Abstract
There is widespread involvement of purinergic signalling in endocrine biology. Pituitary cells express P1, P2X and P2Y receptor subtypes to mediate hormone release. Adenosine 5′-triphosphate (ATP) regulates insulin release in the pancreas and is involved in the secretion of thyroid hormones. ATP plays a major role in the synthesis, storage and release of catecholamines from the adrenal gland. In the ovary purinoceptors mediate gonadotrophin-induced progesterone secretion, while in the testes, both Sertoli and Leydig cells express purinoceptors that mediate secretion of oestradiol and testosterone, respectively. ATP released as a cotransmitter with noradrenaline is involved in activities of the pineal gland and in the neuroendocrine control of the thymus. In the hypothalamus, ATP and adenosine stimulate or modulate the release of luteinising hormone-releasing hormone, as well as arginine-vasopressin and oxytocin. Functionally active P2X and P2Y receptors have been identified on human placental syncytiotrophoblast cells and on neuroendocrine cells in the lung, skin, prostate and intestine. Adipocytes have been recognised recently to have endocrine function involving purinoceptors.
Keywords: Pituitary, Thyroid, Pancreas, Ovary, Testes, Hypothalamus
Synopsis
Introduction
Pituitary gland (hypophysis)
Pancreas
β-Cells
α-Cells
δ-Cells
Thyroid gland
Parathyroid gland
Adrenal gland
-
Adrenal chromaffin cells
Co-storage and release of NA and ATP
from chromaffin cells
Purinoceptor subtypes in adrenal
chromaffin cells
Second messenger transduction
mechanisms
Ectonucleotidases
Diadenosine polyphosphates
Medullary endothelial cells
Purinergic signalling in development and ageing
Adrenocortical cells
Ovary
Testis
Pineal gland
Thymus
Neuroendocrine hypothalamus
Placenta
Neuroendocrine cells
Adipocytes
P1 receptors
P2 receptors
Concluding comments
Introduction
Physiological events in the periphery are locally as well as centrally regulated. The local regulation is concerned with precise functional adjustments according to local needs and is executed predominantly by exocrine/paracrine cells and local neurons. Endocrine/paracrine cells, which secrete bioactive peptides, are found in epithelial structures almost everywhere in the body, including the thyroid (parafollicular cells), epithelium of the airways, the gastro-entero-pancreatic region and the genito-urinary tract. The peptide hormone-producing endocrine cells have an endodermal origin. There is a growing number of reports that purinoceptors on endocrine cells mediate release of hormones (see [65,338,382,411,487,511,513,514]).
Pituitary gland (hypophysis)
The pituitary gland is the master endocrine gland lying beneath the hypothalamus. It has an anterior lobe that secretes: thyroid-stimulating hormone (TSH), which stimulates growth of the thyroid gland and releases its hormone; adrenocorticotropic hormone (ACTH), which regulates the endocrine activities of the adrenal cortex which produces cortisol; follicle stimulating hormone (FSH), which promotes secretion of oestrogen and the development of eggs and sperm cells; gonadotrophins; growth hormone; prolactin; luteinising hormone (LH) that releases oestrogen, progesterone and testosterone; lipotropin and melanocyte-stimulating hormone (MSH). The posterior lobe (neurohypophysis) secretes vasopressin (VP) and oxytocin (OT), which are synthesised in the hypothalamus and transported to the pituitary, where they are stored before release. The anterior pituitary hormones do not act on endocrine glands, but directly affect specific tissues; prolactin causes breast development and milk production and MSH stimulates pigment cells. There are five cell types in the anterior pituitary, namely lactotrophs, somatotrophs, corticotrophs, gonadotrophs and thyrotrophs, as well as pituitary stem cells [161].
Adenosine triphosphatase activity was identified in the neural lobe of the bovine pituitary gland, giving an early indication for the presence of purinergic signalling [574]. Adenosine 5′-triphosphate (ATP) was reported early to induce release of VP from neurohypophysial neurosecretory granules [403,424]. In another early paper, intraperitoneal injection of caffeine was shown to cause a rise in plasma corticosterone and stimulated ACTH release, suggesting that events in the pituitary-adrenal axis were modulated (at least in part) by an effect on adenosine receptors [373,474]. Later, adenosine was shown to regulate the release of ACTH from cultured anterior pituitary cells [10]. In electron microscopic studies, Ca2+-ATPase was shown to be present on the plasma membranes on the granular, but not the non-granular, folliculo-stellate cells (FSC) of the rat anterior pituitary [490] and nerve endings [539]. A more recent study has shown that ATP is released from pituitary cells and then broken down by ecto-NTPDase1-3 [218]. Inhibiting the activity of ecto-NTPDases with ARL 67151 led to an increase in ATP release from perfused pituitary cells and apyrase enhanced the degradation of released ATP. Pannexins mediate ATP release in the pituitary gland; pannexin 1 was dominantly expressed in the anterior lobe, while pannexin 2 expression was dominant in the intermediate and posterior pituitary [308]. Pannexin 1 isoforms have been shown to be present in rat pituitary cells and appear to be associated with P2X2, P2X3 and P2X4, as well as P2X7 receptor channels and ATP release [309].
In the cloned pituitary cell line GH3 and rat anterior pituitary cells, adenosine activity via A1 receptors inhibits prolactin release [121,353,416]. A regulatory role for adenosine in modulating adenylate cyclase activity and reducing prolactin release from primary cultures of rat anterior pituitary cells in both basal and vasoactive intestinal peptide (VIP)-stimulated conditions has been suggested [284]. Adenosine, acting through A1 receptors, however, was claimed to stimulate the release of prolactin from the anterior pituitary in vitro [609]. More recently studies show that hormone-containing endocrine cells express mostly A1 receptors, while non-endocrine follicle stimulating cells express mostly A2B receptors [438]. Adenosine regulates thrombomodulin and endothelial protein C receptor expression in FSC [437]. Adenosine stimulated cells of the hypothalamus-pituitary-adrenal cortical axis [519]. The involvement of A1 receptors has been described in the inhibition of gonadotrophin secretion of LH and FSH induced by adenosine acting via A2 in rat hemipituitaries in vitro [414]. A2 receptors have also been implicated in the stimulatory effects of adenosine on prolactin secretion [415]. ATP, acting after breakdown to adenosine via A1 receptors, induces stellation of 37 % of pituicytes and it was suggested that there is purinergic regulation of pituicyte morphological plasticity and subsequent modulation of hormone release [461]. Further VP and OT reverse adenosine-induced pituicyte stellation [462]. A2B receptors mediate adenosine inhibition of taurine efflux from pituicytes [417]. It has been claimed that adenosine increases interleukin (IL) 6 and decreases release of tumour necrosis factor from anterior pituitary cells [445]. Adenosine signalling pathways in the pituitary gland have been reviewed, highlighting the effects of adenosine on pituitary cell proliferation and the evidence for opposing actions on endocrine and FSC [438–440]. Briefly, A1 receptors are expressed in rodent pituitary endocrine cell lines mediating hormone release, whereas A2B receptors appear to be predominant in primary anterior pituitary cell cultures consisting mainly of FSC mediating stimulation of IL-6 secretion.
Growth hormone releasing hormone (GHRH) is secreted by arcuate neurons into the hypothalamic portal vessels and stimulates growth hormone (GH) release by activating GHRH receptors on somatotrophs. Pulsatile release of GH involves P1 receptors expressed on somatotroph cells [489]. A2A receptor gene expression has been reported to occur transiently during the embryological development of the anterior and intermediate lobes of the pituitary gland [581]. There are no reports of A3 receptors in the pituitary gland. Adenosine, acting via A1 receptors, specifically blocks the terminal N-type Ca2+ channel in isolated rat neurohypophysial terminals, leading to inhibition of the release of both VP and OT [580]. The functions of the pituitary gland are tightly controlled by neuronal and hormonal afferents of the brain. The roles of melatonin and adenosine in rodent pituitary function have been discussed [258]. Adenosine stimulates connexin 43 expression and gap-junctional communication in FSC [305].
Adenosine is an important regulator of the functions of pituitary tumour GH4 cells, which secrete prolactin and growth hormone, by modulating, in an autocrine manner, the activity of L-type voltage-dependent calcium channels [439,612].
Adenosine increased release of IL-6 from primary anterior pituitary cell cultures [445] and the implications of this finding for inflammation and tumorigenesis were discussed [439]. Adenosine-induced IL-6 expression in FSC is mediated via A2B receptors coupled to protein kinase (PK) C and p38 mitogen-activated protein kinase (MAPK) [440].
Extracellular ATP was shown to activate phospholipase (PL) C and mobilise intracellular calcium in primary cultures of sheep anterior pituitary cells [566]. Later it was shown that uridine 5′-triphosphate (UTP), as well as ATP, were potent agonists on these cells [117], suggestive of P2Y2 (and/or P2Y4) receptors on lactotrophs in the rat adenohypophysis [71]. ATP, adenosine 5′-diphosphate (ADP) and UTP stimulate cultured gonadotrophs from rat pituitary gland and gonadotroph-derived αT3-1 cells, probably mediated by P2Y2 and/or P2Y4 receptors [91,92]. It was proposed that ATP represents a paracrine/autocrine factor in the regulation of Ca2+ signalling and secretion of gonadotrophs consistent with mediation by P2X2 and/or P2X5 receptor channels [542].
Molecular cloning and functional characterisation of rat pituitary P2Y2 receptors were carried out and shown to be located on rat primary gonadotrophs, GH3 cells, and mixed sheep pituitary cells [93,94]. An autocrine/paracrine role of ATP in the regulation of release of prolactin from most (if not all) mammotrophs was proposed [383].
Evidence was presented for the presence of at least two types of purinoceptor on all five types of cells in the anterior pituitary, namely P2Y2 and P2X1, although the existence of a subpopulation of cells expressing P2X2/3 and P2Y1 was not excluded [575]. P2X2 receptors have been shown to be localised at the electron microscope level on pituicytes and a subpopulation of neurosecretory axons in the rat neurohypophysis [321]. The primary P2X2 receptor transcript in rat pituitary cells undergoes extensive alternative splicing, with generation of six isoforms [276]. A heteropolymeric P2X2 receptor has been claimed to mediate hormone release from lactotrophs, somatotrophs and gonadotrophs [512]. The mRNAs for wild-type and spliced channels were identified in enriched somatotrophs, where they were shown to be functional, but not gonadotroph or lactotroph fractions.
It has been proposed that ATP, coreleased with neuropeptides from neurohypophysial nerve terminals, acts as a paracrine/autocrine messenger, stimulating Ca2+ entry via a P2X2 receptor and secretion of VP, but not OT [550]. ATP was shown to be released stimulation-dependently from the rat isolated posterior lobe of the hypophysis to act via P2 receptors for local control of hormone secretion [502]. In addition, ATP, cosecreted with VP and OT from cells in the hypothalamus, has been claimed to play a role in the regulation of stimulus-secretion coupling in the neurohypophysis [299]. A recent study has shown that endogenous ATP potentiates VP, but not OT, secretion from neurohypophysial terminals [268]. The output of the neurohypophysial hormones VP and OT depends on the frequency and pattern of firing of their synthesising neurons in the hypothalamus. ATP released from pituicytes and/or nerve terminals in the hypophysis, when broken down by ecto-nucleotidases to adenosine, acts on A1 receptors to modulate release of VP [460]. ATP, acting via P2Y receptors, triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes) ([551]; see [549], for a review of the multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis).
Mixed populations of rat anterior pituitary cells express mRNA transcripts for P2Y2, P2X2, P2X3, P2X4 and P2X7 receptors ([277]; Table 1). The transcripts and functional P2Y2 receptors were identified in lactotrophs and GH3 cells, but not in somatotrophs or gonadotrophs. Lactotrophs and GH3 cells also express transcripts of P2X3, P2X4 and P2X7 receptors. Functional P2X2 receptors were found in somatotrophs and gonadotrophs, but not in lactotrophs. A recent study reported that mRNA transcripts for all P2X receptor subunits (except for P2X5) were expressed in rat anterior pituitary, and of these the P2X4 mRNA transcripts were the most abundant [614,615]. They showed that thyrotropin-releasing hormone-responsive cells, including lactotrophs, express homomeric and/or heteromeric P2X4 receptors, which facilitate Ca2+ influx and hormone secretion. Another study also described P2X7 receptors on GH3 cells and showed that they mediated increase in [Ca2+]i and depolarisation [101]. ATP, operating via P2X2 receptors controls the pacemaker activity, voltage-gated Ca2+ influx and basal LH release in gonadotrophs [613]. A valuable review discusses the complexity of purinergic signalling in lactotrophs, which express multiple purinoceptors and also reports the presence of P2X receptors in thyrotrophs and corticotrophs, although the subtypes were not identified ([510]; Fig. 1a). Transcripts for P2Y1, P2Y4, P2Y6 and P2Y12, as well as P2Y2 receptors, were identified in mixed anterior pituitary cells [217]. It was shown further that P2Y1 receptors mediated the stimulatory actions of ADP (and ATP) for prolactin secretion and that of the P2X receptor subtypes previously recognised, the P2X4 receptors provided the major pathway for Ca2+ influx-dependent signalling and prolactin secretion. In the neurohypophysis, extracellular ATP released from nerve terminals may act directly on pituicytes to induce K+ efflux via a P2Y receptor [552]. Thus, ATP can act as a neuron-glial signalling molecule within the neurohypophysis.
Table 1.
Purinoceptor subtypes expressed by different endocrine cell types
| Cell type | Purinergic receptor subtypes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X1 | P2X2 | P2X3 | P2X4 | P2X6 | P2X7 | P2Y1 | P2Y2 | P2Y4 | A1 | A2A | |
| Lactotrophs | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| GH3 cells | - | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Somatotrophs | ✓ | X | X | X | X | ||||||
| Gonadotrophs | ✓ | X | X | X | X | ✓ | ✓ | ||||
| Melanotrophs | ✓ | ||||||||||
| Thyrotrophs | (P2X✓) | ||||||||||
| Corticotrophs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Folliculo-stellate cells (FSC) | – | – | – | – | ✓ | ✓ | |||||
| Hypophyseal pituicytes | |||||||||||
| (astrocytes) | ✓ | ✓ | |||||||||
| GH4Cl cell line | ✓ | ||||||||||
✓receptors present, X receptors absent
Fig. 1.
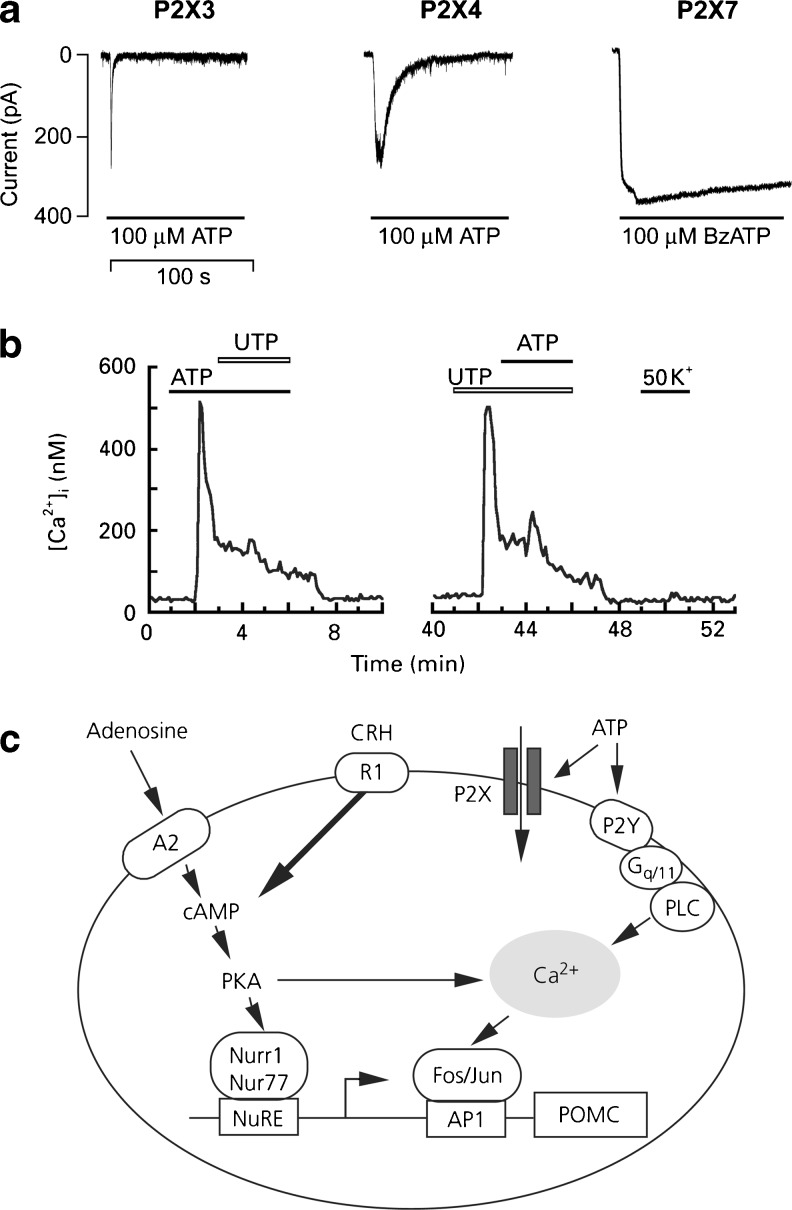
a Characterization of ion-conducting purinergic receptors expressed in pituitary cells. Pattern of current signals in GT1 cells expressing recombinant P2X3, P2X4 and P2X7 receptors. (Reproduced from [510], with permission from Elsevier.) b Responses of rat pituitary folliculo-stellate cells in primary culture to ATP (10 μm), UTP (10 μm) and K+ (50 mm) applied as indicated with horizontal bars above the traces. The trace is not shown during 10–40 min. The same cell responded to ATP and to UTP with a 30-min wash. (Reproduced from [558], with permission from Wiley.) c Schematic representation of the putative molecular mechanism for the purinergic regulation of pro-opiomelanocortin (POMC) gene expression in AtT20 mouse corticotroph cells. ATP, adenosine and corticotrophin-releasing hormone (CRH) stimulate the 5′-promoter activity of the POMC gene in a more than additive manner, suggesting an enhancing role of these compounds in CRH-mediated adrenocorticotropic hormone (ACTH) synthesis. The ligands also stimulate the expression of transcription factors of the regulation of the POMC gene, without enhancing ACTH secretion. The effect of adenosine and CRH, but not ATP, can be inhibited by a protein kinase A (PKA) inhibitor, indicating mediation via different intracellular signalling pathways. NuRE Nurr1/Nur77 response element, PLC phospholipase C. (Reproduced from [617], with permission from Blackwell.)
The Tpit/F1 cell line derived from pituitary FSC (glia-like cells in the anterior pituitary) exhibits responses to ATP consistent with those of normal FSC [89]. It was shown that ATP, acting via P2Y2 receptors increased both nitric oxide (NO) secretion and NO synthase (NOS) mRNA in these cells. ATP actions on FSC in primary culture have also been shown to act via P2Y receptors in response to ATP coreleased with pituitary hormones ([558]; Fig. 1b). In a recent study, P2Y1 and P2Y4 receptors were shown to be expressed in the majority of gonadotrophs and thyrotrophs; P2Y2 receptors were expressed in a small subpopulation of lactotrophs and almost all of the FSC; P2Y6 receptors were expressed on macrophages; and P2Y12 receptors were expressed on a small subpopulation of unidentified cells in the rat anterior pituitary [607]. P2X2 receptors were identified on corticotropin-releasing and thyrotropin-releasing hormone producing neurons [105]. Corticotrophs and somatotrophs were found not to express P2Y receptors. Cultures of stably transfected GH4C1 rat pituitary cells express P2X7 receptors [264,348]. Purinergic receptor ligands stimulate pro-opiomelanocortin (POMC) gene expression in AtT-20 mouse pituitary corticotroph cells. ATP, adenosine and corticotrophin-releasing hormone act synergistically to promote the expression of transcription factors of the POMC gene and ACTH synthesis via different intracellular signalling pathways ([617]; see Fig. 1c). mRNA for A1, A2A, P2X1, P2X3, P2X4, P2X6, P2X7, P2Y1, P2Y2 and P2Y4 receptors was identified in corticotroph cells.
Reviews about purinergic regulation of hypothalamic and pituitary functions are available ([509,513,514]; and see schematic Fig. 2).
Fig. 2.
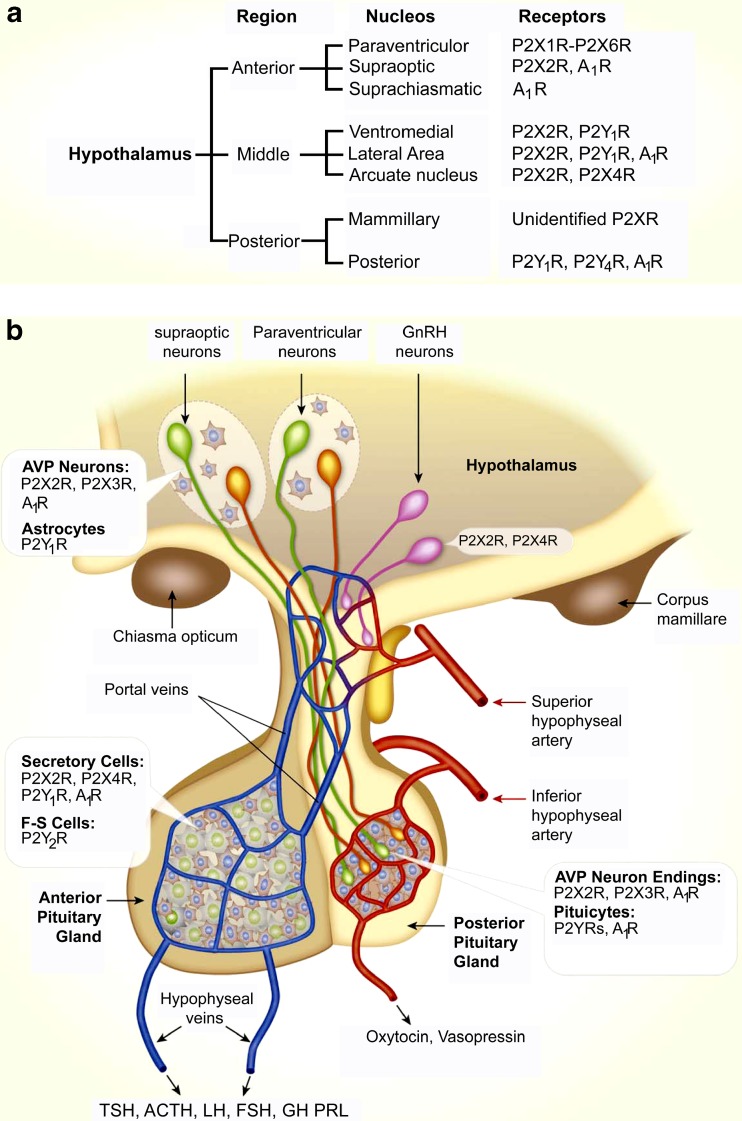
Expression of purinergic receptors in the hypothalamus and pituitary. a Receptors and receptor channels expressed in neurons of nuclei of the hypothalamus. For paraventricular and supraoptic nuclei, receptors expressed in parvocellular areas are listed. b Schematic representation of the hypothalamopituitary system. Insets indicate expression of purinergic receptors in secretory and supporting cells in three compartments. Note the pattern of expression of purinergic receptors: P2X2R are expressed in a majority of secretory cells (in anterior and middle hypothalamic neurons, vasopressinergic nerve endings and anterior pituitary (AP) cells). Supporting cells (astrocytes in the hypothalamus, pituicytes in the posterior pituitary (PP) and folliculostellate (F-S) cells in the anterior lobe) do not express P2XRs. Many cells co-express P2XRs, which facilitate electrical activity, and A1Rs, which silence electrical activity. P2X7R are also expressed in hypothalamopituitary cells, but the cell types expressing these channels have not been identified. In other brain regions, astroglial cells express P2X7Rs. ATP is co-secreted by neurons making synapses with magnocellular neurons in the hypothalamus and by both vasopressin and oxytocin-secreting neurons in the PP. ATP is also released by AP cells through still not well-characterized pathways. Green cells, vasopressin (AVP)-secreting neurons; orange cells, oxytocin-secreting neurons; pink cells, GnRH neurons. (Reproduced from [509], with permission from Elsevier.)
Pancreas
The pancreas performs both exocrine and endocrine functions. It regulates the metabolic states of the body by sensing changes in fatty acids and glucose and responds by secreting insulin and glucagon. Most of the pancreas is exocrine, consisting of 70-90 % acinar cells and 5-25 % duct cells, varying between species. Endocrine cells in the islets of Langerhans consist of only 3-5 % of the pancreas. Pancreatic stellate cells consist of less than 5 % of the pancreas mass.
The first reports on the role of purinergic signalling in the endocrine pancreas appeared 50 years ago. Secretion of insulin by ATP was reported in 1963 for rabbit pancreas slices [449], confirmed later in primates [304]. Experiments on ATP-induced insulin release were carried out on isolated perfused pancreas (e.g. [150,518]).
ATP released together with insulin from pancreatic secretary granules by exocytosis was reported in 1975, comparable to the release of ATP with noradrenaline (NA) from adrenal chromaffin granules [298]. ATP was next shown to stimulate glucagon and insulin secretion from isolated perfused rat pancreas in 1976, which was dependent on low and high glucose concentrations, respectively [328]. The ATP released from secretary granules is broken down to ADP and adenosine monophosphate (AMP) [517] and ectoATPases are present [303]. Adenosine, resulting from ATP breakdown, inhibited insulin secretion stimulated by glucose [240]. Adenosine, ADP and 5′-AMP elicit release of glucagon in isolated perfused rat pancreas [582].
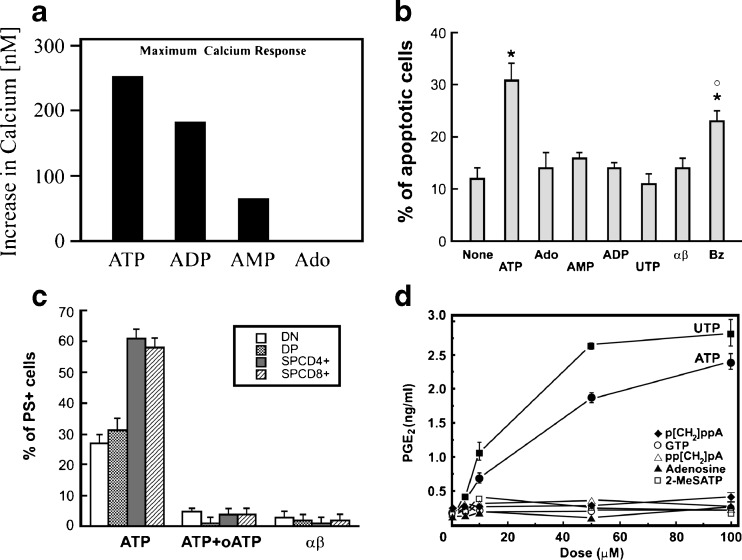
Early studies on the role of nucleotides on insulin secretion came from the laboratory of Mme Marie-Madelaine Loubatières-Mariani. It was shown, for example, that the relative potencies of nucleotides that caused insulin release induced by glucose was ATP ≥ ADP > AMP. Adenosine had only weak activity and guanosine triphosphate (GTP), inosine triphosphate, cytosine triphosphate and UTP were virtually inactive [329]. It was shown that 2-2′'pyridylisatogen tosylate, a P2 receptor antagonist, inhibited the insulin secreting action of ATP [82]. Stimulation of the secretion of glucagon, but not insulin, by adenosine suggested that α-cells were more sensitive to adenosine than β-cells [330]. There have been some valuable reviews about various aspects of purinergic endocrine signalling in the pancreas over the years [50,66,133,219,228,337,382,411,479,515,524]. A recent one is available about purinergic signalling in diabetes ([67]; Fig. 3).
Fig. 3.
Role of purinergic receptors in regulation of insulin secretion and β-cell survival. The facilitative GLUT-2 transporter mediates glucose entry. Glucose metabolism results in production of ATP, which closes the ATP-sensitive channel, KATP. The channel comprises of four Kir6.2 and SUR1 subunits. Closure of KATP depolarises the cell membrane potential and thus opens voltage-gated L-type Ca2+ channels eventually leading to generation of Ca2+ action potentials. Exocytosis of secretory vesicles containing insulin (and ATP) is triggered by increases in the cellular Ca2+. ATP can be also released from parasympathetic and sympathetic nerves. P2 receptors can boost and amplify signals associated with the glucose effect on insulin secretion and on proliferation or apoptosis of β-cells. P2X receptors facilitate Ca2+/Na+ influx and membrane depolarisation, and as a result, they can elicit insulin secretion even at low glucose concentrations. Some P2Y receptors increase cellular Ca2+ and activate protein kinase C (PKC) pathways. In addition, other P2Y and adenosine receptors affect the cyclic AMP pathway and possibly Epac signalling. At high adenosine concentrations, adenosine would be transported into the β-cell and exert metabolic effects. Receptors leading to increased insulin secretion are shown in green, those inhibiting insulin secretion are in red. Receptors affecting cell proliferation are in blue and those stimulating apoptosis purple. Receptors depicted here are taken from functional studies and the prefixes refer to rat, mouse or human receptors. (Reproduced from [66], updated from [382], with permission from The Society of Endocrinology.)
Both endocrine and exocrine cell activities are regulated by parasympathetic and sympathetic nerves, in addition to hormones, and autocrine and paracrine mediators [350]. Intrapancreatic parasympathetic nerves are present at day 14 of gestation in the foetal rat pancreas, but there was no sympathetic innervation at that stage [119]. ATP and acetylcholine (ACh) act synergistically to regulate insulin release [28] and islet oscillations [207], in keeping with their roles as cotransmitters from parasympathetic nerves. Intrapancreatic ganglia are involved in the regulation of periodic insulin secretions and studies of insulin release from the perfused pancreas after nerve blockade led to the proposal that the islets communicate via non-adrenergic, non-cholinergic neurotransmission [505]. Effector cells are innervated when they form close relationships with axonal varicosities [64]. Such relationships have been shown between sympathetic nerve varicosities and both α- and δ- cells, although less so with β-cells [451]. Sympathetic nerve stimulation inhibited insulin secretion, probably via α2A receptor mediated opening of ATP-dependent K+ channels [132,324]. Another study showed that over-expression of the α2A adrenoceptor contributed to development of type 2 diabetes [457]. Sympathetic nerve stimulation regulated exocrine ducts and acinar cells via β-adrenergic receptors [314,315,238,381], although its major effect was on blood vessels where it caused vasoconstriction [238]. Further, sympathetic nerves (releasing NA and ATP as cotransmitters) indirectly regulate pancreatic endocrine and exocrine secretion via actions on parasympathetic ganglionic neurons in the pancreas [605]. Different pancreatic cell types possess a number of purinergic and adenosine receptors and ectonucleotidases, implicating ATP as a parasympathetic/sympathetic cotransmitter.
Several types of nucleotide-/nucleoside-modifying enzymes are expressed in various pancreatic cells. Membrane Mg2+- or Ca2+-activated adenosine triphosphatase activity in rat pancreas has been reported [211,214,283,343]. ATP diphosphohydrolase was identified in pig pancreas, hydrolysing ATP to ADP and AMP [282]. An early study of rat pancreas showed ATPase, ADPase, 5′-nucleotidase and alkaline phosphatase activity in the vasculature, endocrine and exocrine cells [44]. ATPase was present on both endocrine and exocrine cells, while endocrine but not exocrine cells expressed alkaline phosphatase (see [187]). ATP-pyrophosphohydrolase (ecto-NPP) and alkaline phosphatase were shown in isolated mouse pancreatic islets [69]. Later, type-1 ecto-nucleoside triphosphate diphosphohydrolase (denoted NTPDase/CD39) was purified from pig pancreas [480]. A monoclonal antibody was prepared as a specific inhibitor of human NTPDase-3, which was expressed in all Langerhans islet cells [364]. Later, NTPDase-3 was shown to be expressed in endocrine cells of several species, and ecto-5′-nucleotidase (CD73) was expressed in rat, but not in human and mouse [288]. It was also shown that NTPDase-3 modulated insulin secretion.
Islets of Langerhans are situated throughout the pancreas, comprising of four cell types, α-cells containing glucagon, β-cells containing insulin and amylin and δ-cells containing somatostatin and pancreatic polypeptide-containing cells.
β-Cells
Extracellular ATP stimulation of β-cells results in insulin secretion (see [109,411,450]) and ATP released from nerves was proposed to regulate insulin secretion [524]. In 1963, it was reported that ATP added to the medium surrounding pieces of rabbit pancreas increased insulin secretion into the medium [449]. Stimulation of insulin secretion also occured when ATP was applied to the isolated perfused rat pancreas [327–329,518] and hamster pancreas [150]. ATP increases [Ca2+]i in clonal insulin-producing RINm5F cells [15]. ATP action was found to be glucose-dependent and was exerted via two different types of P2 receptors: P2X receptors on rat pancreatic β-cells transiently stimulated insulin release at low glucose concentrations and P2Y receptors potentiated glucose-stimulated insulin secretion ([410]; see [479]). Electrophysiological and immunocytochemical evidence has been presented that P2X1 and P2X3 receptors are expressed by mouse pancreatic β-cells [484]. It has been shown that the mitochondrial Ca2+ uniporter is required for sustained increase in cytosolic ATP/ADP ratio and is essential for glucose-induced ATP increases in pancreatic β-cells [532]. The concentration-response relationship for different P2 receptor agonists with different glucose backgrounds were summarised in a review [411]. Later studies indicated that ATP also had inhibitory effects on insulin release, perhaps via specific P2 receptor subtypes with different binding sites, and/or different intracellular signalling pathways, or even indirectly via adenosine receptors after ATP breakdown. Pancreatic β-cells act as glucose sensors, where intracellular ATP is altered with glucose concentration change. It has been reported that elevated cytosolic ATP enhanced the activity of Na+ channels, which lead to modulation of β-cell excitability and insulin release when blood glucose concentration increases [621]. There also appear to be significant species differences. ATP, via P2X and/or P2Y receptors, increases [Ca2+]i in many β-cell preparations and models, including human insulin-secreting β-cells, where ATP enhances sensitivity and responsiveness of β-cells to glucose fluctuations ([242,503]; see Fig. 4). Intracellular signalling pathways, including KATP channel open/closed state, membrane voltage and Ca2+ influx, lead to release of insulin. The initial phase of the biphasic insulin response to glucose was potentiated by endogenous ATP [85]. Comparative effects of ATP and related analogues on insulin secretion in rat pancreas have been reported [86]. ATP triggers synchronization of β-cell rhythmicity after increasing [Ca2+]i [197].
Fig. 4.
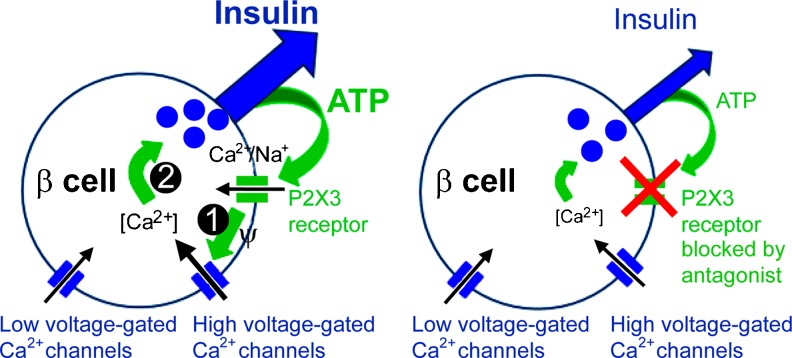
Proposed model for the positive autocrine feedback loop mediated by ATP in human β cells. Left hand panel: ATP, coreleased with insulin, activates ionotropic P2X3 receptors in the β-cell plasma membrane. This opens the cation selective P2X3 channel pore to let Na+ and Ca2+ flow into the cell (1). The resultant membrane depolarization and increase in action potential frequency increases Ca2+ flux through high voltage-gated Ca2+ channels. Increased [Ca2+]i (2) stimulates insulin secretion. Right hand panel: In the absence of P2X3 activation (using a P2X receptor antagonist), insulin secretion is diminished, revealing a strong contribution of ATP receptor activation to the response. (Modified and reproduced from [242], with permission from the National Academy of Sciences of the United States of America.)
Insulin granules contain ATP (and ADP) [239,298]. These granules are secreted and were detected as quantal exocytotic release from rat β-cells expressing P2X2 receptors acting as ATP biosensors; ATP concentrations up to 25 μmol/l close to plasma cell membranes have been detected [216,251]. ATP was shown to be released by exocytosis, while insulin was retained in the granule [384], suggesting that basal release of ATP may have a role as an autocrine regulator. The vesicular nucleotide transporter (VNUT) is expressed in pancreatic β-cells and VNUT-mediated ATP release is part of the mechanism that controls glucose-induced secretion [181]. They showed further that P2X receptors are critical in mediating the effect of ATP on insulin secretion when VNUT is over-expressed. Evidence has been presented to suggest that P2Y1 as well as P2X receptors play a role in the modulation of insulin secretion, proliferation and cell viability in mouse pancreatic β-cells [391]. ATP is also co-released with 5-hydroxythryptamine (5-HT), γ-aminobutyric acid, glutamate and zinc, which have further autocrine coregulatory functions on insulin secretion [49,251,444]. Extracellular nucleotides inhibit insulin receptor signalling [87].
The molecular identities of P2 receptors on various preparations of β-cells are summarised in Table 2 and their role in regulation of insulin secretion is shown in Fig. 3. α,β-Methylene ATP (α,β-meATP) mimicked the ATP effects on insulin secretion [408], indicating that P2X1 or P2X3 receptor subtypes might be involved. RT-PCR and Western blots showed that most of the P2X1 - P2X7 receptors were expressed in rat primary islet β-cells and the INS-1 cell line [444,470]. The characteristics of the P2X7-like receptor activated by ATP were described in the hamster β-cell line, HIT-T15 cells [291]. Mouse, human and porcine β-cells express rapidly desensitising P2X1 and P2X3 receptors, and it was proposed that paracrine and/or neural ATP activation of these receptors contribute to the initial outburst of glucose- or ACh-evoked insulin release [484]. Further, ATP liberated together with insulin, might participate in positive feedback control of insulin release [41,116]. P2X3 receptors were shown to constitute a positive autocrine and amplifying signal for insulin release in the human pancreatic β-cell [242]. In the rat INS-1 cell line, the P2X3 receptor inhibited insulin secretion at all glucose concentrations tested [470].
Table 2.
Molecular identity of P2 receptor subtypes expressed in pancreatic β-cells (Reproduced from [66], with permission from the Society of Endocrinology)
| Receptor subtype | Tissue origin | Technique | Reference |
|---|---|---|---|
| P2X1 | Rat and mouse pancreas (progressively upregulated) | Immunohistochemistry | [109] |
| Mouse islet cells | Immunocytochemistry | [484] | |
| Rat INS-1e | RT-PCR | [470] | |
| P2X2 | Rat islets, rat (INS-1) and mouse (βTC3) β-cell models | RT-PCR, Western blot analysis and immunohistochemistry | [444] |
| Rat INS-1e | RT-PCR | [470] | |
| P2X3 | Mouse islet cells | Immunocytochemistry | [484] |
| Rat islets, rat (INS-1) and mouse (βTC3) β-cell models | RT-PCR, Western blot analysis and immunohistochemistry | [444] | |
| Rat INS-1e | RT-PCR, siRNA | [470] | |
| Human islets | Immunohistochemistry, RT-PCR, Western blot analysis and in-situ hybridization | [242] | |
| P2X4 | Rat islets, RINm5F and HIT-T15 cells | mRNA blot analysis | [579] |
| Rat and mouse pancreas (progressively upregulated) | Immunohistochemistry | [109] | |
| Rat islets, rat (INS-1) and mouse (βTC3) β-cell models | RT-PCR, Western blot analysis and immunohistochemistry | [444] | |
| Rat INS-1e | RT-PCR | [470] | |
| P2X5 | Human islets | in-situ hybridization | [242] |
| P2X6 | Rat islets, rat (INS-1) and mouse (βTC3) β-cell models | RT-PCR, Western blot analysis and immunohistochemistry | [444] |
| Rat INS-1e | RT-PCR | [470] | |
| P2X7 P2Y1 | HIT-T15 cells | Western blot analysis | [292] |
| Rat INS-1e | RT-PCR | [470] | |
| Human islets | in-situ hybridization | [242] | |
| Mouse WT and KO islets and pancreas | RT-PCR, Western blot analysis, immunohistochemistry and functional studies | [188] | |
| Human islets | |||
| INS-1 β-cells | RT-PCR and Western blot analysis | [332] | |
| Mouse islets and β-cells | RT-PCR | [405] | |
| Mouse β-TC6 insulinoma cells | RT-PCR | [390] | |
| Rat INS-1e | RT-PCR | [470] | |
| Mouse MIN6 | RT-PCR | [17] | |
| Mouse WT and KO whole body | Functional studies | [301] | |
| P2Y2 | INS-1 β-cells | RT-PCR and Western blot analysis | [332] |
| Rat INS-1e | RT-PCR | [470] | |
| P2Y4 | Pancreatic β-cells (normal and diabetic rats) | Immunohistochemistry | [109] |
| Rat islets, INS-1 and RIN cells | RT-PCR and Western blot analysis | [470] | |
| INS-1 β-cells | RT-PCR and Western blot analysis | [332] | |
| Rat INS-1e | RT-PCR, siRNA | [470] | |
| P2Y6 | INS-1 β-cells | RT-PCR and Western blot analysis | [332] |
| Mouse islets and β-cells | RT-PCR | [405] | |
| Mouse β-TC6 insulinoma cells | RT-PCR | [390] | |
| Rat INS-1e | RT-PCR | [470] | |
| Mouse MIN6 | RT-PCR | [17] | |
| P2Y11 | Human β-cells | RT-PCR, Western blot analysis, immunofluorescence | [333] |
| HIT-T15 cells | Western blot analysis | [292] | |
| P2Y12 | INS-1 β-cells | RT-PCR and Western blot analysis | [332] |
| Human β-cells | RT-PCR, Western blot analysis, immunofluorescence | [333] | |
| Rat INS-1e | RT-PCR | [470] | |
| P2Y13 | Mouse islets and β-cells | RT-PCR | [9] |
Other functional and pharmacological evidence for P2 receptors is given in the text
Evidence for P2Y receptors mediating the biphasic response in insulin secretion from β-cells has been presented [29,153,306]. Extracellular ATP increases [Ca2+]i in β-cells, mainly by triggering Ca2+ release from intracellular stores [196,597], implicating P2Y receptors. Adenosine-5′-(β-thio)-diphosphate (ADPβS) was a potent agonist mediating insulin secretion from perfused rat pancreas and isolated islets [34,410], indicating that P2Y1, P2Y12 or P2Y13 receptors might be involved. This ADP analogue also enhanced insulin secretion and reduced hyperglycemia after oral administration to rats and dogs [227]. β-Cell apoptosis is induced by high glucose and free fatty acids via the autocrine action of ATP acting via P2Y13 receptors [531]. Several studies focussed on P2Y1 receptors and pharmacological agents were developed [147,159,230]. P2Y1 receptor knockout mouse experiments indicated that the receptor was involved in glucose homeostasis, although insulin secretion was decreased in islets isolated from P2Y1 knockout mice [301]. Pancreatic β-cells also express other P2Y receptors. The P2U (i.e. P2Y2 or P2Y4) receptor was cloned and characterised from human pancreas [506]. The P2Y4 receptor was demonstrated immunohistochemically in rat β-cells [109,110]. mRNA and protein expression showed that rat insulinoma INS-1 cells express P2Y1, P2Y2, P2Y4, P2Y6 and P2Y12 receptors [332,470]. Further, the P2Y4 receptor stimulated insulin secretion at all glucose concentrations tested [470]. However, mouse β-cells did not express P2Y2 and P2Y4 receptors [390,405].
Although most studies have shown that ATP/ADP increase insulin release, some early studies showed that ADP could also decrease insulin release [409,428]. Later studies showed that P2Y receptors, possibly P2Y1, mediated inhibition of L-type Ca2+ channels in rat pancreatic β-cells [194]. Another study showed that in mice β-cells ADP inhibited insulin secretion by activation of P2Y13 receptors, but increased insulin secretion via P2Y1 receptors [9].
P2Y1 and P2Y6 receptors in mouse β-cells mediated inhibition of insulin secretion at high glucose concentrations, but were slightly stimulant at 5 mM glucose [390]. Other studies showed clear stimulation of insulin secretion via these receptors at glucose concentrations >8 mM [17,405]. A further two receptors were identified, P2Y11 and P2Y12, in human pancreatic islets and their involvement in stimulation of insulin secretion was postulated [333]. In the hamster β-cell line HIT-T15, P2Y11 receptors stimulated insulin secretion while P2X7 receptors inhibited it; the net effect depending on the glucose concentration [292]. P2X7 receptors mediate IL-1 receptor antagonist secretion and it has been suggested that this in turn regulates β-cell mass and function [188].
P2 receptors are also involved in β-cell survival. Pancreatic islet cells express NTPDase-3 and ecto-5′-nucleotidase is present in some species, leading to accumulation of adenosine [288]. While rat islets express 5′-nucleotidase for breakdown of extracellular ATP to adenosine, mouse islets do not [604]. Microelectrode recordings from mouse pancreatic β-cells showed that theophylline (a non-selective P1 receptor antagonist) depolarised the β-cell membrane leading to insulin release; further, in 10 mM glucose, β-cells exhibited slow waves with bursts of spikes in the plateau and increased insulin secretion [223]. In perfused dog pancreas, the adenosine analogue 5′-N-ethylcarboxamidoadenosine (NECA) inhibited insulin release, the effect being concentration-dependent [16]. A1 receptors mediating inhibition were pharmacologically identified on β-cells [32,226,572] and in INS-1 cells [543]. A1 receptor antagonism in rat pancreatic islets potentiates insulin secretion [623]. The ectonucleotidases and A1 receptors might explain some of the dual effects of ATP.
The physiological roles of all these P1 and P2 receptor subtypes and their different effects on insulin secretion are being investigated. Studies of both in vivo and in vitro pancreas and in isolated islets with coupled β-cells showed that secretion of insulin (and glucagon and somatostatin) is pulsatile. Pulsatility is reflected by intracellular Ca2+ oscillations and membrane potential changes. It has been suggested that purinergic signalling is one of the coordinating mechanisms [219,221,382]. Activation of P2Y receptors enhanced insulin release from β-cells by triggering the cyclic AMP (cAMP)/PKA pathways [98]. Inhibition of the P2Y1 receptor attenuated glucose-induced insulin oscillations, but increased the total amount of insulin secreted [466]. Glucose stimulation of mouse β-cells triggers oscillations of the ATP concentration in the sub-plasma membrane space and it was suggested that a dynamic interplay between ATP and [Ca2+]i in β-cells may be important for the generation of pulsatile insulin secretion [307]. A1 receptor deletion increased insulin pulses and prolonged glucagon and somatostatin pulses and they lost their antisynchronous action [245,468]. Endothelial cells in the islets had a tonic inhibitory action on β-cell P2 receptors, resulting in impaired synchronisation of the insulin release pulses [222]. Figure 3 illustrates the pulsatility of ATP release and differential regulation via various P2 receptors and shows that P1 receptors could contribute to the pattern of insulin release [11]. It was claimed that adenosine inhibited insulin release from rat β-cells [31].
It has been suggested that P2Y receptors mediating stimulation of Gs proteins could have similar roles as incretins, glucagon-like peptide and gastric inhibitory peptide, both by augmenting insulin release and by maintaining the β-cell number [601]. An important signalling pathway of incretin action involves Epac (exchange proteins activated by cAMP). Whether P2Y or adenosine receptors also stimulate Epac in β-cells has not yet been investigated.
α-Cells
ATP stimulated secretion of glucagon from α-cells in isolated perfused rat pancreas in one study, though in another study adenosine and ADP, but not ATP, were effective [328,582]. The presence of A2 receptors on glucagon-secreting α-cells was reported in several studies [16,83,84]. Adenosine stimulation of glucagon secretion via A2 receptors was potentiated by an α2-adrenergic agonist [203]. NECA, an A2 receptor agonist, potentiated ACh-induced glucagon secretion [30]. Both A1 and A2A receptors on mouse α-cells were shown by immunohistochemistry and stimulation of A2A receptors with CGS-21680 to increase glucagon release, while adenosine decreased it [554]. Pulses of glucagon (and somatostatin) were prolonged in A1 receptor knockout mice, indicating that these α-cells (and δ-cells) possessed A1 receptors [468].
Diadenosine tetraphosphate stimulated glucagon and insulin secretion in perfused rat pancreas [486]. Studies on mice α-cells showed that they expressed P2 receptors. P2Y6 receptors, activated by uridine 5′-O-thiodiphosphate, increased glucagon release [405]. In contrast, P2Y1 receptors mediated inhibition of Ca2+ signalling and glucagon secretion in mice α-cells [554]. In the presence of high concentrations of glucose, insulin secretion was significantly greater in islets from P2Y1 receptor knockout mice, indicating that P2Y1 receptors play a physiological role in the maintenance of glucose homeostasis, at least in part, by regulating insulin secretion [198,301]. Glucagon secretion in rat islets was inhibited by the selective P2Y1 receptor antagonist MRS 2179 [198]. P2X7 receptors are expressed on α-cells, perhaps responding to ATP released from β-cells [109]. P2X7 receptors were shown to be expressed early in a subpopulation of glucagon- and insulin-immunopositive cells in developing islets, which later became restricted to glucagon-expressing α-cells [97,109].
δ-Cells
It was recognised early that δ-cells had local inhibitory effects, via somatostatin, on the release of insulin and glucagon from adjacent α- and β-cells [220]. Stimulation of somatostatin secretion by P2 receptor agonists from dog pancreas was reported [33], especially by ADPβS [229]. Pulses of somatostatin (and glucagon) were removed by addition of the P2Y1 receptor antagonist MRS 2179, although the regularity of insulin secretion was maintained [467].
Thyroid gland
The thyroid gland is a large endocrine gland situated at the base of the neck, consisting of two lobes on each side of the trachea. The thyroid gland is concerned with regulation of the metabolic rate, by the secretion of thyroid hormone, which is stimulated by TSH from the pituitary gland and requires trace amounts of iodine. Sympathetic nerves supply blood vessels in the thyroid and various nerve terminals have also been seen in close apposition to the bases of thyroid follicular epithelial cells [540,559]. Parasympathetic and sensory nerves are also present in the thyroid gland [204].
An early paper reported that ATP stimulated, while adenosine inhibited, PK activity in bovine thyroid [252]. Adenosine was shown to inhibit thyroidal T4 release, through receptor-mediated cAMP activated PK [166,335,591].
The in vitro action of thyroid-releasing hormone (TRH) on iodine metabolism in dog thyroid appears to be modulated by adenosine, but not ATP [122]. Intralysosomal hydrolysis of thyroglobulin, which promotes thyroid hormonal secretion, requires an acidic pH. Addition of ATP to the incubation medium prevented alkalinization and it was argued that an ATP-driven proton pump is present in the membranes of thyroid lysosomes [165].
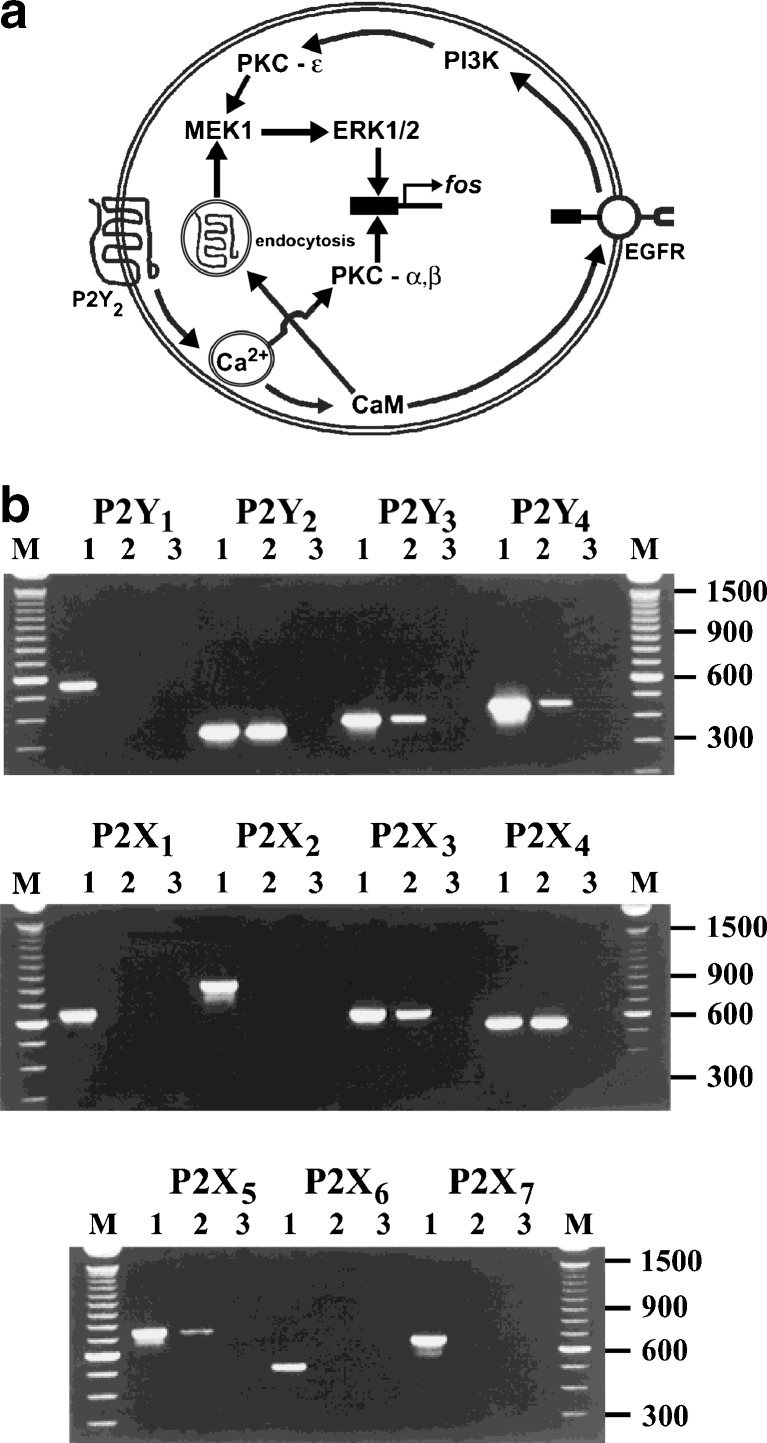
ATP has been claimed to activate Ca2+-dependent nicotinamide adenine dinucleotide phosphate-oxidase, generating hydrogen peroxide in thyroid plasma membranes, which regulates hormone synthesis through the activation of H2O2 production, a substrate for peroxidase [368]. Signals arising from ATP occupation of P2 receptors on rat FRTL-5 thyrocyte cell line leads, via PLC and adenylate cyclase, to iodide efflux [393]. ATP increases [Ca2+]i in dog thyroid cells [432], suggestive of P2 receptor involvement. P2 receptor stimulation also led to arachidonate release from FRTL-5 thyroid cells [395]. ATP, as well as TRH, regulates [Ca2+]i in human thyrocytes in primary culture [434]. However, extracellular ATP has been shown to completely reverse the TSH-induced morphological change in FRTL-5 cells [369]. P2Y receptors have been identified on the PC-C13 rat thyroid cell line that mediates increase in [Ca2+]i via PLC activation, Ca2+ store depletion and L-type voltage-dependent Ca2+ channel activation [340]. In a later study by this group, P2Y2 receptor mRNA was shown on both PC-C13 cells and a transformed cell line (C-ElAraf) derived from PC-C13 cells [140]. However, no mitogenic selective P2Y2 receptor activation occurred in PC-C13 cells ([141]; Fig. 5a).
Fig. 5.
a A proposed model for the control of ERK1/2 phosphorylation and fos induction by thyroid P2Y2 receptors in PC Cl3 cells. The P2Y2 activation provokes intracellular Ca2+ signalling and activation of calmodulin (CaM) and calcium-dependent PKCs. CaM is responsible for the epidermal growth factor receptor (EGFR) transactivation and P2Y2 endocytosis. These two events coordinate the phosphorylation of ERK1/2 through the activity of phosphoinositide 3-kinase (PI3K), novel PKC-ε and mitogen-activated protein kinase (MEK). ERK1/2 and PKCα/β induce the expression of fos protein. (Reproduced from [141], with permission from Elsevier.) b RT-PCR analysis of P2 receptor transcripts present in thyroid FRTL5 cells. Agarose gel electrophoresis of PCR products. M size markers: 100 bp ladder (Gibco), appropriate sizes are indicated. For each receptor amplification, lane 1 is a PCR reaction using the appropriate plasmid construct as template, lanes 2 and 3 incorporated cDNA synthesis where reverse transcriptase was present or absent, respectively. PCR amplifications with no added template were also carried out for each primer set and resulted in no amplification products (data not shown). The figure is representative of three independent experiments. (Reproduced from [137], with permission from Wiley.)
Atrial natriuretic peptide-induced cyclic guanosine monophosphate accumulation by purinergic agonists occurs in FRTL-5 thyroid cells [392]. Porcine thyroid cells produced H2O2, but not O2, when stimulated by extracellular ATP [367]. ATP increased the generation of inositol phosphates in dog thyrocytes [435,436], again suggesting that P2Y receptors might be involved. From a pharmacological study, it was concluded that a G protein is involved in the nucleotide-induced activation of FRTL-5 cells [394]. ATP activates a Ca2+-dependent Cl- current in rat FRTL-5 cells [341]. In an electrophysiological study, it was shown that depolarisation of rat thyroid FRTL-5 cells decreased the ATP-induced Ca2+ influx [544,545], raising the possibility that P2X receptors are also present.
An important advance was made when it was suggested that at least three different purinergic receptors were involved in the responses of FRTL-5 thyroid cells to ATP and probably also its breakdown product, adenosine, coupled to different signal transduction systems, namely activation of PLC, inhibition and activation of adenylate cyclase [473]. The relative order of potencies of nucleotides on the P2 receptors located on FRTL-5 cells was: adenosine-5′-(γ-thio)-triphosphate (ATPγS) ≥ ATP ≫ ADP ≫ GTP [125] perhaps suggestive of a P2X receptor subtype. ATP as low as 10-7 M specifically increased [Ca2+]i; this was duplicated by ATPγS, but not by adenosine, AMP, ADP or α,β-meATP [7]. The ATP-induced rise in [Ca2+]i was biphasic, with the second component related to the opening of a channel, since it required extracellular Ca2+ and was abolished by SC38249, an inhibitor of voltage operated channels [39], consistent with a P2X receptor subtype. On the other hand, P2 receptor stimulation of iodide efflux from FRTL-5 rat thyroid cells involves parallel activation of PLC and PLA2 [488], a clear indication of P2Y receptor involvement. Since extracellular UTP as well as ATP increase [Ca2+]i in single human thyrocytes [478], this suggests that P2Y2 and P2Y4 receptors are involved. A UTP sensitive receptor has also been located on the apical membrane of thyroid epithelial cells that mediates inhibition of Na+ absorption [47]. RT-PCR analysis and pharmacological studies revealed the presence of P2Y2, P2Y4, P2Y6, P2X3, P2X4 and P2X5 receptor mRNA on rat FRTL-5 cells involved in control of DNA synthesis ([137]; Fig. 5b). An immunohistochemical study of the localisation of P2X receptor subtype proteins in adult rat thyroid showed that: P2X1, P2X2 and P2X6 receptors were found exclusively on vascular smooth muscle, endothelial cells stained for P2X3, P2X4 and P2X7 and thyroid follicular cells showed immunoreactivity for P2X3, P2X4 and P2X5 receptors [189]. No immunostaining of P2X receptors was observed on C-cells. P2X7 receptors mediate stimulation of plasma membrane trafficking and internalisation in rat FRTL cells [271,272].
It has been suggested that extracellular ATP, in the presence of insulin, may be a cofactor (comitogen) in the regulation of thyroid cell proliferation, probably by phosphorylating MAPK and stimulating the expression of c-fos [546]. ATP regulates PLA2 activation by a Gi/Go protein-dependent mechanism and Ca2+, PKC and MAPK are also involved in its regulatory process [136].
Sympathetic nervous control of thyroid hormone secretion has been reported [201]. ATP released as a cotransmitter with NA from sympathetic nerves is likely to stimulate P2 receptors on thyroid follicular cells. Another source of ATP may be calcitonin-secreting C-cells, which stain with quinacrine that recognises high levels of ATP bound to peptides in vesicles [135]. ATP may also be released from thyroid follicular epithelial cells by paracrine or autocrine mechanisms [271].
Adenosine A1 receptors were identified on rat FRTL-5 thyroid cells [279,603] and P2 receptor activation of phosphoinositide turnover shown to be potentiated by A1 receptor stimulation of thyroid cells [370]. The P1 receptor agonist phenyliospropyladenosine strongly inhibited thyrotropin (TSH)-induced cAMP accumulation and H2O2 generation in FRTL-5 cells [40]. Adenosine is a potent stimulator of endothelin-1 secretion from rat thyroid FRTL-5 cells [562]. P1 receptor-mediated modulation of TSH actions on FRTL-5 thyroid cells has also been described [273].Thyroid-specific expression of the A2 adenosine receptor transgene promoted gland hyperplasia and severe hyperthyroidism, causing premature death in mice [290]. Adenosine inhibits DNA synthesis stimulated with TSH, insulin or phorbol 12-myristrate 13-acetate in rat thyroid FRTL-5 cells [563]. Extracellular adenosine increased Na+/iodide (I-) supporter gene expression in rat thyroid FRTL-5 cells and stimulates I- transport via the adenosine A1 receptor [212]. Thyrotropin regulates A1 receptor expression in FRTL-5 cells [564]. Thyroid hormone stimulates 5′-ecto-nucleotidase (CD73) of neonatal rat ventricular myocytes [73] and in cultured vascular smooth muscle cells [529].
The parafollicular cell of the mammalian thyroid gland is a neural crest derivative, which is capable of expressing neural characteristics when stimulated by nerve growth factor. Parafollicular cells produce 5-HT, which is stored in the same secretary granules as the peptide hormone, calcitonin. There is ATP-dependent uptake of 5-HT by secretary granules isolated from sheep thyroid parafollicular cells [104].
Hypothyroidism occurs with subnormal activity of the thyroid gland with low testosterone levels. If present at birth and untreated, it leads to cretinism. In adult life, it causes mental and physical slowing, undue sensitivity to cold, slowing of the pulse, weight gain and coarsening of the skin; this can be treated with thyroxine (T4). Thyroid hormones have profound effects on cardiovascular function in both hypothyroidism and hyperthyroidism [23]. It has been suggested that in hyperthyroidism, increase in ATP hydrolysis by E-NTPDase 3 and subsequent decrease in extracellular ATP levels is an important factor for prevention of the excessive contractility of cardiomyocytes induced by an overproduction of triiodothyronine (T3) [22]. Hyperthyroidism increases platelet 5′-nucleotidase activity, while hypothyroidism decreases it [54]. Hyperthyroidism reduces ecto-nucleotidase activity in synaptosomes from hippocampus and cerebral cortex of rats [53,55]. Evidence has been presented to suggest that both excess and deficiency of thyroid hormones can modulate the activities of both diphosphohydrolase (CD39) and CD73 ectoenzyme activities in rat blood serum with effects on vascular activity [56]. It has been claimed that both purinergic signalling and reactive oxygen species participate in thyroid hormone-induced vasorelaxation, and that there is a diminution of P2Y6 receptor expression in hyperthyroid rats [24]. Hypothyroidism has been shown to lead to impotence in some men. In an experimental rabbit model of hypothyroidism, relaxations to ATP, α,β-meATP and electrical field stimulation of corpus cavernosum strips were reduced, while relaxation to adenosine was unchanged [606].
Purinergic stimulation by ATP is able to induce rapid cytoplasm to nucleus translocation of APEI Ref-I protein initially and its neosynthesis later in a human thyroid tumour cell line (ARO) which expresses high levels of the APEI Ref-I protein involved in both base excision repair pathways of DNA lesions and in eukaryotic transcriptional regulation of gene expression [418]. In thyroid papillary carcinoma cells, P2X7 receptor mRNA and protein was increased and it was suggested that it may be a useful marker for this disease [491]. A recent review discusses the role of purinergic signalling in thyroid hormone activities in both heath and disease [485].
Parathyroid gland
Two pairs of parathyroid glands are situated behind or sometimes embedded within the thyroid gland. They are stimulated to produce parathyroid hormone by a decrease in the amount of calcium in the blood. A high level of parathyroid hormone causes transfer of calcium from bones to the blood. A deficiency lowers blood calcium levels causing tetany, a condition relieved by treatment with the hormone. ATP and ATPγS mobilise cellular Ca2+ and inhibit parathyroid hormone secretion [371]. It has been suggested that the ATP may be released from sympathetic nerve terminals in the parathyroid gland and/or by autocrine release from parathyroid secretory vesicles [106]. Parathyroid hormone potentiates nucleotide-induced [Ca2+]i in rat osteoblasts; it is suggested that this may explain how systemic parathyroid hormone can initiate bone remodelling [57]. Human parathyroid hormone secretion is inhibited by caffeine, suggesting that P1 receptors are also involved [331].
Adrenal gland
Adrenal chromaffin cells
Co-storage and release of NA and ATP from chromaffin cells
Chromaffin cells of the adrenal medulla can be regarded as a highly specialised form of sympathetic nerve cell, both have a common embryological origin in the neural crest. Well before NA and ATP were recognised as cotransmitters in sympathetic nerves, NA and ATP were shown to be co-stored in a ratio of about 4:1 [42,46,232,280,587] and coreleased [72,74,507] from adrenal chromaffin cells by vesicular exocytosis [205,237]. It was also suggested that chromagranines and dopamine-β-hydroxylase were stored together with NA and ATP in these cells [422,589,590]. NA and ATP were shown to be localised in chromaffin granules within the chromaffin cells [589] and the ATP stored in the granules is not synthesised in them, but is taken up from the cytoplasm [278,407].
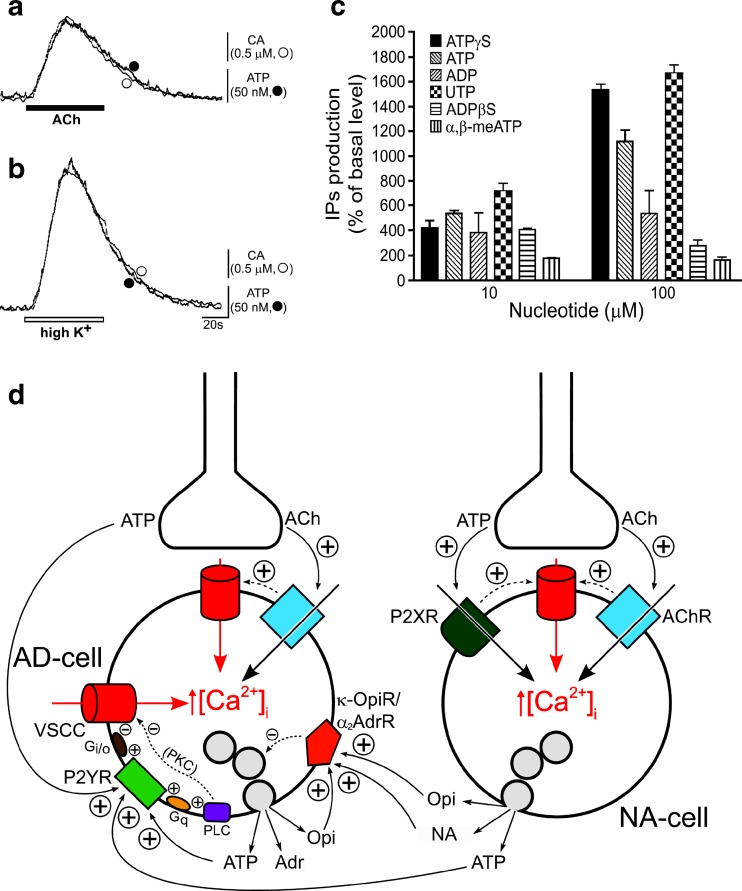
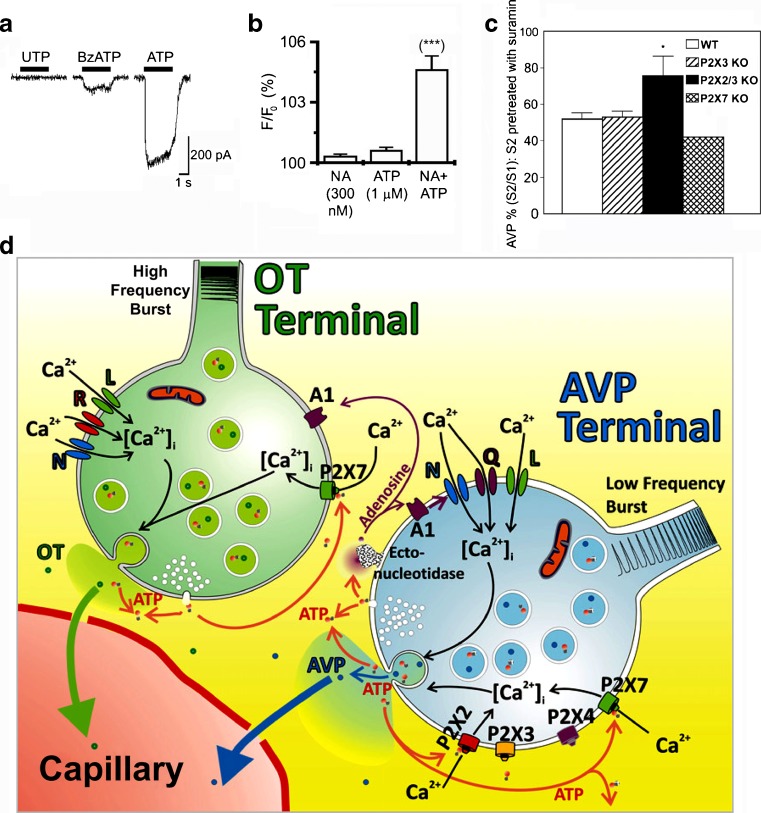
Early studies considered that the major role of ATP was to regulate the synthesis, storage and release of catecholamines (CA) from chromaffin cells (see [231,262,360,536,588]). It was only later that it was recognised as an equal partner in hormonal activities by analogy with the roles of NA and ATP as cotransmitters in sympathetic neurotransmission (see [62]). ATP and CA are released in parallel from adrenal chromaffin cells in response to stimulation by ACh, K+ or Ba2+ ([253]; Fig. 6a and b). ACh and nicotine caused exocytotic release of both CA (mainly adrenaline) and ATP from bovine adrenal chromaffin cells [454,583]. This response was blocked by mecamylamine, a nicotine receptor blocker [186]. Later it was shown that methacholine, a selective muscarinic agonist, as well as nicotine, induced CA and ATP secretion, via increasing [Ca2+]i, in porcine adrenal chromaffin cells, indicating that both nicotinic and muscarinic receptors were expressed by chromaffin cells [600]. Diadenosine tetraphosphate (Ap4A) is co-released with ATP and CA from bovine adrenal medulla [75,483].
Fig. 6.
a and b Typical recordings of on-line measurement of ATP and catecholamine (CA) released from cultured adrenal chromaffin cells in response to ACh and high K+. ACh (a, 100 mM, ■) or high K+ (b, 60 mM, □) was applied for 1 min. The responses of ATP (filled circle) and CA (open circle) are superimposed. Vertical bars indicate the amplitude of peak oxidative currents and luminescence induced by ATP (50 nM) and NA (0.5 mM). (Reproduced from [253], with permission from Elsevier.) c Effect of nucleotides on production of inositol phosphates in bovine adrenocortical fasciculata cells. (Reproduced from [379], with permission from Elsevier.) d Simplified model for inhibitory regulation of adrenaline secretion involving transmitters released from both nerve terminals and chromaffin cells of bovine adrenal gland. Auto-inhibitory feedback loops related to cholinergic transmission are not considered for simplicity. Inhibitory transmitters acting on receptors preferentially located to adrenergic chromaffin cells (i.e. P2Y receptors and κ-opioid receptors) have been considered, as well as nor adrenaline, which inhibits adrenaline release via α2-adrenoceptors. Activation of P2Y, κ-opioid and α2-adrenergic receptors inhibits voltage-sensitive Ca2+ channels (VSCCs) via Gi/o proteins (not depicted for the latter two receptors for simplicity) and, consequently, exocytosis. Protein kinase C (PKC) is negatively coupled to VSCCs in an isoform-specific fashion. AD-cell adrenergic chromaffin cell, NA-cell noradrenergic chromaffin cell, ACh acetylcholine, VSCC voltage-sensitive Ca2+channels, AChR nicotinic cholinergic receptors, P2XR P2X receptors, P2YR P2Y receptors, κ-OpiR/α2 AdrR κ-opioid and α2-adrenergic receptors (represented as a single entity for simplicity), PLC phospholipase C, PKC protein kinase C, G q and G i/o G proteins, Adr adrenaline, NA noradrenaline, Opi opioid peptides. For simplicity, and because [Ca2+]i rises induced by PLC activation do not evoke catecholamine secretion from bovine chromaffin cells, they are not made explicit in the scheme. Also for simplicity, granule exocytosis is not depicted as occurring preferentially in the vicinity of VSCC hot-spots. Positive and negative signs indicate stimulatory and inhibitory interactions, respectively. (Reproduced from [541], with permission.)
In bovine chromaffin cells, the Ca2+ channels involved in exocytosis are effectively inhibited by ATP and opioids that are coreleased with CA during cell activity [70]. Uptake of met-enkephalin by chromaffin cells was shown to be dependent on the presence of ATP in the incubation medium [528]. Chromaffin cells take up adenosine and convert it into ATP [352]. Tricyclic antidepressants block cholinergic nicotinic receptors and ATP secretion in bovine chromaffin cells [241].
Purinoceptor subtypes in adrenal chromaffin cells
ATP was shown early to depolarise adrenal chromaffin cells and it was suggested that this may be related to hormone release from granules and regulation of CA secretion in vivo [313,385,427] via cAMP [236]. CA secretion from bovine chromaffin cells can also be inhibited by extracellular ATP, probably after being converted to adenosine [96].
The presence of P2 receptors on adrenal chromaffin cells was first suggested in 1990 [6]. ATP can produce at least three different effects on adrenal chromaffin cells: inhibition of voltage-gated Ca2+ channels [113,127,225,311], release of Ca2+ from internal stores [441] and activation of a non-selective cation channel [402]. While the first two effects are most probably mediated by P2Y receptors, the third effect has the characteristics for the activation of P2X receptors. A biphasic rise in [Ca2+]i was shown in response to extracellular ATP, one phase due to release of Ca2+ from intracellular sites, the other from extracellular sites which was lost in Ca2+-free solutions [347]. This important study was a clear hint for the recognition that both P2X and P2Y receptors are expressed by chromaffin cells [127,402,441].
The P2 receptors on adrenaline-containing chromaffin cells were claimed to differ from those found on NA-containing chromaffin cells ([79,541]; Fig. 6d). The suggestion was that the inhibitory effect of ATP on NA-containing cells appeared to be largely mediated by P2X receptors, while the adenosine-containing cells were activated by both UTP and ATP and appeared to be largely mediated by P2U (probably P2Y2 or P2Y4) receptors. It was proposed that P2Y receptors on adrenal chromaffin cells mediate negative feedback of hormone secretion and that ATP inhibited both N- and P/Q-type Ca2+ channels [113,311]. Neuropeptide Y (NPY) and ATP may be co-modulators of this feedback pathway [618].
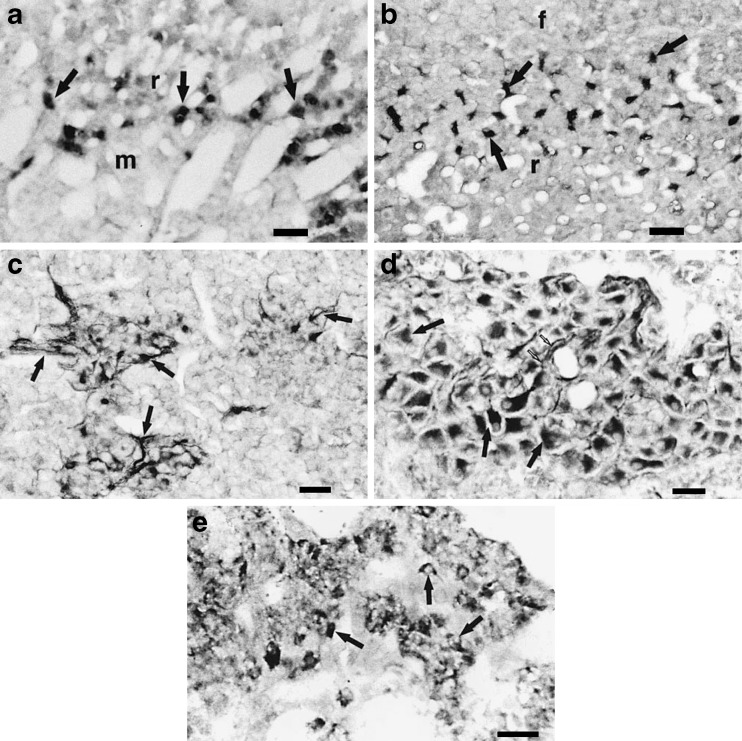
In one of the first immunohistochemical studies of P2X receptors, P2X1 and P2X2 immunoreactivity on chromaffin cells of the adrenal medulla was reported [577]. Later immunohistochemical studies ([2,3]; Fig. 7) showed limited expression of P2X5 and P2X7 receptors in rat chromaffin cells, while P2X6 immunoreactivity was detected in the guinea-pig. Brake et al. [48] cloned the P2X2 receptor from PC12 cells and detected weak expression of the mRNA in the adrenal gland by Northern blotting. P2X4 mRNA has also been detected [43]. However, in both studies, it was not certain whether the mRNA was present in the medullary or cortical cells.
Fig. 7.
a and b Guinea pig adrenal gland sections immunoreacted with P2X1 or P2X2 receptor antibodies. a P2X1 receptor-immunoreactive cortical cells (arrows) of the inner part of the zona reticularis (r) at corticomedullary junction (m medulla). b P2X2 receptor-immunoreacted section showing immunoreactive elements (arrows) located in the outer region of the zona reticularis. Note the irregular shape of the immunoreactive elements and their location between groups of non-immunoreactive cortical cells (f zona fasciculata). Note that whereas the two panels are at the same magnification, a appears of higher magnification due to the presence of large vascular plexus and a more network-like arrangement of the cortical cells in the inner region of zona reticularis. c–e Sections of guinea pig adrenal medulla immunoreacted with P2X5 or P2X6 receptor antibodies. c P2X5 receptor-immunoreactive nerve fibres that form plexuses (arrows) around the chromaffin cells. d P2X5 receptor-immunoreactive intrinsic neurones (black arrows) located in the adrenal medulla. Note the proximal parts of processes (small white arrows) projecting out of some of the cells, which indicate their neural identity. e P2X6 receptor-immunoreactive chromaffin cells (arrows). All scale bars = 40 μm. (Reproduced from [3], with permission from Karger.)
Functional studies have demonstrated the presence of P2X receptors on bovine [441] and guinea-pig [316,402] chromaffin cells. However, these receptors appear to be absent in the rat [237,316]. The P2X receptor present on chromaffin cells can be activated by ATP and 2-methylthio ATP, but is much less sensitive or insensitive to α,β-meATP [316,441]. To date, the only detailed pharmacological study of P2X receptors on chromaffin cells has been carried out on the guinea-pig. Here, the receptor is antagonised by pyridoxalphosphate-6-azonphenyl-2′,4′-disulphonic acid, but suramin and Cibacron blue are quite weak antagonists. The response is potentiated by low pH, but inhibited by Zn2+. Thus, while this receptor has some properties in common with the rat P2X2 receptor (agonist profile, effect of pH), the lack of potentiation by Zn2+ and the low sensitivity to the antagonists suramin and Cibacron blue are not. Although three spliced variants of the guinea-pig P2X2 receptor have been cloned, and some pharmacological characterisation has been carried out, there is at present insufficient information to identify the native P2X receptor present on guinea-pig chromaffin cells. The pharmacological properties of the P2X receptor present on guinea-pig chromaffin cells are very similar to that of the α,β-meATP-insensitive receptor found on pelvic ganglion neurons. It therefore seems likely that it is in fact the homomeric P2X2 receptor. Evidence has been presented that voltage-dependent Ca2+ channels are regulated in a paracrine fashion by ATP acting on P2X receptors in porcine adrenal chromaffin cells [389].
P2Y receptors mediate inhibition of exocytotic release of CA from adrenal chromaffin cells by modulation of voltage-operated Ca2+ channels, rather than by a direct effect on the secretory machinery [213,429,560]. Exposure of bovine chromaffin cells to NPY results in a long-lasting increase in subsequent stimulation of inositol phosphate formation by ATP acting on P2Y receptors [130]. P2Y2 receptors have been identified immunohistochemically on rat chromaffin cells [5], which is consistent with this effect. ATP stimulation also appears to act through adenylate cyclase to stimulate cAMP formation in bovine chromaffin cells [616], so it is interesting that P2Y12 receptors which use this second messenger system, have since been demonstrated in these cells [142].
Second messenger transduction mechanisms
Extracellular ATP leads to increase in [Ca2+]i and accumulation of inositol 1,4,5-trisphosphate (InsP3) in cultured adrenal chromaffin cells [471]. A recent paper suggests that UTP and ATP acting through P2Y2 receptors increase extracellular signal-regulated kinase 1/2 phosphorylation in bovine chromaffin cells through epithelial growth factor receptor (EGFR) transactivation [334]. The EGFR inhibitor, AG1478, decreased ATP-mediated extracellular-signal-regulated kinase (ERK)1/2 phosphorylation.
Ectonucleotidases
ATPase activity in hydrolysing ATP in chromaffin cells was implicated in the uptake of CA [535] and the release of both amines and ATP from the chromaffin granules membrane [413]. The presence of ecto-nucleotidases responsible for the hydrolysis of released ATP was first described in cultured chromaffin cells [547] and were later localised and characterised in intact pig adrenal glands [27]. ARL 67156 is an effective inhibitor of ecto-nucleotidase activity in bovine chromaffin cells [131].
Diadenosine polyphosphates
Ap4A, diadenosine pentaphosphate (Ap5A) and diadenosine hexaphosphate have been identified on bovine adrenal medullary tissue [421,452]. More recently diadenosine diphosphate, adenosine guanosine polyphosphate (ApnG) and diguanosine polyphosphates (GpnG) have also been identified in chromaffin granules [243]. CA secretion evoked by K+-rich solutions was further enhanced by diadenosine triphosphate and Ap5A, while Ap4A inhibited it [76]. It was speculated that P2Y receptors were likely to mediate the extracellular action of Ap4A [77,419]. Carbachol-induced release of Ap4A and Ap5A from perfused bovine adrenal medulla and isolated chromaffin cells was reported [420]. Ecto-dinucleotide polyphosphate hydrolase was identified, in addition to ecto-nucleotidases, in cultured chromaffin cells [453].
Medullary endothelial cells
CA and ATP and other factors released by chromaffin cells must pass through an endothelial cell barrier to enter the bloodstream. ATP has been shown to stimulate prostacyclin formation via production of the second messenger InsP3 [164]. An intracellular Ca2+-releasing P2U receptor (probably P2Y2 or P2Y4) has been identified on adrenal endothelial cells [78].
Purinergic signalling in development and ageing
There is abundant expression of P2Y2 receptors in NA-containing adrenal chromaffin cells and very little on adrenaline-containing cells in mature rats. However, in new-born rats, P2Y2 receptors are expressed equally on both NA and adrenaline-containing cells and by one week the majority of P2Y receptor labelled cells contain adrenaline [5]. There is a dramatic loss of P2Y2 receptor expression on both NA- and adrenaline-containing cells in the adrenal gland of old (22 month) rats compared to newborn animals. ATP, acting via P2Y2 receptors, may influence the phenotypic expression of chromaffin cells into NA- or adrenaline-containing cells during early development and ageing. Age-related changes in the localisation of P2X receptors in the rat adrenal gland have also been reported [4].
Adrenocortical cells
Extracellular ATP stimulates steroidogenesis in bovine adrenocortical cells via P2Y receptors and Ca2+ mobilization [256]. In contrast, adenosine inhibits secretion of corticosteroids [598]. Calcium is essential for ATP-induced steroidogenesis in bovine adrenocortical fasciculata cells [375]. Later UTP and ADP, as well as ATP, were shown to stimulate cortisol secretion in these cells, suggesting more than one P2 receptor subtype is involved [235]. The mechanism of ATP-stimulated cortisol secretion depends on depolarization-dependent Ca2+ entry and may be linked to stress-induced chromaffin cell secretion to corticosteroid production [599].
The rat adrenal cortex is more densely innervated in the capsule-glomerulosa and in the juxta-medullary regions. Electron microscopic studies have shown autonomic axons supplying adrenal cortical tissue, which sometimes penetrate the basal lamina of the cortical cells and come with close (200 nm) contact with their plasma membranes [448,561]. It has been suggested that the nerve fibres in the superficial cortex are mainly of extrinsic origin in contrast to a major contribution of intrinsic neurons in the medulla [401].
Activation of the splanchnic sympathetic innervation strongly potentiates the steroidogenic action of ACTH from the anterior pituitary and there is compelling evidence that the innervation normally plays an important part in cortisol secretion [134]. Neural release of ATP acting on cortical cells has been considered [247], although the possibility that there is a paracrine non-synaptic modulatory role for CA and ATP in the regulation of adrenocortical steroid secretion has also been raised [520]. It has been suggested that the suprachiasmatic nucleus utilises neuronal pathways to spread its time of the day message, not only to the pineal to control melatonin secretion, but also to the adrenal cortex to influence corticosterone secretion [58]. The cotransmitters released by nerve varicosities influence the production of aldosterone [520]. ATP potentiates both ACTH- and angiotensin II-induced steroidogenesis in bovine adrenocortical fasciculata cells [257].
Both ATP and NA were released in response to electrical field stimulation in superfused rat adrenal capsule-glomerulosa preparations and ecto ATPases identified around nerve profiles at the border of capsule and zona glomerulosa tissue [247]. Angiotensin II and ATP provoke K+ efflux from perfused bovine glomerulosa cells and quinine and apamin significantly reduce the effect of ATP [319].
Two different P2Y receptors (one likely to be a P2Y2 or P2Y4 receptor since it was activated by both UTP and ATP) have been shown to be linked to steroidogenesis in bovine adrenocortical cells [377]. They showed further that mRNA for P2Y2, but not P2Y4 receptors, or for P2Y1, P2Y11 and P2Y12 receptors, although ADP did stimulate steroidogenesis, perhaps via an unidentified P2Y receptor subtype ([378,379]; Fig. 6c). In a recent study, a human adrenal cortex-derived cell line, NCl-H295R, which expresses all the key enzymes needed for steroidogenesis, was shown to express receptor mRNA and protein for A2A and A2B, P2X5 and P2X7, and P2Y1, P2Y2, P2Y6, P2Y12 P2Y13 and P2Y14 subtypes [380]. They claimed further that the P2Y1 receptor was linked to Ca2+-mobilization and cortisol secretion.
Adenosine-stimulated adrenal steroidogenesis involves A2A and A2B receptors, activation of which triggers the Janus kinase 2-MAPK-activated PK-ERK signalling pathway [90]. Foetal cortisol concentrations are suppressed by A1 receptor activation and restrict the increase in ACTH during moderate hypoxia [244].
Ovary
Ovaries produce oocytes and are the principal source of oestradiol and a source of progesterone and androgens in females. In addition to oocytes of different stages of maturation, there are specialised mesenchymal granulose and theca cells that engulf oocytes to form ovarian follicles. Oocyte maturation in the mouse is stimulated by a surge of LH 12 hours prior to ovulation. ATP was shown to inhibit LH-stimulated testosterone accumulation by isolated ovarian follicles from rabbits [325]. Adenosine produced a seven-fold amplification of LH-stimulated cAMP accumulation and progesterone secretion in rat luteal cells, but did not show a similar effect on LH-stimulated cAMP accumulation and androgen secretion in luteal cells [208]. Adenosine exerts predominantly inhibitory actions on hormone-induced granulosa cell differentiation [266]. Adenosine stimulates adenylate cyclase in rat ovarian membrane preparations and preovulatory granulosa cells via A2 receptors [36]. AMP-activated PK regulates progesterone secretion in rat granulose cells [548]. It was suggested that adenosine and prostaglandin F2α may be regulators of luteal cell function by acute and local control of the action of LH [25]. In a later study, this group showed that there was no effect of adenosine on androgen secretion in Leydig cells, but that adenosine produced a marked amplification of FSH-stimulated cAMP accumulation and steroid secretion from granulosa cells from rat and human ovaries [26,425].
LH rapidly depletes luteal cell ATP, which appears to be a physiological action, since it occurs during functional luteolysis at the end of the pseudopregnant cycle [501]. The authors suggest that during functional luteolysis, the rising levels of LH that occurs during follicular development and ovulation cause depletion of luteal ATP levels to ensure irreversible regression and eventual death of the corpora lutea of the previous cycle. ATP levels in granulosa-luteal cells can be influenced by gonadotrophins as well as by adenosine [35]. Recognition of the presence of P2U receptor mRNA in the human granulosa cells followed and ATP/UTP was shown to cause rapid and transient increase in [Ca2+]i [526]. ATP was shown to have an antigonadotrophic action in human granulosa cells [525]. In a later publication from this group, they showed that ATP induced nuclear translocation of phosphorylated ERKs and the induction of egr-1 and c-raf-i expression in the human ovary, supporting the notion that the MAPK signalling pathway plays a role in mediating the effects of ATP on gonadotrophin-induced progesterone secretion in the human ovary [527]. P2, but not P1, receptors were also identified on chicken granulosa cells [361]. P2Y2 and/or P2Y4 receptors in human granulosa-luteal cells mediate calcium oscillations [294,504]. Granulosa cells in contact with the oocyte, respond to ATP via a mechanism that involves P2Y2 receptor stimulation and the participation of ryanodine receptors [357]. Regulation of proliferation of cultured thecal/interstitial cells and steroidogenesis via UTP-sensitive P2Y receptors is relevant in ovarian pathophysiology, since theca hyperplasia is involved in polycystic ovarian syndrome [569]. Purinergic signalling to ovarian perifollicular smooth muscle changed from P2X2 to P2X1 receptors during pregnancy, while there was an increase in P2X2 receptor expression on ovarian vascular smooth muscle [255]. Menopause is associated with decline in ovarian function. P2X2 receptor protein levels were shown to increase with ageing (menopause model), perhaps contributing changes in ageing-relates decline in ovarian function [620]. The theca (or ovarian surface epithelium) is the external layer surrounding the ovarian follicle involved in the synthesis of androgens, the substrate for oestradiol and progesterone synthesis in granulosa cells. ATP causes apoptotic cell death of porcine ovarian theca cells via P2X7 receptor activation ([568]; Fig. 8).
Fig. 8.
Purinergic agonist-evoked [Ca2+]i increase in porcine ovarian theca cells. Cultured theca cells were loaded with fluo-4/AM, and [Ca2+]i was monitored with fluorescence microscopy. Plots show the mean (±SEM) of maximum fluorescence increase in response to 1 mM ATP, UTP, or 250 μM Bz-ATP (20 sec applications). (Reproduced from [568], with permission from Wiley Liss.)
The mammalian ovary is directly innervated by sympathetic nerves, which appear to play major roles in regulating ovarian functions, such as follicular maturation, steroid secretion and ovulation [286]. There are also intrinsic neurons in the rat ovary, but it is not known which cells they innervate or whether ATP is a cotransmitter [115]. Ovarian sympathetic activity increases during the ovulatory process, but the neuronal content of NA and ATP decreases after ovulation. ATP evokes Ca2+ oscillations in isolated human granulosa-luteal cells [504]. Granulosa cells secrete oestradiol and luteal cells secrete both oestradiol and progesterone. P2Y receptors are expressed by human and porcine granulosa–luteal cells; ATP has been shown to decrease the production of progesterone and oestradiol and the authors favoured a neuronal origin of ATP [526]. It has been proposed that P2Y2 and P2Y4 receptors on granulosa cells modulate Cl- permeability by regulating Ca2+ release [37]. ATP, probably released from sympathetic nerves, has been shown to activate nuclear translocation of kinases (MAPKs) leading to the induction of early growth response 1 and Raf expression in human granulosa-luteal cells [527].
At least 99 % of follicles in the mammalian ovary undergo follicular atresia, a cellular degeneration that involves apoptosis in both somatic and germinal follicular cells. ATP-induced apoptotic cell death in porcine ovarian theca cells has been shown to be mediated by P2X7 receptors [568], which is part of the regulation of folliculogenesis, known to be modulated by sympathetic cotransmitters. ATP suppresses the K+ current responses to FSH or adenosine in monolayers of the small follicular cells surrounding a single large oocyte of Xenopus [176]. The follicular cells of Xenopus have a P2 receptor [265,356] and since UTP and ATP are equipotent, this may be a P2Y2 or P2Y4 receptor subtype [176].
Ovariectomy and oestradiol replacement therapy significantly decreased the hydrolysis of ATP and ADP [423]. Ovarian tumours appear to arise mainly from the ovarian surface epithelium, which is a simple squamous-to-cuboid mesothelium that covers the ovary. ATP stimulates mitogen-activated kinase in pre-neoplastic and neoplastic surface epithelial cells and it was suggested that co-released ATP from sympathetic nerves may play a role in regulating cell proliferation in both normal and neoplastic ovarian surface epithelial cells [99]. Ovarian stimulation is a significant risk factor for arterial and venous thrombosis. It has been shown that FSH has a stimulatory effect on ATP release and platelet aggregation [19]. Functional phosphodiesterase 8 has been identified in the mammalian ovarian follicle and it was suggested that it is involved in hormonal regulation of folliculogenesis, indicating a potential application of inhibitors as novel contraceptives [472].
Testis
The testis is the primary source of testosterone production. It consists of seminiferous tubules, within which spermatogenesis takes place, and interstitial spaces between these tubules, containing Leydig cells (testosterone-producing cells), as well as supporting tissue and blood or lymphatic vessels. Germ cells and Sertoli cells are the only cell types present within the seminiferous tubules and they are in close contact with each other. The germ cells migrate within the seminiferous tubules and differentiate from stem spermatogonia, through spermatocytes, to spermatids. The changes in Sertoli cell and germ cell morphology during the repetitive cycle of germ cell development in the rat have been categorised into the 14 different developmental stages. P2X2 and P2Y2 receptors have been described on mouse Sertoli cells and a paper identifies mitochondria as essential components of Sertoli cell signalling that control the purinergic-mediated Ca2+ responses [570]. Activation of AMP-activated PK by adenosine promotes lactate offer to germ cells, thus contributing to successful spermatogenesis [178]. There is sympathetic innervation of the testis with predominant supply to blood vessels; sensory nerve fibres are also present.
There is ultrastructural evidence for sympathetic innervation of Leydig and interstitial cells, which secrete androgens in the testis of various animals and hormones [430]. ATP was shown to act via P2 receptors to increase [Ca2+]i in mouse Leydig cells [412]. P2X2 receptors were later described on Leydig cells [426] and ATP shown to increase testosterone secretion [163]. Leydig cells express pannexin hemichannels, which may account for ATP release [555]. Various P2X receptor subtypes, namely, P2X1, P2X2, P2X3, P2X5 and P2X7 (but not P2X4 or P2X6) receptors, are expressed on germ cells during spermatogenesis [191]. No evidence for a role of sympathetic innervation in the control of sperm development has been presented. Multiple purinergic receptors lead to intracellular calcium increases in rat Sertoli cells [270].
A1 receptors were identified in rat testis [365,508] and adenosine caused steroid production in isolated Leydig cells [455]. The A1 receptors were also localised in Sertoli cells of the seminiferous tubules [354]. Pertussis toxin treatment of cultured Sertoli cells reversed the adenosine-mediated inhibition of cAMP accumulation and potentiated the cAMP response to FSH [249,355].
The Sertoli cells from the mammalian testis are multifunctional cells that release several proteins and fluid into the lumen of the seminiferous tubules and play a key role in germ cell development. FSH is the main messenger of the response of immature Sertoli cells. When Sertoli cells were exposed to ATP, a fast and biphasic increase in [Ca2+]i was obtained [281]. Sertoli cells express P2 receptors that are associated with phosphoinositide turnover and are activated equally by ATP and UTP suggesting that P2Y2 or P2Y4 receptors are involved; they have profound effects on FSH responsiveness [157]. ATP stimulates accumulation of InsP3 in primary cultures of rat and mouse Sertoli calls, consistent with P2Y2 or P2Y4 receptor activation [162,463]. Extracellular ATP stimulates oestradiol secretion in rat Sertoli cells via both P2X and P2Y receptors, which leads to increases in both [Ca2+]i and [Na+]i and membrane depolarisation leading to oestradiol secretion ([459]; Fig. 9). RT-PCR studies revealed mRNA for P2Y1, P2Y2 and P2X4 and P2X7 receptors in cultured rat Sertoli cells [270].
Fig. 9.
Effects of extracellular ATP (ATPe) on oestradiol production in rat Sertoli cells: cells were cultured for 4 days in control medium. On the fourth day in culture, cells were stimulated with ATPe (1, 10, 100 and 1000 μM). After 24 h, media were collected and oestradiol production determined by radioimmunoassay. For evaluation of ATPe-induced oestradiol secretion, Sertoli cells were incubated in different experimental conditions as reported in figure legend. Values are expressed as mean ± S.D. of three separate experiments performed in duplicate: a p < 0.05; b p < 0.01; c p < 0.001 vs. control and Na+-free medium. (Reproduced from [459], with permission from Elsevier.)
Leydig cells are interposed between the seminiferous tubules in the testis. They secrete androgens in response to LH from the anterior pituitary gland. Rat Leydig cells express P2 receptors and their activation by ATP leads to testosterone secretion via a mechanism dependent on the influx of Ca2+ from the external medium [163], consistent with mediation via a P2X receptor subtype. The pharmacological features suggested that the P2X2 receptor subtype was involved [426]. Production of androgens by Leydig cells is dependent on androstenedione, the precursor of testosterone synthesis and the activation of the microsomal enzyme 17β-hydroxysteroid dehydrogenase (17βHSD). ATP generation is required for the activation of 17βHSD in the final step of androgen biosynthesis [260]. The activity of 17βHSD is modulated by extracellular pyridine dinucleotides and adenosine [152]. Evidence for sympathetic innervation of human Leydig cells has been presented and their influence on the secretion of testosterone, perhaps involving ATP release as a cotransmitter with NA [162].
Thyroid hormones are regulators of the male reproductive system. They modulate extracellular ATP levels in hypothyroid cultured Sertoli cells and congenital hypothyroidism and thyroid hormone supplementation on NTPDase activities in Sertoli cells can influence the actions of ATP and adenosine on reproductive functions during development [611].
There is sympathetic and sensory innervation of the rodent testicular artery and the pampiniform plexus, a venous network that surrounds it. The innervation is largely restricted to the capsule of the testes and most superficial blood vessels, suggesting a role in the control of temperature. The testicular capsule of the rat, mouse, rabbit and man all contain contractible smooth muscle. ATP released as a cotransmitter from sympathetic nerves can stimulate contraction of testicular smooth muscle, probably mediated through P2X1 and/or P2X2 receptors [18]. Mouse Leydig cells express P2X4, P2X6 and P2X7 receptor subunits as well as P2X2 receptors and it was suggested that heteromeric P2X2/4/6 receptors may also be present [12].
Pineal gland
The pineal gland is a pea-sized mass of tissue attached by a stalk to the third ventricle of the brain, deep between the cerebral hemispheres at the back of the skull. It contains neurons, glia and special secretary cells called pinealocytes. It functions as an endocrine gland, synthesising, storing and secreting the hormone melatonin.
Endogenous adenosine was shown to be involved in the regulation of melatonin output in the chick pineal gland [145]. Adenosine, acting by A2 receptors, elevated both N-acetylserotonin and melatonin in rat pineal gland [182], probably via A2B receptors [183,372]. A1 receptors and later A2A receptors were identified in the pineal of sheep [146,602]. A2B and A3 receptors were both claimed to be present on mouse pineal tumour cells [516].
It was believed for many years that pineal function was regulated by release of NA from sympathetic nerve terminals. However, when it was established that ATP was released as a cotransmitter with NA from sympathetic nerves (see [62]), evidence was presented that ATP was also involved in regulation of pineal activities by sympathetic nerves [362,376]. The presence of P2 receptors in the rat pineal gland was later reported, and claimed that their main role was to mediate potentiation of the effect of NA-induced N′-acetyl-5-HT production [155]. A P2Y1 receptor was identified in cultured rat pineal glands [154] and later shown to mediate enhancement of the rate of pinealocyte-induced extracellular acidification via a calcium-dependent mechanism [156].
Chick pineal glands exhibit persistent circadian rhythms in the rate of formation of melatonin. It has been claimed that purinergic receptors play no major role in control of this circadian rhythm in the rate of thymidine uptake [578].
Thymus
The thymus is a bilobed organ in the base of the neck, above and in front of the heart. It is enclosed in a capsule and divided internally by cross walls into many lobules, each full of T-lymphocytes. It doubles in size by puberty, after which it gradually shrinks, being replaced by adipose tissue. In infancy, the thymus controls the development of lymphoid tissue and immune responses related to autoimmunity. The thymus is important in immunological function because it contains the active hormone thymosin, which helps to stimulate the production and development of T-lymphocytes. The purine degradation enzymes adenosine deaminase and purine nucleoside phosphohydrolase are linked to lymphocyte differentiation and formation and there is evidence for deficiencies in these enzymes in some combined immunodeficiency diseases. T-lymphocytes migrate from the bone marrow to the thymus, where they mature and differentiate until activated by antigen. The thymus gland is innervated by sympathetic nerves that supply the subcapsular cortex, particularly the major blood vessels that run to the corticomedullary junction, but are sparse in the medulla, although there is an increase in β-adrenoceptor expression in the medulla during maturation. There is also evidence that nerve fibres containing ACh and VIP also supply the thymus. There is an increase in sympathetic innervation of the thymus with age, suggesting that these nerves may play a role in age-associated immune dysregulation.
Evidence for stimulation of thymocytes by adenosine, leading to increase in cAMP was presented early [45,172], to enhance DNA synthesis [202] and regulate thymocyte proliferation [469]. The adenosine receptor involved was claimed to be the A2 subtype, based on agonist potencies [168]. There is cross-talk between A2A receptors and T cell receptors in both directions, supporting a possible role of A2A receptors in the mechanism of immunosuppression in vivo, under adenosine deaminase deficiency and hypoxic conditions such as solid tumours [275].
ATP stimulates calf thymus DNA α-polymerase [584] and enhances calcium influx in intact thymocytes [139,312], suggesting the involvement of P2X receptors. Extracellular ATP increases [Ca2+]i in mouse thymocytes, but they vary in sensitivity depending on the degree of maturation [458]. It was suggested that extracellular ATP may be involved in the processes that control cellular proliferation within the thymus. P2X4 receptor mRNA was identified in the rat thymus [43]. ATP and adenosine are selective in targeting different thymocyte subsets and they have additive and/or antagonistic effects with T cell receptor- and steroid-induced thymocyte death ([13]; Fig. 10a).
Fig. 10.
a Extracellular ATP increases intracellular [Ca2+]i in mouse thymocytes in culture. Comparison of effects of ATP with effects of its catabolites [ADP, AMP and adenosine (Ado)] on elevation of [Ca2+]i. Thymocytes were loaded with the [Ca2+]i-sensitive indicator indo-I and incubated with (1 mM) or without nucleotides, and the concentration of [Ca2+], was continuously measured. (Reproduced from [13], with permission from the American Association of Immunologists.) b and c ATP-induced apoptosis is mediated by P2X7 receptors in thymocytes from BALB/c mice. b Thymocytes were incubated for 5 h with purinoceptor agonists. Apoptosis was evaluated from the percentage of cells with apoptotic nuclei (means ± S.E. of four to five experiments). ATP and the P2X7 receptor agonist 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP) were the most potent agonists. *Significantly different from corresponding control, °significantly different from ATP. c Thymocytes were incubated for 30 min with 1 mM ATP or 5 μM α,β-methylene ATP (αβ), for 2 h with 500 μM oxidised ATP (oATP), followed by 30 min with 1 mM ATP. Data shown are the percentages of phosphatidylserine (PS) + propidium iodide (PI) cells (treated minus control), determined from fluorescence microscopy after the binding of annexin-V-FITC in PI-cells (means ± S.E. of three to four experiments). The most mature thymocytes, the single positive cells (SP: CD4 + CD8- and CD4-CD8+), were the most sensitive to ATP, whereas the double positive (DP: CD4 + CD8+) and double negative (DN: CD4-CD8-) cells exhibited a lower sensitivity. (Reproduced from [302], with permission from Elsevier.) d Dose-dependent increase in prostaglandin E2 (PGE 2) production by ATP and UTP in TEA3A1 rat thymic epithelial cells. Confluent TEA3A1 cells were incubated for 15 min at 37 °C with increasing doses of adenosine 5′-[βγ-methylene]triphosphate (p[CH2]ppA), guanosine 5′-triphosphate (GTP), adenosine 5′-[αβ-methylene]triphosphate (pp[CH2]pA) and 2-methylthioadenosine triphosphate (2-MeSATP). At the end of the experiment, media were collected and the level of PGE2 produced by the cells was determined by radioimmunoassay. Each point represents the mean + S.D. (n = 3). (Reproduced from [317], with permission from Portland Press.)
ATP has been shown to produce apoptotic cell death of thymocytes [366,619], implicating the presence of P2X7 receptors, which were later identified on phagocytic cells of the thymic reticulum [108]. It has been suggested that P2X7 receptor-mediated signalling is involved in the regulation of differentiation as well as cell death in the thymus and purified T, but not B, lymphocytes [102]. P2X7 receptor-mediated apoptosis of thymocytes involves de novo ceramide synthesis and mitochondria alterations ([302]; Fig. 10b and c). P2X1 receptors have also been claimed to play a role in apoptosis of thymocytes [103].
ATP had a biphasic effect on mouse thymocyte consisting of hyperpolarisation followed by depolarisation [345]. There is transient upregulation of P2Y2, but not P2X1, receptor mRNA expression in mouse thymocytes after the addition of steroid hormone [274]. It was suggested that there may be a common early event in responses of T cells to different activating stimuli. mRNA for P2X1, P2X2, P2X6 and P2X7 receptors has been described on mouse thymocytes [173].
In an immunohistochemical and in situ hybridization study of P2 receptors in the rat thymus, it was confirmed that P2X4 receptors were expressed in thymocytes and P2X1 and P2Y2 receptors on subpopulations of lymphocytes (see [65]). It was also shown that P2X1, P2X2 and P2X4 receptors were present in thymic blood vessel smooth muscle, P2X3 receptors on endothelial cells and P2X5 receptors on fibroblasts in the adventitia ([190]; Fig. 11a). Further, P2X2 and P2X3 receptors were abundant on medullary epithelial cells, while P2X6 receptors were prominent in Hassall's capsules. P2X2 receptors were found on subcapsular and perivascular epithelial cells and P2X2, P2X6 and P2X7 receptors on epithelial cells along the thymic septa. In a functional study of three preparations of thymic epithelial cells: 2BH4 murine cell line, IT45-R1 rat cell line, and primary murine cells derived from the Nurse cell lympho-epithelial complex, it was shown that extracellular ATP increases [Ca2+]i probably largely via P2Y2 receptors activated by both ATP and UTP [38]. They showed further that murine 2BH4 cells also expressed P2X7 receptors. P2Y2 receptor mRNA was identified at the electron microscopic level in the rat thymus and shown to be localised on cortical T cells and endothelial cells of thymic blood vessels ([322]; Fig. 11b).
Fig. 11.
a and b P2X4 and P2X1 receptor immunoreactivity in rat thymocytes: immunofluorescence with Texas Red. a Clusters of P2X4 receptor-expressing thymocytes along the cortico-medullary junction and within the medulla. b Thymocytes staining for P2X1 in the subcapsular area. Scale bar in a 20 μm and b 40 μm. c Ultrastructural identification of P2Y2 receptor mRNA in the cortex of rat thymus. Note intense labelling (numerous ‘black’ gold-silver grains: arrows) localised in the cytoplasm of resting T-cells (T) of the specimen that was hybridised to the DIG-labelled rat P2Y2 receptor antisense oligonucleotide probe. ×11,000. (a and b Reproduced from [190] with permission from Springer. c Reproduced from [322], with permission from Karger.)
In the thymus, prostaglandin E2 (PGE2) is produced and maintained at a high level, largely by thymic epithelial cells. ATP, acting via P2Y receptors, leads to production of PGE2 and it has been suggested that ATP released as a cotransmitter from sympathetic and parasympathetic nerves may be responsible for the high levels of PGE2 in the thymus ([317,318]; Fig. 10d).
IL-6 is an important factor for thymic proliferation and differentiation, produced by thymic epithelial cells. It has been suggested that ATP released as a cotransmitter from sympathetic nerves leads to IL-6 production [576], implicating the presence of P2X7 receptors. Extracellular ATP induces phosphatidylserine externalisation earlier than nuclear apoptotic events in thymocytes [107]. Intercellular calcium waves have been identified between thymic epithelial cells and shown to depend on both gap junctions and P2 receptors [374].
Adenosine triphosphatase was localised histochemically intracellularly in thymocytes and shown to be more prominent in thymocyte precursors than in mature thymocytes [363]. ATP, and to a lesser extent ADP, but not AMP, GTP or inosine triphosphate, increased [Ca2+]i and initiated blastogenesis [138]. Adenosine deaminase was localised in the human thymus [88]. Phorbol esters regulate adenosine deaminase mRNA in human thymocytes [344]. Studies of transgenic mice over-expressing CD73, suggest that adenosine accumulation may play a role in adenosine deaminase-deficiency severe combined immunodeficiency [442]. It is known that the thymus and other lymphoid tissues react to nutritional disorders more rapidly than most other organs. Re-feeding with a 20 % protein diet for 9 days is enough to reverse the effect produced by severe protein malnutrition and adenosine deaminase and purine nucleoside phosphorylase activities [151]. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signalling [14].
There is a valuable review discussing the roles of extracellular ATP in the neuroendocrine control of the thymus [8].
Neuroendocrine hypothalamus
Mg2+ATP has been shown to stimulate the release of luteinising hormone-releasing hormone (LHRH) from isolated hypothalamic granules [68]. ATP facilitates the action of chelated copper, perhaps released endogenously, to stimulate the release of LHRH from explants of the median eminence via interaction with a purinergic receptor [21]. ATP stimulated LHRH release and increased [Ca2+]i levels in both neurons and glia; LHRH neurons express P2X2 and P2X4 receptors, while glia express P2Y1 and P2Y2 receptors and interactions between neurons and glia appear to be involved in the initiation of Ca2+ oscillations and pulsatile LHRH release in vivo in primates [537]. P2X2, P2X4, P2X5 and P2X6 receptor subunits were shown by immunohistochemistry to be expressed on the perykarya of LHRH-producing neurons, and P2X2 and P2X6 receptors on the axon terminals [175,320,321,595]. NTPDase3 has been identified in the neuroendocrine hypothalamus and it has been suggested that it plays a role in the initiation of the LH surge and ATP involvement in the regulation of pituitary LH release [622].
ATP injected into the paraventricular and supraoptic nuclei leads to a release of the antidiuretic hormone, arginine-vasopressin (AVP) [358,359]. It was later proposed that ATP was released as a cotransmitter with NA from neurons in the caudal medulla that project to supraoptic VP cells [118]. Application of ATP and UTP (but not adenosine) produced depolarisations of supraoptic neurosecretory cells in superfused explants of rat hypothalamus, via P2X and P2Y2 receptors [233]. ATP appears to act via P2X receptors both on the cell bodies and dendrites of vasopressinergic neurons in the supraoptic nucleus of the hypothalamus [481]. ATP produces inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors [267]. RT-PCR studies showed that mRNAs for P2X3, P2X4 and P2X7 receptors were predominant in rat supraoptic nucleus and functionally expressed, leading to increase in [Ca2+]i ([481]; Fig. 12a). Evidence has been presented that ATP-induced currents in AVP neurons in the supraoptic nucleus may be mediated, at least in part, by pannexin channels associated with P2X receptors [386]. Adenosine, probably resulting from the breakdown of ATP released from nerves in the supraoptic nucleus, inhibits the release of γ-aminobutyric acid and glutamate via activation of presynaptic A1 receptors leading to modulation of AVP and OT release [396].
Fig. 12.
a Patch-clamp analysis of ATP-induced currents in rat supraoptic nucleus (SON) neurons. Representative current responses to UTP (10-3 M), BzATP (10-4 M) and ATP (10-3 M) obtained from a single SON neuron. The breaks in the trace are 3–5 min. The holding potential was −80 mV. The major salt in the pipette used in experiments shown in this figure was caesium methanesulphonate. (Reproduced from [481], with permission from Wiley.) b Rat SON astrocytes respond to ATP and noradrenaline (NA) and the response is synergistic. Averaged values show that the amplitude of the response to the co-application of the two transmitters is significantly greater than the sum of the responses to individual applications (*** P < 0.001, n = 22). (Reproduced from [143], with permission from Elsevier.) c Summary of effects of suramin on electrically-stimulated vasopressin (AVP) release from wild-type (WT), P2X3, P2X2⁄3 and P2X7 receptor knockout (KO) mice. These data indicate that P2X2 is the primary receptor responsible for the facilitation of electrically-stimulated AVP release by endogenous release of ATP. The inhibitory effect of suramin on endogenous ATP facilitation of AVP release was significantly (P < 0.05) reduced only in the P2X2⁄3 KO mice. * Indicates significant difference P < 0.05 compared to WT control. (Reproduced from [114], with permission from Wiley.) d Model of the established exogenous and the proposed endogenous purinergic effects on neurohypophysial terminals. Different physiological burst patterns regulate oxytocin (OT; high frequency) vs. AVP (low frequency) release. The biophysical properties of the VGCC (N, L, R on OT and N, L, Q on AVP terminals) alone, however, cannot explain the differential effects of such bursts. Thus, we propose that endogenous co-released ATP activates P2X2, P2X3, P2X4 and P2X7 receptors localised on AVP terminals, while activating only P2X7 receptors on OT terminals. The flux of Ca2+ through these receptors increases [Ca2+]i and, thus, neuropeptide release. The ATP is then broken down to adenosine by ecto-nucleotidases, which are present only on AVP terminals. Adenosine, which acts on A1 receptors, present on both terminal types, directly inhibits N-type Ca2+ channels and subsequent neuropeptide release. (Reproduced from [300], with permission from Elsevier.)
In keeping with the features of cotransmission, ATP (via P2X receptors) and phenylephrine (via α1 adrenoceptors) act synergistically to stimulate AVP release [487,497,500]. Synergistic activation of astrocytes by ATP and NA in the rat supraoptic nucleus has also been described ([143]; Fig. 12b). ATP, acting via P2X2 receptors (which do not show desensitization), caused rapid, sustained release of AVP and OT into perfused explants of the rat hypothalamus-neurohypophysial system [193], while substance P potentiated these responses [250]. P2X5 receptors were shown to be expressed on neurons containing AVP and NOS in the rat hypothalamus ([596]; Fig. 13). Evidence was presented to show that P2Y as well as P2X receptors mediate ATP-stimulated increase in [Ca2+]i in the supraoptic nucleus, the P2Y1 receptor subtype being more prominent than the P2Y2, P2Y4 or P2Y6 subtypes [498]. In a later paper from this group, it was suggested that P2Y1 receptors may regulate VP release by mediating stretch-inactivated cation channels [499]. A recent study has shown that AVP-containing neurons to the rat paraventricular nucleus expressed P2X4, P2X5 and P2X6 receptors, while OT-containing neurons only expressed P2X4 receptors; in the supraoptic nucleus, AVP neurons expressed P2X2, P2X4, P2X5 and P2X6 receptors and OT-containing neurons expressed P2X2, P2X4 and P2X5 receptors [206]. It was concluded in recent papers that P2X4 receptors were found only on AVP terminals, while P2X7 receptors were expressed on both AVP and OT terminals and somata and this suggested that this is controlled by hypothalamic neurohypophysial neurons to form a positive feedback mechanism for hormone release (Fig. 12c) [114,269]. A model was proposed to explain how purinergic and/or opioid feedback modulation during bursts can mediate differences in the control of neurohypophysial AVP and OT release ([300]; Fig. 12d). Adenosine, acting via P1 receptors, reduces ATP-stimulated AVP release from hypothalamo-neurohypophysial explants [496].
Fig. 13.
Coexistence of P2X5 receptor immunoreactivity and vasopressin (AVP) in rat hypothalamus. a P2X5 receptor-immunoreactive (ir) neurons and fibers in the paraventricular hypothalamic nucleus, lateral magnocellular area (PaLM; green). b AVP-ir neurons and fibers in the PaLM at the same section of a (red). c Coexistence of P2X5 receptor-ir and AVP-ir in the supraoptic nucleus (SON). Note that nearly all the AVP-ir neurons also expressed P2X5 receptor immunoreactivity (yellow), but a number of the P2X5 receptor-ir neurons (green) did not express AVP. d Coexistence (yellow) of P2X5 receptor immunoreactivity (green) and AVP (red) in the retrochiasmatic part of supraoptic nucleus (SOR). Scale bar for all figures 80 μm. In each figure, the dorsal aspect of the nuclei is at the top and the ventral aspect of the nuclei is at the bottom. (Reproduced from [596], with permission from Elsevier.)
Orexin/hypocretin neurons in the hypothalamus, involved in arousal and feeding behaviours, express A1 adenosine receptors [538,594]. P2X2 receptor mRNA has also been shown to be expressed on orexin/hypocretin neurons in the rat perifornical hypothalamus [160] and ATP, released from neurons and/or glia, leads to increased activity of the hypocretin arousal system via P2X2 receptors [592].
Placenta
The placenta and umbilical vessels are involved in steroidogenesis as well as regulation of blood flow and control of transport of materno-foetal fluid and solutes.
NO, released from endothelial cells following occupation of P2 receptors in response to ATP, ADP and UTP, may regulate the release of corticotrophin-releasing hormone from human placental syncytiotrophoblast cells. An increase in placental 5′-nucleotidase was described in late human pregnancy and duration of labour and it was suggested that this may reflect enhanced oestrogen synthesis and facilitation of uterine contractions during labour [61]. Immunocytochemical localisation of 5′-nucleotidase was shown on the external surface of the microvillous plasma membrane of the syncytiotrophoblast, where it may play a role in regulating foeto-placental-maternal microcirculation in the human term placenta [346]. P2X7 receptors mediate regulation of PLD in human placental trophoblasts [126].
P2X1, P2X4, P2X5, P2X6 and P2X7 receptor mRNA has been described in human placental vessels, which contribute to humoral regulation of placental blood flow [565]. The syncytiotrophoblast is the solute-transporting epithelium of the human placenta that facilitates maternal-foetal nutrient exchange. Since the human placenta is not innervated, autocrine, paracrine and endocrine modulation of syncytiotrophoblast transport function is of pivotal importance. Functionally active P2X4, P2X7, P2Y2 and P2Y6 receptors have been identified on human placental syncytiotrophoblast cells [446]. This group showed later that post-translational modifications of the syncytiotrophoblast P2X4 receptor are altered in preeclampsia [447].
Neuroendocrine cells
The neuroepithelial bodies (NEBs) consist of pulmonary neuroendocrine cells that are usually arranged in innervated clusters in the airway mucosa. They are O2 sensors, of particular importance in early life before the carotid body O2 sensory system is fully established. They also appear to mediate reflex activities in response to hyperventilation and noxious substances, by releasing ATP to act on P2X3 receptors on sensory nerves arising from the nodose ganglia, which innervate NEBs [51,52]. Parasympathetic efferent fibres also innervate NEBs [1].
Merkel cells in the skin are also regarded as neuroendocrine cells. They are innervated largely by sensory nerves, which are likely to be activated by ATP, which is stored in high concentrations and probably released from these cells by mechanical distortion [112].
Rat prostate neuroendocrine cells express both P2X and P2Y receptor subtypes, which mediate marked increase in [Ca2+]i [59,261]. The authors speculate ATP is released as a cotransmitter with NA in sympathetic reviews innervating the prostate.
The gastrointestinal tract is, in size at least, the largest endocrine organ in the body. Endocrine cells in the intestinal mucosa release a number of putative hormones [259,476]. For example, the intestinal hormone cholecystokinin acts on primary afferent nerve fibres in the vagal trunk [128]. OT is expressed by intrinsic sensory and secretomotor neurons in the guinea-pig enteric nervous system, suggesting that OT in the gut is involved in both motility and the balance of absorption and secretion of water and electrolytes [608].
Adipocytes
Adipocytes were long considered to be an inert tissue for fat storage, but it is now recognised that it has endocrine functions [80,224,263,456]. Adipocytes secrete adipokines, including adiponectin, leptin, tumour necrosis factor-α and IL-6, as well as adenosine and fasting-induced adipose factor. Leptin is produced by white adipocytes and acts on the brain to maintain body weight by suppressing food intake [431]. Adiponectin has an anti-inflammatory role, protecting against insulin resistant type 2 diabetes, fatty liver disorder and atherosclerosis.
P1 receptors
Adenosine was shown to inhibit adenylate cyclase activity in fat cell ghosts [144,323] and lipolysis in adipose cells stimulated by NA or sympathetic nerve stimulation [169,234,493,556]. Insulin and adenosine are both antilipolytic; they are additive, but not synergistic [494]. Both insulin and adenosine have major roles in regulating adipose tissue mobilisation [351]. Adenosine also plays a role in the regulation of adipose tissue blood flow [342,492,557]. Fat cell plasma membranes were shown to contain sites which bind [3H]adenosine with high affinity [339]. Adenosine receptors on fat cells that mediate inhibition of cAMP accumulation and lipolysis were identified [553]. CD73-derived adenosine is an insulin-independent modulator of lipolysis in fat tissue under in vivo conditions [60]. They were claimed first to be Ra, Ri and then P receptors [179] and later as A1 receptors in rats [199,406], pigs [349] and humans [200,287,534]. Adenosine inhibited lipolysis in vivo in obese premenopausal women [180]. White adipocytes were found to be more responsive than brown adipocytes to inhibition of lipolysis by A1 receptor agonists [464]. Lipolysis of mature brown fat cells is significantly increased by activation by A2A receptor agonists or by A1 receptor antagonists [192]. The A2 receptor subtype, which is positively coupled to adenylate cyclase, is expressed in adipocyte precursor cells, but not mature adipocytes [567]. However, in later papers A1 receptors expressed in human pre-adipocytes were shown to initiate differentiation while A2B receptors mediated inhibition of apidogenesis [185,533]. Adipocyte A1 receptors are tonically activated by endogenous adenosine at nanomolar concentrations [310]. A partial agonist of the A1 receptor was identified and evidence presented that the rat epididymal A1 receptors are a homogenous receptor population with regard to affinities for ligands [148]. There is a deficient lipolytic response to CA in hypothyroidism and it was suggested that this may be due to an increased influence of adenosine [170]. Short-term hyperthyroidism modulates the expression of adenosine receptors in adipocytes [433].
In subcutaneous abdominal fat cells from obese subjects, the antilipolytic effect of an adenosine analogue was markedly attenuated [387,388], with decreased adenosine receptor numbers [248]. Insulin resistance in Obese Zucker rats is tissue specific and signalling via adenosine receptors may be a factor contributing to tissue specific insulin resistance [111]. Overexpression of A1 receptors in adipose tissue protects mice from obesity-related insulin resistance [129]. Data has been presented to suggest that inhibition of lipolysis by adenosine is greater in obese African-American women and this may explain why obese African-American women have more difficulty in losing weight than obese Caucasian women [20]. It has been claimed recently that promotion of brown adipose tissue development in white adipose tissue by physiological activation of AMP kinase may have potential for treating obesity [573]. Adenosine had different effects on the actions of OT and insulin on glucose oxidation and lipogenesis [195]. Adenosine greatly enhanced lipolysis in isolated fat cells from streptozotocin-diabetic rats compared to controls [495]. The maximal rate of lipolysis of adipocytes from exercise-trained rats was increased compared to controls, but inhibition by adenosine was comparable in the two groups [482]. Lactation results in an increased responsiveness of adipocytes to β-agonists which stimulate lipolysis and paradoxically, to adenosine which inhibits lipolysis [571]. Activation of A1 receptors, which have a dominant expression in adipocytes, increases leptin secretion [95,443], as well as inhibition of lipolysis and protection against obesity-related insulin resistance [185]. They suggest that targeting A1 and A2B receptors could be considered for the management of obesity and diabetes (see also [123,124]). Leptin-induced lipolysis opposes the tonic inhibition by endogenous adenosine in white adipocytes [174]. AMP kinase has been claimed to have fat-reducing effects on adipose tissue [177]. In a study using A1 receptor knockout mice, increase in lipolysis and decrease in lipogenesis was expected, but in fact an increased fat mass was observed [246]. The authors suggested that this might indicate that other actions of A1 receptors, possibly outside adipose tissue, may also be important. However, partial antagonism of A1 receptors increased lipolysis in cells incubated with adrenaline and adenosine with insulin [523]. It was concluded that the adenosine that accumulates in human adipocyte suspensions is almost exclusively derived from ATP released from cells [254]. A1 receptor signalling contributes to insulin-controlled glucose homeostasis and insulin sensitivity and is involved in the metabolic regulation of adipose tissue [149]. An early review about adenosine and lipolysis is available [167]. AMP is a selective inhibitor of brown adipocyte non-selective cation channels [209].
There is recent interest in the differentiation of mesenchymal stem cells (MSCs) into adipocytes and purinoceptors appear to be involved. For example, differentiation of MSCs into adipocytes was accompanied by significant increases in A1 and A2A receptor expression and their activation was associated with adipogenesis [184].
P2 receptors
ATP inhibition of insulin-stimulated glucose transport in fat cells was recognised early [81,158,210]. ATP also inhibited insulin-stimulated glucose oxidation [530]. Insulin-stimulated D-allose transport, into or out of the cell, but not basal transport, is inhibited by brief exposure of isolated fat cells to exogenous ATP and ADP [326]. It was suggested that ATP blocks transmission of signal from the insulin receptor to the carrier system. Sympathetic nerve stimulation induces a rapid fall in ATP in subcutaneous adipose tissue, perhaps secondary to the hypoxia produced by vasoconstriction [171]. Evidence was presented to suggest that extracellular ATP may partially inhibit the binding of insulin to its surface receptor and, at the same time, may strongly block the degradative pathways for the processing of insulin [215]. Chronic inflammation in adipose tissue is an important etiologic factor for the development of insulin-resistance, particularly in obesity. In a recent paper, it has been shown that high doses of ATP induce inflammatory responses and insulin resistance in rat adipocytes [610]. The authors suggest that defects in ATP-induced insulin signalling play a major role for the impaired glucose uptake in response to insulin treatment. Echinocytosis by glucose depletion, where erythrocytes shrink, has been attributed to ATP depletion, although other mechanisms may also be involved [593].
High fat diets are associated with a reduction in sympathetic activity in brown adipose tissue [465], bearing in mind that it is now well established that ATP is released as a cotransmitter from sympathetic nerves (see [63]). In obesity, sympathetic nerve activity is increased relating to obesity hypertension, while sympathetic nerve activity to adipose tissue is reduced and unresponsive to stimulation by feeding [289]. It was further suggested that local sympathetic nerve dysfunction may contribute to abnormal adipose tissue behaviour in obesity and body fat accumulation.
From a study of brown adipocytes of rats it was suggested that secretion, mobilization of membrane transporters, and/or membrane expression of receptors may be regulated by ATP released as a cotransmitter from sympathetic nerves acting via P2Y receptors [295,404]. In a later study, these authors concluded that white adipocytes are very similar to brown adipocytes in their response to extracellular ATP [296]. ATP, acting via P2 receptors, is involved in the regulation of the key enzyme of oestrogen biosynthesis, aromatase, in stromal cells from human adipose tissue [475]. They suggest that P2 receptors might provide a direct link between sympathetic nerve activity and oestrogen biosynthesis. ATP not only mobilises Ca2+ from intracellular stores (probably via P2Y receptors), but also exerts a potent inhibitory effect on the store-operated Ca2+ entry process in adult rat brown adipocytes [397,398]. ATP, probably released from sympathetic nerves, modulates via P2 receptor activation, the amount and voltage dependence of voltage-gated K+ currents in brown adipocytes [585], and increases membrane conductance in single rat adipocytes [100].
ATP also mediates long-term signalling, for example it modulates proliferation of brown adipocytes [586]. Evidence has been presented that extracellular ATP redistributes actin filaments towards the plasma membrane of brown adipocytes via P2 receptors [399].
Genes expressing P2X1, P2X4, P2X5 and P2X7, in addition to P2Y1, P2Y2, P2Y4 and P2Y6 receptor mRNA identified by RT-PCR, have been described in rat adipocytes [398]. P2Y2 and P2Y11 receptors have been identified on white adipocytes and it has been suggested that P2Y11 receptors might be involved in inhibition of insulin-mediated leptin production and stimulation of lipolysis [293]. In a more recent paper, leptin production by white adipocytes was decreased in P2Y1 receptor knockout mice [285]. It was suggested that the P2Y1 receptor may regulate plasma leptin in lean mice, but is overcome in obese mice. P2Y2, P2Y6 and P2Y12 receptors, and all P2X receptor subtypes except P2X6, were identified as the nucleotide receptors on brown fat cells [297]. Human adipocytes express functionally active P2X7 receptors that mediate release of inflammatory cytokines; adipocytes from patients with metabolic syndrome show enhanced P2X7 receptor expression [336].
ATP enhanced 3 T3-L1 pre-adipocyte cell migration into fat cell clusters, one of the essential processes of adipose tissue development, by activating P2Y receptors, as well as enhancing the differentiation of adipocytes by adipogenic hormones [400]. Deficits in receptor regulation, transporter mobilization and adipocyte hormone secretion are all thought to contribute to the pathology of obesity. Stimulation of lipogenesis in rat adipocytes by ATP, which regulates fat stores independently from established hormones, has been reported [477].
Ca2+ ATPase in mitochondria, that is brown adipose tissue-specific, has been described that can generate heat in the presence of Ca2+ concentrations similar to those generated by adrenergic stimulation [120]. Resveratrol and genistein, naturally occurring plant-derived compounds present in red wine and said to have anti-adipogenic effects, deplete ATP from adipocytes [521].
Increase in release of ATP in adipocytes appears to be an important factor increasing leptin gene expression and enhancing leptin secretion after a meal (see [522]).
Concluding comments
In most other areas, the recent emphasis has been on the pathophysiology and therapeutic potential of purinergic signalling. Surprisingly, this has not yet happened in relation to endocrine biology, but hopefully with the recent development of purinoceptor subtype antagonists that are orally bioavailable and stable in vivo, this aspect will be explored.
Acknowledgments
Andrea Nistri made helpful suggestions that improved the first draft of this review. The author is very grateful to Dr Gillian E. Knight for her invaluable assistance in the preparation of this review article.
References
- 1.Adriaensen D, Timmermans JP. Purinergic signalling in the lung: important in asthma and COPD? Curr Opin Pharmacol. 2004;4:207–214. doi: 10.1016/j.coph.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Afework M, Burnstock G. Distribution of P2X receptors in the rat adrenal gland. Cell Tissue Res. 1999;298:449–456. doi: 10.1007/s004419900103. [DOI] [PubMed] [Google Scholar]
- 3.Afework M, Burnstock G. Localization of P2X receptors in the guinea pig adrenal gland. Cells Tissues Organs. 2000;167:297–302. doi: 10.1159/000016793. [DOI] [PubMed] [Google Scholar]
- 4.Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. Int J Dev Neurosci. 2000;18:515–520. doi: 10.1016/s0736-5748(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 5.Afework M, Burnstock G. Changes in P2Y2 receptor localization on adrenaline- and noradrenaline containing chromaffin cells in the rat adrenal gland during development and ageing. Int J Dev Neurosci. 2005;23:567–573. doi: 10.1016/j.ijdevneu.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Allsup DJ, Boarder MR. Comparison of P2 purinergic receptors of aortic endothelial cells with those of adrenal medulla: evidence for heterogeneity of receptor subtype and of inositol phosphate response. Mol Pharmacol. 1990;38:84–91. [PubMed] [Google Scholar]
- 7.Aloj SM, Liguoro D, Kiang JG, Smallridge RC. Purinergic (P2) receptor-operated calcium entry into rat thyroid cells. Biochem Biophys Res Commun. 1993;195:1–7. doi: 10.1006/bbrc.1993.2000. [DOI] [PubMed] [Google Scholar]
- 8.Alves LA, Coutinho-Silva R, Savino W. Extracellular ATP: a further modulator in neuroendocrine control of the thymus. Neuroimmunomodulation. 1999;6:81–89. doi: 10.1159/000026367. [DOI] [PubMed] [Google Scholar]
- 9.Amisten S, Meidute-Abaraviciene S, Tan C, Olde B, Lundquist I, Salehi A, Erlinge D. ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia. 2010;53:1927–1934. doi: 10.1007/s00125-010-1807-8. [DOI] [PubMed] [Google Scholar]
- 10.Anand-Srivastava MB, Cantin M, Gutkowska J. Adenosine regulates the release of adrenocorticotropic hormone (ACTH) from cultured anterior pituitary cells. Mol Cell Biochem. 1989;89:21–28. doi: 10.1007/BF00228276. [DOI] [PubMed] [Google Scholar]
- 11.Andersson A. Nucleoside-stimulated insulin production by isolated mouse pancreatic islets. Horm Metab Res Suppl. 1980;10:14–19. [PubMed] [Google Scholar]
- 12.Antonio LS, Costa RR, Gomes MD, Varanda WA. Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal. 2009;5:277–287. doi: 10.1007/s11302-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apasov SG, Koshiba M, Chused TM, Sitkovsky MV. Effects of extracellular ATP and adenosine on different thymocyte subsets: possible role of ATP-gated channels and G protein-coupled purinergic receptor. J Immunol. 1997;158:5095–5105. [PubMed] [Google Scholar]
- 14.Apasov SG, Blackburn MR, Kellems RE, Smith PT, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. J Clin Invest. 2001;108:131–141. doi: 10.1172/JCI10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arkhammar P, Hallberg A, Kindmark H, Nilsson T, Rorsman P, Berggren PO. Extracellular ATP increases cytoplasmic free Ca2+ concentration in clonal insulin-producing RINm5F cells. A mechanism involving direct interaction with both release and refilling of the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. Biochem J. 1990;265:203–211. doi: 10.1042/bj2650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacher S, Kraupp O, Conca W, Raberger G. The effects of NECA (adenosine-5′N-ethylcarboxamide) and of adenosine on glucagon and insulin release from the in situ isolated blood-perfused pancreas in anesthetized dogs. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:67–71. doi: 10.1007/BF00499075. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramanian R, de Azua IR, Wess J, Jacobson KA. Activation of distinct P2Y receptor subtypes stimulates insulin secretion in MIN6 mouse pancreatic β cells. Biochem Pharmacol. 2010;79:1317–1326. doi: 10.1016/j.bcp.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks FCL, Knight GE, Calvert RC, Turmaine M, Thompson CS, Mikhailidis DP, Morgan RJ, Burnstock G. Smooth muscle and purinergic contraction of the human, rabbit, rat, and mouse testicular capsule. Biol Reprod. 2006;74:473–480. doi: 10.1095/biolreprod.105.044602. [DOI] [PubMed] [Google Scholar]
- 19.Bar J, Orvieto R, Lahav J, Hod M, Kaplan B, Fisch B. Effect of urinary versus recombinant follicle-stimulating hormone on platelet function and other hemostatic variables in controlled ovarian hyperstimulation. Fertil Steril. 2004;82:1564–1569. doi: 10.1016/j.fertnstert.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 20.Barakat H, Davis J, Lang D, Mustafa SJ, McConnaughey MM. Differences in the expression of the adenosine A1 receptor in adipose tissue of obese black and white women. J Clin Endocrinol Metab. 2006;91:1882–1886. doi: 10.1210/jc.2005-2109. [DOI] [PubMed] [Google Scholar]
- 21.Barnea A, Cho G, Katz BM. A putative role for extracellular ATP: facilitation of 67copper uptake and of copper stimulation of the release of luteinizing hormone- releasing hormone from median eminence explants. Brain Res. 1991;541:93–97. doi: 10.1016/0006-8993(91)91079-g. [DOI] [PubMed] [Google Scholar]
- 22.Barreto-Chaves ML, Carneiro-Ramos MS, Cotomacci G, Junior MB, Sarkis JJ. E-NTPDase 3 (ATP diphosphohydrolase) from cardiomyocytes, activity and expression are modulated by thyroid hormone. Mol Cell Endocrinol. 2006;251:49–55. doi: 10.1016/j.mce.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Barreto-Chaves ML, de Souza MP, Fürstenau CR. Acute actions of thyroid hormone on blood vessel biochemistry and physiology. Curr Opin Endocrinol Diabetes Obes. 2011;18:300–303. doi: 10.1097/MED.0b013e32834a785c. [DOI] [PubMed] [Google Scholar]
- 24.Basso CR, Barreto-Chaves ML. Mechanisms related to the thyroid hormone (TH)-induced vasorelaxation: contribution of reactive oxygen species (ROS) and purinergic signalling. FASEJ J. 2012;26:1140.11. [Google Scholar]
- 25.Behrman HR, Hall AK, Preston SL, Gore SD. Antagonistic interactions of adenosine and prostaglandin F2α modulate acute responses of luteal cells to luteinizing hormone. Endocrinology. 1982;110:38–46. doi: 10.1210/endo-110-1-38. [DOI] [PubMed] [Google Scholar]
- 26.Behrman HR, Polan ML, Ohkawa R, Laufer N, Luborsky JL, Williams AT, Gore SD. Purine modulation of LH action in gonadal cells. J Steroid Biochem. 1983;19:789–793. doi: 10.1016/0022-4731(83)90013-4. [DOI] [PubMed] [Google Scholar]
- 27.Benrezzak O, Grondin G, Proulx J, Rousseau E, D'Orléans-Juste P, Beaudoin AR. Characterization and immunohistochemical localization of nucleoside triphosphate diphosphohydrolase (NTPDase) in pig adrenal glands (presence of a non-sedimentable isoform) Biochim Biophys Acta. 2000;1524:94–101. doi: 10.1016/s0304-4165(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand G, Chapal J, Loubatières-Mariani MM. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am J Physiol. 1986;251:E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand G, Chapal J, Loubatières-Mariani MM, Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br J Pharmacol. 1987;91:783–787. doi: 10.1111/j.1476-5381.1987.tb11276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand G, Gross R, Petit P, Loubatières-Mariani MM. An A2-purinoceptor agonist, NECA, potentiates acetylcholine-induced glucagon secretion. Br J Pharmacol. 1989;96:500–502. doi: 10.1111/j.1476-5381.1989.tb11844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertrand G, Nenquin M, Henquin JC. Comparison of the inhibition of insulin release by activation of adenosine and alpha 2-adrenergic receptors in rat β-cells c. Biochem J. 1989;259:223–228. doi: 10.1042/bj2590223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand G, Petit P, Bozem M, Henquin JC. Membrane and intracellular effects of adenosine in mouse pancreatic β-cells. Am J Physiol. 1989;257:E473–E478. doi: 10.1152/ajpendo.1989.257.4.E473. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand G, Gross R, Ribes G, Loubatières-Mariani MM. P2 purinoceptor agonists stimulate somatostatin secretion from dog pancreas. Eur J Pharmacol. 1990;182:369–373. doi: 10.1016/0014-2999(90)90296-i. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand G, Chapal J, Puech R, Loubatières-Mariani MM. Adenosine-5′-O-(2-thiodiphosphate) is a potent agonist at P2 purinoceptors mediating insulin secretion from perfused rat pancreas. Br J Pharmacol. 1991;102:627–630. doi: 10.1111/j.1476-5381.1991.tb12223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billig H, Rosberg S. Gonadotropin depression of adenosine triphosphate levels and interaction with adenosine in rat granulosa cells. Endocrinology. 1986;118:645–652. doi: 10.1210/endo-118-2-645. [DOI] [PubMed] [Google Scholar]
- 36.Billig H, Thelander H, Rosberg S. Adenosine receptor-mediated effects by nonmetabolizable adenosine analogs in preovulatory rat granulosa cells: a putative local regulatory role of adenosine in the ovary. Endocrinology. 1988;122:52–61. doi: 10.1210/endo-122-1-52. [DOI] [PubMed] [Google Scholar]
- 37.Bintig W, Baumgart J, Walter WJ, Heisterkamp A, Lubatschowski H, Ngezahayo A. Purinergic signalling in rat GFSHR-17 granulosa cells: an in vitro model of granulosa cells in maturing follicles. J Bioenerg Biomembr. 2009;41:85–94. doi: 10.1007/s10863-009-9199-5. [DOI] [PubMed] [Google Scholar]
- 38.Bisaggio RD, Nihei OK, Persechini PM, Savino W, Alves LA. Characterization of P2 receptors in thymic epithelial cells. Cell Mol Biol (Noisy-le-grand) 2001;47:19–31. [PubMed] [Google Scholar]
- 39.Bizzarri C, Corda D. Norepinephrine, unlike ATP, induces all-or-none increase in cytosolic calcium in thyroid cells. The role of inositol-trisphosphate-sensitive stores and calcium channels. Eur J Biochem. 1994;219:837–844. doi: 10.1111/j.1432-1033.1994.tb18565.x. [DOI] [PubMed] [Google Scholar]
- 40.Björkman U, Ekholm R. Effect of P1-purinergic agonist on thyrotropin stimulation of H2O2 generation in FRTL-5 and porcine thyroid cells. Eur J Endocrinol. 1994;130:180–186. doi: 10.1530/eje.0.1300180. [DOI] [PubMed] [Google Scholar]
- 41.Blachier F, Malaisse WJ. Effect of exogenous ATP upon inositol phosphate production, cationic fluxes and insulin release in pancreatic islet cells. Biochim Biophys Acta. 1988;970:222–229. doi: 10.1016/0167-4889(88)90182-6. [DOI] [PubMed] [Google Scholar]
- 42.Blaschko H, Born GV, D'Iorio A, Eade NR. Observations on the distribution of catechol amines and adenosinetriphosphate in the bovine adrenal medulla. J Physiol. 1956;133:548–557. doi: 10.1113/jphysiol.1956.sp005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 44.Böck P. Fate of ATP in secretory granules: phosphohydrolase studies in pancreatic vascular bed. Arch Histol Cytol. 1989;52(Suppl):85–90. [PubMed] [Google Scholar]
- 45.Bonnafous JC, Dornand J, Mani JC. Hormone-like action of adenosine in mouse thymocytes and splenocytes: evidence for the existence of membrane adenosine receptors coupled to adenylate cyclase. FEBS Lett. 1979;107:95–99. doi: 10.1016/0014-5793(79)80471-8. [DOI] [PubMed] [Google Scholar]
- 46.Borges R. The ATP or the natural history of neurotransmission. Purinergic Signal. 2013;9:5–6. doi: 10.1007/s11302-012-9330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourke J, Abel K, Huxham G, Cooper V, Manley S. UTP-preferring P2 receptor mediates inhibition of sodium transport in porcine thyroid epithelial cells. Br J Pharmacol. 1999;127:1787–1792. doi: 10.1038/sj.bjp.0702733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 49.Braun M, Wendt A, Karanauskaite J, Galvanovskis J, Clark A, MacDonald PE, Rorsman P. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic β cells. J Gen Physiol. 2007;129:221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun M, Ramracheya R, Rorsman P. Autocrine regulation of insulin secretion. Diabetes Obes Metab. 2012;14(Suppl 3):143–151. doi: 10.1111/j.1463-1326.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- 51.Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am J Respir Cell Mol Biol. 2000;23:52–61. doi: 10.1165/ajrcmb.23.1.3936. [DOI] [PubMed] [Google Scholar]
- 52.Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans J-P, Adriaensen D. Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol. 2003;28:275–285. doi: 10.1165/rcmb.2002-0117OC. [DOI] [PubMed] [Google Scholar]
- 53.Bruno AN, Da Silva RS, Bonan CD, Battastini AM, Barreto-Chaves ML, Sarkis JJ. Hyperthyroidism modifies ecto-nucleotidase activities in synaptosomes from hippocampus and cerebral cortex of rats in different phases of development. Int J Dev Neurosci. 2003;21:401–408. doi: 10.1016/s0736-5748(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 54.Bruno AN, Pochmann D, Ricachenevsky FK, Bonan CD, Barreto-Chaves ML, Freitas Sarkis JJ. 5′-nucleotidase activity is altered by hypo- and hyperthyroidism in platelets from adult rats. Platelets. 2005;16:25–30. doi: 10.1080/0953710042000260164. [DOI] [PubMed] [Google Scholar]
- 55.Bruno AN, Ricachenevsky FK, Pochmann D, Bonan CD, Battastini AM, Barreto-Chaves ML, Sarkis JJ. Hypothyroidism changes adenine nucleotide hydrolysis in synaptosomes from hippocampus and cerebral cortex of rats in different phases of development. Int J Dev Neurosci. 2005;23:37–44. doi: 10.1016/j.ijdevneu.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Bruno AN, Carneiro-Ramos MS, Buffon A, Pochmann D, Ricachenevsky FK, Barreto-Chaves ML, Sarkis JJ. Thyroid hormones alter the adenine nucleotide hydrolysis in adult rat blood serum. Biofactors. 2011;37:40–45. doi: 10.1002/biof.133. [DOI] [PubMed] [Google Scholar]
- 57.Buckley KA, Wagstaff SC, McKay G, Gaw A, Hipskind RA, Bilbe G, Gallagher JA, Bowler WB. Parathyroid hormone potentiates nucleotide-induced [Ca2+]i release in rat osteoblasts independently of Gq activation or cyclic monophosphate accumulation. A mechanism for localizing systemic responses in bone. J Biol Chem. 2001;276:9565–9571. doi: 10.1074/jbc.M005672200. [DOI] [PubMed] [Google Scholar]
- 58.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 59.Buljubasich R, Ventura S. Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol. 2004;499:335–344. doi: 10.1016/j.ejphar.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 60.Burghoff S, Bongardt S, Burkart V, Roden M, Flögel U, Schrader J. CD73-derived adenosine modulates lipolysis un vivo. Purinergic Signal. 2012;8:162–163. [Google Scholar]
- 61.Burns JK (1987) Relation between elevated serum 5-nucleotidase in late human pregnancy and duration of labour. Proc Physiol Soc Suppl 392:57P
- 62.Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int. 1990;17:357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- 63.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 64.Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem Int. 2008;52:14–25. doi: 10.1016/j.neuint.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 66.Burnstock G, Novak I. Purinergic signalling in the pancreas in health and disease. J Endocrinol. 2012;213:123–141. doi: 10.1530/JOE-11-0434. [DOI] [PubMed] [Google Scholar]
- 67.Burnstock G, Novak I (2013) Purinergic signalling and diabetes. Purinergic Signalling 9:307–324 [DOI] [PMC free article] [PubMed]
- 68.Burrows GH, Barnea A. Comparison of the effects of ATP, Mg2+, and MgATP on the release of luteinizing hormone-releasing hormone from isolated hypothalamic granules. J Neurochem. 1982;38:569–573. doi: 10.1111/j.1471-4159.1982.tb08664.x. [DOI] [PubMed] [Google Scholar]
- 69.Capito K, Hansen SE, Hedeskov CJ, Thams P. Presence of ATP-pyrophosphohydrolase in mouse pancreatic islets. Diabetes. 1986;35:1096–1100. doi: 10.2337/diab.35.10.1096. [DOI] [PubMed] [Google Scholar]
- 70.Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- 71.Carew MA, Wu M, Law GJ, Tseng YZ, Mason WT. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium. 1994;16:227–235. doi: 10.1016/0143-4160(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 72.Carlsson A, Hillarp N, Hökfelt B. The concomitant release of adenosine triphosphate and catechol amines from the adrenal medulla. J Biol Chem. 1957;227:243–252. [PubMed] [Google Scholar]
- 73.Carneiro-Ramos MS, da Silva VB, Coutinho MB, Jr, Battastini AM, Sarkis JJ, Barreto-Chaves ML. Thyroid hormone stimulates 5′-ecto-nucleotidase of neonatal rat ventricular myocytes. Mol Cell Biochem. 2004;265:195–201. doi: 10.1023/b:mcbi.0000044396.31443.a8. [DOI] [PubMed] [Google Scholar]
- 74.Casey RP, Njus D, Radda GK, Sehr PA. Adenosine triphosphate-evoked catecholamine release in chromatin granules. Osmotic lysis as a consequence of proton translocation. Biochem J. 1976;158:583–588. doi: 10.1042/bj1580583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castillo CJ, Moro MA, Del Valle M, Sillero A, García AG, Sillero MA. Diadenosine tetraphosphate is co-released with ATP and catecholamines from bovine adrenal medulla. J Neurochem. 1992;59:723–732. doi: 10.1111/j.1471-4159.1992.tb09428.x. [DOI] [PubMed] [Google Scholar]
- 76.Castro E, Torres M, Miras-Portugal MT, Gonzalez MP. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990;100:360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castro E, Pintor J, Miras-Portugal MT. Ca2+-stores mobilization by diadenosine tetraphosphate, Ap4A, through a putative P2Y purinoceptor in adrenal chromaffin cells. Br J Pharmacol. 1992;106:833–837. doi: 10.1111/j.1476-5381.1992.tb14421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro E, Tomé AR, Miras-Portugal MT, Rosário LM. Single-cell fura-2 microfluorometry reveals different purinoceptor subtypes coupled to Ca2+ influx and intracellular Ca2+ release in bovine adrenal chromaffin and endothelial cells. Pflugers Arch. 1994;426:524–533. doi: 10.1007/BF00378530. [DOI] [PubMed] [Google Scholar]
- 79.Castro E, Mateo J, Tomé AR, Barbosa RM, Miras-Portugal MT, Rosário LM. Cell-specific purinergic receptors coupled to Ca2+ entry and Ca2+ release from internal stores in adrenal chromaffin cells. Differential sensitivity to UTP and suramin. J Biol Chem. 1995;270:5098–5106. doi: 10.1074/jbc.270.10.5098. [DOI] [PubMed] [Google Scholar]
- 80.Chaldakov GM, Tunçel N, Beltowski J, Fiore M, Rancic G, Tonchev A, Panayotov P, Evtimov N, Hinev A, Anakievski D, Ghenev P, Aloe L. Adipoparacrinology: an emerging field in biomedical research. Balkan Med J. 2012;29:2–9. [Google Scholar]
- 81.Chang KJ, Cuatrecasas P. Adenosine triphosphate-dependent inhibition of insulin-stimulated glucose transport in fat cells. Possible role of membrane phosphorylation. J Biol Chem. 1974;249:3170–3180. [PubMed] [Google Scholar]
- 82.Chapal J, Loubatières-Mariani MM. Attempt to antagonized the stimulatory effect or ATP on insulin secretion. Eur J Pharmacol. 1981;74:127–134. doi: 10.1016/0014-2999(81)90522-7. [DOI] [PubMed] [Google Scholar]
- 83.Chapal J, Loubatieres-Mariani MM, Roye M, Zerbib A. Effects of adenosine, adenosine triphosphate and structural analogues on glucagon secretion from the perfused pancreas of rat in vitro. Br J Pharmacol. 1984;83:927–933. doi: 10.1111/j.1476-5381.1984.tb16533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chapal J, Loubatières-Mariani MM, Petit P, Roye M. Evidence for an A2-subtype adenosine receptor on pancreatic glucagon secreting cells. Br J Pharmacol. 1985;86:565–569. doi: 10.1111/j.1476-5381.1985.tb08932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapal J, Bertrand G, Hillaire-Buys D, Gross R, Loubatièeres-Mariani MM. Prior glucose deprivation increases the first phase of glucose-induced insulin response: possible involvement of endogenous ATP and (or) ADP. Can J Physiol Pharmacol. 1993;71:611–614. doi: 10.1139/y93-086. [DOI] [PubMed] [Google Scholar]
- 86.Chapal J, Hillaire-Buys D, Bertrand G, Pujalte D, Petit P, Loubatieres-Mariani MM. Comparative effects of adenosine-5′-triphosphate and related analogues on insulin secretion from the rat pancreas. Fundam Clin Pharmacol. 1997;11:537–545. doi: 10.1111/j.1472-8206.1997.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 87.Chatterjee C, Sparks DL. Extracellular nucleotides inhibit insulin receptor signaling, stimulate autophagy and control lipoprotein secretion. PLoS One. 2012;7:e36916. doi: 10.1371/journal.pone.0036916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chechik BE, Schrader WP, Minowada J. An immunomorphologic study of adenosine deaminase distribution in human thymus tissue, normal lymphocytes, and hematopoietic cell lines. J Immunol. 1981;126:1003–1007. [PubMed] [Google Scholar]
- 89.Chen L, Maruyama D, Sugiyama M, Sakai T, Mogi C, Kato M, Kurotani R, Shirasawa N, Takaki A, Renner U, Kato Y, Inoue K. Cytological characterization of a pituitary folliculo-stellate-like cell line, Tpit/F1, with special reference to adenosine triphosphate-mediated neuronal nitric oxide synthase expression and nitric oxide secretion. Endocrinology. 2000;141:3603–3610. doi: 10.1210/endo.141.10.7710. [DOI] [PubMed] [Google Scholar]
- 90.Chen YC, Huang SH, Wang SM. Adenosine-stimulated adrenal steroidogenesis involves the adenosine A2A and A2B receptors and the Janus kinase 2-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase signaling pathway. Int J Biochem Cell Biol. 2008;40:2815–2825. doi: 10.1016/j.biocel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 91.Chen ZP, Levy A, McArdle CA, Lightman SL. Pituitary ATP receptors: characterization and functional localization to gonadotropes. Endocrinology. 1994;135:1280–1283. doi: 10.1210/endo.135.3.8070374. [DOI] [PubMed] [Google Scholar]
- 92.Chen ZP, Kratzmeier M, Levy A, McArdle CA, Poch A, Day A, Mukhopadhyay AK, Lightman SL. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc Natl Acad Sci U S A. 1995;92:5219–5223. doi: 10.1073/pnas.92.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen ZP, Kratzeirer M, Poch A, Xu S, McCardle CA, Levy A, Mukhopadhyay AK, Lightman SL. Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived αT3-1 cells. Endocrinology. 1996;137:248–256. doi: 10.1210/endo.137.1.8536620. [DOI] [PubMed] [Google Scholar]
- 94.Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- 95.Cheng JT, Liu IM, Chi TC, Shinozuka K, Lu FH, Wu TJ, Chang CJ. Role of adenosine in insulin-stimulated release of leptin from isolated white adipocytes of Wistar rats. Diabetes. 2000;49:20–24. doi: 10.2337/diabetes.49.1.20. [DOI] [PubMed] [Google Scholar]
- 96.Chern YJ, Herrera M, Kao LS, Westhead EW. Inhibition of catecholamine secretion from bovine chromaffin cells by adenine nucleotides and adenosine. J Neurochem. 1987;48:1573–1576. doi: 10.1111/j.1471-4159.1987.tb05703.x. [DOI] [PubMed] [Google Scholar]
- 97.Cheung KK, Coutinho-Silva R, Chan WY, Burnstock G. Early expression of adenosine 5′-triphosphate-gated P2X7 receptors in the developing rat pancreas. Pancreas. 2007;35:164–168. doi: 10.1097/MPA.0b013e318053e00d. [DOI] [PubMed] [Google Scholar]
- 98.Chevassus H, Roig A, Belloc C, Lajoix AD, Broca C, Manteghetti M, Petit P. P2Y receptor activation enhances insulin release from pancreatic beta-cells by triggering the cyclic AMP/protein kinase A pathway. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:464–469. doi: 10.1007/s00210-002-0620-4. [DOI] [PubMed] [Google Scholar]
- 99.Choi JY, Namkung W, Shin JH, Yoon JH. Uridine-5′-triphosphate and adenosine triphosphate γS induce mucin secretion via Ca2+-dependent pathways in human nasal epithelial cells. Acta Otolaryngol. 2003;123:1080–1086. doi: 10.1080/00016480310002528. [DOI] [PubMed] [Google Scholar]
- 100.Chowdhury HH, Grilc S, Zorec R. Correlated ATP-induced changes in membrane area and membrane conductance in single rat adipocytes. Ann N Y Acad Sci. 2005;1048:281–286. doi: 10.1196/annals.1342.025. [DOI] [PubMed] [Google Scholar]
- 101.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca2+]i changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–1852. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chused TM, Apasov S, Sitkovsky M. Murine T lymphocytes modulate activity of an ATP-activated P2Z-type purinoceptor during differentiation. J Immunol. 1996;157:1371–1380. [PubMed] [Google Scholar]
- 103.Chvatchko Y, Valera S, Aubry JP, Renno T, Buell G, Bonnefoy JY. The involvement of an ATP-gated ion channel, P2X1, in thymocyte apoptosis. Immunity. 1996;5:275–283. doi: 10.1016/s1074-7613(00)80322-2. [DOI] [PubMed] [Google Scholar]
- 104.Cidon S, Tamir H, Nunez EA, Gershon MD. ATP-dependent uptake of 5-hydroxytryptamine by secretory granules isolated from thyroid parafollicular cells. J Biol Chem. 1991;266:4392–4400. [PubMed] [Google Scholar]
- 105.Colldén G, Mangano C, Meister B. P2X2 purinoreceptor protein in hypothalamic neurons associated with the regulation of food intake. Neuroscience. 2010;171:62–78. doi: 10.1016/j.neuroscience.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 106.Conigrave AD, Delbridge L, Cook DI. Extracellular ATP elevates cytosolic free Ca2+ concentration in human parathyroid cells. Proc Aust Physiol Pharmacol Soc. 1992;23:60P. [Google Scholar]
- 107.Courageot MP, Lépine S, Giraud F, Sulpice JC. Extracellular ATP induces phosphatidylserine externalization earlier than nuclear apoptotic events in thymocytes. Ann N Y Acad Sci. 2002;973:186–189. doi: 10.1111/j.1749-6632.2002.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 108.Coutinho-Silva R, Alves LA, de Carvalho AC, Savino W, Persechini PM. Characterization of P2Z purinergic receptors on phagocytic cells of the thymic reticulum in culture. Biochim Biophys Acta. 1996;1280:217–222. doi: 10.1016/0005-2736(95)00293-6. [DOI] [PubMed] [Google Scholar]
- 109.Coutinho-Silva R, Parsons M, Robson T, Burnstock G. Changes in expression of P2 receptors in rat and mouse pancreas during development and aging. Cell Tissue Res. 2001;306:373–383. doi: 10.1007/s004410100458. [DOI] [PubMed] [Google Scholar]
- 110.Coutinho-Silva R, Parsons M, Robson T, Lincoln J, Burnstock G. P2X and P2Y purinoceptor expression in pancreas from streptozotocin-diabetic rats. Mol Cell Endocrinol. 2003;204:141–154. doi: 10.1016/s0303-7207(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 111.Crist GH, Xu B, Lanoue KF, Lang CH. Tissue-specific effects of in vivo adenosine receptor blockade on glucose uptake in Zucker rats. FASEB J. 1998;12:1301–1308. doi: 10.1096/fasebj.12.13.1301. [DOI] [PubMed] [Google Scholar]
- 112.Crowe R, Whitear M. Quinacrine fluorescence of Merkel cells in Xenopus laevis. Cell Tissue Res. 1978;190:273–283. doi: 10.1007/BF00218175. [DOI] [PubMed] [Google Scholar]
- 113.Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 114.Custer EE, Knott TK, Cuadra AE, Ortiz-Miranda S, Lemos JR. P2X purinergic receptor knockout mice reveal endogenous ATP modulation of both vasopressin and oxytocin release from the intact neurohypophysis. J Neuroendocrinol. 2012;24:674–680. doi: 10.1111/j.1365-2826.2012.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.D'Albora H, Lombide P, Ojeda SR. Intrinsic neurons in the rat ovary: an immunohistochemical study. Cell Tissue Res. 2000;300:47–56. doi: 10.1007/s004419900130. [DOI] [PubMed] [Google Scholar]
- 116.da Silva MCJ, Cabrera O, Ricordi C, Berggren PO, Caicedo A. Extracellular ATP is a positive autocrine signal for insulin release in the human pancreatic beta-cell. FASEB J. 2007;21:A829–A830. [Google Scholar]
- 117.Davidson JS, Wakefield IK, Sohnius U, van der Merwe PA, Millar RP. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990;126:80–87. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- 118.Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–344. doi: 10.1016/0006-8993(93)91528-z. [DOI] [PubMed] [Google Scholar]
- 119.de Gasparo M, Krinke G, Milner GR, Milner RD. Influence of autonomic innervation on the foetal rat pancreas in vitro. J Endocrinol. 1978;79:49–58. doi: 10.1677/joe.0.0790049. [DOI] [PubMed] [Google Scholar]
- 120.de Meis L, Arruda AP, da Costa RM, Benchimol M. Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+ J Biol Chem. 2006;281:16384–16390. doi: 10.1074/jbc.M600678200. [DOI] [PubMed] [Google Scholar]
- 121.Delahunty TM, Cronin MJ, Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988;255:69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Delbeke D, Van Sande J, Cochaux P, Decoster C, Dumont JE. Effect of thyrotropin-releasing hormone on dog thyroid in vitro. Biochim Biophys Acta. 1983;761:262–268. doi: 10.1016/0304-4165(83)90075-2. [DOI] [PubMed] [Google Scholar]
- 123.Dhalla AK, Chisholm JW, Reaven GM, Belardinelli L (2009a) A1 adenosine receptor: role in diabetes and obesity. Handb Exp Pharmacol 193:271–295 [DOI] [PubMed]
- 124.Dhalla AK, Santikul M, Chisholm JW, Belardinelli L, Reaven GM. Comparison of the antilipolytic effects of an A1 adenosine receptor partial agonist in normal and diabetic rats. Diabetes Obes Metab. 2009;11:95–101. doi: 10.1111/j.1463-1326.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 125.Di Jeso B, Laviola L, Liguoro D, Formisano S, Consiglio E. P2 purinergic agonists and 12-O-tetradecanoylphorbol-13-acetate, as well as protein kinase A activators, stimulate thyroglobulin secretion in FRTL-5 cells. Biochem Biophys Res Commun. 1993;191:385–391. doi: 10.1006/bbrc.1993.1229. [DOI] [PubMed] [Google Scholar]
- 126.Divald A, Karl PI, Fisher SE. Regulation of phospholipase D in human placental trophoblasts by the P2 purinergic receptor. Placenta. 2002;23:584–593. doi: 10.1053/plac.2002.0844. [DOI] [PubMed] [Google Scholar]
- 127.Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:1261–1265. doi: 10.1073/pnas.88.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. [PubMed] [Google Scholar]
- 129.Dong Q, Ginsberg HN, Erlanger BF. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes Metab. 2001;3:360–366. doi: 10.1046/j.1463-1326.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- 130.Drakulich DA, Walls AM, Toews ML, Hexum TD. Neuropeptide Y receptor-mediated sensitization of ATP-stimulated inositol phosphate formation. J Pharmacol Exp Ther. 2003;307:559–565. doi: 10.1124/jpet.103.053082. [DOI] [PubMed] [Google Scholar]
- 131.Drakulich DA, Spellmon C, Hexum TD. Effect of the ecto-ATPase inhibitor, ARL 67156, on the bovine chromaffin cell response to ATP. Eur J Pharmacol. 2004;485:137–140. doi: 10.1016/j.ejphar.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 132.Drews G, Krippeit-Drews P, Dufer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–163. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- 133.Dubyak GR. Focus on “multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells”. Am J Physiol. 1999;277:C202–C204. doi: 10.1152/ajpcell.1999.277.2.C202. [DOI] [PubMed] [Google Scholar]
- 134.Edwards AV, Jones CT. Autonomic control of adrenal function. J Anat. 1993;183:291–307. [PMC free article] [PubMed] [Google Scholar]
- 135.Ekelund M, Ahren B, Håkanson R, Lundquist I, Sundler F. Quinacrine accumulates in certain peptide hormone-producing cells. Histochemistry. 1980;66:1–9. doi: 10.1007/BF00493240. [DOI] [PubMed] [Google Scholar]
- 136.Ekokoski E, Dugué B, Vainio M, Vainio PJ, Törnquist K. Extracellular ATP-mediated phospholipase A2 activation in rat thyroid FRTL-5 cells: regulation by a Gi /Go protein, Ca2+, and mitogen-activated protein kinase. J Cell Physiol. 2000;183:155–162. doi: 10.1002/(SICI)1097-4652(200005)183:2<155::AID-JCP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 137.Ekokoski E, Webb TE, Simon J, Tornquist K. Mechanisms of P2 receptor-evoked DNA synthesis in thyroid FRTL-5 cells. J Cell Physiol. 2001;187:166–175. doi: 10.1002/jcp.1070. [DOI] [PubMed] [Google Scholar]
- 138.el-Moatassim C, Dornand J, Mani JC. Extracellular ATP increases cytosolic free calcium in thymocytes and initiates the blastogenesis of the phorbol 12-myristate 13-acetate-treated medullary population. Biochim Biophys Acta. 1987;927:437–444. doi: 10.1016/0167-4889(87)90110-8. [DOI] [PubMed] [Google Scholar]
- 139.el-Moatassim C, Bernad N, Mani JC, Dornand J. Extracellular ATP induces a nonspecific permeability of thymocyte plasma membranes. Biochem Cell Biol. 1989;67:495–502. doi: 10.1139/o89-080. [DOI] [PubMed] [Google Scholar]
- 140.Elia MG, Muscella A, Greco S, Vilella S, Storelli C, Marsigliante S. Disturbances in purinergic [Ca2+]i signaling pathways in a transformed rat thyroid cell line. Cell Calcium. 2003;33:59–68. doi: 10.1016/s0143-4160(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 141.Elia MG, Muscella A, Romano S, Greco S, Di Jeso B, Verri T, Storelli C, Marsigliante S. Effects of extracellular nucleotides in the thyroid: P2Y2 receptor-mediated ERK1/2 activation and c-Fos induction in PC Cl3 cells. Cell Signal. 2005;17:739–749. doi: 10.1016/j.cellsig.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 142.Ennion SJ, Powell AD, Seward EP. Identification of the P2Y12 receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol. 2004;66:601–611. doi: 10.1124/mol.104.000224. [DOI] [PubMed] [Google Scholar]
- 143.Espallergues J, Solovieva O, Técher V, Bauer K, Alonso G, Vincent A, Hussy N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience. 2007;148:712–723. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 144.Fain JN, Pointer RH, Ward WF. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972;247:6866–6872. [PubMed] [Google Scholar]
- 145.Falcón J, Brun-Marmillon J, Claustrat B, Collin JP. Melatonin production in organ cultured chicken pineal: modulation by adenosine and its analogs. Pflugers Arch. 1988;413:93–95. doi: 10.1007/BF00581234. [DOI] [PubMed] [Google Scholar]
- 146.Falcón J, Privat K, Ravault JP. Binding of an adenosine A1 receptor agonist and adenosine A1 receptor antagonist to sheep pineal membranes. Eur J Pharmacol. 1997;337:325–331. doi: 10.1016/s0014-2999(97)01305-8. [DOI] [PubMed] [Google Scholar]
- 147.Farret A, Filhol R, Linck N, Manteghetti M, Vignon J, Gross R, Petit P. P2Y receptor mediated modulation of insulin release by a novel generation of 2-substituted-5′-O-(1-boranotriphosphate)-adenosine analogues. Pharm Res. 2006;23:2665–2671. doi: 10.1007/s11095-006-9112-4. [DOI] [PubMed] [Google Scholar]
- 148.Fatholahi M, Xiang Y, Wu Y, Li Y, Wu L, Dhalla AK, Belardinelli L, Shryock JC. A novel partial agonist of the A1-adenosine receptor and evidence of receptor homogeneity in adipocytes. J Pharmacol Exp Ther. 2006;317:676–684. doi: 10.1124/jpet.105.099119. [DOI] [PubMed] [Google Scholar]
- 149.Faulhaber-Walter R, Jou W, Mizel D, Li L, Zhang J, Kim SM, Huang Y, Chen M, Briggs JP, Gavrilova O, Schnermann JB. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes. 2011;60:2578–2587. doi: 10.2337/db11-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feldman JM, Jackson TB. Specificity of nucleotide-induced insulin secretion. Endocrinology. 1974;94:388–394. doi: 10.1210/endo-94-2-388. [DOI] [PubMed] [Google Scholar]
- 151.Feliu MS, Slobodianik NH. Protein feeding and the activity of adenosine deaminase and purine nucleoside phosphorylase in rat thymus. Nutr Res. 1998;18:1973–1979. [Google Scholar]
- 152.Ferguson SE, Pallikaros Z, Michael AE, Cooke BA. The effects of different culture media, glucose, pyridine nucleotides and adenosine on the activity of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Mol Cell Endocrinol. 1999;158:37–44. doi: 10.1016/s0303-7207(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 153.Fernandez-Alvarez J, Hillaire-Buys D, Loubatieres-Mariani MM, Gomis R, Petit P. P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas. 2001;22:69–71. doi: 10.1097/00006676-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 154.Ferreira ZS, Markus RP. Characterisation of P2Y1-like receptor in cultured rat pineal glands. Eur J Pharmacol. 2001;415:151–156. doi: 10.1016/s0014-2999(01)00823-8. [DOI] [PubMed] [Google Scholar]
- 155.Ferreira ZS, Cipolla-Neto J, Markus RP. Presence of P2-purinoceptors in the rat pineal gland. Br J Pharmacol. 1994;112:107–110. doi: 10.1111/j.1476-5381.1994.tb13037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ferreira ZS, Garcia CR, Spray DC, Markus RP. P2Y1 receptor activation enhances the rate of rat pinealocyte-induced extracellular acidification via a calcium-dependent mechanism. Pharmacology. 2003;69:33–37. doi: 10.1159/000071264. [DOI] [PubMed] [Google Scholar]
- 157.Filippini A, Riccioli A, De Cesaris P, Paniccia R, Teti A, Stefanini M. Activation of inositol phospholipid turnover and calcium signaling in rat sertoli cells be P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 1994;134:1537–1545. doi: 10.1210/endo.134.3.8119196. [DOI] [PubMed] [Google Scholar]
- 158.Filkins JP. Effects of exogenous ATP on glucoregulation in vivo. Proc Soc Exp Biol Med. 1978;158:554–556. doi: 10.3181/00379727-158-40244. [DOI] [PubMed] [Google Scholar]
- 159.Fischer B, Chulkin A, Boyer JL, Harden KT, Gendron FP, Beaudoin AR, Chapal J, Hillaire-Buys D, Petit P. 2-Thioether 5′-O-(1-thiotriphosphate)adenosine derivatives as new insulin secretagogues acting through P2Y-Receptors. J Med Chem. 1999;42:3636–3646. doi: 10.1021/jm990158y. [DOI] [PubMed] [Google Scholar]
- 160.Florenzano F, Viscomi MT, Mercaldo V, Longone P, Bernardi G, Bagni C, Molinari M, Carrive P. P2X2R purinergic receptor subunit mRNA and protein are expressed by all hypothalamic hypocretin/orexin neurons. J Comp Neurol. 2006;498:58–67. doi: 10.1002/cne.21013. [DOI] [PubMed] [Google Scholar]
- 161.Florio T. Adult pituitary stem cells: from pituitary plasticity to adenoma development. Neuroendocrinology. 2011;94:265–277. doi: 10.1159/000330857. [DOI] [PubMed] [Google Scholar]
- 162.Foresta C, Rossato M, Bordon P, Di Virgilio F. Extracellular ATP activates different signalling pathways in rat Sertoli cells. Biochem J. 1995;311:269–274. doi: 10.1042/bj3110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Foresta C, Rossato M, Nogara A, Gottardello F, Bordon P, Di Virgilio F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem J. 1996;320:499–504. doi: 10.1042/bj3200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forsberg EJ, Feuerstein G, Shohami E, Pollard HB. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987;84:5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fouchier F, Mego JL, Dang J, Simon C. ATP-induced stimulation of the intralysosomal hydrolysis of thyroglobulin. Evidence for an ATP-driven proton pump in thyroid lysosomes. Horm Metab Res. 1984;16:359–362. doi: 10.1055/s-2007-1014790. [DOI] [PubMed] [Google Scholar]
- 166.Fradkin JE, Hardy W, Wolff J. Adenosine receptor-mediated accumulation of adenosine 3′,5′-monophosphate in guinea pig thyroid tissue. Endocrinology. 1982;110:2018–2023. doi: 10.1210/endo-110-6-2018. [DOI] [PubMed] [Google Scholar]
- 167.Fredholm BB. Adenosine and lipolysis. Int J Obes. 1981;5:643–649. [PubMed] [Google Scholar]
- 168.Fredholm BB, Sandberg G. Inhibition by xanthine derivatives of adenosine receptor-stimulated cyclic adenosine 3′,5′-monophosphate accumulation in rat and guinea-pig thymocytes. Br J Pharmacol. 1983;80:639–644. doi: 10.1111/j.1476-5381.1983.tb10053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fredholm BB, Sollevi A. Antilipolytic effect of adenosine in dog adipose tissue in situ. Acta Physiol Scand. 1977;99:254–256. doi: 10.1111/j.1748-1716.1977.tb10377.x. [DOI] [PubMed] [Google Scholar]
- 170.Fredholm BB, Vernet L. Accumulation and inactivation of adenosine by fat cells from hypothyroid rats. Acta Physiol Scand. 1984;121:155–163. doi: 10.1111/j.1748-1716.1984.tb07442.x. [DOI] [PubMed] [Google Scholar]
- 171.Fredholm BB, Belfrage E, Blaschke E. Changes in ATP and cyclic nucleotide levels during sympathetic nerve stimulation in canine subcutaneous adipose tissue in situ. Acta Physiol Scand. 1977;99:313–322. doi: 10.1111/j.1748-1716.1977.tb10384.x. [DOI] [PubMed] [Google Scholar]
- 172.Fredholm BB, Sandberg G, Ernström U. Cyclic AMP in freshly prepared thymocyte suspensions, evidence for stimulation by endogenous adenosine. Biochem Pharmacol. 1978;27:2675–2682. doi: 10.1016/0006-2952(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 173.Freedman BD, Liu QH, Gaulton G, Kotlikoff MI, Hescheler J, Fleischmann BK. ATP-evoked Ca2+ transients and currents in murine thymocytes: possible role for P2X receptors in death by neglect. Eur J Immunol. 1999;29:1635–1646. doi: 10.1002/(SICI)1521-4141(199905)29:05<1635::AID-IMMU1635>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 174.Frühbeck G, Gómez-Ambrosi J, Salvador J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001;15:333–340. doi: 10.1096/fj.00-0249com. [DOI] [PubMed] [Google Scholar]
- 175.Fu J, Yu Q, Guo W, He C, Burnstock G, Xiang Z. P2X receptors are expressed on the neurons containing luteinizing hormone-releasing hormone in the mouse hypothalamus. Neurosci Lett. 2009;458:32–36. doi: 10.1016/j.neulet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 176.Fujita R, Kimura S, Kawasaki S, Takashima K, Matsumoto M, Hirano H, Sasaki K. ATP suppresses the K+ current responses to FSH and adenosine in the follicular cells of Xenopus oocyte. Jpn J Physiol. 2001;51:491–500. doi: 10.2170/jjphysiol.51.491. [DOI] [PubMed] [Google Scholar]
- 177.Gaidhu MP, Ceddia RB. The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exerc Sport Sci Rev. 2011;39:102–108. doi: 10.1097/JES.0b013e31820ac03e. [DOI] [PubMed] [Google Scholar]
- 178.Galardo MN, Riera MF, Pellizzari EH, Sobarzo C, Scarcelli R, Denduchis B, Lustig L, Cigorraga SB, Meroni SB. Adenosine regulates Sertoli cell function by activating AMPK. Mol Cell Endocrinol. 2010;330:49–58. doi: 10.1016/j.mce.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 179.García-Sáinz JA, Torner ML. Rat fat-cells have three types of adenosine receptors (Ra, Ri and P). Differential effects of pertussis toxin. Biochem J. 1985;232:439–443. doi: 10.1042/bj2320439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gavin KM, Geyer GH, LaFavor JD, Hickner RC, Choi MD. Adenosine suppression of in-vivo lipolysis in obese premenopausal women. Med Sci Sports Sci. 2010;42:565. [Google Scholar]
- 181.Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology. 2013;154:675–684. doi: 10.1210/en.2012-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gharib A, Reynaud D, Sarda N, Vivien-Roels B, Pévet P, Pacheco H. Adenosine analogs elevate N-acetylserotonin and melatonin in rat pineal gland. Neurosci Lett. 1989;106:345–349. doi: 10.1016/0304-3940(89)90188-2. [DOI] [PubMed] [Google Scholar]
- 183.Gharib A, Delton I, Lagarde M, Sarda N. Evidence for adenosine A2b receptors in the rat pineal gland. Eur J Pharmacol. 1992;225:359–360. doi: 10.1016/0922-4106(92)90113-a. [DOI] [PubMed] [Google Scholar]
- 184.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26:2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 185.Gharibi B, Abraham AA, Ham J, Evans BA. Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes (Lond) 2012;36:397–406. doi: 10.1038/ijo.2011.129. [DOI] [PubMed] [Google Scholar]
- 186.Giniatullin RA, Sokolova EM, Di AS, Skorinkin A, Talantova MV, Nistri A. Rapid relief of block by mecamylamine of neuronal nicotinic acetylcholine receptors of rat chromaffin cells in vitro: an electrophysiological and modeling study. Mol Pharmacol. 2000;58:778–787. doi: 10.1124/mol.58.4.778. [DOI] [PubMed] [Google Scholar]
- 187.Githens S. Localization of alkaline phosphatase and adenosine triphosphatase in the mammalian pancreas. J Histochem Cytochem. 1983;31:697–705. doi: 10.1177/31.5.6221048. [DOI] [PubMed] [Google Scholar]
- 188.Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K. Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetologia. 2009;52:1579–1588. doi: 10.1007/s00125-009-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Glass R, Burnstock G. Immunohistochemical identification of cells expressing ATP-gated cation channels (P2X receptors) in the adult rat thyroid. J Anat. 2001;198:569–579. doi: 10.1046/j.1469-7580.2001.19850569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Glass R, Townsend-Nicholson A, Burnstock G. P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res. 2000;300:295–306. doi: 10.1007/s004410000206. [DOI] [PubMed] [Google Scholar]
- 191.Glass R, Bardini M, Robson T, Burnstock G. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells Tissues Organs. 2001;169:377–387. doi: 10.1159/000047905. [DOI] [PubMed] [Google Scholar]
- 192.Gnad T, Mutlu S, Müller CE, Pfeifer A. The biological role of adenosine receptors in brown adipose tissue. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:S29. [Google Scholar]
- 193.Gomes DA, Song Z, Stevens W, Sladek CD. Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R940–R949. doi: 10.1152/ajpregu.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gong Q, Kakei M, Koriyama N, Nakazaki M, Morimitsu S, Yaekura K, Tei C. P2Y-purinoceptor mediated inhibition of L-type Ca2+ channels in rat pancreatic β-cells. Cell Struct Funct. 2000;25:279–289. doi: 10.1247/csf.25.279. [DOI] [PubMed] [Google Scholar]
- 195.Goren HJ, Hanif K, Dudley R, Hollenberg MD, Lederis K. Adenosine modulation of fat cell responsiveness to insulin and oxytocin. Regul Pept. 1986;16:125–134. doi: 10.1016/0167-0115(86)90056-x. [DOI] [PubMed] [Google Scholar]
- 196.Grapengiesser E, Dansk H, Hellman B. Pulses of external ATP aid to the synchronization of pancreatic beta-cells by generating premature Ca2+ oscillations. Biochem Pharmacol. 2004;68:667–674. doi: 10.1016/j.bcp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 197.Grapengiesser E, Dansk H, Hellman B. External ATP triggers Ca2+ signals suited for synchronization of pancreatic β-cells. J Endocrinol. 2005;185:69–79. doi: 10.1677/joe.1.06040. [DOI] [PubMed] [Google Scholar]
- 198.Grapengiesser E, Salehi A, Qader SS, Hellman B. Glucose induces glucagon release pulses antisynchronous with insulin and sensitive to purinoceptor inhibition. Endocrinology. 2006;147:3472–3477. doi: 10.1210/en.2005-1431. [DOI] [PubMed] [Google Scholar]
- 199.Green A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1987;262:15702–15707. [PubMed] [Google Scholar]
- 200.Green A, Swenson S, Johnson JL, Partin M. Characterization of human adipocyte adenosine receptors. Biochem Biophys Res Commun. 1989;163:137–142. doi: 10.1016/0006-291x(89)92110-4. [DOI] [PubMed] [Google Scholar]
- 201.Green ST. Intrathyroidal autonomic nerves can directly influence hormone release from rat thyroid follicles: a study in vitro employing electrical field stimulation and intracellular microelectrodes. Clin Sci (Lond) 1987;72:233–238. doi: 10.1042/cs0720233. [DOI] [PubMed] [Google Scholar]
- 202.Gregory S, Kern M. Adenosine and adenine nucleotides are mitogenic for mouse thymocytes. Biochem Biophys Res Commun. 1978;83:1111–1116. doi: 10.1016/0006-291x(78)91510-3. [DOI] [PubMed] [Google Scholar]
- 203.Gross R, Bertrand G, Ribes G, Loubatières-Mariani MM. α2-Adrenergic potentiation of adenosine-stimulating effect on glucagon secretion. Endocrinology. 1987;121:765–769. doi: 10.1210/endo-121-2-765. [DOI] [PubMed] [Google Scholar]
- 204.Grunditz T, Hakanson R, Sundler F, Uddman R. Neuronal pathways to the rat thyroid revealed by retrograde tracing and immunocytochemistry. Neuroscience. 1988;24:321–335. doi: 10.1016/0306-4522(88)90334-x. [DOI] [PubMed] [Google Scholar]
- 205.Gualix J, Abal M, Pintor J, Garcia-Carmona F, Miras-Portugal MT. Nucleotide vesicular transporter of bovine chromaffin granules. Evidence for a mnemonic regulation. J Biol Chem. 1996;271:1957–1965. doi: 10.1074/jbc.271.4.1957. [DOI] [PubMed] [Google Scholar]
- 206.Guo W, Sun J, Xu X, Burnstock G, He C, Xiang Z. P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurons in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem Cell Biol. 2009;131:29–41. doi: 10.1007/s00418-008-0493-9. [DOI] [PubMed] [Google Scholar]
- 207.Gylfe E, Grapengiesser E, Dansk H, Hellman B. The neurotransmitter ATP triggers Ca2+ responses promoting coordination of pancreatic islet oscillations. Pancreas. 2012;41:258–263. doi: 10.1097/MPA.0b013e3182240586. [DOI] [PubMed] [Google Scholar]
- 208.Hall AK, Preston SL, Behrman HR. Purine amplification of luteinizing hormone action in ovarian luteal cells. J Biol Chem. 1981;256:10390–10398. [PubMed] [Google Scholar]
- 209.Halonen J, Nedergaard J. Adenosine 5′-monophosphate is a selective inhibitor of the brown adipocyte nonselective cation channel. J Membr Biol. 2002;188:183–197. doi: 10.1007/s00232-001-0184-0. [DOI] [PubMed] [Google Scholar]
- 210.Halperin ML, Mak ML, Taylor WM. Control of glucose transport in adipose tissue of the rat: role of insulin, ATP, and intracellular metabolites. Can J Biochem. 1978;56:708–712. doi: 10.1139/o78-106. [DOI] [PubMed] [Google Scholar]
- 211.Hamlyn JM, Senior AE. Evidence that Mg2+- or Ca2+-activated adenosine triphosphatase in rat pancreas is a plasma-membrane ecto-enzyme. Biochem J. 1983;214:59–68. doi: 10.1042/bj2140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Harii N, Endo T, Ohmori M, Onaya T. Extracellular adenosine increases Na+/I- symporter gene expression in rat thyroid FRTL-5 cells. Mol Cell Endocrinol. 1999;157:31–39. doi: 10.1016/s0303-7207(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 213.Harkins AB, Fox AP. Activation of purinergic receptors by ATP inhibits secretion in bovine adrenal chromaffin cells. Brain Res. 2000;885:231–239. doi: 10.1016/s0006-8993(00)02952-8. [DOI] [PubMed] [Google Scholar]
- 214.Harper F, Lamy F, Calvert R. Some properties of a Ca2 + − and (or) Mg2 + −requiring nucleoside di- and tri-phosphatase(s) associated with the membranes of rat pancreatic zymogen granules. Can J Biochem. 1978;56:565–576. doi: 10.1139/o78-086. [DOI] [PubMed] [Google Scholar]
- 215.Hashimoto N, Robinson FW, Shibata Y, Flanagan JE, Kono T. Diversity in the effects of extracellular ATP and adenosine on the cellular processing and physiologic actions of insulin in rat adipocytes. J Biol Chem. 1987;262:15026–15032. [PubMed] [Google Scholar]
- 216.Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 217.He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem. 2003;278:46270–46277. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- 218.He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1–3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hellman B. Pulsatility of insulin release–a clinically important phenomenon. Ups J Med Sci. 2009;114:193–205. doi: 10.3109/03009730903366075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hellman B, Lernmark A. Inhibition of the in vitro secretion of insulin by an extract of pancreatic α1−cells. Endocrinology. 1969;84:1484–1488. doi: 10.1210/endo-84-6-1484. [DOI] [PubMed] [Google Scholar]
- 221.Hellman B, Dansk H, Grapengiesser E. Pancreatic β-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab. 2004;286:E759–E765. doi: 10.1152/ajpendo.00452.2003. [DOI] [PubMed] [Google Scholar]
- 222.Hellman B, Jansson L, Dansk H, Grapengiesser E. Effects of external ATP on Ca2+ signalling in endothelial cells isolated from mouse islets. Endocrine. 2007;32:33–40. doi: 10.1007/s12020-007-9004-3. [DOI] [PubMed] [Google Scholar]
- 223.Henquin JC, Meissner HP. Effects of theophylline and dibutyryl cyclic adenosine monophosphate on the membrane potential of mouse pancreatic β-cells. J Physiol. 1984;351:595–612. doi: 10.1113/jphysiol.1984.sp015265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Henry SL, Bensley JG, Wood-Bradley RJ, Cullen-McEwen LA, Bertram JF, Armitage JA. White adipocytes: more than just fat depots. Int J Biochem Cell Biol. 2012;44:435–440. doi: 10.1016/j.biocel.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 225.Hernández A, Segura-Chama P, Jiménez N, García AG, Hernández-Guijo JM, Hernández-Cruz A. Modulation by endogenously released ATP and opioids of chromaffin cell calcium channels in mouse adrenal slices. Am J Physiol Cell Physiol. 2011;300:C610–C623. doi: 10.1152/ajpcell.00380.2010. [DOI] [PubMed] [Google Scholar]
- 226.Hillaire-Buys D, Bertrand G, Gross R, Loubatières-Mariani MM. Evidence for an inhibitory A1 subtype adenosine receptor on pancreatic insulin-secreting cells. Eur J Pharmacol. 1987;136:109–112. doi: 10.1016/0014-2999(87)90786-2. [DOI] [PubMed] [Google Scholar]
- 227.Hillaire-Buys D, Bertrand G, Chapal J, Puech R, Ribes G, Loubatières-Mariani MM. Stimulation of insulin secretion and improvement of glucose tolerance in rat and dog by the P2y-purinoceptor agonist, adenosine-5′-O-(2-thiodiphosphate) Br J Pharmacol. 1993;109:183–187. doi: 10.1111/j.1476-5381.1993.tb13551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hillaire-Buys D, Bertrand G, Petit P, Loubatières-Mariani MM. Purinergic receptors on insulin-secreting cells. Fundam Clin Pharmacol. 1994;8:117–127. doi: 10.1111/j.1472-8206.1994.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 229.Hillaire-Buys D, Gross R, Pares-Herbute N, Ribes G, Loubatieres-Mariani MM. In vivo and in vitro effects of adenosine-5′-O-(2-thiodiphosphate) on pancreatic hormones in dogs. Pancreas. 1994;9:646–651. [PubMed] [Google Scholar]
- 230.Hillaire-Buys D, Shahar L, Fischer B, Chulkin A, Linck N, Chapal J, Loubatiéres-Mariani MM, Petit P. Pharmacological evaluation and chemical stability of 2-benzylthioether-5′-O-(1-thiotriphosphate)-adenosine, a new insulin secretagogue acting through P2Y receptors. Drug Dev Res. 2001;53:33–43. [Google Scholar]
- 231.Hillarp NA, Thieme G. Nucleotides in the catechol amine granules of the adrenal medulla. Acta Physiol Scand. 1959;45:328–338. doi: 10.1111/j.1748-1716.1959.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 232.Hillarp NA, Nilson B, Högberg B. Adenosine triphosphate in the adrenal medulla of the cow. Nature. 1955;176:1032–1033. doi: 10.1038/1761032a0. [DOI] [PubMed] [Google Scholar]
- 233.Hiruma H, Bourque CW. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol. 1995;489:805–811. doi: 10.1113/jphysiol.1995.sp021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hjemdahl P, Fredholm BB. Cyclic AMP-dependent and independent inhibition of lipolysis by adenosine and decreased pH. Acta Physiol Scand. 1976;96:170–179. doi: 10.1111/j.1748-1716.1976.tb10186.x. [DOI] [PubMed] [Google Scholar]
- 235.Hoey ED, Nicol M, Williams BC, Walker SW. Primary cultures of bovine inner zone adrenocortical cells secrete cortisol in response to adenosine triphosphate, adenosine diphosphate, and uridine triphosphate via a nucleotide receptor which may be coupled to two signal generation systems. Endocrinology. 1994;134:1553–1560. doi: 10.1210/endo.134.3.8119198. [DOI] [PubMed] [Google Scholar]
- 236.Hoffman PG, Zinder O, Nikodijevic O, Pollard HB. ATP-stimulated transmitter release and cyclic AMP synthesis in isolated chromaffin granules. J Supramol Struct. 1976;4:181–184. doi: 10.1002/jss.400040205. [DOI] [PubMed] [Google Scholar]
- 237.Hollins B, Ikeda SR. Heterologous expression of a P2x-purinoceptor in rat chromaffin cells detects vesicular ATP release. J Neurophysiol. 1997;78:3069–3076. doi: 10.1152/jn.1997.78.6.3069. [DOI] [PubMed] [Google Scholar]
- 238.Holst JJ. Neural regulation of pancreatic exocrine function. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas. Biology, pathobilogy, and disease. New York: Raven Press; 1993. pp. 381–402. [Google Scholar]
- 239.Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ismail NA, El Denshary EE, Montague W. Adenosine and the regulation of insulin secretion by isolated rat islets of Langerhans. Biochem J. 1977;164:409–413. doi: 10.1042/bj1640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Izaguirre V, Fernández-Fernández JM, Ceña V, González-García C. Tricyclic antidepressants block cholinergic nicotinic receptors and ATP secretion in bovine chromaffin cells. FEBS Lett. 1997;418:39–42. doi: 10.1016/s0014-5793(97)01343-4. [DOI] [PubMed] [Google Scholar]
- 242.Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM, Kenyon NS, Ricordi C, Pileggi A, Molano RD, Berggren PO, Caicedo A. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic β cell. Proc Natl Acad Sci U S A. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jankowski J, Jankowski V, Seibt B, Henning L, Zidek W, Schlüter H. Identification of dinucleoside polyphosphates in adrenal glands. Biochem Biophys Res Commun. 2003;304:365–370. doi: 10.1016/s0006-291x(03)00596-5. [DOI] [PubMed] [Google Scholar]
- 244.Jensen EC, Bennet L, Fraser M, Power GG, Hunter CJ, Gunn AJ. Adenosine A1 receptor mediated suppression of adrenal activity in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2010;298:R700–R706. doi: 10.1152/ajpregu.00474.2009. [DOI] [PubMed] [Google Scholar]
- 245.Johansson SM, Salehi A, Sandstrom ME, Westerblad H, Lundquist I, Carlsson PO, Fredholm BB, Katz A. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem Pharmacol. 2007;74:1628–1635. doi: 10.1016/j.bcp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 246.Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol. 2008;597:92–101. doi: 10.1016/j.ejphar.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 247.Jurányi Z, Orsó E, Jánossy A, Szalay KS, Sperlágh B, Windisch K, Vinson GP, Vizi ES. ATP and [3H]noradrenaline release and the presence of ecto-Ca2+-ATPases in the capsule-glomerulosa fraction of the rat adrenal gland. J Endocrinol. 1997;153:105–114. doi: 10.1677/joe.0.1530105. [DOI] [PubMed] [Google Scholar]
- 248.Kaartinen JM, Hreniuk SP, Martin LF, Ranta S, Lanoue KF, Ohisalo JJ. Attenuated adenosine-sensitivity and decreased adenosine-receptor number in adipocyte plasma membranes in human obesity. Biochem J. 1991;279:17–22. doi: 10.1042/bj2790017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kangasniemi M. Effects of adenosine analog PIA (n-phenylisopropyladenosine) on FSH-stimulated cyclic AMP (cAMP) production in the rat seminiferous epithelium. Mol Cell Endocrinol. 1993;96:141–146. doi: 10.1016/0303-7207(93)90104-r. [DOI] [PubMed] [Google Scholar]
- 250.Kapoor JR, Sladek CD. Substance P and NPY differentially potentiate ATP and adrenergic stimulated vasopressin and oxytocin release. Am J Physiol. 2001;280:R69–R78. doi: 10.1152/ajpregu.2001.280.1.R69. [DOI] [PubMed] [Google Scholar]
- 251.Karanauskaite J, Hoppa MB, Braun M, Galvanovskis J, Rorsman P. Quantal ATP release in rat β-cells by exocytosis of insulin-containing LDCVs. Pflugers Arch. 2009;458:389–401. doi: 10.1007/s00424-008-0610-6. [DOI] [PubMed] [Google Scholar]
- 252.Kariya T, Field JB. Effects of adenosine and its derivatives on protein kinase activity of beef thyroid. Biochim Biophys Acta. 1976;451:41–47. doi: 10.1016/0304-4165(76)90255-5. [DOI] [PubMed] [Google Scholar]
- 253.Kasai Y, Ito S, Kitamura N, Ohta T, Nakazato Y. On-line measurement of adenosine triphosphate and catecholamine released from adrenal chromaffin cells. Comp Biochem Physiol A Mol Integr Physiol. 1999;122:363–368. doi: 10.1016/s1095-6433(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 254.Kather H. Purine accumulation in human fat cell suspensions. Evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J Biol Chem. 1988;263:8803–8809. [PubMed] [Google Scholar]
- 255.Katugampola H, Burnstock G. Purinergic signalling to rat ovarian smooth muscle: changes in P2X receptor expression during pregnancy. Cells Tissues Organs. 2004;178:33–47. doi: 10.1159/000081091. [DOI] [PubMed] [Google Scholar]
- 256.Kawamura M, Matsui T, Niitsu A, Kondo T, Ohno Y, Nakamichi N. Extracellular ATP stimulates steroidogenesis in bovine adrenocortical fasciculata cells via P2 purinoceptors. Jpn J Pharmacol. 1991;56:543–545. doi: 10.1254/jjp.56.543. [DOI] [PubMed] [Google Scholar]
- 257.Kawamura M, Niitsu A, Nishi H, Masaki E. Extracellular ATP potentiates steroidogenic effect of adrenocorticotropic hormone in bovine adrenocortical fasciculata cells. Jpn J Pharmacol. 2001;85:376–381. doi: 10.1254/jjp.85.376. [DOI] [PubMed] [Google Scholar]
- 258.Kell CA, Stehle JH. Just the two of us: melatonin and adenosine in rodent pituitary function. Ann Med. 2005;37:105–120. doi: 10.1080/07853890510007296. [DOI] [PubMed] [Google Scholar]
- 259.Khan WI, Ghia JE (2010) Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol 161:12–27 [DOI] [PMC free article] [PubMed]
- 260.Khanum A, Buczko E, Dufau ML. Essential role of adenosine triphosphate in activation of 17β-hydroxysteroid dehydrogenase in the rat Leydig cell. Endocrinology. 1997;138:1612–1620. doi: 10.1210/endo.138.4.5062. [DOI] [PubMed] [Google Scholar]
- 261.Kim JH, Nam JH, Kim MH, Koh DS, Choi SJ, Kim SJ, Lee JE, Min KM, Uhm DY, Kim SJ. Purinergic receptors coupled to intracellular Ca2+ signals and exocytosis in rat prostate neuroendocrine cells. J Biol Chem. 2004;279:27345–27356. doi: 10.1074/jbc.M313575200. [DOI] [PubMed] [Google Scholar]
- 262.Kim KT, Westhead EW. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989;86:9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr. 2000;130:3110S–3115S. doi: 10.1093/jn/130.12.3110S. [DOI] [PubMed] [Google Scholar]
- 264.Kimm-Brinson KL, Moeller PD, Barbier M, Glasgow H, Jr, Burkholder JM, Ramsdell JS. Identification of a P2X7 receptor in GH4C1 rat pituitary cells: a potential target for a bioactive substance produced by Pfiesteria piscicida. Environ Health Perspect. 2001;109:457–462. doi: 10.1289/ehp.01109457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.King BF, Wang S, Burnstock G. P2 purinoceptor-activated inward currents in folliculated oocytes of Xenopus laevis. J Physiol. 1996;494:17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Knecht M, Darbon JM, Ranta T, Baukal A, Catt KJ. Inhibitory actions of adenosine on follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Biol Reprod. 1984;30:1082–1090. doi: 10.1095/biolreprod30.5.1082. [DOI] [PubMed] [Google Scholar]
- 267.Knott TK, Velázquez-Marrero C, Lemos JR. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch. 2005;450:381–389. doi: 10.1007/s00424-005-1471-x. [DOI] [PubMed] [Google Scholar]
- 268.Knott TK, Marrero HG, Custer EE, Lemos JR. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol. 2008;217:155–161. doi: 10.1002/jcp.21485. [DOI] [PubMed] [Google Scholar]
- 269.Knott TK, Hussy N, Cuadra AE, Lee RH, Ortiz-Miranda S, Custer EE, Lemos JR. Adenosine trisphosphate appears to act via different receptors in terminals versus somata of the hypothalamic neurohypophysial system. J Neuroendocrinol. 2012;24:681–689. doi: 10.1111/j.1365-2826.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ko WH, Au CL, Yip CY. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci. 2003;72:1519–1535. doi: 10.1016/s0024-3205(02)02410-4. [DOI] [PubMed] [Google Scholar]
- 271.Kochukov MY, Ritchie AK. A P2X7 receptor stimulates plasma membrane trafficking in the FRTL rat thyrocyte cell line. Am J Physiol Cell Physiol. 2004;287:C992–C1002. doi: 10.1152/ajpcell.00538.2003. [DOI] [PubMed] [Google Scholar]
- 272.Kochukov MY, Ritchie AK. P2X7 receptor stimulation of membrane internalization in a thyrocyte cell line. J Membr Biol. 2005;204:11–21. doi: 10.1007/s00232-005-0742-y. [DOI] [PubMed] [Google Scholar]
- 273.Kondo Y, Sho K, Majid MA, Okajima F. P1-Purinergic receptor-mediated modulation of TSH actions on FRTL-5 thyroid cells: possible switching from cAMP pathway to inositol phosphate-Ca system. Nucleosid Nucleotid. 1991;10:1217–1218. [Google Scholar]
- 274.Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci U S A. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 276.Koshimizu T, Tomic M, Van Goor F, Stojilkovic SS. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol. 1998;12:901–913. doi: 10.1210/mend.12.7.0129. [DOI] [PubMed] [Google Scholar]
- 277.Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology. 2000;141:4091–4099. doi: 10.1210/endo.141.11.7737. [DOI] [PubMed] [Google Scholar]
- 278.Kostron H, Winkler H, Peer LJ, König P. Uptake of adenosine triphosphate by isolated adrenal chromaffin granules: a carrier-mediated transport. Neuroscience. 1977;2:159–166. doi: 10.1016/0306-4522(77)90077-x. [DOI] [PubMed] [Google Scholar]
- 279.Kosugi S, Mori T, Iwamori M, Nagai Y, Imura H. α2- and β-adrenergic receptors and adenosine A1 receptor of FRTL-5 rat thyroid cells in relation to fucosyl GM1 ganglioside. Endocrinology. 1989;124:2707–2710. doi: 10.1210/endo-124-6-2707. [DOI] [PubMed] [Google Scholar]
- 280.Lagercrantz H. On the composition and function of large dense cored vesicles in sympathetic nerves. Neuroscience. 1976;1:81–92. doi: 10.1016/0306-4522(76)90002-6. [DOI] [PubMed] [Google Scholar]
- 281.Lalevee N, Rogier C, Becq F, Joffre M. Acute effects of adenosine triphosphates, cyclic 3′,5′-adenosine monophosphates, and follicle-stimulating hormone on cytosolic calcium level in cultured immature rat Sertoli cells. Biol Reprod. 1999;61:343–352. doi: 10.1095/biolreprod61.2.343. [DOI] [PubMed] [Google Scholar]
- 282.Laliberte JF, Beaudoin AR. Sequential hydrolysis of the γ- and β-phosphate groups of ATP by the ATP diphosphohydrolase from pig pancreas. Biochim Biophys Acta. 1983;742:9–15. doi: 10.1016/0167-4838(83)90352-7. [DOI] [PubMed] [Google Scholar]
- 283.Lambert M, Christophe J. Characterization of (Mg, Ca)-ATPase activity in rat pancreatic plasma membranes. Eur J Biochem. 1978;91:485–492. doi: 10.1111/j.1432-1033.1978.tb12701.x. [DOI] [PubMed] [Google Scholar]
- 284.Landolfi E, Florio T, Rapanà A, Cocozza E, Schettini G, Marino A. Purinergic modulation of adenylate cyclase activity and prolactin secretion in rat adenohypophysis. Eur J Pharmacol. 1990;183:483. [Google Scholar]
- 285.Laplante MA, Monassier L, Freund M, Bousquet P, Gachet C. The purinergic P2Y1 receptor supports leptin secretion in adipose tissue. Endocrinology. 2010;151:2060–2070. doi: 10.1210/en.2009-1134. [DOI] [PubMed] [Google Scholar]
- 286.Lara HE, Belmar J. Release of norepinephrine from the cat ovary: changes after ovulation. Biol Reprod. 1991;44:752–759. doi: 10.1095/biolreprod44.5.752. [DOI] [PubMed] [Google Scholar]
- 287.Larrouy D, Galitzky J, Lafontan M. A1 adenosine receptors in the human fat cell: tissue distribution and regulation of radioligand binding. Eur J Pharmacol. 1991;206:139–147. doi: 10.1016/0922-4106(91)90022-a. [DOI] [PubMed] [Google Scholar]
- 288.Lavoie EG, Fausther M, Kauffenstein G, Kukulski F, Kunzli BM, Friess H, Sevigny J. Identification of the ectonucleotidases expressed in mouse, rat, and human Langerhans islets: potential role of NTPDase3 in insulin secretion. Am J Physiol Endocrinol Metab. 2010;299:E647–E656. doi: 10.1152/ajpendo.00126.2010. [DOI] [PubMed] [Google Scholar]
- 289.Lawrence VJ, Patel JN, Eisenhofer G, Coppack SW. Sympathetic nervous system dysfunction in obesity: regional and gobal abnormalities. Diabetes. 2002;51:A407. [Google Scholar]
- 290.Ledent C, Dumont JE, Vassart G, Parmentier M. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. EMBO J. 1992;11:537–542. doi: 10.1002/j.1460-2075.1992.tb05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee DH, Kim EG, Park KS, Jeong SW, Kong ID, Lee JW. Characteristics of P2X7-like receptor activated by adenosine triphosphate in HIT-T15 cells. Pancreas. 2007;35:53–62. doi: 10.1097/01.mpa.0000278676.58491.ef. [DOI] [PubMed] [Google Scholar]
- 292.Lee DH, Park KS, Kim DR, Lee JW, Kong ID. Dual effect of ATP on glucose-induced insulin secretion in HIT-T15 cells. Pancreas. 2008;37:302–308. doi: 10.1097/MPA.0b013e318168daaa. [DOI] [PubMed] [Google Scholar]
- 293.Lee H, Jun DJ, Suh BC, Choi BH, Lee JH, Do MS, Suh BS, Ha H, Kim KT. Dual roles of P2 purinergic receptors in insulin-stimulated leptin production and lipolysis in differentiated rat white adipocytes. J Biol Chem. 2005;280:28556–28563. doi: 10.1074/jbc.M411253200. [DOI] [PubMed] [Google Scholar]
- 294.Lee PS, Squires PE, Buchan AM, Yuen BH, Leung PC. P2-purinoreceptor evoked changes in intracellular calcium oscillations in single isolated human granulosa-lutein cells. Endocrinology. 1996;137:3756–3761. doi: 10.1210/endo.137.9.8756543. [DOI] [PubMed] [Google Scholar]
- 295.Lee SC, Pappone PA. Effects of P2 purinergic receptor stimulation in brown adipocytes. Am J Physiol. 1997;273:C679–C686. doi: 10.1152/ajpcell.1997.273.2.C679. [DOI] [PubMed] [Google Scholar]
- 296.Lee SC, Pappone PA. Membrane responses to extracellular ATP in rat isolated white adipocytes. Pflugers Arch. 1997;434:422–428. doi: 10.1007/s004240050416. [DOI] [PubMed] [Google Scholar]
- 297.Lee SC, Vielhauer NS, Leaver EV, Pappone PA. Differential regulation of Ca2+ signaling and membrane trafficking by multiple P2 receptors in brown adipocytes. J Membr Biol. 2005;207:131–142. doi: 10.1007/s00232-005-0808-x. [DOI] [PubMed] [Google Scholar]
- 298.Leitner JW, Sussman KE, Vatter AE, Schneider FH. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 299.Lemos JR, Wang G. Excitatory versus inhibitory modulation by ATP of neurohypophysial terminal activity in the rat. Exp Physiol. 2000;85:67S–74S. doi: 10.1111/j.1469-445x.2000.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 300.Lemos JR, Ortiz-Miranda SI, Cuadra AE, Velázquez-Marrero C, Custer EE, Dad T, Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Léon C, Freund M, Latchoumanin O, Farret A, Petit P, Cazenave JP, Gachet C. The P2Y1 receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal. 2005;1:145–151. doi: 10.1007/s11302-005-6209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lépine S, Le Stunff H, Lakatos B, Sulpice JC, Giraud F. ATP-induced apoptosis of thymocytes is mediated by activation of P2X7 receptor and involves de novo ceramide synthesis and mitochondria. Biochim Biophys Acta. 2006;1761:73–82. doi: 10.1016/j.bbalip.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 303.Levin SR, Kasson BG, Driessen JF. Adenosine triphosphatases of rat pancreatic islets: comparison with those of rat kidney. J Clin Invest. 1978;62:692–701. doi: 10.1172/JCI109177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Levine RA, Oyama S, Kagan A, Glick SM. Stimulation of insulin and growth hormone secretion by adenine nucleotides in primates. J Lab Clin Med. 1970;75:30–36. [PubMed] [Google Scholar]
- 305.Lewis BM, Pexa A, Francis K, Verma V, McNicol AM, Scanlon M, Deussen A, Evans WH, Rees DA, Ham J. Adenosine stimulates connexin 43 expression and gap junctional communication in pituitary folliculostellate cells. FASEB J. 2006;20:2585–2587. doi: 10.1096/fj.06-6121fje. [DOI] [PubMed] [Google Scholar]
- 306.Li GD, Milani D, Dunne MJ, Pralong WF, Theler JM, Petersen OH, Wollheim CB. Extracellular ATP causes Ca2+-dependent and -independent insulin secretion in RINm5F cells. Phospholipase C mediates Ca2+ mobilization but not Ca2+ influx and membrane depolarization. J Biol Chem. 1991;266:3449–3457. [PubMed] [Google Scholar]
- 307.Li J, Gylfe E, Tengholm A. Interplay between sub-plasma membrane oscillations of Ca2+ and ATP in mouse beta cells. Diabetologia. 2011;54:S201. [Google Scholar]
- 308.Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology. 2011;152:2342–2352. doi: 10.1210/en.2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Li S, Tomic M, Stojilkovic SS. Characterization of novel pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011;174:202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liang HX, Belardinelli L, Ozeck MJ, Shryock JC. Tonic activity of the rat adipocyte A1-adenosine receptor. Br J Pharmacol. 2002;135:1457–1466. doi: 10.1038/sj.bjp.0704586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lim W, Kim SJ, Yan HD, Kim J. Ca2+-channel-dependent and -independent inhibition of exocytosis by extracellular ATP in voltage-clamped rat adrenal chromaffin cells. Pflugers Arch. 1997;435:34–42. doi: 10.1007/s004240050481. [DOI] [PubMed] [Google Scholar]
- 312.Lin J, Krishnaraj R, Kemp RG. Exogenous ATP enhances calcium influx in intact thymocytes. J Immunol. 1985;135:3403–3410. [PubMed] [Google Scholar]
- 313.Lin LF, Bott MC, Kao LS, Westhead EW. ATP stimulated catecholamine secretion: response in perfused adrenal glands and a subpopulation of cultured chromaffin cells. Neurosci Lett. 1995;183:147–150. doi: 10.1016/0304-3940(94)11136-7. [DOI] [PubMed] [Google Scholar]
- 314.Lingard JM, Young JA. β-Adrenergic control of exocrine secretion by perfused rat pancreas in vitro. Am J Physiol. 1983;245:G690–G696. doi: 10.1152/ajpgi.1983.245.5.G690. [DOI] [PubMed] [Google Scholar]
- 315.Lingard JM, Young JA. Adrenergic secromotor control of the rat pancreas. In: Case RM, Lingard JM, Young JA, editors. Secretion: mechanism and control. Manchester: Manchester University Press; 1984. pp. 271–276. [Google Scholar]
- 316.Liu M, Dunn PM, King BF, Burnstock G. Rat chromaffin cells lack P2X receptors while those of the guinea-pig express a receptor with novel pharmacology. Br J Pharmacol. 1999;128:61–68. doi: 10.1038/sj.bjp.0702790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Liu P, Wen M, Hayashi J. Characterization of ATP receptor responsible for the activation of phospholipase A2 and stimulation of prostaglandin E2 production in thymic epithelial cells. Biochem J. 1995;308:399–404. doi: 10.1042/bj3080399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Liu P, Lalor D, Bowser SS, Hayden JH, Wen M, Hayashi J. Regulation of arachidonic acid release and prostaglandin E2 production in thymic epithelial cells by ATPgammaS and transforming growth factor-alpha. Cell Immunol. 1998;188:81–88. doi: 10.1006/cimm.1998.1343. [DOI] [PubMed] [Google Scholar]
- 319.Lobo MV, Marusic ET. Effect of angiotensin II, ATP, and ionophore A23187 on potassium efflux in adrenal glomerulosa cells. Am J Physiol. 1986;250:E125–E130. doi: 10.1152/ajpendo.1986.250.2.E125. [DOI] [PubMed] [Google Scholar]
- 320.Loesch A, Burnstock G. Immunoreactivity to P2X6 receptors in the rat hypothalamo-neurohypophysial system: an ultrastructural study with ExtrAvidin and colloidal gold-silver immunolabelling. Neuroscience. 2001;106:621–631. doi: 10.1016/s0306-4522(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 321.Loesch A, Miah S, Burnstock G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamo-neurohypophysial system. J Neurocytol. 1999;28:495–504. doi: 10.1023/a:1007009222518. [DOI] [PubMed] [Google Scholar]
- 322.Loesch A, Glass R, Burnstock G. Ultrastructural identification of P2Y2 receptor mRNA in the rat thymus. Cells Tissues Organs. 2002;172:255–264. doi: 10.1159/000067199. [DOI] [PubMed] [Google Scholar]
- 323.Londos C, Cooper DM, Schlegel W, Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978;75:5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lorrain J, Angel I, Duval N, Eon MT, Oblin A, Langer SZ. Adrenergic and nonadrenergic cotransmitters inhibit insulin secretion during sympathetic stimulation in dogs. Am J Physiol. 1992;263:E72–E78. doi: 10.1152/ajpendo.1992.263.1.E72. [DOI] [PubMed] [Google Scholar]
- 325.Losier AJ, Armstrong RW, Younglai EV. Adenosine triphosphate inhibits LH stimulated testosterone accumulation by isolated rabbit ovarian follicles. IRCS Med Sci. 1980;8:322. [Google Scholar]
- 326.Loten EG, Regen DM, Park CR. Transport of D-allose by isolated fat-cells: an effect of adenosine triphosphate on insulin stimulated transport. J Cell Physiol. 1976;89:651–660. doi: 10.1002/jcp.1040890423. [DOI] [PubMed] [Google Scholar]
- 327.Loubatières AL, Loubatières-Mariani MM, Chapal J. Adenosine triphosphate (ATP), cyclic adenosine 3′5′ monophosphate (cycl 3′5′ AMP) and insulin secretion. C R Seances Soc Biol Fil. 1972;166:1742–1746. [PubMed] [Google Scholar]
- 328.Loubatières-Mariani MM, Loubatières AL, Chapal J, Valette G. Adenosine triphosphate (ATP) and glucose. Action on insulin and glucagon secretion. C R Seances Soc Biol Fil. 1976;170:833–836. [PubMed] [Google Scholar]
- 329.Loubatières-Mariani MM, Chapal J, Lignon F, Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979;59:277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- 330.Loubatières-Mariani MM, Chapal J, Roye M. Effects of adenosine on the secretions of glucagon and insulin of isolated ad perfused pancreas of the rat. C R Seances Soc Biol Fil. 1982;176:663–669. [PubMed] [Google Scholar]
- 331.Lu M, Farnebo LO, Bränström R, Larsson C. Inhibition of parathyroid hormone secretion by caffeine in human parathyroid cells. J Clin Endocrinol Metab. 2013;98:E1345–E1351. doi: 10.1210/jc.2013-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Lugo-Garcia L, Filhol R, Lajoix AD, Gross R, Petit P, Vignon J. Expression of purinergic P2Y receptor subtypes by INS-1 insulinoma β-cells: a molecular and binding characterization. Eur J Pharmacol. 2007;568:54–60. doi: 10.1016/j.ejphar.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 333.Lugo-Garcia L, Nadal B, Gomis R, Petit P, Gross R, Lajoix AD. Human pancreatic islets express the purinergic P2Y11 and P2Y12 receptors. Horm Metab Res. 2008;40:827–830. doi: 10.1055/s-0028-1082050. [DOI] [PubMed] [Google Scholar]
- 334.Luke TM, Hexum TD. UTP and ATP increase extracellular signal-regulated kinase 1/2 phosphorylation in bovine chromaffin cells through epidermal growth factor receptor transactivation. Purinergic Signal. 2008;4:323–330. doi: 10.1007/s11302-008-9098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Maayan ML, Volpert EM, Dawry F. Inhibition by adenosine of thyroidal T4 release in vitro. Endocrinology. 1978;103:652–655. doi: 10.1210/endo-103-2-652. [DOI] [PubMed] [Google Scholar]
- 336.Madec S, Rossi C, Chiarugi M, Santini E, Salvati A, Ferrannini E, Solini A. Adipocyte P2X7 receptors expression: a role in modulating inflammatory response in subjects with metabolic syndrome? Atherosclerosis. 2011;219:552–558. doi: 10.1016/j.atherosclerosis.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 337.Makino H, Manganiello VC, Kono T. Roles of ATP in insulin actions. Annu Rev Physiol. 1994;56:273–295. doi: 10.1146/annurev.ph.56.030194.001421. [DOI] [PubMed] [Google Scholar]
- 338.Malavasi F, Deaglio S, Zaccarello G, Horenstein AL, Chillemi A, Audrito V, Serra S, Gandione M, Zitella A, Tizzani A. The hidden life of NAD+-consuming ectoenzymes in the endocrine system. J Mol Endocrinol. 2010;45:183–191. doi: 10.1677/JME-10-0082. [DOI] [PubMed] [Google Scholar]
- 339.Malbon CC, Hert RC, Fain JN. Characterization of [3H]adenosine binding to fat cell membranes. J Biol Chem. 1978;253:3114–3122. [PubMed] [Google Scholar]
- 340.Marsigliante S, Elia MG, Di JB, Greco S, Muscella A, Storelli C. Increase of [Ca2+]i via activation of ATP receptors in PC-Cl3 rat thyroid cell line. Cell Signal. 2002;14:61–67. doi: 10.1016/s0898-6568(01)00208-x. [DOI] [PubMed] [Google Scholar]
- 341.Martin SC. ATP activates a Ca2+ -dependent Cl- current in the rat thyroid cell line, FRTL-5. J Membr Biol. 1992;125:243–253. doi: 10.1007/BF00236437. [DOI] [PubMed] [Google Scholar]
- 342.Martin SE, Bockman EL. Adenosine regulates blood flow and glucose uptake in adipose tissue of dogs. Am J Physiol. 1986;250:H1127–H1135. doi: 10.1152/ajpheart.1986.250.6.H1127. [DOI] [PubMed] [Google Scholar]
- 343.Martin SS, Senior AE. Membrane adenosine triphosphatase activities in rat pancreas. Biochim Biophys Acta. 1980;602:401–418. doi: 10.1016/0005-2736(80)90320-x. [DOI] [PubMed] [Google Scholar]
- 344.Martinez-Valdez H, Cohen A. Coordinate regulation of mRNAs encoding adenosine deaminase, purine nucleoside phosphorylase, and terminal deoxynucleotidyltransferase by phorbol esters in human thymocytes. Proc Natl Acad Sci U S A. 1988;85:6900–6903. doi: 10.1073/pnas.85.18.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Matkó J, Nagy P, Panyi G, Vereb G, Jr, Bene L, Mátyus L, Damjanovich S. Biphasic effect of extracellular ATP on the membrane potential of mouse thymocytes. Biochem Biophys Res Commun. 1993;191:378–384. doi: 10.1006/bbrc.1993.1228. [DOI] [PubMed] [Google Scholar]
- 346.Matsubara S, Tamada T, Kurahashi K, Saito T. Ultracytochemical localizations of adenosine nucleotidase activities in the human term placenta, with special reference to 5′-nucleotidase activity. Acta Histochem Cytochem. 1987;20:409–419. [Google Scholar]
- 347.Matsui T. Biphasic rise caused by extracellular ATP in intracellular calcium concentration in bovine adrenocortical fasciculata cells. Biochem Biophys Res Commun. 1991;178:1266–1272. doi: 10.1016/0006-291x(91)91030-g. [DOI] [PubMed] [Google Scholar]
- 348.Melo AC, Moeller PD, Glasgow H, Burkholder JM, Ramsdell JS. Microfluorimetric analysis of a purinergic receptor (P2X7) in GH4C1 rat pituitary cells: effects of a bioactive substance produced by Pfiesteria piscicida. Environ Health Perspect. 2001;109(Suppl 5):731–737. doi: 10.1289/ehp.01109s5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mersmann HJ, Carey GB, Smith EO. Adipose tissue β-adrenergic and A1 adenosine receptors in suckling pigs. J Anim Sci. 1997;75:3161–3168. doi: 10.2527/1997.75123161x. [DOI] [PubMed] [Google Scholar]
- 350.Miller RE. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981;2:471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- 351.Mills SE. Regulation of porcine adipocyte metabolism by insulin and adenosine. J Anim Sci. 1999;77:3201–3207. doi: 10.2527/1999.77123201x. [DOI] [PubMed] [Google Scholar]
- 352.Miras-Portugal MT, Rotllan P, Aunis D. Incorporation of adenosine into nucleotides of chromaffin cells maintained in primary cultures. Neurochem Int. 1985;7:89–93. doi: 10.1016/0197-0186(85)90012-9. [DOI] [PubMed] [Google Scholar]
- 353.Mollard P, Guerineau N, Chiavaroli C, Schlegel W, Cooper DM. Adenosine A1 receptor-induced inhibition of Ca2+ transients linked to action potentials in clonal pituitary cells. Eur J Pharmacol. 1991;206:271–277. doi: 10.1016/0922-4106(91)90109-u. [DOI] [PubMed] [Google Scholar]
- 354.Monaco L, Conti M. Localization of adenosine receptors in rat testicular cells. Biol Reprod. 1986;35:258–266. doi: 10.1095/biolreprod35.2.258. [DOI] [PubMed] [Google Scholar]
- 355.Monaco L, DeManno DA, Martin MW, Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988;122:2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- 356.Montiel-Herrera M, Zaske AM, Garcia-Colunga J, Martinez-Torres A, Miledi R. Ion currents induced by ATP and angiotensin II in cultured follicular cells of Xenopus laevis. Mol Cells. 2011;32:397–404. doi: 10.1007/s10059-011-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Morales-Tlalpan V, Arellano RO, Diíz-Muñoz M. Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium. 2005;37:203–213. doi: 10.1016/j.ceca.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 358.Mori M, Tsushima H, Matsuda T. Antidiuretic effects of purinoceptor agonists injected into the hypothalamic paraventricular nucleus of water-loaded, ethanol-anesthetized rats. Neuropharmacology. 1992;31:585–592. doi: 10.1016/0028-3908(92)90191-q. [DOI] [PubMed] [Google Scholar]
- 359.Mori M, Tsushima H, Matsuda T. Antidiuretic effects of ATP induced by microinjection into the hypothalamic supraoptic nucleus in water-loaded and ethanol-anesthetized rats. Jpn J Pharmacol. 1994;66:445–450. doi: 10.1254/jjp.66.445. [DOI] [PubMed] [Google Scholar]
- 360.Morita K, Ishii S, Uda H, Oka M. Requirement of ATP for exocytotic release of catecholamines from digitonin-permeabilized adrenal chromaffin cells. J Neurochem. 1988;50:644–648. doi: 10.1111/j.1471-4159.1988.tb02959.x. [DOI] [PubMed] [Google Scholar]
- 361.Morley P, Vanderhyden BC, Tremblay R, Mealing GAR, Durkin JP, Whitfield JF. Purinergic receptor-mediated intracellulat Ca2+ oscillations in chicken granulosa cells. Endocrinology. 1994;134:1269–1276. doi: 10.1210/endo.134.3.8119167. [DOI] [PubMed] [Google Scholar]
- 362.Mortani Barbosa EJ, Ferreira ZS, Markus RP. Purinergic and noradrenergic cotransmission in the rat pineal gland. Eur J Pharmacol. 2000;401:59–62. doi: 10.1016/s0014-2999(00)00416-7. [DOI] [PubMed] [Google Scholar]
- 363.Mughal S, Cuschieri A, Kharbat BA. Histochemical localization of adenosine triphosphatase activity in thymus: a light microscopical and ultrastructural study. Histochem J. 1986;18:341–350. doi: 10.1007/BF01675214. [DOI] [PubMed] [Google Scholar]
- 364.Munkonda MN, Pelletier J, Ivanenkov VV, Fausther M, Tremblay A, Künzli B, Kirley TL, Sévigny J. Characterization of a monoclonal antibody as the first specific inhibitor of human NTP diphosphohydrolase-3: partial characterization of the inhibitory epitope and potential applications. FEBS J. 2009;276:479–496. doi: 10.1111/j.1742-4658.2008.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Murphy KM, Snyder SH. Adenosine receptors in rat testes: labeling with 3H-cyclohexyladenosine. Life Sci. 1981;28:917–920. doi: 10.1016/0024-3205(81)90054-0. [DOI] [PubMed] [Google Scholar]
- 366.Nagy PV, Fehér T, Morga S, Matkó J. Apoptosis of murine thymocytes induced by extracellular ATP is dose- and cytosolic pH-dependent. Immunol Lett. 2000;72:23–30. doi: 10.1016/s0165-2478(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 367.Nakamura Y, Ohtaki S. Extracellular ATP-induced production of hydrogen peroxide in porcine thyroid cells. J Endocrinol. 1990;126:283–287. doi: 10.1677/joe.0.1260283. [DOI] [PubMed] [Google Scholar]
- 368.Nakamura Y, Ogihara S, Ohtaki S. Activation by ATP of calcium-dependent NADPH-oxidase generating hydrogen peroxide in thyroid plasma membranes. J Biochem. 1987;102:1121–1132. doi: 10.1093/oxfordjournals.jbchem.a122150. [DOI] [PubMed] [Google Scholar]
- 369.Nazarea M, Okajima F, Sho K, Inoue K, Kondo Y. Extracellular adenosine triphosphate completely reverses the thyrotropin-induced morphological change in FRTL-5 cells. Endocrinology. 1989;125:100–108. doi: 10.1210/endo-125-1-100. [DOI] [PubMed] [Google Scholar]
- 370.Nazarea M, Okajima F, Kondo Y. P2 -purinergic activation of phosphoinositide turnover is potentiated by A1-receptor stimulation in thyroid cells. Eur J Pharmacol. 1991;206:47–52. doi: 10.1016/0922-4106(91)90145-8. [DOI] [PubMed] [Google Scholar]
- 371.Nemeth EF, Kosz LM. Adenine nucleotides mobilize cellular Ca2+ and inhibit parathyroid hormone secretion. Am J Physiol. 1989;257:E505–E513. doi: 10.1152/ajpendo.1989.257.4.E505. [DOI] [PubMed] [Google Scholar]
- 372.Nicholls J, Skene DJ, Hourani SM. Use of a newly developed technique to isolate rat pinealocytes and study the effects of adenosine agonists on melatonin production. J Pineal Res. 1997;23:164–168. doi: 10.1111/j.1600-079x.1997.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 373.Nicholson SA. The effect of caffeine on plasma corticosterone and pituitary adrenocorticotrophin (ACTH) release in the rat is antagonised by adenosine. J Physiol. 1987;394:124P. [Google Scholar]
- 374.Nihei OK, Campos de Carvalho AC, Spray DC, Savino W, Alves LA. A novel form of cellular communication among thymic epithelial cells: intercellular calcium wave propagation. Am J Physiol Cell Physiol. 2003;285:C1304–C1313. doi: 10.1152/ajpcell.00568.2002. [DOI] [PubMed] [Google Scholar]
- 375.Niitsu A. Calcium is essential for ATP-induced steroidogenesis in bovine adrenocortical fasciculata cells. Jpn J Pharmacol. 1992;60:269–274. doi: 10.1254/jjp.60.269. [DOI] [PubMed] [Google Scholar]
- 376.Nikodijevic O, Klein DC. Adenosine stimulates adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate accumulation in rat pinealocytes: evidence for a role for adenosine in pineal neurotransmission. Endocrinology. 1989;125:2150–2157. doi: 10.1210/endo-125-4-2150. [DOI] [PubMed] [Google Scholar]
- 377.Nishi H. Two different P2Y receptors linked to steroidogenesis in bovine adrenocortical cells. Jpn J Pharmacol. 1999;81:194–199. doi: 10.1254/jjp.81.194. [DOI] [PubMed] [Google Scholar]
- 378.Nishi H, Kato F, Masaki E, Kawamura M. ADP-sensitive purinoceptors induce steroidogenesis via adenylyl cyclase activation in bovine adrenocortical fasciculata cells. Br J Pharmacol. 2002;137:177–184. doi: 10.1038/sj.bjp.0704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Nishi H, Hori S, Niitsu A, Kawamura M. Adenosine 5′-(γ-thio) triphosphate (ATPγS) stimulates both P2Y receptors linked to inositol phosphates production and cAMP accumulation in bovine adrenocortical fasciculata cells. Life Sci. 2004;74:1181–1190. doi: 10.1016/j.lfs.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 380.Nishi H, Arai H, Momiyama T. NCl-H295R, a human adrenal cortex-derived cell line, expresses purinergic receptors linked to Ca2+-mobilization/influx and cortisol secretion. PLoS One. 2013;8:e71022. doi: 10.1371/journal.pone.0071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Novak I. β-Adrenergic regulation of ion transport in pancreatic ducts: patch-clamp study of isolated rat pancreatic ducts. Gastroenterology. 1998;115:1–9. doi: 10.1016/s0016-5085(98)70151-9. [DOI] [PubMed] [Google Scholar]
- 382.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Nuñez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Physiol. 1997;272:E1117–E1123. doi: 10.1152/ajpendo.1997.272.6.E1117. [DOI] [PubMed] [Google Scholar]
- 384.Obermüller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- 385.Ogawa M, Inouye A. Responses of the transmembrane potential coupled to the ATP-evoked catecholamine release in isolated chromaffin granules. Jpn J Physiol. 1979;29:309–325. doi: 10.2170/jjphysiol.29.309. [DOI] [PubMed] [Google Scholar]
- 386.Ohbuchi T, Yokoyama T, Saito T, Ohkubo J, Suzuki H, Ishikura T, Katoh A, Fujihara H, Hashimoto H, Suzuki H, Ueta Y. Possible contribution of pannexin channel to ATP-induced currents in vitro in vasopressin neurons isolated from the rat supraoptic nucleus. Brain Res. 2011;1394:71–78. doi: 10.1016/j.brainres.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 387.Ohisalo JJ, Ranta S, Huhtaniemi IT. Attenuated adenosine R-site effect in adipocytes in obesity. Metabolism. 1986;35:143–146. doi: 10.1016/0026-0495(86)90115-0. [DOI] [PubMed] [Google Scholar]
- 388.Ohisalo JJ, Kaartinen JM, Ranta S, Mustajoki P, Hreniuk SP, Lanoue KF, Martin LF. Weight loss normalizes the inhibitory effect of N6-(phenylisopropyl)adenosine on lipolysis in fat cells of massively obese human subjects. Clin Sci (Lond) 1992;83:589–592. doi: 10.1042/cs0830589. [DOI] [PubMed] [Google Scholar]
- 389.Ohta T, Kai T, Ito S. Evidence for paracrine modulation of voltage-dependent calcium channels by amperometric analysis in cultured porcine adrenal chromaffin cells. Brain Res. 2004;1030:183–192. doi: 10.1016/j.brainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 390.Ohtani M, Suzuki J, Jacobson KA, Oka T. Evidence for the possible involvement of the P2Y6 receptor in Ca2+ mobilization and insulin secretion in mouse pancreatic islets. Purinergic Signal. 2008;4:365–375. doi: 10.1007/s11302-008-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ohtani M, Ohura K, Oka T. Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic β-cells. Cell Physiol Biochem. 2011;28:355–366. doi: 10.1159/000331752. [DOI] [PubMed] [Google Scholar]
- 392.Okajima F, Kondo Y. Inhibition of atrial natriuretic peptide-induced cGMP accumulation by purinergic agonists in FRTL-5 thyroid cells. Involvement of both pertussis toxin-sensitive and insensitive mechanisms. J Biol Chem. 1990;265:21741–21748. [PubMed] [Google Scholar]
- 393.Okajima F, Sho K, Kondo Y. Inhibition by islet-activating protein, pertussis toxin, of P2-purinergic receptor-mediated iodide efflux and phosphoinositide turnover in FRTL-5 cells. Endocrinology. 1988;123:1035–1043. doi: 10.1210/endo-123-2-1035. [DOI] [PubMed] [Google Scholar]
- 394.Okajima F, Sato K, Kondo Y. P2-purinergic agonists activate phospholipase C in a guanine nucleotide- and Ca2+-dependent manner in FRTL-5 thyroid cell membranes. FEBS Lett. 1989;253:132–136. doi: 10.1016/0014-5793(89)80945-7. [DOI] [PubMed] [Google Scholar]
- 395.Okajima F, Sato K, Nazarea M, Sho K, Kondo Y. A permissive role of pertussis toxin substrate G-protein in P2-purinergic stimulation of phosphoinositide turnover and arachidonate release in FRTL-5 thyroid cells. Cooperative mechanism of signal transduction systems. J Biol Chem. 1989;264:13029–13037. [PubMed] [Google Scholar]
- 396.Oliet SHR, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520:815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Omatsu-Kanbe M, Matsuura H. Inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. J Physiol. 1999;521(Pt 3):601–615. doi: 10.1111/j.1469-7793.1999.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Omatsu-Kanbe M, Isono T, Matsuura H. Multiple P2 receptors contribute to a transient increase in intracellular Ca2+ concentration in ATP-stimulated rat brown adipocytes. Exp Physiol. 2002;87:643–652. doi: 10.1113/eph8702455. [DOI] [PubMed] [Google Scholar]
- 399.Omatsu-Kanbe M, Shibata M, Yamamoto T, Isono T, Matsuura H. Actin filaments play a permissive role in the inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. Biochem J. 2004;381:389–396. doi: 10.1042/BJ20040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Omatsu-Kanbe M, Inoue K, Fujii Y, Yamamoto T, Isono T, Fujita N, Matsuura H. Effect of ATP on preadipocyte migration and adipocyte differentiation by activating P2Y receptors in 3 T3-L1 cells. Biochem J. 2006;393:171–180. doi: 10.1042/BJ20051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Oomori Y, Okuno S, Fujisawa H, Iuchi H, Ishikawa K, Satoh Y, Ono K. Ganglion cells immunoreactive for catecholamine-synthesizing enzymes, neuropeptide Y and vasoactive intestinal polypeptide in the rat adrenal gland. Cell Tissue Res. 1994;275:201–213. doi: 10.1007/BF00319418. [DOI] [PubMed] [Google Scholar]
- 402.Otsuguro K, Asano T, Ohta T, Ito S, Nakazato Y. ATP-evoked membrane current in guinea pig adrenal chromaffin cells. Neurosci Lett. 1995;187:145–148. doi: 10.1016/0304-3940(95)11359-5. [DOI] [PubMed] [Google Scholar]
- 403.Overgaard K, Torp-Pedersen C, Thorn NA. ATP-induced release of vasopressin from isolated bovine neurohypophyseal secretory granules. Dependency on chloride and effects of analogues of ATP. Acta Endocrinol (Copenh) 1979;90:609–615. doi: 10.1530/acta.0.0900609. [DOI] [PubMed] [Google Scholar]
- 404.Pappone PA, Lee SC. Purinergic receptor stimulation increases membrane trafficking in brown adipocytes. J Gen Physiol. 1996;108:393–404. doi: 10.1085/jgp.108.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Parandeh F, Abaraviciene SM, Amisten S, Erlinge D, Salehi A. Uridine diphosphate (UDP) stimulates insulin secretion by activation of P2Y6 receptors. Biochem Biophys Res Commun. 2008;370:499–503. doi: 10.1016/j.bbrc.2008.03.119. [DOI] [PubMed] [Google Scholar]
- 406.Parsons WJ, Stiles GL. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. Regulation of both Ns and Ni. J Biol Chem. 1987;262:841–847. [PubMed] [Google Scholar]
- 407.Peer LJ, Winkler H, Snider SR, Gibb JW, Baumgartner H. Synthesis of nucleotides in adrenal medulla and their uptake into chromaffin granules. Biochem Pharmacol. 1976;25:311–315. doi: 10.1016/0006-2952(76)90220-3. [DOI] [PubMed] [Google Scholar]
- 408.Petit P, Manteghetti M, Puech R, Loubatières-Mariani MM. ATP and phosphate-modified adenine nucleotide analogues. Effects on insulin secretion and calcium uptake. Biochem Pharmacol. 1987;36:377–380. doi: 10.1016/0006-2952(87)90297-8. [DOI] [PubMed] [Google Scholar]
- 409.Petit P, Bertrand G, Schmeer W, Henquin JC. Effects of extracellular adenine nucleotides on the electrical, ionic and secretory events in mouse pancreatic beta-cells. Br J Pharmacol. 1989;98:875–882. doi: 10.1111/j.1476-5381.1989.tb14616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Petit P, Hillaire-Buys D, Manteghetti M, Debrus S, Chapal J, Loubatières-Mariani MM. Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br J Pharmacol. 1998;125:1368–1374. doi: 10.1038/sj.bjp.0702214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Petit P, Lajoix AD, Gross R. P2 purinergic signalling in the pancreatic β-cell: control of insulin secretion and pharmacology. Eur J Pharm Sci. 2009;37:67–75. doi: 10.1016/j.ejps.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 412.Pérez-Armendariz EM, Nadal A, Fuentes E, Spray DC. Adenosine 5′-triphosphate (ATP) receptors induce intracellular calcium changes in mouse leydig cells. Endocrine. 1996;4:239–247. doi: 10.1007/BF02738690. [DOI] [PubMed] [Google Scholar]
- 413.Phillips JH, Morton AG. Adenosine triphosphate in the bovine chromaffin granule. J Physiol Paris. 1978;74:503–508. [PubMed] [Google Scholar]
- 414.Picanço-Diniz DL, Valença M, Favaretto AL, McCann SM, Antunes-Rodrigues J. Possible involvement of A1 receptors in the inhibition of gonadotropin secretion induced by adenosine in rat hemipituitaries in vitro. Braz J Med Biol Res. 1999;32:1167–1173. doi: 10.1590/s0100-879x1999000900017. [DOI] [PubMed] [Google Scholar]
- 415.Picanço-Diniz DL, Valença MM, Favaretto AL, Antunes-Rodrigues J. Stimulatory effects of adenosine on prolactin secretion in the pituitary gland of the rat. Braz J Med Biol Res. 2002;35:855–860. doi: 10.1590/s0100-879x2002000700015. [DOI] [PubMed] [Google Scholar]
- 416.Picanço-Diniz DL, Valenca MM, Antunes-Rodrigues J. Adenosine A1 receptor-mediated inhibition of in vitro prolactin secretion from the rat anterior pituitary. Braz J Med Biol Res. 2006;39:1493–1499. doi: 10.1590/s0100-879x2006001100013. [DOI] [PubMed] [Google Scholar]
- 417.Pierson PM, Peteri-Brunbäck B, Pisani DF, Abbracchio MP, Mienville JM, Rosso L. A2b receptor mediates adenosine inhibition of taurine efflux from pituicytes. Biol Cell. 2007;99:445–454. doi: 10.1042/BC20070028. [DOI] [PubMed] [Google Scholar]
- 418.Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Pintor J, Torres M, Castro E, Miras-Portugal MT. Characterization of diadenosine tetraphosphate (Ap4A) binding sites in cultured chromaffin cells: evidence for a P2y site. Br J Pharmacol. 1991;103:1980–1984. doi: 10.1111/j.1476-5381.1991.tb12363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Pintor J, Torres M, Miras-Portugal MT. Carbachol induced release of diadenosine polyphosphates - Ap4A and Ap5A - from perfused bovine adrenal medulla and isolated chromaffin cells. Life Sci. 1991;48:2317–2324. doi: 10.1016/0024-3205(91)90268-g. [DOI] [PubMed] [Google Scholar]
- 421.Pintor J, Rotllán P, Torres M, Miras-Portugal MT. Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem. 1992;200:296–300. doi: 10.1016/0003-2697(92)90469-n. [DOI] [PubMed] [Google Scholar]
- 422.Pletscher A, Da PM, Berneis KH, Steffen H, Lutold B, Weder HG. Molecular organization of amine storage organelles of blood platelets and adrenal medulla. Adv Cytopharmacol. 1974;2:257–264. [PubMed] [Google Scholar]
- 423.Pochmann D, Rucker B, Battastini AM, Sarkis JJ. Ovariectomy and estradiol replacement therapy alters the adenine nucleotide hydrolysis in rat blood serum. Thromb Res. 2004;114:275–281. doi: 10.1016/j.thromres.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 424.Poisner AM, Douglas WW. A possible mechanism of release of posterior pituitary hormones involving adenosine triphosphate and an adenosine triphosphatase in the neurosecretory granules. Mol Pharmacol. 1968;4:531–540. [PubMed] [Google Scholar]
- 425.Polan ML, DeCherney AH, Haseltine FP, Mezer HC, Behrman HR. Adenosine amplifies follicle-stimulating hormone action in granulosa cells and luteinizing hormone action in luteal cells of rat and human ovaries. J Clin Endocrinol Metab. 1983;56:288–294. doi: 10.1210/jcem-56-2-288. [DOI] [PubMed] [Google Scholar]
- 426.Poletto Chaves LA, Pontelli EP, Varanda WA. P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol. 2006;290:C1009–C1017. doi: 10.1152/ajpcell.00506.2005. [DOI] [PubMed] [Google Scholar]
- 427.Pollard HB, Zinder O, Hoffman PG, Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976;251:4544–4550. [PubMed] [Google Scholar]
- 428.Poulsen CR, Bokvist K, Olsen HL, Hoy M, Capito K, Gilon P, Gromada J. Multiple sites of purinergic control of insulin secretion in mouse pancreatic beta-cells. Diabetes. 1999;48:2171–2181. doi: 10.2337/diabetes.48.11.2171. [DOI] [PubMed] [Google Scholar]
- 429.Powell AD, Teschemacher AG, Seward EP. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci. 2000;20:606–616. doi: 10.1523/JNEUROSCI.20-02-00606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Prinster SC, Hague C, Hall RA. Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 431.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 432.Rani CS, Schilling WP, Field JB. Intracellular Ca2+ mobilization by thyrotropin, carbachol, and adenosine triphosphate in dog thyroid cells. Endocrinology. 1989;125:1889–1897. doi: 10.1210/endo-125-4-1889. [DOI] [PubMed] [Google Scholar]
- 433.Rapiejko PJ, Malbon CC. Short-term hyperthyroidism modulates adenosine receptors and catalytic activity of adenylate cyclase in adipocytes. Biochem J. 1987;241:765–771. doi: 10.1042/bj2410765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Raspé E, Andry G, Dumont JE. Adenosine triphosphate, bradykinin, and thyrotropin-releasing hormone regulate the intracellular Ca2+ concentration and the 45Ca2+ efflux of human thyrocytes in primary culture. J Cell Physiol. 1989;140:608–614. doi: 10.1002/jcp.1041400328. [DOI] [PubMed] [Google Scholar]
- 435.Raspé E, Laurent E, Andry G, Dumont JE. ATP, bradykinin, TRH and TSH activate the Ca2+-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol. 1991;81:175–183. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- 436.Raspé E, Laurent E, Corvilain B, Verjans B, Erneux C, Dumont JE. Control of the intracellular Ca2+-concentration and the inositol phosphate accumulation in dog thyrocyte primary culture: evidence for different kinetics of Ca2+-phosphatidylinositol cascade activation and for involvement in the regulation of H2O2 production. J Cell Physiol. 1991;146:242–250. doi: 10.1002/jcp.1041460208. [DOI] [PubMed] [Google Scholar]
- 437.Rees D, Giles P, Lewis M, Ham J. Adenosine regulates thrombomodulin and endothelial protein C receptor expression in folliculostellate cells of the pituitary gland. Purinergic Signal. 2010;6:19–29. doi: 10.1007/s11302-009-9172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Rees DA, Lewis MD, Lewis BM, Smith PJ, Scanlon MF, Ham J. Adenosine-regulated cell proliferation in pituitary folliculostellate and endocrine cells: differential roles for the A1 and A2B adenosine receptors. Endocrinology. 2002;143:2427–2436. doi: 10.1210/endo.143.6.8837. [DOI] [PubMed] [Google Scholar]
- 439.Rees DA, Scanlon MF, Ham J. Novel insights into how purines regulate pituitary cell function. Clin Sci (Lond) 2003;104:467–481. doi: 10.1042/CS20030053. [DOI] [PubMed] [Google Scholar]
- 440.Rees DA, Lewis BM, Lewis MD, Francis K, Scanlon MF, Ham J. Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol. 2003;140:764–772. doi: 10.1038/sj.bjp.0705488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Reichsman F, Santos S, Westhead EW. Two distinct ATP receptors activate calcium entry and internal calcium release in bovine chromaffin cells. J Neurochem. 1995;65:2080–2086. doi: 10.1046/j.1471-4159.1995.65052080.x. [DOI] [PubMed] [Google Scholar]
- 442.Resta R, Hooker SW, Laurent AB, Jamshedur Rahman SM, Franklin M, Knudsen TB, Nadon NL, Thompson LF. Insights into thymic purine metabolism and adenosine deaminase deficiency revealed by transgenic mice overexpressing ecto-5′-nucleotidase (CD73) J Clin Invest. 1997;99:676–683. doi: 10.1172/JCI119211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Rice AM, Fain JN, Rivkees SA. A1 adenosine receptor activation increases adipocyte leptin secretion. Endocrinology. 2000;141:1442–1445. doi: 10.1210/endo.141.4.7423. [DOI] [PubMed] [Google Scholar]
- 444.Richards-Williams C, Contreras JL, Berecek KH, Schwiebert EM. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal. 2008;4:393–405. doi: 10.1007/s11302-008-9126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Ritchie PK, Spangelo BL, Krzymowski DK, Rossiter TB, Kurth E, Judd AM. Adenosine increases interleukin 6 release and decreases tumour necrosis factor release from rat adrenal zona glomerulosa cells, ovarian cells, anterior pituitary cells, and peritoneal macrophages. Cytokine. 1997;9:187–198. doi: 10.1006/cyto.1996.0153. [DOI] [PubMed] [Google Scholar]
- 446.Roberts VH, Greenwood SL, Elliott AC, Sibley CP, Waters LH. Purinergic receptors in human placenta: evidence for functionally active P2X4, P2X7, P2Y2, and P2Y6. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1374–R1386. doi: 10.1152/ajpregu.00612.2005. [DOI] [PubMed] [Google Scholar]
- 447.Roberts VH, Webster RP, Brockman DE, Pitzer BA, Myatt L. Post-translational modifications of the P2X4 purinergic receptor subtype in the human placenta are altered in preeclampsia. Placenta. 2007;28:270–277. doi: 10.1016/j.placenta.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 448.Robinson PM, Perry RA, Hardy KJ, Coghlan JP, Scoggins BA. The innervation of the adrenal cortex in the sheep, Ovis ovis. J Anat. 1977;124:117–129. [PMC free article] [PubMed] [Google Scholar]
- 449.Rodrigue-Candela JL, Martin-Hernandez D, Castilla-Cortazar T. Stimulation of insulin secretion in vitro by adenosine triphosphate. Nature. 1963;197:1304. doi: 10.1038/1971304a0. [DOI] [PubMed] [Google Scholar]
- 450.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT. Subcellular distribution studies of diadenosine polyphosphates-Ap4A and Ap5A-in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 453.Rodríguez-Pascual F, Torres M, Miras-Portugal MT. Studies on the turnover of ecto-nucleotidases and ecto-dinucleoside polyphosphate hydrolase in cultured chromaffin cells. Neurosci Res Commun. 1992;11:101–107. [Google Scholar]
- 454.Rojas E, Pollard HB, Heldman E. Real-time measurements of acetylcholine-induced release of ATP from bovine medullary chromaffin cells. FEBS Lett. 1985;185:323–327. doi: 10.1016/0014-5793(85)80931-5. [DOI] [PubMed] [Google Scholar]
- 455.Rommerts FF, Molenaar R, Hoogerbrugge JW, van der Molen HJ. Development of adenosine responsiveness after isolation of Leydig cells. Biol Reprod. 1984;30:842–847. doi: 10.1095/biolreprod30.4.842. [DOI] [PubMed] [Google Scholar]
- 456.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue. Clin Endocrinol (Oxf) 2005;5:293–296. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 457.Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, Groop L, Rorsman P, Salehi A, Lyssenko V, Luthman H, Renstrom E. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 458.Ross PE, Ehring GR, Cahalan MD. Dynamics of ATP-induced calcium signaling in single mouse thymocytes. J Cell Biol. 1997;138:987–998. doi: 10.1083/jcb.138.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Rossato M, Merico M, Bettella A, Bordon P, Foresta C. Extracellular ATP stimulates estradiol secretion in rat Sertoli cells in vitro: modulation by external sodium. Mol Cell Endocrinol. 2001;178:181–187. doi: 10.1016/s0303-7207(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 460.Rosso L, Mienville JM. Pituicyte modulation of neurohormone output. Glia. 2009;57:235–243. doi: 10.1002/glia.20760. [DOI] [PubMed] [Google Scholar]
- 461.Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Troadec JD, Thirion S, Van Obberghen-Schilling E, Mienville JM. RhoA inhibition is a key step in pituicyte stellation induced by A1-type adenosine receptor activation. Glia. 2002;38:351–362. doi: 10.1002/glia.10072. [DOI] [PubMed] [Google Scholar]
- 462.Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Van Obberghen-Schilling E, Mienville JM. Vasopressin and oxytocin reverse adenosine-induced pituicyte stellation via calcium-dependent activation of Cdc42. Eur J Neurosci. 2002;16:2324–2332. doi: 10.1046/j.1460-9568.2002.02401.x. [DOI] [PubMed] [Google Scholar]
- 463.Rudge SA, Hughes PJ, Brown GR, Michell RH, Kirk CJ. Inositol lipid-mediated signalling in response to endothelin and ATP in the mammalian testis. Mol Cell Biochem. 1995;149–150:161–174. doi: 10.1007/BF01076574. [DOI] [PubMed] [Google Scholar]
- 464.Saggerson ED, Jamal Z. Differences in the properties of A1-type adenosine receptors in rat white and brown adipocytes. Biochem J. 1990;269:157–161. doi: 10.1042/bj2690157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Sakaguchi T, Arase K, Fisler JS, Bray GA. Effect of a high-fat diet on firing rate of sympathetic nerves innervating brown adipose tissue in anesthetized rats. Physiol Behav. 1989;45:1177–1182. doi: 10.1016/0031-9384(89)90106-6. [DOI] [PubMed] [Google Scholar]
- 466.Salehi A, Qader SS, Grapengiesser E, Hellman B. Inhibition of purinoceptors amplifies glucose-stimulated insulin release with removal of its pulsatility. Diabetes. 2005;54:2126–2131. doi: 10.2337/diabetes.54.7.2126. [DOI] [PubMed] [Google Scholar]
- 467.Salehi A, Qader SS, Grapengiesser E, Hellman B. Pulses of somatostatin release are slightly delayed compared with insulin and antisynchronous to glucagon. Regul Pept. 2007;144:43–49. doi: 10.1016/j.regpep.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 468.Salehi A, Parandeh F, Fredholm BB, Grapengiesser E, Hellman B. Absence of adenosine A1 receptors unmasks pulses of insulin release and prolongs those of glucagon and somatostatin. Life Sci. 2009;85:470–476. doi: 10.1016/j.lfs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 469.Sandberg G, Fredholm BB. Regulation of thymocyte proliferation: effects of L-alanine, adenosine and cyclic AMP in vitro. Thymus. 1981;3:63–75. [PubMed] [Google Scholar]
- 470.Santini E, Cuccato S, Madec S, Chimenti D, Ferrannini E, Solini A. Extracellular adenosine 5′-triphosphate modulates insulin secretion via functionally active purinergic receptors of X and Y subtype. Endocrinology. 2009;150:2596–2602. doi: 10.1210/en.2008-1486. [DOI] [PubMed] [Google Scholar]
- 471.Sasakawa N, Nakaki T, Yamamoto S, Kato R. Stimulation by ATP of inositol trisphosphate accumulation and calcium mobilization in cultured adrenal chromaffin cells. J Neurochem. 1989;52:441–447. doi: 10.1111/j.1471-4159.1989.tb09140.x. [DOI] [PubMed] [Google Scholar]
- 472.Sasseville M, Albuz FK, Cote N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian follicle. Biol Reprod. 2009;81:415–425. doi: 10.1095/biolreprod.108.074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Sato K, Okajima F, Kondo Y. Extracellular ATP stimulates three different receptor-signal transduction systems in FRTL-5 thyroid cells. Biochem J. 1992;283:281–287. doi: 10.1042/bj2830281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Scaccianoce S, Navarra D, Di Sciullo A, Angelucci L, Endröczi E. Adenosine and pituitary-adrenocortical axis activity in the rat. Neuroendocrinology. 1989;50:464–468. doi: 10.1159/000125264. [DOI] [PubMed] [Google Scholar]
- 475.Schmidt M, Löffler G. Induction of aromatase activity in human adipose tissue stromal cells by extracellular nucleotides. Evidence for P2-purinoceptors in adipose tissue. Eur J Biochem. 1998;252:147–154. doi: 10.1046/j.1432-1327.1998.2520147.x. [DOI] [PubMed] [Google Scholar]
- 476.Schneider DA, Sayegh AI. Gastrointestinal neuroendocrinology. Vet Clin North Am Equine Pract. 2002;18:205–217. doi: 10.1016/s0749-0739(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 477.Schödel J, Weise I, Klinger R, Schmidt M. Stimulation of lipogenesis in rat adipocytes by ATP, a ligand for P2-receptors. Biochem Biophys Res Commun. 2004;321:767–773. doi: 10.1016/j.bbrc.2004.06.179. [DOI] [PubMed] [Google Scholar]
- 478.Schöfl C, Rossig L, Potter E, von zur Muhlen A, Brabant G. Extracellular ATP and UTP increase cytosolic free calcium by activating a common P2u-receptor in single human thyrocytes. Biochem Biophys Res Commun. 1995;213:928–934. doi: 10.1006/bbrc.1995.2218. [DOI] [PubMed] [Google Scholar]
- 479.Seino S. Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea. Diabetologia. 2012;55:2096–2108. doi: 10.1007/s00125-012-2562-9. [DOI] [PubMed] [Google Scholar]
- 480.Sévigny J, Côté YP, Beaudoin AR. Purification of pancreas type-I ATP diphosphohydrolase and identification by affinity labelling with the 5′-p-fluorosulphonylbenzoyladenosine ATP analogue. Biochem J. 1995;312:351–356. doi: 10.1042/bj3120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514:351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Shinoda S, Izawa T, Komabayashi T, Suda K, Tsuboi M, Iwane H. Effects of adenosine and pertussis toxin on lipolysis in adipocytes from exercise-trained male rats. Res Commun Chem Pathol Pharmacol. 1989;66:397–410. [PubMed] [Google Scholar]
- 483.Sillero MA, Del VM, Zaera E, Michelena P, García AG, Sillero A. Diadenosine 5′,5″-P1, P4-tetraphosphate (Ap4A), ATP and catecholamine content in bovine adrenal medulla, chromaffin granules and chromaffin cells. Biochimie. 1994;76:404–409. doi: 10.1016/0300-9084(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 484.Silva AM, Rodrigues RJ, Tome AR, Cunha RA, Misler S, Rosario LM, Santos RM. Electrophysiological and immunocytochemical evidence for P2X purinergic receptors in pancreatic β cells. Pancreas. 2008;36:279–283. doi: 10.1097/MPA.0b013e31815a8473. [DOI] [PubMed] [Google Scholar]
- 485.Silveira GF, Buffon A, Bruno AN. New approaches to thyroid hormones and purinergic signaling. J Thyroid Res. 2013;2013:434727. doi: 10.1155/2013/434727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Silvestre RA, Rodríguez-Gallardo J, Egido EM, Marco J. Stimulatory effect of exogenous diadenosine tetraphosphate on insulin and glucagon secretion in the perfused rat pancreas. Br J Pharmacol. 1999;128:795–801. doi: 10.1038/sj.bjp.0702837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Sladek CD, Song Z. Regulation of vasopressin release by co-released neurotransmitters: mechanisms of purinergic and adrenergic synergism. Prog Brain Res. 2008;170:93–107. doi: 10.1016/S0079-6123(08)00409-3. [DOI] [PubMed] [Google Scholar]
- 488.Smallridge RC, Gist ID. P2-purinergic stimulation of iodide efflux in FRTL-5 rat thyroid cells involves parallel activation of PLC and PLA2. Am J Physiol. 1994;267:E323–E330. doi: 10.1152/ajpendo.1994.267.2.E323. [DOI] [PubMed] [Google Scholar]
- 489.Smith RG, Griffin PR, Xu Y, Smith AG, Liu K, Calacay J, Feighner SD, Pong C, Leong D, Pomés A, Cheng K, Van der Ploeg LH, Howard AD, Schaeffer J, Leonard RJ. Adenosine: A partial agonist of the growth hormone secretagogue receptor. Biochem Biophys Res Commun. 2000;276:1306–1313. doi: 10.1006/bbrc.2000.3610. [DOI] [PubMed] [Google Scholar]
- 490.Soji T, Nishizono H, Yashiro T, Herbert DC. Cytochemistry of Ca++-dependent adenosine triphosphatase (Ca-ATPase) in rat anterior pituitary cells. Tissue Cell. 1991;23:1–6. doi: 10.1016/0040-8166(91)90061-w. [DOI] [PubMed] [Google Scholar]
- 491.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149:389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 492.Sollevi A, Fredholm BB. Role of adenosine in adipose tissue circulation. Acta Physiol Scand. 1981;112:293–298. doi: 10.1111/j.1748-1716.1981.tb06819.x. [DOI] [PubMed] [Google Scholar]
- 493.Sollevi A, Hjemdahl P, Fredholm BB. Endogenous adenosine inhibits lipolysis induced by nerve stimulation without inhibiting noradrenaline release in canine subcutaneous adipose tissue in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:112–119. doi: 10.1007/BF00505303. [DOI] [PubMed] [Google Scholar]
- 494.Solomon SS, Turpin BP, Duckworth WC. Comparative studies of the antilipolytic effect of insulin and adenosine in the perifused isolated fat cell. Horm Metab Res. 1980;12:601–604. doi: 10.1055/s-2007-999209. [DOI] [PubMed] [Google Scholar]
- 495.Solomon SS, Schwartz Y, Rawlinson T. Lipolysis in diabetic adipocytes: differences in response to growth hormone and adenosine. Endocrinology. 1987;121:1056–1060. doi: 10.1210/endo-121-3-1056. [DOI] [PubMed] [Google Scholar]
- 496.Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–111. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 497.Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the -hypothalamo-neurohypophyseal system. J Neuroendocrinol. 2006;18:266–272. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 498.Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–R431. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- 499.Song Z, Gomes DA, Stevens W. Role of purinergic P2Y1 receptors in regulation of vasopressin and oxytocin secretion. Am J Physiol Regul Integr Comp Physiol. 2009;297:R478–R484. doi: 10.1152/ajpregu.00163.2009. [DOI] [PubMed] [Google Scholar]
- 500.Song Z, Gomes DA, Stevens W, Sladek CD. Multiple α1-adrenergic receptor subtypes support synergistic stimulation of vasopressin and oxytocin release by ATP and phenylephrine. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1529–R1537. doi: 10.1152/ajpregu.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Soodak LK, MacDonald GJ, Behrman HR. Luteolysis is linked to luteinizing hormone-induced depletion of adenosine triphosphate in vivo. Endocrinology. 1988;122:187–193. doi: 10.1210/endo-122-1-187. [DOI] [PubMed] [Google Scholar]
- 502.Sperlágh B, Mergl Z, Jurányi Z, Vizi ES, Makara GB. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J Endocrinol. 1999;160:343–350. doi: 10.1677/joe.0.1600343. [DOI] [PubMed] [Google Scholar]
- 503.Squires PE, James RF, London NJ, Dunne MJ. ATP-induced intracellular Ca2+ signals in isolated human insulin-secreting cells. Pflugers Arch. 1994;427:181–183. doi: 10.1007/BF00585959. [DOI] [PubMed] [Google Scholar]
- 504.Squires PE, Lee PSN, Ho Yuen B, Leung PCK, Buchan AMJ. Mechanisms involved in ATP-evoked Ca2+ oscillations in isolated human granulosa-luteal cells. Cell Calcium. 1997;21:365–374. doi: 10.1016/s0143-4160(97)90030-0. [DOI] [PubMed] [Google Scholar]
- 505.Stagner JI, Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol. 1985;248:E522–E530. doi: 10.1152/ajpendo.1985.248.5.E522. [DOI] [PubMed] [Google Scholar]
- 506.Stam NJ, Klomp J, Van de Heuvel N, Olijve W. Molecular cloning and characterization of a novel orphan receptor (P2P) expressed in human pancreas that shows high structural homology to the P2U purinoceptor. FEBS Lett. 1996;384:260–264. doi: 10.1016/0014-5793(96)00321-3. [DOI] [PubMed] [Google Scholar]
- 507.Stevens P, Robinson RL, Van Dyke K, Stitxel R. Synthesis, storage and drug-induced release of atp-8-3 h in the perfused bovine adrenal gland. Pharmacology. 1975;13:40–55. doi: 10.1159/000136883. [DOI] [PubMed] [Google Scholar]
- 508.Stiles GL, Pierson G, Sunay S, Parsons WJ. The rat testicular A1 adenosine receptor-adenylate cyclase system. Endocrinology. 1986;119:1845–1851. doi: 10.1210/endo-119-4-1845. [DOI] [PubMed] [Google Scholar]
- 509.Stojilkovic SS. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab. 2009;20:460–468. doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/s1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- 511.Stojilkovic SS, Zemkova H. P2X receptor channels in endocrine glands. WIREs Membr Transp Signal. 2013;2:173–180. doi: 10.1002/wmts.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Stojilkovic SS, Tomic M, Van Goor F, Koshimizu T. Expression of purinergic P2X2 receptor-channels and their role in calcium signaling in pituitary cells. Biochem Cell Biol. 2000;78:393–404. [PubMed] [Google Scholar]
- 513.Stojilkovic SS, He ML, Koshimizu TA, Balik A, Zemkova H. Signaling by purinergic receptors and channels in the pituitary gland. Mol Cell Endocrinol. 2010;314:184–191. doi: 10.1016/j.mce.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21:599–609. doi: 10.1016/j.tem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 516.Suh BC, Kim TD, Lee JU, Seong JK, Kim KT. Pharmacological characterization of adenosine receptors in PGT-β mouse pineal gland tumour cells. Br J Pharmacol. 2001;134:132–142. doi: 10.1038/sj.bjp.0704218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Sussman KE, Leitner JW. Conversion of ATP into other adenine nucleotides within isolated islet secretory vesicles. Effect of cyclic AMP on phosphorus translocation. Endocrinology. 1977;101:694–701. doi: 10.1210/endo-101-3-694. [DOI] [PubMed] [Google Scholar]
- 518.Sussman KE, Vaughan GD, Stjernholm MR. Factors controlling insulin secretion in the perfused isolated rat pancreas. In: Ostman J, editor. Diabetes: Proceedings of the 6th Congress of the International Diabetes Federation. Amsterdam: Excerpta Medica Foundation; 1969. p. 123. [Google Scholar]
- 519.Szabó J, Kósa E, Tóth IE, Bruckner GG. Effect of adenosine and its metabolites on the hypothamamo-pituitary-adrenal axis. Nutr Biochem. 1995;6:334–339. [Google Scholar]
- 520.Szalay KS, Orso E, Juranyi Z, Vinson GP, Vizi ES. Local non-synaptic modulation of aldosterone production by catecholamines and ATP in rat: implications for a direct neuronal fine tuning. Horm Metab Res. 1998;30:323–328. doi: 10.1055/s-2007-978892. [DOI] [PubMed] [Google Scholar]
- 521.Szkudelska K, Nogowski L, Szkudelski T. Resveratrol and genistein as adenosine triphosphate-depleting agents in fat cells. Metabolism. 2011;60:720–729. doi: 10.1016/j.metabol.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 522.Szkudelski T. Intracellular mediators in regulation of leptin secretion from adipocytes. Physiol Res. 2007;56:503–512. doi: 10.33549/physiolres.931038. [DOI] [PubMed] [Google Scholar]
- 523.Szkudelski T, Szkudelska K, Nogowski L. Effects of adenosine A1 receptor antagonism on lipogenesis and lipolysis in isolated rat adipocytes. Physiol Res. 2009;58:863–871. doi: 10.33549/physiolres.931467. [DOI] [PubMed] [Google Scholar]
- 524.Tahani HM. The purinergic nerve hypothesis and insulin secretion. Z Ernahrungswiss. 1979;18:128–138. doi: 10.1007/BF02023727. [DOI] [PubMed] [Google Scholar]
- 525.Tai CJ, Kang SK, Choi KC, Tzeng CR, Leung PC. Antigonadotropic action of adenosine triphosphate in human granulosa-luteal cells: involvement of protein kinase Cα. J Clin Endocrinol Metab. 2001;86:3237–3242. doi: 10.1210/jcem.86.7.7691. [DOI] [PubMed] [Google Scholar]
- 526.Tai CJ, Kang SK, Leung PC. Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab. 2001;86:773–777. doi: 10.1210/jcem.86.2.7231. [DOI] [PubMed] [Google Scholar]
- 527.Tai CJ, Chang SJ, Leung PC, Tzeng CR. Adenosine 5′-triphosphate activates nuclear translocation of mitogen-activated protein kinases leading to the induction of early growth response 1 and raf expression in human granulosa-luteal cells. J Clin Endocrinol Metab. 2004;89:5189–5195. doi: 10.1210/jc.2003-032111. [DOI] [PubMed] [Google Scholar]
- 528.Takeda F, Takeda M, Shimada A, Konno K. ATP-dependent [3H]Met-enkephalin uptake by bovine adrenal chromaffin granule membrane. Brain Res. 1985;344:220–226. doi: 10.1016/0006-8993(85)90798-x. [DOI] [PubMed] [Google Scholar]
- 529.Tamajusuku AS, Carrillo-Sepúlveda MA, Braganhol E, Wink MR, Sarkis JJ, Barreto-Chaves ML, Battastini AM. Activity and expression of ecto-5′-nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Mol Cell Biochem. 2006;289:65–72. doi: 10.1007/s11010-006-9148-0. [DOI] [PubMed] [Google Scholar]
- 530.Tamura S, Dubler RE, Larner J. Stimulation of maximal intracellular insulin action on glycogen synthase by preincubation of adipocytes with adenosine 5′-triphosphate. J Biol Chem. 1983;258:719–724. [PubMed] [Google Scholar]
- 531.Tan C, Voss U, Svensson S, Erlinge D, Olde B. High glucose and free fatty acids induce beta cell apoptosis via autocrine effects of ADP acting on the P2Y13 receptor. Purinergic Signal. 2013;9:67–79. doi: 10.1007/s11302-012-9331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS One. 2012;7:e39722. doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Tatsis-Kotsidis I, Erlanger BF. A1 adenosine receptor of human and mouse adipose tissues: cloning, expression, and characterization. Biochem Pharmacol. 1999;58:1269–1277. doi: 10.1016/s0006-2952(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 534.Tatsis-Kotsidis I, Erlanger BF. Initiation of a process of differentiation by stable transfection of ob17 preadipocytes with the cDNA of human A1 adenosine receptor. Biochem Pharmacol. 1999;58:167–170. doi: 10.1016/s0006-2952(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 535.Taugner G, Wunderlich I, John F. Distribution and metabolic fate of adenosine nucleotides in the membrane of storage vesicles from bovine adrenal medulla. Naunyn Schmiedebergs Arch Pharmacol. 1979;309:29–43. doi: 10.1007/BF00498754. [DOI] [PubMed] [Google Scholar]
- 536.Teraoka K, Morita K, Oka M, Hamano S. Influence of cytoplasmic ATP reduction on catecholamine synthesis in cultured bovine adrenal chromaffin cells. Neurochem Int. 1991;18:283–289. doi: 10.1016/0197-0186(91)90196-k. [DOI] [PubMed] [Google Scholar]
- 537.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 538.Thakkar MM, Winston S, McCarley RW. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–194. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- 539.Thirion S, Troadec JD, Nicaise G. Cytochemical localization of ecto-ATPases in rat neurohypophysis. J Histochem Cytochem. 1996;44:103–111. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- 540.Tice LW, Creveling CR. Electron microscopic identification of adrenergic nerve endings on thyroid epithelial cells. Endocrinology. 1975;97:1123–1129. doi: 10.1210/endo-97-5-1123. [DOI] [PubMed] [Google Scholar]
- 541.Tomé ÂR, Castro E, Santos RM, Rosário LM. Functional distribution of Ca2+-coupled P2 purinergic receptors among adrenergic and noradrenergic bovine adrenal chromaffin cells. BMC Neurosci. 2007;8:39. doi: 10.1186/1471-2202-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Tomic M, Jobin RM, Vergara LA, Tojilkovic S. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 543.Töpfer M, Burbiel CE, Müller CE, Knittel J, Verspohl EJ. Modulation of insulin release by adenosine A1 receptor agonists and antagonists in INS-1 cells: the possible contribution of 86Rb+ efflux and 45Ca2+ uptake. Cell Biochem Funct. 2008;26:833–843. doi: 10.1002/cbf.1514. [DOI] [PubMed] [Google Scholar]
- 544.Törnquist K. Depolarization of the membrane potential decreases the ATP-induced influx of extracellular Ca2+ and the refilling of intracellular Ca2+ stores in rat thyroid FRTL-5 cells. J Cell Physiol. 1991;149:485–491. doi: 10.1002/jcp.1041490318. [DOI] [PubMed] [Google Scholar]
- 545.Törnquist K. Calcium fluxes in rat thyroid FRTL-5 cells. Evidence for Ca2+ entry after stimulation with ATP. Mol Cell Endocrinol. 1991;79:147–156. doi: 10.1016/0303-7207(91)90105-2. [DOI] [PubMed] [Google Scholar]
- 546.Törnquist K, Ekokoski E, Dugué B. Purinergic agonist ATP is a comitogen in thyroid FRTL-5 cells. J Cell Physiol. 1996;166:241–248. doi: 10.1002/(SICI)1097-4652(199602)166:2<241::AID-JCP1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 547.Torres M, Pintor J, Miras-Portugal MT. Presence of ectonucleotidases in cultured chromaffin cells: hydrolysis of extracellular adenine nucleotides. Arch Biochem Biophys. 1990;279:37–44. doi: 10.1016/0003-9861(90)90460-g. [DOI] [PubMed] [Google Scholar]
- 548.Tosca L, Froment P, Solnais P, Ferre P, Foufelle F, Dupont J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology. 2005;146:4500–4513. doi: 10.1210/en.2005-0301. [DOI] [PubMed] [Google Scholar]
- 549.Troadec JD, Thirion S. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuroendocrinol Lett. 2002;23:273–280. [PubMed] [Google Scholar]
- 550.Troadec JD, Thirion S, Nicaise G, Lemos JR, Dayanithi G. ATP-evoked increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals via a P2X2 purinoceptor. J Physiol. 1998;511:89–103. doi: 10.1111/j.1469-7793.1998.089bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Troadec JD, Thirion S, Petturiti D, Bohn MT, Poujeol P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes) Pflugers Arch. 1999;437:745–753. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- 552.Troadec JD, Thirion S, Petturiti D, Poujeol P. Potassium efflux triggered by P2Y purinoceptor activation in cultured pituicytes. Pflugers Arch. 2000;440:770–777. doi: 10.1007/s004240000343. [DOI] [PubMed] [Google Scholar]
- 553.Trost T, Schwabe U. Adenosine receptors in fat cells. Identification by (-)-N6-[3H]phenylisopropyladenosine binding. Mol Pharmacol. 1981;19:228–235. [PubMed] [Google Scholar]
- 554.Tudurí E, Filiputti E, Carneiro EM, Quesada I. Inhibition of Ca2+ signaling and glucagon secretion in mouse pancreatic alpha-cells by extracellular ATP and purinergic receptors. Am J Physiol Endocrinol Metab. 2008;294:E952–E960. doi: 10.1152/ajpendo.00641.2007. [DOI] [PubMed] [Google Scholar]
- 555.Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, Cyr DG. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78:124–138. doi: 10.1002/mrd.21280. [DOI] [PubMed] [Google Scholar]
- 556.Turpin BP, Duckworth WC, Solomon SS. Perifusion of isolated rat adipose cells. Modulation of lipolysis by adenosine. J Clin Invest. 1977;60:442–448. doi: 10.1172/JCI108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Uchida Y, Nomoto T. Intravenously infused adenosine increases the blood flow to brown adipose tissue in rats. Eur J Pharmacol. 1990;184:223–231. doi: 10.1016/0014-2999(90)90613-b. [DOI] [PubMed] [Google Scholar]
- 558.Uchiyama M, Nakajima Y, Sakuma Y, Kato M. Purinergic regulation of intracellular Ca2+ concentration of rat pituitary folliculo-stellate cells in primary culture. J Neuroendocrinol. 2001;13:378–385. doi: 10.1046/j.1365-2826.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 559.Uchiyama Y, Murakami G, Ohno Y. The fine structure of nerve endings on rat thyroid follicular cells. Cell Tissue Res. 1985;242:457–460. doi: 10.1007/BF00214563. [DOI] [PubMed] [Google Scholar]
- 560.Ulate G, Scott SR, Gilabert JA, Artalejo AR. Purinergic modulation of Ca2+ channels and exocytosis in bovine chromaffin cells. Drug Dev Res. 2001;52:89–94. [Google Scholar]
- 561.Unsicker K. On the innervation of the rat and pig adrenal cortex. Z Zellforsch Mikrosk Anat. 1971;116:151–156. doi: 10.1007/BF00332863. [DOI] [PubMed] [Google Scholar]
- 562.Vainio M, Saijonmaa O, Fyhrquist F, Törnquist K. Purinergic agonists stimulate the secretion of endothelin-1 in rat thyroid FRTL-5 cells. J Cell Physiol. 1996;169:538–543. doi: 10.1002/(SICI)1097-4652(199612)169:3<538::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 563.Vainio M, Saarinen P, Törnquist K. Adenosine inhibits DNA synthesis stimulated with TSH, insulin, and phorbol 12-myristate 13-acetate in rat thyroid FRTL-5 cells. J Cell Physiol. 1997;171:336–342. doi: 10.1002/(SICI)1097-4652(199706)171:3<336::AID-JCP12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 564.Vainio M, Fredholm BB, Törnquist K. Thyrotropin regulates adenosine A1 receptor expression in rat thyroid FRTL-5 cells. Br J Pharmacol. 2000;130:471–477. doi: 10.1038/sj.bjp.0703325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Valdecantos P, Briones R, Moya P, Germain A, Huidobro-Toro JP. Pharmacological identification of P2X1, P2X4 and P2X7 nucleotide receptors in the smooth muscles of human umbilical cord and chorionic blood vessels. Placenta. 2003;24:17–26. doi: 10.1053/plac.2002.0862. [DOI] [PubMed] [Google Scholar]
- 566.van der Merwe PA, Wakefield IK, Fine J, Millar RP, Davidson JS. Extracellular adenosine triphosphate activates phospholipase C and mobilizes intracellular calcium in primary cultures of sheep anterior pituitary cells. FEBS Lett. 1989;243:333–336. doi: 10.1016/0014-5793(89)80156-5. [DOI] [PubMed] [Google Scholar]
- 567.Vassaux G, Gaillard D, Mari B, Ailhaud G, Negrel R. Differential expression of adenosine A1 and A2 receptors in preadipocytes and adipocytes. Biochem Biophys Res Commun. 1993;193:1123–1130. doi: 10.1006/bbrc.1993.1742. [DOI] [PubMed] [Google Scholar]
- 568.Vázquez-Cuevas FG, Juárez B, Garay E, Arellano RO. ATP-induced apoptotic cell death in porcine ovarian theca cells through P2X7 receptor activation. Mol Reprod Dev. 2006;73:745–755. doi: 10.1002/mrd.20447. [DOI] [PubMed] [Google Scholar]
- 569.Vázquez-Cuevas FG, Zárate-Diaz EP, Garay E, Arellano RO. Functional expression and intracellular signaling of UTP-sensitive P2Y receptors in theca-interstitial cells. Reprod Biol Endocrinol. 2010;8:88. doi: 10.1186/1477-7827-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Veitinger S, Veitinger T, Cainarca S, Fluegge D, Engelhardt CH, Lohmer S, Hatt H, Corazza S, Spehr J, Neuhaus EM, Spehr M. Purinergic signalling mobilizes mitochondrial Ca2+ in mouse Sertoli cells. J Physiol. 2011;589:5033–5055. doi: 10.1113/jphysiol.2011.216309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Vernon RG, Finley E, Watt PW. Adenosine and the control of adrenergic regulation of adipose tissue lipolysis during lactation. J Dairy Sci. 1991;74:695–705. doi: 10.3168/jds.S0022-0302(91)78216-7. [DOI] [PubMed] [Google Scholar]
- 572.Verspohl EJ, Johannwille B, Waheed A, Neye H. Effect of purinergic agonists and antagonists on insulin secretion from INS-1 cells (insulinoma cell line) and rat pancreatic islets. Can J Physiol Pharmacol. 2002;80:562–568. doi: 10.1139/y02-079. [DOI] [PubMed] [Google Scholar]
- 573.Vila-Bedmar R, Lorenzo M, Fernández-Veledo S. Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology. 2010;151:980–992. doi: 10.1210/en.2009-0810. [DOI] [PubMed] [Google Scholar]
- 574.Vilhardt H, Hope DB. Adenosine triphosphatase activity in the neural lobe of the bovine pituitary gland. Biochem J. 1974;143:181–190. doi: 10.1042/bj1430181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Villalobos C, Alonse-Torre SR, Nuñez L, García-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol. 1997;273:C1963–C1971. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- 576.von Patay B, Kurz B, Mentlein R. Effect of transmitters and co-transmitters of the sympathetic nervous system on interleukin-6 synthesis in thymic epithelial cells. Neuroimmunomodulation. 1999;6:45–50. doi: 10.1159/000026363. [DOI] [PubMed] [Google Scholar]
- 577.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by imunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Wainwright SD, Wainwright LK. Purinergic receptors have no major role in control of the circadian rhythm in rate of thymidine incorporation by cultured chick pineal glands. J Pineal Res. 1991;10:186–189. doi: 10.1111/j.1600-079x.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 579.Wang CZ, Namba N, Gonoi T, Inagaki N, Seino S. Cloning and pharmacological characterization of a fourth P2X receptor subtype widely expressed in brain and peripheral tissues including various endocrine tissues. Biochem Biophys Res Commun. 1996;220:196–202. doi: 10.1006/bbrc.1996.0380. [DOI] [PubMed] [Google Scholar]
- 580.Wang G, Dayanithi G, Custer EE, Lemos JR. Adenosine inhibition via A1 receptor of N-type Ca2+ current and peptide release from isolated neurohypophysial terminals of the rat. J Physiol. 2002;540:791–802. doi: 10.1113/jphysiol.2002.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–327. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 582.Weir GC, Knowlton SD, Martin DB. Nucleotide and nucleoside stimulation of glucagon secretion. Endocrinology. 1975;97:932–936. doi: 10.1210/endo-97-4-932. [DOI] [PubMed] [Google Scholar]
- 583.White TD, Bourke JE, Livett BG. Direct and continuous detection of ATP secretion from primary monolayer cultures of bovine adrenal chromaffin cells. J Neurochem. 1987;49:1266–1273. doi: 10.1111/j.1471-4159.1987.tb10019.x. [DOI] [PubMed] [Google Scholar]
- 584.Wierowski JV, Lawton KG, Hockensmith JW, Bambara RA. Stimulation of calf thymus DNA α-polymerase by ATP. J Biol Chem. 1983;258:6250–6254. [PubMed] [Google Scholar]
- 585.Wilson SM, Pappone PA. P2 receptor modulation of voltage-gated potassium currents in brown adipocytes. J Gen Physiol. 1999;113:125–138. doi: 10.1085/jgp.113.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Wilson SM, Barsoum MJ, Wilson BW, Pappone PA. Purine nucleotides modulate proliferation of brown fat preadipocytes. Cell Prolif. 1999;32:131–140. doi: 10.1046/j.1365-2184.1999.32230131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1:65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- 588.Winkler H, Hortnagl H, Asamer H, Plattner H. Membrane proteins of catecholamine-storing vesicles in adrenal medulla and sympathetic nerves. Adv Exp Med Biol. 1972;32:69–81. doi: 10.1007/978-1-4684-6979-0_7. [DOI] [PubMed] [Google Scholar]
- 589.Winkler H, Schopf JA, Hortnagl H. Bovine adrenal medulla: subcellular distribution of newly synthesised catecholamines, nucleotides and chromogranins. Naunyn Schmiedebergs Arch Pharmacol. 1972;273:43–61. doi: 10.1007/BF00508079. [DOI] [PubMed] [Google Scholar]
- 590.Winkler H, Schmidt W, Fischer-Colbrie R, Weber A. Molecular mechanisms of neurotransmitter storage and release: a comparison of the adrenergic and cholinergic systems. Prog Brain Res. 1983;58:11–20. doi: 10.1016/S0079-6123(08)60002-3. [DOI] [PubMed] [Google Scholar]
- 591.Wolff J, Londos C, Cook GH. Adenosine interactions with thyroid adenylate cyclase. Arch Biochem Biophys. 1978;191:161–168. doi: 10.1016/0003-9861(78)90078-4. [DOI] [PubMed] [Google Scholar]
- 592.Wollmann G, Acuna-Goycolea C, van den Pol AN. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J Neurophysiol. 2005;94:2195–2206. doi: 10.1152/jn.00035.2005. [DOI] [PubMed] [Google Scholar]
- 593.Wong P. The basis of echinocytosis of the erythrocyte by glucose depletion. Cell Biochem Funct. 2011;29:708–711. doi: 10.1002/cbf.1806. [DOI] [PubMed] [Google Scholar]
- 594.Xia J, Chen F, Ye J, Yan J, Wang H, Duan S, Hu Z. Activity-dependent release of adenosine inhibits the glutamatergic synaptic transmission and plasticity in the hypothalamic hypocretin/orexin neurons. Neuroscience. 2009;162:980–988. doi: 10.1016/j.neuroscience.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 595.Xiang Z, Bo X, Oglesby IB, Ford APDW, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813:390–397. doi: 10.1016/s0006-8993(98)01073-7. [DOI] [PubMed] [Google Scholar]
- 596.Xiang Z, He C, Burnstock G. P2X5 receptors are expressed on neurons containing arginine vasopressin and nitric oxide synthase in the rat hypothalamus. Brain Res. 2006;1099:56–63. doi: 10.1016/j.brainres.2006.04.126. [DOI] [PubMed] [Google Scholar]
- 597.Xie L, Zhang M, Zhou W, Wu Z, Ding J, Chen L, Xu T. Extracellular ATP stimulates exocytosis via localized Ca2+ release from acidic stores in rat pancreatic beta cells. Traffic. 2006;7:429–439. doi: 10.1111/j.1600-0854.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 598.Xu L, Enyeart JJ. Adenosine inhibits a non-inactivating K + current in bovine adrenal cortical cells by activation of multiple P1 receptors. J Physiol. 1999;521:81–97. doi: 10.1111/j.1469-7793.1999.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Xu L, Enyeart JJ. Purine and pyrimidine nucleotides inhibit a noninactivating K+ current and depolarize adrenal cortical cells through a G protein-coupled receptor. Mol Pharmacol. 1999;55:364–376. doi: 10.1124/mol.55.2.364. [DOI] [PubMed] [Google Scholar]
- 600.Xu YP, Duarte EP, Forsberg EJ. Calcium dependency of muscarinic and nicotinic agonist-induced ATP and catecholamine secretion from porcine adrenal chromaffin cells. J Neurochem. 1991;56:1889–1896. doi: 10.1111/j.1471-4159.1991.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 601.Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and beta cell preservation. Prog Biophys Mol Biol. 2011;107:248–256. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 602.Yan X, Koos BJ, Kruger L, Linden J, Murray TF. Characterization of [125I]ZM 241385 binding to adenosine A2A receptors in the pineal of sheep brain. Brain Res. 2006;1096:30–39. doi: 10.1016/j.brainres.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 603.Yanagita Y, Okajima F, Sho K, Nagamachi Y, Kondo Y. An adenosine derivative cooperates with TSH and Graves' IgG to induce Ca2+ mobilization in single human thyroid cells. Mol Cell Endocrinol. 1996;118:47–56. doi: 10.1016/0303-7207(96)03765-3. [DOI] [PubMed] [Google Scholar]
- 604.Yang GK, Squires PE, Tian F, Kieffer TJ, Kwok YN, Dale N (2012) Glucose decreases extracellular adenosine levels in isolated mouse and rat pancreatic islets. Islets 4:64–70 [DOI] [PMC free article] [PubMed]
- 605.Yi E, Smith TG, Love JA. Noradrenergic innervation of rabbit pancreatic ganglia. Auton Neurosci. 2005;117:87–96. doi: 10.1016/j.autneu.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 606.Yildirim MK, Bagcivan I, Sarac B, Kilicarslan H, Yildirim S, Kaya T. Effect of hypothyroidism on the purinergic responses of corpus cavernosal smooth muscle in rabbits. Int Urol Nephrol. 2008;40:691–699. doi: 10.1007/s11255-008-9332-0. [DOI] [PubMed] [Google Scholar]
- 607.Yu Q, Guo W, Sun X, Xiang Z, He C, Burnstock G. Expression of P2Y receptors in the rat anterior pituitary. Purinergic Signal. 2011;7:207–219. doi: 10.1007/s11302-011-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, Zhao X, Burnstock G, Shi X, He C, Xiang Z. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res. 2011;344:222–237. doi: 10.1007/s00441-011-1155-0. [DOI] [PubMed] [Google Scholar]
- 609.Yu WH, Kimura M, Walczewska A, Porter JC, McCann SM. Adenosine acts by A1 receptors to stimulate release of prolactin from anterior-pituitaries in vitro. Proc Natl Acad Sci U S A. 1998;95:7795–7798. doi: 10.1073/pnas.95.13.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Yu Z, Jin T. Extracellular high dosages of adenosine triphosphate induce inflammatory response and insulin resistance in rat adipocytes. Biochem Biophys Res Commun. 2010;402:455–460. doi: 10.1016/j.bbrc.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 611.Zamoner A, Bruno AN, Casali EA, Corbelini PF, Diniz GP, Barreto-Chaves ML, Silva FR, Sarkis JJ, Pessoa-Pureur R. Genomic-independent action of thyroid hormones on NTPDase activities in Sertoli cell cultures from congenital hypothyroid rats. Life Sci. 2006;80:51–58. doi: 10.1016/j.lfs.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 612.Zapata R, Navarro A, Canela EI, Franco R, Lluis C, Mallol J. Regulation of L-type calcium channels in GH4 cells via A1 adenosine receptors. J Neurochem. 1997;69:2546–2554. doi: 10.1046/j.1471-4159.1997.69062546.x. [DOI] [PubMed] [Google Scholar]
- 613.Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol. 2006;20:1423–1436. doi: 10.1210/me.2005-0508. [DOI] [PubMed] [Google Scholar]
- 614.Zemková H, Balik A, Jindrichová M, Vávra V. Molecular structure of purinergic P2X receptors and their expression in the hypothalamus and pituitary. Physiol Res. 2008;57(Suppl 3):S23–S38. doi: 10.33549/physiolres.931599. [DOI] [PubMed] [Google Scholar]
- 615.Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–E651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Zhang P, Zheng J, Bradley ME, Hexum TD. ATP stimulated cyclic AMP formation in bovine chromaffin cells is enhanced by neuropeptide Y. Peptides. 2001;22:439–444. doi: 10.1016/s0196-9781(01)00354-0. [DOI] [PubMed] [Google Scholar]
- 617.Zhao LF, Iwasaki Y, Oki Y, Tsugita M, Taguchi T, Nishiyama M, Takao T, Kambayashi M, Hashimoto K. Purinergic receptor ligands stimulate pro-opiomelanocortin gene expression in AtT-20 pituitary corticotroph cells. J Neuroendocrinol. 2006;18:273–278. doi: 10.1111/j.1365-2826.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 618.Zheng J, Zhang P, Toews M, Hexum TD. Neuropeptide Y enhances ATP-induced formation of inositol phosphates in chromaffin cells. Biochem Biophys Res Commun. 1997;239:287–290. doi: 10.1006/bbrc.1997.7456. [DOI] [PubMed] [Google Scholar]
- 619.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Zimon A, Erat A, Von Wald T, Bissell B, Koulova A, Choi CH, Bachvarov D, Reindollar RH, Usheva A. Genes invoked in the ovarian transition to menopause. Nucleic Acids Res. 2006;34:3279–3287. doi: 10.1093/nar/gkl387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Zou N, Wu X, Jin YY, He MZ, Wang XX, Su LD, Rupnik M, Wu ZY, Liang L, Shen Y. ATP regulates sodium channel kinetics in pancreatic islet beta cells. J Membr Biol. 2013;246:101–107. doi: 10.1007/s00232-012-9506-7. [DOI] [PubMed] [Google Scholar]
- 622.Zsarnovszky A, Bartha T, Frenyo LV, Diano S. NTPDases in the neuroendocrine hypothalamus: possible energy regulators of the positive gonadotrophin feedback. Reprod Biol Endocrinol. 2009;7:63. doi: 10.1186/1477-7827-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Zywert A, Szkudelska K, Szkudelski T. Effects of adenosine A1 receptor antagonism on insulin secretion from rat pancreatic islets. Physiol Res. 2011;60:905–911. doi: 10.33549/physiolres.932165. [DOI] [PubMed] [Google Scholar]