Abstract
Tumor suppressor microRNA-126 (miR-126) is often down-regulated in cancer cells, and its over-expression is found to inhibit cancer metastasis. To elucidate the mechanism of tumor suppression by miR-126, we analyzed the proteomic response to miR-126 over-expression in the human metastatic breast cancer cell line MDA-MB-231. To acquire quantitative, time-resolved information, we combined two complementary proteomic methods, BONCAT and SILAC. We discovered a new direct target of miR-126: CD97, a pro-metastatic G-protein-coupled receptor (GPCR) that has been reported to promote tumor cell invasion, endothelial cell migration and tumor angiogenesis. This discovery establishes a link between down-regulation of miR-126 and over-expression of CD97 in cancer, and provides new mechanistic insight into the role of miR-126 in inhibiting both cell-autonomous and non-cell-autonomous cancer progression.
microRNAs (miRNAs), small noncoding RNAs that regulate gene expression post-transcriptionally, have been predicted to control more than 60% of all protein-coding genes in mammals.1 miRNAs play essential roles in many biological processes, including angiogenesis and tumorigenesis.2, 3 Unsurprisingly, dysregulation of miRNAs has been observed in various human cancers.3 For example, miR-126, a microRNA involved in angiogenesis,4 has been reported to exhibit reduced expression in many human cancers.5 miR-126 has been defined as a metastasis suppressor because its over-expression was found to suppress metastasis of breast cancer cells to lung and bone.6 Substantial effort has been devoted to understanding the role of miR-126 in suppression of metastasis; however, the underlying mechanism of regulation remains incompletely understood.
In this study, we investigated the regulatory effects of miR-126 in human breast cancer cells by combining two complementary methods of proteomic analysis: SILAC and BONCAT. BONCAT (bioorthogonal noncanonical amino acid tagging) is used to isolate proteins synthesized within specified time intervals, and provides the temporal resolution needed to elucidate time-dependent proteomic responses to cellular stimuli.7 Moreover, BONCAT reduces sample complexity, an important limitation in protein identification by mass spectrometry (MS), by removing the pre-existing proteome.8–11 To quantify the proteomic changes observed upon over-expression of miR126, we combined BONCAT with SILAC (stable isotope labeling by amino acids in cell culture), a widely used method for MS-based quantitative proteomics.12–12 This approach led us to the discovery that CD97, a pro-metastatic adhesion G-protein coupled receptor (GPCR), is a direct target of miR-126. This result sheds new light on the role of miR-126 in tumor suppression.
Results and Discussion
Inducible Expression of miR-126 in Human Breast Cancer Cells
To probe the cellular response to miR-126 expression, we modified the human metastatic breast cancer cell line MDA-MB-231 by lentiviral transduction with the SparQ™ Cumate Switch system. The resulting cell line (designated MDA-CuO-miR) is characterized by constitutive expression of GFP as a selection marker and by cumate-inducible expression13 of a miR-126 precursor (SI Figure S1a). Because endogenous miR-126 is encoded by intron 7 of the epidermal growth-factor-like domain 7 (egfl7) gene,14 we designed the precursor sequence to include the pre-miR-126 and flanking regions from intron 7 to ensure proper transcription and processing. Cells were separated on the basis of GFP fluorescence to obtain cell populations with similar numbers of transgene integrations (SI Figure S2).
We assessed cumate-inducible expression of miR-126 by using the reverse transcriptase polymerase chain reaction (RT-PCR) (SI Figure S1b). At 2, 8, 24, and 72 h after induction with various concentrations of cumate, cells were harvested and total RNA was isolated. Expression of miR-126 increased with induction time; significant over-expression was apparent 8 h after induction. For proteomic studies, we raised the level of expression of miR-126 approximately seven-fold by inducing with 300 Hg/mL cumate for 24 h. We believe such conditions to be relevant to the biology of metastasis; the weakly metastatic human breast cancer cell line MCF-7 expresses miR-126 at levels approximately three-fold higher than MDA-MB-23115 and roughly nine-fold higher than more aggressively metastatic MDA derivatives.6
Combining BONCAT and SILAC
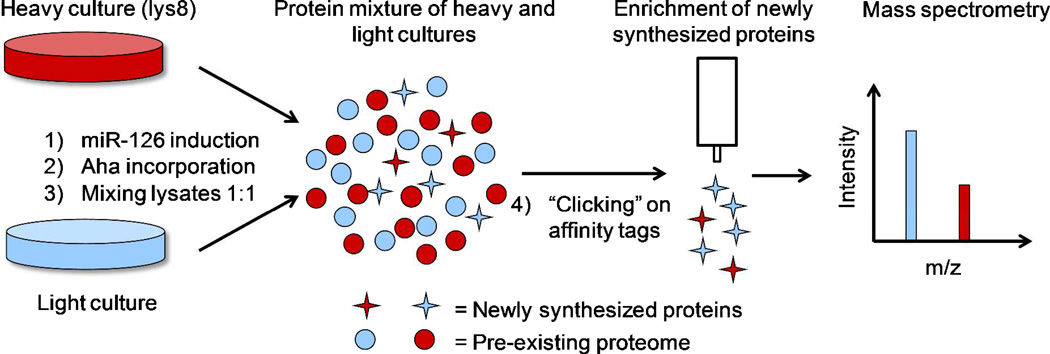
We investigated the effects of miR-126 over-expression on protein synthesis by combining BONCAT and SILAC (Figure 1). MDA-CuO-miR cells were adapted in SILAC medium containing either L-lysine (Lys) or [13C6, 15N2]L-lysine (Lys8) for five doubling times to ensure near-quantitative incorporation of Lys8 in the “heavy” culture. Expression of miR-126 was induced in the heavy culture by addition of cumate. A label-swap experiment was performed to control for any changes in protein expression that might be caused by isotope-labeling. At 24 h post induction, both cultures were treated with azidohomoalanine (Aha), a translationally-active methionine analogue that renders proteins susceptible to tagging with affinity probes via the copper-catalyzed azide-alkyne cycloaddition reaction.16 Cells were incubated with Aha for 4 h, harvested and lysed, and lysates were mixed at equal protein concentrations. The mixed lysates were treated with an acid-cleavable alkyne-biotin tag17 to enable affinity enrichment of Aha-labeled proteins for MS analysis.
Figure 1. Quantitative, time-resolved proteomic analysis of miR-126 over-expression by combining BONCAT and SILAC.
Cells were cultured in SILAC medium containing either L-lysine (light) or [13C6, 15N2]L-lysine (heavy, lys8) for five doubling times prior to induction of miR-126 expression for 24 h, followed by a 4 h Aha pulse. Lysates from the two cultures were mixed at equal protein concentrations and conjugated to an alkyne-biotin tag. Newly synthesized proteins were enriched prior to mass spectrometry analysis.
MS analysis revealed 91 proteins that exhibited significant changes of at least 20% in expression upon miR-126 induction. To address the possible effects of the inducer, control experiments were performed in which cumate was added both to MDA-CuO-miR cells and to a control cell line (MDA-CuO) transduced with empty vector. A set of 33 responsive proteins was identified by applying two criteria: the protein must 1) be quantified on the basis of six or more peptide ratio measurements, and 2) exhibit at least a 20% change in expression with consistent direction of regulation in both sets of experiments described above (SI Table S1). We imagined that this set of proteins would include some that are directly regulated by miR-126, and others that are affected indirectly. The most prevalent mechanism of regulation by human miRNAs involves translational repression as a consequence of miRNA binding in the 3’-untranslated region (UTR) of the target transcript.1 To identify potential direct targets, we cross-referenced the down-regulated proteins with the lists of predicted targets of miR-126 obtained from the following resources: MicroCosm, Target Scan, and microRNA.org, and identified one predicted target, cd97. The fact that CD97 is an established promoter of tumor metastasis18 made this observation especially intriguing. Quantitative mass spectrometry, based on 48 independent peptide measurements, indicated 26% down-regulation of CD97 upon cumate induction of miR-126 (p-value = 6.4×10−7 with the null hypothesis that CD97 is unchanged upon over-expression; SI Figure S3).
cd97 is a Direct Target of miR-126
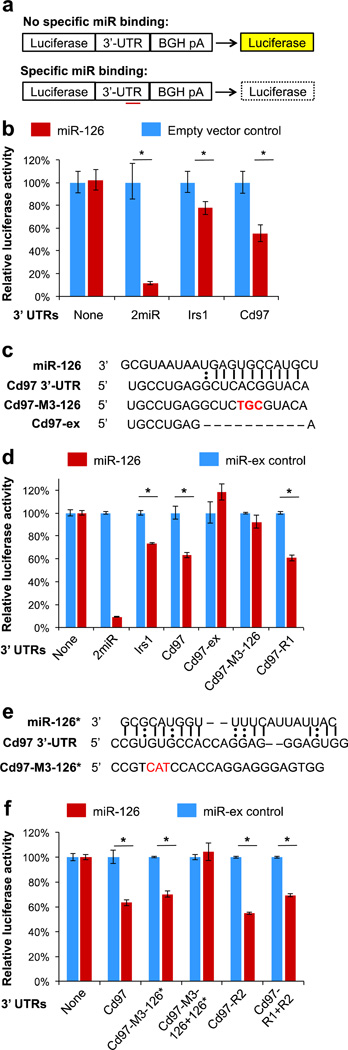
We used a luciferase reporter assay to determine whether cd97 is a direct target of miR-126 (Figure 2a). The miR-126 precursor sequence used for MS studies was cloned downstream of the CMV promoter in expression vector pcDNA™3.1(+) to generate pcDNA™3.1(+)-miR126. Next, the entire 3’-UTR of cd97 was cloned into the firefly luciferase reporter construct pMIR-REPORT™ and co-transfected with either pcDNA™3.1(+)-miR126 (miR-126) or pcDNA™3.1(+) (empty vector control) into human embryonic kidney (HEK293) cells. Positive and negative controls were used to validate the assay: pMIR-REPORT ™, which contains no 3’-UTR downstream of luciferase; 2miR, which carries two miR-126 binding sites; and IRS1, which includes the 3’-UTR of insulin receptor substrate-1 (irs1), a known direct target of miR-126.19 As expected, luciferase activity in the reporter constructs 2miR and IRS1 was reduced by over-expression of miR-126. Notably, cells bearing the reporter construct containing the cd97 3’-UTR exhibited a ~40% decrease in luciferase activity, roughly twice the extent of knockdown observed for the known target irs1 (Figure 2b). These results provide the first experimental evidence that cd97 is a direct target of miR-126.
Figure 2. miR-126 regulates cd97 by directly targeting its 3’-UTR.
(a) pMIR-REPORT™ constructs used for the luciferase assay. Specific miRNA binding in the 3’-UTR (untranslated region) should suppress reporter expression. (b) Human embryonic kidney (HEK293) cells were co-transfected with pMIR-REPORT™ carrying the indicated 3’-UTRs and pcDNA3.1™(+)-miR126 (miR-126) or pcDNA3.1™(+) (empty vector control). Relative luciferase activity for the construct bearing the cd97 3’-UTR decreased by 40% upon expression of miR-126, indicating that cd97 is a direct target of miR126. 2miR contains two miR-126 binding sites; Irs1 is a known target of miR-126. (c) Predicted miR-126 interaction sites in the cd97 3’-UTR and in cd97 3’-UTR mutants. (d) Either removing the predicted binding site or mutating three nucleotides within the binding site abolished miR-126-dependent suppression of the luciferase activity of the cd97 3’-UTR construct, confirming the miR-126 binding site within the cd97 3’-UTR. Mutations at a random site within the cd97 3’-UTR had no effect on suppression of luciferase activity. miR-ex control was taken as 100%. (e) The most favorable miR-126* interaction site in the cd97 3’-UTR as predicted by RNAhybrid algorithm, and the cd97 3’-UTR mutant used for luciferase assay. (f) Neither mutations at the predicted binding site for miR-126* nor at any other random position within the cd97 3’-UTR reversed suppression of luciferase activity, suggesting that miR-126* does not target the cd97 3’-UTR. miR-ex control was taken as 100%. P values were obtained using one-sided Student’s t-tests. *P < 0.01.
To identify possible miR-126 binding sites, we aligned the 3’-UTR of cd97 with the mature miR-126 sequence by using the microRNA target prediction algorithm RNAhybrid.20 We found extensive sequence complementarity, including a 5-nucleotide seed-matched site (Figure 2c). We created several luciferase reporter constructs carrying mutations in the cd97 3’-UTR, and evaluated the effects of miR-126 on expression (Figure 2d). Guided by the predicted binding site, we made cd97-ex by removing all 11 interacting nucleotides and cd97-M3-126 by introducing three point mutations in the predicted binding region. A control construct, cd97-R1, has three point mutations at a random site that is not predicted to bind miR-126. To address the possibility that the flanking sequences may produce false-positive results, we did not use empty vector as the control for miR-126 over-expression. Instead, we constructed pcDNA™3.1(+)-miR-ex (miR-ex control), which lacks the pre-miR-126 sequence and leaves the flanking regions intact. Compared to cells expressing the miR-ex control, cells co-transfected with miR-126 and the luciferase construct containing the cd97 3’-UTR exhibited a knockdown of approximately 40% in luciferase activity. Luciferase expression was rescued either by removing the predicted binding site or by introducing three point mutations within the predicted binding sequence (Figure 2d). In contrast, mutating three nucleotides at an arbitrary site within the cd97 3’-UTR had little effect on luciferase activity. These results confirm the binding site for miR-126 in the 3’-UTR of cd97.
In addition to miR-126, pre-miR-126 contains another known microRNA, miR-126*. Because it has been shown that miR-126 and miR-126* can suppress expression of the same target by binding to different sites in the 3’-UTR,21 we wondered whether miR-126* also targets cd97. In contrast to miR-126, the cd97 3’-UTR does not share strong sequence complementarity with the seed region of miR-126* (Figure 2e). We constructed three luciferase reporter constructs: cd97-M3-126* contains three mutations within the predicted, most favorable interacting region for miR-126*, cd97-M3-126+126* has mutations both in the binding region predicted for miR-126 and in that predicted for miR-126*, and cd97-M3-R1+R2 is a control construct with mutations at two random sites. As expected, mutations at random sites within the 3’-UTR of cd97 did not affect suppression of luciferase activity by miR-126 (Figure 2f). While mutations in the predicted binding site for miR-126* also had little effect on the luciferase signal, the additional mutation in the miR-126 binding site abolished suppression of luciferase activity. These results indicate that miR-126, not miR-126*, controls expression of cd97.
Implications for the Mechanism of Tumor Suppression by miR-126
Reduced expression of miR-126 is observed in many cancers, identifying it as a putative tumor suppressor. To better understand its role in cancer metastasis, we investigated the regulatory effects of miR-126 on protein synthesis in human breast cancer cells, and found consistent down-regulation of CD97 upon over-expression of miR-126. Further, we discovered that miR-126 suppresses expression of cd97 by binding directly to its 3’-UTR. This discovery establishes a link between two welldocumented observations in cancer biology: the down-regulation of tumor suppressor miR-1266, 19 and the over-expression of cd97.22–26
CD97 is an adhesion G-protein coupled receptor (GPCR) involved in cell adhesion and migration.27 Expression levels of CD97 were found to correlate with the in vitro migration and invasion capacity of many colorectal tumor cell lines.25 Tumor cells at the invasion front of colorectal and gastric carcinomas exhibited elevated CD97 expression as compared to other cells within the same tumor.25, 28 Furthermore, over-expression of CD97 has been shown to stimulate cell motility in vitro and to promote tumor growth in vivo.29 All of these observations suggest that CD97 plays a role in promoting tumor invasion by stimulating tumor cell migration. Similar functions have also been reported for a known target of miR-126, crk.15 Crk is a component of the focal adhesion network, and decreased Crk expression has been shown to suppress tumor cell migration.15 Furthermore, it has been reported that miR-126 inhibits gastric cancer metastasis, partially through the down-regulation of Crk.30 The discovery that miR-126 targets cd97, a pro-metastatic factor that is found to be elevated in a majority of gastric carcinomas,22 suggests that miR-126 may suppress metastasis through down-regulation of both Crk and CD97.
In addition to promoting tumor progression in a cell-autonomous manner, CD97 has also been shown to function non-cell-autonomously. CD97 stimulates the motility and invasion of endothelial cells by binding to cell surface integrins to promote angiogenesis,31 an essential process in cancer metastasis. Tumor cells expressing CD97 have been shown to induce tumors in mice with greater vessel density than tumors derived from cells that didn’t express CD97.31 In addition to CD97, three recently identified miR-126 targets also regulate endothelial recruitment. Knockdown of genes encoding insulin-like growth factor binding protein 2 (Igfbp2), c-Mer tyrosine kinase (Mertk), and phosphatidylinositol transfer protein (Pitpnc1) significantly suppressed the ability of metastatic breast cancer cells to recruit endothelial cells, leading to inhibition of metastatic colonization in vivo.32 The suppressive role of miR-126 in non-cell-autonomous cancer progression is further illustrated by the discovery that miR-126 also inhibits recruitment of mesenchymal stem cells and inflammatory monocytes by targeting stromal cell-derived factor-1 alpha (Sdf-1α).21
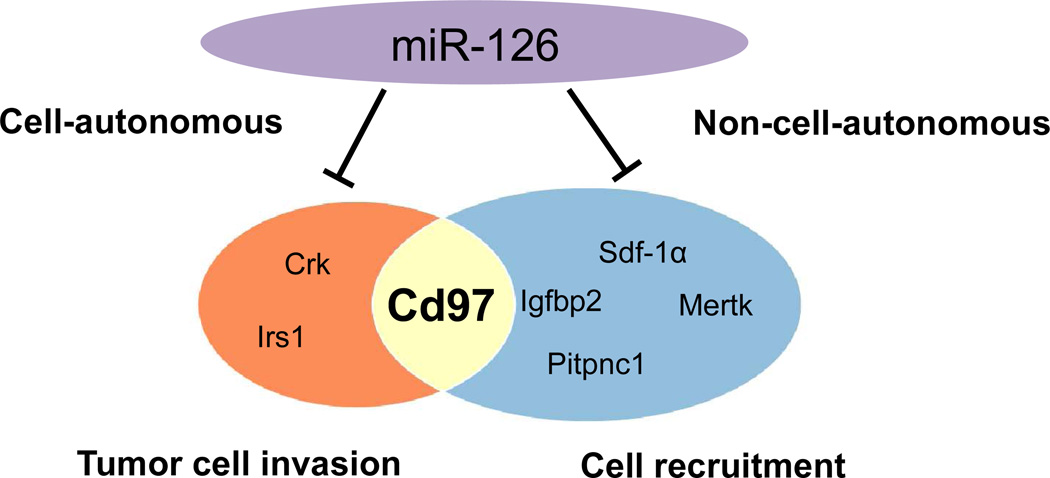
miR-126 has been shown to regulate genes involved in both cell-autonomous and non-cell-autonomous cancer progression (Figure 3). miR-126 can suppress cell-autonomous cancer progression by targeting irs1 and crk to inhibit tumor cell proliferation, migration, and invasion. miR-126 also inhibits metastasis in a non-cell autonomous manner by down-regulating Igfbp2, Mertk, Pitpnc1, and Sdf-1α to limit cell recruitment. In addition, we now know that miR-126 targets CD97, which has been shown to promote metastasis both by stimulating tumor cell invasion and by inducing angiogenesis through recruitment of endothelial cells. This discovery provides new insight into the mechanism of tumor suppression by miR-126 and identifies a potential therapeutic target for controlling both cell-autonomous and non-cell autonomous cancer progression.
Figure 3. Direct targets of miR-126 in cancer.
CD97, a pro-metastatic GPCR, has been reported to promote tumor cell invasion cell-autonomously by increasing tumor cell mobility and to induce angiogenesis non-cell-autonomously by recruiting endothelial cells. The identification of CD97 as a direct target of miR-126 sheds new light on the tumor suppressive roles of miR-126 in both cell-autonomous and non-cell-autonomous cancer progression.
Methods
Methods for creating transduced cell lines, FACS analysis, quantifying miR-126 expression, mass spectrometry analysis, and luciferase assays are provided in the Supporting Information.
Supplementary Material
Acknowledgment
We thank R. Graham, A. Moradian, and G. Smith at the Proteome Exploration Laboratory of the Beckman Institute at Caltech for assistance with proteomic studies. We thank K. Fang, K. Yuet, and L. Dooling for cloning advice, and R. Diamond for assistance with flow cytometry. This work was supported by National Institutes of Health grant NIH R01 GM062523. The Proteome Exploration Laboratory is supported by the Caltech Beckman Institute and by the Gordon and Betty Moore Foundation through Grant GBMF775.
Footnotes
Supporting Information
This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Y.Y.L. and D.A.T conceived the project and wrote the paper; M.J.S. and S.H. participated in the discussion and analysis of mass spectrometry results; D.H. and R.L. generated the MDA-Cuo-miR, MDA-Cuo, and MDA-CymR cell lines.
Competing Financial Interests Statement
The authors declare no competing financial interests.
Contributor Information
Ying Y. Lu, Email: yingl@caltech.edu, Division of Chemistry and Chemical Engineering, MC 210-41, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA.
Michael J. Sweredoski, Email: msweredo@caltech.edu, Proteome Exploration Laboratory of the Beckman Institute, MC 139-74, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA.
David Huss, Email: dhuss@usc.edu, Department of Radiology, Children’s Hospital Las Angeles, Keck School of Medicine, MS 135, University of Southern California, 4650 Sunset Boulevard, Los Angeles, CA 90027, USA.
Rusty Lansford, Email: Lansford@usc.edu, Department of Radiology, Children’s Hospital Las Angeles, Keck School of Medicine, MS 135, University of Southern California, 4650 Sunset Boulevard, Los Angeles, CA 90027, USA.
Sonja Hess, Email: shess@caltech.edu, Proteome Exploration Laboratory of the Beckman Institute, MC 139-74, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA.
David A. Tirrell, Email: tirrell@caltech.edu, Division of Chemistry and Chemical Engineering, MC 210-41, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA.
References
- 1.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meister J, Schmidt MH. miR-126 and miR-126*: new players in cancer. ScientificWorldJournal. 2010;10:2090–2100. doi: 10.1100/tsw.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT) Proc Natl Acad Sci U S A. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elschenbroich S, Ignatchenko V, Sharma P, Schmitt-Ulms G, Gramolini AO, Kislinger T. Peptide separations by on-line MudPIT compared to isoelectric focusing in an off-gel format: application to a membrane-enriched fraction from C2C12 mouse skeletal muscle cells. J Proteome Res. 2009;8:4860–4869. doi: 10.1021/pr900318k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilar M, Olivova P, Chakraborty AB, Jaworski A, Geromanos SJ, Gebler JC. Comparison of 1-D and 2-D LC MS/MS methods for proteomic analysis of human serum. Electrophoresis. 2009;30:1157–1167. doi: 10.1002/elps.200800630. [DOI] [PubMed] [Google Scholar]
- 10.Horvatovich P, Hoekman B, Govorukhina N, Bischoff R. Multidimensional chromatography coupled to mass spectrometry in analysing complex proteomics samples. J Sep Sci. 2010;33:1421–1437. doi: 10.1002/jssc.201000050. [DOI] [PubMed] [Google Scholar]
- 11.Schirle M, Heurtier MA, Kuster B. Profiling core proteomes of human cell lines by one-dimensional PAGE and liquid chromatography-tandem mass spectrometry. Mol Cell Proteomics. 2003;2:1297–1305. doi: 10.1074/mcp.M300087-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nat Protoc. 2006;1:2650–2660. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 13.Mullick A, Xu Y, Warren R, Koutroumanis M, Guilbault C, Broussau S, Malenfant F, Bourget L, Lamoureux L, Lo R, Caron AW, Pilotte A, Massie B. The cumate gene-switch: a system for regulated expression in mammalian cells. BMC Biotechnol. 2006;6:43. doi: 10.1186/1472-6750-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren G, Kang Y. A one-two punch of miR-126/126* against metastasis. Nat Cell Biol. 2013;15:231–233. doi: 10.1038/ncb2703. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Shen Y, Ichikawa H, Antes T, Goldberg GS. Regulation of miRNA expression by Src and contact normalization: effects on nonanchored cell growth and migration. Oncogene. 2009;28:4272–4283. doi: 10.1038/onc.2009.278. [DOI] [PubMed] [Google Scholar]
- 16.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: coppeRI)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Szychowski J, Mahdavi A, Hodas JJ, Bagert JD, Ngo JT, Landgraf P, Dieterich DC, Schuman EM, Tirrell DA. Cleavable biotin probes for labeling of biomolecules via azide-alkyne cycloaddition. J Am Chem Soc. 2010;132:18351–18360. doi: 10.1021/ja1083909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Trojanowicz B, Ye L, Li C, Zhang L, Li X, Li G, Zheng Y, Chen L. The invasion and metastasis promotion role of CD97 small isoform in gastric carcinoma. PLoS One. 2012;7:e39989. doi: 10.1371/journal.pone.0039989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Du YY, Lin YF, Chen YT, Yang L, Wang HJ, Ma D. The cell growth suppressor, mir-126, targets IRS-1. Biochem Biophys Res Commun. 2008;377:136–140. doi: 10.1016/j.bbrc.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 20.Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y, Li C, Chong M, Ibrahim T, Mercatali L, Amadori D, Lu X, Xie D, Li QJ, Wang XF. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–294. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aust G, Steinert M, Schutz A, Boltze C, Wahlbuhl M, Hamann J, Wobus M. CD97, but not its closely related EGF-TM7 family member EMR2, is expressed on gastric, pancreatic, and esophageal carcinomas. Am J Clin Pathol. 2002;118:699–707. doi: 10.1309/A6AB-VF3F-7M88-C0EJ. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chen L, Peng S, Chen Z, Gimm O, Finke R, Hoang-Vu C. The expression of CD97EGF and its ligand CD55 on marginal epithelium is related to higher stage and depth of tumor invasion of gastric carcinomas. Oncol Rep. 2005;14:1413–1420. [PubMed] [Google Scholar]
- 24.Aust G, Eichler W, Laue S, Lehmann I, Heldin NE, Lotz O, Scherbaum WA, Dralle H, Hoang-Vu C. CD97: a dedifferentiation marker in human thyroid carcinomas. Cancer Res. 1997;57:1798–1806. [PubMed] [Google Scholar]
- 25.Steinert M, Wobus M, Boltze C, Schutz A, Wahlbuhl M, Hamann J, Aust G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am J Pathol. 2002;161:1657–1667. doi: 10.1016/S0002-9440(10)64443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACS Chem BiolHoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, Kohrle J, Dralle H. Regulation of CD97 protein in thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:1104–1109. doi: 10.1210/jcem.84.3.5557. [DOI] [PubMed] [Google Scholar]
- 27.Kwakkenbos MJ, Kop EN, Stacey M, Matmati M, Gordon S, Lin HH, Hamann J. The EGF-TM7 family: a postgenomic view. Immunogenetics. 2004;55:655–666. doi: 10.1007/s00251-003-0625-2. [DOI] [PubMed] [Google Scholar]
- 28.Wobus M, Huber O, Hamann J, Aust G. CD97 overexpression in tumor cells at the invasion front in colorectal cancer (CC) is independently regulated of the canonical Wnt pathway. Mol Carcinog. 2006;45:881–886. doi: 10.1002/mc.20262. [DOI] [PubMed] [Google Scholar]
- 29.Galle J, Sittig D, Hanisch I, Wobus M, Wandel E, Loeffler M, Aust G. Individual cell-based models of tumor-environment interactions: Multiple effects of CD97 on tumor invasion. Am J Pathol. 2006;169:1802–1811. doi: 10.2353/ajpath.2006.060006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng R, Chen X, Yu Y, Su L, Yu B, Li J, Cai Q, Yan M, Liu B, Zhu Z. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Ward Y, Tian L, Lake R, Guedez L, Stetler-Stevenson WG, Kelly K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood. 2005;105:2836–2844. doi: 10.1182/blood-2004-07-2878. [DOI] [PubMed] [Google Scholar]
- 32.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.