian spermatozoa: effects of freezing and thawing rates on turkey and sandhill crane sperm cryosurvival. Anim Reprod Sci. 2012;131:1–8. doi: 10.1016/j.anireprosci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Blaug S, Rymer J, Jalickee S, Miller SS. P2 purinoceptors regulate calcium-activated chloride and fluid transport in 31EG4 mammary epithelia. Am J Physiol Cell Physiol. 2003;284:C897–C909. doi: 10.1152/ajpcell.00238.2002. [DOI] [PubMed] [Google Scholar]
- 44.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 45.Bo X, Sexton A, Xiang Z, Nori SL, Burnstock G. Pharmacological and histochemical evidence for P2X receptors in human umbilical vessels. Eur J Pharmacol. 1998;353:59–65. doi: 10.1016/s0014-2999(98)00383-5. [DOI] [PubMed] [Google Scholar]
- 46.Bodas E, Aleu J, Pujol G, Martin-Satue M, Marsal J, Solsona C. ATP crossing the cell plasma membrane generates an ionic current in Xenopus oocytes. J Biol Chem. 2000;275:20268–20273. doi: 10.1074/jbc.M000894200. [DOI] [PubMed] [Google Scholar]
- 47.Boland B, Himpens B, Vincent MF, Gillis JM, Casteels R. ATP activates P2x-contracting and P2y-relaxing purinoceptors in the smooth muscle of mouse vas deferens. Br J Pharmacol. 1992;107:1152–1158. doi: 10.1111/j.1476-5381.1992.tb13422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boryshpolets S, Dzyuba B, Rodina M, Li P, Hulak M, Gela D, Linhart O. Freeze-thawing as the factor of spontaneous activation of spermatozoa motility in common carp (Cyprinus carpio L.) Cryobiology. 2009;59:291–296. doi: 10.1016/j.cryobiol.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Boselli C, Govoni S. Alcohol differentially affects noradrenergic and purinergic responses in the bisected rat vas deferens. Alcohol. 2000;22:91–96. doi: 10.1016/s0741-8329(00)00104-x. [DOI] [PubMed] [Google Scholar]
- 50.Bowman A, Gillespie JS. Neurogenic vasodilatation in isolated bovine and canine penile arteries. J Physiol. 1983;341:603–616. doi: 10.1113/jphysiol.1983.sp014827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]
- 52.Breton S, Smith PJS, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470–472. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- 53.Brock JA, Cunnane TC. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987;326:605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- 54.Brunette MG, Bastani B, Leclerc M, Narbaitz R. Detection of different adenosine triphosphatases in human placental brush border membranes. J Membr Biol. 1995;145:285–293. doi: 10.1007/BF00232720. [DOI] [PubMed] [Google Scholar]
- 55.Bülbring E, Casteels R, Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968;34:388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buljubasich R, Ventura S. Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol. 2004;499:335–344. doi: 10.1016/j.ejphar.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 57.Bulloch JM, McGrath JC. Blockade of vasopressor and vas deferens responses by α, β-methylene ATP in the pithed rat. Br J Pharmacol. 1988;94:103–108. doi: 10.1111/j.1476-5381.1988.tb11504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bültmann R, Klebroff W, Starke K. A contraction-mediating receptor for UTP, presumably P2Y2, in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:196–201. doi: 10.1007/s002109900049. [DOI] [PubMed] [Google Scholar]
- 59.Burns JK (1987) Relation between elevated serum 5-nucleotidase in late human pregnancy and duration of labour. Proc Physiol Soc Suppl:57P
- 60.Burnstock G (1997) Commentary on paper by S. Hukovic (1961) entitled “Responses of the isolated sympathetic nerve-ductus deferens preparation of the guinea-pig”. In: Birmingham AT, Brown DA (eds) Landmarks in pharmacology. Br. J. Pharmacol. (Golden Jubilee 1946–1996), pp 192–201 [DOI] [PMC free article] [PubMed]
- 61.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burnstock G, Di Virgilio F (2013) Purinergic signalling in cancer. Purinergic Signal [Epub ahead of print 25/6/13] [DOI] [PMC free article] [PubMed]
- 63.Burnstock G, Holman ME. Autonomic nerve-smooth muscle transmission. Nature. 1960;187:951–952. doi: 10.1038/187951a0. [DOI] [PubMed] [Google Scholar]
- 64.Burnstock G, Holman ME. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnstock G, Holman ME. Spontaneous potentials at sympathetic nerve endings in smooth muscle. J Physiol. 1962;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal. 2013;9:307–324. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burnstock G, Verkhratsky A. Vas deferens — a model used to establish sympathetic cotransmission. Trends Pharmacol Sci. 2010;31:131–139. doi: 10.1016/j.tips.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Burton LD, Housley GD, Salih SG, Järlebark L, Christie DL, Greenwood D. P2X2 receptor expression by interstitial cells of Cajal in vas deferens implicated in semen emission. Auton Neurosci. 2000;84:147–161. doi: 10.1016/S1566-0702(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 69.Buvinic S, Poblete MI, Donoso MV, Delpiano AM, Briones R, Miranda R, Huidobro-Toro JP. P2Y1 and P2Y2 receptor distribution varies along the human placental vascular tree: role of nucleotides in vascular tone regulation. J Physiol. 2006;573:427–443. doi: 10.1113/jphysiol.2006.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calamera JC, Quirós MC, Brugo S, Nicholson RF, Vilar O. Adenosine 5′-triphosphate (ATP) activity in normal and pathological human semen. Relationship with round cells of the ejaculate. Andrologia. 1986;18:214–219. doi: 10.1111/j.1439-0272.1986.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 71.Calamera JC, Buffone MG, Doncel GF, Brugo-Olmedo S, de Vincentiis S, Calamera MM, Storey BT, Alvarez JG. Effect of thawing temperature on the motility recovery of cryopreserved human spermatozoa. Fertil Steril. 2010;93:789–794. doi: 10.1016/j.fertnstert.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Calvert RC, Khan MA, Thompson CS, Mikhailidis DP, Burnstock G. A functional study of purinergic signalling in the normal and pathological rabbit corpus cavernosum. BJU Int. 2008;101:1043–1047. doi: 10.1111/j.1464-410X.2007.07385.x. [DOI] [PubMed] [Google Scholar]
- 73.Casali EA, da Silva TR, Gelain DP, Kaiser GR, Battastini AM, Sarkis JJ, Bernard EA. Ectonucleotidase activities in Sertoli cells from immature rats. Braz J Med Biol Res. 2001;34:1247–1256. doi: 10.1590/s0100-879x2001001000003. [DOI] [PubMed] [Google Scholar]
- 74.Chan HC, Fu WO, Chung YW, Chan PS, Wong PY. An ATP-activated cation conductance in human epididymal cells. Biol Reprod. 1995;52:645–652. doi: 10.1095/biolreprod52.3.645. [DOI] [PubMed] [Google Scholar]
- 75.Chan HC, Zhou WL, Wong PY. Extracellular ATP activates both Ca2+- and cAMP-dependent Cl− conductances in rat epididymal cells. J Membr Biol. 1995;147:185–193. doi: 10.1007/BF00233546. [DOI] [PubMed] [Google Scholar]
- 76.Chang SJ, Wang TY, Lee YH, Tai CJ. Extracellular ATP activates nuclear translocation of ERK1/2 leading to the induction of matrix metalloproteinases expression in human endometrial stromal cells. J Endocrinol. 2007;193:393–404. doi: 10.1677/JOE-06-0168. [DOI] [PubMed] [Google Scholar]
- 77.Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20:1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, He HY, Li HM, Zheng J, Heng WJ, You JF, Fang WG. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett. 2004;215:239–247. doi: 10.1016/j.canlet.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 79.Chiang PH, Wu SN, Tsai EM, Wu CC, Shen MR, Huang CH, Chiang CP. Adenosine modulation of neurotransmission in penile erection. Br J Clin Pharmacol. 1994;38:357–362. doi: 10.1111/j.1365-2125.1994.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi KC, Tai CJ, Tzeng CR, Auersperg N, Leung PC. Adenosine triphosphate activates mitogen-activated protein kinase in pre-neoplastic and neoplastic ovarian surface epithelial cells. Biol Reprod. 2003;68:309–315. doi: 10.1095/biolreprod.102.006551. [DOI] [PubMed] [Google Scholar]
- 81.Christoforidis S, Papamarcaki T, Galaris D, Kellner R, Tsolas O. Purification and properties of human placental ATP diphosphohydrolase. Eur J Biochem. 1995;234:66–74. doi: 10.1111/j.1432-1033.1995.066_c.x. [DOI] [PubMed] [Google Scholar]
- 82.Chuang YC, Chancellor MB. The application of botulinum toxin in the prostate. J Urol. 2006;176:2375–2382. doi: 10.1016/j.juro.2006.07.127. [DOI] [PubMed] [Google Scholar]
- 83.Cordeaux Y, Middleton RJ, Myatt A, Kellam B, Hill SJ. ABEA-BY630 is a fluorescent agonist at A2B adenosine receptors in human prostatic stromal cells. Br J Pharmacol. 2003;14:16P. [Google Scholar]
- 84.Cox CI, Leese HJ. Characterization of a functional purinergic receptor in primary cultures of bovine oviduct epithelia. J Reprod Fertil. 1994;13:5. [Google Scholar]
- 85.Cox CI, Leese HJ. Effect of purinergic stimulation on intracellular calcium concentration and transepithelial potential difference in cultured bovine oviduct cells. Biol Reprod. 1995;52:1244–1249. doi: 10.1095/biolreprod52.6.1244. [DOI] [PubMed] [Google Scholar]
- 86.Dai Y, Zhang Y, Phatarpekar P, Mi T, Zhang H, Blackburn MR, Xia Y. Adenosine signaling, priapism and novel therapies. J Sex Med. 2009;6(Suppl 3):292–301. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 87.Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Jr, Ojcius DM. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 88.De Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa: II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl. 1992;13:379–386. [PubMed] [Google Scholar]
- 89.de Souza LF, Gelain DP, Jardim FR, Ribeiro GR, Zim M, Bernard EA. Extracellular inosine participates in tumor necrosis factor-alpha induced nitric oxide production in cultured Sertoli cells. Mol Cell Biochem. 2006;281:123–128. doi: 10.1007/s11010-006-0639-9. [DOI] [PubMed] [Google Scholar]
- 90.Di Iulio JL, Gude NM, Brennecke SP, King RG. Effects of ATP, ADP, thrombin, vasopressin and U46619 on human placental nitric oxide synthase activity. Methods Find Exp Clin Pharmacol. 1997;19:509–514. [PubMed] [Google Scholar]
- 91.Diaz JW, Gonzalez MA, Avedo F, Mallea L, Rodriguez N. Use of an ATP-supplemented medium for the conservation of human semen and the effect of caffeine on the motility of preserved sperm. Results in artificial insemination. Andrologia. 1992;24:131–133. doi: 10.1111/j.1439-0272.1992.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 92.Dickens CJ, Comer MT, Southgate J, Leese HJ. Human Fallopian tubal epithelial cells in vitro: establishment of polarity and potential role of intracellular calcium and extracellular ATP in fluid secretion. Hum Reprod. 1996;11:212–217. doi: 10.1093/oxfordjournals.humrep.a019021. [DOI] [PubMed] [Google Scholar]
- 93.Divald A, Karl PI, Fisher SE. Regulation of phospholipase D in human placental trophoblasts by the P2 purinergic receptor. Placenta. 2002;23:584–593. doi: 10.1053/plac.2002.0844. [DOI] [PubMed] [Google Scholar]
- 94.Dobronyi I, Gontero E, Maguire MH (1992) Vascular actions of ATP, α,β-methylene-ATP and β,γ-methylene-ATP in the perfused human placenta cotyledon. FASEB J Supplement:A1007
- 95.Dobronyi I, Hung KS, Satchell DG, Maguire MH. Evidence for a novel P2X purinoceptor in human placental chorionic surface arteries. Eur J Pharmacol. 1997;320:61–64. doi: 10.1016/s0014-2999(96)00967-3. [DOI] [PubMed] [Google Scholar]
- 96.Donoso MV, Montes CG, Lewin J, Fournier A, Calixto JB, Huidobro-Toro JP. Endothelin-1 (ET)-induced mobilization of intracellular Ca2+ stores from the smooth muscle facilitates sympathetic cotransmission by potentiation of adenosine 5′-triphosphate (ATP) motor activity: studies in the rat vas deferens. Peptides. 1992;13:831–840. doi: 10.1016/0196-9781(92)90193-7. [DOI] [PubMed] [Google Scholar]
- 97.Donoso MV, Bates F, Montiel J, Huidobro-Toro JP. Adenosine 5′-triphosphate (ATP), the neurotransmitter in the prostatic portion of the longitudinal muscle layer of the rat vas deferens. Neurosci Lett. 1994;169:59–62. doi: 10.1016/0304-3940(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 98.Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol. 2005;288:H2439–H2449. doi: 10.1152/ajpheart.00548.2004. [DOI] [PubMed] [Google Scholar]
- 99.Donoso MV, Hermosilla D, Navarrete C, Álvarez P, Lillo JG, Huidobro-Toro JP. Reciprocal sympatho-sensory control: functional role of nucleotides and calcitonin gene-related peptide in a peripheral neuroeffector junction. Neuroscience. 2012;203:216–229. doi: 10.1016/j.neuroscience.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 100.Dozi-Vassiliades J, Tsiamitas C, Kokolis N. The effect of PGE2 and PGF2α on the response of guinea pig myometrium to adenine nucleotides in vitro. Prostaglandins. 1976;12:515–524. doi: 10.1016/0090-6980(76)90032-0. [DOI] [PubMed] [Google Scholar]
- 101.Dunn PM. Fertility: purinergic receptors and the male contraceptive pill. Curr Biol. 2000;10:R305–R307. doi: 10.1016/s0960-9822(00)00436-x. [DOI] [PubMed] [Google Scholar]
- 102.Dunn PM, Blakeley AGH. Suramin: a reversible P2-purinceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edwards SE, Buffone MG, Knee GR, Rossato M, Bonanni G, Masiero S, Ferasin S, Gerton GL, Moss SB, Williams CJ. Effects of extracellular adenosine 5′-triphosphate on human sperm motility. Reprod Sci. 2007;14:655–666. doi: 10.1177/1933719107306227. [DOI] [PubMed] [Google Scholar]
- 104.Ellis JL, Burnstock G. Modulation of neurotransmission in the guinea-pig vas deferens by capsaicin: involvement of calcitonin gene-related peptide and substance P. Br J Pharmacol. 1989;98:707–713. doi: 10.1111/j.1476-5381.1989.tb12646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ennion SJ, Evans RJ. Agonist-stimulated internalisation of the ligand-gated ion channel P2X1 in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/s0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- 106.Erkkila K, Kyttanen S, Wikstrom M, Taari K, Hikim AP, Swerdloff RS, Dunkel L. Regulation of human male germ cell death by modulators of ATP production. Am J Physiol Endocrinol Metab. 2006;290:E1145–E1154. doi: 10.1152/ajpendo.00142.2005. [DOI] [PubMed] [Google Scholar]
- 107.Escudero C, Sobrevia L. A hypothesis for preeclampsia: adenosine and inducible nitric oxide synthase in human placental microvascular endothelium. Placenta. 2008;29:469–483. doi: 10.1016/j.placenta.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Escudero C, Casanello P, Sobrevia L. Human equilibrative nucleoside transporters 1 and 2 may be differentially modulated by A2B adenosine receptors in placenta microvascular endothelial cells from pre-eclampsia. Placenta. 2008;29:816–825. doi: 10.1016/j.placenta.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 109.Fabiani R, Ronquist G. Abundance of guanine, guanosine, inosine and adenosine in human seminal plasma. Int J Clin Lab Res. 1995;25:47–51. doi: 10.1007/BF02592577. [DOI] [PubMed] [Google Scholar]
- 110.Faria M, Magalhães-Cardoso T, Lafuente-de-Carvalho JM, Correia-de-Sá P. Corpus cavernosum from men with vasculogenic impotence is partially resistant to adenosine relaxation due to endothelial A2B receptor dysfunction. J Pharmacol Exp Ther. 2006;319:405–413. doi: 10.1124/jpet.106.107821. [DOI] [PubMed] [Google Scholar]
- 111.Faria M, Magalhães-Cardoso T, Lafuente-de-Carvalho JM, Correia-de Sá P. Decreased ecto-NTPDase1/CD39 activity leads to desensitization of P2 purinoceptors regulating tonus of corpora cavernosa in impotent men with endothelial dysfunction. Nucleosides Nucleotides Nucleic Acids. 2008;27:761–768. doi: 10.1080/15257770802145744. [DOI] [PubMed] [Google Scholar]
- 112.Faria M, Valente MJ, Magalhães-Cardoso T, Lafuente-de-Carvalho JM, Correia-de Sá P. Dual regulation of human penile smooth muscle tone by P2 purinoceptors: possible implications in erectile dysfunction. Purinergic Signal. 2008;4:S4. [Google Scholar]
- 113.Faria M, Timóteo MA, Lafuente-de-Carvalho M, Correia-de-Sá P. P2 purinoceptor subtype changes in patients with vasculogenic erectile dysfunction. Purinergic Signal. 2010;6:S115. [Google Scholar]
- 114.Fedan JS, Hogaboom GK, O'Donnell JP, Colby J, Westfall DP. Contributions by purines to the neurogenic response of the vas deferens of the guinea-pig. Eur J Pharmacol. 1981;69:41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- 115.Fedan JS, Hogaboom GK, Westfall DP, O'Donnell JP. Comparison of the effects of arylazido aminopropionyl ATP (ANAPP3), an ATP antagonist, on responses of the smooth muscle of the guinea-pig vas deferens to ATP and related nucleotides. Eur J Pharmacol. 1982;85:277–290. doi: 10.1016/0014-2999(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 116.Felder CC, Williams HL, Axelrod J. A transduction pathway associated with receptors coupled to the inhibitory guanine nucleotide binding protein Gi that amplifies ATP-mediated arachidonic acid release. Proc Natl Acad Sci U S A. 1991;88:6477–6480. doi: 10.1073/pnas.88.15.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng YH, Wang L, Wang Q, Li X, Zeng R, Gorodeski GI. ATP stimulates GRK-3 – phosphorylation and β-arrestin-2-dependent internalization of P2X7-receptor. Am J Physiol. 2005;288:C1342–C1356. doi: 10.1152/ajpcell.00315.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fénichel P, Gharib A, Emiliozzi C, Donzeau M, Ménézo Y. Stimulation of human sperm during capacitation in vitro by an adenosine agonist with specificity for A2 receptors. Biol Reprod. 1996;54:1405–1411. doi: 10.1095/biolreprod54.6.1405. [DOI] [PubMed] [Google Scholar]
- 120.Ferguson SE, Pallikaros Z, Michael AE, Cooke BA. The effects of different culture media, glucose, pyridine nucleotides and adenosine on the activity of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Mol Cell Endocrinol. 1999;158:37–44. doi: 10.1016/s0303-7207(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 121.Figenschau Y, Bertheussen K. Enzymatic treatment of spermatozoa with a trypsin solution, SpermSolute: improved motility and enhanced ATP concentration. Int J Androl. 1999;22:342–344. doi: 10.1046/j.1365-2605.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 122.Filippi S, Amerini S, Maggi M, Natali A, Ledda F. Studies on the mechanisms involved in the ATP-induced relaxation in human and rabbit corpus cavernosum. J Urol. 1999;161:326–331. [PubMed] [Google Scholar]
- 123.Filippini A, Riccioli A, De Cesaris P, Paniccia R, Teti A, Stefanini M, Conti M, Ziparo E. Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 1994;134:1537–1545. doi: 10.1210/endo.134.3.8119196. [DOI] [PubMed] [Google Scholar]
- 124.Fleet IR, Heap RB. Uterine blood flow, myometrial activity and their response to adenosine during the peri-implantation period in sheep. J Reprod Fertil. 1982;65:195–205. doi: 10.1530/jrf.0.0650195. [DOI] [PubMed] [Google Scholar]
- 125.Foresta C, Rossato M, Di Virgilio F. Extracellular ATP is a trigger for the acrosome reaction in human spermatozoa. J Biol Chem. 1992;267:19443–19447. [PubMed] [Google Scholar]
- 126.Foresta C, Rossato M, Bordon P, Di Virgilio F. Extracellular ATP activates different signalling pathways in rat Sertoli cells. Biochem J. 1995;311:269–274. doi: 10.1042/bj3110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Foresta C, Rossato M, Chiozzi P, Di Virgilio F. Mechanism of human sperm activation by extracellular ATP. Am J Physiol. 1996;270:C1709–C1714. doi: 10.1152/ajpcell.1996.270.6.C1709. [DOI] [PubMed] [Google Scholar]
- 128.Foresta C, Rossato M, Nogara A, Gottardello F, Bordon P, Di Virgilio F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem J. 1996;320:499–504. doi: 10.1042/bj3200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fourie MH, du Toit D, Bornman MS, van der Merwe MP, du Plessis DJ. α-Glucosidase, sperm ATP concentrations, and epididymal function. Arch Androl. 1991;26:139–141. doi: 10.3109/01485019108987636. [DOI] [PubMed] [Google Scholar]
- 130.Fox IH, Kurpis L. Binding characteristics of an adenosine receptor in human placenta. J Biol Chem. 1983;258:6952–6955. [PubMed] [Google Scholar]
- 131.Francavilla S, Moscardelli S, Properzi G, De Matteis MA, Scorza BP, Natali PG, De MC. Postnatal development of epididymis and ductus deferens in the rat. A correlation between the ultrastructure of the epithelium and tubule wall, and the fluorescence-microscopic distribution of actin, myosin, fibronectin, and basement membrane. Cell Tissue Res. 1987;249:257–265. doi: 10.1007/BF00215508. [DOI] [PubMed] [Google Scholar]
- 132.Fraser LR. Adenosine and its analogues, possibly acting at A2 receptors, stimulate mouse sperm fertilizing ability during early stages of capacitation. J Reprod Fertil. 1990;89:467–476. doi: 10.1530/jrf.0.0890467. [DOI] [PubMed] [Google Scholar]
- 133.Fraser LR. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev. 2010;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- 134.Fraser LR, Duncan AE. Adenosine analogues with specificity for A2 receptors bind to mouse spermatozoa and stimulate adenylate cyclase activity in uncapacitated suspensions. J Reprod Fertil. 1993;98:187–194. doi: 10.1530/jrf.0.0980187. [DOI] [PubMed] [Google Scholar]
- 135.Fu W, McCormick T, Qi X, Zhou L, Li X, Wang BC, Gibbons HE, Abdul-Karim FW, Gorodeski GI. Activation of P2X7-mediated apoptosis Inhibits DMBA/TPA-induced formation of skin papillomas and cancer in mice. BMC Cancer. 2009;9:114. doi: 10.1186/1471-2407-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fujita R, Kimura S, Kawasaki S, Takashima K, Matsumoto M, Hirano H, Sasaki K. ATP suppresses the K+ current responses to FSH and adenosine in the follicular cells of Xenopus oocyte. Jpn J Physiol. 2001;51:491–500. doi: 10.2170/jjphysiol.51.491. [DOI] [PubMed] [Google Scholar]
- 137.Funahashi H, Romar R. Reduction of the incidence of polyspermic penetration into porcine oocytes by pretreatment of fresh spermatozoa with adenosine and a transient co-incubation of the gametes with caffeine. Reproduction. 2004;128:789–800. doi: 10.1530/rep.1.00295. [DOI] [PubMed] [Google Scholar]
- 138.Funahashi H, Asano A, Fujiwara T, Nagai T, Niwa K, Fraser LR. Both fertilization promoting peptide and adenosine stimulate capacitation but inhibit spontaneous acrosome loss in ejaculated boar spermatozoa in vitro. Mol Reprod Dev. 2000;55:117–124. doi: 10.1002/(SICI)1098-2795(200001)55:1<117::AID-MRD16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 139.Furness JB, McLean JR, Burnstock G. Distribution of adrenergic nerves and changes in neuromuscular transmission in the mouse vas deferens during postnatal development. Dev Biol. 1970;21:491–505. doi: 10.1016/0012-1606(70)90074-6. [DOI] [PubMed] [Google Scholar]
- 140.Furuya K, Nakano H, Enomoto K, Okuda A, Ichikawa J. Mechanically induced calcium waves mediating purinergic coupling between myo- and secretory epithelial cells in mammary gland. Drug Dev Res. 2000;50:54. [Google Scholar]
- 141.Gelain DP, de Souza LF, Bernard EA. Extracellular purines from cells of seminiferous tubules. Mol Cell Biochem. 2003;245:1–9. doi: 10.1023/a:1022857608849. [DOI] [PubMed] [Google Scholar]
- 142.Gessi S, Merighi S, Stefanelli A, Mirandola P, Bonfatti A, Fini S, Sensi A, Marci R, Varani K, Borea PA, Vesce F. Downregulation of A1 and A2B adenosine receptors in human trisomy 21 mesenchymal cells from first-trimester chorionic villi. Biochim Biophys Acta. 2012;1822:1660–1670. doi: 10.1016/j.bbadis.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 143.Ghildyal P, Palani D, Manchanda R. Post- and prejunctional consequences of ecto-ATPase inhibition: electrical and contractile studies in guinea-pig vas deferens. J Physiol. 2006;575:469–480. doi: 10.1113/jphysiol.2006.109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gillman TA, Pennefather JN. Evidence for the presence of both P1 and P2 purinoceptors in the rat myometrium. Clin Exp Pharmacol Physiol. 1998;25:592–599. doi: 10.1111/j.1440-1681.1998.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 145.Glass R, Bardini M, Robson T, Burnstock G. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells Tissues Org. 2001;169:377–387. doi: 10.1159/000047905. [DOI] [PubMed] [Google Scholar]
- 146.Gorodeski GI. Regulation of transcervical permeability by two distinct P2 purinergic receptor mechanisms. Am J Physiol Cell Physiol. 2002;282:C75–C83. doi: 10.1152/ajpcell.2002.282.1.C75. [DOI] [PubMed] [Google Scholar]
- 147.Gorodeski GI. Expression, regulation, and function of P2X4 purinergic receptor in human cervical epithelial cells. Am J Physiol Cell Physiol. 2002;282:C84–C93. doi: 10.1152/ajpcell.2002.282.1.C84. [DOI] [PubMed] [Google Scholar]
- 148.Gorodeski GI. Regulation of paracellular permeability in low-resistance human vaginal–cervical epithelia. In: Ehrhardt C, Kim KJ, editors. Biotechnology, pharmaceutical aspects, drug absorption studies in situ, in vitro, and in silico Models. Berlin: Springer; 2008. pp. 339–367. [Google Scholar]
- 149.Gorodeski GI. P2X7-mediated chemoprevention of epithelial cancers. Exp Opin Therap Targ. 2009;13:1313–1332. doi: 10.1517/14728220903277249. [DOI] [PubMed] [Google Scholar]
- 150.Gorodeski GI. P2X7 receptors and epithelial cancers: focus article. WIREs Membr Transp Signal. 2012;1:349–371. [Google Scholar]
- 151.Gorodeski GI, Goldfarb J. Extracellular ATP regulates transcervical permeability by modulating two distinct paracellular pathways. Am J Physiol. 1997;272:C1602–C1610. doi: 10.1152/ajpcell.1997.272.5.C1602. [DOI] [PubMed] [Google Scholar]
- 152.Gorodeski GI, Goldfarb J. Seminal fluid factor increases the resistance of the tight junctional complex of the cultured human cervical epithelium CaSki. Fertil Steril. 1998;69:309–317. doi: 10.1016/s0015-0282(97)00471-8. [DOI] [PubMed] [Google Scholar]
- 153.Gorodeski GI, Peterson DE, De Santis BJ, Hopfer U. Nucleotide receptor-mediated decrease of tight-junctional permeability in cultured human cervical epithelium. Am J Physiol. 1996;270:C1715–C1725. doi: 10.1152/ajpcell.1996.270.6.C1715. [DOI] [PubMed] [Google Scholar]
- 154.Gorodeski GI, Hopfer U, Liu CC, Margles E. Estrogen acidifies vaginal pH by upregulation of proton secretion via the apical membrane of vaginal–ectocervical epithelial cells. Endocrinology. 2005;146:816–824. doi: 10.1210/en.2004-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gray KT, Short JL, Ledent C, Ventura S. Targeted disruption of the A2A adenosine receptor reduces in-vitro prostate contractility in mature mice. Eur J Pharmacol. 2008;592:151–157. doi: 10.1016/j.ejphar.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 156.Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999;296:599–605. doi: 10.1007/s004410051321. [DOI] [PubMed] [Google Scholar]
- 157.Gür S, Kadowitz PJ, Hellstrom WJ. Purinergic (P2) receptor control of lower genitourinary tract function and new avenues for drug action: an overview. Curr Pharm Des. 2007;13:3236–3244. doi: 10.2174/138161207782341277. [DOI] [PubMed] [Google Scholar]
- 158.Gür S, Kadowitz PJ, Abdel-Mageed AS, Kendirci M, Sikka SC, Burnstock G, Hellstrom WJG. Management of erectile function by purinergic P2 receptors in diabetic rat penis. J Urol. 2009;181:2382. doi: 10.1016/j.juro.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Hall AK, Preston SL, Behrman HR. Purine amplification of luteinizing hormone action in ovarian luteal cells. J Biol Chem. 1981;256:10390–10398. [PubMed] [Google Scholar]
- 160.Hammami S, Willumsen NJ, Meinild AK, Klaerke DA, Novak I. Purinergic signalling — a possible mechanism for KCNQ1 channel response to cell volume challenges. Acta Physiol (Oxf) 2013;207:503–515. doi: 10.1111/j.1748-1716.2012.02460.x. [DOI] [PubMed] [Google Scholar]
- 161.Hashitani H. Neuroeffector transmission to different layers of smooth muscle in the rat penile bulb. J Physiol. 2000;524(Pt 2):549–563. doi: 10.1111/j.1469-7793.2000.t01-2-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haynes JM, Alexander SP, Hill SJ. A1 and A2 adenosine receptor modulation of contractility in the cauda epididymis of the guinea-pig. Br J Pharmacol. 1998;125:570–576. doi: 10.1038/sj.bjp.0702095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hedlund H, Andersson KE, Larsson B. Effect of drugs interacting with adrenoreceptors and muscarinic receptors in the epididymal and prostatic parts of the human isolated vas deferens. J Auton Pharmacol. 1985;5:261–270. doi: 10.1111/j.1474-8673.1985.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 164.Henry D, Ferino F, Tomova S, Ferry N, Stengel D, Hanoune J. Inhibition of the catalytic subunit of ram sperm adenylate cyclase by adenosine. Biochem Biophys Res Commun. 1986;137:970–977. doi: 10.1016/0006-291x(86)90320-7. [DOI] [PubMed] [Google Scholar]
- 165.Holck MI, Marks BH. Purine nucleoside and nucleotide interactions on normal and subsensitive alpha adrenoreceptor responsiveness in guinea-pig vas deferens. J Pharmacol Exp Ther. 1978;205:104–117. [PubMed] [Google Scholar]
- 166.Holman ME. The gamma controversy. Clin Exp Pharmacol Physiol. 1987;14:415–422. doi: 10.1111/j.1440-1681.1987.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 167.Honoré E, Martin C, Mironneau C, Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989;257:C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- 168.Hourani SM. Postnatal development of purinoceptors in rat visceral smooth muscle preparations. Gen Pharmacol. 1999;32:3–7. doi: 10.1016/s0306-3623(98)00112-8. [DOI] [PubMed] [Google Scholar]
- 169.Hourani SMO, Nicholls J, Lee BS, Halfhide EJ, Kitchen I. Characterization and ontogeny of P1-purinoceptors on rat vas deferens. Br J Pharmacol. 1993;108:754–758. doi: 10.1111/j.1476-5381.1993.tb12873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huidobro-Toro JP, Briones R, Buvinic S, Valdecantos P, Germain A (2002) Nucleotide receptor subtypes regulate flow in the human placenta. Vascular Neuroeffector Mechanisms, Lake Tahoe, July 12–15, 2002
- 171.Hukovic S. Responses of the isolated sympathetic nerveductus deferens preparation of the guinea-pig. Br J Pharmacol Chemother. 1961;16:188–194. doi: 10.1111/j.1476-5381.1961.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hupertan V, Neuzillet Y, Stücker O, Pons C, Leammel E, Lebret T. Effects of nucleotides adenosine monophosphate and adenosine triphosphate in combination with l-arginine on male rabbit corpus cavernosum tissue. Int J Androl. 2012;35:860–866. doi: 10.1111/j.1365-2605.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 173.Hutchings G, Gevaert T, Deprest J, Nilius B, Williams O, De Ridder D. The effect of extracellular adenosine triphosphate on the spontaneous contractility of human myometrial strips. Eur J Obstet Gynecol Reprod Biol. 2009;143:79–83. doi: 10.1016/j.ejogrb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 174.Hutchings G, Williams O, Cretoiu D, Ciontea SM. Myometrial interstitial cells and the coordination of myometrial contractility. J Cell Mol Med. 2009;13:4268–4282. doi: 10.1111/j.1582-4934.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hutton RA, Chow FP, Craft IL, Dandona P. Inhibitors of platelet aggregation in the fetoplacental unit and myometrium with particular reference to the ADP-degrading property of placenta. Placenta. 1980;1:125–130. doi: 10.1016/s0143-4004(80)80020-8. [DOI] [PubMed] [Google Scholar]
- 176.Illes P, Rickmann H, Brod I, Bucher B, Stoclet J-C. Subsensitivity of presynaptic adenosine A1-receptors in caudal arteries of spontaneously hypertensive rats. Eur J Pharmacol. 1989;174:237–251. doi: 10.1016/0014-2999(89)90316-6. [DOI] [PubMed] [Google Scholar]
- 177.Insler V, Glazerman M, Zeidel L, Bernstein D, Misgav N. Sperm storage in the human cervix. Fertil Steril. 1980;33:288–229. doi: 10.1016/s0015-0282(16)44596-6. [DOI] [PubMed] [Google Scholar]
- 178.Iredale PA, Hill SJ. Increases in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993;110:1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–432. doi: 10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- 180.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132:536–546. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems JM. Cloning and tissue distribution of the human P2Y1 receptor. Biochem Biophys Res Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- 182.Jeulin C, Soufir JC. Reversible intracellular ATP changes in intact rat spermatozoa and effects on flagellar sperm movement. Cell Motil Cytoskeleton. 1992;21:210–222. doi: 10.1002/cm.970210305. [DOI] [PubMed] [Google Scholar]
- 183.Jiang L, Bardini M, Keogh A, dos Remedios CG, Burnstock G. P2X1 receptors are closely associated with connexin 43 in human ventricular myocardium. Int J Cardiol. 2005;98:291–297. doi: 10.1016/j.ijcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 184.Kamada S, Blackmore PF, Oehninger S, Gordon K, Hodgen GD. Existence of P2-purinoceptors on human and porcine granulosa cells. J Clin Endocrinol Metab. 1994;78:650–656. doi: 10.1210/jcem.78.3.8126137. [DOI] [PubMed] [Google Scholar]
- 185.Kangasniemi M. Effects of adenosine analog PIA (n-phenylisopropyladenosine) on FSH-stimulated cyclic AMP (cAMP) production in the rat seminiferous epithelium. Mol Cell Endocrinol. 1993;96:141–146. doi: 10.1016/0303-7207(93)90104-r. [DOI] [PubMed] [Google Scholar]
- 186.Karunanithi S, Lavidis NA, Bennett MR. Evidence that each nerve varicosity on the surface of the mouse vas deferens secretes ATP. Neurosci Lett. 1993;161:157–160. doi: 10.1016/0304-3940(93)90283-q. [DOI] [PubMed] [Google Scholar]
- 187.Kasakov L, Ellis J, Kirkpatrick K, Milner P, Burnstock G. Direct evidence for concomitant release of noradrenaline, adenosine 5′-triphosphate and neuropeptide Y from sympathetic nerve supplying the guinea-pig vas deferens. J Auton Nerv Syst. 1988;22:75–82. doi: 10.1016/0165-1838(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 188.Kaschube M, Möller-Hartmann H, Zetler G. The field-stimulated vas deferens of the streptozotocin-diabetic mouse: effects of prazosin, α, β-methylene ATP, and variation of stimulation parameters. J Neural Transm. 1989;77:171–180. doi: 10.1007/BF01248930. [DOI] [PubMed] [Google Scholar]
- 189.Kataoka K, Furukawa K, Nagao K, Ishii N, Tsuru H. The participation of adenosine receptors in the adenosine 5′-triphosphate-induced relaxation in the isolated rabbit corpus cavernosum penis. Int J Urol. 2007;14:764–768. doi: 10.1111/j.1442-2042.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 190.Katoh K, Komatsu T, Yonekura S, Ishiwata H, Hagino A, Obara Y. Effects of adenosine 5′-triphosphate and growth hormone on cellular H+ transport and calcium ion concentrations in cloned bovine mammary epithelial cells. J Endocrinol. 2001;169:381–388. doi: 10.1677/joe.0.1690381. [DOI] [PubMed] [Google Scholar]
- 191.Katugampola H, Burnstock G. Purinergic signalling to rat ovarian smooth muscle: changes in P2X receptor expression during pregnancy. Cells Tissues Org. 2004;178:33–47. doi: 10.1159/000081091. [DOI] [PubMed] [Google Scholar]
- 192.Kaya T, Sarioglu Y, Göksu OC, Yildirim S, Yildirim K. Effect of additional testosterone on purinergic responses in isolated rabbit corpus cavernosum strips. Pharmacol Res. 1998;37:227–232. doi: 10.1006/phrs.1997.0285. [DOI] [PubMed] [Google Scholar]
- 193.Keating N, Quinlan LR. Effect of basolateral adenosine triphosphate on chloride secretion by bovine oviductal epithelium. Biol Reprod. 2008;78:1119–1126. doi: 10.1095/biolreprod.107.065508. [DOI] [PubMed] [Google Scholar]
- 194.Keating N, Quinlan LR. Small conductance potassium channels drive ATP-activated chloride secretion in the oviduct. Am J Physiol Cell Physiol. 2012;302:C100–C109. doi: 10.1152/ajpcell.00503.2010. [DOI] [PubMed] [Google Scholar]
- 195.Kempinas WD, Suarez JD, Roberts NL, Strader L, Ferrell J, Goldman JM, Klinefelter GR. Rat epididymal sperm quantity, quality, and transit time after guanethidine-induced sympathectomy. Biol Reprod. 1998;59:890–896. doi: 10.1095/biolreprod59.4.890. [DOI] [PubMed] [Google Scholar]
- 196.Kerr KP. The effect of histamine on field-stimulated contractions of the guinea-pig prostate. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:237–244. doi: 10.1007/s00210-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 197.Kesim M, Kadioglu M, Yaris E, Kalyoncu NI, Ozyavuz R, Ulku C. Paroxetine alters KCl- or ATP-induced contractile responses of isolated vas deferens in rats chronically treated with ethanol. Pharmacol Res. 2004;49:51–57. doi: 10.1016/j.phrs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 198.Kettlun AM, Alvarez A, Quintar R, Valenzuela MA, Collados L, Aranda E, Banda A, Chayet L, Chiong M, Mancilla M. Human placental ATP-diphosphohydrolase: biochemical characterization, regulation and function. Int J Biochem. 1994;26:437–448. doi: 10.1016/0020-711x(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 199.Khanam T, Burnstock G. Changes in expression of P2X1 receptor and connexin 43 in the rat myometrium during pregnancy. Fertil Steril. 2007;88:1174–1179. doi: 10.1016/j.fertnstert.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 200.Khanum A, Buczko E, Dufau ML. Essential role of adenosine triphosphate in activation of 17β-hydroxysteroid dehydrogenase in the rat Leydig cell. Endocrinology. 1997;138:1612–1620. doi: 10.1210/endo.138.4.5062. [DOI] [PubMed] [Google Scholar]
- 201.Khattab MM, Al-Rawi MB, Aleisa AM. Postjunctional synergism of norepinephrine with ATP and diadenosine tetraphosphate in guinea pig vas deferens. Role of protein kinase C and Myosin light chain phosphatase. Pharmacology. 2007;80:27–32. doi: 10.1159/000102778. [DOI] [PubMed] [Google Scholar]
- 202.Khera M, Mohamed O, Avila D, Ekeruo W, Gangitano DA, Lipshultz LI, Somogyi GT. ATP release from cavernosal tissue is greater in patients following prostatectomy as compared to patients with organic ED. J Urol. 2008;179:232. [Google Scholar]
- 203.Kilicarslan H, Yildirim S, Bagcivan I, Ayan S, Sarac B, Sarioglu Y. Effect of chronic renal failure on the purinergic responses of corpus cavernosal smooth muscle in rabbits. BJU Int. 2002;90:596–600. doi: 10.1046/j.1464-410x.2002.02979.x. [DOI] [PubMed] [Google Scholar]
- 204.Kim JH, Hong EK, Choi HS, Oh SJ, Kim KM, Uhm DY, Kim SJ. K+ channel currents in rat ventral prostate epithelial cells. Prostate. 2002;51:201–210. doi: 10.1002/pros.10090. [DOI] [PubMed] [Google Scholar]
- 205.Kim JH, Nam JH, Kim MH, Koh DS, Choi SJ, Kim SJ, Lee JE, Min KM, Uhm DY, Kim SJ. Purinergic receptors coupled to intracellular Ca2+ signals and exocytosis in rat prostate neuroendocrine cells. J Biol Chem. 2004;279:27345–27356. doi: 10.1074/jbc.M313575200. [DOI] [PubMed] [Google Scholar]
- 206.Kimoto Y, Ito Y. Autonomic innervation of the canine penile artery and vein in relation to neural mechanisms involved in erection. Br J Urol. 1987;59:463–472. doi: 10.1111/j.1464-410x.1987.tb04847.x. [DOI] [PubMed] [Google Scholar]
- 207.King BF, Wang S, Burnstock G. P2 purinoceptor-activated inward currents in folliculated oocytes of Xenopus laevis. J Physiol. 1996;494:17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kirkpatrick K, Burnstock G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur J Pharmacol. 1987;138:207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- 209.Klinge E, Sjöstrand NO. Comparative study of some isolated mammalian smooth muscle effectors of penile erection. Acta Physiol Scand. 1977;100:354–367. doi: 10.1111/j.1748-1716.1977.tb05961.x. [DOI] [PubMed] [Google Scholar]
- 210.Knecht M, Darbon JM, Ranta T, Baukal A, Catt KJ. Inhibitory actions of adenosine on follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Biol Reprod. 1984;30:1082–1090. doi: 10.1095/biolreprod30.5.1082. [DOI] [PubMed] [Google Scholar]
- 211.Knight D, D'Arbe M, Liang S, Phillips WD, Lavidis NA. Regional differences in sympathetic purinergic transmission along the length of the mouse vas deferens. Synapse. 2003;47:225–235. doi: 10.1002/syn.10119. [DOI] [PubMed] [Google Scholar]
- 212.Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 213.Knudsen TB, Blackburn MR, Chinsky JM, Airhart MJ, Kellems RE. Ontogeny of adenosine deaminase in the mouse decidua and placenta: immunolocalization and embryo transfer studies. Biol Reprod. 1991;44:171–184. doi: 10.1095/biolreprod44.1.171. [DOI] [PubMed] [Google Scholar]
- 214.Ko WH, Au CL, Yip CY. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci. 2003;72:1519–1535. doi: 10.1016/s0024-3205(02)02410-4. [DOI] [PubMed] [Google Scholar]
- 215.Konrad L, Schiemann P, Renneberg H, Wennemuth G, Fini C, Aumuller G. Expression and enzymic activity of ecto 5′-nucleotidase in the human male genital tract. Biol Reprod. 1998;59:190–196. doi: 10.1095/biolreprod59.1.190. [DOI] [PubMed] [Google Scholar]
- 216.Koshi R, Coutinho-Silva R, Cascabulho CM, Henrique-Pons A, Knight GE, Loesch A, Burnstock G. Presence of the P2X7 purinergic receptor on immune cells that invade the rat endometrium during oestrus. J Reprod Immunol. 2005;66:127–140. doi: 10.1016/j.jri.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 217.Kumcu EK, Büyüknacar HS, Göçmen C, Evrüke IC, Önder S. Differential effect of neocuproine, a copper(I) chelator, on contractile activity in isolated ovariectomized non-pregnant rat, pregnant rat and pregnant human uterus. Eur J Pharmacol. 2009;605:158–163. doi: 10.1016/j.ejphar.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 218.Kupitz Y, Atlas D. A putative ATP-activated Na+ channel involved in sperm-induced fertilization. Science. 1993;261:484–486. doi: 10.1126/science.8392753. [DOI] [PubMed] [Google Scholar]
- 219.Kwan CY, Ramlal T. 5′-Nucleotidase activity in smooth muscle membranes of rat vas deferens. Mol Physiolo. 1985;8:277–292. [Google Scholar]
- 220.Lahooti H, Thorsen T, Aakvaag A. Effect of ATP on the regulation of the steroid binding activity of the oestradiol receptor. J Steroid Biochem. 1990;36:333–343. doi: 10.1016/0022-4731(90)90226-i. [DOI] [PubMed] [Google Scholar]
- 221.Lalevee N, Rogier C, Becq F, Joffre M. Acute effects of adenosine triphosphates, cyclic 3′,5′-adenosine monophosphates, and follicle-stimulating hormone on cytosolic calcium level in cultured immature rat Ssertoli cells. Biol Reprod. 1999;61:343–352. doi: 10.1095/biolreprod61.2.343. [DOI] [PubMed] [Google Scholar]
- 222.Lara HE, Belmar J. Release of norepinephrine from the cat ovary: changes after ovulation. Biol Reprod. 1991;44:752–759. doi: 10.1095/biolreprod44.5.752. [DOI] [PubMed] [Google Scholar]
- 223.Larriba S, Bassas L, Egozcue S, Giménez J, Ramos MD, Briceño O, Estivill X, Casals T. Adenosine triphosphate-binding cassette superfamily transporter gene expression in severe male infertility. Biol Reprod. 2001;65:394–400. doi: 10.1095/biolreprod65.2.394. [DOI] [PubMed] [Google Scholar]
- 224.Lau DH, Metcalfe MJ, Mumtaz FH, Mikhailidis DP, Thompson CS. Purinergic modulation of human corpus cavernosum relaxation. Int J Androl. 2009;32:149–155. doi: 10.1111/j.1365-2605.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 225.Lau WA, Ventura S, Pennefather JN. Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J Auton Pharmacol. 1998;18:349–356. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- 226.Lavidis NA, Bennett MR. Probabilistic secretion of quanta from visualized sympathetic nerve varicosities in mouse vas deferens. J Physiol. 1992;454:9–26. doi: 10.1113/jphysiol.1992.sp019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee HY, Bardini M, Burnstock G. P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res. 2000;300:321–330. doi: 10.1007/s004410000207. [DOI] [PubMed] [Google Scholar]
- 228.Lee PS, Squires PE, Buchan AM, Yuen BH, Leung PC. P2-purinoreceptor evoked changes in intracellular calcium oscillations in single isolated human granulosa-lutein cells. Endocrinology. 1996;137:3756–3761. doi: 10.1210/endo.137.9.8756543. [DOI] [PubMed] [Google Scholar]
- 229.Lee SY, Palmer ML, Maniak PJ, Jang SH, Ryu PD, O'Grady SM. P2Y receptor regulation of sodium transport in human mammary epithelial cells. Am J Physiol Cell Physiol. 2007;293:C1472–C1480. doi: 10.1152/ajpcell.00068.2007. [DOI] [PubMed] [Google Scholar]
- 230.León D, Albasanz JL, Ruíz MA, Martín M. Chronic caffeine or theophylline intake during pregnancy inhibits A1 receptor function in the rat brain. Neuroscience. 2005;131:481–489. doi: 10.1016/j.neuroscience.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 231.Leung AY, Tai HL, Wong PY. ATP stimulates Ca 2+ release from a rapidly exchanging pool in cultured rat epididymal cells. Am J Physiol. 1993;264:C1388–C1394. doi: 10.1152/ajpcell.1993.264.6.C1388. [DOI] [PubMed] [Google Scholar]
- 232.Levin RM, Hypolite JA, Broderick GA. Comparison of the pharmacological response of human corpus cavernosal tissue with the response of rabbit cavernosal tissue. Gen Pharmacol. 1995;26:1107–1111. doi: 10.1016/0306-3623(94)00260-t. [DOI] [PubMed] [Google Scholar]
- 233.Levine RF, Derks R, Beaman K. Regeneration and tolerance factor. A vacuolar ATPase with consequences. Chem Immunol Allergy. 2005;89:126–134. doi: 10.1159/000087954. [DOI] [PubMed] [Google Scholar]
- 234.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic–fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 235.Lew MJ, White TD. Release of endogenous ATP during sympathetic nerve stimulation. Br J Pharmacol. 1987;92:349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li X, Qi X, Zhou L, Catera D, Rote NS, Potashkin J, Abdul-Karim FW, Gorodeski GI. Decreased expression of P2X7 in endometrial epithelial pre-cancerous and cancer cells. Gynecol Oncol. 2007;106:233–243. doi: 10.1016/j.ygyno.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li X, Qi X, Zhou L, Fu W, Abdul-Karim FW, MacLennan G, Gorodeski GI. P2X7 receptor expression is decreased in epithelial cancer cells of ectodermal, uro-genital sinus, and distal paramesonephric-duct origin. Purinergic Signal. 2009;5:351–368. doi: 10.1007/s11302-009-9161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liang SX, Phillips WD, Lavidis N. Development of fast purinergic transmission in the mouse vas deferens. Proc Aust Neurosci Soc. 1998;9:119. doi: 10.1002/1098-2396(20000915)37:4<283::AID-SYN5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 239.Liang SX, D'Arbe M, Phillips WD, Lavidis NA. Development of fast purinergic transmission in the mouse vas deferens. Synapse. 2000;37:283–291. doi: 10.1002/1098-2396(20000915)37:4<283::AID-SYN5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 240.Lin WY, Mannikarottu A, Chichester P, Neuman P, Johnson A, Pérez-Martínez FC, Levin RM. The effect of chronic partial bladder outlet obstruction on corpus cavernosum smooth muscle and Rho-kinase in rabbits. Neurourol Urodyn. 2008;27:826–831. doi: 10.1002/nau.20607. [DOI] [PubMed] [Google Scholar]
- 241.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu DY, Jennings MG, Baker HW. Correlation between defective motility (asthenospermia) and ATP reactivation of demembranated human spermatozoa. J Androl. 1987;8:349–353. doi: 10.1002/j.1939-4640.1987.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 243.Loir M. Spermatogonia of rainbow trout: III. In vitro study of the proliferative response to extracellular ATP and adenosine. Mol Reprod Dev. 1999;53:443–450. doi: 10.1002/(SICI)1098-2795(199908)53:4<443::AID-MRD10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 244.Longhurst PA, Schwegel T, Folander K, Swanson R. The human P2X1 receptor: molecular cloning, tissue distribution, and localization to chromosome 17. Biochim Biophys Acta. 1996;1308:185–188. doi: 10.1016/0167-4781(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 245.Losier AJ, Armstrong RW, Younglai EV. Adenosine triphosphate inhibits LH stimulated testosterone accumulation by isolated rabbit ovarian follicles. IRCS Med Sci. 1980;8:322. [Google Scholar]
- 246.Luciano LG, Orleans-Juste P, Calixto JB, Rae GA. Endothelin-1 contracts and increases motor responses to ATP in rat seminal vesicle. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S91–S94. [PubMed] [Google Scholar]
- 247.Luria A, Rubinstein S, Lax Y, Breitbart H. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol Reprod. 2002;66:429–437. doi: 10.1095/biolreprod66.2.429. [DOI] [PubMed] [Google Scholar]
- 248.MacDonald A, McGrath JC. Post-natal development of functional neurotransmission in rat vas deferens. Br J Pharmacol. 1984;82:25–34. doi: 10.1111/j.1476-5381.1984.tb16438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mackenzie I, Burnstock G, Dolly JO. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982;7:997–1006. doi: 10.1016/0306-4522(82)90056-2. [DOI] [PubMed] [Google Scholar]
- 250.Mackenzie I, Manzini S, Burnstock G. Regulation of voltage-dependent excitatory responses to α, β-methylene ATP, ATP and non-adrenergic nerve stimulation by dihydropyridines in the guinea-pig vas deferens. Neuroscience. 1988;27:317–332. doi: 10.1016/0306-4522(88)90241-2. [DOI] [PubMed] [Google Scholar]
- 251.Macleod GT, Lavidis NA, Bennett MR. Calcium dependence of quantal secretion from visualized sympathetic nerve varicosities on the mouse vas deferens. J Physiol. 1994;480(Pt 1):61–70. doi: 10.1113/jphysiol.1994.sp020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Magocsi M, Penniston JT. Ca2+ or Mg2+ nucleotide phosphohydrolases in myometrium: two ecto-enzymes. Biochim Biophys Acta. 1991;1070:163–172. doi: 10.1016/0005-2736(91)90159-6. [DOI] [PubMed] [Google Scholar]
- 253.Maguire MH, Kitagawa H, Hosokawa T, Howard RB. ATP and adenosine. Ann N Y Acad Sci. 1990;603:443–444. [Google Scholar]
- 254.Maguire MH, Szabo I, Slegel P, King CR. Determination of concentrations of adenosine and other purines in human term placenta by reversed-phase high-performance liquid chromatography with photodiode-array detection: evidence for pathways of purine metabolism in the placenta. J Chromatogr. 1992;575:243–253. doi: 10.1016/0378-4347(92)80152-g. [DOI] [PubMed] [Google Scholar]
- 255.Major TC, Weishaar RE, Taylor DG. Two phases of contractile response in rat isolated vas deferens and their regulation by adenosine and α-receptors. Eur J Pharmacol. 1989;167:323–331. doi: 10.1016/0014-2999(89)90441-x. [DOI] [PubMed] [Google Scholar]
- 256.Majumder GC, Biswas R. Evidence for the occurrence of an ecto-(adenosine triphosphatase) in rat epididymal spermatozoa. Biochem J. 1979;183:737–743. doi: 10.1042/bj1830737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Malek A, Miller RK, Mattison DR, Ceckler T, Panigel M, di Sant'Agnese PA, Jessee LN. Continuous measurement of ATP by 31P-NMR in term human dually perfused placenta in vitro: response to ischemia. J Appl Physiol. 1995;78:1778–1786. doi: 10.1152/jappl.1995.78.5.1778. [DOI] [PubMed] [Google Scholar]
- 258.Mallard N, Marshall R, Sithers A, Spriggs B. Suramin: a selective inhibitor of purinergic neurotransmission in the rat isolated vas deferens. Eur J Pharmacol. 1992;220:1–10. doi: 10.1016/0014-2999(92)90004-n. [DOI] [PubMed] [Google Scholar]
- 259.Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- 260.Martín-Satué M, Lavoie ÉG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sévigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol. 2009;131:615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Martín-Satué M, Lavoie EG, Fausther M, Lecka J, Aliagas E, Kukulski F, Sévigny J. High expression and activity of ecto-5′-nucleotidase/CD73 in the male murine reproductive tract. Histochem Cell Biol. 2010;133:659–668. doi: 10.1007/s00418-010-0704-z. [DOI] [PubMed] [Google Scholar]
- 262.Matsubara S, Tamada T, Kurahashi K, Saito T. Ultracytochemical localizations of adenosine nucleotidase activities in the human term placenta, with special reference to 5′-nucleotidase activity. Acta Histochem Cytochem. 1987;20:409–419. [Google Scholar]
- 263.Matsumoto M, Sakurai Y, Kokubo T, Yagi H, Makita K, Matsui T, Titani K, Fujimura Y, Narita N. The cDNA cloning of human placental ecto-ATP diphosphohydrolases I and II. FEBS Lett. 1999;453:335–340. doi: 10.1016/s0014-5793(99)00751-6. [DOI] [PubMed] [Google Scholar]
- 264.Matsuoka I, Ohkubo S, Kimura J, Uezono Y. Adenine nucleotide-induced activation of adenosine A2B receptors expressed in Xenopus laevis oocytes: involvement of a rapid and localized adenosine formation by ectonucleotidases. Mol Pharmacol. 2002;61:606–613. doi: 10.1124/mol.61.3.606. [DOI] [PubMed] [Google Scholar]
- 265.Mazzoni MR, Buffoni RS, Giusti L, Lucacchini A. Characterization of a low affinity binding site for N6-substituted adenosine derivatives in rat testis membranes. J Recept Signal Transduct Res. 1995;15:905–929. doi: 10.3109/10799899509049864. [DOI] [PubMed] [Google Scholar]
- 266.McLaren GJ, Lambrecht G, Mutschler E, Bäumert HG, Sneddon P, Kennedy C. Investigation of the actions of PPADS, a novel P2x-purinoceptor antagonist, in the guinea-pig isolated vas deferens. Br J Pharmacol. 1994;111:913–917. doi: 10.1111/j.1476-5381.1994.tb14825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Megory E, Shoham Z, Madgar I, Lunenfeld B, Modan M, Weissenberg R. ATP content in human semen and sperm quality. Arch Androl. 1987;19:243–247. doi: 10.3109/01485018708986823. [DOI] [PubMed] [Google Scholar]
- 268.Meldrum LA, Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983;92:161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- 269.Meldrum LA, Burnstock G. Evidence that ATP is involved as a co-transmitter in the hypogastric nerve supplying the seminal vesicle of the guinea-pig. Eur J Pharmacol. 1985;110:363–366. doi: 10.1016/0014-2999(85)90565-5. [DOI] [PubMed] [Google Scholar]
- 270.Menzies JRW, Kennedy C. Perinuclear P2X7-like immunoreactivity in the guinea-pig vas deferens. Br J Pharmacol. 2002;135:50P. [Google Scholar]
- 271.Meroni SB, Canepa DF, Pellizzari EH, Schteingart HF, Cigorraga SB. Effects of purinergic agonists on aromatase and gamma-glutamyl transpeptidase activities and on transferrin secretion in cultured Sertoli cells. J Endocrinol. 1998;157:275–283. doi: 10.1677/joe.0.1570275. [DOI] [PubMed] [Google Scholar]
- 272.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mieusset R, Parinaud J, Chap H, Pontonnier G. Absence of correlation between the levels of ATP and other seminal compounds in semen and the results of human in-vitro fertilization. Int J Androl. 1989;12:346–353. doi: 10.1111/j.1365-2605.1989.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 274.Mihaylova-Todorova ST, Todorov LD, Westfall DP. Enzyme kinetics and pharmacological characterization of nucleotidases released from the guinea pig isolated vas deferens during nerve stimulation: evidence for a soluble ecto-nucleoside triphosphate diphosphohydrolase-like ATPase and a soluble ecto-5′-nucleotidase-like AMPase. J Pharmacol Exp Ther. 2002;302:992–1001. doi: 10.1124/jpet.102.033332. [DOI] [PubMed] [Google Scholar]
- 275.Milošević M, Petrović S, Veličković N, Grković I, Ignjatović M, Horvat A. ATP and ADP hydrolysis in cell membranes from rat myometrium. Mol Cell Biochem. 2012;371:199–208. doi: 10.1007/s11010-012-1436-2. [DOI] [PubMed] [Google Scholar]
- 276.Min K, Munarriz R, Yerxa BR, Goldstein I, Shaver SR, Cowlen MS, Traish AM. Selective P2Y2 receptor agonists stimulate vaginal moisture in ovariectomized rabbits. Fertil Steril. 2003;79:393–398. doi: 10.1016/s0015-0282(02)04677-0. [DOI] [PubMed] [Google Scholar]
- 277.Minelli A, Miscetti P, Allegrucci C, Mezzasoma I. Evidence of A1 adenosine receptor on epidydimal bovine spermatozoa. Arch Biochem Biophys. 1995;322:272–276. doi: 10.1006/abbi.1995.1462. [DOI] [PubMed] [Google Scholar]
- 278.Minelli A, Allegrucci C, Mezzasoma I. [3H]-(R)-N6-Phenylisopropyladenosine agonist binding to the solubilized A1 adenosine receptor from bovine epididymal spermatozoa. Arch Biochem Biophys. 1997;337:54–61. doi: 10.1006/abbi.1996.9737. [DOI] [PubMed] [Google Scholar]
- 279.Minelli A, Allegrucci C, Piomboni P, Mannucci R, Lluis C, Franco R. Immunolocalization of A1 adenosine receptors in mammalian spermatozoa. J Histochem Cytochem. 2000;48:1163–1171. doi: 10.1177/002215540004800901. [DOI] [PubMed] [Google Scholar]
- 280.Minelli A, Liguori L, Bellazza I, Mannucci R, Johansson B, Fredholm BB. Involvement of A1 adenosine receptors in the acquisition of fertilizing capacity. J Androl. 2004;25:286–292. doi: 10.1002/j.1939-4640.2004.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 281.Minelli A, Bellezza I, Collodel G, Fredholm BB. Promiscuous coupling and involvement of protein kinase C and extracellular signal-regulated kinase 1/2 in the adenosine A1 receptor signalling in mammalian spermatozoa. Biochem Pharmacol. 2008;75:931–941. doi: 10.1016/j.bcp.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 282.Miyoshi H, Yamaoka K, Urabe S, Kodama M, Kudo Y. Functional expression of purinergic P2X7 receptors in pregnant rat myometrium. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1117–R1124. doi: 10.1152/ajpregu.00507.2009. [DOI] [PubMed] [Google Scholar]
- 283.Miyoshi H, Yamaoka K, Urabe S, Kudo Y. ATP-induced currents carried through P2X7 receptor in rat myometrial cells. Reprod Sci. 2012;19:1285–1291. doi: 10.1177/1933719112450333. [DOI] [PubMed] [Google Scholar]
- 284.Monaco L, Conti M. Localization of adenosine receptors in rat testicular cells. Biol Reprod. 1986;35:258–266. doi: 10.1095/biolreprod35.2.258. [DOI] [PubMed] [Google Scholar]
- 285.Monaco L, DeManno DA, Martin MW, Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988;122:2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- 286.Montiel-Herrera M, Zaske AM, Garcia-Colunga J, Martínez-Torres A, Miledi R. Ion currents induced by ATP and angiotensin II in cultured follicular cells of Xenopus laevis. Mol Cells. 2011;32:397–404. doi: 10.1007/s10059-011-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Morales B, Barrera N, Uribe P, Mora C, Villalon M. Functional cross talk after activation of P2 and P1 receptors in oviductal ciliated cells. Am J Physiol Cell Physiol. 2000;279:C658–C669. doi: 10.1152/ajpcell.2000.279.3.C658. [DOI] [PubMed] [Google Scholar]
- 288.Morales-Tlalpan V, Arellano RO, Diíz-Muñoz M. Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium. 2005;37:203–213. doi: 10.1016/j.ceca.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 289.Morikawa T, Tanaka N, Kubota Y, Mizuno H, Nakamura K, Kunitomo M, Shinozuka K. ATP modulates the release of noradrenaline through two different prejunctional receptors on the adrenergic nerves of rat prostate. Clin Exp Pharmacol Physiol. 2007;34:601–605. doi: 10.1111/j.1440-1681.2007.04627.x. [DOI] [PubMed] [Google Scholar]
- 290.Morishita H, Katsuragi T. Existence and pharmacological properties of dopamine D4 receptors in guinea pig vas deferens. Eur J Pharmacol. 1999;374:255–261. doi: 10.1016/s0014-2999(99)00312-x. [DOI] [PubMed] [Google Scholar]
- 291.Moritoki H, Takei M, Kasai T, Matsumura Y, Ishida Y. Possible involvement of prostaglandins in the action of ATP on guinea-pig uterus. J Pharmacol Exp Ther. 1979;211:104–111. [PubMed] [Google Scholar]
- 292.Morley P, Vanderhyden BC, Tremblay R, Mealing GAR, Durkin JP, Whitfield JF. Purinergic receptor-mediated intracellular Ca2+ oscillations in chicken granulosa cells. Endocrinology. 1994;134:1269–1276. doi: 10.1210/endo.134.3.8119167. [DOI] [PubMed] [Google Scholar]
- 293.Movahed E, Sadrkhanlou R, Ahmadi K, Nejati V, Zamani Z. Effect of purine nucleoside analogue-acyclovir on the sperm parameters and testosterone production in rats. Int J Fertil Steril. 2013;7:49–56. [PMC free article] [PubMed] [Google Scholar]
- 294.Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 295.Munch E, Munoz A, Boone TB, Smith CP, Somogyi GT. Release of ATP and NO from rat prostate: an in vitro and in vivo assessment of adrenergic receptor stimulation. J Urol. 2009;181:503. [Google Scholar]
- 296.Murphy KM, Snyder SH. Adenosine receptors in rat testes: labeling with 3H-cyclohexyladenosine. Life Sci. 1981;28:917–920. doi: 10.1016/0024-3205(81)90054-0. [DOI] [PubMed] [Google Scholar]
- 297.Nakanishi H, Takeda H. The possible role of adenosine triphosphate in chemical transmission between the hypogastric nerve terminal and seminal vesicle in the guinea-pig. Jpn J Pharmacol. 1973;23:479–490. doi: 10.1254/jjp.23.479. [DOI] [PubMed] [Google Scholar]
- 298.Nakano H, Furuya K, Furuya S, Yamagishi S. Involvement of P2-purinergic receptors in intracellular Ca2+ responses and the contraction of mammary myoepithelial cells. Pflugers Arch. 1997;435:1–8. doi: 10.1007/s004240050477. [DOI] [PubMed] [Google Scholar]
- 299.Nakano H, Furuya K, Yamagishi S. Synergistic effects of ATP on oxytocin-induced intracellular Ca2+ response in mouse mammary myoepithelial cells. Pflugers Arch. 2001;442:57–63. doi: 10.1007/s004240100521. [DOI] [PubMed] [Google Scholar]
- 300.Navarro B, Miki K, Clapham DE. ATP-activated P2X2 current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011;108:14342–14347. doi: 10.1073/pnas.1111695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Neild TO, Hirst GDS. The γ-connection': a reply. Trends Pharmacol Sci. 1984;5:56–57. [Google Scholar]
- 302.Nelson L, Olsson T, Emanuelson U, Holmberg O, Sandholm M, Åstrom G (1985) Adenosine-triphosphate (ATP) as a detection method of mastitis. Presented at IDF Seminar ‘Progress in the Control of Bovine Mastitis’, 21–24 May, 1985
- 303.Ning C, Qi L, Wen J, Zhang Y, Zhang W, Wang W, Blackburn M, Kellems R, Xia Y. Excessive penile norepinephrine level underlies impaired erectile function in adenosine A1 receptor deficient mice. J Sex Med. 2012;9:2552–2561. doi: 10.1111/j.1743-6109.2012.02896.x. [DOI] [PubMed] [Google Scholar]
- 304.Ning C, Wen J, Zhang Y, Blackburn M, Kellems R, Xia Y. Impaired adenosine signaling contributes to erectile dysfunction. J Sex Med. 2012;9:228. doi: 10.1111/j.1743-6109.2012.02896.x. [DOI] [PubMed] [Google Scholar]
- 305.Ning C, Zhang Y, Wen J, Blackburn M, Kellems R, Xia Y. Adenosine signaling in priapism and novel therapies. J Sex Med. 2012;9:223. doi: 10.1111/j.1743-6109.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 306.Ninomiya JG, Suzuki H. Electrical responses of smooth muscle cells of the mouse uterus to adenosine triphosphate. J Physiol. 1983;342:499–515. doi: 10.1113/jphysiol.1983.sp014865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Noto T, Inoue H, Mochida H, Kikkawa K. Role of adenosine and P2 receptors in the penile tumescence in anesthetized dogs. Eur J Pharmacol. 2001;425:51–55. doi: 10.1016/s0014-2999(01)01167-0. [DOI] [PubMed] [Google Scholar]
- 308.Obara K, Lepor H, Walden PD. Localization of P 2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J Urol. 1998;160:587–591. [PubMed] [Google Scholar]
- 309.Ohkawa H. Excitatory junction potentials recorded from the circular smooth muscles of the guinea-pig seminal vesicle. Tohoku J Exp Med. 1982;136:89–102. doi: 10.1620/tjem.136.89. [DOI] [PubMed] [Google Scholar]
- 310.Okada Y, Taniguchi T, Akagi Y, Muramatsu I. Two-phase response of acid extrusion triggered by purinoceptor in Chinese hamster ovary cells. Eur J Pharmacol. 2002;455:19–25. doi: 10.1016/s0014-2999(02)02556-6. [DOI] [PubMed] [Google Scholar]
- 311.Osa T, Maruta K. The mechanical response of the isolated longitudinal muscle of pregnant rat myometrium to adenosine triphosphate in the Ca-free solution containing various polyvalent cations. Jpn J Physiol. 1987;37:821–836. doi: 10.2170/jjphysiol.37.821. [DOI] [PubMed] [Google Scholar]
- 312.Page KR, Abramovich DR, Bush P, Clarke S, Dacke CG, Pereira J, Willoughby D. ATP action on calcium uptake by human placental microvillous border vesicles. J Physiol. 1993;459:495P. [Google Scholar]
- 313.Palmer ML, Lee SY, Carlson D, Fahrenkrug S, O'Grady SM. Stable knockdown of CFTR establishes a role for the channel in P2Y receptor-stimulated anion secretion. J Cell Physiol. 2006;206:759–770. doi: 10.1002/jcp.20519. [DOI] [PubMed] [Google Scholar]
- 314.Palmer ML, Schiller KR, O'Grady SM. Estrogen and progesterone differentially regulate UTP-stimulated anion secretion in endometrial epithelial cells by altering expression of P2Y receptors and basolateral K+ channels. FASEB J. 2007;21:A956. [Google Scholar]
- 315.Palmer ML, Peitzman ER, Maniak PJ, Sieck GC, Prakash YS, O'Grady SM. KCa3.1 channels facilitate K+ secretion or Na+ absorption depending on apical or basolateral P2Y receptor stimulation. J Physiol. 2011;589:3483–3494. doi: 10.1113/jphysiol.2011.207548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Papamarcaki T, Tsolas O. Identification of ATP diphosphohydrolase activity in human term placenta using a novel assay for AMP. Mol Cell Biochem. 1990;97:1–8. doi: 10.1007/BF00231696. [DOI] [PubMed] [Google Scholar]
- 317.Papka RE, Hafemeister J, Storey-Workley M. P2X receptors in the rat uterine cervix, lumbosacral dorsal root ganglia, and spinal cord during pregnancy. Cell Tissue Res. 2005;321:35–44. doi: 10.1007/s00441-005-1114-8. [DOI] [PubMed] [Google Scholar]
- 318.Peachey JA, Brownhill VR, Hourani SMO, Kitchen I. The ontogenetic profiles of the pre- and postjunctional adenosine receptors in the rat vas deferens. Br J Pharmacol. 1996;117:1105–1110. doi: 10.1111/j.1476-5381.1996.tb16703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Petit A, Bélisle S. Stimulation of intracellular calcium concentration by adenosine triphosphate and uridine 5′-triphosphate in human term placental cells: evidence for purinergic receptors. J Clin Endocrinol Metab. 1995;80:1809–1815. doi: 10.1210/jcem.80.6.7775628. [DOI] [PubMed] [Google Scholar]
- 320.Pinna C, Rubino A, Burnstock G. Age-related changes in purinergic and adrenergic components of sympathetic neurotransmission in guinea-pig seminal vesicles. Br J Pharmacol. 1997;122:1411–1416. doi: 10.1038/sj.bjp.0701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Piper AS, Hollingsworth M. P2-purinoceptors mediating spasm of the isolated uterus of the non-pregnant guinea-pig. Br J Pharmacol. 1996;117:1721–1729. doi: 10.1111/j.1476-5381.1996.tb15345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pochmann D, Rucker B, Battastini AM, Sarkis JJ. Ovariectomy and estradiol replacement therapy alters the adenine nucleotide hydrolysis in rat blood serum. Thromb Res. 2004;114:275–281. doi: 10.1016/j.thromres.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 323.Polan ML, DeCherney AH, Haseltine FP, Mezer HC, Behrman HR. Adenosine amplifies follicle-stimulating hormone action in granulosa cells and luteinizing hormone action in luteal cells of rat and human ovaries. J Clin Endocrinol Metab. 1983;56:288–294. doi: 10.1210/jcem-56-2-288. [DOI] [PubMed] [Google Scholar]
- 324.Poletto Chaves LA, Pontelli EP, Varanda WA. P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol. 2006;290:C1009–C1017. doi: 10.1152/ajpcell.00506.2005. [DOI] [PubMed] [Google Scholar]
- 325.Pousette Å, Åkerlöf E, Lundin A, Rosenborg L, Fredricsson B. On the use of adenosine triphosphate for estimation of motility and energy status in human spermatozoa. Int J Androl. 1986;9:331–340. doi: 10.1111/j.1365-2605.1986.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 326.Prapong S, Reinhardt TA, Goff JP, Horst RL. Short communication: Ca2+-adenosine triphosphatase protein expression in the mammary gland of periparturient cows. J Dairy Sci. 2005;88:1741–1744. doi: 10.3168/jds.S0022-0302(05)72847-2. [DOI] [PubMed] [Google Scholar]
- 327.Preston A, Lau WA, Pennefather JN, Ventura S. Effects of adenine nucleosides and nucleotides on neuromuscular transmission to the prostatic stroma of the rat. Br J Pharmacol. 2000;131:1073–1080. doi: 10.1038/sj.bjp.0703652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Preston A, Frydenberg M, Haynes JM. A1 and A2A adenosine receptor modulation of α1-adrenoceptor-mediated contractility in human cultured prostatic stromal cells. Br J Pharmacol. 2004;141:302–310. doi: 10.1038/sj.bjp.0705535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Preston SL, Parmer TG, Behrman HR. Adenosine reverses calcium-dependent inhibition of follicle-stimulating hormone action and induction of maturation in cumulus-enclosed rat oocytes. Endocrinology. 1987;120:1346–1353. doi: 10.1210/endo-120-4-1346. [DOI] [PubMed] [Google Scholar]
- 330.Queiroz G, Talaia C, Gonçalves J. ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J Pharmacol Exp Ther. 2003;307:809–815. doi: 10.1124/jpet.103.054809. [DOI] [PubMed] [Google Scholar]
- 331.Quintas LE, Noël F. Mechanisms of adaptive supersensitivity in vas deferens. Auton Neurosci. 2009;146:38–46. doi: 10.1016/j.autneu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 332.Ragazzi E, Chinellato A, Italiano G, Pagano F, Calabrò A. Characterization of in vitro relaxant mechanisms in erectile tissue from rabbits of different ages. Urol Res. 1996;24:317–322. doi: 10.1007/BF00389786. [DOI] [PubMed] [Google Scholar]
- 333.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 334.Ralevic V, Burrell S, Kingdom J, Burnstock G. Characterization of P2 receptors for purine and pyrimidine nucleotides in human placental cotyledons. Br J Pharmacol. 1997;121:1121–1126. doi: 10.1038/sj.bjp.0701262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ratnasooriya WD. Intra-epidiymal administration of α, β-methylene ATP impairs fertility of male rats. Med Sci Res. 1993;21:417–419. [Google Scholar]
- 336.Read MA, Boura AL, Walters WA. Vascular actions of purines in the foetal circulation of the human placenta. Br J Pharmacol. 1993;110:454–460. doi: 10.1111/j.1476-5381.1993.tb13832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Reid DL, Davidson SR, Phernetton TM, Rankin JH. Adenosine causes a biphasic response in the ovine fetal placental vasculature. J Dev Physiol. 1990;13:237–240. [PubMed] [Google Scholar]
- 338.Ren LM, Hoyle CH, Burnstock G. Developmental changes in sympathetic contraction of the circular muscle layer in the guinea-pig vas deferens. Eur J Pharmacol. 1996;318:411–417. doi: 10.1016/s0014-2999(96)00791-1. [DOI] [PubMed] [Google Scholar]
- 339.Reviriego J, Alonso MJ, Ibañez C, Marín J. Action of adenosine and characterization of adenosine receptors in human placental vasculature. Gen Pharmacol. 1990;21:227–233. doi: 10.1016/0306-3623(90)90906-3. [DOI] [PubMed] [Google Scholar]
- 340.Roberts VH, Clarson LH. Expression of the P2X receptor family in human placental tissue and trophoblast cells in culture. J Physiol. 2002;539:125P. [Google Scholar]
- 341.Roberts VH, Greenwood SL, Elliott AC, Sibley CP, Waters LH. Purinergic receptors in human placenta: evidence for functionally active P2X4, P2X7, P2Y2, and P2Y6. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1374–R1386. doi: 10.1152/ajpregu.00612.2005. [DOI] [PubMed] [Google Scholar]
- 342.Roberts VH, Waters LH, Powell T. Purinergic receptor expression and activation in first trimester and term human placenta. Placenta. 2007;28:339–347. doi: 10.1016/j.placenta.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 343.Roberts VH, Webster RP, Brockman DE, Pitzer BA, Myatt L. Post-translational modifications of the P2X4 purinergic receptor subtype in the human placenta are altered in preeclampsia. Placenta. 2007;28:270–277. doi: 10.1016/j.placenta.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 344.Rodríguez-Miranda E, Buffone MG, Edwards SE, Ord TS, Lin K, Sammel MD, Gerton GL, Moss SB, Williams CJ. Extracellular adenosine 5′-triphosphate alters motility and improves the fertilizing capability of mouse sperm. Biol Reprod. 2008;79:164–171. doi: 10.1095/biolreprod.107.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rohde GC, Venezian E, Huidobro-Toro JP. Asymmetric distribution of purinergic and adrenergic neurotransmission cooperates in the motor activity along the rat vas deferens. Neurosci Lett. 1986;71:197–202. doi: 10.1016/0304-3940(86)90558-6. [DOI] [PubMed] [Google Scholar]
- 346.Romac P, Zanic Grubisic T, Jezek D, Vucic M, Culic O. Hydrolysis of extracellular adenine nucleotides by human permatozoa: regulatory role of ectonucleotidases in sperm function. Croat Chem Acta. 2008;81:169–176. [Google Scholar]
- 347.Rommerts FF, Molenaar R, Hoogerbrugge JW, van der Molen HJ. Development of adenosine responsiveness after isolation of Leydig cells. Biol Reprod. 1984;30:842–847. doi: 10.1095/biolreprod30.4.842. [DOI] [PubMed] [Google Scholar]
- 348.Ronca-Testoni S, Galbani P, Gambacciani M. Some properties of a purinergic receptor solubilized from human uterus membranes. FEBS Lett. 1984;172:335–338. doi: 10.1016/0014-5793(84)81152-7. [DOI] [PubMed] [Google Scholar]
- 349.Ronquist KG, Ek B, Stavreus-Evers A, Larsson A, Ronquist G. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am J Physiol Endocrinol Metab. 2013;304:E576–E582. doi: 10.1152/ajpendo.00511.2012. [DOI] [PubMed] [Google Scholar]
- 350.Rossato M, La Sala GB, Balasini M, Taricco F, Galeazzi C, Ferlin A, Foresta C. Sperm treatment with extracellular ATP increases fertilization rates in in-vitro fertilization for male factor infertility. Hum Reprod. 1999;14:694–697. doi: 10.1093/humrep/14.3.694. [DOI] [PubMed] [Google Scholar]
- 351.Rossato M, Merico M, Bettella A, Bordon P, Foresta C. Extracellular ATP stimulates estradiol secretion in rat Sertoli cells in vitro: modulation by external sodium. Mol Cell Endocrinol. 2001;178:181–187. doi: 10.1016/s0303-7207(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 352.Rossato M, Ferigo M, Galeazzi C, Foresta C. Estradiol inhibits the effects of extracellular ATP in human sperm by a non genomic mechanism of action. Purinergic Signal. 2005;1:369–375. doi: 10.1007/s11302-005-1172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ruan YC, Wang Z, Du JY, Zuo WL, Guo JH, Zhang J, Wu ZL, Wong HY, Chung YW, Chan HC, Zhou WL. Regulation of smooth muscle contractility by the epithelium in rat vas deferens: role of ATP-induced release of PGE2. J Physiol. 2008;586:4843–4857. doi: 10.1113/jphysiol.2008.154096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ruan YC, Shum WW, Belleannée C, Da Silva N, Breton S. ATP secretion in the male reproductive tract: essential role of CFTR. J Physiol. 2012;590:4209–4222. doi: 10.1113/jphysiol.2012.230581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rudge SA, Hughes PJ, Brown GR, Michell RH, Kirk CJ. Inositol lipid-mediated signalling in response to endothelin and ATP in the mammalian testis. Mol Cell Biochem. 1995;149–150:161–174. doi: 10.1007/BF01076574. [DOI] [PubMed] [Google Scholar]
- 356.Saldaña C, Vázquez-Cuevas F, Garay E, Arellano RO. Epithelium and/or theca are required for ATP-elicited K+ current in follicle-enclosed Xenopus oocytes. J Cell Physiol. 2005;202:814–821. doi: 10.1002/jcp.20184. [DOI] [PubMed] [Google Scholar]
- 357.Salvetti NR, Panzani CG, Gimeno EJ, Neme LG, Alfaro NS, Ortega HH. An imbalance between apoptosis and proliferation contributes to follicular persistence in polycystic ovaries in rats. Reprod Biol Endocrinol. 2009;7:68. doi: 10.1186/1477-7827-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Samuelson UE, Wiklund NP, Gustafsson LE. Dual effects of adenosine and adenosine analogues on motor activity of the human fallopian tube. Acta Physiol Scand. 1985;125:369–376. doi: 10.1111/j.1748-1716.1985.tb07731.x. [DOI] [PubMed] [Google Scholar]
- 359.Sasseville M, Albuz FK, Côté N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian follicle. Biol Reprod. 2009;81:415–425. doi: 10.1095/biolreprod.108.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sawa R, Asakura H, Power GG. Changes in plasma adenosine during simulated birth of fetal sheep. J Appl Physiol. 1991;70:1524–1528. doi: 10.1152/jappl.1991.70.4.1524. [DOI] [PubMed] [Google Scholar]
- 361.Schiemann WP, Westfall DP, Buxton IL. Smooth muscle adenosine A1 receptors couple to disparate effectors by distinct G proteins in pregnant myometrium. Am J Physiol. 1991;261:E141–E150. doi: 10.1152/ajpendo.1991.261.1.E141. [DOI] [PubMed] [Google Scholar]
- 362.Shaban M, Smith RA, Stone TW. Purine suppression of proliferation of Sertoli-like TM4 cells in culture. Cell Prolif. 1995;28:673–682. doi: 10.1111/j.1365-2184.1995.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 363.Shabbir M, Ryten M, Thompson CS, Mikhailidis DP, Burnstock G. Characterisation of calcium-independent purinergic receptor-mediated apoptosis in hormone refractory prostate cancer. BJU Int. 2008;101:352–359. doi: 10.1111/j.1464-410X.2007.07293.x. [DOI] [PubMed] [Google Scholar]
- 364.Shalev M, Staerman F, Allain H, Lobel B, Saïag B. Stimulation of P2y purinoceptors induces, via nitric oxide production, endothelium-dependent relaxation of human isolated corpus cavernosum. J Urol. 1999;161:955–959. [PubMed] [Google Scholar]
- 365.Shariatmadari R, Sipila P, Vierula M, Tornquist K, Huhtaniemi I, Poutanen M. Adenosine triphosphate induces Ca2+ signal in epithelial cells of the mouse caput epididymis through activation of P2X and P2Y purinergic receptors. Biol Reprod. 2003;68:1185–1192. doi: 10.1095/biolreprod.102.007419. [DOI] [PubMed] [Google Scholar]
- 366.Shen MR, Linden J, Chen SS, Wu SN. Identification of adenosine receptors in human spermatozoa. Clin Exp Pharmacol Physiol. 1993;20:527–534. doi: 10.1111/j.1440-1681.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 367.Shi D, Winston JH, Blackburn MR, Datta SK, Hanten G, Kellems RE. Diverse genetic regulatory motifs required for murine adenosine deaminase gene expression in the placenta. J Biol Chem. 1997;272:2334–2341. [PubMed] [Google Scholar]
- 368.Slater M, Barden JA, Murphy CR. Distributional changes in purinergic receptor subtypes (P2X1-7) in uterine epithelial cells during pregnancy. Histochem J. 2000;32:365–372. doi: 10.1023/a:1004017714702. [DOI] [PubMed] [Google Scholar]
- 369.Slater M, Barden JA, Murphy CR. The purinergic calcium channels P2X1,2,5,7 are down-regulated while P2X3,4,6 are up-regulated during apoptosis in the ageing rat prostate. Histochem J. 2000;32:571–580. doi: 10.1023/a:1004110529753. [DOI] [PubMed] [Google Scholar]
- 370.Slater M, Murphy CR, Barden JA. Purinergic receptor expression in the apical plasma membrane of rat uterine epithelial cells during implantation. Cell Calcium. 2002;31:201–207. doi: 10.1016/S0143-4160(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 371.Slater M, Danieletto S, Barden JA. Expression of the apoptotic calcium channel P2X7 in the glandular epithelium. J Mol Histol. 2005;36:159–165. doi: 10.1007/s10735-004-6166-7. [DOI] [PubMed] [Google Scholar]
- 372.Smith MA, Buxton IL, Westfall DP. Pharmacological classification of receptors for adenyl purines in guinea pig myometrium. J Pharmacol Exp Ther. 1988;247:1059–1063. [PubMed] [Google Scholar]
- 373.Smith NCE, Burnstock G. Mechanism underlying postjunctional synergism between responses of the vas deferens to noradrenaline and ATP. Eur J Pharmacol. 2004;498:241–248. doi: 10.1016/j.ejphar.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 374.Sneddon P. Suramin inhibits excitatory junction potentials in guinea-pig isolated vas deferens. Br J Pharmacol. 1992;107:101–103. doi: 10.1111/j.1476-5381.1992.tb14469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by α, β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100:85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- 376.Sneddon P, Burnstock G. Do we need γ-receptors? Trends Pharmacol Sci. 1984;5:264–265. [Google Scholar]
- 377.Sneddon P, Westfall DP. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol Lond. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sneddon P, Westfall TD, Todorov LD, Todorova SM, Westfall DP, Nickel P, Kennedy C. The effect of P2 receptor antagonists and ATPase inhibition on sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens. Br J Pharmacol. 2000;129:1089–1094. doi: 10.1038/sj.bjp.0703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Söderquist L. ATP content and sperm motility of extended bovine semen under different storage conditions. Acta Vet Scand. 1991;32:511–518. doi: 10.1186/BF03546952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Söderquist L, Stålhammar EM. Relationship between ATP content and motility in bovine spermatozoa with reference to the effects of the bull and the A.I. centre. Acta Vet Scand. 1991;32:353–359. doi: 10.1186/BF03546965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Somers GR, Bradbury R, Trute L, Conigrave A, Venter DJ. Expression of the human P2Y6 nucleotide receptor in normal placenta and gestational trophoblastic disease. Lab Invest. 1999;79:131–139. [PubMed] [Google Scholar]
- 382.Soodak LK, MacDonald GJ, Behrman HR. Luteolysis is linked to luteinizing hormone-induced depletion of adenosine triphosphate in vivo. Endocrinology. 1988;122:187–193. doi: 10.1210/endo-122-1-187. [DOI] [PubMed] [Google Scholar]
- 383.Souza LF, Horn AP, Gelain DP, Jardim FR, Lenz G, Bernard EA. Extracellular inosine modulates ERK 1/2 and p38 phosphorylation in cultured Sertoli cells: possible participation in TNF-alpha modulation of ERK 1/2. Life Sci. 2005;77:3117–3126. doi: 10.1016/j.lfs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 384.Squires PE, Hill CJ, Pacey AA, Li TC, Cooke ID, Warren MA, Dunne MJ. Purinergic receptor-mediated increases in intracellular Ca2+ in single isolated epithelial cells of the human uterine tube. Proc Physiol Soc. 1995;482P:PC57. [Google Scholar]
- 385.Squires PE, Lee PSN, Ho Yuen B, Leung PCK, Buchan AMJ. Mechanisms involved in ATP-evoked Ca2+ oscillations in isolated human granulosa-luteal cells. Cell Calcium. 1997;21:365–374. doi: 10.1016/s0143-4160(97)90030-0. [DOI] [PubMed] [Google Scholar]
- 386.Stein DM, Fraser LR, Monks NJ. Adenosine and Gpp(NH)p modulate mouse sperm adenylate cyclase. Gamete Res. 1986;13:151–158. [Google Scholar]
- 387.Stiles GL, Pierson G, Sunay S, Parsons WJ. The rat testicular A1 adenosine receptor-adenylate cyclase system. Endocrinology. 1986;119:1845–1851. doi: 10.1210/endo-119-4-1845. [DOI] [PubMed] [Google Scholar]
- 388.Stjärne L. Novel dual ‘small’ vesicle model of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission. Auton Neurosci. 2001;87:16–36. doi: 10.1016/S1566-0702(00)00246-0. [DOI] [PubMed] [Google Scholar]
- 389.Stone TW. Differential blockade of ATP, noradrenaline and electrically evoked contractions of the rat vas deferens by nifedipine. Eur J Pharmacol. 1981;74:373–376. doi: 10.1016/0014-2999(81)90058-3. [DOI] [PubMed] [Google Scholar]
- 390.Suzuki Y. Contraction and prostaglandin biosynthesis by myometrium from non-pregnant and pregnant rabbits in response to adenosine 5′-triphosphate. Eur J Pharmacol. 1991;195:93–99. doi: 10.1016/0014-2999(91)90385-4. [DOI] [PubMed] [Google Scholar]
- 391.Tai CJ, Kang SK, Cheng KW, Choi KC, Nathwani PS, Leung PC. Expression and regulation of P2U-purinergic receptor in human granulosa-luteal cells. J Clin Endocrinol Metab. 2000;85:1591–1597. doi: 10.1210/jcem.85.4.6558. [DOI] [PubMed] [Google Scholar]
- 392.Tai CJ, Kang SK, Choi KC, Tzeng CR, Leung PC. Antigonadotropic action of adenosine triphosphate in human granulosa-luteal cells: involvement of protein kinase Cα. J Clin Endocrinol Metab. 2001;86:3237–3242. doi: 10.1210/jcem.86.7.7691. [DOI] [PubMed] [Google Scholar]
- 393.Tai CJ, Chang SJ, Leung PC, Tzeng CR. Adenosine 5′-triphosphate activates nuclear translocation of mitogen-activated protein kinases leading to the induction of early growth response 1 and raf expression in human granulosa-luteal cells. J Clin Endocrinol Metab. 2004;89:5189–5195. doi: 10.1210/jc.2003-032111. [DOI] [PubMed] [Google Scholar]
- 394.Takahashi Y, Ishii N, Lue TF, Tanagho EA. Pharmacological effects of adenosine on canine penile erection. Tohoku J Exp Med. 1991;165:49–58. doi: 10.1620/tjem.165.49. [DOI] [PubMed] [Google Scholar]
- 395.Takahashi Y, Ishii N, Lue TF, Tanagho EA. Effects of adenosine on canine penile erection. J Urol. 1992;148:1323–1325. doi: 10.1016/s0022-5347(17)36901-x. [DOI] [PubMed] [Google Scholar]
- 396.Tassell W, Slater M, Barden JA, Murphy CR. Endometrial cell death during early pregnancy in the rat. Histochem J. 2000;32:373–379. doi: 10.1023/a:1004069731540. [DOI] [PubMed] [Google Scholar]
- 397.Tiemann U, Neels P, Küchenmeister U, Walzel H, Spitschak M. Effect of ATP and platelet-activating factor on intracellular calcium concentrations of cultured oviductal cells from cows. J Reprod Fertil. 1996;108:1–9. doi: 10.1530/jrf.0.1080001. [DOI] [PubMed] [Google Scholar]
- 398.Todorov L, Tashiro K, Hakoda H, Ito Y. Possible role of the α1-adrenoceptor in the modulation of adrenergic co-transmission in the guinea-pig vas deferens. Biomed Res. 1991;12:365–376. [Google Scholar]
- 399.Todorov LD, Mihaylova Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- 400.Todorov LD, Mihaylova-Todorova ST, Bjur RA, Westfall DP. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J Pharmacol Exp Ther. 1999;290:241–246. [PubMed] [Google Scholar]
- 401.Tomiyama T, Ohashi K, Tsutsui T, Saji F, Tanizawa O. Acrosome reaction induced in a limited population of human spermatozoa by progesterone (Ca2+-dependent) and ATP (Ca2+-independent) Hum Reprod. 1995;10:2052–2055. doi: 10.1093/oxfordjournals.humrep.a136235. [DOI] [PubMed] [Google Scholar]
- 402.Tong YC, Broderick G, Hypolite J, Levin RM. Correlations of purinergic, cholinergic and adrenergic functions in rabbit corporal cavernosal tissue. Pharmacology. 1992;45:241–249. doi: 10.1159/000139007. [DOI] [PubMed] [Google Scholar]
- 403.Tosca L, Froment P, Solnais P, Ferre P, Foufelle F, Dupont J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology. 2005;146:4500–4513. doi: 10.1210/en.2005-0301. [DOI] [PubMed] [Google Scholar]
- 404.Tostes RC, Giachini FR, Carneiro FS, Leite R, Inscho EW, Webb RC. Determination of adenosine effects and adenosine receptors in murine corpus cavernosum. J Pharmacol Exp Ther. 2007;322:678–685. doi: 10.1124/jpet.107.122705. [DOI] [PubMed] [Google Scholar]
- 405.Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, Cyr DG. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78:124–138. doi: 10.1002/mrd.21280. [DOI] [PubMed] [Google Scholar]
- 406.Urabe S, Miyoshi H, Fujiwara H, Yamaoka K, Kudo Y. Enhanced expression of P2X4 and P2X7 purinergic receptors in the myometrium of pregnant rats in preterm delivery models. Reprod Sci. 2009;16:1186–1192. doi: 10.1177/1933719109344630. [DOI] [PubMed] [Google Scholar]
- 407.Urbach V, Harvey BJ. Regulation of intracellular Ca2+ by CFTR in Chinese hamster ovary cells. J Membr Biol. 1999;171:255–265. doi: 10.1007/s002329900576. [DOI] [PubMed] [Google Scholar]
- 408.Valdecantos P, Briones R, Moya P, Germain A, Huidobro-Toro JP. Pharmacological identification of P2X1, P2X4 and P2X7 nucleotide receptors in the smooth muscles of human umbilical cord and chorionic blood vessels. Placenta. 2003;24:17–26. doi: 10.1053/plac.2002.0862. [DOI] [PubMed] [Google Scholar]
- 409.Valenzuela MA, Collados L, Kettlun AM, Mancilla M, Lara H, Puente J, Aranda E, Chayet L, Alvarez A, Traverso-Cori A. Changes in apyrase activity in uterus and mammary gland during the lactogenic cycle. Comp Biochem Physiol B. 1992;103:113–118. doi: 10.1016/0305-0491(92)90421-m. [DOI] [PubMed] [Google Scholar]
- 410.Vander Kooy D, Dubyak GR, Moore RM, Moore JJ. Adenosine triphosphate activates the phospholipase-C cascade system in human amnion cells without increasing prostaglandin production. Endocrinology. 1989;124:2005–2012. doi: 10.1210/endo-124-4-2005. [DOI] [PubMed] [Google Scholar]
- 411.Vasudevan K, Sztein JM. Treatment of sperm with extracellular adenosine 5′-triphosphate improves the in vitro fertility rate of inbred and genetically modified mice with low fertility. Theriogenology. 2011;76:729–736. doi: 10.1016/j.theriogenology.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Vázquez-Cuevas FG, Juárez B, Garay E, Arellano RO. ATP-induced apoptotic cell death in porcine ovarian theca cells through P2X7 receptor activation. Mol Reprod Dev. 2006;73:745–755. doi: 10.1002/mrd.20447. [DOI] [PubMed] [Google Scholar]
- 413.Vázquez-Cuevas FG, Zárate-Diaz EP, Garay E, Arellano RO. Functional expression and intracellular signaling of UTP-sensitive P2Y receptors in theca-interstitial cells. Reprod Biol Endocrinol. 2010;8:88. doi: 10.1186/1477-7827-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Veitinger S, Veitinger T, Cainarca S, Fluegge D, Engelhardt CH, Lohmer S, Hatt H, Corazza S, Spehr J, Neuhaus EM, Spehr M. Purinergic signalling mobilizes mitochondrial Ca2+ in mouse Sertoli cells. J Physiol. 2011;589:5033–5055. doi: 10.1113/jphysiol.2011.216309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Venkova KM, Radomirov R, Petkov V. Contractile responses of vas deferens from spontaneously hypertensive rats to electrical stimulation. C R Acad Bulg Sci. 1985;38:277. [Google Scholar]
- 416.Ventura S, Pennefather JN. Sympathetic co-transmission to the cauda epididymis of the rat: characterization of postjunctional adrenoceptors and purinoceptors. Br J Pharmacol. 1991;102:540–544. doi: 10.1111/j.1476-5381.1991.tb12207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ventura S, Pennefather JN. Inhibition of field stimulation-induced contractions of rat cauda epididymis by purinoceptor agonists but not by adrenoceptor agonists. J Auton Pharmacol. 1992;12:299–309. doi: 10.1111/j.1474-8673.1992.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 418.Ventura S, Dewalagama RK, Lau LC. Adenosine 5′-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland. Br J Pharmacol. 2003;138:1277–1284. doi: 10.1038/sj.bjp.0705167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Vigue C, Vigue L, Huszar G. Adenosine triphosphate (ATP) concentrations and ATP/adenosine diphosphate ratios in human sperm of normospermic, oligospermic, and asthenospermic specimens and in their swim-up fractions: lack of correlation between ATP parameters and sperm creatine kinase concentrations. J Androl. 1992;13:305–311. [PubMed] [Google Scholar]
- 420.Villalon MJ, Carolina Danovaro M, Hinds TR. Intracellular calcium concentration increases in monkey oviductal ciliated cells after stimulation with extracellular ATP. Biol Reprod. 1995;52:145. [Google Scholar]
- 421.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford APDW, Burnstock G. P2X3 knockout mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.von Kügelgen I, Bültmann R, Starke K. Effects of suramin and α, β-methylene ATP indicate noradrenaline–ATP co-transmission in the response of the mouse vas deferens to single and low frequency pulses. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:760–763. doi: 10.1007/BF00169686. [DOI] [PubMed] [Google Scholar]
- 423.von Versen-Höynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta. 2009;30:434–442. doi: 10.1016/j.placenta.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wali FA, Greenidge E. Evidence that ATP and noradrenaline are released during electrical field stimulation of the rat isolated seminal vesicle. Pharmacol Res. 1989;21:397–404. doi: 10.1016/1043-6618(89)90157-6. [DOI] [PubMed] [Google Scholar]
- 425.Wang XF, Chan HC. Adenosine triphosphate induces inhibition of Na+ absorption in mouse endometrial epithelium: a Ca2+-dependent mechanism. Biol Reprod. 2000;63:1918–1924. doi: 10.1095/biolreprod63.6.1918. [DOI] [PubMed] [Google Scholar]
- 426.Wang XF, Tsang LL, So SC, Chan HC. Suppression of ATP-induced Cl− secretion by enhanced expression of epithelial Na+ channels in mouse endometrial epithelium. Cell Biol Int. 2001;25:1017–1020. doi: 10.1006/cbir.2001.0754. [DOI] [PubMed] [Google Scholar]
- 427.Wang YT, Takiguchi Y, Uematsu T, Nakashima M. Augmented responsiveness to sympathetic nerve stimulation of isolated vas deferens of diabetic rats. Arch Int Pharmacodyn Ther. 1991;309:160–169. [PubMed] [Google Scholar]
- 428.Wasilenko WJ, Cooper J, Palad AJ, Somers KD, Blackmore PF, Rhim JS, Wright GL, Jr, Schellhammer PF. Calcium signaling in prostate cancer cells: evidence for multiple receptors and enhanced sensitivity to bombesin/GRP. Prostate. 1997;30:167–173. doi: 10.1002/(sici)1097-0045(19970215)30:3<167::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 429.Welter-Stahl L, da Silva CM, Schachter J, Persechini PM, Souza HS, Ojcius DM, Coutinho-Silva R. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNγ in human epithelial cells. Biochim Biophys Acta. 2009;1788:1176–1187. doi: 10.1016/j.bbamem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 430.Wen J, Xia Y. Adenosine signaling: good or bad in erectile function? Arterioscler Thromb Vasc Biol. 2012;32:845–850. doi: 10.1161/ATVBAHA.111.226803. [DOI] [PubMed] [Google Scholar]
- 431.Wen J, Dai Y, Zhang Y, Zhang W, Kellems RE, Xia Y. Impaired erectile function in CD73-deficient mice with reduced endogenous penile adenosine production. J Sex Med. 2011;8:2172–2180. doi: 10.1111/j.1743-6109.2011.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J. 2011;25:2823–2830. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wen X, Perrett D, Jones N, Tozer AJ, Docherty SM, Iles RK. High follicular fluid adenosine levels may be pivotal in the metabolism and recycling of adenosine nucleotides in the human follicle. Metabolism. 2010;59:1145–1155. doi: 10.1016/j.metabol.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 434.Westfall DP, Stitzel RE, Rowe JN. The postjunctional effects and neural release of purine compounds in the guinea-pig vas deferens. Eur J Pharmacol. 1978;50:27–38. doi: 10.1016/0014-2999(78)90250-9. [DOI] [PubMed] [Google Scholar]
- 435.Westfall TD, Westfall DP. Pharmacological techniques for the in vitro study of the vas deferens. J Pharmacol Toxicol Methods. 2001;45:109–122. doi: 10.1016/s1056-8719(01)00144-7. [DOI] [PubMed] [Google Scholar]
- 436.Westfall TD, Kennedy C, Sneddon P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. Br J Pharmacol. 1996;117:867–872. doi: 10.1111/j.1476-5381.1996.tb15273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Westfall TD, Menzies JR, Liberman R, Waterston S, Ramphir N, Westfall DP, Sneddon P, Kennedy C. Release of a soluble ATPase from the rabbit isolated vas deferens during nerve stimulation. Br J Pharmacol. 2000;131:909–914. doi: 10.1038/sj.bjp.0703662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wheeler CP, Yudilevich DL. Transport and metabolism of adenosine in the perfused guinea-pig placenta. J Physiol. 1988;405:511–526. doi: 10.1113/jphysiol.1988.sp017345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.White DR, Aitken RJ. Relationship between calcium, cyclic AMP, ATP, and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res. 1989;22:163–177. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- 440.Wiklund NP, Samuelson UE, Brundin J. Adenosine modulation of adrenergic neurotransmission in the human fallopian tube. Eur J Pharmacol. 1986;123:11–18. doi: 10.1016/0014-2999(86)90681-3. [DOI] [PubMed] [Google Scholar]
- 441.Wong PY. Control of anion and fluid secretion by apical P2-purinoceptors in the rat epididymis. Br J Pharmacol. 1988;95:1315–1321. doi: 10.1111/j.1476-5381.1988.tb11770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.World Health Organization Task Force on the Prevention and Management of Infertility Adenosine triphosphate in semen and other sperm characteristics: their relevance for fertility prediction in men with normal sperm concentration. Fertil Steril. 1992;57:877–881. [PubMed] [Google Scholar]
- 443.Wu HY, Broderick GA, Suh JK, Hypolite JA, Levin RM. Effects of purines on rabbit corpus cavernosum contractile activity. Int J Impot Res. 1993;5:161–167. [PubMed] [Google Scholar]
- 444.Xue J, Ruddock NT, Tecirlioglu RT, French AJ (2004) Effect of exogenous ATP on the development of parthenogenetically activated bovine oocytes. Biol Reprod SP ISS:217
- 445.Yamashiro H, Toyomizu M, Toyama N, Aono N, Sakurai M, Hiradate Y, Yokoo M, Moisyadi S, Sato E. Extracellular ATP and dibutyryl cAMP enhance the freezability of rat epididymal sperm. J Am Assoc Lab Anim Sci. 2010;49:167–172. [PMC free article] [PubMed] [Google Scholar]
- 446.Yang K, Hardy DB, Doumouras MA, van Beek JP, Rocha E. ATP stimulates human placental 11beta-hydroxysteroid dehydrogenase type 2 activity by a novel mechanism independent of phosphorylation. J Cell Biochem. 2002;84:295–300. doi: 10.1002/jcb.10016. [DOI] [PubMed] [Google Scholar]
- 447.Yeung CH. Temporary inhibition of the initiation of motility of demembranated hamster sperm by high concentrations of ATP. Int J Androl. 1986;9:359–370. doi: 10.1111/j.1365-2605.1986.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 448.Yildirim MK, Bagcivan I, Sarac B, Kilicarslan H, Yildirim S, Kaya T. Effect of hypothyroidism on the purinergic responses of corpus cavernosal smooth muscle in rabbits. Int Urol Nephrol. 2008;40:691–699. doi: 10.1007/s11255-008-9332-0. [DOI] [PubMed] [Google Scholar]
- 449.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 1996;174:267–271. doi: 10.1016/s0002-9378(96)70406-4. [DOI] [PubMed] [Google Scholar]
- 450.Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Power GG, Araki T. Plasma adenosine levels increase in women with normal pregnancies. Am J Obstet Gynecol. 2000;182:1200–1203. doi: 10.1067/mob.2000.104832. [DOI] [PubMed] [Google Scholar]
- 451.Yoneyama Y, Suzuki S, Sawa R, Araki T. Plasma adenosine concentrations increase in women with hyperemesis gravidarum. Clin Chim Acta. 2004;342:99–103. doi: 10.1016/j.cccn.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 452.Young JS, Brain KL, Cunnane TC. Electrical and optical study of nerve impulse-evoked ATP-induced, P2X-receptor-mediated sympathetic neurotransmission at single smooth muscle cells in mouse isolated vas deferens. Neuroscience. 2007;148:82–91. doi: 10.1016/j.neuroscience.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–680. doi: 10.1002/jcp.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Yuh IS, Jang BB, Hong BJ. Effects of adenosine and epidermal growth factor on the growth of mouse mammary epithelial cells. Cell Biol Int. 1998;22:131–135. doi: 10.1006/cbir.1998.0231. [DOI] [PubMed] [Google Scholar]
- 455.Zamoner A, Bruno AN, Casali EA, Corbelini PF, Diniz GP, Barreto-Chaves ML, Silva FR, Sarkis JJ, Pessoa-Pureur R. Genomic-independent action of thyroid hormones on NTPDase activities in Sertoli cell cultures from congenital hypothyroid rats. Life Sci. 2006;80:51–58. doi: 10.1016/j.lfs.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 456.Zhou L, Qi X, Potashkin JA, Abdul-Karim FW, Gorodeski GI. Micro-RNAs miR-186 and miR-150 downregulate expression of the pro-apoptotic purinergic P2X7 receptor by activation of instability sites at the 3′-untranslated region of the gene that decrease steady-state levels of the transcript. J Biol Chem. 2008;283:28274–28286. doi: 10.1074/jbc.M802663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zhou L, Luo L, Qi X, Li X, Gorodeski GI. Regulation of P2X7 gene transcription. Purinergic Signal. 2009;5:409–426. doi: 10.1007/s11302-009-9167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Zhu L, Li X, Zeng R, Gorodeski GI. Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kilodalton and 50- kilodalton forms. Endocrinology. 2006;147:977–989. doi: 10.1210/en.2005-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Ziessen T, Cellek S. Purines and pyrimidines are not involved in NANC relaxant responses in the rabbit vaginal wall. Br J Pharmacol. 2002;137:513–521. doi: 10.1038/sj.bjp.0704898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Ziessen T, Moncada S, Cellek S. Characterization of the non-nitrergic NANC relaxation responses in the rabbit vaginal wall. Br J Pharmacol. 2002;135:546–554. doi: 10.1038/sj.bjp.0704481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Ziganshin AU, Zaitsev AP, Shamsutdinov AF. Pharmacological characteristics of P2 receptors in human uterus. Bull Exp Biol Med. 2002;133:255–257. doi: 10.1023/a:1015886701248. [DOI] [PubMed] [Google Scholar]
- 462.Ziganshin AU, Zaitzev AP, Khasanov AA, Shamsutdinov AF, Burnstock G. Term-dependency of P2 receptor-mediated contractile responses of isolated human pregnant uterus. Eur J Obstet Gynecol Reprod Biol. 2006;129:128–134. doi: 10.1016/j.ejogrb.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 463.Ziganshin AU, Vafina ZR, Fatkullin IF. Contrasting effects of P2 receptor agonists on spontaneous contractility of human fallopian tubes with and without acute inflammation. Pharmacol Res. 2008;57:56–59. doi: 10.1016/j.phrs.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 464.Zimon A, Erat A, Von Wald T, Bissell B, Koulova A, Choi CH, Bachvarov D, Reindollar RH, Usheva A. Genes invoked in the ovarian transition to menopause. Nucleic Acids Res. 2006;34:3279–3287. doi: 10.1093/nar/gkl387. [DOI] [PMC free article] [PubMed] [Google Scholar]