Abstract
Background:
The Doppler Tei index is an independent predictor of outcomes in adult heart failure. Tissue Doppler imaging (TDI) may be a superior method to measure the Tei index in children, as it is less affected by heart rate variability. We hypothesized that the TDI Tei index reflects severity of illness in pediatric heart failure.
Methods:
Twenty-five pediatric heart failure patients were prospectively enrolled. Listing for heart transplantation or death were the outcomes used to define severity of illness. Baseline demographics, brain natriuretic peptide (BNP), standard echocardiographic and TDI-derived parameters were analyzed to determine outcome indicators.
Results:
Ten of the 25 patients (40%) were listed for transplantation. There were no deaths. Multivariate analysis combining age, heart rate, standard echocardiographic parameters, and BNP, resulted in shortening fraction (p=0.002) as the best indicator of listing for transplantation (R2 = 0.32). A second multivariate analysis combining age, heart rate, TDI parameters and BNP, resulted in age (p = 0.03) and septal Tei index (p = 0.03) as the best predictive model (R2 = 0.36). The area under the receiver operating characteristic (ROC) curve for septal Tei index was 0.84 (0.64-0.96, 95% confidence interval) and it was comparable to the ROC curve for shortening fraction, p=0.76. Optimal values of sensitivity (100%) and specificity (60%) were obtained with septal Tei index values > 0.51.
Conclusion:
The TDI septal Tei index is an indicator of disease severity in pediatric heart failure patients and offers potential advantages in comparison with standard echocardiographic measures of left ventricular ejection.
Keywords: Tissue Doppler, Tei index, heart failure
INTRODUCTION
The assessment of heart failure severity in pediatric patients is primarily based upon symptoms. Standard echocardiographic estimates of left ventricular (LV) systolic ejection and brain natriuretic peptide (BNP) levels are also routinely used to guide clinical care. The echocardiographic evaluation of cardiac function in children is confounded by the effects of age, body composition, heart rate, loading conditions and cardiac geometry. The standard linear and volumetric echocardiographic methods used to quantify systolic function have the disadvantage of being load- and geometry-dependent[1,2]. The standard Doppler measures of diastolic dysfunction are also limited by load-dependency, heart rate and “pseudonormalization” of the mitral inflow patterns[3-5].
Tissue Doppler imaging (TDI) quantifies myocardial tissue velocities and allows a direct estimation of the longitudinal systolic and diastolic ventricular performance[6]. TDI is less load-dependent and more sensitive to diastolic function than standard Doppler methods. It also differentiates pseudonormal from normal diastolic filling patterns[3,4]. Studies in adults with heart failure have shown that TDI parameters have greater prognostic value when compared with standard echocardiographic measurements[7,8].
The myocardial performance index (Tei index) evaluates the global LV systolic and diastolic function. Its original description utilized Doppler mitral valve inflow and aortic outflow velocities[9]. It has been reported as an indicator of adverse outcomes in pediatric and adult patients with dilated cardiomyopathy (DCM)[10-12]. With TDI, the Tei index can be determined from myocardial velocities despite limited 2-dimensional acoustic windows and may be more accurate in pediatric patients as it is less altered by heart rate variability[13]. However, the TDI Tei index has not been evaluated as an outcome predictor in pediatric heart failure.
BNP is a hormonal marker of the severity of LV dysfunction. Increased wall stress induces its release from the ventricular myocardium[14]. BNP levels predict re-hospitalization and death in pediatric patients who have decompensated heart failure[15].
This prospective pilot study evaluates whether the TDI Tei index reflects disease severity in pediatric patients with heart failure.
MATERIALS AND METHODS
Study design and patient selection
This was a prospective, cohort study performed at Saint Louis Children’s Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. It was part of a study designed to assess insulin resistance in pediatric heart failure patients. Institutional review board approval was obtained at both institutions.
Twenty-five pediatric patients with congestive heart failure were prospectively enrolled. All had complete clinical, echocardiographic and laboratory evaluation at the time of entry. The echocardiograms and BNP levels were obtained within 10 days for 20 patients and within 60 days for the other 5 patients.
The inclusion criteria were: 1) age less than 18 years, 2) heart failure secondary to congenital heart disease, myocarditis or cardiomyopathy, and 3) New York Heart Association (NYHA) symptomatology classification II, III or IV.
Exclusion criteria were: 1) single ventricle physiology, 2) inability to fast overnight, 3) diabetes, medications that affect insulin metabolism, and first-degree relative with maturity onset diabetes of the young, 4) lipodystrophy, 5) mitochondrial myopathy, 6) metabolic disorders, 7) co-existing liver, lung, gastrointestinal or kidney disease, 8) obesity (Body mass index Z-score > 2).
Primary End Points: Listing for heart transplantation or death from heart failure were the primary endpoints used to define severity of illness. The cardiologists who made the decision to list for heart transplantation were blinded to the TDI measurements. At the time of study enrollment, none of the patients were actively listed for transplantation. The medical charts were reviewed at a single arbitrary time of 3.3 years after the enrollment of the first patient. All the patients were alive at the end of the study. For the patients who were not listed for transplantation, a minimum follow-up of 6 months was required for inclusion.
Echocardiography
All studies were performed using commercially available echocardiography systems. Images were taken with patients in the decubitus position. Standard 2-dimensional (2D), M-mode and Doppler studies were performed according to the American Society of Echocardiography guidelines[1]. The parameters included were: 2-D ejection fraction from the 4-chamber apical view, M-mode shortening fraction and relative wall thickness (LV posterior wall x 2/LV diastolic dimension), mitral E and A waves, E/A wave ratio and E-wave deceleration time.
Pulse wave TDI velocities were obtained in the apical 4- chamber view, at the septal and lateral mitral annulus (LV free wall). The Doppler beam was aligned as parallel as possible to the direction of the maximum annular motion. The sample volumes were less than 5mm. The lowest filter settings were used to keep the Nyquist limit between 10 and 30 cm/s. The sweep speed was at least 100 mm/sec. In each segment, the peak systolic (S’), peak early diastolic (E’) and peak late diastolic (A’) velocities were measured. The isovolumic relaxation time (IVRT’) was measured from the end of the S’ wave to the onset of the E’ wave, and the isovolumic contraction time (IVCT’) was measured from the end of the A’ wave to the onset of the S’ wave. The E/E’ ratio was also calculated. Each TDI velocity or time interval was measured on 2-3 consecutive cardiac cycles and subsequently averaged.
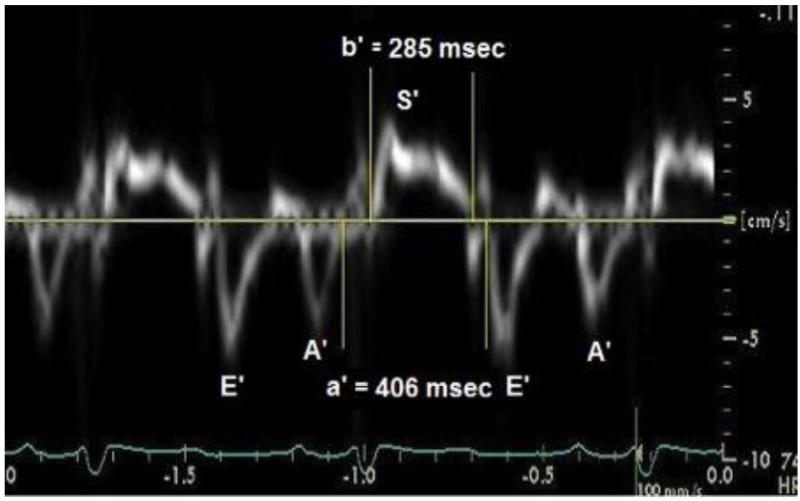
The Doppler Tei index was calculated based on the time interval between the end and the start of the transmitral flow (a component) and the LV ejection time, obtained by Doppler interrogation of the LV outflow tract (b component). Its value was the difference of a minus b, divided by b[16]. The TDI Tei index was calculated in a similar way, but the a’ component was measured from the trailing edge of the A’ wave to the leading edge of the subsequent E’ wave. The b’ component, was measured from the leading edge to the trailing edge of the S’ wave (Figure 1)[13].
Fig. 1.
Pulsed tissue Doppler imaging (TDI) tracing obtained at the septal mitral annulus of a 6 year old patient with dilated cardiomyopathy who was not listed for heart transplantation. The a’ component was measured from the trailing edge of the A’ wave to the leading edge of the E’ wave. The b’ component, was measured from the leading edge to the trailing edge of the S’ wave. TDI septal Tei index =(a’-b’)/b’=0.42.
A single experienced pediatric echocardiographer, blinded to the clinical and listing status of the patients, made all the echocardiographic measurements at the time of study entry.
Plasma BNP levels
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes. The BNP levels were measured by chemiluminescence immunoassay using ADVIA® Centaur Assay[17,18]. The normal value ranged from 0 to100 pg/ml.
Statistical analysis
As there were no deaths during the study period, the patients were divided in two groups; listed and non-listed for heart transplantation. The echocardiographic data and BNP levels at the time of enrollment were compared between the groups to identify predictors of the study outcome. Data distributions were tested for normality with the Shapiro-Wilk W test. Because most data were non-normal (Shapiro-Wilk W test, p < 0.05), medians and interquartile ranges (25th to 75th percentiles) were used as descriptive statistics. Univariate analysis to assess the predictive value of individual variables for listing for heart transplantation was done. Exact p values were calculated for the Fisher exact test to assess associations in r × c contingency tables (cross tabulations). Mann-Whitney test and t-test were used for non-normally and normally distributed continuous variables, respectively. For variables with p values ≤ 0.05, multivariate stepwise logistic regression analysis was used to build predictive models, with the minimum corrected Akaike information criterion being used to choose the best predictive model. Receiver operating characteristic (ROC) curves were created for TDI septal Tei index and shortening fraction based on the results of the univariate analysis. Regression/correlation analysis was used to assess the association between TDI septal Tei index and shortening fraction (residuals were normally distributed). The Spearman’s ρ non-parametric correlation coefficient was used to test the association between TDI septal Tei index and NYHA classification (ordinal variable).
To assess measurement reproducibility, the TDI septal Tei index and septal E/E’ ratio were re-measured in 14 patients by a junior investigator (A.S.) who was blinded to the results obtained by the senior echocardiographer (M.J.). The difference between the two averaged readings was divided by the mean, to calculate the mean percent error as an estimate of inter-observer variability[8,19]. Statistical analyses were performed with JMP Statistical Software Release 10.0.0 (SAS Institute, Inc., Cary, NC), StatXact 10 Statistical Software for Exact Nonparametric Inference (Cytel, Inc., Cambridge, MA) and MedCalc Statistics for Biomedical Research Version 12.3.0.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
A total of 25 patients were enrolled with a median age of 5.3 years (range: 1.6 to 18.4). Ten patients were listed at a median of 10.6 months (range: 0.2-12) after their enrollment. The remaining fifteen patients were followed for a median of 22.6 months (range: 6.9-39). There were no deaths. Table 1 shows the demographic and clinical characteristics of the listed and non-listed groups at the time of study entry. The patients that were listed were older and had worse clinical status based on NYHA classification and need for inotropic support.
Table 1.
Comparison of the demographic and underlying diagnosis between the listed and non-listed groups.
| Listed (N=10) | Non-listed (N=15) | p value | |||
|---|---|---|---|---|---|
| Median | (25%-75%) | Median | (25%-75%) | ||
| Age at entry (years) | 11.4 | (5.6-13.9) | 3.8 | (2.1-6.2) | 0.01 |
|
Time from diagnosis
to entry (months) |
9.5 | (1.8-133.7) | 36 | (24.8-46.2) | 0.37 |
| Height (Z-score) | −0.8 | (−1.5- −0.0) | 0 | (−1- 0.9) | 0.25 |
| Weight (Z-score) | 0 | (−1.0- 0.5) | 0 | (−0.9- 2.5) | 0.86 |
| * BSA (m2) | 1.2 | (0.8-1.6) | 0.6 | (0.5-0.8) | 0.03 |
|
Gender
(Male/Female) |
5/6 | 10/5 | 0.37 | ||
| Diagnosis: | 0.09 | ||||
| †CHD (%) | 2 | (20) | 0 | (0) | |
| ‡DCM (%) | 5 | (50) | 13 | (87) | |
| Myocarditis (%) | 3 | (30) | 2 | (13) | |
| NYHA classification | 0.0002 | ||||
| II (%) | 2 | (20) | 14 | (93) | |
| III (%) | 3 | (30) | 1 | (7) | |
| IV (%) | 5 | (50) | 0 | (0) | |
| £ N° oral medications | 3.5 | (2-4) | 2 | (2-3) | 0.67 |
| * N° IV Inotropes | 1 | (0-1) | 0 | 0 | 0.0015 |
Numbers in parentheses are the ranges expressed as 25th and 75th percentiles, except for diagnosis where the numbers in parentheses are percentages.
BSA: Body surface area.
CHD: Congenital heart disease. Atrioventricular canal with Shone’s complex (1 patient) and multiple ventricular septal defects status post pulmonary artery banding (1 patient).
DCM: Dilated cardiomyopathy. Idiopathic (11 non-transplanted patients, 4 listed patients), familial (2 non-listed patients), congenital (1 listed patient).
N° oral medications: Number of oral medications (Diuretics, angiotensin-converting-enzyme inhibitors, carvedilol and digoxin).
§N° IV Inotropes: Number of intravenous intropic agents (Milrinone and dopamine).
Table 2 contains the results of the univariate logistic regressions for heart rate, standard M-mode, 2D, Doppler measurements and BNP for the two groups. The heart rate did not differ between the groups. The listed patients had significantly worse LV systolic function, as assessed by M-mode shortening fraction and 2D ejection fraction. Similarly, they had higher Doppler Tei index values. There was no difference between groups with respect to the M-mode LV dimensions, relative wall thickness, E-wave deceleration time and BNP.
Table 2.
Comparison of heart rate, standard echocardiographic parameters and BNP levels between the listed and non-listed groups.
| Listed (N=10) | Non-listed (N=15) | p value | |||
|---|---|---|---|---|---|
| Median | (25%-75%) | Median | (25%-75%) | ||
| Heart rate | 88 | (71-106) | 88 | (80-109) | 0.56 |
| M-mode | |||||
| *LVDD Z-score | 6.3 | (5.1-8.5) | 3.4 | (2.1-6.1) | 0.16 |
| ‡RWT | 0.23 | (0.16-0.25) | 0.24 | (0.19-0.30) | 0.88 |
| Shortening fraction | 0.15 | (0.13-0.22) | 0.25 | (0.20-0.27) | 0.0012 |
| Doppler | |||||
| Mitral E (cm/s) | 92.3 | (79-97.0) | 94.0 | (78.0-114.0) | 0.89 |
| Mitral A (cm/s) | 47.0 | (31-86) | 50.0 | (41.0-77.0) | 0.94 |
| Mitral E/A | 2 | (1.3-2.9) | 1.7 | (1.2-2.2) | 0.41 |
| Doppler Tei index | 0.67 | (0.54-0.78) | 0.48 | (0.35-0.51) | 0.009 |
| £EDT (ms) | 116 | (109-153) | 108 | (99-115) | 0.17 |
| 2-Dimension | |||||
| Ejection fraction | 0.28 | (0.18-0.40) | 0.44 | (0.39-0.50) | 0.0014 |
| §BNP (pg/ml) | 684 | (160-1122) | 19 | (12-202) | 0.16 |
Numbers in parentheses are the ranges expressed as 25th and 75th percentiles.
LVDD: Left ventricular diastolic dimension.
RWT: Relative wall thickness.
EDT: E-wave deceleration time (EDT).
BNP: Brain natriuretic peptide.
The results of the univariate analysis for the TDI-derived parameters are shown in Table 3. Measured at the lateral mitral valve annulus, E’ and S’ were significantly lower while the E/E’ ratio was significantly greater in the listed group. At the septal annulus, E’ and S’ were significantly decreased, while the E/E’ ratio, septal and LV free wall Tei indices were significantly elevated in the listed group.
Table 3.
Comparison of the TDI-derived parameters between the listed and non-listed groups.
| Listed (N=10) | Non-listed (N=15) | p value | |||
|---|---|---|---|---|---|
| Median | (25%-75%) | Median | (25%-75%) | ||
| LV free wall TDI | |||||
| LV E' (cm/s) | 7.2 | (5-10.3) | 12.0 | (10.7-14.0) | 0.003 |
| LV A' (cm/s) | 3.5 | (2.5-5) | 4.5 | (3.0-6.5) | 0.37 |
| LV S' (cm/s) | 4.6 | (4.3-5) | 6.0 | (5.0-6.5) | 0.05 |
| LV E'/A' | 2.0 | (1.1-3.4) | 2.4 | (2.0-3.8) | 0.26 |
| LV E/E' | 10.9 | (8.6-29.3) | 7.9 | (6.0-10.8) | 0.03 |
| LV Tei index | 0.72 | (0.49-0.83) | 0.51 | (0.49-0.63) | 0.08 |
| Septum TDI | |||||
| Septal E' (cm/s) | 6.0 | (4.0-7.0) | 9.3 | (8.0-10.0) | 0.0005 |
| Septal A' (cm/s) | 3.5 | (2.5-4) | 5.0 | (4.0-6.0) | 0.18 |
| Septal S' (cm/s) | 4.2 | (4.0-5.0) | 6.0 | (5.0-7.0) | 0.007 |
| Septal E'/A' | 1.7 | (0.9-2.8) | 1.9 | (1.4-2.4) | 0.30 |
| Septal E/E' | 16.4 | (11.3-24.7) | 9.4 | (8.4-12.3) | 0.015 |
| Septal Tei index | 0.70 | (0.57-78) | 0.49 | (0.46-0.57) | 0.012 |
Numbers in parentheses are the ranges expressed as 25th and 75th percentiles.
LV: Left ventricle.
TDI: Tissue Doppler Imaging.
The statistically significant standard echocardiographic measurements (shortening fraction, ejection fraction, Doppler Tei index) and BNP were entered into a stepwise multivariate logistic regression, with listing for heart transplantation as the dependent variable. Age at entry and heart rate were also included, as both variables have been demonstrated to influence several echocardiographic parameters. The best predictive model (R2 = 0.32) selected by the minimum corrected Akaike information criterion contained only shortening fraction (p= 0.002).
A second stepwise multivariate logistic regression analysis was run using only the statistically significant TDI-derived measurements (LV E/E′, LV Tei index, septal E/E′, septal Tei index), BNP, age at entry and heart rate as independent variables and listing for heart transplantation as the dependent variable. Septal Tei index (p=0.03) and age (p=0.03) were the only parameters retained in the best predictive model (R2= 0.36).
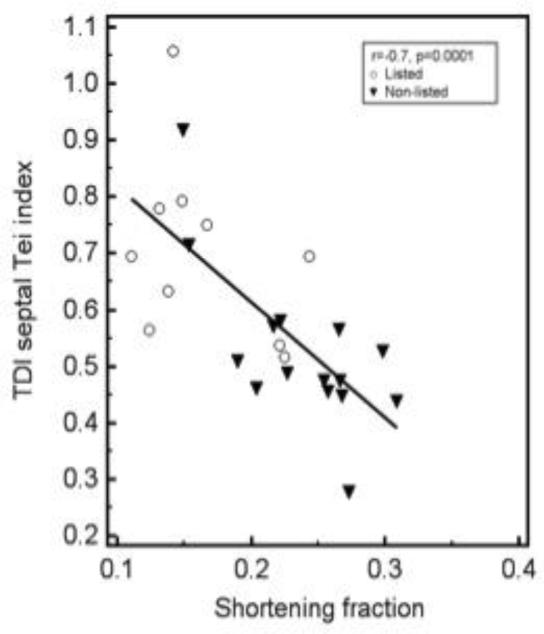
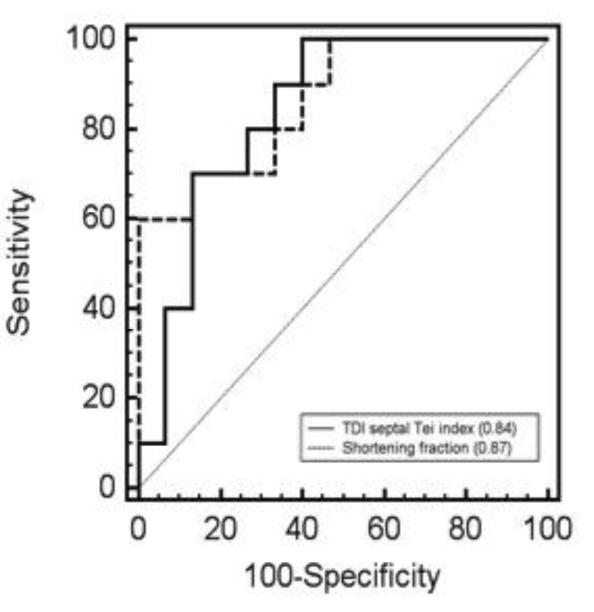
There was a significant negative correlation between shortening fraction and septal Tei index (r=− 0.7, p=0.0001, figure 2). The results of the univariate analysis of the septal Tei index and shortening fraction were used to create ROC curves (Figure 3). The areas under the curves were statistically similar; 0.84 (0.64-0.96, 95% confidence interval) for septal Tei index and 0.87 (0.67-0.97, 95% confidence interval) for shortening fraction, p=0.76. Under the assumption that false negatives and false positives have similar costs, a TDI septal Tei index > 0.51 results in optimal values for sensitivity (100%) and specificity (60%).
Fig. 2.
Scattered diagram showing the correlation between TDI septal Tei index and shortening. Different markers were used to identify the listed and non-listed patients. The correlation coefficient (r) was −0.7, p=0.0001.
Fig. 3.
Comparison of the receiver operating characteristic curves (ROC) for TDI septal Tei index and shortening fraction. The area under the ROC curves are in parentheses.
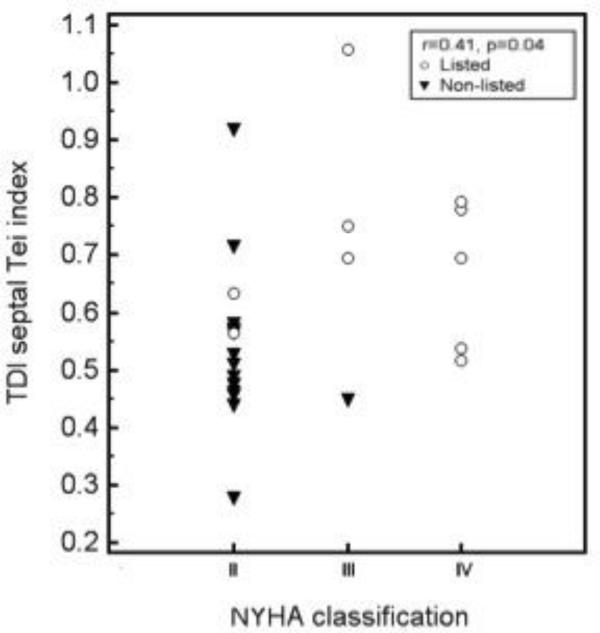
Figure 4 shows the distribution of TDI septal Tei index values in relation to the NYHA classification in this group of patients. The correlation (Spearman’s ρ) between these variables was significant (rρ =0.41, p=0.04).
Fig. 4.
Scattered diagram showing the distribution of TDI septal Tei index in relation to the NYHA classification. Different markers were used to identify the listed and non-listed patients.
The TDI measurements were reproducible between observers. The mean percent error for inter-observer variability was 2% for the septal Tei index and 3% for the septal E/E’ ratio.
DISCUSSION
This prospective pilot study demonstrates that the TDI Tei index is an indicator of the severity of heart failure in pediatric patients. This study was not powered to demonstrate the superiority of this index in comparison to standard echocardiographic measurements of left ventricular ejection. The TDI septal Tei index offers practical advantages because it can be quickly obtained and measured in patients with acoustic windows that may preclude accurate 2-dimensional ejection measurements. Furthermore, the TDI Tei index may offer advantages for longitudinal follow-up of heart failure patients with varying preload and afterload because of its relative load independence.
Previous studies in pediatric patients with DCM identified shortening fraction as an indicator of disease severity[20,21]. However, there are a number of advantages to the TDI Tei index. It reflects a combination of systolic and diastolic dysfunction, which is present in the majority of patients with heart failure[3,22-24]. In the setting of heart failure with preserved ejection fraction, it remains sensitive to diastolic dysfunction in adult and pediatric patients[19,25]. Most relevant to the pediatric population, TDI Tei indices are less sensitive to heart rate and loading conditions[13].
The routine assessment of diastolic dysfunction has been based on parameters derived from Doppler interrogation of the mitral valve, including E/A ratio and E wave deceleration time[25]. These parameters are limited by their dependence on age, heart rate and loading conditions[26,27]. In contrast, TDI-derived myocardial tissue velocities are less load-dependent than the standard echocardiographic-derived measurements[3,4]. TDI mitral E’ and E/E’ ratios are stronger predictors of adverse outcomes in adults with heart failure when compared with standard clinical and echocardiographic variables[7,8,28]. The E/E’ ratio correlates directly with elevated left ventricular filling pressures, and values above 12.5 in adults are associated with cardiac death, urgent transplantation and re-hospitalization[8,29]. In our univariate analysis, the septal and LV free wall E/E’ ratios were associated with listing for heart transplantation, but after stepwise multivariate regression of the TDI parameters, only septal Tei index remained in the predictive model.
The classic Tei index is determined from pulse Doppler interrogation of the mitral inflow and aortic outflow[9]. It is an independent predictor of worsening outcomes in adult and pediatric patients with DCM[10-12]. In a study of 161 healthy children, the Doppler Tei index decreased until 3 years of age, after which it did not change[2]. Variations in preload affect its value by less than 10% in normal subjects[30]. In a study of 289 healthy children, age, heart rate, and body size had no significant impact on the Tei index values obtained by Doppler, M-mode or TDI[13].
This study shows that TDI septal Tei index is a more sensitive indicator of disease severity than the TDI LV free wall Tei index. The enhanced sensitivity of the septal Tei index may reflect the combined dysfunction of the left and right ventricles. Furthermore, the interrogation of the septum might be more accurate than the free wall. Displacement of the free wall as a result of left ventricular dilatation may cause a suboptimal Doppler angle of incidence.
There was overlap in the BNP values between the groups, which impacted its association with disease severity in this sample. A previous study suggested that a BNP ≥300 pg/ml increased the risk of hospitalization, death and transplantation in children with chronic left ventricular dysfunction[31]. If this cutoff is used in our study population, four of the patients that were listed for heart transplant were under this cutoff, while three of the non-listed patients were above the cutoff.
The groups compared in this study were different regarding age and disease severity at the time of entry. These variables have been shown to predict adverse outcomes in children with heart failure[21]. This study was not primarily designed to determine the predictive power of the TDI Tei index. Additional limitations include a small sample size and relative short-term follow-up. Age was a predictor of disease severity in our multivariable analysis including TDI variables. A larger study with age stratification of the TDI Tei index would allow investigation of potential confounding by age. Our hypothesis was not tested in infants. This age group may have greater variability in this measurement[2].
This prospective study shows that the TDI septal Tei index is an echocardiographic marker of severity of illness in children with biventricular physiology and heart failure. Currently, the decision to list a child for heart transplantation is primarily based on symptomatology. Larger prospective, longitudinal studies are warranted to test the superiority of the TDI septal Tei index over ejection fraction, shortening fraction, and BNP in regard to the long-term follow-up of children with heart failure. Additional studies of the TDI Tei index in patients with single ventricle physiology may prove productive given the limitations of 2-dimensional imaging in this population.
ACKNOWLEDGEMENTS
Support for this project was provided by an interdisciplinary research initiative grant from the Children’s Discovery Institute, a partnership of Washington University and St. Louis Children’s Hospital. Additional support was provided by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
REFERENCES
- 1.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. quiz 576-467. [DOI] [PubMed] [Google Scholar]
- 2.Eto G, Ishii M, Tei C, Tsutsumi T, Akagi T, Kato H. Assessment of global left ventricular function in normal children and in children with dilated cardiomyopathy. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1999;12:1058–1064. doi: 10.1016/s0894-7317(99)70102-1. [DOI] [PubMed] [Google Scholar]
- 3.De Boeck BWL, Cramer M-JM, Oh JK, van der Aa RPLM, Jaarsma W. Spectral pulsed tissue doppler imaging in diastole: A tool to increase our insight in and assessment of diastolic relaxation of the left ventricle. American heart journal. 2003;146:411–419. doi: 10.1016/S0002-8703(03)00322-3. [DOI] [PubMed] [Google Scholar]
- 4.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. Journal of the American College of Cardiology. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 5.Harada K, Takahashi Y, Shiota T, Suzuki T, Tamura M, Ito T, Takada G. Effect of heart rate on left ventricular diastolic filling patterns assessed by Doppler echocardiography in normal infants. The American journal of cardiology. 1995;76:634–636. doi: 10.1016/s0002-9149(99)80175-1. [DOI] [PubMed] [Google Scholar]
- 6.Galiuto L, Ignone G, DeMaria AN. Contraction and relaxation velocities of the normal left ventricle using pulsed-wave tissue Doppler echocardiography. The American journal of cardiology. 1998;81:609–614. doi: 10.1016/s0002-9149(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Yip GWK, Wang AYM, Zhang Y, Ho PY, Tse MK, Lam PKW, Sanderson JE. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. Journal of the American College of Cardiology. 2003;41:820–826. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 8.Acil T, Wichter T, Stypmann J, Janssen F, Paul M, Grude M, Scheld HH, Breithardt G, Bruch C. Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. International journal of cardiology. 2005;103:175–181. doi: 10.1016/j.ijcard.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. Journal of cardiology. 1995;26:357–366. [PubMed] [Google Scholar]
- 10.Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. The American journal of cardiology. 1998;82:1071–1076. doi: 10.1016/s0002-9149(98)00559-1. [DOI] [PubMed] [Google Scholar]
- 11.Petko C, Minich LL, Everitt MD, Holubkov R, Shaddy RE, Tani LY. Echocardiographic evaluation of children with systemic ventricular dysfunction treated with carvedilol. Pediatric cardiology. 2010;31:780–784. doi: 10.1007/s00246-010-9700-2. [DOI] [PubMed] [Google Scholar]
- 12.Azevedo VM, Albanesi Filho FM, Santos MA, Castier MB, Tura BR, Amino JG, Da Cunha MO. Is myocardial performance index an independent echocardiographic marker of death in children with idiopathic dilated cardiomyopathy? Clinical cardiology. 2008;31:424–430. doi: 10.1002/clc.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui W, Roberson DA. Left ventricular Tei index in children: comparison of tissue Doppler imaging, pulsed wave Doppler, and M-mode echocardiography normal values. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2006;19:1438–1445. doi: 10.1016/j.echo.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Tobias JD. B-type natriuretic peptide: diagnostic and therapeutic applications in infants and children. Journal of intensive care medicine. 2011;26:183–195. doi: 10.1177/0885066610387993. [DOI] [PubMed] [Google Scholar]
- 15.Tan LH, Jefferies JL, Liang JF, Denfield SW, Dreyer WJ, Mott AR, Grenier MA, Dickerson HA, Price JF, Towbin JA, Ou CN, Chang AC. Concentrations of brain natriuretic peptide in the plasma predicts outcomes of treatment of children with decompensated heart failure admitted to the Intensive Care unit. Cardiology in the young. 2007;17:397–406. doi: 10.1017/S1047951107000601. [DOI] [PubMed] [Google Scholar]
- 16.Lakoumentas JA, Panou FK, Kotseroglou VK, Aggeli KI, Harbis PK. The Tei index of myocardial performance: applications in cardiology. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2005;46:52–58. [PubMed] [Google Scholar]
- 17.Wu AH, Packer M, Smith A, Bijou R, Fink D, Mair J, Wallentin L, Johnston N, Feldcamp CS, Haverstick DM, Ahnadi CE, Grant A, Despres N, Bluestein B, Ghani F. Analytical and clinical evaluation of the Bayer ADVIA Centaur automated B-type natriuretic peptide assay in patients with heart failure: a multisite study. Clinical chemistry. 2004;50:867–873. doi: 10.1373/clinchem.2003.026138. [DOI] [PubMed] [Google Scholar]
- 18.Elkhateeb, Bata, Jackson Meta-analysis of B-type natriuretic peptide in diagnosis of congestive heart failure in different clinical settings. Research Reports in Clinical Cardiology. 2010:11. [Google Scholar]
- 19.Sasaki N, Garcia M, Lytrivi I, Ko H, Nielsen J, Parness I, Srivastava S. Utility of Doppler tissue imaging-derived indices in identifying subclinical systolic ventricular dysfunction in children with restrictive cardiomyopathy. Pediatric cardiology. 2011;32:646–651. doi: 10.1007/s00246-011-9948-1. [DOI] [PubMed] [Google Scholar]
- 20.Pahl E, Sleeper LA, Canter CE, Hsu DT, Lu M, Webber SA, Colan SD, Kantor PF, Everitt MD, Towbin JA, Jefferies JL, Kaufman BD, Wilkinson JD, Lipshultz SE, Pediatric Cardiomyopathy Registry I. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: a report from the Pediatric Cardiomyopathy Registry. Journal of the American College of Cardiology. 2012;59:607–615. doi: 10.1016/j.jacc.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towbin JALA, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA : the journal of the American Medical Association. 2006:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez JA, Orav EJ, Wilkinson JD, Fleming LE, Lee DJ, Sleeper LA, Rusconi PG, Colan SD, Hsu DT, Canter CE, Webber SA, Cox GF, Jefferies JL, Towbin JA, Lipshultz SE, Pediatric Cardiomyopathy Registry I. Competing risks for death and cardiac transplantation in children with dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. Circulation. 2011;124:814–823. doi: 10.1161/CIRCULATIONAHA.110.973826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman W. Diastolic dysfunction and congestive heart failure. Circulation. 1990;81:III1–7. [PubMed] [Google Scholar]
- 24.Border WL, Michelfelder EC, Glascock BJ, Witt SA, Spicer RL, Beekman RH, Kimball TR. Color m-mode and doppler tissue evaluation of diastolic function in children: simultaneous correlation with invasive indices. Journal of the American Society of Echocardiography. 2003;16:988–994. doi: 10.1016/S0894-7317(03)00511-X. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Yoon HJ, Park HS, Cho YK, Nam CW, Hur SH, Kim YN, Kim KB. Usefulness of tissue Doppler imaging-myocardial performance index in the evaluation of diastolic dysfunction and heart failure with preserved ejection fraction. Clinical cardiology. 2011;34:494–499. doi: 10.1002/clc.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 27.Choong CY, Herrmann HC, Weyman AE, Fifer MA. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. Journal of the American College of Cardiology. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 28.Gardin JM, Leifer ES, Kitzman DW, Cohen G, Landzberg JS, Cotts W, Wolfel EE, Safford RE, Bess RL, Fleg JL. Usefulness of Doppler echocardiographic left ventricular diastolic function and peak exercise oxygen consumption to predict cardiovascular outcomes in patients with systolic heart failure (from HF-ACTION) The American journal of cardiology. 2012;110:862–869. doi: 10.1016/j.amjcard.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. The American journal of cardiology. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 30.Moller JEPS, Egstrup K. Effect of preload alternations on a new doppler echocardiographic index of combined systolic and diastolic performance. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1999;12:1065–1072. doi: 10.1016/s0894-7317(99)70103-3. [DOI] [PubMed] [Google Scholar]
- 31.Price JF, Thomas AK, Grenier M, Eidem BW, O’Brian Smith E, Denfield SW, Towbin JA, Dreyer WJ. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114:1063–1069. doi: 10.1161/CIRCULATIONAHA.105.608869. [DOI] [PubMed] [Google Scholar]