Abstract
Purinergic signalling is involved in both the physiology and pathophysiology of the liver. Hepatocytes, Kupffer cells, vascular endothelial cells and smooth muscle cells, stellate cells and cholangiocytes all express purinoceptor subtypes activated by adenosine, adenosine 5′-triphosphate, adenosine diphosphate, uridine 5′-triphosphate or UDP. Purinoceptors mediate bile secretion, glycogen and lipid metabolism and indirectly release of insulin. Mechanical stress results in release of ATP from hepatocytes and Kupffer cells and ATP is also released as a cotransmitter with noradrenaline from sympathetic nerves supplying the liver. Ecto-nucleotidases play important roles in the signalling process. Changes in purinergic signalling occur in vascular injury, inflammation, insulin resistance, hepatic fibrosis, cirrhosis, diabetes, hepatitis, liver regeneration following injury or transplantation and cancer. Purinergic therapeutic strategies for the treatment of these pathologies are being explored.
Keywords: Purinoceptors, Diabetes, Cirrhosis, Hepatitis, Cancer, Fibrosis
Synopsis
Introduction
Receptor subtypes for purines and pyrimidines on hepatocytes
Actions of purines and pyrimidines in liver
ATP release from hepatocytes and biliary epithelium
Kupffer cells
Bile canaliculi and hepatic couplets
ATP breakdown and phosphohydrolysis in liver
Metabolism of glucose
Lipid metabolism and fatty acids
Cell volume regulation
Vascularity of liver
Purinergic signalling in liver disease
Inflammation, liver injury and immune regulation
Diabetes
Fibrosis and hepatic stellate cells
Cirrhosis
Cancer
Hepatitis
Ischaemia and vascular injury
Hepato-renal syndrome
Regeneration of liver
Liver transplantation
Conclusions and future directions
Introduction
The purinergic signalling hypothesis, i.e. adenosine 5′-triphosphate (ATP) acting as an extracellular signalling molecule, was proposed in 1972 [32]. In 1978, separate families of receptors for adenosine (P1) and ATP (P2) were identified [33]. Throughout the 1990s, various receptors for purines and pyrimidines were cloned and characterised. Nucleotide receptors were separated into P2X ligand-gated ion channel and P2Y G protein-coupled receptors [206]. Currently, four subtypes of P1 receptors (A1, A2A, A2B and A3), seven subtypes of P2X (P2X1-7) and eight subtypes of P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) are recognised [34]. ATP is established as a cotransmitter with classical transmitters in most, if not all, nerves in the peripheral and central nervous systems (see [35]), and receptors are expressed on many non-neuronal, as well as neuronal cell types (see [36]).
The liver is the largest internal organ in the body. The liver is a vital organ with a diverse range of functions including crucial metabolic pathways, carbohydrate metabolism, protein synthesis and detoxification and bile secretion, among others. These functions are absolutely necessary for survival and are chiefly performed by the parenchymal cells or hepatocytes. The necessity of these functions is best appreciated in the absence of adequate liver function. Here, roles of carbohydrate and glucose metabolism, detoxification and immunologic function are lost, and hyperglycemia, hepatic encephalopathy bleeding and infection inevitably result in demise without liver transplantation. Through this, one begins to appreciate the complexity of the liver in homeostasis in health and the resulting loss in disease.
Relevant to this review is that the liver synthesises most of the nucleotides in the body. These molecular compounds are released or can leak from cells and extracellular nucleotides [e.g. ATP, adenosine diphosphate (ADP), uridine 5′-triphosphate (UTP) and uridine diphosphate (UDP)] are key signalling molecules recognised by hepatocytes impacting metabolic processes. Specifically, extracellular nucleotides bind type-2 purinergic/pyrimidinergic (P2Y G protein-coupled receptors and P2X ATP-gated cation channels), whilst the phosphohydrolytic product adenosine is recognised by P1 adenosine receptors, as detailed above. These purinergic effects are closely regulated by ecto-enzymes termed ectonucleotidases (ecto-ADPases, ecto-ATPases, etc.) that hydrolyse extracellular nucleotides, ultimately to the respective nucleosides that often exert opposing effects.
There are a growing number of papers concerned with purinergic signalling in the liver, but only a few reviews [14, 22, 89, 120, 146]. Purinergic signalling regulates important hepatic processes such as bile secretion, glycogen metabolism and, indirectly, release of insulin. Biological stress may lead to alteration of release of nucleotides or uptake of nucleosides or may decrease enzyme function of ectonucleotidases [211].
Perturbations in purinergic signalling responses result in heightened inflammation, insulin resistance, vascular injury and abnormal liver cell regeneration. We have shown that high levels of extracellular nucleotides promote injury while adenosine can be protective during acute inflammatory settings as in vascular reperfusion [14] or with acetaminophen (APAP) toxicity [130]. In the liver, like many tissues, ethanol or fructose ingestion leads to an increase in adenine nucleotide release with both CD39 and ecto-5′-nucleotidase (CD73)-dependent extracellular increases in adenosine concentration. Chronic adenosine exposure as a direct result of ethanol or fructose metabolism and two of the adenosine receptors mediate ethanol-induced fatty liver disease by direct effects on lipid metabolism. Adenosine, however, also activates those cells that cause hepatic fibrosis and participates in a final common pathway leading not only to hepatic steatosis but also to fibrosis and ultimately to cirrhosis.
Thus, the liver is of major importance for both system nucleotide homeostasis as well as for local intercellular signalling within canalicular networks and biliary systems. In addition to hepatocyte parenchymal cells, the liver contains Kupffer, vascular endothelial and smooth muscle cells, stellate cells (fat stroma) and biliary canaliculi (cholangiocytes) all of which express purinoceptors. This review will examine purinergic signalling in first health and then in disease. Following recent advances, several of these divergent elements of the purinergic response are now susceptible to intervention. Non-selective adenosine receptor antagonists are in common clinical use (e.g. caffeine and aminophylline), and the more specific targeting of the adenosine receptor subtypes involved in liver injury and cirrhosis may reduce the toxicities associated with such nonselective antagonists as caffeine, theophylline and aminophylline.
Receptor subtypes for purines and pyrimidines on hepatocytes
Evidence for two Ca2+-mobilising purinoceptors on rat hepatocytes was reported [58], and ATP and 2-methylthio ATP (2-MeSATP) were claimed to act via different P2 receptors [149] probably by P2Y2 and/or P2Y4 receptors [219]. One is suramin-sensitive, coupled to phospholipase C (PLC) in a stimulatory manner; the other is suramin-insensitive and coupled to adenylate cyclase in an inhibitory manner [245]. 5′-[α,β-Methylene]-triphosphate potentiates oscillations in cytosolic [Ca2+]i of hepatocytes induced by ATP, but not ADP, again suggest two different P2 receptors are involved [59].
ATP and UTP have similar effects on activation of glycogen phosphorylase, generation of inositol 1,4,5-triphosphate (InsP3) and inhibition of glycogen synthase [152], suggesting that P2Y2 or P2Y4 receptors were involved. However, ADP and 2-MeSATP produced different effects from those of ATP [60], suggesting that P2Y1, P2Y12 or P2Y13 receptors might be present. In a later paper, it was shown that functional P2Y1 and P2Y2 receptors were expressed on rat hepatocytes [61]. Three different receptors to purines and pyrimidines were proposed to be present on liver plasma membranes, one involving activation of PLC, another activation of phospholipase D (PLD) and a third the inhibition of adenylate cyclase [146, 172]. The P2Y13 receptor is a key regulator of hepatocyte high-density lipoprotein (HDL) endocytosis by cultured hepatocytes as well as in situ in perfused mouse livers [137]. It was also shown that the P2Y13 receptor antagonist, AR-C69931MX, is a stimulator of this pathway and opens up the way for the design of new drugs able to increase HDL-cholesterol clearance, thus increasing the atheroprotective effect of the HDL [171]. ADP, acting via P2Y13 receptors, controls insulin signalling as well as lipoprotein secretion [44].
ATP-activated cation currents via P2X receptors have also been identified in hepatocytes [40]. In more recent papers, P2X4 and P2X7 receptor mRNA and protein were detected on rat hepatocytes and functional roles established [75, 103]. In a study of P2X receptors on immune cells in the rat liver during postnatal development, it was shown that P2X6 receptors were up-regulated by 15-fold on hepatic sinusoid cells during postnatal days P1 to P30, subpopulations of Kupffer cells co-expressed P2X4 and P2X6 receptor subtypes, and dendritic cells co-expressed P2X4 and P2X7 receptors [258]. The P2X6 receptor on Kupffer cells was substantially up-regulated by exposure of animals to lipopolysaccharide, suggesting that they may be evoked by endotoxin. ATP mediates calcium-dependent killing of isolated rat hepatocytes via P2X7 receptors [269]. Table 1 summarises the expression and function of P2 receptor subtypes by cellular compartments of the liver.
Table 1.
Expression and function of P2 receptors and ectonucleotidases by cellular components of the liver
| Cellular component | Expression | Function |
|---|---|---|
| Hepatocytes | P2Y1, P2Y2, P2Y4, P2Y6, P2Y13, NTPDase8 (apical membrane) | Glycogen metabolism, insulin resistance |
| Cholangiocytes | P2Y1, P2Y2, P2Y4, P2Y6, P2X2, P2X3, P2X4, P2X6 | Bile (anion) secretion, nucleotide salvage, canalicular contraction, interaction with hepatocytes, adenosine resorption from bile |
| Endothelial cells | P2Y1, P2Y2, P2Y6, P2X4, P2X7; NTPDase1 | NO release, secretion of prostaglandins E2 |
| Vascular smooth muscle cells | P2Y1, P2Y2, P2Y6, P2X4, P2X7 | Portal vein contraction |
| Hepatic stellate cells | P2Y2, P2Y4, P2Y6, NTPDase2 | Secretion of prostaglandin F2 and D2, cell contraction |
| Portal fibroblasts | NTPDase2 | Hepatic fibrosis |
| Kupffer cell/macrophages | P2Y1, P2Y2, P2Y4, P2Y6, P2X1, P2X4, P2X7; NTPDase1 | Killing of intracellular pathogens, secretion of prostaglandin E2, interleukin-6 |
| Liver-associated lymphocytes: NK, NKT, T cells, B cells | P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y14, P2X1, P2X2, P2X4, P2X7 NTPDase1 | Modulate concanavalin A-mediated hepatitis |
| Neutrophils | P2Y2 | Chemotaxis |
NK natural killer cells, NKT natural killer T cells.
Modified from [14].
There was early recognition of the effect of adenosine on hepatic cell activity [52, 212]. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues was shown in perfused rat liver [39, 227]. Adenosine produced dose-dependent stimulation of urea biosynthesis in hepatocytes [109] via all three P1 receptor subtypes, A1, A2 and A3 [110], although different second messengers are involved [112]. Adenosine reverses in vivo hepatic responsiveness to insulin [178]. Evidence for adenosine A2 subtype receptors on Kupffer cells was presented [209]. It was claimed that adenosine stimulates cyclic adenosine monophosphate (cAMP) formation and regulation of glycogenolysis and gluconeogenesis most likely through the A2B receptor subtype in rat hepatocytes [265]. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells (HSC; [45]).
Actions of purines and pyrimidines in liver
Early studies showed that ATP and ADP hyperpolarise guinea-pig liver cells and enhance the response to β-agonists that probably involve steps subsequent to receptor activation [138], perhaps by increasing K+ permeability [30]. Later, this group showed that quinine and apamin greatly reduced the effect of ATP on K+ content [31]. Extracellular ATP and ADP induce Ca2+ uptake into rat liver cells [131, 157, 166, 219]. Single cell measurements have shown increases in intracellular Ca2+ in T51B rat liver epithelial cells stimulated by ATP [26]. ATP induces intercellular Ca2+ waves in the Fischer 344 rat liver epithelial cell line (WB-F344) derived from normal liver [96]. Purine nucleotides increase the cellular level of InsP3 [43], suggesting that P2Y receptors might be involved. On bile epithelial cells, ATP has been shown to stimulate transepithelial secretion of potassium. This effect can be inhibited by suramin and 2-aminoethoxydiphenyl borate (InsP3 receptor inhibitor), suggesting that the mechanism is operative through P2Y-InsP3 coupled pathways. Adenosine did not change [Ca2+]i, but did increase cAMP, suggesting that P1 receptors were also involved.
Evidence has been presented that P2X4 receptors are functionally important in mediating ATP control of Na+ and Ca2+ transport and may be a mechanism for autocrine regulation of hepatic glycogen metabolism [75]. ATP (rather than ADP and AMP) was shown to cause hepatic cell degeneration [142] before the presence of P2X7 receptors (which are known to mediate apoptotic cell death on hepatocytes) was recognised. Hypoxic injury to hepatocytes is associated with ATP and blebbing occurs [116], another feature of P2X7 receptor activation. The role of P2X7 receptors in the control of liver homeostasis is discussed in recent reviews [81, 83].
UTP was more effective than ATP in regulating hepatocyte metabolism, ion fluxes and haemodynamics [121], suggesting that P2Y2 or P2Y4 receptors might be involved. Using ATPα35S as a radioligand, high-affinity P2Y receptors were identified in both human and rat liver plasma membranes [150], perhaps suggesting that they might be P2Y11 receptors.
ATP release from hepatocytes and biliary epithelium
ATP can be released by hepatocytes into different extracellular compartments via basolateral, sinusoidal or apical exocrine routes. Secretion of ATP into the bile is mediated by an increase in cholangiocyte cell volume, which stimulates nucleotide release by vesicular exocytosis [90, 99]. Movement of ATP from the intracellular compartment to the extracellular compartment in liver epithelium may occur via ATP channels or exocytosis, although it has been claimed that sustained release of ATP from liver cells is not mediated by vesicular exocytosis [66]. Exocytosis of ATP-containing vesicles can be in response to cell volume changes [91]. Hypotonicity resulted in cell-swelling triggering release of ATP from human Huh-7 hepatoma cells, followed by volume regulatory decrease [77]. Two ATP transport mechanisms were identified, one of which was vesicular exocytosis. Aside from volume changes, mechanical stress seems to cause release of ATP from both Kupffer cells and hepatocytes. It has been suggested in this scenario that ATP from hepatocytes is released from lysosomes by exocytosis [104]. The source of ATP involving various actions in the liver is likely to be by paracrine or autocrine release from hepatocytes [165, 251], but the possibility that ATP released as a cotransmitter from sympathetic nerves is another source of ATP has also been proposed [35].
Extracellular nucleotides are released into the canaliculus and modulate bile secretion. The biliary epithelium and hepatocytes constitutively release ATP into the bile [46]. In biliary epithelium, ATP is stored in vesicles and is released in response to cell swelling [99]; concentrations of canalicular adenine nucleotides in bile samples are estimated to be 5 ± 0.9 μM [88, 89]. Extracellular nucleotides entering the bile ducts are potent stimuli for secretion of bile fluid including anions, and canalicular contraction, which is, in part, explained by interaction and communication between hepatocytes and bile duct via local ATP release [216]. These effects are mediated and coordinated by apical P2Y2 receptors and NTPDase8 [69].
Bile acid ursodeoxycholic acid stimulated secretion of ATP by isolated hepatocytes and perfusion of ATP into bile duct segments induced Ca2+ signalling in bile duct epithelia [187]. The authors suggest that this may be used as a strategy for the treatment of secretary disorders of the liver.
Kupffer cells
Stimulation of parenchymal cell glycogenolysis by adenosine involves release of ATP from parenchymal cells and stimulation of eicosanoid release from Kupffer cells [190]. Nucleotide receptors responsive to both ATP and UTP are present on stellate (fat storing) cells in the rat that mediate contraction of the cells [237]. ATP release from Kupffer cells stimulated after mechanical stress promotes liver regeneration [104].
Bile canaliculi and hepatic couplets
Fluid absorption and secretion across intrahepatic bile duct units (IBDUs) play a key role in modifying the volume and composition of bile. Bile formation by the liver results from the combined complementary interactions and functions of two distinct liver cell types. Secretion is initiated by hepatic parenchymal cells (about 80 % of liver mass) that actively transport bile salts and other organic solutes into the canalicular space between cells. Subsequently, canalicular bile flows into the lumen of an extensive network of intrahepatic ducts, where it undergoes dilution and alkalinisation as a result of cholangiocyte Cl- and HCO3- secretion. P2Y1, P2Y2, P2Y4, P2Y6 and P2X4 receptor mRNA was identified in isolated, microperfused IBDUs using RT-PCR [69]. cAMP-induced secretion of bicarbonate from IBDUs involves apical release of ATP and stimulation of apical P2 receptors [180]. An interesting correlation is cystic liver disease. P2X4 receptors are expressed in liver cyst epithelia, and it was speculated that they mediate fluid secretion leading to increase in luminal pressure, causing cell proliferation and cyst expansion [64].
Contraction of bile canaliculi is ATP-dependent [154, 252]. External ATP regulates canalicular bile formation in isolated perfused rat liver, lowering bile flow, whilst inducing release of glucose and lactate [158]. ATP and UTP increase [Ca2+]i in human intrahepatic biliary epithelial cell lines [255], probably via P2Y2 or P2Y4 receptors. Cholangiocytes, which secrete Cl- and HCO3- in the intrahepatic bile ducts, are activated by purinergic receptors, which were assumed to be activated via autocrine and/or paracrine release of ATP [213]. Recent data suggests that vesicles containing ATP within the biliary epithelial cells are, in part, responsible for the initiation of purinergic signalling in the biliary system [215].
ATP breakdown and phosphohydrolysis in liver
Among all tissues, the liver has one of the highest ATPase and ADPase activities. Histochemical studies showed that most of liver ectonucleotidase activity was associated with the canalicular domain of hepatocytes [78]. Further studies revealed that NTPDase1 (CD39) is expressed by Kupffer cells and vascular endothelial cells [224], whereas NTPDase2 is produced by portal fibroblasts and activated HSC [70]. NTPDase2 is a preferential ATPase. This ectoenzyme expressed by these adventitial cells may have differential effects in inflammation and fibrogenesis that are related to generation of ADP.
More recently, cloning and biochemical characterisation of NTPDase8 in human and rat species and its identification as the hepatic canalicular ecto-ATPase/ATPDase with potential roles in nucleoside salvage from bile as well as biliary electrolyte/fluid secretion have been reported [82]. Deletion of CD39 results in hepatic insulin resistance [76]. Other ATPases, such as NTPDase3 and NTPDase5, have been found in hepatic tissue, but their functional relevance is not yet known.
Nucleotide pyrophosphatase/phosphodiesterase (NPP) has been localised on the basolateral membranes of hepatocytes and associated with hepatocellular growth [221]. NPP1 is absent during the foetal period and is decreased during liver degeneration [228]. Ecto-5′-nucleotidase (CD73) has been detected in the canalicular plasma membrane and on hepatic satellite cells [217]. Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis [199]. Coexpression of CD73 with specific NTPDases (NTPDase 1, 2 and 8) differentially regulated adenosine formation in rat liver [84]. A recent presentation has reported the presence of ATP synthase in rat hepatocyte plasma membrane as well as in mitochondria [167].
Metabolism of glucose
One of the major functions of the liver is the storage and production of glucose. Purinergic signalling has been shown to have an impact on almost every element glucose production and storage including glycogenolysis, gluconeogenesis, and glycolysis. Glycogenolysis is mainly mediated via the actions of glucagon. Noradrenaline (NA) and ATP released via the splanchnic nerve also serve to stimulate glycogenolysis. In fact glucagon hyperpolarises the liver cell membrane, partly by inducing the release of ATP to act on P2 receptors [93]. Extracellular ATP comes not only from the splanchnic nerve but also from surrounding hepatocytes and activated platelets [24]. Activation of P2Y1 receptors on rat and human hepatocytes stimulates glycogen phosphorylase [62, 63]. The mechanism involves increases in intracellular calcium as well as activating PLD, which may enhance hepatic glycogenolysis. In hepatocytes and perused livers, extracellular ATP stimulates glycogenolysis [38, 122, 147, 148, 151]. ATP, however, is rabidly degraded to adenosine, which, via the action of P1 receptors, increases both cAMP and intracellular calcium. This results in the activation of glycogen phosphorylase. Despite similar levels of activation of glycogen phosphorylase, adenosine is inferior to glucagon at increasing glucose production and even antagonises the stimulation of glycogenolysis by glucagon or cAMP [24]. Thus, whilst the net effect of ATP and adenosine in the liver is to stimulate glycogenolysis, it is unclear to what extent that plays a role in intracellular glucose concentrations. Hepatocyte heterogeneity in response to ATP in perfused rat liver has been described, including glycogenolysis to glucose predominantly in the periportal area, ATP is predominantly hydrolysed by a small hepatocyte population located at the perivenous outflow of the acinus, contractile elements (sphincters) exist near the inflow of the sinusoidal bed, and a portion of the Ca2+ mobilised by ATP is derived from liver cells that do not contribute to hepatocyte glucose output [122].
In isolated rat liver cells exposed to ATP (10μM), gluconeogenesis is increased, an effect that was mimicked to a lesser degree with adenosine [226]. The initial transient rise in [Ca2+]i evoked by ATP plays a significant role in triggering gluconeogenesis [155]. The effect, however, may be dependent on the carbon source for gluconeogenesis or the concentration of extracellular ATP. In isolated hepatocyte cells, gluconeogenesis from pyruvate and lactate (but not glycerol or fructose) is inhibited by ATP at high extracellular concentrations (1 mM), with adenosine producing a similar effect [9].
Stimulation of glycolysis by insulin in cultured hepatocytes is attenuated by ATP via inhibition of phosphofructokinase 2 [205]. The mammalian target of rapamycin (mTOR) pathway is one pathway utilised in hepatocytes to control glucose metabolism. Purinergic regulation (mainly P2Y1 and P2Y2) of certain hepatocyte functions, such as glucose metabolism, may be controlled by the mTOR pathway [47]. It has been claimed that activation of A2B receptors modulates glucose homeostasis and obesity [141].
Lipid metabolism and fatty acids
The effects of ATP on hepatic fatty acid metabolism have been studied and it was shown that inhibition of acetyl-CoA carboxylase activity by ATP may be mediated by elevation of [Ca2+]i, whereas carnitine O-palmitoyltransferase may be inhibited through a protein kinase C-dependent mechanism [113]. A slight decrease in intracellular ATP coincided with stimulation of fatty acid biosynthesis from glucose in rat hepatocytes, whilst further lowering of intracellular ATP led to a gradual inhibition [100]. It has been reported recently that A1 receptors do not play a major role in the regulation of lipogenic gene expression in hepatocytes [262].
Reverse cholesterol transport has been shown to be, in part, modulated by purinergic signalling. Chronic activation of P2Y13 receptors decreases HDL-cholesterol levels in mice [223]. P2Y13-deficient mice had decreased hepatic HDL cholesterol uptake. In addition to decreased HDL uptake, hepatic cholesterol transport and biliary cholesterol output (but not plasma HDL) were also decreased [79]. P2Y13 knockout mice have also been shown to have lower faecal concentrations of sterols indicating a role for purinergic signalling in cholesterol transport to the faeces [23].
Cell volume regulation
ATP, acting via P2 receptors, is a critical determinant of membrane Cl- permeability and cell volume regulation [90]. In response to hypotonic solution, rat hepatoma cells release ATP into the extracellular environment and rapidly activate volume-sensitive outward-rectifying Cl- channels [246].
Vascularity of liver
Portally infused ATP leads to trans-hepatic vasodilatation via P2Y receptors on the endothelium, whilst ATP released from sympathetic nerves leads to vasoconstriction of hepatic arteries via P2X receptors on the muscle [27, 28, 203]. In the sinusoidal vascular bed, ATP impairs the flow-limited distribution of [3H]water [92]. Both endothelium-dependent and endothelium-independent vasodilation of rabbit hepatic artery mediated by purines has been described [28]. In the ATP perfused liver, both P2X vasoconstrictor and P2Y vasodilator receptors [leading to release of nitric oxide (NO)] were identified in the hepatic vascular bed [177, 207]. Adenosine-induced dilation of the rabbit hepatic arterial bed is mediated by A2 receptors [176]. ATP-magnesium chloride (MgCl2) restores depressed hepatocellular function and hepatic blood flow following haemorrhage and resuscitation [249].
There is evidence for two types of P2 receptor on the longitudinal muscle wall of the portal vein [145]. ATP dilates the portal circulation via P2Y receptors on endothelial cells leading to release of NO [238]. The non-adrenergic contractile response of the guinea-pig portal vein to electrical field stimulation mimics the response to UTP, but not ATP [136], perhaps indicative of the presence of a P2Y2 or P2Y4 receptor on smooth muscle. P2X1 receptors have also been identified on rat portal vein myocytes [183, 196].
Purinergic signalling in liver disease
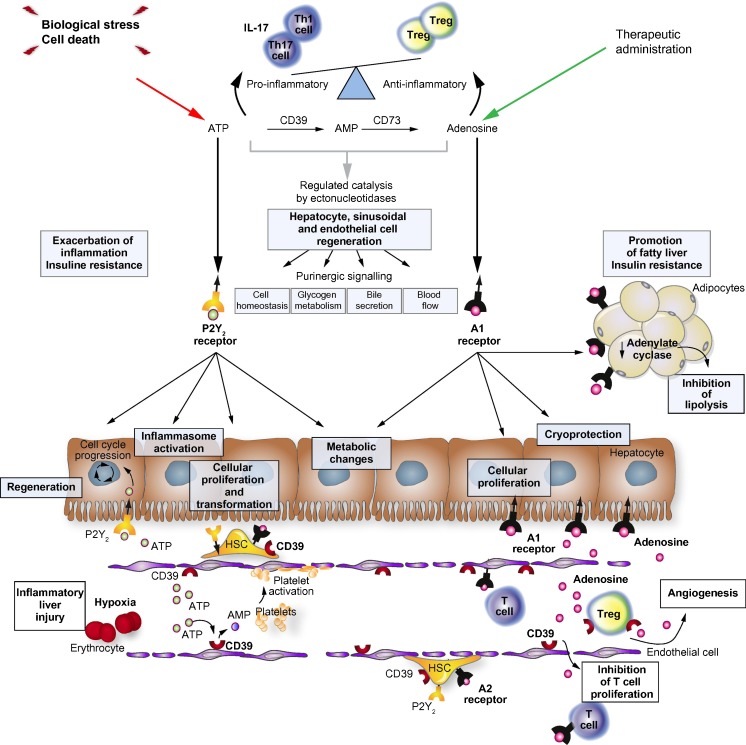
Readers are referred to a recent review entitled ‘Pathological roles of purinergic signalling in the liver’ with an emphasis on potential future clinical applications ([247]; see Fig. 1).
Fig. 1.
Schematic overview of the complex interplay mediated between extracellular ATP and adenosine by ectonucleotidases within the liver. As illustrated, purinergic signalling influences multiple cell types in the liver and in the hepatic sinusoids to impact processes of inflammation, metabolism and parameters of insulin signalling, cellular regeneration, healing, fibrosis and cell transformation. As an example, extracellular ATP arising from biologic stress can incite hepatocytic inflammation mediated by P2X7 and the inflammasome, whilst driving parenchymal cell proliferation via P2Y2. With ATP metabolized to adenosine by ectonucleotidases expressed by non-parenchymal sinusoidal cellular surfaces, the inflammatory response is down-regulated and tissue remodelling develops. Induction of CD39 expression by immune suppressive T regulatory cells as well as endothelial cells with associated alterations in sinusoidal vascular responsiveness and hepatic stellate cell (HSC) responses. Heightened levels of adenosine generated over a protracted time may result in increased fibrosis, as formed by myofibroblasts and activated stellate cells. These considerations and the crucially important levels of nucleotide:nucleoside balance controlled by CD39 have important implications for the development of purinergic therapies in liver transplantation and inflammatory fibrotic liver diseases. (Reproduced from [247] with permission—License number: 3172040541217; license date: 6/18/2013; Journal of Hepatology)
Inflammation, liver injury and immune regulation
Purinergic signalling is one mechanism by which the immune response is regulated in the liver. UTP and ATP stimulate thromboxane release from perfused liver [123]. Adenosine, resulting from the breakdown of ATP, inhibits the incorporation of radiolabelled leucine into proteins in isolated hepatocytes [244]. Alcohol-induced liver injury is associated with enhanced inflammatory responses and it has been reported that adenosine, acting via A2A receptors, may be a useful anti-inflammatory pathway for reducing these effects [204]. In another study, it was claimed that endogenous A1 receptor activation protects mice against acute ethanol-induced liver injury by reducing oxidative stress and decreasing lipid accumulation [264]. In cholestasis, bile flow from the liver is impaired, resulting in liver injury. In mice lacking the A1 receptor, there was attenuation of α-naphthylisothiocyanate-induced cholestatic liver injury [263]. Prostaglandin (PG) F2α and PGD2 are released from rat liver cells after stimulation by ATP, but not adenosine [10]. Prostanoid secretion by rat hepatic sinusoidal endothelial cells is regulated by ATP [119].
Liver plasma membrane properties have been used for studies of nucleotide signalling. For example, phosphatidylcholine breakdown by rat liver plasma membrane was increased by guanosine triphosphate (GTP; [135]). Ca2+-ATPase is located in hepatocyte plasma membranes and is involved in calcium homeostasis [57]. A functionally active ecto-F0F1-ATP synthase has been identified on the plasma membrane of hepatocytes that mediates extracellular formation from ADP and inorganic phosphate [173]. Membrane-bound ATP synthesis has a role in modulating the concentrations of extracellular ADP and is regulated by a plasma apoliproprotein [175]. Synergistic activation of mitogen-activated protein kinase by insulin and ATP in liver cells has been demonstrated [114]. Autophagy, i.e. autophage-lysosomal degradation, in isolated hepatocytes is inhibited by purine nucleotides and nucleosides [156]. Activation of P2Y2 receptors plays a key role in endotoxin-induced acute liver injury in mice [214]. P2Y2 receptors mediate neutrophil infiltration, thereby regulating immune responses associated with hepatocyte death in mice with acute liver injury, and it was suggested that P2Y2 receptor antagonists might be used to treat inflammatory liver disease [11]. A review about purinergic signalling during sterile liver injury has been published recently [195].
Diadenosine triphosphate and tetraphosphate, probably acting via P2Y receptors, like ATP regulate hepatic haemodynamics and metabolism involving complex interactions between parenchymal and non-parenchymal cells [37, 74]. Adenine dinucleotide-related cytosolic free Ca2+ oscillations in single hepatocytes have been reported [107]. Adenine dinucleotides activate glycogen phosphorylase in isolated liver cells [53]. The metabolic and haemodynamic effects of extracellular nicotine adenine dinucleotide are caused mainly by interactions with purinergic receptors with a highly significant participation of its main transformation product ADP-ribose [29].
Hepatocyte lipoapoptosis induced by saturated free fatty acids contributes to hepatic inflammation in lipotoxic liver injury. It has been suggested that pannexin1 may play an important role in hepatic inflammation by mediating an increase in ATP release in lipotoxic liver injury [259]. APAP is often used to treat fever and pain, but it can cause damage to hepatocytes. In mouse models of APAP-induced inflammation, full injury after overdose required P2X7 receptor activation [4]. P2X7 is required for hepatic caspase-1 activation and migration of neutrophils into the liver. This suggests that extracellular ATP may play a pivotal role in development of the inflammasome after APAP overdose [130]. The P2X7 receptor antagonist A438079 is protective against APAP-induced liver injury, and it was claimed that it acted by inhibiting P450 isoenzymes rather than by inflammasome activation [260].
It has been suggested that ATP affects polymerisation of cytoskeletal elements [143]. Extracellular ATP induces rapid cell rounding in cultured human Chang liver cells (ATCC CCL B) [225]. Roles of natural killer (NK) T cells and relationships with stellate cells as modulated by local mediators is controversial and of great interest in both murine models and in clinical studies. Early studies suggest that T-, NK and NKT cells are exquisitely susceptible to regulation by purinergic and the adenosinergic microenvironment within inflamed tissues.
Hepatic encephalopathy is a neuropsychiatric syndrome that is a complication of either acute or chronic impaired liver function. It is the result of the inability of the liver to clear various “toxins” (often amino acids) from the portal tract. Adenosine had been postulated to have anticonvulsive effects and potentially anti-ischaemic effects in the brain [68]. Thus, it was postulated that adenosine could have a therapeutic effect [218].
Diabetes
Liver dysfunction can produce diabetic syndrome or may be secondary to it. ATP has been shown to increase insulin secretion in normal and alloxan diabetic rats and influence liver function [235]. An increase in adenosine A1 receptor expression was claimed in the liver of streptozotocin (STZ)-induced diabetic rats [168]. A later paper showed a significant increase in A2A and A3 receptor mRNA levels, whilst A2B receptor mRNA decreased and A1 receptors were unchanged; administration of insulin for 4 days to the STZ rats led to return to control levels of P1 receptor expression [106]. In addition, CD39 knockout mice have higher plasma insulin levels following a glucose challenge. Whilst the mice do not develop obesity, they do have higher leptin levels. It is suggested that CD39 acts in the metabolic pathway directly via insulin receptor substrate-2 phosphorylation; however, it is also likely that there are indirect mechanisms through altered inflammatory responses. The end result, however, is that aberrations in the ability to process extracellular ATP lead to insulin resistance and likely is involved in the complex pathogenesis of diabetes [76]. This process may indicate an important metabolic cross-link between inflammation and insulin resistance in other disease states.
Fibrosis and HSCs
Liver fibrosis with subsequent cirrhosis is the most common cause of liver failure. Cell types implicated in hepatic fibrosis are becoming better defined. The most relevant effector cells are activated HSC, ductular epithelial cells and (portal and perivascular) fibroblasts. More recently, it has become evident that a minor proportion of fibroblastic cells originate from bone marrow-derived circulating fibrocytes. HSC appear to be the primary fibrogenic cells of the liver, and these express functional nucleotide receptors [159, 237], which mediate PLD activity [20]. Adenosine A2B receptors have also been identified in human HSC, which play pro-fibrotic roles [266]. During fibrosis, HSC undergo proliferation and senescence and it has been reported that A2A receptors mediate both these key processes, making A2A receptor antagonists potential antifibrotics [1]. Quiescent stellate cells express P2Y2 and P2Y4 receptors activated by UTP and ATP, whereas activated stellate cells express P2Y6 receptors responding to UDP [71]. These authors have also shown that activation of these P2Y receptors regulates procollagen-1 transcription. They suggest that these cells may be a target to intervene therapeutically to prevent liver fibrosis and preclude development of cirrhosis and chronic liver failure. The P2 receptor antagonist, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate, inhibited proliferation of HSC and prevented non-biliary liver fibrosis [72]. NTPDase2 is produced by portal fibroblasts and activated HSC [71]. NTPDase2 is a preferential ATPase that appears to have major effects in inflammation and biliary type fibrogenesis that are related to generation of ADP. Ecto-5′-nucleotidase (CD73) activity was found to be higher in the quiescent, rather than in the activated phenotype of a HSC line, suggesting that ecto-5′-nucleotidase-dependent adenosine production may play a role in the regulation of quiescent HSC functions [6]. However, it has been claimed recently that ecto-5′-nucleotidase (CD73) gene expression increased in both HSC and portal fibroblasts during myofibroblastic differentiation and represents a promising target for antifibrotic therapy [85].
A2A receptors play an active role in the pathogenesis of hepatic fibrosis and it has been proposed that A2A receptor antagonists will inhibit ethanol-induced fibrosis and stellate cell activation [48, 49, 234]. Fatty liver is associated with alcohol abuse and it has been reported that adenosine, generated by ethanol metabolism, plays a role in ethanol-induced hepatic steatosis via both A1 and A2B receptors and it was suggested that targeting these adenosine receptors may be effective for the prevention of alcohol-induced fatty liver [210]. The A2B receptor antagonist, MRS1754, reduces hepatic collagen deposition during fibrosis progression [229].
In a recent paper, it has been claimed that ATP released from human platelets contributes to suppression of both human HSC activation and type I collagen production in vitro [132]. It was suggested further that an adenosine-cAMP signalling pathway mediated this process. In progressive fibrosis, eicosapentaenoic acid replenishes hepatic ATP levels resulting in reduction of inflammation and steatosis [140].
Cirrhosis
ATP-MgCl2 has been used to improve survival following massive hepatectomy among cirrhotic rats [127]. It has also been used to treat trauma-haemorrhage and resuscitation, which may be due, in part, to the restoration of P2 receptor binding capacity and the enhancement of receptor affinity [170]. Blockade of intrahepatic adenosine receptors improved urine excretion in cirrhotic rats induced by thioacetamide [182]. Adenosine partially reversed cirrhosis induced by carbon tetrachloride in rats [124] and reversed induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation [125]. A2A receptors play an active role in the pathogenesis of hepatic cirrhosis [42]. During fulminant hepatitis, A2A receptors play an important role in the physiological anti-inflammatory mechanisms that limit liver damage [50]. Adenosine receptor blockade reduces splanchnic hyperaemia in cirrhotic rats [162]. Adenosine deaminase activity was elevated during tuberculous peritonitis in patients with underlying liver cirrhosis [164].
As cirrhosis progresses, the normal response to increased portal venous vascular resistance of decreasing hepatic artery vascular resistance is decreased. The process is mediated by adenosine in normal livers. In cirrhotic livers, the adenosine-mediated vasodilation of the hepatic artery is exaggerated leading to a greater vasodilator effect of the hepatic artery to adenosine [268].
Platelet dysfunction in cirrhosis may also be mediated, in part, by purinergic signalling. Patients who had variceal bleeding had platelets that displayed low levels of impaired actin polymerisation compared to both non-bleeders and controls. The authors suggest this may be related to cytosolic calcium levels [7]. Cerebral A1 receptors have been implicated in liver cirrhosis [25]. Ectonucleotidase NTPDase 2 is selectively down-regulated in biliary cirrhosis [70].
Cancer
Liver cancer exhibits chronic inflammation, which is associated with aberrant cell proliferation, disordered metabolism and immune dysregulation. Deletion of CD39 promotes the development of both induced and spontaneous liver cancer in contrast to the effects on transplanted tumours to the liver [233]. These manifestations are comparable to what has been observed with Entpd5 studies and knockout mice [80, 208].
Calcium uptake by rat hepatoma cells is increased by ATP [13]. Nucleotide receptors mediated activation of cation, potassium and chloride currents in HTC cells from a rat liver tumour line [94]. There was increased incidence of spontaneous and induced hepatocellular carcinoma with associated metabolic perturbations in CD39 knockout mice [256].
ENTPD5/CD39L4 is a related ectoenzyme to CD39 and is a soluble endoplasmic reticulum UDPase involved in intracellular purine metabolism, which promotes glycolysis as well as proliferation in cancer cells via the PTEN signalling pathway [80]. Interestingly, there are also contrasting and somewhat overlooked roles of this ectonucleotidase in the suppression of liver cancer development [208] vs. the promotion of transplanted tumour growth in mice [80]. ATP infusions into the intraperitoneal space of a two-stage rat model of hepatocarcinogenesis displayed an increase of preneoplastic foci in the liver. In this experiment, ATP and adenosine altered the balance of apoptosis and proliferation towards malignancy [98]. In developed malignancy, however, ATP plays in important role as an early danger signal to the immune system. ATP released from necrotic cells stimulates neighbouring cells to die. CD39 is an ectonucleotidase that converts ATP to AMP. CD39 expression on endothelial cells suppresses the anti-tumour effect of ATP [87]. Swelling-induced ATP release results in activation of P2X4 receptors, which leads to modulation of volume-sensitive outwardly rectifying chloride channels in rat hepatoma cells [246]. Ecto-NTPDase2, which converts ATP to ADP, was expressed on human Huh-7 hepatoma cells and ADP then activates P2Y13 receptors, which mediate volume regulatory decrease [77]. The generation of adenosine both inhibits T cell proliferation and promotes angiogenesis, which ultimately is permissive of the growth of malignant cells [231].
Metastatic melanoma from mouse livers that were CD39 null had more ATP and less tumour cell growth. In addition, CD39 expression on T regulatory cells plays a suppressive role on NK cell-mediated tumour suppression of metastatic tumours to the liver. Thus, it further supports the role of purinergic signalling as an important and potentially therapeutic modulator in tumour biology in the liver. High concentrations of ATP switched autophagy to apoptosis in anchored and non-anchored human hepatoma cell lines [253]. The authors suggest that this work provides evidence that explains how hepatoma cells escape from ATP-induced cytotoxicity as well as offering another clue about effective manipulation of liver cancer.
The hepatoma call line N1S1-67 has been used to study signal transduction activated by ATP via P2Y2 or P2Y4 subtypes [201]. Increase in intracellular calcium leads to the opening of Ca2+-activated K+ channels and membrane hyperpolarisation. Intra-arterial injection of an inhibitor of ATP production has been proposed as a novel therapy for liver cancer [101]. Release of ATP is, at least in part, by vesicular exocytosis from HTC cells, and a Cl- channel inhibitor has been used to specifically stimulate ATP release through exocytotic mechanisms [65]. Hepatoma cell growth inhibition by adenosine was reported [115]. The A3 receptor agonist, CF101, caused inhibition of liver metastasis (following colon carcinoma) [12]. Human hepatocellular carcinoma HepG2 cells expressed high-affinity A1 receptors, which mediated decreased AMP and erythropoietin production [193]. ATP, via the A3 adenosine receptor, induced cell apoptosis of the human hepatoma cell line Li-7A [12, 254]. A2B receptors were highly expressed in human hepatoma carcinoma [257].
Hepatitis
Suramin was shown to inhibit in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus [202]. An influence of sympathetic nerves in immune-mediated experimental hepatitis has been demonstrated [188], and ATP released as a cotransmitter might be involved. P2X7 receptors regulate NKT cells in autoimmune hepatitis [144]. In fact, while working with concanavalin A models for NKT cell-mediated inflammation used to study immune liver disease, deletion of CD39 was noted to be protective against liver injury [15]. This suggested that modulation of NKT cell activation by novel pharmacologic therapies could quell inflammation and injury. P2X7 receptor-mediated responses are needed for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus [241]. Chronic hepatitis C virus (HCV) infection results in progressive liver disease including fibrosis, cirrhosis, insulin resistance and eventually hepatocellular carcinoma. The mechanism of ATP binding has been explored to facilitate targeting of the ATP-binding site for potential therapeutic development for hepatitis C [197]. It has been suggested that P2X4 receptors are a major component of the purinergic signalling complex in HCV-induced liver pathogenesis [174].
Inosine triphosphate (ITP) is broken down by ITPase (ITPA). A protective effect of ITPA gene variants against ribavirin associated anaemia has been reported [86]. ITPA deficiency results in the build-up of ITP that may alter the pharmacokinetics of ribavirin. Ribavirin has been associated with low levels of intracellular ATP which is part of the pathogenesis of anaemia. High levels of ITP, such as those from deficiency ITPA, allow ITP to substitute for GTP in the generation of AMP, which may be how high ITP levels attenuate the ribavirin-induced anaemia [128].
A2a receptor activation prevents hepatocyte lipotoxicity and non-alcoholic steatohepatitis in rats [134].
Ischaemia and vascular injury
That infusion of ATP-MgCl2 improved hepatic function and survival after hepatic ischaemia was recognised early [97, 126, 194]. It was also effective following reperfusion [51]. The beneficial effect of ATP-MgCl2 treatment following trauma-haemorrhage may be associated with a down-regulation of the circulating levels of the inflammatory cytokines tumour necrosis factor and interleukin-6 [250]. It was also suggested that reduction of ischaemic damage by ATP-MgCl2 infusion may be mediated through improvement in mitochondrial energy metabolism [139]. Treatment of ischaemia by ATP was particularly effective in old mice; aging of the liver is related to mitochondrial dysfunction [222]. During 60 min of ischaemia, there is a 90 % ATP loss from hepatocytes [108]. Hepatocyte resistance to hypoxia is promoted via P2Y2 receptors by down-modulating ERK1/2-mediated signals that promote Na+ influx through the Na+/H+ exchanger [41]. Vascular NTPDase activity was lost after hepatic ischaemia and reperfusion injury and deletion of NTPDase1 in mice led to increased injury and decreased survival [133]. Also deletion of CD39 in NK cells attenuated hepatic ischaemia/reperfusion injury in mice, suggesting that ATP modulates NK cell function during liver regeneration. NK cells that lack the CD39 gene had less secretion of interferon gamma in response to inflammatory mediators. This probably, in part, accounts for the decrease in tissue damage after ischaemia reperfusion injury [17]. Interestingly, vascular CD39, however, seems to have a protective role in hepatic ischaemia reperfusion injury. CD39-null and heterogeneous mice had decreased survival compared to wildtype after an induced model of ischaemia. The CD39 deficient mice that received adenosine were protected from reperfusion injury [232].
Adenosine can also play a protective role against ischaemia reperfusion injury [73, 189] probably by activation of A2 receptors [8, 200], especially A2A receptors [18, 55, 56, 160]. Administration of an adenosine A1 receptor antagonist before ischaemia attenuated ischaemia-reperfusion injury [153, 169]. Oxidative preconditioning by ozone was mediated by A1 receptors in a rat model of liver ischaemia reperfusion [163].
Pharmacologic preconditioning is a potential mechanism to protect against hepatic ischaemic reperfusion injury. Blockage of A1 receptors abolished ischaemic preconditioning whilst activation of A1 receptors decreased the effect of ischaemic reperfusion injury [2]. Hepatic ischaemic preconditioning is associated with up-regulation of CD39. This is likely mediated by transcription factor Sp1 and is a potential therapeutic target for the treatment of liver ischaemia [117]. This also has renal implications as well, as one study demonstrated that activation of renal A1 receptors was protective for the liver as well as kidney after liver ischaemia reperfusion injury [198].
Administration of UTP before induction of ischaemia can attenuate, via P2Y2 and/or P2Y4 receptors, post-ischaemic hepatocyte apoptosis and thereby reverse liver damage [19]. The authors suggest that the UTP-mediated protective effect may be regulated through nuclear factor-κB inactivation. Inosine is an endogenous nucleotide that may be useful in maintaining homeostasis after tissue ischaemia. Through the action of adenosine receptors (A3), high extracellular inosine stimulated gluconeogenesis [111].
Interestingly, ADP-dependent platelet aggregation was shown to correlate with reperfusion injury as well as thrombocytopenia and early graft survival. ADP-triggered platelet function may have a role in ischaemia reperfusion injury [220].
Metallothionein protein (MT) is induced in vivo in rat liver by P1 adenosine agonists, probably via A2 receptors [261]. The authors suggest that adenosine via modulation of transcription of MT genes may be important in stress situations involving tissue damage, hypoxia and haemorrhage shock.
Hepato-renal syndrome
Purinergic signalling may play a role in hepato-renal syndrome. The administration of intra-hepatic caffeine into the portal vein of rats has been shown to increase urine output. This effect was not seen with intravenous caffeine or after the liver was denervated, which suggests a porto-renal effect of adenosine [181]. This effect is mediated by hepatic A1 receptors. This presents a potentially novel therapeutic option for a difficult to treat complication of cirrhosis. In fact, selective blockade of the hepatorenal reflux with SLV329 (an A1 receptor antagonist) resulted in a diuretic and natriuretic effect without a change of creatinine clearance in a rat model of cirrhosis [129].
Regeneration of liver
It is interesting that the sympathetic nervous system has been implicated in the regulation of liver repair (see [191]). Since ATP is now well established as a cotransmitter with NA in sympathetic nerves, it may be a source of ATP involved in liver regeneration. ATP, released from nerves, from hepatocytes or after synthesis of inorganic phosphate and ADP by the cell membrane via kinases, may participate in the transmembrane signal transduction from growth factors to the cell effector system [102]. ATP activates c-jun N-terminal kinase signalling and cell cycle progression in hepatocytes, with involvement in the initiation of regeneration, liver growth and development [242]. Gene expression profile analysis of regenerating liver using a cDNA microarray system suggests that increase in ATP metabolism is associated with rapid regeneration of liver [185]. Hepatocellular proliferation is impaired in P2Y2 receptor knockout mice, establishing a trophic role for ATP in hepatocyte proliferation with implications for liver regeneration and growth after injury [243]. Regulated catalysis of extracellular nucleotides by vascular CD39 (NTPDase1) is required for both hepatocyte and endothelial cell proliferation during liver regeneration [16]. Adenosine, perhaps via A2B or A3 receptors, has been reported to accelerate the cell cycle during rat liver regeneration induced by partial hepatectomy [179]. A selective A2A agonist, ATL-146e, has been claimed to prevent concanavalin A-induced acute liver injury in mice [192]. A3 receptor activation decreases mortality and hepatic injury in murine septic peritonitis [161]. After a partial hepatectomy adenine nucleotides have been noted to undergo a rapid decrease in the remnant liver. The onset of liver regeneration occurs after seconds, possibly related to this loss of nucleotides [54]. ATP release after partial hepatectomy regulates liver regeneration in the rat [104].
A recent insight into this process is the modulation of extracellular ATP on NK cells after partial hepatectomy. Immediately after partial hepatectomy, extracellular ATP is increased and will bind to P2 receptors on NK cells that, in turn, inhibit their function. Administration of exogenous apyrase (CD39/NTPDase1) depletes extracellular ATP and allows NK cells to regulate the immune response and improves liver regeneration [105]. Liver regeneration is enhanced by the ATP-sensitive K+ channel opener, diazoxide, after partial hepatectomy [186].
Liver transplantation
Accurate, rapid, non-invasive markers of graft viability have valuable clinical uses. A combination of liver ATP levels and serum hyaluronic acid has been recommended as a measure of graft viability [230]. Whilst the human liver has been successfully maintained under hypothermic conditions for up to 10 h using solutions with high concentrations of adenosine, organ preservation to overcome ischaemic damage is a major obstacle to liver transplantation. Infused ATP preserves sublethally injured cells by enhancing their recovery after ischaemic injury; this action is enhanced by the synergistic effect of superoxide dismutase [95]. Purinergic receptor antagonists prevent cold preservation-induced cell death [5]. Hepatocyte viability and ATP content decrease linearly over time during conventional cold storage of rat liver grafts [21].
With organ transplantation, NTPDase1 activity is lost with reperfusion or rejection and up-regulation occurs with graft survival [133]. Administration of soluble NTPDase in the bloodstream or up-regulation of CD39 post-adenoviral infection has been shown to prolong transplant graft survival. Regeneration of the donor liver after transplantation is important. ATP activates cell cycle progression and proliferation of hepatocytes in vitro and in vivo and modulates growth factor activities probably via P2Y2 receptors [242]. A2A receptor activation has been claimed to have a protective effect in small-for-size liver transplantation [239]. This group have demonstrated that A2A receptor activation down-regulated proinflammatory cytokines, adhesion molecules and ultimately improved liver function in small for size liver transplantation in rats [240].
Another development from purinergic signalling is the ability to monitor and predict progression of fibrosis and rejection in the post-transplant allograft. The ImmuKnow assay is measure of peripheral blood CD4+ total ATP. After living donor transplantation, the ImmuKnow assay was studied as a tool of immune response. Based on the results of the ImmuKnow assay, there was a correlation between immune response and required immune suppression with tacrolimus [184]. It was concluded that it had an excellent ability to monitor immune response especially in combination with assessment of the CYP3A5 allele. Blood from patients who had acute rejection displayed a significantly elevated level of intracellular ATP in CD4+ lymphocytes compared to those without acute rejection. This may be developed as a useful clinical tool to diagnose early rejection [67, 236].
The ImmuKnow assay has also showed potential in areas other than acute rejection. Recurrence of hepatitis C after transplant is a difficult to predict problem. In patients with HCV after orthotopic liver transplant, low CD4+ T cell ATP levels based on the ImmuKnow assay were significantly associated with progression to fibrosis. Thus, the greater suppression of cellular immunity measured by the ImmuKnow assay, the greater the risk of development of fibrosis [3].
In a separate study, in patients with HCV who had been transplanted, the ImmuKnow assay was assessed to distinguish acute cellular rejection from recurrent HCV. Recipients with recurrent HCV had a significantly lower immune response compared to those with acute cellular rejection. Interestingly, those patients with overlap features of both HCV and acute cellular rejection who had a low immune response were more often found to have HCV. Thus, the ImmuKnow assay has potential to serve as a clinical tool to distinguish recurrent HCV after transplantation or acute cellular rejection [118]. Clinical utility of the ImmuKnow assay, which determines immunosuppression levels, is limited in children with kidney transplants, but it is very valuable with serious infections [248]. The A2A receptor agonist, regadenoson, increases hepatic artery flow in the recipients of small-for-size liver grafts, giving some improved outcome [267].
Conclusions and future directions
As reviewed above, there are now substantial data implicating extracellular nucleotides and nucleosides in a variety of liver functions in both health and disease. Purinergic signalling is involved in the vascular and immune responses to liver transplantation and can influence the pathophysiological responses to ischaemic injury, disordered bile flow and metabolic disorders such as insulin resistance. All of these entities are critical to optimal clinical outcomes following hepatic allografting.
In terms of other therapeutic non-transplant strategies, the expectation is for the development and study of non-toxic drugs that can modulate breakdown of ATP by ectonucleotidases, in addition to selective agonists and antagonists for purinoceptor subtypes that are orally bioavailable and stable in vivo. Such therapies might be employed for several of the more common liver diseases to not only improve hepatic steatosis but also ameliorate the progression to scarring, disordered regeneration and cirrhosis.
Acknowledgement
The authors are very grateful to Dr Gillian E. Knight for her invaluable assistance in the preparation of this review article.
References
- 1.Ahsan MK. The adenosine A2 receptor enhances primary rat HSC proliferation and inhibits senescence by down-regulation of P53 and RB. Hepatology. 2011;54:750A–751A. [Google Scholar]
- 2.Ajamieh HH, Candelario-Jalil E, Fernandez OS, Gerbes AL. Ischaemic and pharmacological preconditionings protect liver via adenosine and redox status following hepatic ischaemia/reperfusion in rats. Clin Sci (Lond) 2008;115:69–77. doi: 10.1042/CS20070415. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouri N, Hanouneh IA, Lopez R, Zein NN. Monitoring peripheral blood CD4+ adenosine triphosphate activity in recurrent hepatitis C and its correlation to fibrosis progression. Liver Transpl. 2010;16:155–162. doi: 10.1002/lt.21939. [DOI] [PubMed] [Google Scholar]
- 4.Amaral SS, Oliveira AG, Marques PE, Quintão JL, Pires DA, Resende RR, Sousa BR, Melgaco JG, Pinto MA, Russo RC, Gomes AK, Andrade LM, Zanin RF, Pereira RV, Bonorino C, Soriani FM, Lima CX, Cara DC, Teixeira MM, Leite MF, Menezes GB. Altered responsiveness to extracellular ATP enhances acetaminophen hepatotoxicity. Cell Commun Signal. 2013;11:10. doi: 10.1186/1478-811X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CD, Pierce J, Nicoud IB, Belous AE, Jones CM, Chari RS. Purinergic receptor antagonism prevents cold preservation-induced cell death independent of cellular ATP levels. J Surg Res. 2007;141:234–240. doi: 10.1016/j.jss.2006.12.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade CM, Roesch GC, Wink MR, Guimarães EL, Souza LF, Jardim FR, Guaragna RM, Bernard EA, Margis R, Borojevic R, Battastini AM, Guma FC. Activity and expression of ecto-5′-nucleotidase/CD73 are increased during phenotype conversion of a hepatic stellate cell line. Life Sci. 2008;82:21–29. doi: 10.1016/j.lfs.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Annie-Jeyachristy S, Geetha A, Surendran R, Kumar SJ, Arulprakash A. Role of cytosolic calcium and actim polymerization on agonist-induced secretion by the platelets of liver cirrhosis patients. Turk J Hematol. 2009;26:82–89. [PubMed] [Google Scholar]
- 8.Arai M, Thurman RG, Lemasters JJ. Contribution of adenosine A2 receptors and cyclic adenosine monophosphate to protective ischemic preconditioning of sinusoidal endothelial cells against Storage/Reperfusion injury in rat livers. Hepatology. 2000;32:297–302. doi: 10.1053/jhep.2000.8896. [DOI] [PubMed] [Google Scholar]
- 9.Asensi M, Lopez-Rodas A, Sastre J, Viña J, Estrela JM. Inhibition of gluconeogenesis by extracellular ATP in isolated rat hepatocytes. Am J Physiol. 1991;261:R1522–R1526. doi: 10.1152/ajpregu.1991.261.6.R1522. [DOI] [PubMed] [Google Scholar]
- 10.Athari A, Hänecke K, Jungermann K. Prostaglandin F2α and D2 release from primary Ito cell cultures after stimulation with noradrenaline and ATP but not adenosine. Hepatology. 1994;20:142–148. doi: 10.1016/0270-9139(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 11.Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M, Robaye B, Boeynaems JM, Di Virgilio F, Pellegatti P, Diefenbach A, Idzko M, Hasselblatt P. Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143:1620–1629. doi: 10.1053/j.gastro.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-κB signal transduction pathways. Int J Oncol. 2008;33:287–295. [PubMed] [Google Scholar]
- 13.Bear CE, Li CH. Calcium-permeable channels in rat hepatoma cells are activated by extracellular nucleotides. Am J Physiol. 1991;261:C1018–C1024. doi: 10.1152/ajpcell.1991.261.6.C1018. [DOI] [PubMed] [Google Scholar]
- 14.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M, Robson SC. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, Junger WG, Candinas D, Robson SC. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Ari Z, Pappo O, Sulkes J, Cheporko Y, Vidne BA, Hochhauser E. Effect of adenosine A2A receptor agonist (CGS) on ischemia/reperfusion injury in isolated rat liver. Apoptosis. 2005;10:955–962. doi: 10.1007/s10495-005-0440-3. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ari Z, Pappo O, Yitzhaki S, Cheporko Y, Shainberg A, Zinman T, Ravid A, Zemel R, Bachmatov L, Kurtzwald E, Mor E, Hochhauser E. Uridine-5′-triphosphate protects against hepatic–ischemic/reperfusion injury in mice. Transplantation. 2009;87:1155–1162. doi: 10.1097/TP.0b013e31819e3cdc. [DOI] [PubMed] [Google Scholar]
- 20.Benitez-Rajal J, Lorite MJ, Burt AD, Day CP, Thompson MG. Phospholipase D and extracellular signal-regulated kinase in hepatic stellate cells: effects of platelet-derived growth factor and extracellular nucleotides. Am J Physiol Gastrointest Liver Physiol. 2006;291:G977–G986. doi: 10.1152/ajpgi.00041.2006. [DOI] [PubMed] [Google Scholar]
- 21.Berendsen TA, Izamis ML, Xu H, Liu Q, Hertl M, Berthiaume F, Yarmush ML, Uygun K. Hepatocyte viability and adenosine triphosphate content decrease linearly over time during conventional cold storage of rat liver grafts. Transplant Proc. 2011;43:1484–1488. doi: 10.1016/j.transproceed.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnard A, Julien B, Gonzales E, Tordjmann T. Innate immunity, purinergic system, and liver regeneration: a trip in complexity. Hepatology. 2013;57:1688–1690. doi: 10.1002/hep.26312. [DOI] [PubMed] [Google Scholar]
- 23.Blom D, Yamin TT, Champy MF, Selloum M, Bedu E, Carballo-Jane E, Gerckens L, Luell S, Meurer R, Chin J, Mudgett J, Puig O. Altered lipoprotein metabolism in P2Y13 knockout mice. Biochim Biophys Acta. 2010;1801:1349–1360. doi: 10.1016/j.bbalip.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J. 1998;336:19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boy C, Meyer PT, Kircheis G, Holschbach MH, Herzog H, Elmenhorst D, Kaiser HJ, Coenen HH, Haussinger D, Zilles K, Bauer A. Cerebral A1 adenosine receptors (A1AR) in liver cirrhosis. Eur J Nucl Med Mol Imaging. 2008;35:589–597. doi: 10.1007/s00259-007-0586-z. [DOI] [PubMed] [Google Scholar]
- 26.Boynton AL, Cooney RV, Hill TD, Nilsson T, Arkhammar P, Berggren PO. Extracellular ATP mobilizes intracellular Ca2+ in T51B rat liver epithelial cells: a study involving single cell measurements. Exp Cell Res. 1989;181:245–255. doi: 10.1016/0014-4827(89)90198-5. [DOI] [PubMed] [Google Scholar]
- 27.Brizzolara AL, Burnstock G. Evidence for noradrenergic–purinergic cotransmission in the hepatic artery of the rabbit. Br J Pharmacol. 1990;99:835–839. doi: 10.1111/j.1476-5381.1990.tb13016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brizzolara AL, Burnstock G. Endothelium-dependent and endothelium-independent vasodilatation of the hepatic artery of the rabbit. Br J Pharmacol. 1991;103:1206–1212. doi: 10.1111/j.1476-5381.1991.tb12325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broetto-Biazon AC, Bracht F, Babeto de Sá-Nakanishi A, Lopez CH, Constantin J, Kelmer-Bracht AM, Bracht A. Transformation products of extracellular NAD+ in the rat liver: kinetics of formation and metabolic action. Mol Cell Biochem. 2008;307:41–50. doi: 10.1007/s11010-007-9582-7. [DOI] [PubMed] [Google Scholar]
- 30.Burgess GM, Claret M, Jenkinson DH. Effects of catecholamines, ATP and ionophore A23187 on potassium and calcium movements in isolated hepatocytes. Nature. 1979;279:544–546. doi: 10.1038/279544a0. [DOI] [PubMed] [Google Scholar]
- 31.Burgess GM, Claret M, Jenkinson DH. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 33.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- 34.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 36.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 37.Busshardt E, Gerok W, Häussinger D. Regulation of hepatic parenchymal and non-parenchymal cell function by the diadenine nucleotides Ap3A and Ap4A. Biochim Biophys Acta. 1989;1010:151–159. doi: 10.1016/0167-4889(89)90155-9. [DOI] [PubMed] [Google Scholar]
- 38.Buxton DB, Robertson SM, Olson MS. Stimulation of glycogenolysis by adenine nucleotides in the perfused rat liver. Biochem J. 1986;237:773–780. doi: 10.1042/bj2370773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buxton DB, Fisher RA, Robertson SM, Olson MS. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem J. 1987;248:35–41. doi: 10.1042/bj2480035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capiod T. ATP-activated cation currents in single guinea-pig hepatocytes. J Physiol. 1998;507:795–805. doi: 10.1111/j.1469-7793.1998.795bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carini R, Alchera E, Grazia De Cesaris M, Splendore R, Piranda D, Baldanzi G, Albano E. Purinergic P2Y2 receptors promote hepatocyte resistance to hypoxia. J Hepatol. 2006;45:236–245. doi: 10.1016/j.jhep.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charest R, Blackmore PF, Exton JH. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985;260:15789–15794. [PubMed] [Google Scholar]
- 44.Chatterjee C, Sparks DL. Extracellular nucleotides inhibit insulin receptor signaling, stimulate autophagy and control lipoprotein secretion. PLoS ONE. 2012;7:e36916. doi: 10.1371/journal.pone.0036916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Che J, Chan ES, Cronstein BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.038760. [DOI] [PubMed] [Google Scholar]
- 46.Che M, Gatmaitan Z, Arias IM. Ectonucleotidases, purine nucleoside transporter, and function of the bile canalicular plasma membrane of the hepatocyte. FASEB J. 1997;11:101–108. doi: 10.1096/fasebj.11.2.9039951. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Z, Dixon J, Boarder MR. Dominance of P2Y receptors in the control of hepatocyte Akt/mTOR/S6K pathway: EGF-stimulated, but not UTP-stimulated, phosphorylation of p70S6K is blocked by glucagon. Purinergic Signal. 2011;7:147. [Google Scholar]
- 48.Chiang DJ, Pritchard MT, Roychowdhury S, McMullen MR, Pratt B, Nagy L. Adenosine 2A receptor antagonist improves ethanol-induced impaired angiogenesis and liver fibrosis in mice. Hepatology. 2010;52:1277A. [Google Scholar]
- 49.Chiang DJ, Roychowdhury S, Bush K, McMullen MR, Pisano S, Niese K, Olman MA, Pritchard MT, Nagy LE. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS ONE. 2013;8:e69114. doi: 10.1371/journal.pone.0069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choukèr A, Thiel M, Lukashev D, Ward JM, Kaufmann I, Apasov S, Sitkovsky MV, Ohta A. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol Med. 2008;14:116–123. doi: 10.2119/2007-00075.Chouker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemens MG, McDonagh PF, Chaudry IH, Baue AE. Hepatic microcirculatory failure after ischemia and reperfusion: improvement with ATP-MgCl2 treatment. Am J Physiol. 1985;248:H804–H811. doi: 10.1152/ajpheart.1985.248.6.H804. [DOI] [PubMed] [Google Scholar]
- 52.Cooper DM, Londos C. Evaluation of the effects of adenosine on hepatic and adipocyte adenylate cyclase under conditions where adenosine is not generated endogenously. J Cyclic Nucleotide Res. 1979;5:289–302. [PubMed] [Google Scholar]
- 53.Craik KM, McLennan AG, Fisher MJ. Adenine dinucleotide-mediated activation of glycogen phosphorylase in isolated liver cells. Cell Signal. 1993;5:89–96. doi: 10.1016/0898-6568(93)90011-a. [DOI] [PubMed] [Google Scholar]
- 54.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: an early signal for regeneration after partial hepatectomy. Hepatology. 2008;48:898–908. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 56.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia–reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 57.Delgado-Coello B, Trejo R, Mas-Oliva J. Is there a specific role for the plasma membrane Ca2+ -ATPase in the hepatocyte? Mol Cell Biochem. 2006;285:1–15. doi: 10.1007/s11010-005-9060-z. [DOI] [PubMed] [Google Scholar]
- 58.Dixon CJ, Woods NM, Cuthbertson KS, Cobbold PH. Evidence for two Ca2+-mobilizing purinoceptors on rat hepatocytes. Biochem J. 1990;269:499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon CJ, Cobbold PH, Green AK. Adenosine 5′-[α, β-methylene]triphosphate potentiates the oscillatory cytosolic Ca2+ responses of hepatocytes to ATP, but not to ADP. Biochem J. 1993;293:757–760. doi: 10.1042/bj2930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon CJ, Cobbold PH, Green AK. Actions of ADP, but not ATP, on cytosolic free Ca2+ in single rat hepatocytes mimicked by 2-methylthioATP. Br J Pharmacol. 1995;116:1979–1984. doi: 10.1111/j.1476-5381.1995.tb16401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dixon CJ, Hall JF, Webb TE, Boarder MR. Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5′-diphosphate. J Pharmacol Exp Ther. 2004;311:334–341. doi: 10.1124/jpet.104.067744. [DOI] [PubMed] [Google Scholar]
- 63.Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther. 2005;313:1305–1313. doi: 10.1124/jpet.104.082743. [DOI] [PubMed] [Google Scholar]
- 64.Doctor RB, Johnson S, Brodsky KS, Amura CR, Gattone V, Fitz JG. Regulated ion transport in mouse liver cyst epithelial cells. Biochim Biophys Acta. 2007;1772:345–354. doi: 10.1016/j.bbadis.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Dolovcak S, Waldrop SL, Fitz JG, Kilic G. 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) stimulates cellular ATP release through exocytosis of ATP-enriched vesicles. J Biol Chem. 2009;284:33894–33903. doi: 10.1074/jbc.M109.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolovcak S, Waldrop SL, Xiao F, Kilic G. Evidence for sustained ATP release from liver cells that is not mediated by vesicular exocytosis. Purinergic Signal. 2011;7:435–446. doi: 10.1007/s11302-011-9240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong JY, Yin H, Li RD, Ding GS, Fu ZR, Wu YM, Wang ZX. The relationship between adenosine triphosphate within CD4(+) T lymphocytes and acute rejection after liver transplantation. Clin Transplant. 2011;25:E292–E296. doi: 10.1111/j.1399-0012.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 68.Dragunow M, Faull RL. Neuroprotective effects of adenosine. Trends Pharmacol Sci. 1988;9:193–194. doi: 10.1016/0165-6147(88)90079-x. [DOI] [PubMed] [Google Scholar]
- 69.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1059–G1067. doi: 10.1152/ajpgi.2001.281.4.G1059. [DOI] [PubMed] [Google Scholar]
- 70.Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D, Knowles AF, Sevigny J. Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Invest Med. 2004;52:475–482. doi: 10.1136/jim-52-07-42. [DOI] [PubMed] [Google Scholar]
- 71.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dranoff JA, Kruglov EA, Abreu-Lanfranco O, Nguyen T, Arora G, Jain D. Prevention of liver fibrosis by the purinoceptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) In Vivo. 2007;21:957–965. [PubMed] [Google Scholar]
- 73.Dunne JB, Alexander B, Williams R, Tredger JM. Evidence that S-adenosyl-l-methionine diastereoisomers may reduce ischaemia-reperfusion injury by interacting with purinoceptors in isolated rat liver. Br J Pharmacol. 1998;125:225–233. doi: 10.1038/sj.bjp.0702043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edgecombe M, McLennan AG, Fisher MJ. Characterization of the binding of diadenosine 5′,5'''-P1, P4-tetraphosphate (Ap4A) to rat liver cell membranes. Biochem J. 1996;314:687–693. doi: 10.1042/bj3140687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, McWilliams R, Doctor RB, Fitz JG. Characterization of ionotropic purinergic receptors in hepatocytes. Hepatology. 2008;47:698–705. doi: 10.1002/hep.22035. [DOI] [PubMed] [Google Scholar]
- 76.Enjyoji K, Kotani K, Thukral C, Blumel B, Sun X, Wu Y, Imai M, Friedman D, Csizmadia E, Bleibel W, Kahn BB, Robson SC. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Espelt MV, de Tezanos PF, Alvarez CL, Alberti GS, Incicco J, Leal Denis MF, Davio C, Schwarzbaum PJ. On the role of ATP release, ectoATPase activity, and extracellular ADP in the regulatory volume decrease of Huh-7 human hepatoma cells. Am J Physiol Cell Physiol. 2013;304:C1013–C1026. doi: 10.1152/ajpcell.00254.2012. [DOI] [PubMed] [Google Scholar]
- 78.Essner E, Novikoff AB, Masek B. Adenosinetriphosphatase and 5-nucleotidase activities in the plasma membrane of liver cells as revealed by electron microscopy. J Biophys Biochem Cytol. 1958;4:711–716. doi: 10.1083/jcb.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabre AC, Malaval C, Ben AA, Verdier C, Pons V, Serhan N, Lichtenstein L, Combes G, Huby T, Briand F, Collet X, Nijstad N, Tietge UJ, Robaye B, Perret B, Boeynaems JM, Martinez LO. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology. 2010;52:1477–1483. doi: 10.1002/hep.23897. [DOI] [PubMed] [Google Scholar]
- 80.Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, Wang X. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. doi: 10.1016/j.cell.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Fausther M, Sévigny J. Extracellular nucleosides and nucleotides regulate liver functions via a complex system of membrane proteins. C R Biol. 2011;334:100–117. doi: 10.1016/j.crvi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Fausther M, Lecka J, Kukulski F, Lévesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sévigny J. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292:G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fausther M, Gonzales E, Dranoff JA. Role of purinergic P2X receptors in the control of liver homeostasis. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:341–348. doi: 10.1002/wmts.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fausther M, Lecka J, Soliman E, Kauffenstein G, Pelletier J, Sheung N, Dranoff JA, Sévigny J. Coexpression of ecto-5′-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2012;302:G447–G459. doi: 10.1152/ajpgi.00165.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fausther M, Sheung N, Saiman Y, Bansal MB, Dranoff JA. Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am J Physiol Gastrointest Liver Physiol. 2012;303:G904–G914. doi: 10.1152/ajpgi.00015.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M, Warner A, Muir AJ, Brass C, Albrecht J, Sulkowski M, McHutchison JG, Goldstein DB. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405–408. doi: 10.1038/nature08825. [DOI] [PubMed] [Google Scholar]
- 87.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis. 2002;22:251–262. doi: 10.1055/s-2002-34503. [DOI] [PubMed] [Google Scholar]
- 89.Feranchak AP, Fitz JG. Purinergic receptors and hepatobiliary function. Curr Top Membr. 2003;54:395–414. [Google Scholar]
- 90.Feranchak AP, Fitz JG, Roman RM. Volume-sensitive purinergic signaling in human hepatocytes. J Hepatol. 2000;33:174–182. doi: 10.1016/s0168-8278(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 91.Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandes TR, Suzuki-Kemmelmeier F, Bracht A. The hemodynamic effects of ATP in retrograde perfusion of the bivascularly perfused rat liver. Liver Int. 2003;23:371–378. doi: 10.1034/j.1478-3231.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 93.Fischer L, Haag-Diergarten S, Scharrer E, Lutz TA. Leukotriene and purinergic receptors are involved in the hyperpolarizing effect of glucagon in liver cells. Biochim Biophys Acta. 2005;1669:26–33. doi: 10.1016/j.bbamem.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Fitz JG, Sostman AH. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. Am J Physiol. 1994;266:G544–G553. doi: 10.1152/ajpgi.1994.266.4.G544. [DOI] [PubMed] [Google Scholar]
- 95.Flye MW, Yu S. The synergistic effect of superoxide dismutase and adenosine triphosphate-MgCl2 on acute hepatic ischemia. Transplant Proc. 1987;19:1324–1326. [PubMed] [Google Scholar]
- 96.Frame MK, de Feijter AW. Propagation of mechanically induced intercellular calcium waves via gap junctions and ATP receptors in rat liver epithelial cells. Exp Cell Res. 1997;230:197–207. doi: 10.1006/excr.1996.3409. [DOI] [PubMed] [Google Scholar]
- 97.Frederiks WM, Fronik GM. Quantitative analysis of the effect of ATP-MgCl2 and adenosine-MgCl2 on the extent of necrosis in rat liver after ischemia. J Surg Res. 1986;41:518–523. doi: 10.1016/0022-4804(86)90170-8. [DOI] [PubMed] [Google Scholar]
- 98.Frontini AV, De La Vega Elena CD, Nicolorich MV, Naves A, Schwarzbaum P, Venera GD. In vivo effects of adenosine 5′-triphosphate on rat preneoplastic liver. Medicina (B Aires) 2011;71:139–145. [PubMed] [Google Scholar]
- 99.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- 100.Geelen MJ, Harris RA, Van den Bergh SG. Enigmatic effect of cellular ATP on fatty acid biosynthesis. Stimulation by moderate decrease and inhibition by increase of cellular ATP. FEBS Lett. 2008;582:2242–2246. doi: 10.1016/j.febslet.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 101.Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- 102.Globa AG, Vishnevskii VA, Demidova VS, Abakumova OI, Karelin AA. Accumulation of ATP in rat and human hepatocyte cell membranes exposed to certain growth factors and phosphatidylcholine. Biull Eksp Biol Med. 1996;121:271–274. [PubMed] [Google Scholar]
- 103.Gonzales E, Prigent S, Abou-Lovergne A, Boucherie S, Tordjmann T, Jacquemin E, Combettes L. Rat hepatocytes express functional P2X receptors. FEBS Lett. 2007;581:3260–3266. doi: 10.1016/j.febslet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 104.Gonzales E, Julien B, Serriere-Lanneau V, Nicou A, Doignon I, Lagoudakis L, Garcin I, Azoulay D, Duclos-Vallee JC, Castaing D, Samuel D, Hernandez-Garcia A, Awad SS, Combettes L, Thevananther S, Tordjmann T. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52:54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Graubardt N, Fahrner R, Trochsler M, Keogh A, Breu K, Furer C, Stroka D, Robson SC, Slack E, Candinas D, Beldi G. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology. 2013;57:1969–1979. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 106.Grdeñ M, Podgorska M, Szutowicz A, Pawelczyk T. Diabetes-induced alterations of adenosine receptors expression level in rat liver. Exp Mol Pathol. 2007;83:392–398. doi: 10.1016/j.yexmp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Green AK, Dixon CJ, McLennan AG, Cobbold PH, Fisher MJ. Adenine dinucleotide-mediated cytosolic free Ca2+ oscillations in single hepatocytes. FEBS Lett. 1993;322:197–200. doi: 10.1016/0014-5793(93)81567-j. [DOI] [PubMed] [Google Scholar]
- 108.Grune T, Müller K, Zöllner S, Haseloff R, Blasig IE, David H, Siems W. Evaluation of purine nucleotide loss, lipid peroxidation and ultrastructural alterations in post-hypoxic hepatocytes. J Physiol. 1997;498:511–522. doi: 10.1113/jphysiol.1997.sp021877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guinzberg R, Laguna I, Zentella A, Guzman R, Pina E. Effect of adenosine and inosine on ureagenesis in hepatocytes. Biochem J. 1987;245:371–374. doi: 10.1042/bj2450371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guinzberg R, Díaz-Cruz A, Uribe S, Piña E. Ca2+ dependence of the response of three adenosine type receptors in rat hepatocytes. Eur J Pharmacol. 1997;340:243–247. doi: 10.1016/s0014-2999(97)01384-8. [DOI] [PubMed] [Google Scholar]
- 111.Guinzberg R, Cortes D, Diaz-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Pina E. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab. 2006;290:E940–E951. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- 112.Guinzberg R, Uribe S, Díaz-Cruz A, Hernandez Cruz A, Piña E. In rat hepatocytes, different adenosine receptor subtypes use different secondary messengers to increase the rate of ureagenesis. Life Sci. 2006;79:382–390. doi: 10.1016/j.lfs.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 113.Guzmán M, Velasco G, Castro J. Effects of extracellular ATP on hepatic fatty acid metabolism. Am J Physiol. 1996;270:G701–G707. doi: 10.1152/ajpgi.1996.270.4.G701. [DOI] [PubMed] [Google Scholar]
- 114.Haddad PS, Vallerand D, Mathé L, Benzeroual K, Van de Werve G. Synergistic activation of mitogen-activated protein kinase by insulin and adenosine triphosphate in liver cells: permissive role of Ca2+ Metabolism. 2003;52:590–598. doi: 10.1053/meta.2003.50094. [DOI] [PubMed] [Google Scholar]
- 115.Hargrove JL, Granner DK. Inhibition of hepatoma cell growth by analogs of adenosine and cyclic AMP and the influence of enzymes in mammalian sera. J Cell Physiol. 1982;111:232–238. doi: 10.1002/jcp.1041110303. [DOI] [PubMed] [Google Scholar]
- 116.Harman AW, Nieminen AL, Lemasters JJ, Herman B. Cytosolic free magnesium, ATP and blebbing during chemical hypoxia in cultured rat hepatocytes. Biochem Biophys Res Commun. 1990;170:477–483. doi: 10.1016/0006-291x(90)92116-h. [DOI] [PubMed] [Google Scholar]
- 117.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hashimoto K, Miller C, Hirose K, Diago T, Aucejo F, Quintini C, Eghtesad B, Corey R, Yerian L, Lopez R, Zein N, Fung J. Measurement of CD4+ T-cell function in predicting allograft rejection and recurrent hepatitis C after liver transplantation. Clin Transplant. 2010;24:701–708. doi: 10.1111/j.1399-0012.2009.01169.x. [DOI] [PubMed] [Google Scholar]
- 119.Hashimoto N, Watanabe T, Shiratori Y, Ikeda Y, Kato H, Han K, Yamada H, Toda G, Kurokawa K. Prostanoid secretion by rat hepatic sinusoidal endothelial cells and its regulation by exogenous adenosine triphosphate. Hepatology. 1995;21:1713–1718. [PubMed] [Google Scholar]
- 120.Häussinger D. Regulation of hepatic metabolism by extracellular nucleotides and eicosanoids. The role of cell heterogeneity. J Hepatol. 1989;8:259–266. doi: 10.1016/0168-8278(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 121.Häussinger D, Stehle T, Gerok W. Actions of extracellular UTP and ATP in perfused rat liver. A comparative study. Eur J Biochem. 1987;167:65–71. doi: 10.1111/j.1432-1033.1987.tb13304.x. [DOI] [PubMed] [Google Scholar]
- 122.Häussinger D, Stehle T, Gerok W, Tran-Thi TA, Decker K. Hepatocyte heterogeneity in response to extracellular ATP. Eur J Biochem. 1987;169:645–650. doi: 10.1111/j.1432-1033.1987.tb13656.x. [DOI] [PubMed] [Google Scholar]
- 123.Häussinger D, Busshardt E, Stehle T, Stoll B, Wettstein M, Gerok W. Stimulation of thromboxane release by extracellular UTP and ATP from perfused rat liver. Role of icosanoids in mediating the nucleotide responses. Eur J Biochem. 1988;178:249–256. doi: 10.1111/j.1432-1033.1988.tb14450.x. [DOI] [PubMed] [Google Scholar]
- 124.Hernández-Muñoz R, Díaz-Muñoz M, Suárez J, Chagoya de Sánchez V. Adenosine partially prevents cirrhosis induced by carbon tetrachloride in rats. Hepatology. 1990;12:242–248. doi: 10.1002/hep.1840120210. [DOI] [PubMed] [Google Scholar]
- 125.Hernández-Muñoz R, Díaz-Muñoz M, Suárez-Cuenca JA, Trejo-Solis C, Lopez V, Sanchez-Sevilla L, Yanez L, De Sanchez V. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 126.Hirasawa H, Chaundry IH, Baue AE. Improved hepatic function and survival with adenosine triphosphate-magnesium chloride after hepatic ischemia. Surgery. 1978;83:655–662. [PubMed] [Google Scholar]
- 127.Hirasawa H, Ohtake Y, Odaka M, Sato H. ATP-MgCl2 improves hepatic cellular energy metabolism, reticuloendothelial system function, and survival following massive hepatectomy among cirrhotic rats. Am Coll Surg. 1984;35:12–14. [Google Scholar]
- 128.Hitomi Y, Cirulli ET, Fellay J, McHutchison JG, Thompson AJ, Gumbs CE, Shianna KV, Urban TJ, Goldstein DB. Inosine triphosphate protects against ribavirin-induced adenosine triphosphate loss by adenylosuccinate synthase function. Gastroenterology. 2011;140:1314–1321. doi: 10.1053/j.gastro.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 129.Hocher B, Heiden S, von WK, Arafat AM, Rahnenfuhrer J, Alter M, Kalk P, Ziegler D, Fischer Y, Pfab T. Renal effects of the novel selective adenosine A1 receptor blocker SLV329 in experimental liver cirrhosis in rats. PLoS ONE. 2011;6:e17891. doi: 10.1371/journal.pone.0017891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoque R, Sohail MA, Salhanick S, Malik AF, Ghani A, Robson SC, Mehal WZ. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ikari A, Sakai H, Takeguchi N. ATP, thapsigargin and cAMP increase Ca2+ in rat hepatocytes by activating three different Ca2+ influx pathways. Jpn J Physiol. 1997;47:235–239. doi: 10.2170/jjphysiol.47.235. [DOI] [PubMed] [Google Scholar]
- 132.Ikeda N, Murata S, Maruyama T, Tamura T, Nozaki R, Kawasaki T, Fukunaga K, Oda T, Sasaki R, Homma M, Ohkohchi N. Platelet-derived adenosine 5′-triphosphate suppresses activation of human hepatic stellate cell: in vitro study. Hepatol Res. 2012;42:91–102. doi: 10.1111/j.1872-034X.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 133.Imai M, Takigami K, Guckelberger O, Lin Y, Sevigny J, Kaczmarek E, Goepfert C, Enjyoji K, Bach FH, Rosenberg RD, Robson SC. CD39/vascular ATP diphosphohydrolase modulates xenograft survival. Transplant Proc. 2000;32:969. doi: 10.1016/s0041-1345(00)01065-4. [DOI] [PubMed] [Google Scholar]
- 134.Imarisio C, Alchera E, Sutti S, Valente G, Boccafoschi F, Albano E, Carini R. Adenosine A2a receptor stimulation prevents hepatocyte lipotoxicity and non-alcoholic steatohepatitis (NASH) in rats. Clin Sci (Lond) 2012;123:323–332. doi: 10.1042/CS20110504. [DOI] [PubMed] [Google Scholar]
- 135.Irving HR, Exton JH. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987;262:3440–3443. [PubMed] [Google Scholar]
- 136.Ishizaki M, Iizuka Y, Suzuki-Kusaba M, Kimura T, Satoh S. Nonadrenergic contractile response of guinea pig portal vein to electrical field stimulation mimics response to UTP but not to ATP. J Cardiovasc Pharmacol. 1997;29:360–366. doi: 10.1097/00005344-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 137.Jacquet S, Malaval C, Martinez LO, Sak K, Rolland C, Perez C, Nauze M, Champagne E, Tercé F, Gachet C, Perret B, Collet X, Boeynaems JM, Barbaras R. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jenkinson DH, Koller K. Interactions between the effects of α- and β-adrenoceptor agonists and adenine nucleotides on the membrane potential of cells in guinea-pig liver slices. Br J Pharmacol. 1977;59:163–175. doi: 10.1111/j.1476-5381.1977.tb06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeong C, Lee SM. The beneficial effect of ATP-MgCl2 on hepatic ischemia/reperfusion-induced mitochondrial dysfunction. Eur J Pharmacol. 2000;403:243–250. doi: 10.1016/s0014-2999(00)00485-4. [DOI] [PubMed] [Google Scholar]
- 140.Jia X, Naito H, Yetti H, Tamada H, Kitamori K, Hayashi Y, Yamagishi N, Wang D, Yanagiba Y, Ito Y, Wang J, Tanaka N, Ikeda K, Yamori Y, Nakajima T. The modulation of hepatic adenosine triphosphate and inflammation by eicosapentaenoic acid during severe fibrotic progression in the SHRSP5/Dmcr rat model. Life Sci. 2012;90:934–943. doi: 10.1016/j.lfs.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 141.Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS ONE. 2012;7:e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katayama T, Kimura T, Miura S, Shimamura M. A histological study on hepatic changes induced by the administration of ATP, ADP and AMP. Hepatogastroenterol. 1980;27:259–265. [PubMed] [Google Scholar]
- 143.Katz J, Wals PA. The role of ATP in the cytostructure of the hepatocytes. J Cell Biochem. 1987;33:127–136. doi: 10.1002/jcb.240330207. [DOI] [PubMed] [Google Scholar]
- 144.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–2160. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 145.Kennedy C, Burnstock G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol. 1985;111:49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- 146.Keppens S. The complex interaction of ATP and UTP with isolated hepatocytes. How many receptors? Gen Pharmacol. 1993;24:283–289. doi: 10.1016/0306-3623(93)90304-g. [DOI] [PubMed] [Google Scholar]
- 147.Keppens S, De Wulf H. P2-purinergic control of liver glycogenolysis. Biochem J. 1985;231:797–799. doi: 10.1042/bj2310797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keppens S, De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986;240:367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keppens S, De Wulf H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br J Pharmacol. 1991;104:301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keppens S, Vandekerckhove A, De Wulf H. Characterization of purinoceptors present on human liver plasma membranes. FEBS Lett. 1989;248:137–140. doi: 10.1016/0014-5793(89)80448-x. [DOI] [PubMed] [Google Scholar]
- 151.Keppens S, Vandekerckhove A, De Wulf H. Characterization of the purinoceptors present in rabbit and guinea pig liver. Eur J Pharmacol. 1990;182:149–153. doi: 10.1016/0014-2999(90)90504-y. [DOI] [PubMed] [Google Scholar]
- 152.Keppens S, Vandekerckhove A, De Wulf H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br J Pharmacol. 1992;105:475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim J, Kim M, Song JH, Lee HT. Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl. 2008;14:845–854. doi: 10.1002/lt.21432. [DOI] [PubMed] [Google Scholar]
- 154.Kitamura T, Brauneis U, Gatmaitan Z, Arias IM. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991;14:640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- 155.Koike M, Kashiwagura T, Takeguchi N. Gluconeogenesis stimulated by extracellular ATP is triggered by the initial increase in the intracellular Ca2+ concentration of the periphery of hepatocytes. Biochem J. 1992;283:265–272. doi: 10.1042/bj2830265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kovács AL, Gordon PB, Grotterød EM, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, adenosine analogs and AMP. Biol Chem. 1998;379:1341–1347. doi: 10.1515/bchm.1998.379.11.1341. [DOI] [PubMed] [Google Scholar]
- 157.Krell H, Ermisch N, Kasperek S, Pfaff E. On the mechanisms of ATP-induced and succinate-induced redistribution of cations in isolated rat liver cells. Eur J Biochem. 1983;131:247–254. doi: 10.1111/j.1432-1033.1983.tb07256.x. [DOI] [PubMed] [Google Scholar]
- 158.Krell H, Jaeschke H, Pfaff E. Regulation of canalicular bile formation by α-adrenergic action and by external ATP in the isolated perfused rat liver. Biochem Biophys Res Commun. 1985;131:139–145. doi: 10.1016/0006-291x(85)91781-4. [DOI] [PubMed] [Google Scholar]
- 159.Kruglov EA, Correa PR, Arora G, Yu J, Nathanson MH, Dranoff JA. Molecular basis for calcium signaling in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G975–G982. doi: 10.1152/ajpgi.00401.2006. [DOI] [PubMed] [Google Scholar]
- 160.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 162.Lee SS, Chilton EL, Pak JM. Adenosine receptor blockade reduces splanchnic hyperemia in cirrhotic rats. Hepatology. 1992;15:1107–1111. doi: 10.1002/hep.1840150622. [DOI] [PubMed] [Google Scholar]
- 163.León Fernández OS, Ajamieh HH, Berlanga J, Menéndez S, Viebahn-Hánsler R, Re L, Carmona AM. Ozone oxidative preconditioning is mediated by A1 adenosine receptors in a rat model of liver ischemia/ reperfusion. Transpl Int. 2008;21:39–48. doi: 10.1111/j.1432-2277.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 164.Liao YJ, Wu CY, Lee SW, Lee CL, Yang SS, Chang CS, Lee TY. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol. 2012;18:5260–5265. doi: 10.3748/wjg.v18.i37.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lidofsky S. Adenosine triphosphate mediates intercellular communication in liver: talk ain't exactly cheap. Hepatology. 1997;25:778–779. doi: 10.1002/hep.510250350. [DOI] [PubMed] [Google Scholar]
- 166.Lin SH. Novel ATP-dependent calcium transport component from rat liver plasma membranes. The transporter and the previously reported (Ca2+-Mg2+)-ATPase are different proteins. J Biol Chem. 1985;260:7850–7856. [PubMed] [Google Scholar]
- 167.Lippe G, Rai AK, Harris DA, Dabbeni-Sala F. ATP synthase from rat liver plasma membrane. Biochim Biophys Acta. 2012;1817:S18–S19. [Google Scholar]
- 168.Liu IM, Tzeng TF, Tsai CC, Lai TY, Chang CT, Cheng JT. Increase in adenosine A1 receptor gene expression in the liver of streptozotocin-induced diabetic rats. Diabetes Metab Res Rev. 2003;19:209–215. doi: 10.1002/dmrr.369. [DOI] [PubMed] [Google Scholar]
- 169.Magata S, Taniguchi M, Suzuki T, Shimamura T, Fukai M, Furukawa H, Fujita M, Todo S. The effect of antagonism of adenosine A1 receptor against ischemia and reperfusion injury of the liver. J Surg Res. 2007;139:7–14. doi: 10.1016/j.jss.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 170.Mahmoud MS, Wang P, Hootman SR, Reich SS, Chaudry IH. ATP-MgCl2 treatment after trauma-hemorrhage/resuscitation increases hepatocyte P2-purinoceptor binding capacity. Am J Physiol. 1994;266:R1810–R1815. doi: 10.1152/ajpregu.1994.266.6.R1810. [DOI] [PubMed] [Google Scholar]
- 171.Malaval C, Laffargue M, Barbaras R, Rolland C, Peres C, Champagne E, Perret B, Tercé F, Collet X, Martinez LO. RhoA/ROCK I signalling downstream of the P2Y13 ADP-receptor controls HDL endocytosis in human hepatocytes. Cell Signal. 2009;21:120–127. doi: 10.1016/j.cellsig.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 172.Malcolm KC, Trammell SE, Exton JH. Purinergic agonist and G protein stimulation of phospholipase D in rat liver plasma membranes. Independence from phospholipase C activation. Biochim Biophys Acta. 1995;1268:152–158. doi: 10.1016/0167-4889(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 173.Mangiullo R, Gnoni A, Leone A, Gnoni GV, Papa S, Zanotti F. Structural and functional characterization of FoF1-ATP synthase on the extracellular surface of rat hepatocytes. Biochim Biophys Acta. 2008;1777:1326–1335. doi: 10.1016/j.bbabio.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 174.Manzoor S, Idrees M, Ashraf J, Mehmood A, Butt S, Fatima K, Akbar H, Rehaman IU, Qadri I. Identification of ionotropic purinergic receptors in Huh-7 cells and their response towards structural proteins of HCV genotype 3a. Virol J. 2011;8:431. doi: 10.1186/1743-422X-8-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F, Collet X, Perret B, Barbaras R. Ectopic -chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 176.Mathie RT, Alexander B, Ralevic V, Burnstock G. Adenosine-induced dilatation of the rabbit hepatic arterial vasculature is mediated by A2-purinoceptors. Br J Pharmacol. 1991;103:1103–1107. doi: 10.1111/j.1476-5381.1991.tb12307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mathie RT, Ralevic V, Alexander B, Burnstock G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br J Pharmacol. 1991;103:1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McLane MP, Black PR, Law WR, Raymond RM. Adenosine reversal of in vivo hepatic responsiveness to insulin. Diabetes. 1990;39:62–69. doi: 10.2337/diacare.39.1.62. [DOI] [PubMed] [Google Scholar]
- 179.Mendieta-Condado E, Chagoya de Sanchez V, Hernandez-Munoz R. Adenosine can accelerate the cell cycle during rat liver regeneration induced by partial hepatectomy. J Hepatol. 2007;46:S142–S143. doi: 10.1124/jpet.109.156620. [DOI] [PubMed] [Google Scholar]
- 180.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, LaRusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ming Z, Lautt WW. Caffeine-induced natriuresis and diuresis via blockade of hepatic adenosine-mediated sensory nerves and a hepatorenal reflex. Can J Physiol Pharmacol. 2010;88:1115–1121. doi: 10.1139/y10-090. [DOI] [PubMed] [Google Scholar]
- 182.Ming Z, Fan YJ, Yang X, Lautt WW. Blockade of intrahepatic adenosine receptors improves urine excretion in cirrhotic rats induced by thioacetamide. J Hepatol. 2005;42:680–686. doi: 10.1016/j.jhep.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 183.Mironneau J, Coussin F, Morel JL, Barbot C, Jeyakumar LH, Fleischer S, Mironneau C. Calcium signalling through nucleotide receptor P2X1 in rat portal vein myocytes. J Physiol. 2001;536:339–350. doi: 10.1111/j.1469-7793.2001.0339c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mizuno S, Hamada T, Nakatani K, Kishiwada M, Usui M, Sakurai H, Tabata M, Sakamoto Y, Nishioka J, Muraki Y, Okuda M, Nobori T, Isaji S. Monitoring peripheral blood CD4+ adenosine triphosphate activity after living donor liver transplantation: impact of combination assays of immune function and CYP3A5 genotype. J Hepatobiliary Pancreat Sci. 2011;18:226–234. doi: 10.1007/s00534-010-0335-8. [DOI] [PubMed] [Google Scholar]
- 185.Nagano Y, Nagahori K, Yoshiro F, Hamaguchi Y, Ishikawa T, Ichikawa Y, Togo S, Okazaki Y, Hayashizaki Y, Shimada H. Gene expression profile analysis of regenerating liver after portal vein ligation in rats by a cDNA microarray system. Liver Int. 2004;24:253–258. doi: 10.1111/j.1478-3231.2004.0912.x. [DOI] [PubMed] [Google Scholar]
- 186.Nakagawa Y, Yoshioka M, Abe Y, Uchinami H, Ohba T, Ono K, Yamamoto Y. Enhancement of liver regeneration by adenosine triphosphate-sensitive K+ channel opener (diazoxide) after partial hepatectomy. Transplantation. 2012;93:1094–1100. doi: 10.1097/TP.0b013e31824ef1d1. [DOI] [PubMed] [Google Scholar]
- 187.Nathanson MH, Burgstahler AD, Masyuk A, LaRusso NF. Stimulation of ATP secretion in the liver by therapeutic bile acids. Biochem J. 2001;358:1–5. doi: 10.1042/0264-6021:3580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Neuhuber WL, Tiegs G. Innervation of immune cells: evidence for neuroimmunomodulation in the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:884–892. doi: 10.1002/ar.a.20093. [DOI] [PubMed] [Google Scholar]
- 189.Nilsson B, Friman S, Wallin M, Gustafsson B, Delbro D. The liver protective effect of ischemic preconditioning may be mediated by adenosine. Transpl Int. 2000;13(Suppl 1):S558–S561. doi: 10.1007/s001470050402. [DOI] [PubMed] [Google Scholar]
- 190.Nukina S, Fusaoka T, Thurman RG. Glycogenolytic effect of adenosine involves ATP from hepatocytes and eicosanoids from Kupffer cells. Am J Physiol. 1994;266:G99–G105. doi: 10.1152/ajpgi.1994.266.1.G99. [DOI] [PubMed] [Google Scholar]
- 191.Oben JA, Diehl AM. Sympathetic nervous system regulation of liver repair. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:874–883. doi: 10.1002/ar.a.20081. [DOI] [PubMed] [Google Scholar]
- 192.Odashima M, Otaka M, Jin M, Horikawa Y, Matsuhashi T, Ohba R, Linden J, Watanabe S. A selective adenosine A2A receptor agonist, ATL-146e, prevents concanavalin A-induced acute liver injury in mice. Biochem Biophys Res Commun. 2006;347:949–954. doi: 10.1016/j.bbrc.2006.06.185. [DOI] [PubMed] [Google Scholar]
- 193.Ohigashi T, Brookins J, Fisher JW. Adenosine A1 receptors and erythropoietin production. Am J Physiol. 1993;265:C934–C938. doi: 10.1152/ajpcell.1993.265.4.C934. [DOI] [PubMed] [Google Scholar]
- 194.Ohkawa M, Clemens MG, Chaudry IH. Studies on the mechanism of beneficial effects of ATP-MgCl2 following hepatic ischemia. Am J Physiol. 1983;244:R695–R702. doi: 10.1152/ajpregu.1983.244.5.R695. [DOI] [PubMed] [Google Scholar]
- 195.Oliveira AG, Marques PE, Amaral SS, Quintão JL, Cogliati B, Dagli ML, Rogiers V, Vanhaecke T, Vinken M, Menezes GB. Purinergic signalling during sterile liver injury. Liver Int. 2013;33:353–361. doi: 10.1111/liv.12109. [DOI] [PubMed] [Google Scholar]
- 196.Orre M, Pennefather JN, Story ME, Haynes JM. The effects of P2 purinoceptor agonists on the isolated portal vein of the guinea pig. Eur J Pharmacol. 1996;316:229–236. doi: 10.1016/s0014-2999(96)00687-5. [DOI] [PubMed] [Google Scholar]
- 197.Palla M, Chen CP, Zhang Y, Li J, Ju J, Liao JC. Mechanism of flexibility control for ATP access of hepatitis C virus NS3 helicase. J Biomol Struct Dyn. 2013;31:129–141. doi: 10.1080/07391102.2012.698236. [DOI] [PubMed] [Google Scholar]
- 198.Park SW, Chen SW, Kim M, Brown KM, D'Agati VD, Lee HT. Protection against acute kidney injury via A1 adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2010;333:736–747. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN. Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 200.Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- 201.Peres A, Giovannardi S. Characteristics of the signal transduction system activated by ATP receptors in the hepatoma cell line N1S1-67. Biochim Biophys Acta. 1995;1265:33–39. doi: 10.1016/0167-4889(94)00192-h. [DOI] [PubMed] [Google Scholar]
- 202.Petcu DJ, Aldrich CE, Coates L, Taylor JM, Mason WS. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988;167:385–392. [PubMed] [Google Scholar]
- 203.Phillips JK, McLean AJ, Hill CE. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pritchard M, Mandal P, Chiang DJ, Ndum O, Nagy LE. Adenosine and adenosine signaling contribute to the anti-inflammatory effect of globular adiponectin in macrophages. Hepatology. 2011;54:1099A–1100A. [Google Scholar]
- 205.Probst I, Quentmeier A, Schweickhardt C, Unthan-Fechner K. Stimulation by insulin of glycolysis in cultured hepatocytes is attenuated by extracellular ATP and puromycin through purine-dependent inhibition of phosphofructokinase 2 activation. Eur J Biochem. 1989;182:387–393. doi: 10.1111/j.1432-1033.1989.tb14843.x. [DOI] [PubMed] [Google Scholar]
- 206.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 207.Ralevic V, Mathie RT, Alexander B, Burnstock G. Characterization of P2X- and P2Y-purinoceptors in the rabbit hepatic arterial vasculature. Br J Pharmacol. 1991;103:1108–1113. doi: 10.1111/j.1476-5381.1991.tb12308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Read R, Hansen G, Kramer J, Finch R, Li L, Vogel P. Ectonucleoside triphosphate diphosphohydrolase type 5 (Entpd5)-deficient mice develop progressive hepatopathy, hepatocellular tumors, and spermatogenic arrest. Vet Pathol Online. 2009;46:491–504. doi: 10.1354/vp.08-VP-0201-R-AM. [DOI] [PubMed] [Google Scholar]
- 209.Reinstein LJ, Lichtman SN, Currin RT, Wang J, Thurman RG, Lemasters JJ. Suppression of lipopolysaccharide-stimulated release of tumor necrosis factor by adenosine: evidence for A2 receptors on rat Kupffer cells. Hepatology. 1994;19:1445–1452. [PubMed] [Google Scholar]
- 210.Robson SC, Schuppan D. Adenosine: tipping the balance towards hepatic steatosis and fibrosis. J Hepatol. 2010;52:941–943. doi: 10.1016/j.jhep.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 212.Rodbell M, Londos C. Regulation of hepatic adenylate cyclase by glucagon, GTP, divalent cations, and adenosine. Metabolism. 1976;25:1347–1349. doi: 10.1016/s0026-0495(76)80139-4. [DOI] [PubMed] [Google Scholar]
- 213.Salter KD, Fitz JG, Roman RM. Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2000;278:G492–G500. doi: 10.1152/ajpgi.2000.278.3.G492. [DOI] [PubMed] [Google Scholar]
- 214.Samuel SS, Mani A, Tachett B, Desai M, Thevananther S. P2Y2 purinergic receptor activation is essential for endotoxin-induced acute liver injury in mice. Hepatology. 2010;52:608A. [Google Scholar]
- 215.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci U S A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schmid TC, Loffing J, Le Hir M, Kaissling B. Distribution of ecto-5′-nucleotidase in the rat liver: effect of anaemia. Histochemistry. 1994;101:439–447. doi: 10.1007/BF00269494. [DOI] [PubMed] [Google Scholar]
- 218.Schmidt W, Wolf G, Grüngreiff K, Linke K. Adenosine influences the high-affinity uptake of transmitter glutamate and aspartate under conditions of hepatic encephalopathy. Metab Brain Dis. 1993;8:73–80. doi: 10.1007/BF00996890. [DOI] [PubMed] [Google Scholar]
- 219.Schöfl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Mühlen A, Mix H, Cornberg M, Böker KH, Manns MP, Wagner S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164–G172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- 220.Schulte am Esch J, Akyildiz A, Tustas RY, Ganschow R, Schmelzle M, Krieg A, Robson SC, Topp SA, Rogiers X, Knoefel WT, Fischer L. ADP-dependent platelet function prior to and in the early course of pediatric liver transplantation and persisting thrombocytopenia are positively correlated with ischemia/reperfusion injury. Transpl Int. 2010;23:745–752. doi: 10.1111/j.1432-2277.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 221.Scott LJ, Delautier D, Meerson NR, Trugnan G, Goding JW, Maurice M. Biochemical and molecular identification of distinct forms of alkaline phosphodiesterase I expressed on the apical and basolateral plasma membrane surfaces of rat hepatocytes. Hepatology. 1997;25:995–1002. doi: 10.1002/hep.510250434. [DOI] [PubMed] [Google Scholar]
- 222.Selzner M, Selzner N, Jochum W, Graf R, Clavien PA. Increased ischemic injury in old mouse liver: an ATP-dependent mechanism. Liver Transpl. 2007;13:382–390. doi: 10.1002/lt.21100. [DOI] [PubMed] [Google Scholar]
- 223.Serhan N, Cabou C, Verdier C, Lichtenstein L, Malet N, Perret B, Laffargue M, Martinez LO. Chronic pharmacological activation of P2Y13 receptor in mice decreases HDL-cholesterol level by increasing hepatic HDL uptake and bile acid secretion. Biochim Biophys Acta. 2013;1831:719–725. doi: 10.1016/j.bbalip.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 224.Sévigny J, Robson SC, Waelkens E, Csizmadia E, Smith RN, Lemmens R. Identification and characterization of a novel hepatic canalicular ATP diphosphohydrolase. J Biol Chem. 2000;275:5640–5647. doi: 10.1074/jbc.275.8.5640. [DOI] [PubMed] [Google Scholar]
- 225.Sit KH, Bay BH, Wong KP. Extracellular ATP induces rapid cell rounding in cultured human Chang liver cells. Jpn J Physiol. 1992;42:355–362. doi: 10.2170/jjphysiol.42.355. [DOI] [PubMed] [Google Scholar]
- 226.Staddon JM, McGivan JD. Effects of ATP and adenosine addition on activity of oxoglutarate dehydrogenase and the concentration of cytoplasmic free Ca2+ in rat hepatocytes. Eur J Biochem. 1985;151:567–572. doi: 10.1111/j.1432-1033.1985.tb09141.x. [DOI] [PubMed] [Google Scholar]
- 227.Stanley JC, Markovic J, Gutknecht AM, Lozeman FJ. Stimulation of glycogenolysis in isolated hepatocytes by adenosine and one of its analogues is inhibited by caffeine. Biochem J. 1987;247:779–783. doi: 10.1042/bj2470779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stefan C, Stalmans W, Bollen M. Growth-related expression of the ectonucleotide pyrophosphatase PC-1 in rat liver. Hepatology. 1998;28:1497–1503. doi: 10.1002/hep.510280608. [DOI] [PubMed] [Google Scholar]
- 229.Stoll M, Kim YO, Bebich B, Robson SC, Schuppan D. The selective adenosine 2B receptor antagonist MRS1754 mitigates hepatic collagen deposition during fibrosis progression and induces mild fibrosis regression. Gastroenterology. 2012;142:S-974–S-975. [Google Scholar]
- 230.Sudo Y, Takaya S, Kobayashi M, Fukuda A, Harada O, Suto T, Onozuka N, Suzuki S. Assessment of graft viability using hyaluronic acid and adenosine triphosphate in orthotopic liver transplantation from non-heart-beating donors. Transplant Proc. 2000;32:2114–2115. doi: 10.1016/s0041-1345(00)01594-3. [DOI] [PubMed] [Google Scholar]
- 231.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, Khalpey Z, Berberat P, Munasinghe J, Robson SC. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal. 2011;7:427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, Junger WG, Schmelzle M, Usheva A, Tapper EB, Baffy G, Sukhatme VP, Wu Y, Robson SC. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in D39/Entpd1 null mice. Hepatology. 2012;57:205–216. doi: 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Szuster-Ciesielska A, Sztanke K, Kandefer-Szerszen M. A novel fused 1,2,4-triazine aryl derivative as antioxidant and nonselective antagonist of adenosine A(2A) receptors in ethanol-activated liver stellate cells. Chem Biol Interact. 2012;195:18–24. doi: 10.1016/j.cbi.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 235.Tahani H, Samia M, Rizk S, Habib YA, Tallaat M. Effect of repeated doses of ATP on serum protein pattern and fat content of the liver in experimental diabetes. Z Ernahrungswiss. 1977;16:120–127. doi: 10.1007/BF02021488. [DOI] [PubMed] [Google Scholar]
- 236.Takatsuki M, Eguchi S, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Impact of peripheral blood CD4+ adenosine triphosphate activity in long-term living donor transplantation under weaning of immunosupression. Liver Transpl. 2011;17:S207. [Google Scholar]
- 237.Takemura S, Kawada N, Hirohashi K, Kinoshita H, Inoue M. Nucleotide receptors in hepatic stellate cells of the rat. FEBS Lett. 1994;354:53–56. doi: 10.1016/0014-5793(94)01090-0. [DOI] [PubMed] [Google Scholar]
- 238.Takemura S, Minamiyama Y, Kawada N, Inoue M, Kubo S, Hirohashi K, Kinoshita H. Extracellular nucleotides modulate the portal circulation with generation of nitric oxide. Hepatol Res. 1998;13:29–36. [Google Scholar]
- 239.Tang LM, Wang YP, Wang K, Pu LY, Zhang F, Li XC, Kong LB, Sun BC, Li GQ, Wang XH. Protective effect of adenosine A2A receptor activation in small-for-size liver transplantation. Transpl Int. 2007;20:93–101. doi: 10.1111/j.1432-2277.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 240.Tang LM, Zhu JF, Wang F, Qian J, Zhu J, Mo Q, Lu HH, Li GQ, Wang XH. Activation of adenosine A2A receptor attenuates inflammatory response in a rat model of small-for-size liver transplantation. Transplant Proc. 2010;42:1915–1920. doi: 10.1016/j.transproceed.2010.02.084. [DOI] [PubMed] [Google Scholar]
- 241.Taylor JM, Han Z. Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS ONE. 2010;5:e15784. doi: 10.1371/journal.pone.0015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Thevananther S, Sun H, Li D, Arjunan V, Awad SS, Wyllie S, Zimmerman TL, Goss JA, Karpen SJ. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 243.Thevananther S, Sun H, Hernandez A, Awad SS, Karpen SJ. Impaired hepatocellular proliferation in P2Y2 purinergic receptor knockout mice: mitogenic role of extracellular ATP. Hepatology. 2008;44:206A. [Google Scholar]
- 244.Tinton S, Buc-Calderon P. Inhibition of protein synthesis induced by adenine nucleotides requires their metabolism into adenosine. Biochem Pharmacol. 1995;50:481–488. doi: 10.1016/0006-2952(95)00163-t. [DOI] [PubMed] [Google Scholar]
- 245.Tomura H, Okajima F, Kondo Y. Discrimination between two types of P2 purinoceptors by suramin in rat hepatocytes. Eur J Pharmacol. 1992;226:363–365. doi: 10.1016/0922-4106(92)90054-y. [DOI] [PubMed] [Google Scholar]
- 246.Varela D, Penna A, Simon F, Eguiguren AL, Leiva-Salcedo E, Cerda O, Sala F, Stutzin A. P2X4 activation modulates volume-sensitive outwardly rectifying chloride channels in rat hepatoma cells. J Biol Chem. 2010;285:7566–7574. doi: 10.1074/jbc.M109.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vaughn B, Robson S, Burnstock G. Pathological roles of purinergic signaling in the liver. J Hepatol. 2012;57:916–920. doi: 10.1016/j.jhep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vyas S, Roberti I. Lymphocyte ATP immune cell function assay in pediatric renal transplants: is it useful? Transplant Proc. 2011;43:3675–3678. doi: 10.1016/j.transproceed.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 249.Wang P, Ba ZF, Dean RE, Chaudry IH. ATP-MgCl2 restores the depressed hepatocellular function and hepatic blood flow following hemorrhage and resuscitation. J Surg Res. 1991;50:368–374. doi: 10.1016/0022-4804(91)90205-z. [DOI] [PubMed] [Google Scholar]
- 250.Wang P, Ba ZF, Morrison MH, Ayala A, Dean RE, Chaudry IH. Mechanism of the beneficial effects of ATP-MgCl2 following trauma-hemorrhage and resuscitation: downregulation of inflammatory cytokine (TNF, IL-6) release. J Surg Res. 1992;52:364–371. doi: 10.1016/0022-4804(92)90117-i. [DOI] [PubMed] [Google Scholar]
- 251.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Watanabe N, Tsukada N, Smith CR, Edwards V, Phillips MJ. Permeabilized hepatocyte couplets. Adenosine triphosphate-dependent bile canalicular contractions and a circumferential pericanalicular microfilament belt demonstrated. Lab Invest. 1991;65:203–213. [PubMed] [Google Scholar]
- 253.Wei Q, Zhang Y, Sun L, Jia X, Huai W, Yu C, Wan Z, Han L (2013) High dose of extracellular ATP switched autophagy to apoptosis in anchorage-dependent and anchorage-independent hepatoma cells. Purinergic Signal [DOI] [PMC free article] [PubMed]
- 254.Wen LT, Knowles AF. Extracellular ATP and adenosine induce cell apoptosis of human hepatoma Li-7A cells via the A3 adenosine receptor. Br J Pharmacol. 2003;140:1009–1018. doi: 10.1038/sj.bjp.0705523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wolkoff LI, Perrone RD, Grubman SA, Lee DW, Soltoff SP, Rogers LC, Beinborn M, Fang SL, Cheng SH, Jefferson DM. Purinoceptor P2U identification and function in human intrahepatic biliary epithelial cell lines. Cell Calcium. 1995;17:375–383. doi: 10.1016/0143-4160(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 256.Wu Y, Sun X, Imai M, Sultan B, Csizmadia E, Enjyoji K, Jackson S, Usheva A, Robson SC (2005) Modulation of RAS/ERK signaling by CD39/ENTPD1 during liver regeneration. Hepatology 42:Absr. 1138
- 257.Xiang HJ, Liu ZC, Wang DS, Chen Y, Yang YL, Dou KF. Adenosine A2b receptor is highly expressed in human hepatocellular carcinoma. Hepatol Res. 2006;36:56–60. doi: 10.1016/j.hepres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 258.Xiang Z, Lv J, Jiang P, Chen C, Jiang B, Burnstock G. Expression of P2X receptors on immune cells in the rat liver during postnatal development. Histochem Cell Biol. 2006;126:453–463. doi: 10.1007/s00418-006-0180-7. [DOI] [PubMed] [Google Scholar]
- 259.Xiao F, Waldrop SL, Khimji AK, Kilic G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am J Physiol Cell Physiol. 2012;303:C1034–C1044. doi: 10.1152/ajpcell.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting P450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xiong X, Garrett SH, Arizono K, Brady FO. Purinergic agonist induction of metallothionein. Proc Soc Exp Biol Med. 1992;201:59–65. doi: 10.3181/00379727-201-43480. [DOI] [PubMed] [Google Scholar]
- 262.Yang M, Chu R, Chisholm JW, Doege H, Belardinelli L, Dhalla AK. Adenosine A1 receptors do not play a major role in the regulation of lipogenic gene expression in hepatocytes. Eur J Pharmacol. 2012;683:332–339. doi: 10.1016/j.ejphar.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 263.Yang P, Chen P, Wang T, Zhan Y, Zhou M, Xia L, Cheng R, Guo Y, Zhu L, Zhang J. Loss of A1 adenosine receptor attenuates alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Toxicol Sci. 2013;131:128–138. doi: 10.1093/toxsci/kfs263. [DOI] [PubMed] [Google Scholar]
- 264.Yang P, Wang Z, Zhan Y, Wang T, Zhou M, Xia L, Yang X, Zhang J. Endogenous A1 adenosine receptor protects mice from acute ethanol-induced hepatotoxicity. Toxicology. 2013;309:100–106. doi: 10.1016/j.tox.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 265.Yasuda N, Inoue T, Horizoe T, Nagata K, Minami H, Kawata T, Hoshino Y, Harada H, Yoshikawa S, Asano O, Nagaoka J, Murakami M, Abe S, Kobayashi S, Tanaka I. Functional characterization of the adenosine receptor contributing to glycogenolysis and gluconeogenesis in rat hepatocytes. Eur J Pharmacol. 2003;459:159–166. doi: 10.1016/s0014-2999(02)02832-7. [DOI] [PubMed] [Google Scholar]
- 266.Zhong H, Yang L, Belardinelli L, Zeng D. Pro-fibrotic roles of the A2B adenosine receptor in human primary hepatic stellate cells. J Hepatol. 2007;46:S135. [Google Scholar]
- 267.Zhu X, Shiba H, Fung JJ, Wang LF, Arakawa Y, Irefin S, Demetris AJ, Kelly DM. The role of the A2a receptor agonist, regadenoson, in modulating hepatic artery flow in the porcine small-for-size liver graft. J Surg Res. 2012;174:e37–e45. doi: 10.1016/j.jss.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 268.Zipprich A, Mehal WZ, Ripoll C, Groszmann RJ. A distinct nitric oxide and adenosine A1 receptor dependent hepatic artery vasodilatatory response in the CCl-cirrhotic liver. Liver Int. 2010;30:988–994. doi: 10.1111/j.1478-3231.2010.02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zoetewij JP, van de Water B, de Bont HJ, Nagelkerke JF. The role of a purinergic P2z receptor in calcium-dependent cell killing of isolated rat hepatocytes by extracellular adenosine triphosphate. Hepatology. 1996;23:858–865. doi: 10.1002/hep.510230429. [DOI] [PubMed] [Google Scholar]